Abstract
The mammalian skin is a heterogeneous organ/tissue covering our body, showing regional variations and endowed with neuroendocrine activities. The latter is represented by its ability to produce and respond to neurotransmitters, neuropeptides, hormones and neurohormones, of which expression and phenotypic activities can be modified by ultraviolet radiation, chemical and physical factors, as well as by cytokines. The neuroendocrine contribution to the responses of skin to stress is served, in part, by local synthesis of all elements of the hypothalamo-pituitary-adrenal axis. Skin with subcutis can also be classified as a steroidogenic tissue because it expresses the enzyme, CYP11A1, which initiates steroid synthesis by converting cholesterol to pregnenolone, as in other steroidogenic tissues. Pregnenolone, or steroidal precursors from the circulation, are further transformed in the skin to corticosteroids or sex hormones. Furthermore, in the skin CYP11A1 acts on 7-dehydrocholesterol with production of 7-dehydropregnolone, which can be further metabolized to other Δ7steroids, which after exposure to UVB undergo photochemical transformation to vitamin D like compounds with a short side chain. Vitamin D and lumisterol, produced in the skin after exposure to UVB, are also metabolized by CYP11A1 to several hydroxyderivatives. Vitamin D hydroxyderivatives generated by action of CYP11A1 are biologically active and are subject to further hydroxylations by CYP27B1, CYP27A1 and CP24A. Establishment of which intermediates are produced in the epidermis in vivo and whether they circulate on the systemic level represent a future research challenge. In summary, skin is a neuroendocrine organ endowed with steroid/secosteroidogenic activities
Keywords: Skin, steroids, secosteroids, homeostasis, neuroendocrinology, UVR
1. Skin as a sensory organ endowed with neuroendocrine activities
a) Structure and functions of the skin
The mammalian skin is a heterogeneous organ/tissue covering our body and showing significant regional variations in its structure and functions. Adult human skin has a surface area of approximately 2 m2, is around 2.5 mm thick, and constitutes approximately 6% of our total bodyweight which exceeds all other organs except muscle, bone, adipose tissue and blood systems [1–3].
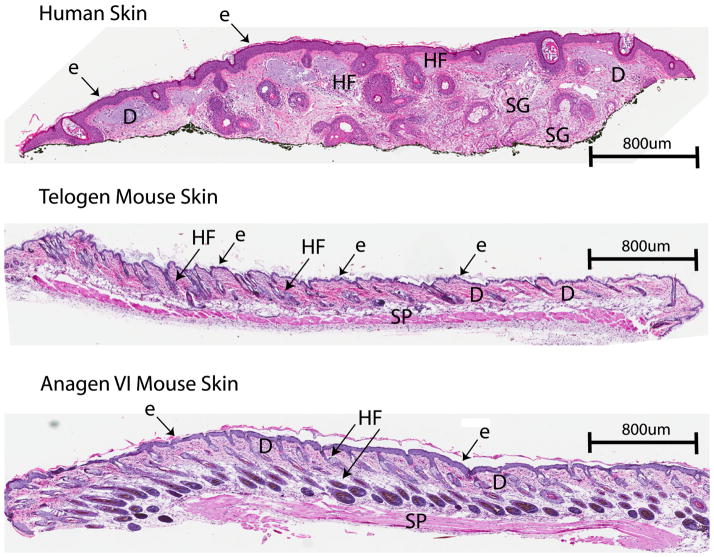
Skin is a multi layered organ composed predominantly of an external stratified, non-vascularized, epithelium (epidermis), underlying connective tissue (dermis), and the subcutaneous adipose tissue called the hypodermis [1–3]. The skin also produces several specialized miniorgans called appendages that include hair follicles, eccrine, sebaceous, apocrine glands, nails and mammary glands in the breast that penetrate deeply into the subcutaneous fat [1–6]. The human skin shows significant differences in histoarchitecture depending on anatomical location. In addition, there are significance differences in skin histology and physiology between humans and primates and furry animals including laboratory mice (Figure 1) [2–6]. In the latter, terminal hair follicles cover most of the body serving as insulating cover and as touch organs, and the histology and physiological activities of their skin are dependent on the phase of the hair cycle [5–7].
Figure 1.
Differences in the histostructure of the skin between human (A) and mouse skin at telogen (B) and anagen VI (C) phases of the hair cycle. E: epidermis, D: dermis, HF: hair follicles: SG: sebaceous glands, SP: panniculus carnosus.
The epidermis stratifies and is a continually keratinizing or cornifying squamous epithelium that terminates at the muco-cutaneous junctions and consists of four major sub-layers composed predominantly of keratin-producing keratinocytes. The basal layer of the epidermis contains keratinocytes with stem cell-like properties and minor populations of Merkel cells (with sensory functions) and melanocytes (melanin-producing cells at a ratio of 1:36 with keratinocytes). Above it is the suprabasal layer followed by the stratum spinosum containing 8–10 sheets of keratinocytes with limited capacity for cell division among which are bone marrow-derived Langerhans cells (at a ratio of 1:53 with keratinocytes) [8]. Differentiating keratinocytes form the stratum granulosum (SG) composed of 3–5 sheets of non-dividing differentiating keratinocytes producing keratinohyalins that further differentiate to form the stratum corneum (SC). Lipids released from lamellar granules in the SG are probarrier lipids that are processed in extracellular spaces as the SC forms. These lipids includes glucosyl ceramides, cholesterol, cholesterol esters, other sterols and long-chain fatty acids, which are further metabolized or spontaneously organized into multiple layers between the corneocytes [9–12]. The SC represents a cornified layer of 15–30 sheets of non-viable, but biochemically-active cells called corneocytes linked by corneodesmosomes and surrounded by lipids, playing a critical role in the barrier function of the epidermis. This physical barrier not only protects against water loss or the harmful action or penetration of physicochemical elements from the environment into the skin and by association, the body, but also protects against microbial invasion by acidic pH (4.0–5.5) or by the presence of antimicrobial peptides and substances with antimicrobial and perhaps antiviral activities [1, 4, 9]. The SC is also desquamating releasing metabolic by-products and sheading corneocytes each day that may have a microbial load or be harmful to local and systemic homeostasis elements. It must be noted that the pores of sweat glands and hair follicles disrupt this epidermal barrier providing potential entry points for environmental factors on one hand and serving as an exist points for secretory activity designed to protect local homeostasis on the other [4].
The epidermis is separated from the underlying dermis by the basement membrane which helps in defining the polarity of continuously renewing and differentiating squamous epithelium (see above) [1–3]. The dermis consists principally of fibroblasts/fibrocytes, a critical mesenchymal cell type which produces dense fibrous/elastic components as well as loose proteoglycans, glycoproteins, water and hyaluronic acid, and other amorphous and hydroscopic but biologically active molecules, altogether called the extracellular matrix (ECM) [1–3]. There are also other mesenchymal in nature cell types including fat cells and other surrounding structures such as vascular, neural and lymphatic systems and networks, excretory and secretary glands (sebaceous, eccrine and apocrine glands), and keratinizing structures including hair follicles and nails. Also, present are sensory nerve receptors of Meissner’s corpuscles (touch), Pacinian corpuscles (pressure), Pilo-Ruffini corpuscles (mechanoreceptors), free nerve terminals which also penetrate the epidermis and hair follicles [1–4, 6]. Additional elements of the dermis and hypodermis are represented by non-resident cell of hematopoietic origin contributing to the functions of the skin immune system. Both resident and circulating dermal cells, as opposed to epidermal and adnexal epithelial cells, are migratory cells communicating via their soluble secreted ECM components. In general, the dermis is divided into the papillary dermis adjacent to the basement membrane and the deeper reticular dermis adjoining subcutaneous adipose tissue/hypodermis. The latter is composed of fat-rich connective tissue connecting the dermis to the skeletal components and hosting the adnexal structures [13]. The thermoregulatory and mechanical functions of the dermis and hypodermis are appreciated. Fat lobules forming the hypodermis are separated by fibrous septae transverse rich in vasculature and play an important function in isolation, cushion and energy storage. Furthermore, the deep and superficial vascular beds deliver oxygen and nutritional and regulatory factors to the dermis/hypodermis (and by diffusion to the epidermis), Also, epidermal and dermal factors can enter the circulation for delivery to other body coordinating centers. These factors include vitamin D, cytokines, leptin, growth factors, neuropeptides, hormones and skin specific metabolites [4, 13, 14]. Furthermore, the extensive neural network collects information from all components of the skin to be processed by local, spinal cord, para-and sympathetic coordinating centers as well as by the brain, to coordinate the body’s responses as well as to regulate homeostatic skin functions in a reciprocal manner [4, 14].
Thus, the skin functions are orchestrated through myriad divisions of labor organized via distinct compartments, components and skin cells defined by the anatomy and histology of the skin, which includes site-associated variations in the presence and density of skin appendages and in epidermis and dermis thickness and functional activity. These functions, in addition to those mentioned above, also include the activities of the epidermal and follicular pigmentary systems protecting the skin against the damaging effects of solar radiation and other physicochemical stresses [15, 16], and by determining skin pigmentation playing a role in social communication and camouflage [7]. The innate and adaptive skin immune systems provide defense against infectious agents (viruses, rickets, bacteria, fungi and parasites), and are also involved in the integration of the response to foreign and self-antigens through interactions with the central immune system, or protect against cancer development [17–19]. Adnexal (secretory in nature) structures participate in thermoregulatory and sensory functions, regulation of electrolyte balance, release of substances strengthening the epidermal barrier, as well as being involved in social communication [1, 6, 9]. The latter can be achieved by secretion of pheromones, odorant and other substances-affecting behavior. Furthermore, the skin is armed with diverse enzymes including cytochromes P450 [20] that are involved in metabolism or activation of hormones and prohormones or activation or inactivation of other substances of exogenous or endogenous origin.
b) Cutaneous neuroendocrine activities
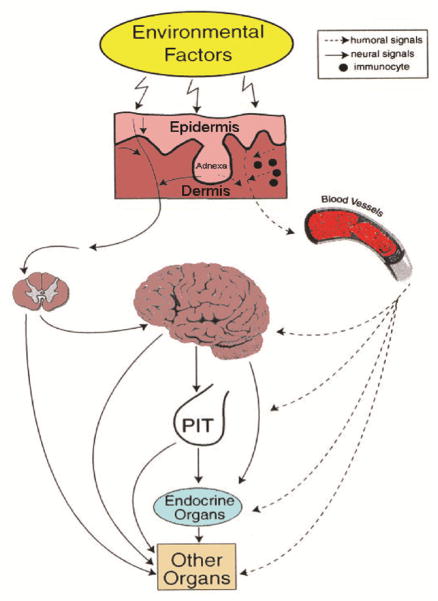
Skin functions are a critical component of survival [9, 14, 21], which requires precise calibration of cutaneous responses and a high degree of local autonomy [14, 21]. To meet these requirements, the skin has developed sophisticated sensory and computing systems to differentially respond to environmental changes represented by biological, chemical, and physical factors in order to regulate local and systemic homeostasis as presented in figure 2 and discussed extensively in [4, 14, 22]. Such responses are coordinated, at least in part by a skin neuro-immuno-endocrine system that can calibrate adaptive mechanisms through neural (rapid) or humoral (tardy) pathways acting at local (epidermis vs dermis vs hypodermis vs adnexa) or systemic levels (reviewed in [4, 14, 22, 23]).
Figure 2. Neuroendocrinology of the skin.
In response to stress, skin transmits humoral or neural signals to the central nervous, endocrine and immune systems, or other organs in order to regulate global homeostasis. Reprinted with permission from Endocrine Society from [14].
Skin or its compartments, through sophisticated communication with central nervous and endocrine systems, also contributes to the maintenance and regulation of body homeostasis [21, 22]. This can be achieved, by skin humoral factors entering the circulation, or local signals activating cutaneous sensory nerve endings that than alert the brain or other coordinating centers through spinal cord neurotransmission as originally proposed in figure 2 [14]. In addition, changes in the skin compartments induced by environmental factors or pathological processes imprint circulating immune cells that act at distant locations as cellular messengers of skin responses to the changes in local homeostasis (Fig. 2)[4, 14].
This sophisticated neuro-immuno-endocrine system is represented by resident and circulating cells of the epidermis and dermis, subcutis/hypodermis and adnexal structures expressing functionally active receptors for neurotransmitters, neuropeptides, hormones, neurohormones and cytokines for which expression and phenotypic activity can be modified by ultraviolet radiation, chemical and physical factors as well as by cytokines and growth factors as extensively discussed in [4, 14, 22, 24–26]. Skin cells also produce several hormones, neurohormones, neurotransmitters and neuropeptides (reviewed in [4, 14, 22, 27]), which is in addition to the production of vitamin D [28–30]. Thus, skin cells can produce biogenic amines such as catecholamines [31–33] and precursors to them such as L-tyrosine and L-DOPA [16, 34], which can also act as hormone-like bioregulators, with melanocytes regulating their local levels and bioactivity [16, 35–37]. They also produce serotonin [38–41], which is further transformed to N-acetyl-serotonin and melatonin [38, 40, 42–46] defining cutaneous serotoninergic and melatoninergic systems [44, 45, 47, 48]. Melatonin is further metabolised locally through kynuric and indolic pathways to metabolites that are biologically active [44, 45, 48–53]. Skin cells also produce acetylcholine [54–57], histamine [58], and cannabinoids [59]. They are capable of producing hypothalamic and pituitary hormones in a cell-type and context dependent fashion. Examples of pituitary hormones include proopiomelanocortin (POMC) which is further processed to ACTH, β-endorphin and MSH peptides in a skin cell type-and context- dependent fashion [22, 60–65]. Again, skin cells can produce thyroid stimulating hormone (TSH) [66, 67], oxytocin [68], growth hormone (GH) [69] and prolactin a manner that is dependent on the cell lineage and cutaneous compartment [69–71]. Examples of hypothalamic peptides include thyroid releasing hormone (TRH) [66, 72] and corticotropin releasing hormone (CRH) [65, 73–80]. Skin cells also produce CRH-related peptides including urocortin 1 and 2 [22, 81] and express the corresponding receptors CRH-R1 and CRH-R2 [65, 73, 74, 82–85]. Other proteins with neurohormonal activities that are produced by skin cells include proenkephalin and dynorphin plus intermediate and final peptide products of their local proteolytic processing (reviewed in [86–90]), tachykinins [91, 92] and various neurotrophins [93, 94].
