Abstract
Myristoylated Alanine-Rich C Kinase Substrate (MARCKS), a substrate of protein kinase C, is a key regulatory molecule controlling mucus granule secretion by airway epithelial cells as well as directed migration of leukocytes, stem cells and fibroblasts. Phosphorylation of MARKCS may be involved in these responses. However, the functionality of MARCKS and its related phosphorylation in lung cancer malignancy have not been characterized. This study demonstrated elevated levels of MARCKS and phospho-MARCKS in highly invasive lung cancer cell lines and lung cancer specimens from non-small-cell lung cancer patients. siRNA knockdown of MARCKS expression in these highly invasive lung cancer cell lines reduced cell migration and suppressed PI3K (phosphatidylinositol 3′-kinase)/Akt phosphorylation and Slug level. Interestingly, treatment with a peptide identical to the MARCKS N-terminus sequence (the MANS peptide) impaired cell migration in vitro and also the metastatic potential of invasive lung cancer cells in vivo. Mechanistically, MANS peptide treatment resulted in a coordination of increase of E-cadherin expression, suppression of MARCKS phosphorylation and AKT/Slug signalling pathway but not the expression of total MARCKS. These results indicate a crucial role for MARCKS, specifically its phosphorylated form, in potentiating lung cancer cell migration/metastasis and suggest a potential use of MARCKS-related peptides in the treatment of lung cancer metastasis.
Keywords: MARCKS, MANS peptide, migration, metastasis, lung cancer
INTRODUCTION
Metastasis is the characteristic that distinguishes benign from malignant tumors. Metastatic spread of solid tumors makes many cancers incurable from surgical resection and in many cases resistant to treatment from radiation and/or chemotherapy. To ultimately improve cancer treatment and survival, inhibition of the metastatic process must be a major part of any drug development and targeting.1 For many years, protein kinase C (PKC) inhibition was considered a strong molecular candidate for targeting in cancer therapy due to its increased activation in many cancers.2 However, the diverse signalling effects of PKCs along with their large number of isoforms has made drug development difficult for these enzymes.3 Accordingly, one consideration is to narrow the inhibition to downstream mediators of PKCs that have more focused mechanisms of action and differential activation in cancers vs normal tissues. One substrate of PKCs, Myristoylated Alanine-Rich C Kinase Substrate (MARCKS), could be an intriguing target for inhibition in cancer therapy.
MARCKS, originally identified as a major target of PKC phosphorylation, is tethered to the plasma membrane through its myristyl group along with ionic interactions between the membrane phospholipids and the MARCKS effector domain.4 Phosphorylation of MARCKS by PKCs leads to its shuttling from the plasma membrane to the cytosol where it has roles in cell migration through actin cytoskeletal remodelling and regulation of exocytic vesicle release in secretory cells, such as neurons and airway goblet cells.5–7 In addition to PKCs, phosphorylation of MARCKS in certain tissues is regulated by other kinases such as rho or mitogen-activated protein kinases.8–10 Particularly, serine 159 in MARCKS may be an important phosphorylation site, because it can be phosphorylated by both PKCs and Rho kinases (ROCK).9,10 Activation of ROCK have been linked to metastasis and their inhibition by ROCK inhibitors has shown promise in cancer therapy.11–13 However, whether ROCK inhibitors alter metastasis through MARCKS phosphorylation is unclear.
There have been limited studies on MARCKS in cancer metastasis, but the results have been conflicting. In cholangiocarcinoma and leukemia cells, MARCKS has been associated with invasion.14,15 In contrast, MARCKS phosphorylation seems to inhibit bladder cancer invasiveness.16 Inactivating mutations of MARCKS is known to be implicated with intestinal adenocarcinoma formation.17 Interestingly, MARCKS has been reported to be not only pro-metastatic18,19 but also a growth suppressor20,21 in glioma and melanoma cells. In particular, there have thus far been no studies on MARCKS in lung cancer. In the lungs, MARCKs has been extensively studied because of its role in regulating mucous granule exocytosis. A pharmacological inhibitor of MARCKS signalling, termed MANS peptide, has been developed and tested in asthma models.22 Inhibition of MARCKS with MANS peptide can reduce airway mucus hypersecretion both in vitro and in vivo.7,22 Inflammatory leukocyte migration and degranulation have more recently also been found to be capable of being inhibited by MANS peptide.23,24 Despite these findings showing the feasibility of physiological inhibition of MARCKS, MANS peptide has not been studied on lung cancer cells to determine whether MARCKS can be a therapeutic target for inhibiting metastasis.
In this report, three aims were defined: (1) to determine whether invasive lung cancer cell’s migratory properties are due to MARCKS and its associated phosphorylation; (2) to test whether pharmacological inhibition of MARCKS by the MANS peptide can be used to inhibit metastatic properties in highly invasive lung cancer cells; and (3) to determine whether MARCKS signalling may demonstrate some crosstalk with other pathways that affect cancer cell metastasis, such as epithelial–mesenchymal transition (EMT). Collectively, our studies point to a promoting role of MARCKS, especially phospho-MARCKS, in lung cancer metastasis, and also identify MANS peptide as a novel pharmacological inhibitor of MARCKS function in lung cancer.
RESULTS
MARCKS expression and phosphorylation are associated with lung cancer cell migration
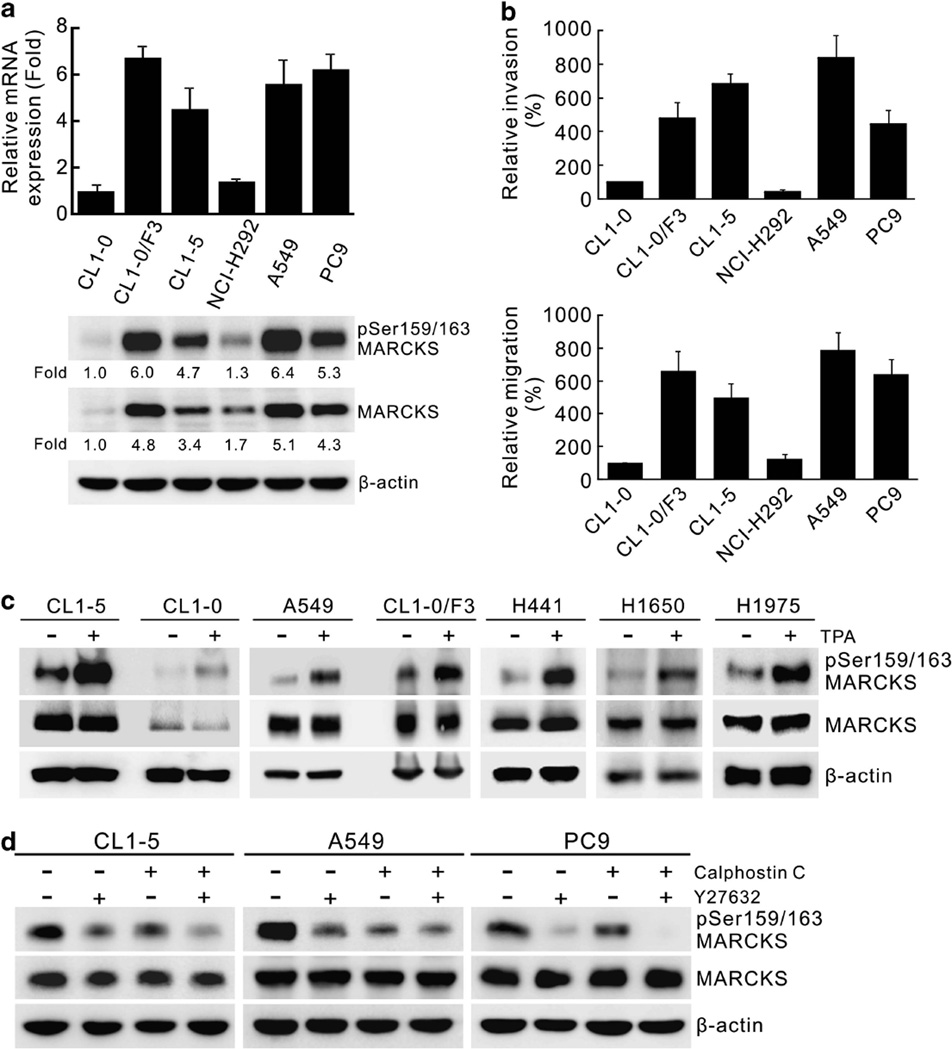
To identify novel genes associated with metastasis, we performed RNA-seq on two cell lines derived from the same patient: the non-metastatic CL1-0 parent line and the highly invasive and metastatic CL1-5 subpopulation.25 MARCKS mRNA expression appeared to be highly elevated in the CL1-5 cells vs the CL1-0 cells. To confirm this, we performed quantitative real-time reverse transcriptase–PCR (RT-PCR) on MARCKS mRNA expression in CL1-0, CL1-5 and three other invasive (CL1-0/F3, A549, PC9) and one non-invasive (NCI-H292) cell lines (Figure 1a, top). The relative invasive and migratory potential of these cells were confirmed by matrigel invasion and transwell migration assays, respectively (Figure 1b, top and bottom). MARCKS expression is low in CL1-0 and NCI-H292 (Figure 1a), which also shows low invasive and migratory potential (Figure 1b). In contrast, cells with high migratory and invasive potential, such as A549, PC9, CL1-0/F3 and CL1-5, also showed higher MARCKS expression. However, activated MARCKS requires phosphorylation at serine 159/163 for its translocation to the cytosol to interact with and remodel the actin cytoskeleton, so we also looked at the levels of phospho-MARCKS. Consistent with the above data, phospho-MARCKS levels also correlated with the cell’s invasive and migratory potential (Figure 1a, bottom). Similarly, higher levels of MARCKS phosphorylation were found in an H441/F3 motile subpopulation compared with the less-motile H441 parental cells (Supplementary Figure S1). These data strongly suggest that increased MARCKS signalling events are important determinants of increased invasive and migratory ability of cancer cell populations.
