Abstract
Hydroxyl radical protein footprinting (HRPF) by Fast Photochemical Oxidation of Proteins (FPOP) is a powerful benchtop tool used to probe protein structure, interactions, and conformational changes in solution. However, the reproducibility of all HRPF techniques is limited by the ability to deliver a defined concentration of hydroxyl radicals to the protein. This ability is impacted by both the amount of radical generated and the presence of radical scavengers in solution. In order to compare HRPF data from sample to sample, a hydroxyl radical dosimeter is needed that can measure the effective concentration of radical that is delivered to the protein, after accounting for both differences in hydroxyl radical generation and non-analyte radical consumption. Here, we test three radical dosimeters (Alexa Fluor 488, terepthalic acid, and adenine) for their ability to quantitatively measure the effective radical dose under the high radical concentration conditions of FPOP. Adenine has a quantitative relationship between UV spectrophotometric response, effective hydroxyl radical dose delivered, and peptide and protein oxidation levels over the range of radical concentrations typically encountered in FPOP. The simplicity of an adenine-based dosimeter allows for convenient and flexible incorporation into FPOP applications, and the ability to accurately measure the delivered radical dose will enable reproducible and reliable FPOP across a variety of platforms and applications.
Introduction
Hydroxyl radical protein footprinting (HRPF) coupled with mass spectrometry is an emerging structural technique that provides a more modest level of structural detail than traditional high resolution structural biology techniques such as X-ray crystallography or multidimensional nuclear magnetic resonance (NMR) spectroscopy, but can be applied to a broader range of protein systems.1–2 HRPF entails oxidizing amino acid side chains in a protein of interest with diffusing hydroxyl radicals and quantifying the oxidation of each amino acid using tandem mass spectrometry. 3–7 HRPF takes advantage of the fact that the rate of oxidation of each amino acid varies directly with the solvent accessibility of that amino acid.2, 8 Due to the hydroxyl radical’s high reactivity and comparable size to a water molecule,9 hydroxyl radicals can effectively probe protein solvent accessibility, monitoring conformational changes and interaction surfaces.1
FPOP is one available hydroxyl radical generation method, which utilizes a 248nm pulsed UV laser beam to photolyze hydrogen peroxide into two hydroxyl radicals on a sub-microsecond time scale to achieve extensive labeling of proteins while retaining their native structure, all in a benchtop setup.3, 10–13 However, while the concentration of radical generated can be controlled by controlling the intensity and width of the laser pulse and the concentration of hydrogen peroxide in solution, the amount of radical delivered to the protein is also influenced by the presence of radical scavengers in solution, including proteins in differing concentrations, buffer components, ligands, cofactors, etc. Additionally, the particular geometry of each FPOP setup can alter the effective amount of radical generation by altering the intensity of UV light delivered to the sample, making comparisons of FPOP data between labs difficult.
One method for measuring the available radical dose is by introducing a radical dosimeter that will compete with the protein and all radical scavengers for reaction with the hydroxyl radical, and that will alter its measurable characteristics after reaction with the radical. An ideal dosimeter for FPOP would be measurable either during or directly after the FPOP irradiation, using a very simple and rapid analysis method that does not require further sample handling or derivatization. Niu et al recently reported the first dosimetry tool specifically for FPOP, an approach using phenylalanine as a dosimeter for FPOP, followed by dosimeter detection using isotope dilution gas chromatography mass spectrometry (GCMS) to quantitatively determine hydroxyl radical concentration.14 However, this method is somewhat cumbersome, requiring derivatization of the sample and separate detection by specialized GCMS. Gupta et al. reported using Alexa Fluor 488 in HRPF by X-ray synchrotron irradiation, monitoring a loss of fluorescence as a function of synchrotron radiation.15 This dosimeter has been used frequently by Chance and co-workers for synchrotron HRPF studies, but the radical concentration in FPOP is considerably higher than in synchrotron radiolysis, which could potentially interfere with the dosimetry. Similar issues exist with dosimeters commonly used to study oxidative stress in biological systems, including terephthalic acid coupled with fluorescence spectroscopy16–22 and adenine coupled with UV absorbance spectroscopy.23 The suitability of these dosimeters for the radical concentrations reached by FPOP must be tested before they can be relied upon in these experiments.