There are also many other proteins/peptides with neurohormonal and neuropeptide activities produced in the skin, as reviewed in [4, 14, 24–26, 95]. Furthermore, the skin and subcutis produce different adipokines including leptin, adiponectin, IL-6, TNFα that act both at systemic and local levels [13, 96]. Importantly, the skin and subcutis/hypodermis can synthetize or activate or inactivate corticosteroids, sex hormones and their precursors as well as produce Δ7 steroids/sterols and different secosteroids (reviewed in [20, 97–101]). Finally, skin can activate and inactivate thyroid hormones (reviewed in [4]), and produces different oxysterols and oxysteroids (reviewed in [20, 102]). Thus, the production and metabolism of these biologically active molecules define neuroendocrine functions of the skin with local and systemic homeostatic implications, as proposed for the first time in [14] and further developed in [4, 22].
It must be noted that the production of neurohormones in the skin is hierarchical, which follows the algorithms of the classical neuroendocrine axes (e.g. hypothalamic pituitary adrenal (HPA) axis, hypothalamic-thyroid axis, serotoninergic/melatoninergic, catecholaminergic, cholinergic, steroidogenic and secosteroidogenic systems (reviewed in [4, 20, 22, 31, 32, 101, 103–105]). Their deregulation may lead to skin pathology and in some cases systemic pathology. These local neuroendocrine systems may represent a record of evolution of regulatory networks to deal with environmental pressure, which originated in the integument and further developed into neuro-endocrine-immune systems regulating global body homeostasis, as proposed previously [22, 106]. Accordingly, other neuroendocrine systems may also have originated in the skin/integument, since biogenic amines, melatonin and precursors to them (reviewed in [31, 35, 36, 45, 107]) as well as oxysterols (reviewed in [20]), acetylcholine [31] and various neuropeptides (reviewed in [4]) can also be produced by simple organisms to deal with stress responses.
c) Skin equivalent of the hypothalamic-pituitary-adrenal (HPA) axis
The neuroendocrine contribution to the responses of skin to stress is served, in part, by local synthesis of all elements of the classical HPA axis. These include CRH, urocortins POMC-derived ACTH, α-MSH and β-endorphin, and glucocorticoids as well as their corresponding receptors, e.g., CRHR1 and 2, melanocortin receptors (MC-Rs), opioid receptors and glucocorticoid receptors (GRs). These are supplemented further by a cytokine network formed by IL-1, IL-6, TNF-α, and adipokins such as leptin, adiponectin, IL-6 and TNFα. All of these molecules are produced in the skin and hypodermis in an organized fashion (reviewed in [4, 13, 22, 60, 82, 96, 103]). The details of cutaneous steroidogenesis will be discussed in following subchapters, and the differences and similarities between the central and peripheral HPA will be outlined with an emphasis on the uniqueness of this signaling system in the skin ([22, 106]).
The original hypothesis that local skin responses to stress utilize an HPA axis homologue [108] was based on seminal discoveries of the production of POMC and POMC peptides by skin cells, and cutaneous expression of the CRH gene and genes coding CRH-R1, MC-2R and crucial enzymes of steroidogenesis [60, 62, 65, 75, 108–112]. Since then, a flurry of papers substantiated the fundamental role of the cutaneous POMC and CRH signaling systems in the regulation of skin homeostasis and its responses to stress (reviewed in [22, 24, 58, 60, 95, 113, 114]), and substantiated the concept of the cutaneous HPA axis (reviewed in [22, 103]).
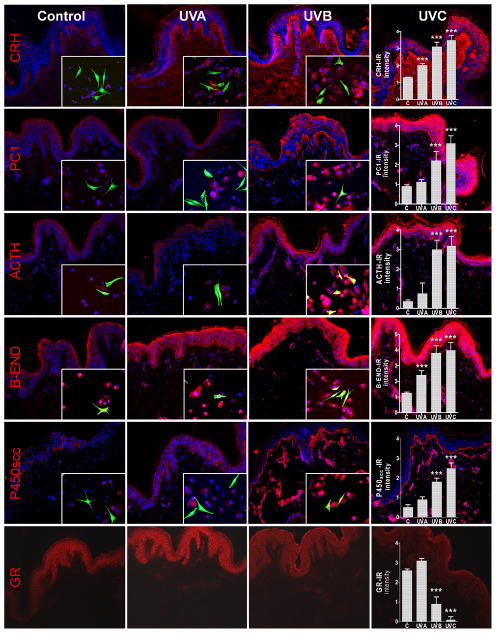
Since all elements of the cutaneous HPA axis as well as cytokines regulating CRH and POMC activities are produced in close proximity or even in the same cells of the skin, the cutaneous equivalent would operate on multiple levels that would also include para-, auto- and intracrine modes of action [4, 22]. In addition, skin cells produce an alternative ligand for the CRHR1, urocortin 1 [81], and also express CRHR2 [60, 74, 83]. Therefore, it is likely that the cutaneous HPA will depart at multiple levels from its central algorithm: CRH→CRHR1→POMC→ACTH→cortisol, in a context dependent fashion. However, evidence for the classical HPA organization operating in the skin has already been provided [61, 115–118](Fig. 3). The possible departures in the skin from the central organization of the HPA axis are discussed in a recent review [22] along with its phenotypic consequences, many of which are addressed at counteracting cutaneous stress and restricting the pro-inflammatory activities of CRH [22, 80, 113, 119–121]. These activities include regulation of melanin pigmentation which protects against environmental stress represented by UVR [15, 122, 123], through direct or indirect effects on elements of the cutaneous hypothalamic-pituitary axis [15].
Fig. 3. UVR can differentially regulate cutaneous equivalent of the HPA axis.
The panel shows UVR-induced changes in in-situ expression of CRH, PC1, ACTH, β-END, P450scc and GR (CY3–red). Double staining shows co-culture of keratinocytes and melanocytes with the use of melanocyte-specific markers [mouse MEL-5 antibody (FITC-green)]. The graphs show the quantitative (means ± SD) comparison of the immunoreactive signal intensity for appropriate antigens as a function of dose and/or wavelength. All viable nuclei were counterstained with DAPI (blue). Magnification 200x (skin) and 400x (co-culture). Reprinted with permission from American Physiology Society from [283].
2. Steroidogenic activities of the skin and subcutis
a) Principles of steroidogenesis in the human skin
Skin with its associated subcutaneous adipose tissue can be classified as a steroidogenic tissue because it expresses the enzyme, CYP11A1 (also known as cytochrome P450scc) [101, 109, 124–127] which initiates steroid synthesis by converting cholesterol to pregnenolone, as in other steroidogenic tissues [99, 128, 129]. This enzyme hydroxylates cholesterol at C22 followed by C20, then cleaves the side chain between C20 and C22 in a third oxidative reaction which produces pregnenolone and isocaproic aldehyde [128–130]. As well as CYP11A1, skin also expresses the necessary electron transport chain partners, adrenodoxin reductase and adrenodoxin [124, 125]. Adrenodoxin reductase is a FAD-containing protein that accepts two electrons at a time from NADPH and delivers them, one at a time, to adrenodoxin [131]. Adrenodoxin contains a Fe2S2 center and is a one electron carrier which shuttles the electrons from adrenodoxin reductase to CYP11A1. CYP11A1 requires two electrons to activate molecular oxygen for each of the three oxidative reactions required to cleave the side chain of cholesterol [131].
The activity of CYP11A1 in the skin has been confirmed with mitochondria prepared from immortalized keratinocytes which were shown to convert cholesterol to pregnenolone, but at a rate only 1% of that for placental mitochondria [124]. CYP11A1 activity has also been demonstrated in cultured skin cells (squamous cell carcinoma, melanoma and keratinocytes) [99, 103, 124] and immortalized sebaceous gland cells [125] where 22R-hydroxycholesterol was converted to pregnenolone and 17α-hydroxypregnenolone, respectively. CYP11A1 expression has been detected at the mRNA or protein level in a range of skin cell types including sebocytes [125, 132] keratinocytes, melanocytes, squamous cell carcinoma, dermal fibroblasts and melanoma cells [124, 133].
In steroidogenic tissues such as the adrenal cortex and gonads, CYP11A1 activity is substrate limited and the rate of cholesterol delivery to the inner mitochondrial membrane where CYP11A1 is bound determines the activity of the enzyme [129, 134]. This delivery of cholesterol is mediated via the Steroidogenic Acute Regulatory Protein (StAR) and is acutely regulated by the tropic hormones, ACTH for the adrenal cortex and LH for the gonads, via cAMP and other pathways [129, 134, 135]. The tropic hormones cause rapid stimulation of the synthesis of StAR as well as its activation by phosphorylation. StAR contains a single binding site for cholesterol and acts at the outer mitochondrial membrane with the end result being increased delivery of cholesterol to the inner mitochondrial membrane for metabolism by CYP11A1 [136–139]. The exact role of StAR and the mechanism of cholesterol transfer from the outer to the inner mitochondrial membrane is unclear. The action of StAR at the outer mitochondrial membrane appears to involve the formation of a “transduceosome” also containing the 18 kDa outer mitochondrial membrane translocator protein (TSPO, also a cholesterol binding protein) and the voltage-dependent anion channel (VDAC), plus the 35 kDa mitochondrial adenine nucleotide translocator (ANT) which is associated with the inner mitochondrial membrane [139]. It has recently been reported that steroidogenesis is not altered in TSPO global knock-out mice, questioning the role of this protein in the transduceosome and cholesterol transport to the inner mitochondrial membrane [140]. Other recent studies have shown that ERK1/2 and the catalytic subunit of protein kinase A (PKA) also associate with the outer mitochondrial membrane [141]. Activation of ERK1/2 by PKA results in phosphorylation of StAR at Ser232 which is necessary for its localization and retention at the outer mitochondrial membrane required for its stimulation of steroidogenesis. Mitochondrial fusion is also stimulated by the tropic hormones via cAMP pathways and Mitofusin 2, and influences both StAR mRNA levels and the localization of the active StAR protein at the outer mitochondrial membrane [141].
In contrast to the adrenal cortex and gonads, pregnenolone synthesis by CYP11A1 in the human placenta which contains a much lower CYP11A1 concentration, is not acutely regulated and delivery of cholesterol appears to be mediated by metastatic lymph node 64 protein (MLN64), also known STARD3 [128, 136]. MLN64, like the StAR protein, belongs to the START-domain family of lipid transfer proteins [136]. Electron transfer to CYP11A1 determined by the concentration of adrenodoxin reductase, rather than cholesterol transport, appears to be rate limiting for placental steroidogenesis [128, 142].
The skin which contains only a low CYP11A1 concentration, has been shown to express both MLN64 [143] and StAR [99, 132] providing a mechanism for cholesterol delivery to CYP11A1 in the inner mitochondrial membrane. Unlike the adrenal cortex and gonads, but similar to the placenta, skin appears to lack an acute response to cAMP where pregnenolone synthesis is increased within 15 min. For example, in HaCaT keratinocytes an increase in StAR protein and pregnenolone synthesis in response to cAMP requires a long period of time [99]. The StAR protein and/or MLN64 may thus serve to maintain saturating or near-saturating cholesterol levels in the inner mitochondrial membrane for CYP11A1 activity, as in the placenta [128], with skin steroidogenesis being chronically regulated by the levels of one or more of the steroidogenic proteins.
In the adrenal cortex, pregnenolone can be acted on by CYP17A1 in the endoplasmic reticulum, producing 17α-hydroxypregnenolone. This then undergoes the lyase reaction between C17 and C20, also catalyzed by CYP17A1, to produce the inactive androgen, dehydroepiandrosterone (DHEA) [144]. Alternatively, the pregnenolone can be converted to progesterone by 3β-hydroxysteroid dehydrogenase (3βHSD) which oxidizes the C3 alcohol to a ketone, and via its isomerase activity, shifts the double bond from the C5-C6 position in the B-ring to the C4-C5 position in the A-ring. CYP17A1 can then act on the progesterone via its 17α-hydroxylase activity to produce 17α-hydroxyprogesterone [129]. CYP17A1 does not express appreciable lyase activity on 17α-hydroxyprogesterone so rather than being converted to androgen, it is acted on by CYP21A2 which hydroxylates it at C21 producing 11-deoxycortisol and commits the steroid to the glucocorticoid biosynthetic pathway. Thus, the level of 3βHSD in steroidogenic tissues plays a key role in determining whether androgens or corticosteroids are produced. For example, the reticularis zone of the adrenal cortex is deficient in 3βHSD and produces predominantly DHEA, whereas the fasciculata zone has abundant 3βHSD and produces mainly cortisol. The lyase activity of CYP17A1 is also stimulated by cytochrome b5, high concentrations of cytochrome P450 oxidoreductase and phosphorylation [129, 144].