Figure 1.
Expression of MARCKS and its phosphorylated molecule are involved in lung cancer cell migration. (a) MARCKS expression in various lung cancer cell lines. Top, cells from near-confluent cultures were harvested for RNA isolation and the level of expression was quantified with real-time RT-PCR and normalized with the β-actin level. Bottom, MARCKS protein and its Ser159/163 phosphorylation were confirmed by western blotting and expressed as fold change relative to CL1-0 cells. (b) The invasion (top) and migratory (bottom) abilities of CL1-0, CL1-0/F3, CL1-5, NCI-H292, A549 and PC9 cells as analyzed by transwell assays with or without matrigel, respectively. (c) Western blot analyses of MARCKS and its Ser159/163 phosphorylated molecule in cells of various lung cancer cell lines with or without treatment with TPA. Cultures were treated with 100 ng/ml TPA or control vehicle for 20 min and harvested for protein lysates. (d) Determination of the major kinase that led to high levels of MARCKS phosphorylation in these malignant lung cancer cell lines. The cell lysates were prepared from CL1-5, A549 and PC9 cultures that were pre-treated with a ROCK inhibitor (Y27632; 10 µm) and/or a PKC inhibitor (Calphostin C; 250 nm) for 2 h. Western blot analysis was carried out to assess MARCKS and its Ser159/163 phosphorylated molecule in these cell lysates.
MARCKS is known to be a major substrate of PKCs. To confirm whether MARCKS phosphorylation is regulated by PKCs in our cell lines, we examined the level of MARCKS phosphorylation in cells treated with the phorbol ester, 12-O-tetradecanoyl phorbol-13-acetate (TPA). As shown in Figure 1c, Ser159/163 phosphorylation of MARCKS is increased upon TPA stimulation in numerous lung adenocarcinoma cell lines. However, ROCK have also been shown to phosphorylate MARCKS at serine 159, and TPA has been reported to also stimulate ROCK.25 To determine whether the high levels of basal MARCKS phosphorylation noted in the invasive cancer cell lines CL1-5, A549 and PC9, were due to PKC or ROCK activation, Calphostin C, a potent PKC inhibitor, at 250 nm, and Y-27632, a ROCK inhibitor, at 10 µm, were used to treat these cells for 2 h. Western blot analysis demonstrated that both inhibitors decreased basal phosphorylation of Ser159/163 of MARCKS in all of these cells, and there was an additive inhibition when both inhibitors were used (Figure 1d). These results suggest that the high basal Ser159/163 phosphorylation of MARCKS in invasive lung cancer cell lines is due to combined contributions from both PKC and ROCK activation. Thus, activated MARCKS may be a key mediator of the increased motility and metastatic potential of cancer cells attributed to the activation of PKCs and ROCK.2,11–13,26–28
Elevated phospho-MARCKS in lung cancer specimens
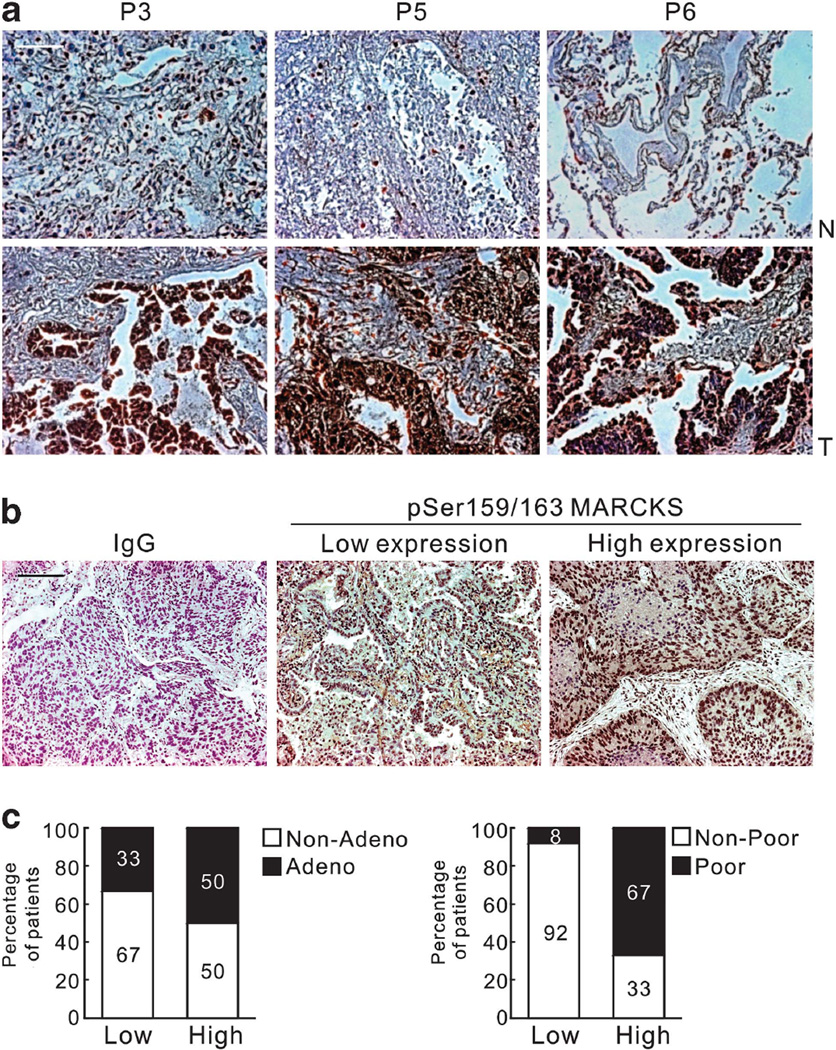
To evaluate the clinical significance of MARCKS phosphorylation, 18 primary cancer specimens of histologically confirmed non-small-cell lung cancer (NSCLC) were studied. Immunohistochemical (IHC) analysis of MARCKS phosphorylation showed that significantly higher intensity and number of cells stained in the tumor vs adjacent non-tumor tissue sections in 14/18 patients. Figure 2a shows representative pictures in 3 of the 14 patients demonstrating this differential IHC staining. In addition, we also found that higher levels of MARCKS phosphorylation occurred in adenocarcinoma vs other histological types of NSCLC at 50% vs 33%, respectively (Figures 2b and c). MARCKS phosphorylation was expressed much higher in NSCLCs with a poorly differentiated histotype (67%) and significantly correlated with differentiated status (P = 0.021; Fisher’s exact test). These observations in primary lung cancer specimens support an association between MARCKS phosphorylation and a more aggressive lung cancer histological grade.
Figure 2.
High levels of MARCKS phosphorylation are found in lung cancer specimens. (a) Higher IHC staining of Ser159/163 phosphorylated MARCKS in tumor (T) vs adjacent non-tumor areas (N) in 14/18 patients. P3, P5 and P6 are three representative stainings from these 14 patients. (b) Representative images of negative control (secondary antibody only) and positive staining of MARCKS phosphorylation at Ser159/163 by using IHC staining in tumor specimens from patients with NSCLC. The low expression (Score = 1) and high expression (Score = 2 or 3) of MARCKS phoshporylation by scoring system as described in Materials and methods. Bar = 20 µm. (c) Percentage of patients with high and low MARCKS phoshporylation with respect to adenocarcinoma (Adeno) vs non-adenocarcinoma (Non-Adeno) (Left), and poorly differentiated vs well differentiated (Right). Numbers in bars represent the percentage of patients for each condition.