In this study, we measured the suitability of Alexa Fluor 488, terephthalic acid and adenine, three optically-responsive hydroxyl radical dosimeters, for FPOP radical dosimetry under a range of conditions. By validating a convenient radical dosimeter for the high radical concentration conditions of modern FPOP that is easily monitored by optical spectroscopy, we introduce a powerful tool for the convenient standardization and comparison of FPOP experiments. The dosimetry can be quickly monitored directly from the sample post-oxidation without the need for further sample handling, minimizing the need for sample workup and time-consuming LC-MS analyses required for MS-based dosimeters.
Experimental
Materials
Lysozyme, angiotensin II, terephthalic acid, catalase, formic acid, and sequencing-grade modified trypsin were obtained from Sigma-Aldrich Corporation (St. Louis, MO, USA). Adenine, dithiothreitol (DTT), and LCMS-grade acetonitrile were purchased from Fisher Scientific (Fair Lawn, NJ, USA). Hydrogen peroxide (30%) was purchased from J. T. Baker (Phillipsburg, NJ, USA). Alex Flour 488 was purchased from Life Technologies (Grand Island, NY, USA). All reagents were used as provided. Purified water (18 MΩ) was obtained from an in-house Milli-Q synthesis system (Millipore, Billerica, MA, USA).
FPOP Dosimetry
Protein samples with hydroxyl radical dosimeter was oxidized using a 248 nm GAM EX100 KrF excimer laser to photolyze hydrogen peroxide as described previously.3 Each reaction was carried out in a 20 μL volume with a final concentration of 20 μM protein or peptide, a dosimeter (5.8 mM terephthalic acid, 5 μM Alexa Fluor 488 or 1mM adenine), freshly prepared hydrogen peroxide at various concentrations, and glutamine sufficient to balance the radical scavenging characteristics of 20 mM glutamine with no dosimeter (0mM for the terephthalic acid dosimeter, 20 mM for the Alexa Fluor 488 dosimeter, or 17 mM for the adenine dosimeter). The appropriate glutamine concentrations were calculated by using the kinetics simulator Tenua to ensure that the concentration of hydroxyl radicals were decreased to 100 nM within 1 μs; see Figure S1 in Supporting Information.15–16, 18, 24 Tris-HCl or sodium phosphate were also added, in concentrations ranging from 0mM to 160 mM. The reaction solution was loaded into a 100 μL syringe and flowed through 100 μm i.d. fused silica tubing using a syringe pump (Harvard Apparatus, Holliston, MA, USA). The flow rate used to introduce solution to laser beam was set at 12.19 μL/min. The laser was foucused through a convex lens with 120 mm focal length (Edmunds Optics, Barrington, NJ, USA) onto the fused silica tubing located 180 mm from the lens. The excimer laser power (GAM Laser Inc., Orlando, FL, USA) at 248 nm was adjusted to ~70 mJ/pulse at a laser pulse repetition rate of 20 Hz to provide approximately 10% of the total volume remaining unirradiated to prevent multiple irradiations per volume. The capillary outflow was collected in a microcentrifuge tube containing 50 nM catalase and 20 mM methionine amide in ammonium bicarbonate buffer (50 mM, pH=7.8) to quench any remaining hydrogen peroxide and other reactive oxygen species. For terephthalic acid and Alexa Fluor 488 combined with fluorescence detection experiment, the irradiated sample solution was diluted to 1mL solution prior to fluorescence analysis. All fluorescence measurements were collected using a Shimadzu RF-5301PC spectrofluorophotometer (Kyoto, Japan). For adenine UV absorbance measurements, 2 μL of irradiated sample solution was introduced into the Thermo NanoDrop 2000c UV spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The absorbance of the buffer blank at 260 nm subjected to FPOP under identical conditions to the adenine-containing sample was subtracted from the adenine UV reading to correct for the UV absorbance of buffer subjected to FPOP. A control solution without photolyzation was also included in each sample set.
Protein Digestion
DTT was added into the remaining FPOP solution after the UV/fluorescence measurement to a final concentration of 5 mg/mL and incubated at 65°C for 30 min to denature and reduce the protein. After cooling to room temperature, a 1:100 weight ratio of trypsin:protein was added to the protein sample and incubated at 37°C overnight with rotation. Another portion of DTT was mixed with the digested sample solution to a final concentration of 1mg/mL fresh DTT and incubated at 65°C for 30 min in waterbath to reduce reformed peptidyl disulfide bonds. The samples were stored at −20°C for LC-MS/MS analysis.