11-Deoxycortisol formed in the zona fasciculata is converted to cortisol by the action of CYP11B1, as the final product of the glucocorticoid biosynthetic pathway. The glomerulosa zone of the adrenal cortex is deficient in CYP17A1 and thus progesterone produced in this zone is acted on by CYP21A2 to produce 11-deoxycorticosterone and then by CYP11B2 producing aldosterone, the final product of the mineralocorticoid biosynthetic pathway [129, 145].
As well as expressing the genes for CYP11A1, the StAR protein and MLN64 required to initiate steroidgenesis, the skin expresses the additional genes required to synthesize glucocorticoids, namely CYP17A1, CYP21A2, CYP11B1 [20, 61, 109, 146] and 3βHSD [147, 148]. Furthermore, the glucosteroidogenic pathway has been demonstrated to occur in skin with production of deoxycorticosteone, corticosterone, cortisol and 18-hydroxydeoxycorticosterone being documented [61, 115–117, 149–156]. The pathways for the synthesis of these steroids appears to be the same as those described above for the adrenal gland [98, 99].
In the gonads, CYP17A1 displays high levels of both 17α-hydroxylase and 17–20 lyase activity ensuring that pregnenolone produced by the action of CYP11A1 is converted to androgen, as in the zona reticularis of the adrenal cortex. However, the DHEA formed is acted on by 3βHSD converting it to androstendione and then 17βHSD1 converts androstendione to testosterone, the final product of the androgen biosynthetic pathway in the Leydig cells of the testis. In the ovary, particularly the granulosa cells of the follicle, much of the testosterone (produced by the theca cells) is converted to estradiol by CYP19A1 (aromatase)[129].
The skin can also synthesize testosterone but in sebocytes at least, most of it is derived from circulating DHEA sulfate [157] and thus does not require CYP17A1 or CYP11A1. This conversion requires steroid sulfatase to produce free DHEA, 3βHSD1 to convert DHEA to androstendione and 17βHSD type 5 to convert androstendione to testosterone, all of which are expressed in skin [132, 147, 158–160]. Testosterone synthesis by sebocytes can be stimulated by the addition of pregnenolone [157], indicating the involvement of CYP17A1 and suggesting that some testosterone synthesis derives from endogenous cutaneous cholesterol. This is supported by the findings of Inoue et al [126] who reported that pregnenolone and testosterone synthesis by human epidermal keratinocytes was stimulates by culturing the cells with exogenous cholesterol in the media. Human skin also expresses CYP19A1 (aromatase) and thus can synthesize estrogens [126, 161], with conversion of androstendione to estrone being demonstrated for a human skin culture system [126].
b) Novel concepts on steroidogenesis in human skin
As mentioned above, skin cells synthesize cholesterol, express the StAR protein, and possess the functional biochemical apparatus for the synthesis of glucocorticoids, androgens, and estrogens, which play crucial roles in the homeostasis of human skin, in addition to their well-known effects on reproductive functions [47, 99, 126, 162–164]. StAR is a rapidly synthesized mitochondrial phosphoprotein whose expression, activation and extinction are mediated by PKA, as well by protein kinase C and a host of other signaling pathways that produce both acute and chronic effects on steroidogenesis [138, 165–168]. In addition to StAR, epidermal skin keratinocytes also express other components of the “transduceosome” such TSPO, as well as the StAR-related protein, MLN64 [124, 154, 169–172]. It has been demonstrated that HaCaT cells (a human keratinocyte cell line) treated with a cAMP analog increases StAR expression and pregnenolone synthesis over their basal levels [99, 164]. However, StAR and steroid levels, in response to cAMP signaling, were considerably lower in HaCaT cells when compared to the responses with classical adrenal and gonadal steroidogenic cells. This suggests the slower onset of StAR localization to mitochondria and rates of steroid synthesis in epidermal keratinocytes. It should be noted that expression of StAR has been detected in sebocytes, outer root sheath of hair follicles, vascular tissues, and eccrine sweat ducts, by RT-PCR and immunohistochemical analyses [154, 173, 174].
An overwhelming amount of evidence indicates that regulation of glucocorticosteroidogenesis in the skin is similar to that operating in classical steroidogenic tissues. Malfunction in skin cholesterol synthesis, involving a global reduction in steroids, is associated with down-regulation of epidermal differentiation leading to many skin complications and disorders [21, 22, 99, 153, 175, 176]. In keeping with this, studies have shown that expression of StAR mRNA is decreased or absent in several inflammatory skin diseases including psoriasis, intertigo, eczema, and atopic dermatitis, and warts (HPV etiology), suggesting steroid biosynthesis is disrupted in these diseased conditions [99, 154, 164, 177]. Nevertheless, StAR and CYP19A1 levels have been shown to correlate with androgen and estrogen levels in male and female skin tissues, respectively, demonstrating the relevance of StAR and sex steroids in the homeostasis of the human skin [126]. It has been proposed that modulation of local steroidogenic activity can serve as a therapeutic approach for treatment of many skin conditions, including inflammatory and autoimmune disorders which are most prevalent in geriatric populations [22, 99, 103, 178]. It is well known that steroidogenesis decreases in aging. Thus, a central unanswered question is whether modulation of the steroidogenic activity can serve as a therapeutic approach in preventing a number of skin complications and diseases. Attempts have been made to reverse the serum hormone levels of elderly individuals to younger levels employing a number of approaches, including growth hormone, dehydroepiandrosterone, testosterone, and androgen; however, the benefit of hormone replacement is still evolving owing to the risk of incurring long-term side effects [179–185].
Hormone sensitive-lipase (HSL), a neutral cholesteryl ester hydrolase (NCEH), catalyzes the hydrolysis of cholesteryl esters in steroidogenic tissues and by doing so facilitates cholesterol availability for steroidogenesis [186–189]. It should be noted that cholesterol used for steroidogenesis derives from a number of sources, i.e. de novo synthesis of cellular cholesterol, lipoprotein-derived cholesteryl esters, and hydrolysis of cholesteryl esters stored in lipid droplets. Hormonal control of HSL activity is primarily mediated by phosphorylation of several serine residues by PKA, PKC and other signaling pathways [187, 189–191]. HSL also mediates retinyl ester hydrolysis and male sterility has been proposed to be due to perturbed retinoid metabolism [192, 193]. It has recently been demonstrated that HSL-dependent regulation of cAMP/PKA-induced steroidogenesis involves modulation of liver X receptor (LXR) target genes, steroid receptor element binding protein 1c (SREBP-1c) and ATP binding cassette transporter A1 (ABCA1), in gonadal, adrenal, and epidermal tissues [164, 189]. Conversely, depletion of HSL attenuates the steroidogenic response mediated by LXR agonists, demonstrating involvement of the LXR regulatory pathway in steroidogenesis. Oxysterols act as ligands for LXRs (LXRα and LXRβ) that heterodimerize with retinoid X receptors (RXRs), eventually with retinoic acid receptors (RARs), and regulate a number of genes involved in controlling intracellular cholesterol trafficking, metabolism, and balance [194–197]. Retinoids (vitamin A and its derivatives; especially all-trans retinoic acid, atRA, and 9-cis RA), acting primarily through RARs and RXRs, were found to elevate HSL-mediated steroidogenesis in gonadal and HaCaT cells [164, 198]. An increase in HSL levels was also found to increase SREBP-1c promoter activity as well as ABCA1 protein expression, which mirrored StAR expression and steroid synthesis in HaCaT cells. Both LXRs and ABCA1, as well as several RAR/RXR isoforms, are expressed in keratinocytes, suggesting they play important roles in epidermal development, differentiation, and function [102, 199, 200]. Studies have demonstrated that heterodimerization partners of RXRs, including RARs, LXRs, peroxisome proliferator-activated receptor, vitamin D receptors and thyroid hormone receptors play important roles in normalizing keratinocyte differentiation, and some are used as therapeutic targets in a number of health concerns [201–204]. LXR induces lipogenesis in skin tissues [205, 206] and LXR activators display keratinocyte differentiation and anti-inflammatory activity [207, 208]. It is likely that HSL-LXR/RXR/RAR-ABCA1 signaling is involved in retinoid-mediated regulation of steroidogenesis in skin physiology and/or pathophysiology.
The therapeutic and preventive effects of retinoids in numerous skin conditions, including premature skin aging, skin cancer prevention, squamous cell carcinoma, skin rejuvenation and hyperpigmentation, have long been recognized [209–215]. Retinoids are widely used to alleviate various problems, ranging from vision to reproduction to homeostasis. The biological action of retinoids is mediated by the two families of nuclear receptors, RARs and RXRs, each of which has three subtypes (α, β and γ) with additional isoforms resulting from alternative splicing [198, 216–218]. Both RARs and RXRs interact with multiple putative co-regulators which results in a large array of combinatorial actions that underlie retinoid’s pleiotropic effects. Mice lacking RARα, RARγ, RXRα, and RXRβ display profound anomalies in gonadal, adrenal, and epidermal functions, including sterility and/or embryonic lethality [219–222]. Also, retinoid metabolism and signaling are frequently decreased with many age-related complications and diseases [223–226]. Systemic administration of RA reverses most reproductive and developmental blocks in vitamin A deficient (VAD) rats/mice induced either by nutritional or genetic approaches, demonstrating that retinoid signaling rescues reproductive defects in VAD animals [220, 222, 227]. Hormonal imbalance, involving a global reduction in steroidogenic output, is linked to numerous health complications together with a host of pathologies that are widespread in aging populations. We recently demonstrated that retinoids, especially RAs, enhance the steroidogenic potential in a number of classical and non-classical steroidogenic tissues [99, 164, 198]. It is conceivable that retinoid-mediated restoration of the steroidogenic machinery may play an important role in a number of physiological abnormalities, including skin complications and diseases, which are paramount to geriatric populations.
The maintenance of a well-balanced endocrine circadian rhythmicity is critical to human health; however, a number of endocrine axes are affected as life progresses from the adult to the elderly. The occurrence of hormone deficiencies (also called endocrinosenescence) is constituted to be the major cause of human senescence and it is associated with numerous complications and disabilities [228–231]. Physiological aging results in most of the phenotypic changes observed in skin. Even so, age-related changes affect morphological and functional properties of all endocrine glands; many are so intertwined that the reduced function in one gland adversely affects the others [21, 99, 232]. Aging also affects POMC and POMC-derived peptides, especially agonists for melanocortin receptor 1 (MC1R) and MC2R, which play key roles in skin biological systems [21, 233, 234]. Regulation of the cutaneous steroidogenic system is important for functions of the epidermis, as well as local immunity. Conversely, breakdown of the steroidogenic potential can lead to many skin complications and diseases. Increasing evidence indicates that abnormal skin cholesterol synthesis, involving a global reduction in steroids, is associated with down-regulation of epidermal differentiation, including keratin filaments and cornified envelope components [11, 99, 153, 235]. We recently analyzed StAR mRNA levels from de-identified formalin fixed paraffin-embedded skin samples from men and women of various ages (14–86 years) obtained from various body sites, which revealed an inverse correlation between StAR expression and age (Manna PR et al., unpublished observations). Furthermore, StAR mRNA levels decreased gradually in skin tissues of elderly people when compared with younger individuals. Retinoids, in particular RAs, were found to enhance/restore StAR expression and pregnenolone synthesis in primary cultures of aged epidermal kearatinocytes. These results suggest that retinoid signaling is capable of reversing the decline in steroid dependent events involved in skin physiology/pathophysiology. Previous studies have shown that RA treatment in epidermal keratinocytes inhibits telomerase activity, a feature that is important for the induction of skin tumors/cancers [236, 237]. Retinoids regulate multiple groups of genes, including those involved in differentiation, apoptosis, cell cycle, MAPK/ERKs, signal transduction and lipid metabolism, which are associated with the structural integrity of the epidermis and its appendages [231, 235, 238–240]. Accumulating evidence indicates that RAs improve wrinkled appearance, post-inflammatory hyperpigmentation and inhibits differentiation of keratinocytes in both mice and humans. As such, local regulation of steroidogenic activity in epidermal keratinocytes is important for skin biological systems, as well as skin homeostasis. Hence, therapeutic strategies involving the use of retinoids will have benefits on restoration of many impaired physiological activities that are important for aging in health and dignity.
3. Involvement of the steroidogenic machinery in production of novel secosteroids
a) Derivatives of 7-dehydrocholesterol, vitamin D3 and lumisterol
Besides cholesterol, there are other known substrates for CYP11A1 that are specific to the skin, or that are present in skin in relatively high concentrations. 7-dehydrocholesterol (7DHC) is an example of the latter being the final intermediate in cholesterol biosynthesis by the Kandutsch-Russel pathway and the immediate substrate for the generation of vitamin D3 mediated by UVB radiation [101]. 7DHC levels in the skin, like other tissues, are largely determined by the enzyme 3β-hydroxysterol Δ7-reductase (DHCR7). The gene encoding this enzyme is one of four genes that correlate with vitamin D insufficiency in genome wide association studies [241]. Experiments, initially performed with purified CYP11A1, demonstrated that this P450 can cleave the side chain of 7DHC to produce 7-dehydropregnenolone (7DHP) [124, 242]. Subsequent studies showed that this occurred by an analogous pathway to that for the conversion of cholesterol to pregnenolone, with 7DHC being converted to 22-hydroxy-7DHC then to 20,22-dihydroxy-7DHC and finally the side chain is cleaved between C20–C22 to produce 7DHP [243, 244]. However, unlike the conversion of cholesterol to pregnenolone, the intermediates of the reaction accumulate to some extent and can be considered as minor products of the pathway. Their production has been detected in epidermal keratinocytes isolated from pig skin and in immortalized HaCaT cells [243]. The conversion of 7DHC to 7DHP has been shown to occur following the addition of 7DHC to mitochondria prepared from the rat adrenal, human skin and human placenta [243, 244].