MARCKS is a potential oncogene in lung cancer
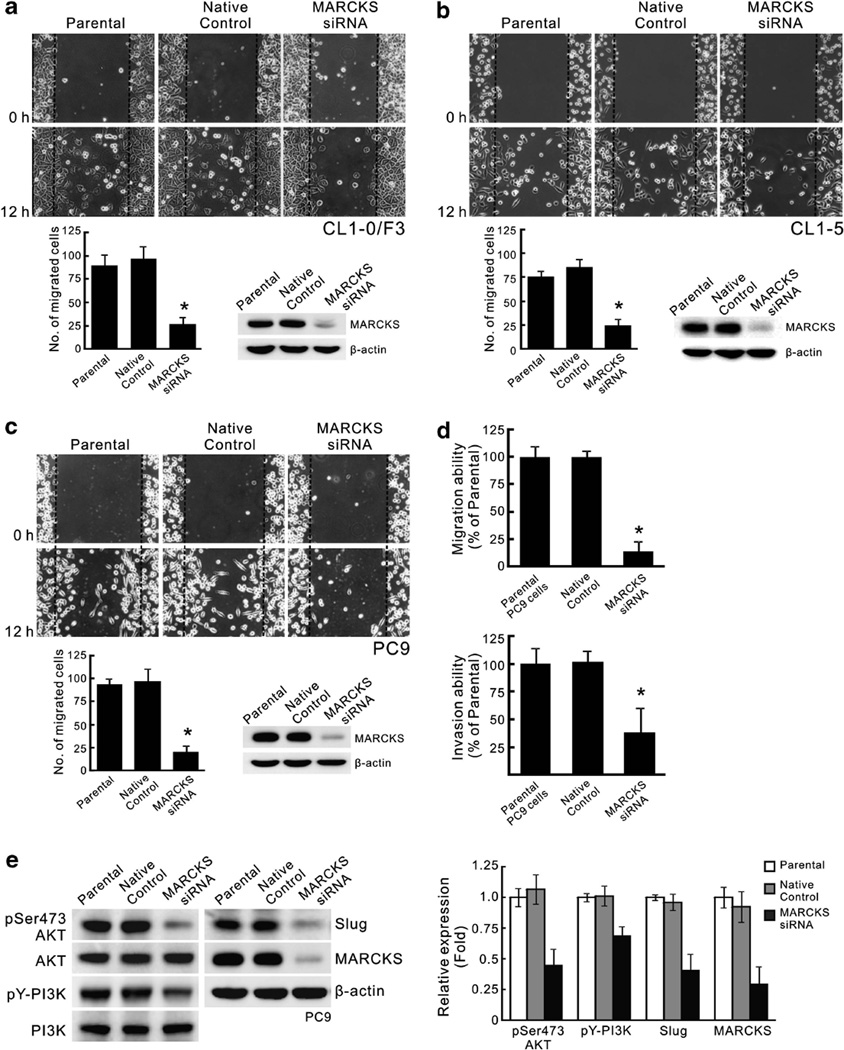
We next investigated potential mechanisms by which MARCKS could affect migration of NSCLC cells. We used the pooling of four different siRNA sequences to silence endogenous MARCKS expression in the highly MARCKS-expressing cell lines, CL1-0/F3, CL1-5 and PC9. Wound-healing assays demonstrated a 70% reduction in migration of MARCKS knockdown cells compared with native control (Figures 3a–c). Consistently, transwell migration ability of PC9 cells was also decreased after silencing MARCKS expression (Figure 3d, top). To observe whether this also reduced invasive potential, we further tested PC9 cells in matrigel invasion assays, and this also showed that MARCKS knockdown could reduce its invasion (Figure 3d, bottom). One of MARCKS’ functions is to sequester phosphatidylinositol 4,5-bisphosphate (PIP2), and PIP2 is a component of phosphatidylinositol 3′-kinase (PI3K)/AKT pathways. Moreover, it has been reported that MARCKS expression is associated with the PI3K/AKT pathway in glioma cells.20 In addition, the EMT transcriptional repressor Slug is known to be an important metastasis enhancer in lung cancer29 and acts downstream of AKT signalling.30,31 Therefore, we hypothesized that there could be a relationship between MARCKS and the AKT/Slug pathway. Our results showed that PI3K and AKT phosphorylation, as well as Slug expression levels, were reduced by siRNA silencing of MARCKS expression (Figure 3e). This indicates that MARCKS functions in PI3K/AKT signaling to alter expression of pro-metastatic genes, such as Slug.
Figure 3.
MARCKS expression is crucial for lung cancer cell migration and invasion. (a–c) siRNA knockdown of MARCKS decreases migration capability of CL1-0/F3 (a), CL1-5 (b) and PC9 (c) cells. Cells were transfected with MARCKS-specific siRNAs or control non-specific siRNA (Native control) as indicated. After 48 h of transfection, cells were subjected to scratching/wound-healing assay, and the numbers of cells migrated to the wound area were quantified at 12 h post scratching. (left; n = 3, *P < 0.05 vs Native control). Cell lysates from these transfectants were prepared and subjected to western blotting (right). (d) Transwell migration (top) and matrigel invasion (bottom) assays confirmed the importance of MARCKS expression in cell motility and invasiveness after silencing MARCKS expression of PC9 cells by MARCKS-specific or non-specific siRNA transfeciton (n = 3, *P < 0.05 vs Native control). (e) siRNA knockdown of MARCKS also diminishes pAKT/Slug pathway. PC9 cells were transfected with MARCKS-specific or non-specific siRNA (Native control), and cell lysates were prepared 72 h after transfection. Western blot analyses were carried out with specific antibodies as shown in the left panel of figure. Right, the mean results for densitometric scans of three blots from multiple experiments were expressed as fold relative to that of parental PC9 cells.
The MANS peptide inhibits migration and invasion of lung cancer cells in vitro
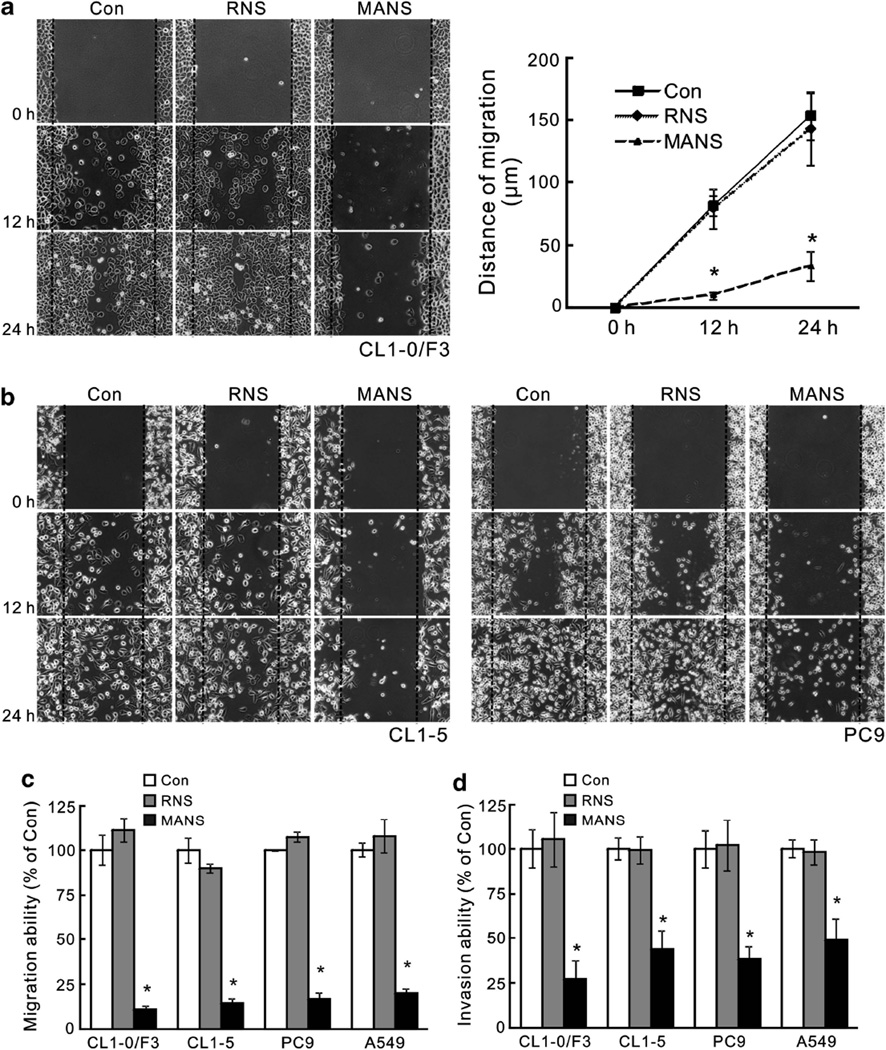
Previously, an N-terminal fragment of MARCKS termed MANS peptide has been shown to inhibit MARCKS signaling and mucin secretion in normal humn bronchial epithelial (NHBE) cells without causing cytotoxicity.7,22 We confirmed this lack of toxicity by treating lung cancer cells with the MANS peptide at concentrations up to 100 µm and noted similar viability to that observed in untreated or the control, RNS peptide-treated cells (Supplementary Figure S2). Treatment of the invasive cell lines CL1-0/F3, CL1-5 and PC9 with MANS at 100 µm impaired cell motility, as observed in wound-healing assays at 12 and 24 h post scratch (Figures 4a and b), as well as in transwell migration assays (Figure 4c). A similar suppression on cell invasive ability in matrigel-coated transwell was seen in these cells after MANS peptide treatment, whereas no suppression was seen with the control RNS peptide treatment (Figure 4d). These results demonstrate that MANS peptide has anti-motility and anti-invasive effects on lung cancer cells without causing toxicity to normal cells.