LC-MS/MS
Mass spectral analyses of oxidized angiotensin II and lysozyme were performed on a Thermo Orbitrap Eilte with an ETD source (Thermo Fisher Scientific, Waltham, MA, USA) coupled with the Ultimate 3000 Nano LC system (Dionex, Sunnyvale, CA, USA). Chromatography was conducted using a 150×0.075 mm PepMap 100 C18 analytical column with 3 μm particle size (Thermo Fisher Scientific, Waltham, MA, USA). The gradient elution was performed from 4% to 40% acetonitrile in 0.1% formic acid over 15 min at a flow rate of 0.3 μL/min for angiotensin; a 4% to 50% acetonitrile in 0.1% formic acid over 50 min at a flow rate of 0.3 μL/min for lysozyme, and then increased to 90% acetonitrile in 0.1% formic acid for 5 min followed by a 15 min re-equilibration step. Oxidation of each peptide or specific amino acid residue was computed as previously reported,25–26 and is briefly described in Supporting Information.
Results and Discussion
Linear response of radical dosimeters in FPOP
In order to test the suitability of three of the previously reported hydroxyl radical dosimeters, we studied the dose response curves of three radical dosimeters (Alexa Fluor 488, terepthalic acid and adenine) used in FPOP experiments both to the amount of radical generated as well as to the amount of radical consumed by radical scavengers. Alexa Fluor 488 is a bright green fluorescent dye that has previously been shown to lose fluorescence upon oxidation by synchrotron radiolysis15. Figure S2 shows the fluorescence of Alexa Fluor 488 after oxidation by FPOP in the presence of either varying concentrations of hydrogen peroxide radical precursor, where increases in hydrogen peroxide concentration result in increased amount of hydroxyl radical generation (Figure S2a, Supporting Information) or with 100 mM hydrogen peroxide and varying concentrations of the hydroxyl radical scavenging buffer tris-HCl where increased tris-HCl concentrations result in decreased amounts of hydroxyl radical available to react with the dosimeter (Figure S2b, Supporting Information). The dose response curves shows a non-linear correlation between the hydroxyl radical dose available to the sample and fluorescence relationship, indicating Alexa Fluor 488 cannot be readily used as a radical dosimeter in FPOP experiments. We suspect that the non-linear response in FPOP as contrasted to X-ray synchrotron radiolysis studies is at least partially due to the higher radical concentrations produced by FPOP resulting in multiple oxidations per molecule of Alexa Fluor with each oxidation product having a different fluorescence profile leading to the observed non-linear response. If correct, this would also prevent the use of Alexa Fluor 488 as a radical dosimeter in other high radical flux applications, such as electron pulse radiolysis.
Terephthalic acid is a radical dosimeter widely used in cell biology because of its symmetry, where oxidation at any ring position results in an identical oxidation product.16 Its oxidized form can be easily traced using fluorescence whereas terephthalic acid itself is non-fluorescent. However, a non-linear relationship between radical dose and fluorescence of terephthalic acid used in FPOP experiment with varying concentrations of hydrogen peroxide (Figure S3a, Supporting Information) and tris-HCl buffer (Figure S3b, Supporting Information) was observed in our study, with very poor response to changing available radical concentrations and an inverse response in the presence of Tris. Again, this non-linear relationship is probably due to multiple oxidations per molecule due to the high radical concentrations of FPOP. As terephthalic acid is a strong radical scavenger, increasing the concentration of the dosimeter to improve the radical dosage response would compromise the radical dose delivered to the analyte. Our data on Alexa Fluor 488 and terephthalic acid indicates both of these previously reported radical dosimeters are not reliable for modern FPOP experiments.
Adenine is a nucleobase with a variety of roles in biochemistry. It was previously reported that adenine decreases its UV absorbance at 260 nm upon oxidation by hydroxyl radicals.23 To determine the suitability of adenine as a radical dosimeter in the high radical concentration conditions of FPOP, several experiments were performed. First, several adenine UV absorbance spectra were collected with adenine under varying FPOP experimental conditions. The results indicated that the loss of adenine UV absorbance upon oxidation is maximal at 260 nm, and this loss in FPOP requires both laser irradiation and hydrogen peroxide indicating that there is no background oxidation from hydrogen peroxide nor laser-induced photobleaching (Figure S4, Supporting Information).