CYP11A1 cleaves the side chain of 7DHC with slightly higher catalytic efficiency than for cholesterol and, like cholesterol, can be transported into the mitochondria for this reaction via StAR [124, 243, 244]. Furthermore, 7DHP is produced by epidermal keratinocytes in culture in the absence of exogenous 7DHC [244] and is present in serum indicating that this reaction does occur in vivo [100]. While its physiological importance remains to be established, 7DHP has been shown to inhibit the proliferation of cultured keratinocytes, melanocytes and leukemia cells, to stimulate the differentiation of leukemia cells and to inhibit TGF-β-induced collagen synthesis [97, 243–245]. It can potentially be converted to other Δ7-steroids in the skin by pathways analogous to those seen with pregnenolone as substrate, with 7-dehydroprogesterone, 17-hydroxy-7DHP and 20-hydroxy-7DHP having been identified to date [97, 244]. Furthermore, 7DHP and its hydroxyderivatives can be converted to pregnavitamin D (pD, vitamin D with 6 carbons of the side chain removed) by UVB irradiation [97, 124, 246–248]. These secosteroids are also known to be biologically active, also inhibiting the proliferation of keratinocytes, normal and malignant melanocytes and leukemia cells, stimulating the differentiation of leukemia cells and inhibiting TGF-β-induced collagen synthesis [97, 245, 247–249]. The intermediates, 22-hydroxy-7DHC and 20,22-dihydroxy-7DHC may also be biologically active as proposed in [244], since the corresponding intermediates of pregnenolone biosynthesis are agonists for the LXR receptor [241] and oxysterols play an important role in epidermal barrier functions [207, 208].
Lumisterol, a photoproduct of UVB irradiation of 7DHC in skin [250] is another substrate for CYP11A1 found from enzymatic analysis of the purified enzyme [251]. UVB irradiation of 7DHC produces previtamin D3 which undergoes thermal isomerization to vitamin D3. With continued UVB irradiation the broken B-ring in previtamin D3 can reform in a 9β,10α-configuration, producing lumisterol 3 which is a stereoisomer isomer of 7DHC [250, 252–254]. Lumisterol 3 is metabolized by purified bovine CYP11A1 with a catalytic efficiency approximately 20% of that for cholesterol metabolism, producing 22-hydroxylumisterol 3, 24-hydroxylumisterol 3 and 20,22-dihydroxylumisterol 3 as major products [251]. These metabolites are also produced from lumisterol 3 by human CYP11A1 [251]. Some cleavage of the side chain of 20,22-dihyrdoxylumisterol 3 does occur producing pregnalumisterol. All these lumisterol products have also been detected following incubation of pig adrenal mitochondria with lumisterol 3, further supporting the potential for this reaction to occur in vivo [251]. Thus, while the physiological importance of this reaction remains to be established, pregnalumisterol is reported to act on cultured leukemia cells, inhibiting proliferation and stimulating differentiation [245]. Given that synthetic 1,25-dihydroxylumisterol is also biologically active with respect to photoprotection of skin against UV radiation [255], and synthetic hydroxyderivatives of pL are also active in cell culture, inhibiting proliferation of keratinocytes and melanoma cells [97, 248, 256], 22-hydroxylumisterol 3, 24-hydroxylumisterol 3 and 20,22-dihydroxylumisterol 3 may also be biologically active, but this remains to be studied.
Vitamin D3, produced in the skin by UVB irradiation of 7DHC, is also a substrate for CYP11A1. As for 7DHC, initial studies on this reaction were carried out using purified bovine CYP11A1 and revealed that the main pathway is conversion of vitamin D3 to 20S-hydroxyvitamin D3 ((20(OH)D3) [242, 257]. This primary and major product accumulates but some undergoes subsequent consecutive hydroxylation to produce 20,23-dihydroxyvitamin D3 (20,23(OH)2D3) and to 17,20,23-trihydroxyvitamin D3. Other products include 22-hydroxyvitamin D3 (22(OH)D3) 17,20-dihydroxyvitamin D3 and 20,22-hydroxyvitamin vitamin D3 (20,22(OH)2D3) [258–260]. Thus, four sites of hydroxylation of the vitamin D3 side chain have been identified, C20 (the preferred carbon), C17, C22 and C23. It appears that there are several minor pathways where the order of hydroxylation is different to that of the major pathway with the potential to produce 15 different products. Many of these minor products have been identified [259, 260]. It is important to note that unlike cholesterol and 7DHC, no cleavage of the vitamin D3 side chain occurs. This is despite the necessary precursor for cleavage, 20,22(OH)2D3, being identified as a product of the reaction [242, 257, 259, 260].
The catalytic efficiency for the metabolism of vitamin D3 by CYP11A1 is similar to that for cholesterol when substrates are dissolved in 2-hydroxypropyl-β-cyclodextrin, but lower when substrates are incorporated into the membrane of phospholipid vesicles [259]. Bovine CYP11A1 catalyzes the hydroxylation of vitamin D3 more efficiently than human CYP11A1 [133, 259, 261]. There is strong evidence that this reaction can occur in vivo in animals and humans. First, incubation of vitamin D3 with bovine or rat adrenal mitochondria, or human placental mitochondria, results in the production of 20(OH)D3 and 1,20(OH)2D3 (the latter resulting from CYP27B1 action on 20(OH)D3) [133, 257]. Second, ex-utero incubation of tissue fragments of human placentae result in the production of 20(OH)D3, 1,20(OH)2D3; 22(OH)D3, 20,22(OH)2D3, 20,23(OH)2D3 and 17,20,23(OH)3D3 [133]. Third, cultured HaCaT keratinocytes produce 20(OH)D3, 22(OH)D3, 20,22/20,23(OH)2D3 (combined peak), and 17,20,23(OH)3D3 even when exogenous vitamin D3 is no added, presumably from the limited D3 present in the serum used to culture the cells [133]. Last, a hydroxyvitamin D3 metabolite with the same mass spectrum and retention time as 20(OH)D3 has been identified in human serum [133].
The physiological importance of the metabolism of vitamin D3 by CYP11A1 in human skin cannot be underestimated, since the products could potentially be important regulators of skin growth, differentiation, inflammation and DNA repair (reviewed in [20, 100, 101]). The production and release of these metabolites from the skin [133], or their production by other steroidogenic tissues [133, 262] could result in systemic actions. These predictions are based on extensive studies on the biological activities of the two major products, 20(OH)D3 and 20,23(OH)2D3 (reviewed in [100, 101, 256]). Both of these secosteroids, as well as their 1α-hydroxyderivatives, 22(OH)D3, 20,22(OH)2D3 and other hydroxyderivatives of 20(OH)D3, inhibit the proliferation of keratinocytes, melanocytes and melanoma cells and stimulate keratinocyte differentiation [100, 256, 260, 263–270]. They also have antileukemic activity [245, 270] and inhibit NF-κB activity in skin cells by stimulating the production of inhibitory IκBα and thus downregulating inflammation [264, 271]. The down regulation of inflammation was further substantiated by the decrease in proinflammatory cytokines by 20(OH)D3 and 20,23(OH)2D3 [100, 272]. Importantly, 20(OH)D3 and 20,23(OH)2D3 inhibit proliferation and fibrotic activity of fibroblasts both in vitro and in vivo [273]. They act, at least in part, as biased agonists on the vitamin D receptor (VDR) displaying many, but not all, of the effects of 1,25-dihydroxyvitamin D3 (1,25OH)2D3) [100, 269]. Furthermore, they also act as reverse agonists on retinoic acid orphan receptors α an γ (RORα and γ), which are expressed in all types of the skin cells [272]. The two key effects of 1,25(OH)2D3 that are absent, or at least weak with 20(OH)D3 and 20,23(OH)2D3, are the regulation of serum calcium levels and the stimulation of the expression of the vitamin D-inactivating enzyme, CYP24A1 [245, 263, 274, 275]. The lack of these properties give these products enhanced therapeutic potential for treatment of hyperproliferative and immune disorders, and sclerosis, compared to 1,25(OH)2D3 [245]. In vivo studies with 20(OH)D3 administration have shown its lack of calcemic activity, up to a dose of 60 μg/kg, in mice [275]. 20(OH)D3 also reduces the symptoms of arthritis in a mouse model of the disease [100] and reduces fibrosis in a mouse model of bleomycin-induces skin scleroderma [273]. Most recently, it has been reported to reduce the damage to DNA resulting from UV-irradiation of mouse skin when the 20(OH)D3 was applied topically to the skin after the irradiation [276] or when it was applied to cultured human keratinocytes and melanocytes [277]. Whether novel CYP11A1-derived secosteroids play a similar protective role on human skin when it is produced endogenously, is an important question which remains to be studied [101].
b) Vitamin D2 and ergosterol
It should be noted that CYP11A1-derived metabolites of plant-derived vitamin D2 [270, 278, 279] and ergosterol [280, 281] can also play a role in the regulation of skin functions when either applied topically or orally [100, 101]. Specifically, vitamin D2 can be hydroxylated by CYP11A1 producing 20(OH)D2, 20,24(OH)2D2 and 17,20,24(OH)3D2 [278, 279], with 20(OH)D2 being further hydroxylated by CYP27B1 to 1,20(OH)2D2 [270]. Importantly, 20(OH)D2 and its metabolites can be produced ex-vivo by keratinocytes, placenta, adrenal glands and colon epithelial Caco-2 cells [262]. Therefore vitamin D2 delivered either orally or topically can be activated by CYP11A1 and further modified by CYP27B1 to exert biological activity on the skin and perhaps other organs [20, 100, 101, 262]. Importantly, 20(OH)D2 acting via the VDR is noncalcemic and nontoxic (like 20(OH)D3) at concentrations as high as 4 μg/kg [270] and can also act as a reverse agonist on RORs [272]. While context dependent production of both hydroxy- and epoxide-derivatives of ergosterol by purified CYP11A1 has been documented [280, 281], determination of whether they can be produced ex-vivo or in vivo remains to be investigated. To date production of 17,24-dihydroxyergosterol by adrenal mitochondria has been demonstrated and this dihydroxyergosterol showed suppression of the proliferative activity of keratinocytes and melanoma cells [280].
4. Regulation of cutaneous and systemic steroidogenesis by skin derived factors
a) Cutaneous neuropeptides and cytokines
As indicated above, the general principles regulating skin steroidogenesis are similar to those operating in classical steroidogenic tissues. Thus, local steroidogenesis can be stimulated by skin derived CRH, ACTH and other factors that enhance intracellular cAMP [74, 99, 115–117, 132, 153, 155] and by cytokines [99, 155]. These factors can either stimulate cutaneous steroidogenesis directly or indirectly through activation of the skin homolog of the HPA axis as discussed above and reviewed recently [22, 98, 99]. The current challenge is to establish whether similar regulators and mechanisms are involved in regulation of cutaneous Δ7-steroidogenesis [244], lumisterol metabolism [251], and sequential hydroxylation of vitamin D through traditional [28, 29, 282] and novel [133, 262] metabolic pathways. With respect to Δ7-steroidogenesis there is good evidence that its regulation involves cAMP dependent pathways [243], which is similar to the classical pathway of steroidogenesis. Similar regulatory mechanisms are predicted for regulation of local metabolism of lumisterol and CYP11A1-dependent metabolism of vitamin D3 since these processes are dependent on CYP11A1 activity and expression of StAR or StAR-like proteins [101, 251, 257]. An exciting area of future research is the role of locally produced neuropeptides and cytokines in the regulation of CYP27B1, CYP27A1 and CYP24A1 activities and their role in modifying products of the novel secosteroidogenic pathways [101].
b) Ultraviolet radiation (UVR) as regulator of cutaneous neuroendocrine activities
UVR has recently been recognized as a regulator of local steroidogenesis with its highly energetic short wavelengths including UVC (λ<280 nm) and UVB (λ=280–320 nm) able to stimulate cutaneous cortisol and/or corticosterone synthesis [118, 156, 283]. Of note, UVA had no significant effect on local cortisol production [156, 283]. The increase in cortisol was associated with stimulation of the expression of CRH (human skin solely), urocortin, POMC, ACTH, MC1R, MC2R, CYP11A1, CYP11B1 and 11βHSD1, and downregulation of 11βHSD2 and glucorticoid receptor (GR) gene expression [118, 156, 283, 284]. This was associated with corresponding changes in the levels of protein or peptide for CRH, urocortin, POMC, ACTH, CYP11A1, 11βHSD1, 11βHSD2 and GR. These results further substantiate previous findings on the stimulatory effect of UVB on MC1, POMC and POMC-derived peptides expression [285–287], and CRH, CRH-related peptides and CRH receptor expression [75, 80, 84]. Similarly, studies by Tiganescu et al [288] showed that there is increased expression of 11βHSD1 in skin samples exposed to ultraviolet radiation. Thus, UVB not only stimulates cutaneous corticosteroidogenesis but also upregulates positive neuroendocrine regulators of corticosteroidogenesis as well as enzymes involved in this metabolic pathway including CYP11A1. The latter raises the challenging question on whether UVB can also stimulate CYP11A1-mediated secosteroidogenesis.