Figure 4.
MANS peptide treatment impairs migration and invasiveness of malignant lung cancer cells. (a) Scratch/wound-healing assay for evaluating the inhibitory effects of MANS peptide on cell migration. Confluent cultures of CL1-0/F3 cells were scratched and wound-healing repair was monitored microscopically examined at 12 and 24 h after the scratch and the addition of RNS or MANS peptide (100 µm). Left, phase-contrast pictures. Right, quantification of the migration distance in cultures after the scratch. Data are representative of three independent experiments, *P < 0.05 compared with untreated culture (Con). (b) MANS peptide (100 µm), but not RNS peptide, also inhibits CL1-5 (Left) and PC9 (Right) cell migration in a scratch/wound-healing assay. These results are representative of three independent experiments. (c) Transwell migration assay also demonstrated the inhibitory effects of MANS peptide on invasive cell migration. Dissociated cells of CL1-0/F3, CL1-5, PC9 and A549 cultures were plated on transwells with or without RNS or MANS peptide (100 µm); 12 h later, cells that migrated to the lower chamber were fixed, stained and counted using light microscopy. Quantification of migrated cells to the lower chamber. Data expressed as mean ± s.d. (n = 4), *P < 0.05 compared with Con (untreated cells). (d) Invasion ability of cells with or without MANS peptide (100 µm) as determined by matrigel invasion assays (n = 3, *P < 0.05 vs Con).
The MANS peptide inhibits lung cancer metastasis in vivo
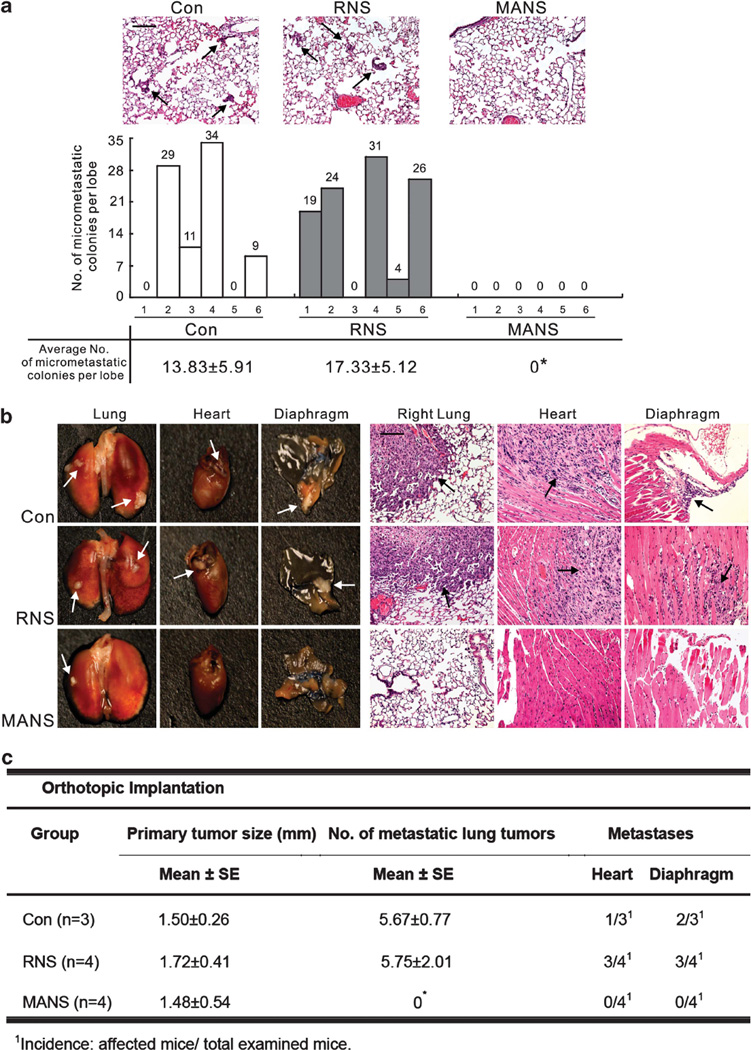
To determine whether the MANS peptide inhibits metastatic activities in vivo, a subcutaneous and orthotopic xenografts model were used. In the first model, subcutaneously grown tumors from PC9 cells were assessed for lung micrometastasis at day 32. These mice were split into three groups that received phosphate-buffered saline (PBS; control), PBS with 50 nmol RNS or MANS peptide every 2 days. As shown in Figure 5a, Hematoxylin and eosin staining revealed that the lungs of mice receiving PBS or RNS peptide treatment contained significantly more micrometastatic lesions than those treated with MANS peptide. To strengthen this finding, we used another metastasis model more specific for lung cancer by using orthotopic lung injections. In this model, after pretreatment with PBS, RNS or MANS peptide for 4 h, PC9 cells were injected percutaneously into the left lung of NOD/SCID (severe combined immunodeficiency) mice. After 7 days, these mice were given additional treatment with PBS, RNS and MANS at 50 nmol per intraperitoneal injection every 3 days. At day 25 (six injections), mice were killed, and the number of metastasized tumor nodules in the contralateral lung and other organs was counted. The MANS-treated group showed no difference in average size of the tumor at the site of injection, suggesting that MANS treatment does not affect tumorigenesis. However, a significant decrease of metastastic nodules was noted in the contralateral lung and other organs compared with the PBS- or RNS-treated groups (Figures 5b and c); in fact, treatment with the MANS peptide essentially blocked totally all metastasis from the tumor to other lung sites as well as to other organs. Similarly, we also found an inhibitory effect of MANS peptide on CL1-5 cell’s metastasis (Supplementary Figure S3). These in vivo results are consistent with the above in vitro findings and support the notion that inhibition of MARCKS function by the MANS peptide can reduce the metastatic spread of lung cancer in vivo.
Figure 5.
The suppressive effect of MANS peptide on cancer metastasis in vivo. (a) The nude mice bearing subcutaneous tumors were treated with intratumoral injections of PBS (Con), RNS or MANS peptide at the dosage of 50 nmol every 2 days. Top, representative hematoxylin and eosin (H&E)-stained sections of the lungs from nude mice (n = 6) with subcutaneous tumors. The black arrow indicates the micrometastasis of subcutaneous PC9 cells to the lung. Bottom, total numbers of lung micrometastatic colonies in individual mice were counted under the dissection scope (*P < 0.05 vs Con). (b, c) Dissociated PC9 cells were orthotopically injected to the left lobe of the mouse lung as described in Materials and methods. After a week, mice were injected intraperitoneally with 500 µl of PBS or with PBS containing either the RNS or MANS peptide (50 nmol) once every 3 days thereafter for a total of six injections up to day 25. At day 25, mice were killed, and the organs were removed and examined. (b), Gross (left) and H&E-stained (right) pictures of various organs removed from mice. The arrows indicate tumor nodules in the organ. Bar = 20 µm. (c) Quantification of the average pulmonary metastasis nodules from mice with injected cancer cells and treated with RNS or MANS peptide as described (*P < 0.05 vs Con).
MANS peptide suppresses cell spreading and increases epithelial cell–cell contacts with upregulation of E-cadherin
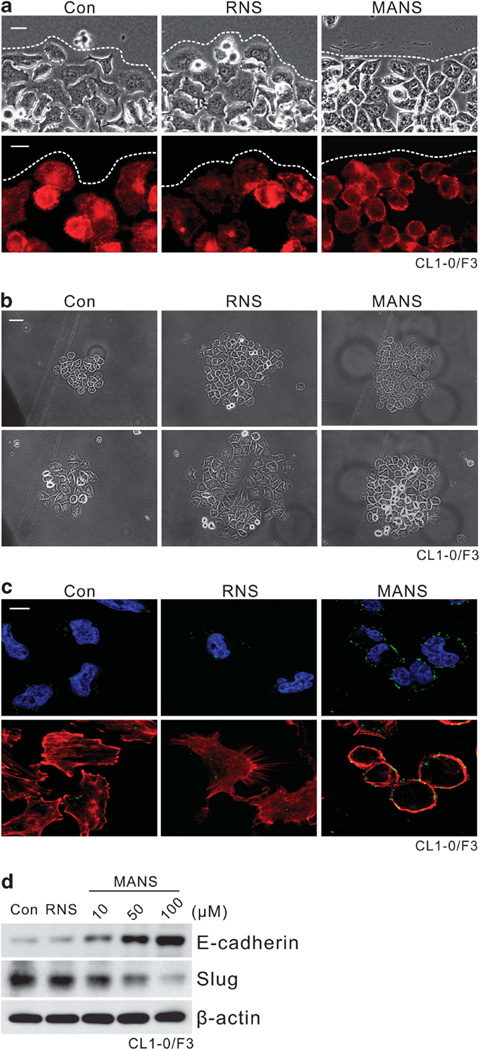
To further elucidate the MANS peptide effects, we noticed a change of fibroblastic morphology to a more epithelial cobblestone-like appearance at the edge of scratch in these lung cancer cultures after MANS peptide treatment, while no change in the appearance was observed after the control RNS peptide treatment (Figure 6a, top). To evaluate F-actin at the cell edges of the scratch, phalloidin staining was carried out. We noticed that the extent of cell spreading and surface protrusions was reduced in MANS peptide-treated cultures, suggesting an inhibition of filopodia and lamellopodia formation (Figure 6a, bottom). To determine effects on cell spreading, a cell-scattering assay was performed. Cell colonies incubated with the MANS peptide were more compact and exhibited more cell-to-cell adhesion than control or RNS peptide-treated colonies (Figure 6b). Consistent with the morphological observation, immunofluorescent staining has shown an increase of E-cadherin’s presence in the periphery of MANS-treated cells (Figure 6c). Western blot analyses of expression of E-cadherin, a major adhesion molecule in epithelial cell junctions, revealed elevated E-cadherin levels in cultures treated with the MANS peptide but not in cultures treated with the control RNS peptide, and this increase appeared to be concentration dependent (Figure 6d). Interestingly, un-parallel with this elevation, Slug level was suppressed in cells treated with MANS peptide.