Next, in order to test the ability of adenine dosimetry coupled with UV absorption detection to monitor changes in available hydroxyl radical concentrations in FPOP experiment, several FPOP experiments using different variables that alter the effective radical dosage were conducted. Adenine dosimetry solutions were mixed with either differing concentrations of hydrogen peroxide (Figure 1a); various concentrations of tris-HCl buffer (Figure 1b); or with varying energy per laser pulse (Figure 1c). As the concentration of hydrogen peroxide or the photolysis laser power increase, more hydroxyl radicals were produced for reacting with the dosimeter, corresponding to a decreased adenine UV absorbance that was linear over the range of FPOP conditions tested. In contrast, higher concentrations of tris-HCl buffer will scavenge more radicals, resulting in a lower effective radical dose available to react with the adenine dosimeter. This decreased effective dose was reflected in an increased amount of adenine UV absorbance, again with a linear response over the range of conditions tested. This linear relationship suggests that adenine may be useful as a radical dosimeter in the high radical concentration conditions present in FPOP.
Figure 1. Dose response curve of adenine in condition of different concentrations of variables.
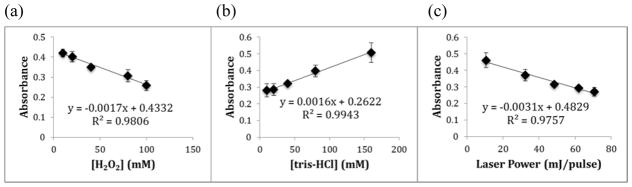
UV absorbance at 260 nm of 1mM adenine, 20 μM ubiquitin, 17 mM glutamine with: (a) different concentrations of hydrogen peroxide (10, 20, 40, 80, 100 mM) in 50 mM sodium phosphate buffer; (b) 100 mM hydrogen peroxide in different concentrations of tris-HCl buffer (10, 20, 40, 80, 160 mM); (c) 100 mM hydrogen peroxide photolyzed in 50 mM sodium phosphate buffer using different photolysis laser power (10.5, 32.4, 48.3, 62, 70.7 mJ/pulse). Each data point represents the average of three FPOP oxidation replicates, with error bars representing one standard deviation.
Correlation of measured adenine radical dose response and sample oxidation in FPOP experiments
To determine if the measured radical dose response of the adenine dosimeter correlated with peptide sample oxidation in FPOP experiments, angiotensin II samples were oxidized in the presence of 1mM adenine dosimeter and varying concentrations of hydrogen peroxide or tris-HCL scavenger. The oxidized peptides were analyzed using LC-MS. The average masses of unoxidized and all oxidized products of doubly charged angiotensin II peptide under different effective radical doses were found and compared to the adenine UV readings over the ranges tested. Two variables, hydrogen peroxide concentration and tris-HCl buffer concentrations, were used in these sets of experiment. As the concentration of hydroxyl radicals available to oxidize the peptide changes, the centroid mass of angiotensin and all angiotensin oxidation products should shift to higher m/z. Increasing the hydrogen peroxide concentration increased the centroid mass of angiotensin, with a corresponding decrease in adenine UV absorption (Figure 2a), whereas increasing the tris-HCl concentration decreased the amount of oxidation of angiotensin, shifting the centroid to lower mass with a corresponding increase in adenine UV absorption (Figure 2b). The correlation between the reactions of adenine and angiotensin with hydroxyl radicals supports adenine as a possible hydroxyl radical dosimeter that can be used in FPOP experiments for monitoring the radical amounts delivered into the biological system.
Figure 2. Relationship between angiotensin oxidation and adenine dosimetry.
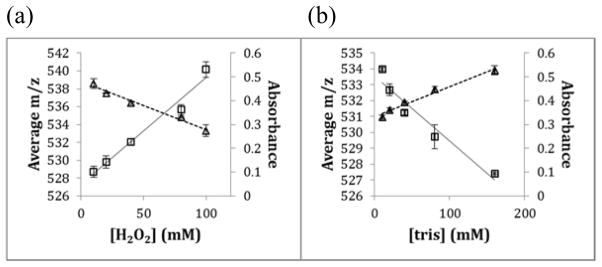
(a) Standard angiotensin Sample with addition of adenine photolyzed in varying concentration of Hydrogen Peroxide. Squares, left y-axis - The centroid mass of oxidized and unoxidized angiotensin, 2+ charge state, with varying concentrations of hydrogen peroxide (10, 20, 40, 80, 100 mM); Triangles, right y-axis - Dose response curve between hydrogen peroxide concentrations and adenine UV reading. (b) Standard Angiotensin Sample with addition of adenine photolyzed in varying concentration of tris-HCl buffer. Square marker - The centroid mass of oxidized and unoxidized angtension, 2+ charge state, with varying concentrations of tris-HCl buffer (10, 20, 40, 80, 160 mM); Triangle marker - Dose response curve between tris-HCl buffer concentrations and adenine UV reading.