UVB upregulation of 11βHSD1 and downregulation of 11βHSD2 in the skin which favors conversion of cortisone to cortisol, deserves special attention [118, 156]. In this context it is important to emphasize that both enzymes are expressed in the skin and their expression level and activity can affect the status and functions of the epidermal and adnexal structures and can lead to pathology when they are dysregulated [288–292]. These considerations mandate further study of whether classical stimulators of de novo steroidogenesis have a similar role in the regulation of 11βHSD1 and 11βHSD2 to control local cortisol levels.
c) UVB can regulate systemic homeostasis via activation of the central HPA axis
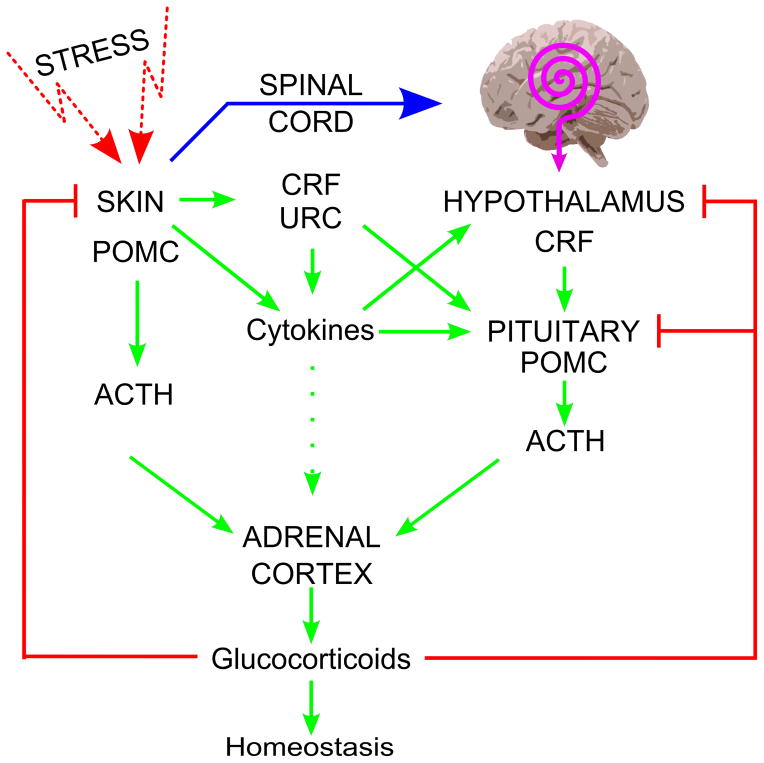
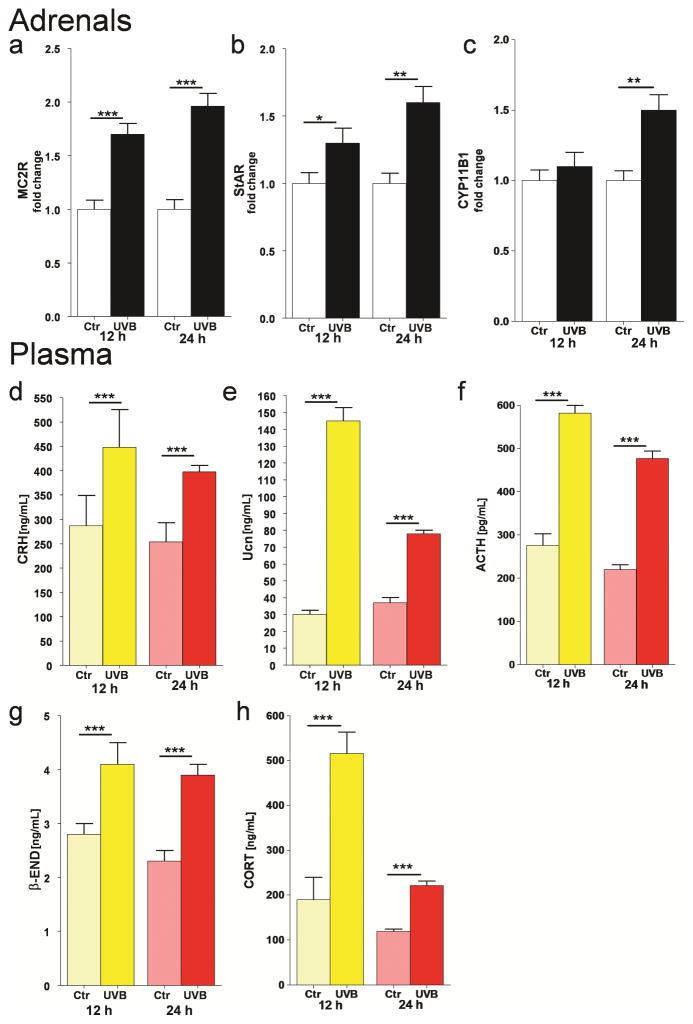
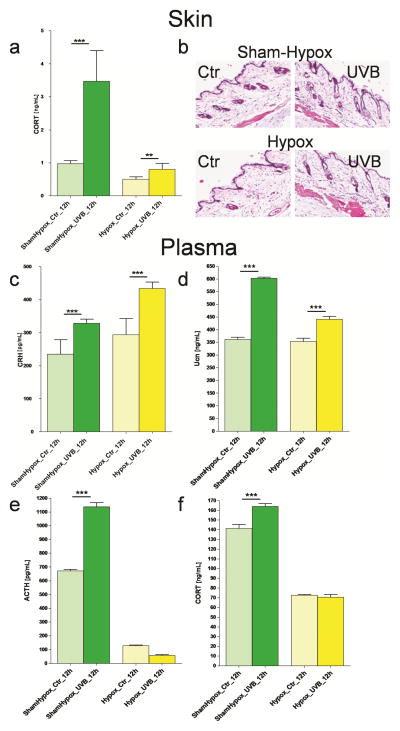
Most recently, we tested the hypothesis that systemic responses to UVB originating in the skin (in addition to production of vitamin D) also involve the activation of the local (cutaneous) and systemic HPA (Figure 4) [22, 293]. We proposed that the skin would activate the systemic HPA via neural transmission to the brain (hypothalamus) or through skin-derived factors delivered by the circulation to act on either the hypothalamus or pituitary [22, 23, 294]. Experimental testing on mice demonstrated that UVB stimulated plasma levels of CRH, urocortin, β-endorphin, ACTH and corticosterone, and increased skin expression of urocortin, β-endorphin and corticosterone, at the gene and protein levels (Figure 5) [293]. UVB stimulated CRH gene and protein expression in the brain that was localized to the paraventricular nucleus of the hypothalamus. In adrenal glands it increased mRNAs for MC2R, StAR and CYP11B1 [293]. This UVB induction of adrenal corticosterone production was associated with stimulation of CRH in the PVN of the hypothalamus and required an intact pituitary, since hypophysectomy abolished UVB stimulation of plasma but not of skin corticosterone levels, and had no effect on UVB stimulation of CRH and urocortin levels in the plasma (Fig. 6)[293]. Thus, we have substantiated the above hypothesis [22, 23] that UVB can regulate body homeostasis through activation of the systemic HPA [23, 293, 295]. These findings are consistent with the well-known phenomenon of systemic immunosuppression caused by UVB [296, 297]. Furthermore, Hiramoto and collaborators demonstrated that exposure of the murine eye to UVR leads to systemic immunomodulation [298–301] that is organ specific, and in the case of UVB is accompanied by increases in serum levels of POMC-derive α-MSH that again requires an intact pituitary [298]. These studies indicate that there are similarities and differences between mechanisms of UVR signal transduction from the skin and eye to the brain [284, 295, 301].
Figure 4. Skin can regulate systemic homeostasis.
Production and secretion of glucocorticoids can be regulated by sequential and/or alternative modes of action originating in the skin that depend on the nature and intensity of the stressor. Reprinted with permission from Endocrine Society from [22].
Figure 5. UVB stimulates the systemic HPA axis in the C57BL/6 mice.
UVB stimulated expression of gene coding MC2R (a), StAR (b) and CYP11B1 (c) in C57Bl/6 adrenals, and enhanced plasma concentrations of CRH (d), Ucn (e), ACTH (f), β-END (g), and CORT (h). The statistical significance was analyzed using t-test, * p<0.05, ** p<0.01, and *** p<0.001. Reprinted with permission of Journal of Investigative Dermatology from [284].
Figure 6. UVB activation of the HPA axis requires an intact pituitary.
The panels show UVB effect on the HPA axis in hypox (pituitary removed) and sham-hypox (pituitary intact) C57BL6 mice. CORT levels were evaluated with ELISA and presented as ng/mL (a). Comparison of histological evaluation between hypox and sham-hypox mouse skin after UVB (400 mJ/cm2) exposure (b). Plasma levels of peptide CRH (c), Ucn (d), ACTH (e) and CORT (f) after exposure of shaved back skin to UVB. Data were analyzed using t-test, ** p<0.01, and *** p<0.001. Reprinted with permission of Journal of Investigative Dermatology from [284].
The above findings may have direct implications in clinical medicine, providing a rationale for UVB-based therapy of autoimmune disorders such as rheumatoid arthritis, multiple sclerosis, inflammatory bowel disease or scleroderma, by stimulation of endogenous production of glucocorticoids in an organized fashion through UVB induction of the HPA axis. Also, our recent [293] and previous findings [4] can provide a mechanistic explanation for the phenomenon of “UVR addiction” [302, 303], because of UVR-induced increases in β-endorphin levels in the skin and circulation [4, 293].
Highlights.
Skin as a sensory organ with neuroendocrine activities
Skin and subcutis demonstrate steroidogenic activities
Cutaneous steroidogenic machinery is involved in production of novel secosteroids
Cutaneous and systemic steroidogenesis are regulated by skin factors and UVR
Acknowledgments
This work was supported by NIH grants 2R01AR052190-A6, R21AR066505-01A1 and 1R01AR056666-01A2 to AS. Help of Dr Tae-Kang Kim in preparation of figure 1 is greatly appreciated.