Figure 6.
Enhancing cell–cell contact with increase of E-cadherin expression in cancer cells after MANS peptide treatment. Experiments were carried out with CL1-0/F3 cultures. (a) MANS peptide decreased cell spreading and increased cell–cell contacts at wound margins. Top, phase-contrast pictures 16 h after scratch and MANS peptide (100 µm) treatment; bottom, fluorescent microscopy pictures on scratch/wound-healing cultures stained with the F-actin-specific fluorescent dye, phalloidin. A white dotted line represents the wound margin. Bar = 10 µm. (b) Effects of the MANS peptide on inhibition of cell spreading. Passage CL1-0/F3 cells were seeded at low cell density (1000 cells per well of six-well plate) and allowed to grow as cell colonies. After 24 h of serum-free starvation, cells were stimulated with complete medium with RNS or MANS peptide (100 µm). Representative phase-contrast pictures were taken at 0 and 16 h after the treatment. Bar = 20 µm. (c) These cells as described in panel (b) were stained for E-cadherin and F-actin. The fluorescence of FITC-conjugated E-cadherin (green), TRITC-conjugated phalloidin (F-actin stained: red) and DAPI (nucleus counter-stained: blue) was visualized under a confocal laser-scanning microscope. Bar = 10 µm (d) Western blot analysis of E-cadherin expression in CL1-0/F3 cells after RNS or MANS peptide (10–100 µm) treatment. Sixteen hours after the treatment, cell lysates were prepared for western blot analysis as indicated.
The MANS peptide suppresses MARCKS phosphorylation and PI3K/AKT signalling
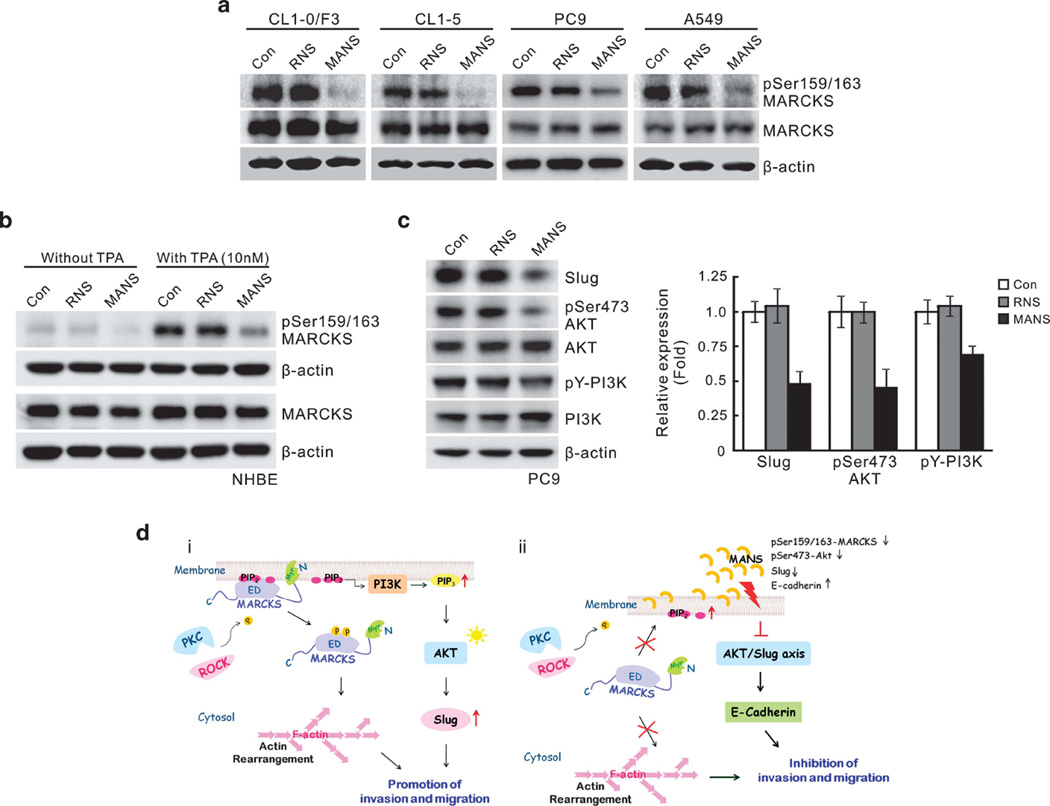
Although the MANS peptide has been shown before to inhibit MARCKS function, it is not clear whether it alters MARCKS phosphorylation in cells. To explore this possibility, lung cancer cell lines CL1-0/F3, CL1-5, PC9 and A549 with high phospho-MARCKS expression (see Figure 1) were treated with MANS or the control RNS peptide. As shown in Figure 7a, treatment with the MANS peptide suppressed levels of phospho-MARCKS (but not the total MARCKS) in these cell lines. To our surprise, a similar inhibition was seen in primary normal NHBE cells after TPA treatment, which induced phospho-MARCKS formation from a low basal level, but the enhancement was suppressed by the MANS peptide (Figure 7b). Again, there was no effect of the MANS peptide on total MARCKS levels. As PI3K/AKT signalling is involved in EMT and Slug level, we carried out western blot analyses of PI3K/AKT phosphorylation and Slug expression in these cells after treatment with MANS. As shown in Figure 7c and Supplementary Figure S4, the MANS peptide was able to suppress phosphorylation of these signalling molecules as well as decrease levels of Slug in these invasive cells. These results suggest that MARCKS, especially its phosphorylated form, has a coordinating role upstream of signaling events associated with the AKT/Slug pathway, which may, in turn, affect cell migration and lung cancer invasiveness.
Figure 7.
MANS peptide coordinately suppressed MARCKS phosphorylation and AKT/Slug pathway in lung cancer cells. (a) The effect of MANS peptide on MARCKS phosphorylation in various lung cancer cell lines. Cells were incubated in a medium containing 100 µm RNS or MANS peptide for 16 h and harvested. Cell lysates were analyzed by western blotting for MARCKS and phospho-MARCKS. (b) MANS peptide suppressed TPA-induced MARCKS phosphorylation of NHBE cells. NHBE cells were treated with or without TPA (10 nm) and also with RNS or MANS (100 µm) at the same time. After co-treatment for 16 h, NHBE cells were harvested and cell lysates prepared for western blot analyses with appropriate antibodies. (c) Western blot analysis of the repression of pAKT/Slug pathway in PC9 cells after MANS peptide (100 µm) treatment. The mean results for densitometric scans of three blots from three separate experiments are shown in right panel. (d) Proposed hypothetical models for MARKS protein-mediated lung cancer cell invasion/migration, under un-treated condition (i), and also the mechanism of MANS peptide-mediated coordinative suppression of MARCKS phosphorylation and pAKT/Slug pathway (ii).
DISCUSSION
The results of this study have identified a novel mechanism to inhibit lung cancer cell migration in vitro and metastasis in vivo. Initially, we found an elevation in expression of MARCKS protein and its phosphorylation in several lung cancer cell lines with high metastatic potential, compared with less invasive lung cancer cell lines and normal NHBE cells. Significantly elevated phospho-MARCKS was also demonstrated IHC in tissue sections from lung cancer patients but not in adjacent non-cancer areas. Using the MANS peptide to pharmacologically inhibit MARCKS phosphorylation and function, lung cancer cell lines showed reduced migration and in vivo metastasis. This effect could be further verified to be due to MARCKS, as siRNA knockdown of MARCKS also reduced migration characteristics of lung cancer cells. In addition, we have identified a potential additional mechanism for MARCKS signalling where it is associated with PI3 kinase/AKT pathways to alter epithelial characteristics in invasive lung cancer cells. Collectively, these findings suggest that the MANS peptide inhibits MARCKS phosphorylation, which then results in reduced signalling to the AKT/Slug axis, which, in turn, ultimately reduces migration, invasiveness and metastasis of lung cancer cells (Figure 7d).