Relationship between adenine dosimetry and protein HRPF oxidation
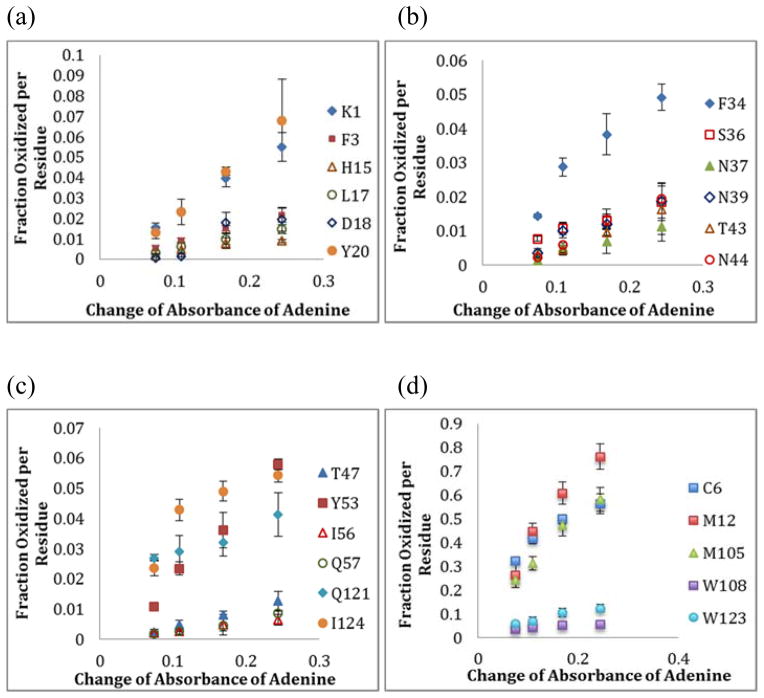
As the primary use of a radical dosimeter in HRPF is to enable the standardization and improve the reproducibility of HRPF experiments on actual protein samples, relating the HRPF data to adenine dosimetry readings is critical. The HRPF data were analyzed on both peptide and residue levels using standard protein lysozyme. FPOP experiments were performed using four different hydrogen peroxide concentrations (10, 25, 50, 100 mM) in order to have various extents of oxidation of both radical dosimeter and protein.
Plotting the amount of oxidation of each lysozyme peptide following FPOP and digestion versus adenine dosimetry yields a linear relationship with a good fit for all peptides detected, including both highly and poorly reactive peptides (Figure S5, Supporting Information). Residue-level quantification of oxidation can be obtained as previously described using ETD MS/MS.25–26 From the residue level quantification data, 23 amino acids from lysozyme were found to be substantially oxidized by FPOP. From the fraction oxidized per residue versus UV absorbance change of adenine plot (Figure 3), most of the oxidized residues showed a linear response over the tested range with the R2 values higher than 0.9 regardless of the inherent chemical reactivity or solvent accessibility of the residue, indicating the reliability of the adenine dosimeter for quantification not only at the peptide level, but for all classes of oxidized amino acids. All oxidized residues showed a linear response compared to adenine UV absorbance (as defined by an R2 > 0.90) with the exception of I124, which was one amino acid that had very poor ETD signal, compromising quantification. Four amino acids (C6, F38, Q121, and W108) show a good fit to a linear regression, but a y-intercept of linear regression significantly different from zero (as defined as an absolute value of y-intercept greater than one tenth of the slope), suggesting either poor quantification due to poor signal-to-noise of the ETD spectrum (C6 for the 50 and 100 mM H2O2 data points only, Q121, and W108), or oxidation processes not induced by hydroxyl radicals (F38). However, the linearity of all amino acids for which good ETD signal could be obtained shows that adenine dosimetry is capable of accurately representing the hydroxyl radical dose delivered by FPOP to each amino acid probed.
Figure 3.
Plots of fraction oxidized per residue versus the change in absorbance for adenine for (a–c) 18 moderately reactive amino acids found to be oxidized by FPOP experiments; (d) five highly reactive amino acids found to be oxidized by FPOP experiments.