List of abbreviations
- ABCA1
ATP binding cassette transporter A1
- DHCR7
3β-hydroxysterol Δ7-reductase
- CRH
corticotropin releasing hormone
- 7DHC
7-dehydrocholesterol
- 7DHP
7-dehydropregnenolone
- HPA
hypothalamic-pituitary-adrenal axis
- GH
growth hormone
- MC-R
melanocortin receptors
- GR
glucocorticoid receptors
- MC-R
melanocortin receptors
- HSL
hormone sensitive-lipase
- LXR
liver X receptor
- MLN64
metastatic lymph node 64 protein
- NCEH
neutral cholesteryl ester hydrolase
- POMC
proopiomelanocortin
- RARs
retinoic acid receptors
- StAR
Steroidogenic Acute Regulatory Protein
- SREBP-1c
steroid receptor element binding protein 1c
- TRH
thyroid releasing hormone
- TSH
thyroid stimulating hormone
- UVR
ultraviolet radiation
- VAD
vitamin A deficient
- VDR
vitamin D receptor
Footnotes
Conflict of Interest:
The authors declare no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bolognia J, Jorizzo JL, Rapini RP. Dermatology. 2. Mosby: Elsevier; 2008. [Google Scholar]
- 2.Fitzpatrick TBEA, Wolff K, Freedberg IM, Austen KF. Dermatology in General Medicine. New York: McGraw Hill; 1993. [Google Scholar]
- 3.Weedon D. Weedon’s skin pathology. 3. Churchill Livingstone Elsevier; 2010. [Google Scholar]
- 4.Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD. Sensing the environment: regulation of local and global homeostasis by the skin’s neuroendocrine system. Advances in Anatomy, Embryology and Cell Biology. 2012;212:1–115. doi: 10.1007/978-3-642-19683-6_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slominski A, Paus R. Melanogenesis is coupled to murine anagen: toward new concepts for the role of melanocytes and the regulation of melanogenesis in hair growth. J Invest Dermatol. 1993;101:90S–7S. doi: 10.1111/1523-1747.ep12362991. [DOI] [PubMed] [Google Scholar]
- 6.Stenn KS, Paus R. What controls hair follicle cycling? Exp Dermatol. 1999;8:229–33. doi: 10.1111/j.1600-0625.1999.tb00376.x. discussion 33–6. [DOI] [PubMed] [Google Scholar]
- 7.Slominski A, Wortsman J, Plonka PM, Schallreuter KU, Paus R, Tobin DJ. Hair follicle pigmentation. J Invest Dermatol. 2005;124:13–21. doi: 10.1111/j.0022-202X.2004.23528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoath SB, Leahy DG. The organization of human epidermis: functional epidermal units and phi proportionality. J Invest Dermatol. 2003;121:1440–6. doi: 10.1046/j.1523-1747.2003.12606.x. [DOI] [PubMed] [Google Scholar]
- 9.Elias PM. Stratum corneum defensive functions: an integrated view. J Invest Dermatol. 2005;125:183–200. doi: 10.1111/j.0022-202X.2005.23668.x. [DOI] [PubMed] [Google Scholar]
- 10.Menon GK, Feingold KR, Moser AH, Brown BE, Elias PM. De novo sterologenesis in the skin. II. Regulation by cutaneous barrier requirements. J Lipid Res. 1985;26:418–27. [PubMed] [Google Scholar]
- 11.Elias PM, Crumrine D, Paller A, Rodriguez-Martin M, Williams ML. Pathogenesis of the cutaneous phenotype in inherited disorders of cholesterol metabolism: Therapeutic implications for topical treatment of these disorders. Dermatoendocrinol. 2011;3:100–6. doi: 10.4161/derm.3.2.14831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feingold KR, Elias PM. Role of lipids in the formation and maintenance of the cutaneous permeability barrier. Biochim Biophys Acta. 2014;1841:280–94. doi: 10.1016/j.bbalip.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Klein J, Permana PA, Owecki M, Chaldakov GN, Bohm M, Hausman G, et al. What are subcutaneous adipocytes really good for? Exp Dermatol. 2007;16:45–70. doi: 10.1111/j.1600-0625.2006.00519_1.x. [DOI] [PubMed] [Google Scholar]
- 14.Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;21:457–87. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- 15.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155–228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 16.Slominski A, Zmijewski MA, Pawelek J. L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2012;25:14–27. doi: 10.1111/j.1755-148X.2011.00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallo RL, Hooper LV. Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol. 2012;12:503–16. doi: 10.1038/nri3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallo RL. The birth of innate immunity. Exp Dermatol. 2013;22:517. doi: 10.1111/exd.12197. [DOI] [PubMed] [Google Scholar]
- 19.Sanford JA, Gallo RL. Functions of the skin microbiota in health and disease. Semin Immunol. 2013;25:370–7. doi: 10.1016/j.smim.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slominski AT, Zmijewski MA, Semak I, Zbytek B, Pisarchik A, Li W, et al. Cytochromes p450 and skin cancer: role of local endocrine pathways. Anticancer Agents Med Chem. 2014;14:77–96. doi: 10.2174/18715206113139990308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD. Sensing the environment: regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv Anat Embryol Cell Biol. 2012;212:v, vii, 1–115. doi: 10.1007/978-3-642-19683-6_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slominski AT, Zmijewski MA, Zbytek B, Tobin DJ, Theoharides TC, Rivier J. Key role of CRF in the skin stress response system. Endocr Rev. 2013;34:827–84. doi: 10.1210/er.2012-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slominski A, Wortsman J, Paus R, Elias PM, Tobin DJ, Feingold KR. Skin as an endocrine organ: implications for its function. Drug Discov Today Dis Mech. 2008;5:137–44. doi: 10.1016/j.ddmec.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tobin DJ. Biochemistry of human skin--our brain on the outside. Chem Soc Rev. 2006;35:52–67. doi: 10.1039/b505793k. [DOI] [PubMed] [Google Scholar]
- 25.Arck PC, Slominski A, Theoharides TC, Peters EM, Paus R. Neuroimmunology of stress: skin takes center stage. J Invest Dermatol. 2006;126:1697–704. doi: 10.1038/sj.jid.5700104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paus R, Arck P, Tiede S. (Neuro-)endocrinology of epithelial hair follicle stem cells. Mol Cell Endocrinol. 2008;288:38–51. doi: 10.1016/j.mce.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 27.Steinhoff M, Bienenstock J, Schmelz M, Maurer M, Wei E, Biro T. Neurophysiological, neuroimmunological, and neuroendocrine basis of pruritus. J Invest Dermatol. 2006;126:1705–18. doi: 10.1038/sj.jid.5700231. [DOI] [PubMed] [Google Scholar]
- 28.Bikle DD. Vitamin D: an ancient hormone. Experimental Dermatology. 2011;20:7–13. doi: 10.1111/j.1600-0625.2010.01202.x. [DOI] [PubMed] [Google Scholar]
- 29.Holick MF. Vitamin D: A millenium perspective. J Cell Biochem. 2003;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 30.Lehmann B, Sauter W, Knuschke P, Dressler S, Meurer M. Demonstration of UVB-induced synthesis of 1 alpha,25-dihydroxyvitamin D3 (calcitriol) in human skin by microdialysis. Arch Dermatol Res. 2003;295:24–8. doi: 10.1007/s00403-003-0387-6. [DOI] [PubMed] [Google Scholar]
- 31.Grando SA, Pittelkow MR, Schallreuter KU. Adrenergic and cholinergic control in the biology of epidermis: physiological and clinical significance. J Invest Dermatol. 2006;126:1948–65. doi: 10.1038/sj.jid.5700151. [DOI] [PubMed] [Google Scholar]
- 32.Gillbro JM, Marles LK, Hibberts NA, Schallreuter KU. Autocrine catecholamine biosynthesis and the beta-adrenoceptor signal promote pigmentation in human epidermal melanocytes. J Invest Dermatol. 2004;123:346–53. doi: 10.1111/j.0022-202X.2004.23210.x. [DOI] [PubMed] [Google Scholar]
- 33.Schallreuter KU, Pittelkow MR, Swanson NN, Beazley WD, Korner C, Ehrke C, et al. Altered catecholamine synthesis and degradation in the epidermis of patients with atopic eczema. Arch Dermatol Res. 1997;289:663–6. doi: 10.1007/s004030050258. [DOI] [PubMed] [Google Scholar]
- 34.Schallreuter KU, Wood JM, Pittelkow MR, Gutlich M, Lemke KR, Rodl W, et al. Regulation of melanin biosynthesis in the human epidermis by tetrahydrobiopterin. Science. 1994;263:1444–6. doi: 10.1126/science.8128228. [DOI] [PubMed] [Google Scholar]
- 35.Slominski A, Paus R. Are L-tyrosine and L-dopa hormone-like bioregulators. J Theor Biol. 1990;143:123–38. doi: 10.1016/s0022-5193(05)80292-9. [DOI] [PubMed] [Google Scholar]
- 36.Slominski A, Paus R, Schadendorf D. Melanocytes as “sensory” and regulatory cells in the epidermis. J Theor Biol. 1993;164:103–20. doi: 10.1006/jtbi.1993.1142. [DOI] [PubMed] [Google Scholar]
- 37.Slominski A. Neuroendocrine activity of the melanocyte. Exp Dermatol. 2009;18:760–3. doi: 10.1111/j.1600-0625.2009.00892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slominski A, Wortsman J, Tobin DJ. The cutaneous serotoninergic/melatoninergic system: securing a place under the sun. FASEB Journal. 2005;19:176–94. doi: 10.1096/fj.04-2079rev. [DOI] [PubMed] [Google Scholar]
- 39.Slominski A, Pisarchik A, Semak I, Sweatman T, Wortsman J, Szczesniewski A, et al. Serotoninergic and melatoninergic systems are fully expressed in human skin. FASEB Journal. 2002;16:896–8. doi: 10.1096/fj.01-0952fje. [DOI] [PubMed] [Google Scholar]
- 40.Slominski A, Semak I, Pisarchik A, Sweatman T, Szczesniewski A, Wortsman J. Conversion of Ltryptophan to serotonin and melatonin in human melanoma cells. FEBS letters. 2002;511:102–6. doi: 10.1016/s0014-5793(01)03319-1. [DOI] [PubMed] [Google Scholar]
- 41.Slominski A, Pisarchik A, Semak I, Sweatman T, Szczesniewski A, Wortsman J. Serotoninergic system in hamster skin. J Invest Dermatol. 2002;119:934–42. doi: 10.1046/j.1523-1747.2002.00156.x. [DOI] [PubMed] [Google Scholar]
- 42.Slominski A, Baker J, Rosano TG, Guisti LW, Ermak G, Grande M, et al. Metabolism of serotonin to Nacetylserotonin, melatonin, and 5-methoxytryptamine in hamster skin culture. Journal of Biological Chemistry. 1996;271:12281–6. doi: 10.1074/jbc.271.21.12281. [DOI] [PubMed] [Google Scholar]
- 43.Kobayashi H, Kromminga A, Dunlop TW, Tychsen B, Conrad F, Suzuki N, et al. A role of melatonin in neuroectodermal-mesodermal interactions: the hair follicle synthesizes melatonin and expresses functional melatonin receptors. FASEB Journal. 2005;19:1710–2. doi: 10.1096/fj.04-2293fje. [DOI] [PubMed] [Google Scholar]
- 44.Slominski A, Tobin DJ, Zmijewski MA, Wortsman J, Paus R. Melatonin in the skin: synthesis, metabolism and functions. Trends in Endocrinology and Metabolism. 2008;19:17–24. doi: 10.1016/j.tem.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 45.Slominski AT, Kleszczynski K, Semak I, Janjetovic Z, Zmijewski MA, Kim TK, et al. Local melatoninergic system as the protector of skin integrity. Int J Mol Sci. 2014;15:17705–32. doi: 10.3390/ijms151017705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gaudet SJ, Slominski A, Etminan M, Pruski D, Paus R, Namboordiri MAA. Identification and characterization of two isozymic forms of arylamine N-acetyltransferase in Syrian hamster skin. J Invest Dermatol. 1993;101:660–5. doi: 10.1111/1523-1747.ep12371672. [DOI] [PubMed] [Google Scholar]
- 47.Slominski A, Wortsman J, Tobin DJ. The cutaneous serotoninergic/melatoninergic system: securing a place under the sun. FASEB J. 2005;19:176–94. doi: 10.1096/fj.04-2079rev. [DOI] [PubMed] [Google Scholar]
- 48.Fischer TW, Sweatman TW, Semak I, Sayre RM, Wortsman J, Slominski A. Constitutive and UVinduced metabolism of melatonin in keratinocytes and cell-free systems. FASEB J. 2006;20:1564–6. doi: 10.1096/fj.05-5227fje. [DOI] [PubMed] [Google Scholar]
- 49.Kim TK, Kleszczynski K, Janjetovic Z, Sweatman T, Lin Z, Li W, et al. Metabolism of melatonin and biological activity of intermediates of melatoninergic pathway in human skin cells. FASEB J. 2013;27:2742–55. doi: 10.1096/fj.12-224691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Janjetovic Z, Nahmias ZP, Hanna S, Jarrett SG, Kim TK, Reiter RJ, et al. Melatonin and its metabolites ameliorate ultraviolet B-induced damage in human epidermal keratinocytes. J Pineal Res. 2014;57:90–102. doi: 10.1111/jpi.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim TK, Lin Z, Tidwell WJ, Li W, Slominski AT. Melatonin and its metabolites accumulate in the human epidermis in vivo and inhibit proliferation and tyrosinase activity in epidermal melanocytes in vitro. Mol Cell Endocrinol. 2015;404:1–8. doi: 10.1016/j.mce.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kleszczynski K, Fischer TW. Melatonin and human skin aging. Dermatoendocrinol. 2012;4:245–52. doi: 10.4161/derm.22344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim TK, Lin Z, Li W, Reiter RJ, Slominski AT. N-acetyl-5-methoxykynuramine (AMK) is produced in the human epidermis and shows anti-proliferative effects. Endocrinology. 2015:en20141980. doi: 10.1210/en.2014-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grando SA, Kist DA, Qi M, Dahl MV. Human keratinocytes synthesize, secrete, and degrade acetylcholine. J Invest Dermatol. 1993;101:32–6. doi: 10.1111/1523-1747.ep12358588. [DOI] [PubMed] [Google Scholar]