MARCKS has been reported to have an important role in several lung diseases.7,22–24 Here, we reveal a novel function for MARCKS in possibly potentiating human lung cancer cell malignancy. First, inhibitor studies demonstrated that PKC and/or ROCK activation contribute to an increase of MARCKS phosphorylation in invasive lung cancer cells, suggesting that at least Ser159 phosphorylation of MARCKS could be a convergence between PKC and ROCK signalling in lung cancer. The other phosphorylation site on MARCKS at Ser163, which is phosphorylated only by PKC, may not be involved here as it would not be phosphorylated by ROCK.9–10 Indeed, studies of lung cancer specimens from NSCLC patients confirmed the clinical significance of MARCKS phosphorylation (phospho-Ser159), and, importantly, we showed that reduced invasiveness of lung cancer cells appeared due to the MANS peptide’s ability to block phosphorylation of the MARCKS at the Ser159 site. Phosphorylation at this site is necessary for MARCKS to shift from its location on the inner face of the plasma membrane into the cytosol, where it can alter F-actin crosslinking that ultimately can affect cell spreading and migration.5 This loss of phosphorylation of MARCKS induced by the MANS peptide was correlated with increased cell-to-cell contact along with increased expression of E-cadherin and reduced expression of Slug. This suggests that inhibiting MARCKS phosphorylation can prevent the loss of cell-to-cell adhesion and decrease in migratory potential seen in many invasive cancers that ultimately lead to less metastasis. In addition, MANS peptide also reduced PI3K and AKT phosphorylation, suggesting that MARCKS is functioned in this pathway that may ultimately lead to downstream changes in Slug and E-cadherin expression levels to alter EMT characteristics of cancer cells. One study in glioma cells shows that MARCKS inhibits AKT activation,20 but this is likely due to intrinsic differences in the tissue origin and phenotype of the cancer cells. For instance, AKT has been reported to be highly expressed in the lung but less expressed in the brain.32 Basing on the findings that loss of phosphatase and tensin homolog and overexpression of AKT frequently occur in lung cancer cells,32,33 we considered that PIP3 pool may be favorable to bind to AKT and then promotes AKT activation in lung cancer after sequestration of PIP2 by MARCKS. Conversely, the binding of PIP3 to phosphatase and tensin homolog may be increased to produce PIP2 in glioma cells, leading to inactivation of AKT. Further experiments are needed to discern the difference.
As MARCKS localized to the plasma membrane is able to sequester PIP2 via electrostatic interactions,34 it is possible that MARCKS may be a potential regulator of the switch between PIP2 and PIP3, depending on MARCKS associations within or outside of the membrane. MARCKS tethers to the plasma membrane via its N-terminal myristyl group and also affects lipid rafts in the membrane.35–37 Based on the theory proposed by Kalwa and Michel,37 it is reasonable that MARCKS recruits PIP2 and facilitates accumulation of PIP2 levels in lipid raft. Following phosphorylation, MARCKS detaches from the membrane and releases PIP2 pool, which becomes available to PI3K to produce PIP3. Such a two-step contribution of MARCKS may explain why the availability of PIP2 is adversely affected both by siRNA knockdown of MARCKS (Figure 3) and by MANS-mediated inhibition of MARCKS phosphorylation (Figure 7). Though MANS peptide has been reported to inhibit MARCKS membrane targeting,24 there is a theoretical possibility that MANS may act not just by excluding MARCKS from certain protein complexes but also by sequestering the proteins that naturally interact with MARCKS. Consequently, loss of the functions of these proteins may contribute to suppression of cancer metastasis. Using MANS peptide to interfere protein complex formations or to antagonize the functions of MARCKS-associated proteins may provide new insights into anticancer therapy.
Cancer metastasis is a complex process, and many steps are involved in the spreading of the cells from the primary tumor to distant sites. This multiple-step process is, for the most part, associated with increased cell spreading and motility, which depend critically on dynamic reorganization of the actin cytoskeleton.1,38,39 MARCKS has long been known to participate in cell migration and regulate actin cytoskeleton remodelling.24,36,40,41 Recently, the role of MARCKS in promoting colon cancer metastasis has been confirmed by Rombouts et al.,42 so it is logical that its inhibition may alter cancer cell migration and metastasis. Indeed, our results here show that MANS peptide could not only repress cancer cell migration/metastasis but also suppress the expression of the EMT regulator, Slug, which is known to be a downstream target of AKT30,31,43 and an important prognostic indicator in lung cancer.44 Correspondingly, increase in the epithelial marker E-cadherin occurs during MARCKS inhibition. These correlated results may suggest an interactively direct or a parallel consequence of MARCKS inhibition by MANS peptide on migration/invasion and PI3K/AKT pathway. Altogether, these results suggest that MANS’ effects may be more complicated than direct actin cytoskeletal remodelling and may include regulating other signalling pathways such as PI3K/AKT and EMT, which also have roles in cancer metastasis. In addition to invasion and migration, metastasis is known to be influenced by complex number of factors, including angiogenesis, inflammatory microenvironment, immune evasion, survival in the circulation and metastatic colonization. It is not clear whether these factors could also be affected by MANS peptide, and future studies should be able to delineate if MANS peptide can alter any of these other properties of cancer metastasis.
Although MANS peptide has been used before pharmacologically to inhibit MARCKS in the airways to reduce mucous hypersecretion in animal models of inflammatory lung disease,22 this is, to our knowledge, the first report where this peptide could be used as an effective treatment in lung cancer by preventing cancer cell migration and metastasis. Previously, the MANS peptide was reported to be efficacious in its inhibition of mucus secretion via its inhibition of MARCKS’ association with membranes of intracellular mucus granules.24 We show here that another novel pharmacological function of MANS peptide is its ability to reduce the phosphorylation of MARCKS at serine 159. Although further studies will be needed to determine the mechanism(s) of how the MANS peptide can inhibit serine 159 phosphorylation, we hypothesize that the MANS peptide, which may act as a competitive inhibitor, may repress the binding of endogenous PKC and/or ROCK with MARCKS and prevent its phosphorylation. Consistent with this notion, we have shown that MANS peptide can also suppress TPA-induced phospho-MARCKS expression in primary NHBE cells (Figure 7b). These observations indicate that the MANS peptide could specifically target cells with high level of MARCKS phosphorylation, including malignant lung cancer cells.
Cancer treatments from conventional chemotherapy drugs have always been problematic because of their toxicity to normal cells. Because our results show significant differences in MARCKS phosphorylation between normal vs cancer cells, the fact that the MANS peptide can inhibit MARCKS phosphorylation suggest that it may have a more selective action on cancer cells while sparing normal cells. Indeed, the narrower spectrum of its inhibitory activity on cellular migration, rather than activating apoptotic pathways, suggest it will have reduced cytotoxicity compared with PKC inhibitors or conventional chemotherapeutic drugs.45,46 The relatively good safety profile demonstrated here in our in vitro NHBE cells as well as in our in vivo mouse studies suggests that MANS or a similar peptide that blocks MARCKS phosphorylation could be further tested in clinical trials as an adjuvant to surgery, chemotherapy and/or radiotherapy for lung cancer.
In summary, our studies here show a novel role for MARCKS, specifically its phosphorylation (Ser159), in potentiating lung cancer cell migration and malignancy. Importantly, we also reveal that a truncated N-terminal portion of MARCKS, termed the MANS peptide, antagonizes the functions of MARCKS, apparently via reducing phosphorylation of MARCKS and of the AKT/Slug axis. The results here form the basis for future studies that can elucidate in more detail exactly how MARCKS is involved in cancer cell migration and whether its inhibition by MANS and MANS-related peptides can be used in clinical settings to treat lung cancer.
MATERIALS AND METHODS
Materials
All reagents and antibodies used in this study are described in the Supplementary Information and Supplementary Methods.
Cell culture and transfection
The CL1-0 and CL1-5 were established as previously described.47 The CL1-0/F3 and H441/F3, respectively, are derived from CL1-0 and H441 through progressive isolation and expansion of cells that pass through an 8-µm Transwell membrane (without overlay of matrigel) similar to previous methods.25 The cell lines PC9, A549, and NCI-H292 cells were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA). Cells were cultured in RPMI-1640 medium with 10% fetal bovine serum and 1% penicillin-streptomycin at 37 °C in a humidified atmosphere of 5% CO2. For siRNAs transfection, ON-TARGETplus MARCKS siRNA and scrambled siRNA sequences (Thermo Scientific, Pittsburgh, PA, USA) were transfected using DharmaFECT (Thermo Scientific Inc.) according to the manufacturer’s protocol.
Quantitative real-time PCR
The mRNA expression level of MARCKS was detected by real-time RT-PCR. The MARCKS primers were as follows: forward primer 5′-TTGTTGAAGAAGCCAGCATGGGTG-3′ and reverse primer 5′-TTACCTTCACGTGGCCATTCTCCT-3′. We used the housekeeping gene β-actin (ACTB) as the reference gene in real-time RT-PCR assay. The relative expression level of MARCKS compared with that of ACTB was defined as −ΔCT = −(CTtarget − CTACTB). The target/ACTB mRNA ratio was calculated as 2−ΔCT × K, where K is a constant.