Conclusion
The aim of this study was to identify a UV-responsive radical dosimeter useful under the conditions typical for FPOP experiments. Given the ease of performing simple, single wavelength absorbance measurements, the large spectrophotometric response of adenine, and the ready availability of adenine to researchers, incorporation of adenine dosimetry into an FPOP workflow is simple and low-cost, requiring little added time, no added sample cleanup, and no specialized equipment. Incorporation of adenine dosimetry directly in line with the FPOP flow path is also feasible, and would essentially allow dynamic radical dosimetry in real-time with the experiment, giving an added level of control to FPOP experiments currently unavailable. Additionally, adenine dosimetry provides the growing FPOP community a simple method to standardize radical dosages applied to their sample, both between experiments within a laboratory as well as between groups, allowing for improved experimental control and reproducibility within the field.
Supplementary Material
Acknowledgments
This research is supported by the National Institute of General Medical Sciences (1R01GM096049). MS instrumentation is supported by the National Institute of General Medical Sciences-funded “Research Resource for Integrated Glycotechnology” (P41 GM103390). The authors would like to thank Dr. Franklin Leach of Photochem Technologies, LLC for his valuable technical insights.
Footnotes
Conflict of Interest Disclosure
J.S.S. discloses a significant ownership share of Photochem Technologies, LLC, a small company that is active in the area of hydroxyl radical protein footprinting.
Additional experimental details and results.
References
- 1.Xu GH, Chance MR. Hydroxyl radical-mediated modification of proteins as probes for structural proteomics. Chem Rev. 2007;107(8):3514–3543. doi: 10.1021/cr0682047. [DOI] [PubMed] [Google Scholar]
- 2.Takamoto K, Chance MR. Radiolytic protein footprinting with mass spectrometry to probe the structure of macromolecular complexes. Annual review of biophysics and biomolecular structure. 2006;35:251–76. doi: 10.1146/annurev.biophys.35.040405.102050. [DOI] [PubMed] [Google Scholar]
- 3.Hambly DM, Gross ML. Laser flash photolysis of hydrogen peroxide to oxidize protein solvent-accessible residues on the microsecond timescale. J Am Soc Mass Spectrom. 2005;16(12):2057–63. doi: 10.1016/j.jasms.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Maleknia SD, Chance MR, Downard KM. Electrospray-assisted modification of proteins: a radical probe of protein structure. Rapid Commun Mass Spectrom. 1999;13(23):2352–8. doi: 10.1002/(SICI)1097-0231(19991215)13:23<2352::AID-RCM798>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 5.Maleknia SD, Brenowitz M, Chance MR. Millisecond radiolytic modification of peptides by synchrotron X-rays identified by mass spectrometry. Anal Chem. 1999;71(18):3965–73. doi: 10.1021/ac990500e. [DOI] [PubMed] [Google Scholar]
- 6.Sharp JS, Becker JM, Hettich RL. Protein surface mapping by chemical oxidation: structural analysis by mass spectrometry. Anal Biochem. 2003;313(2):216–25. doi: 10.1016/s0003-2697(02)00612-7. [DOI] [PubMed] [Google Scholar]
- 7.Kiselar JG, Chance MR. Future directions of structural mass spectrometry using hydroxyl radical footprinting. Journal of Mass Spectrometry. 2010;45(12):1373–1382. doi: 10.1002/jms.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharp JS, Guo JT, Uchiki T, Xu Y, Dealwis C, Hettich RL. Photochemical surface mapping of C14S-Sml1p for constrained computational modeling of protein structure. Anal Biochem. 2005;340(2):201–12. doi: 10.1016/j.ab.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Kiselar JG, Chance MR. Future directions of structural mass spectrometry using hydroxyl radical footprinting. Journal of mass spectrometry : JMS. 2010;45(12):1373–82. doi: 10.1002/jms.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gau BC, Chen J, Gross ML. Fast photochemical oxidation of proteins for comparing solvent-accessibility changes accompanying protein folding: Data processing and application to barstar. Biochim Biophys Acta. 2013;1834(6):1230–8. doi: 10.1016/j.bbapap.