- 55.Grando SA, Zelickson BD, Kist DA, Weinshenker D, Bigliardi PL, Wendelschafer-Crabb G, et al. Keratinocyte muscarinic acetylcholine receptors: immunolocalization and partial characterization. J Invest Dermatol. 1995;104:95–100. doi: 10.1111/1523-1747.ep12613582. [DOI] [PubMed] [Google Scholar]
- 56.Grando SA, Horton RM, Mauro TM, Kist DA, Lee TX, Dahl MV. Activation of keratinocyte nicotinic cholinergic receptors stimulates calcium influx and enhances cell differentiation. J Invest Dermatol. 1996;107:412–8. doi: 10.1111/1523-1747.ep12363399. [DOI] [PubMed] [Google Scholar]
- 57.Grando SA. Biological functions of keratinocyte cholinergic receptors. J Investig Dermatol Symp Proc. 1997;2:41–8. doi: 10.1038/jidsymp.1997.10. [DOI] [PubMed] [Google Scholar]
- 58.Paus R, Theoharides TC, Arck PC. Neuroimmunoendocrine circuitry of the ‘brain-skin connection’. Trends Immunol. 2006;27:32–9. doi: 10.1016/j.it.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 59.Biro T, Toth BI, Hasko G, Paus R, Pacher P. The endocannabinoid system of the skin in health and disease: novel perspectives and therapeutic opportunities. Trends Pharmacol Sci. 2009;30:411–20. doi: 10.1016/j.tips.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80:979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- 61.Skobowiat C, Dowdy JC, Sayre RM, Tuckey RC, Slominski AT. Cutaneous hypothalamic pituitary adrenal (HPA) axis homologue - regulation by ultraviolet radiation. Am J Physiol Endocrinol Metab. 2011 doi: 10.1152/ajpendo.00217.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Slominski A, Paus R, Mazurkiewicz J. Proopiomelanocortin expression in the skin during induced hair growth in mice. Experientia. 1992;48:50–4. doi: 10.1007/BF01923606. [DOI] [PubMed] [Google Scholar]
- 63.Luger T, Paus R, Lipton J, Slominski A. Cutaneous Neuromodulation: the Proopiomelanocortin System. Ann NY Acad Sci. 1999;885:1–479. doi: 10.1111/j.1749-6632.1999.tb08661.x. [DOI] [PubMed] [Google Scholar]
- 64.Slominski A, Wortsman J, Mazurkiewicz JE, Matsuoka L, Dietrich J, Lawrence K, et al. Detection of proopiomelanocortin-derived antigens in normal and pathologic human skin. J Lab Clin Med. 1993;122:658–66. [PubMed] [Google Scholar]
- 65.Slominski A, Ermak G, Hwang J, Chakraborty A, Mazurkiewicz JE, Mihm M. Proopiomelanocortin, corticotropin releasing hormone and corticotropin releasing hormone receptor genes are expressed in human skin. FEBS Lett. 1995;374:113–6. doi: 10.1016/0014-5793(95)01090-2. [DOI] [PubMed] [Google Scholar]
- 66.Slominski A, Wortsman J, Kohn L, Ain KB, Venkataraman GM, Pisarchik A, et al. Expression of hypothalamic-pituitary-thyroid axis related genes in the human skin. J Invest Dermatol. 2002;119:1449–55. doi: 10.1046/j.1523-1747.2002.19617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bodo E, Kany B, Gaspar E, Knuver J, Kromminga A, Ramot Y, et al. Thyroid-stimulating hormone, a novel, locally produced modulator of human epidermal functions, is regulated by thyrotropin-releasing hormone and thyroid hormones. Endocrinology. 2010;151:1633–42. doi: 10.1210/en.2009-0306. [DOI] [PubMed] [Google Scholar]
- 68.Deing V, Roggenkamp D, Kuhnl J, Gruschka A, Stab F, Wenck H, et al. Oxytocin modulates proliferation and stress responses of human skin cells: implications for atopic dermatitis. Exp Dermatol. 2013;22:399–405. doi: 10.1111/exd.12155. [DOI] [PubMed] [Google Scholar]
- 69.Slominski A, Malarkey WB, Wortsman J, Asa SL, Carlson A. Human skin expresses growth hormone but not the prolactin gene. J Lab Clin Med. 2000;136:476–81. doi: 10.1067/mlc.2000.110605. [DOI] [PubMed] [Google Scholar]
- 70.Foitzik K, Langan EA, Paus R. Prolactin and the skin: a dermatological perspective on an ancient pleiotropic peptide hormone. J Invest Dermatol. 2009;129:1071–87. doi: 10.1038/jid.2008.348. [DOI] [PubMed] [Google Scholar]
- 71.Langan EA, Foitzik-Lau K, Goffin V, Ramot Y, Paus R. Prolactin: an emerging force along the cutaneous-endocrine axis. Trends Endocrinol Metab. 2010;21:569–77. doi: 10.1016/j.tem.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 72.Gaspar E, Hardenbicker C, Bodo E, Wenzel B, Ramot Y, Funk W, et al. Thyrotropin releasing hormone (TRH): a new player in human hair-growth control. FASEB Journal. 2010;24:393–403. doi: 10.1096/fj.08-126417. [DOI] [PubMed] [Google Scholar]
- 73.Slominski A, Wortsman J, Pisarchik A, Zbytek B, Linton EA, Mazurkiewicz JE, et al. Cutaneous expression of corticotropin releasing hormone (CRH), urocortin, and CRH receptors. FASEB J. 2001;15:1678–93. doi: 10.1096/fj.00-0850rev. [DOI] [PubMed] [Google Scholar]
- 74.Zouboulis CC, Seltmann H, Hiroi N, Chen W, Young M, Oeff M, et al. Corticotropin-releasing hormone: an autocrine hormone that promotes lipogenesis in human sebocytes. Proc Natl Acad Sci U S A. 2002;99:7148–53. doi: 10.1073/pnas.102180999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Slominski A, Baker J, Ermak G, Chakraborty A, Pawelek J. Ultraviolet B stimulates production of corticotropin releasing factor (CRF) by human melanocytes. FEBS Lett. 1996;399:175–6. doi: 10.1016/s0014-5793(96)01315-4. [DOI] [PubMed] [Google Scholar]
- 76.Slominski A, Ermak G, Mazurkiewicz JE, Baker J, Wortsman J. Characterization of corticotropinreleasing hormone (CRH) in human skin. Journal of Clinical Endocrinology and Metabolism. 1998;83:1020–4. doi: 10.1210/jcem.83.3.4650. [DOI] [PubMed] [Google Scholar]
- 77.Slominski A, Szczesniewski A, Wortsman J. Liquid chromatography-mass spectrometry detection of corticotropin-releasing hormone and proopiomelanocortin-derived peptides in human skin. J Clin Endocrinol Metab. 2000;85:3582–8. doi: 10.1210/jcem.85.10.6863. [DOI] [PubMed] [Google Scholar]
- 78.Kono M, Nagata H, Umemura S, Kawana S, Osamura RY. In situ expression of corticotropin-releasing hormone (CRH) and proopiomelanocortin (POMC) genes in human skin. FASEB Journal. 2001;15:2297–9. doi: 10.1096/fj.01-0254fje. [DOI] [PubMed] [Google Scholar]
- 79.Ito N, Ito T, Betterman A, Paus R. The human hair bulb is a source and target of CRH. J Invest Dermatol. 2004;122:235–7. doi: 10.1046/j.1523-1747.2003.22145.x. [DOI] [PubMed] [Google Scholar]
- 80.Zbytek B, Wortsman J, Slominski A. Characterization of a ultraviolet B-induced corticotropinreleasing hormone-proopiomelanocortin system in human melanocytes. Mol Endocrinol. 2006;20:2539–47. doi: 10.1210/me.2006-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Slominski A, Roloff B, Curry J, Dahiya M, Szczesniewski A, Wortsman J. The skin produces urocortin. Journal of Clinical Endocrinology and Metabolism. 2000;85:815–23. doi: 10.1210/jcem.85.2.6381. [DOI] [PubMed] [Google Scholar]
- 82.Slominski AT, Botchkarev V, Choudhry M, Fazal N, Fechner K, Furkert J, et al. Cutaneous expression of CRH and CRH-R. Is there a “skin stress response system?”. Ann N Y Acad Sci. 1999;885:287–311. doi: 10.1111/j.1749-6632.1999.tb08686.x. [DOI] [PubMed] [Google Scholar]
- 83.Slominski A, Pisarchik A, Tobin DJ, Mazurkiewicz JE, Wortsman J. Differential expression of a cutaneous corticotropin-releasing hormone system. Endocrinology. 2004;145:941–50. doi: 10.1210/en.2003-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pisarchik A, Slominski AT. Alternative splicing of CRH-R1 receptors in human and mouse skin: identification of new variants and their differential expression. FASEB J. 2001;15:2754–6. doi: 10.1096/fj.01-0487fje. [DOI] [PubMed] [Google Scholar]
- 85.Pisarchik A, Slominski A. Corticotropin releasing factor receptor type 1: molecular cloning and investigation of alternative splicing in the hamster skin. J Invest Dermatol. 2002;118:1065–72. doi: 10.1046/j.1523-1747.2002.01770.x. [DOI] [PubMed] [Google Scholar]
- 86.Bigliardi PL, Tobin DJ, Gaveriaux-Ruff C, Bigliardi-Qi M. Opioids and the skin--where do we stand? Exp Dermatol. 2009;18:424–30. doi: 10.1111/j.1600-0625.2009.00844.x. [DOI] [PubMed] [Google Scholar]
- 87.Slominski AT, Zmijewski MA, Zbytek B, Brozyna AA, Granese J, Pisarchik A, et al. Regulated proenkephalin expression in human skin and cultured skin cells. J Invest Dermatol. 2011;131:613–22. doi: 10.1038/jid.2010.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McLaughlin PJ, Zagon IS. The opioid growth factor-opioid growth factor receptor axis: homeostatic regulator of cell proliferation and its implications for health and disease. Biochem Pharmacol. 2012;84:746–55. doi: 10.1016/j.bcp.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 89.Bigliardi PL, Neumann C, Teo YL, Pant A, Bigliardi-Qi M. Activation of the delta-opioid receptor promotes cutaneous wound healing by affecting keratinocyte intercellular adhesion and migration. Br J Pharmacol. 2015;172:501–14. doi: 10.1111/bph.12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Slominski AT. On the role of the endogenous opioid system in regulating epidermal homeostasis. J Invest Dermatol. 2015;135:333–4. doi: 10.1038/jid.2014.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Katayama I, Bae SJ, Hamasaki Y, Igawa K, Miyazaki Y, Yokozeki H, et al. Stress response, tachykinin, and cutaneous inflammation. J Investig Dermatol Symp Proc. 2001;6:81–6. doi: 10.1046/j.0022-202x.2001.00015.x. [DOI] [PubMed] [Google Scholar]
- 92.Severini C, Improta G, Falconieri-Erspamer G, Salvadori S, Erspamer V. The tachykinin peptide family. Pharmacol Rev. 2002;54:285–322. doi: 10.1124/pr.54.2.285. [DOI] [PubMed] [Google Scholar]
- 93.Botchkarev VA, Yaar M, Peters EM, Raychaudhuri SP, Botchkareva NV, Marconi A, et al. Neurotrophins in skin biology and pathology. J Invest Dermatol. 2006;126:1719–27. doi: 10.1038/sj.jid.5700270. [DOI] [PubMed] [Google Scholar]
- 94.Botchkarev VA. Neurotrophins and their role in pathogenesis of alopecia areata. J Investig Dermatol Symp Proc. 2003;8:195–8. doi: 10.1046/j.1087-0024.2003.00808.x. [DOI] [PubMed] [Google Scholar]
- 95.Roosterman D, Goerge T, Schneider SW, Bunnett NW, Steinhoff M. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev. 2006;86:1309–79. doi: 10.1152/physrev.00026.2005. [DOI] [PubMed] [Google Scholar]
- 96.Poeggeler B, Schulz C, Pappolla MA, Bodo E, Tiede S, Lehnert H, et al. Leptin and the skin: a new frontier. Exp Dermatol. 2010;19:12–8. doi: 10.1111/j.1600-0625.2009.00930.x. [DOI] [PubMed] [Google Scholar]
- 97.Slominski A, Kim TK, Zmijewski MA, Janjetovic Z, Li W, Chen J, et al. Novel vitamin D photoproducts and their precursors in the skin. Dermatoendocrinol. 2013;5:7–19. doi: 10.4161/derm.23938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Slominski AT, Manna PR, Tuckey RC. Cutaneous glucocorticosteroidogenesis: securing local homeostasis and the skin integrity. Experimental Dermatology. 2014;23:369–74. doi: 10.1111/exd.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Slominski A, Zbytek B, Nikolakis G, Manna PR, Skobowiat C, Zmijewski M, et al. Steroidogenesis in the skin: implications for local immune functions. J Steroid Biochem Mol Biol. 2013;137:107–23. doi: 10.1016/j.jsbmb.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Slominski AT, Kim TK, Li W, Yi AK, Postlethwaite A, Tuckey RC. The role of CYP11A1 in the production of vitamin D metabolites and their role in the regulation of epidermal functions. J Steroid Biochem Mol Biol. 2014;144PA:28–39. doi: 10.1016/j.jsbmb.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Slominski AT, Li W, Kim TK, Semak I, Wang J, Zjawiony JK, et al. Novel activities of CYP11A1 and their potential physiological significance. J Steroid Biochem Mol Biol. 2014 doi: 10.1016/j.jsbmb.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schmuth M, Jiang YJ, Dubrac S, Elias PM, Feingold KR. Thematic review series: skin lipids. Peroxisome proliferator-activated receptors and liver X receptors in epidermal biology. J Lipid Res. 2008;49:499–509. doi: 10.1194/jlr.R800001-JLR200. [DOI] [PubMed] [Google Scholar]
- 103.Slominski A, Wortsman J, Tuckey RC, Paus R. Differential expression of HPA axis homolog in the skin. Mol Cell Endocrinol. 2007;265–266:143–9. doi: 10.1016/j.mce.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hardman JA, Tobin DJ, Haslam IS, Farjo N, Farjo B, Al-Nuaimi Y, et al. The peripheral clock regulates human pigmentation. J Invest Dermatol. 2015;135:1053–64. doi: 10.1038/jid.2014.442. [DOI] [PubMed] [Google Scholar]
- 105.Slominski AT, Hardeland R, Reiter RJ. When the circadian clock meets the melanin pigmentary system. J Invest Dermatol. 2015;135:943–5. doi: 10.1038/jid.2014.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Slominski A. A nervous breakdown in the skin: stress and the epidermal barrier. J Clin Invest. 2007;117:3166–9. doi: 10.1172/JCI33508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Reiter RJ, Tan DX, Galano A. Melatonin: exceeding expectations. Physiology (Bethesda) 2014;29:325–33. doi: 10.1152/physiol.00011.2014. [DOI] [PubMed] [Google Scholar]