Patient tumor specimens and IHC staining
Lung tumor tissue and adjacent normal tissue specimens were obtained from patients with histologically confirmed NSCLC who underwent surgical resection at the UC Davis Medical Center. None of the patients had received pre-operative adjuvant chemotherapy or radiation therapy. This investigation was approved by the Institutional Review Board of the UC Davis Health System. Written informed consent was obtained from all the patients. The post-surgical pathological stage of each tumor was classified according to the International TNM (Tumor, Node, Metastasis) Classification.48 Formalin-fixed and paraffin-embedded specimens were used, and the level of phospho-MARCKS was analyzed by IHC staining as described previously.49 Detailed information on staining scoring is included in the Supplementary Methods. These results were also reviewed and scored independently by two pathologists.
Scratch wound-healing assay
Cells were seeded to six-well tissue culture dishes and grown to confluence. Each confluent monolayer was then wounded linearly using a pipette tip and washed three times with PBS. Thereafter, cell morphology and migration were observed and photographed at regular intervals for 12 h. The distance of cells migrating into the cell-free zone was acquired under a light microscope and analyzed by Image-Pro Plus software (version 5.1; Media Cybernetics, Inc., Bethesda, MD, USA). The distances of cell migration were calculated by subtracting the distance between the lesions’ edges at indicated time from the distance measured at 0 h.
Transwell migration and invasion assays
An in vitro cell migration assay was performed as previously described25 using transwell chambers (8-µm pore size; Costar, Cambridge, MA, USA). Briefly, 5 × 103 cells were seeded on top of the polycarbonate filters, and 0.5 ml of growth medium at a RNS or MANS peptide (100 µm) was added to both the upper and lower wells. After incubation for 12 h, filters were swabbed with a cotton swab, fixed with methanol and then stained with Giemsa solution (Sigma-Aldrich, St Louis, MO, USA). For the invasion assays, filters were coated with matrigel (Becton Dickinson, Franklin Lakes, NJ, USA), and 2 × 104 cells were seeded onto the matrigel and incubated for 20 h. The cells attached to the lower surface of the filter were counted under a light microscope (× 10 magnification).
Immunoblotting and immunofluorescent staining
Western blot analyses and the preparations of whole-cell lysates have been previously described.18 For whole-cell lysates, cells were lysed in a lysis buffer (50 mm Tris-HCl (pH 7.4), 1% Triton X-100, 10% glycerol, 150 mm NaCl, 1 mm EDTA, 20 µg/ml leupeptin, 1 mm phenylmethanesulfonylfluoride and 20 µg/ml aprotinin) and separated by SDS–PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis). Immunoblotting was conducted with appropriate antibodies followed by chemiluminescent detection. For immunofluorescent staining, cells cultured on 12-mm glass coverslips were fixed for 15 min in PBS containing 4% paraformaldehyde and 2% sucrose and then permeabilized in PBS containing 0.3% Triton X-100 for 2 min. Coverslips were reacted with primary antibody against E-cadherin and FITC (fluorescein isothiocyanate)-labeled anti-mouse secondary antibody. F-actin was stained with TRITC (tetramethyl rhodamine isothiocyanate)-conjugated phalloidin, and nuclei were demarcated with DAPI (4,6-diamidino-2-phenylindole) staining. The cells were mounted onto slides and visualized using fluorescence microscopy (model Axiovert 100; Carl Zeiss, Oberkochen, Germany) or a Zeiss LSM510 laser-scanning confocal microscope image system.
In vivo subcutaneous and orthotopic implantation
Six-week-old nude mice and NOD SCID mice (supplied by Charles River Laboratories, San Diego, CA, USA) were housed (four mice per cage) and fed autoclaved food ad libitum. Detailed information on subcutaneous and orthotopic implantation of tumors is included in the Supplementary Methods. Mouse experiments were approved by the Institutional Animal Care and Use Committee of UC Davis.
Statistical analysis
Data are presented as the mean ± s.d. for at least three independent experiments. The quantitative in vitro and in vivo data were analyzed using the Student’s t-test. The difference in patient characteristics between the high-expression and the low-expression groups was analyzed using the Fisher’s exact test. All analyses were performed using the SPSS software (v10.0; SPSS, Inc., Chicago, IL, USA). All statistical tests were two-sided, and P values < 0.05 were considered statistically significant.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported, in parts, by Grants from the NIH-NHLBI (HL077902, HL096373, and HL36982).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies this paper on the Oncogene website (http://www.nature.com/onc)
REFERENCES
- 1.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teicher BA. Protein kinase C as a therapeutic target. Clin Cancer Res. 2006;12:5336–5345. doi: 10.1158/1078-0432.CCR-06-0945. [DOI] [PubMed] [Google Scholar]
- 3.Roffey J, Rosse C, Linch M, Hibbert A, McDonald NQ, Parker PJ. Protein kinase C intervention: the state of play. Curr Opin Cell Biol. 2009;21:268–279. doi: 10.1016/j.ceb.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 4.Harlan DM, Graff JM, Stumpo DJ, Eddy RL, Jr, Shows TB, Boyle JM, et al. The human myristoylated alanine-rich C kinase substrate (MARCKS) gene (MACS). Analysis of its gene product, promoter, and chromosomal localization. J Biol Chem. 1991;266:14399–14405. [PubMed] [Google Scholar]
- 5.Thelen M, Rosen A, Nairn AC, Aderem A. Regulation by phosphorylation of reversible association of a myristoylated protein kinase C substrate with the plasma membrane. Nature. 1991;351:320–322. doi: 10.1038/351320a0. [DOI] [PubMed] [Google Scholar]
- 6.Nairn AC, Aderem A. Calmodulin and protein kinase C cross-talk: the MARCKS protein is an actin filament and plasma membrane cross-linking protein regulated by protein kinase C phosphorylation and by calmodulin. Ciba Found Symp. 1992;164:145–154. doi: 10.1002/9780470514207.ch10. discussion 154–161. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Martin LD, Spizz G, Adler KB. MARCKS protein is a key molecule regulating mucin secretion by human airway epithelial cells in vitro. J Biol Chem. 2001;276:40982–40990. doi: 10.1074/jbc.M105614200. [DOI] [PubMed] [Google Scholar]
- 8.Ohmitsu M, Fukunaga K, Yamamoto H, Miyamoto E. Phosphorylation of myristoylated alanine-rich protein kinase C substrate by mitogen-activated protein kinase in cultured rat hippocampal neurons following stimulation of glutamate receptors. J Biol Chem. 1999;274:408–417. doi: 10.1074/jbc.274.1.408. [DOI] [PubMed] [Google Scholar]
- 9.Tatsumi S, Mabuchi T, Katano T, Matsumura S, Abe T, Hidaka H, et al. Involvement of Rho-kinase in inflammatory and neuropathic pain through phosphorylation of myristoylated alanine-rich C-kinase substrate (MARCKS) Neuroscience. 2005;131:491–498. doi: 10.1016/j.neuroscience.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 10.Tanabe A, Kamisuki Y, Hidaka H, Suzuki M, Negishi M, Takuwa Y. PKC phosphorylates MARCKS Ser159 not only directly but also through RhoA/ROCK. Biochem Biophys Res Commun. 2006;345:156–161. doi: 10.1016/j.bbrc.2006.04.082. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Wang D, Guo Z, Zhao J, Wu B, Deng H, et al. Rho kinase phosphorylation promotes ezrin-mediated metastasis in hepatocellular carcinoma. Cancer Res. 2011;71:1721–1729. doi: 10.1158/0008-5472.CAN-09-4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu S, Goldstein RH, Scepansky EM, Rosenblatt M. Inhibition of rho-associated kinase signaling prevents breast cancer metastasis to human bone. Cancer Res. 2009;69:8742–8751. doi: 10.1158/0008-5472.CAN-09-1541. [DOI] [PubMed] [Google Scholar]