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J, Rempel DL, Gau BC, Gross ML. Fast photochemical oxidation of proteins and mass spectrometry follow submillisecond protein folding at the amino-acid level. J Am Chem Soc. 2012;134(45):18724–31. doi: 10.1021/ja307606f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gau B, Garai K, Frieden C, Gross ML. Mass spectrometry-based protein footprinting characterizes the structures of oligomeric apolipoprotein E2, E3, and E4. Biochemistry. 2011;50(38):8117–26. doi: 10.1021/bi200911c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gau BC, Sharp JS, Rempel DL, Gross ML. Fast photochemical oxidation of protein footprints faster than protein unfolding. Anal Chem. 2009;81(16):6563–71. doi: 10.1021/ac901054w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niu B, Zhang H, Giblin D, Rempel DL, Gross ML. Dosimetry Determines the Initial OH Radical Concentration in Fast Photochemical Oxidation of Proteins (FPOP) Journal of the American Society for Mass Spectrometry. 2015;26(5):843–6. doi: 10.1007/s13361-015-1087-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta S, Sullivan M, Toomey J, Kiselar J, Chance MR. The Beamline X28C of the Center for Synchrotron Biosciences: a national resource for biomolecular structure and dynamics experiments using synchrotron footprinting. J Synchrotron Radiat. 2007;14(Pt 3):233–43. doi: 10.1107/S0909049507013118. [DOI] [PubMed] [Google Scholar]
- 16.Barreto JC, Smith GS, Strobel NH, McQuillin PA, Miller TA. Terephthalic acid: a dosimeter for the detection of hydroxyl radicals in vitro. Life sciences. 1995;56(4):PL89–96. doi: 10.1016/0024-3205(94)00925-2. [DOI] [PubMed] [Google Scholar]
- 17.Yang XF, Guo XQ. Fe(II)-EDTA chelate-induced aromatic hydroxylation of terephthalate as a new method for the evaluation of hydroxyl radical-scavenging ability. Analyst. 2001;126(6):928–32. doi: 10.1039/b100085n. [DOI] [PubMed] [Google Scholar]
- 18.Saran M, Summer KH. Assaying for hydroxyl radicals: hydroxylated terephthalate is a superior fluorescence marker than hydroxylated benzoate. Free radical research. 1999;31(5):429–36. doi: 10.1080/10715769900300991. [DOI] [PubMed] [Google Scholar]
- 19.Page SE, Arnold WA, McNeill K. Terephthalate as a probe for photochemically generated hydroxyl radical. J Environ Monit. 2010;12(9):1658–65. doi: 10.1039/c0em00160k. [DOI] [PubMed] [Google Scholar]
- 20.Mishin VM, Thomas PE. Characterization of hydroxyl radical formation by microsomal enzymes using a water-soluble trap, terephthalate. Biochem Pharmacol. 2004;68(4):747–52. doi: 10.1016/j.bcp.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 21.McLean JR, Mortimer AJ. A cavitation and free radical dosimeter for ultrasound. Ultrasound in medicine & biology. 1988;14(1):59–64. doi: 10.1016/0301-5629(88)90164-0. [DOI] [PubMed] [Google Scholar]
- 22.Mark G, Tauber A, Laupert R, Schuchmann HP, Schulz D, Mues A, von Sonntag C. OH-radical formation by ultrasound in aqueous solution--Part II: Terephthalate and Fricke dosimetry and the influence of various conditions on the sonolytic yield. Ultrason Sonochem. 1998;5(2):41–52. doi: 10.1016/s1350-4177(98)00012-1. [DOI] [PubMed] [Google Scholar]
- 23.Cohn CA, Fisher SC, Brownawell BJ, Schoonen MA. Adenine oxidation by pyrite-generated hydroxyl radicals. Geochemical transactions. 2010;11(1):2. doi: 10.1186/1467-4866-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalkwarf DR. RATE CONSTANTS OF ORGANIC COMPOUNDS WITH HYDROXYL RADICALS. 1969 [Google Scholar]
- 25.Li Z, Moniz H, Wang S, Ramiah A, Zhang F, Moremen KW, Linhardt RJ, Sharp JS. High structural resolution hydroxyl radical protein footprinting reveals an extended robo1-heparin binding interface. J Biol Chem. 2015;290(17):10729–40. doi: 10.1074/jbc.M115.648410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, Li Z, Xie B, Sharp JS. Improved identification and relative quantification of sites of peptide and protein oxidation for hydroxyl radical footprinting. J Am Soc Mass Spectrom. 2013;24(11):1767–76. doi: 10.1007/s13361-013-0719-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.