- 13.Rath N, Olson MF. Rho-associated kinases in tumorigenesis: re-considering ROCK inhibition for cancer therapy. EMBO Rep. 2012;13:900–908. doi: 10.1038/embor.2012.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Techasen A, Loilome W, Namwat N, Takahashi E, Sugihara E, Puapairoj A, et al. Myristoylated alanine-rich C kinase substrate phosphorylation promotes cholangiocarcinoma cell migration and metastasis via the protein kinase C-dependent pathway. Cancer Sci. 2010;101:658–665. doi: 10.1111/j.1349-7006.2009.01427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reddy MM, Fernandes MS, Salgia R, Levine RL, Griffin JD, Sattler M. NADPH oxidases regulate cell growth and migration in myeloid cells transformed by oncogenic tyrosine kinases. Leukemia. 2011;25:281–289. doi: 10.1038/leu.2010.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yokoyama Y, Ito T, Hanson V, Schwartz GK, Aderem AA, Holland JF, et al. PMA-induced reduction in invasiveness is associated with hyperphosphorylation of MARCKS and talin in invasive bladder cancer cells. Int J Cancer. 1998;75:774–779. doi: 10.1002/(sici)1097-0215(19980302)75:5<774::aid-ijc18>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 17.Michel S, Kloor M, Singh S, Gdynia G, Roth W, von Knebel Doeberitz M, et al. Coding microsatellite instability analysis in microsatellite unstable small intestinal adenocarcinomas identifies MARCKS as a common target of inactivation. Mol Carcinog. 2010;49:175–182. doi: 10.1002/mc.20587. [DOI] [PubMed] [Google Scholar]
- 18.Chen X, Rotenberg SA. PhosphoMARCKS drives motility of mouse melanoma cells. Cell Signal. 2010;22:1097–1103. doi: 10.1016/j.cellsig.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Micallef J, Taccone M, Mukherjee J, Croul S, Busby J, Moran MF, et al. Epidermal growth factor receptor variant III-induced glioma invasion is mediated through myristoylated alanine-rich protein kinase C substrate overexpression. Cancer Res. 2009;69:7548–7556. doi: 10.1158/0008-5472.CAN-08-4783. [DOI] [PubMed] [Google Scholar]
- 20.Jarboe JS, Anderson JC, Duarte CW, Mehta T, Nowsheen S, Hicks PH, et al. MARCKS regulates growth and radiation sensitivity and is a novel prognostic factor for glioma. Clin Cancer Res. 2012;18:3030–3041. doi: 10.1158/1078-0432.CCR-11-3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brooks G, Brooks SF, Goss MW. MARCKS functions as a novel growth suppressor in cells of melanocyte origin. Carcinogenesis. 1996;17:683–689. doi: 10.1093/carcin/17.4.683. [DOI] [PubMed] [Google Scholar]
- 22.Singer M, Martin LD, Vargaftig BB, Park J, Gruber AD, Li Y, et al. A MARCKS-related peptide blocks mucus hypersecretion in a mouse model of asthma. Nat Med. 2004;10:193–196. doi: 10.1038/nm983. [DOI] [PubMed] [Google Scholar]
- 23.Takashi S, Park J, Fang S, Koyama S, Parikh I, Adler KB. A peptide against the N-terminus of myristoylated alanine-rich C kinase substrate inhibits degranulation of human leukocytes in vitro. Am J Respir Cell Mol Biol. 2006;34:647–652. doi: 10.1165/rcmb.2006-0030RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eckert RE, Neuder LE, Park J, Adler KB, Jones SL. Myristoylated alanine-rich C-kinase substrate (MARCKS) protein regulation of human neutrophil migration. Am J Respir Cell Mol Biol. 2010;42:586–594. doi: 10.1165/rcmb.2008-0394OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chu YW, Yang PC, Yang SC, Shyu YC, Hendrix MJ, Wu R, et al. Selection of invasive and metastatic subpopulations from a human lung adenocarcinoma cell line. Am J Respir Cell Mol Biol. 1997;17:353–360. doi: 10.1165/ajrcmb.17.3.2837. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez-Guerrico AM, Meshki J, Xiao L, Benavides F, Conti CJ, Kazanietz MG. Molecular mechanisms of protein kinase C-induced apoptosis in prostate cancer cells. J Biochem Mol Biol. 2005;38:639–645. doi: 10.5483/bmbrep.2005.38.6.639. [DOI] [PubMed] [Google Scholar]
- 27.Kim J, Thorne SH, Sun L, Huang B, Mochly-Rosen D. Sustained inhibition of PKCalpha reduces intravasation and lung seeding during mammary tumor metastasis in an in vivo mouse model. Oncogene. 2011;30:323–333. doi: 10.1038/onc.2010.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naylor TL, Tang H, Ratsch BA, Enns A, Loo A, Chen L, et al. Protein kinase C inhibitor sotrastaurin selectively inhibits the growth of CD79 mutant diffuse large B-cell lymphomas. Cancer Res. 2011;71:2643–2653. doi: 10.1158/0008-5472.CAN-10-2525. [DOI] [PubMed] [Google Scholar]
- 29.Shih JY, Tsai MF, Chang TH, Chang YL, Yuan A, Yu CJ, et al. Transcription repressor slug promotes carcinoma invasion and predicts outcome of patients with lung adenocarcinoma. Clin Cancer Res. 2005;11:8070–8078. doi: 10.1158/1078-0432.CCR-05-0687. [DOI] [PubMed] [Google Scholar]
- 30.Zhang K, Zhang M, Zhao H, Yan B, Zhang D, Liang J. S100A4 regulates motility and invasiveness of human esophageal squamous cell carcinoma through modulating the AKT/Slug signal pathway. Dis Esophagus. 2012;25:731–739. doi: 10.1111/j.1442-2050.2012.01323.x. [DOI] [PubMed] [Google Scholar]
- 31.Garcia J, Sandi MJ, Cordelier P, Binétruy B, Pouysségur J, Iovanna JL, et al. Tie1 deficiency induces endothelial-mesenchymal transition. EMBO Rep. 2012;13:431–439. doi: 10.1038/embor.2012.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lukk M, Kapushesky M, Nikkila J, Parkinson H, Goncalves A, Huber W, et al. A global map of human gene expression. Nat Biotechnol. 2010;28:322–324. doi: 10.1038/nbt0410-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hollander MC, Blumenthal GM, Dennis PA. PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nat Rev Cancer. 2011;11:289–301. doi: 10.1038/nrc3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McLaughlin S, Murray D. Plasma membrane phosphoinositide organization by protein electrostatics. Nature. 2005;438:605–611. doi: 10.1038/nature04398. [DOI] [PubMed] [Google Scholar]
- 35.Botto L, Masserini M, Cassetti A, Palestini P. Immunoseparation of Prion protein-enriched domains from other detergent-resistant membrane fractions, isolated from neuronal cells. FEBS Lett. 2004;557:143–147. doi: 10.1016/s0014-5793(03)01463-7. [DOI] [PubMed] [Google Scholar]
- 36.Yamaguchi H, Shiraishi M, Fukami K, Tanabe A, Ikeda-Matsuo Y, Naito Y, et al. MARCKS regulates lamellipodia formation induced by IGF-I via association with PIP2 and beta-actin at membrane microdomains. J Cell Physiol. 2009;220:748–755. doi: 10.1002/jcp.21822. [DOI] [PubMed] [Google Scholar]
- 37.Kalwa H, Michel T. The MARCKS protein plays a critical role in phosphatidylinositol 4,5-bisphosphate metabolism and directed cell movement in vascular endothelial cells. J Biol Chem. 2011;286:2320–2330. doi: 10.1074/jbc.M110.196022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 39.Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- 40.Yarmola EG, Edison AS, Lenox RH, Bubb MR. Actin filament cross-linking by MARCKS: characterization of two actin-binding sites within the phosphorylation site domain. J Biol Chem. 2001;276:22351–22358. doi: 10.1074/jbc.M101457200. [DOI] [PubMed] [Google Scholar]
- 41.Myat MM, Anderson S, Allen LA, Aderem A. MARCKS regulates membrane ruffling and cell spreading. Curr Biol. 1997;7:611–614. doi: 10.1016/s0960-9822(06)00262-4. [DOI] [PubMed] [Google Scholar]
- 42.Rombouts K, Carloni V, Mello T, Omenetti S, Galastri S, Madiai S, et al. Myristoylated alanine-rich protein kinase C Substrate (MARCKS) expression modulates the metastatic phenotype in human and murine colon carcinoma in vitro and in vivo. Cancer Lett. 2013;333:244–252. doi: 10.1016/j.canlet.2013.01.040. [DOI] [PubMed] [Google Scholar]
- 43.Fenouille N, Tichet M, Dufies M, Pottier A, Mogha A, Soo JK, et al. The epithelial-mesenchymal transition (EMT) regulatory factor SLUG (SNAI2) is a downstream target of SPARC and AKT in promoting melanoma cell invasion. PLoS One. 2012;7:e40378. doi: 10.1371/journal.pone.0040378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shih JY, Yang PC. The EMT regulator slug and lung carcinogenesis. Carcinogenesis. 2011;32:1299–1304. doi: 10.1093/carcin/bgr110. [DOI] [PubMed] [Google Scholar]
- 45.Tauskela JS, Brunette E. Neuroprotection against staurosporine by metalloporphyrins independent of antioxidant capability. Neurosci Lett. 2009;466:41–46. doi: 10.1016/j.neulet.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 46.Hanigan MH, Devarajan P. Cisplatin nephrotoxicity: molecular mechanisms. Cancer Ther. 2003;1:47–61. [PMC free article] [PubMed] [Google Scholar]
- 47.Chen JJ, Peck K, Hong TM, Yang SC, Sher YP, Shih JY, et al. Global analysis of gene expression in invasion by a lung cancer model. Cancer Res. 2001;61:5223–5230. [PubMed] [Google Scholar]
- 48.Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest. 1997;111:1710–1717. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 49.Fan T, Li R, Todd NW, Qiu Q, Fang HB, Wang H, et al. Up-regulation of 14-3-3zeta in lung cancer and its implication as prognostic and therapeutic target. Cancer Res. 2007;67:7901–7906. doi: 10.1158/0008-5472.CAN-07-0090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.