- 108.Slominski A, Mihm MC. Potential mechanism of skin response to stress. Int J Dermatol. 1996;35:849–51. doi: 10.1111/j.1365-4362.1996.tb05049.x. [DOI] [PubMed] [Google Scholar]
- 109.Slominski A, Ermak G, Mihm M. ACTH receptor, CYP11A1, CYP17 and CYP21A2 genes are expressed in skin. J Clin Endocrinol Metab. 1996;81:2746–9. doi: 10.1210/jcem.81.7.8675607. [DOI] [PubMed] [Google Scholar]
- 110.Slominski A, Paus R, Wortsman J. On the potential role of proopiomelanocortin in skin physiology and pathology. Mol Cell Endocrinol. 1993;93:C1–C6. doi: 10.1016/0303-7207(93)90131-3. [DOI] [PubMed] [Google Scholar]
- 111.Schauer E, Trautinger F, Kock A, Schwarz A, Bhardwaj R, Simon M, et al. Proopiomelanocortinderived peptides are synthesized and released by human keratinocytes. J Clin Invest. 1994;93:2258–62. doi: 10.1172/JCI117224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Slominski A, Ermak G, Hwang J, Mazurkiewicz J, Corliss D, Eastman A. The expression of proopiomelanocortin (POMC) and of corticotropin releasing hormone receptor (CRH-R) genes in mouse skin. Biochimica et biophysica acta. 1996;1289:247–51. doi: 10.1016/0304-4165(95)00159-x. [DOI] [PubMed] [Google Scholar]
- 113.Zbytek B, Slominski AT. CRH Mediates Inflammation Induced by Lipopolysaccharide in Human Adult Epidermal Keratinocytes. J Invest Dermatol. 2006 doi: 10.1038/sj.jid.5700607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Slominski A, Zbytek B, Pisarchik A, Slominski RM, Zmijewski MA, Wortsman J. CRH functions as a growth factor/cytokine in the skin. J Cell Physiol. 2006;206:780–91. doi: 10.1002/jcp.20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ito N, Ito T, Kromminga A, Bettermann A, Takigawa M, Kees F, et al. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB Journal. 2005;19:1332–4. doi: 10.1096/fj.04-1968fje. [DOI] [PubMed] [Google Scholar]
- 116.Slominski A, Zbytek B, Semak I, Sweatman T, Wortsman J. CRH stimulates POMC activity and corticosterone production in dermal fibroblasts. Journal of neuroimmunology. 2005;162:97–102. doi: 10.1016/j.jneuroim.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 117.Slominski A, Zbytek B, Szczesniewski A, Semak I, Kaminski J, Sweatman T, et al. CRH stimulation of corticosteroids production in melanocytes is mediated by ACTH. American Journal of Physiology Endocrinology and Metabolism. 2005;288:E701–E6. doi: 10.1152/ajpendo.00519.2004. [DOI] [PubMed] [Google Scholar]
- 118.Skobowiat C, Nejati R, Lu L, Williams RW, Slominski AT. Genetic variation of the cutaneous HPA axis: an analysis of UVB-induced differential responses. Gene. 2013;530:1–7. doi: 10.1016/j.gene.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zbytek B, Slominski AT. Corticotropin-releasing hormone induces keratinocyte differentiation in the adult human epidermis. J Cell Physiol. 2005;203:118–26. doi: 10.1002/jcp.20209. [DOI] [PubMed] [Google Scholar]
- 120.Zbytek B, Pfeffer LM, Slominski AT. CRH inhibits NF-kappaB signaling in human melanocytes. Peptides. 2006;27:3276–83. doi: 10.1016/j.peptides.2006.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Slominski A. On the role of the corticotropin-releasing hormone signalling system in the aetiology of inflammatory skin disorders. Br J Dermatol. 2009;160:229–32. doi: 10.1111/j.1365-2133.2008.08958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Slominski A, Pawelek J. Animals under the sun: effects of ultraviolet radiation on mammalian skin. Clin Dermatol. 1998;16:503–15. doi: 10.1016/s0738-081x(98)00023-6. [DOI] [PubMed] [Google Scholar]
- 123.Slominski A, Postlethwaite AE. Skin under the sun: when melanin pigment meets vitamin D. Endocrinology. 2015;156:1–4. doi: 10.1210/en.2014-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Slominski A, Zjawiony J, Wortsman J, Semak I, Stewart J, Pisarchik A, et al. A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. Eur J Biochem. 2004;271:4178–88. doi: 10.1111/j.1432-1033.2004.04356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Thiboutot D, Jabara S, McAllister JM, Sivarajah A, Gilliland K, Cong Z, et al. Human skin is a steroidogenic tissue: steroidogenic enzymes and cofactors are expressed in epidermis, normal sebocytes, and an immortalized sebocyte cell line (SEB-1) J Invest Dermatol. 2003;120:905–14. doi: 10.1046/j.1523-1747.2003.12244.x. [DOI] [PubMed] [Google Scholar]
- 126.Inoue T, Miki Y, Abe K, Hatori M, Hosaka M, Kariya Y, et al. Sex steroid synthesis in human skin in situ: The roles of aromatase and steroidogenic acute regulatory protein in the homeostasis of human skin. Mol Cell Endocrinol. 2012;362:19–28. doi: 10.1016/j.mce.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 127.Li J, Daly E, Campioli E, Wabitsch M, Papadopoulos V. De novo synthesis of steroids and oxysterols in adipocytes. J Biol Chem. 2014;289:747–64. doi: 10.1074/jbc.M113.534172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tuckey RC. Progesterone synthesis by the human placenta. Placenta. 2005;26:273–81. doi: 10.1016/j.placenta.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 129.Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocrine Reviews. 2011;32:81–151. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Burstein S, Middleditch BS, Gut M. Mass spectrometric study of the enzymatic conversion of cholesterol to (22R)-22-hydroxycholesterol, (20R,22R)-20,22-dihydroxycholesterol, and pregnenolone, and of (22R)-22-hydroxycholesterol to the lgycol and pregnenolone in bovine adrenocortical preparations. Mode of oxygen incorporation. J Biol Chem. 1975;250:9028–37. [PubMed] [Google Scholar]
- 131.Lambeth JD, Seybert DW, Lancaster JR, Jr, Salerno JC, Kamin H. Steroidogenic electron transport in adrenal cortex mitochondria. Mol Cell Biochem. 1982;45:13–31. doi: 10.1007/BF01283159. [DOI] [PubMed] [Google Scholar]
- 132.Azmahani A, Nakamura Y, Felizola SJA, Ozawa Y, Ise K, Inoue T, et al. Steroidogenic enzymes, their related transcription factors and nuclear receptors in human sebaceous glands under normal and pathological conditions. The Journal of Steroid Biochemistry and Molecular Biology. 2014;144(Part B):268–79. doi: 10.1016/j.jsbmb.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 133.Slominski AT, Kim TK, Shehabi HZ, Semak I, Tang EK, Nguyen MN, et al. In vivo evidence for a novel pathway of vitamin D(3) metabolism initiated by P450scc and modified by CYP27B1. FASEB J. 2012;26:3901–15. doi: 10.1096/fj.12-208975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Miller WL. Steroid hormone synthesis in mitochondria. Mol Cell Endocrinol. 2013;379:62–73. doi: 10.1016/j.mce.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 135.Clark BJ, Wells J, King SR, Stocco DM. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR) J Biol Chem. 1994;269:28314–22. [PubMed] [Google Scholar]
- 136.Miller WL, Bose HS. Early steps in steroidogenesis: intracellular cholesterol trafficking. J Lipid Res. 2011;52:2111–35. doi: 10.1194/jlr.R016675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bose HS, Lingappa VR, Miller WL. Rapid regulation of steroidogenesis by mitochondrial protein import. Nature. 2002;417:87–91. doi: 10.1038/417087a. [DOI] [PubMed] [Google Scholar]
- 138.Papadopoulos V, Miller WL. Role of mitochondria in steroidogenesis. Best Pract Res Clin Endocrinol Metab. 2012;26:771–90. doi: 10.1016/j.beem.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 139.Midzak A, Rone M, Aghazadeh Y, Culty M, Papadopoulos V. Mitochondrial protein import and the genesis of steroidogenic mitochondria. Mol Cell Endocrinol. 2011;336:70–9. doi: 10.1016/j.mce.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tu LN, Morohaku K, Manna PR, Pelton SH, Butler WR, Stocco DM, et al. Peripheral benzodiazepine receptor/translocator protein global knock-out mice are viable with no effects on steroid hormone biosynthesis. J Biol Chem. 2014;289:27444–54. doi: 10.1074/jbc.M114.578286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Duarte A, Castillo AF, Podesta EJ, Poderoso C. Mitochondrial fusion and ERK activity regulate steroidogenic acute regulatory protein localization in mitochondria. PLoS One. 2014;9:e100387. doi: 10.1371/journal.pone.0100387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tuckey RC, Sadleir J. The concentration of adrenodoxin reductase limits cytochrome p450scc activity in the human placenta. Eur J Biochem. 1999;263:319–25. doi: 10.1046/j.1432-1327.1999.00483.x. [DOI] [PubMed] [Google Scholar]
- 143.Slominski A, Zjawiony J, Wortsman J, Semak I, Stewart J, Pisarchik A, et al. A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. Eur J Biochem. 2004;271:4178–88. doi: 10.1111/j.1432-1033.2004.04356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rainey WE, Carr BR, Sasano H, Suzuki T, Mason JI. Dissecting human adrenal androgen production. Trends Endocrinol Metab. 2002;13:234–9. doi: 10.1016/s1043-2760(02)00609-4. [DOI] [PubMed] [Google Scholar]
- 145.Strushkevich N, Gilep AA, Shen L, Arrowsmith CH, Edwards AM, Usanov SA, et al. Structural insights into aldosterone synthase substrate specificity and targeted inhibition. Mol Endocrinol. 2013;27:315–24. doi: 10.1210/me.2012-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhu G, Janjetovic Z, Slominski A. On the role of environmental humidity on cortisol production by epidermal keratinocytes. Exp Dermatol. 2014;23:15–7. doi: 10.1111/exd.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Labrie F, Luu-The V, Labrie C, Pelletier G, El-Alfy M. Intracrinology and the skin. Hormone research. 2000;54:218–29. doi: 10.1159/000053264. [DOI] [PubMed] [Google Scholar]
- 148.Labrie F, Luu-The V, Labrie C, Belanger A, Simard J, Lin SX, et al. Endocrine and intracrine sources of androgens in women: inhibition of breast cancer and other roles of androgens and their precursor dehydroepiandrosterone. Endocrine Reviews. 2003;24:152–82. doi: 10.1210/er.2001-0031. [DOI] [PubMed] [Google Scholar]
- 149.Slominski A, Gomez-Sanchez CE, Foecking MF, Wortsman J. Metabolism of progesterone to DOC, corticosterone and 18OHDOC in cultured human melanoma cells. FEBS Lett. 1999;455:364–6. doi: 10.1016/s0014-5793(99)00889-3. [DOI] [PubMed] [Google Scholar]
- 150.Slominski A, Gomez-Sanchez C, Foecking MF, Wortsman J. Active steroidogenesis in the normal rat skin. Biochimica et biophysica acta. 2000;1474:1–4. doi: 10.1016/s0304-4165(99)00215-9. [DOI] [PubMed] [Google Scholar]
- 151.Rogoff D, Gomez-Sanchez CE, Foecking MF, Wortsman J, Slominski A. Steroidogenesis in the human skin: 21-hydroxylation in cultured keratinocytes. J Steroid Biochem Mol Biol. 2001;78:77–81. doi: 10.1016/s0960-0760(01)00076-0. [DOI] [PubMed] [Google Scholar]
- 152.Slominski A, Zbytek B, Szczesniewski A, Wortsman J. Cultured human dermal fibroblasts do produce cortisol. J Invest Dermatol. 2006;126:1177–8. doi: 10.1038/sj.jid.5700204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cirillo N, Prime SS. Keratinocytes synthesize and activate cortisol. J Cell Biochem. 2011;112:1499–505. doi: 10.1002/jcb.23081. [DOI] [PubMed] [Google Scholar]
- 154.Hannen RF, Michael AE, Jaulim A, Bhogal R, Burrin JM, Philpott MP. Steroid synthesis by primary human keratinocytes; implications for skin disease. Biochem Biophys Res Commun. 2011;404:62–7. doi: 10.1016/j.bbrc.2010.11.059. [DOI] [PubMed] [Google Scholar]
- 155.Vukelic S, Stojadinovic O, Pastar I, Rabach M, Krzyzanowska A, Lebrun E, et al. Cortisol synthesis in epidermis is induced by IL-1 and tissue injury. J Biol Chem. 2011;286:10265–75. doi: 10.1074/jbc.M110.188268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Skobowiat C, Sayre RM, Dowdy JC, Slominski AT. Ultraviolet radiation regulates cortisol activity in a waveband-dependent manner in human skin ex vivo. Br J Dermatol. 2013;168:595–601. doi: 10.1111/bjd.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chen W, Tsai SJ, Sheu HM, Tsai JC, Zouboulis CC. Testosterone synthesized in cultured human SZ95 sebocytes derives mainly from dehydroepiandrosterone. Exp Dermatol. 2010;19:470–2. doi: 10.1111/j.1600-0625.2009.00996.x. [DOI] [PubMed] [Google Scholar]
- 158.Milewich L, Sontheimer RD, Herndon JH., Jr Steroid sulfatase activity in epidermis of acne-prone and non-acne-prone skin of patients with acne vulgaris. Arch Dermatol. 1990;126:1312–4. [PubMed] [Google Scholar]
- 159.Dufort I, Rheault P, Huang XF, Soucy P, Luu-The V. Characteristics of a highly labile human type 5 17beta-hydroxysteroid dehydrogenase. Endocrinology. 1999;140:568–74. doi: 10.1210/endo.140.2.6531. [DOI] [PubMed] [Google Scholar]
- 160.Hoffmann R, Rot A, Niiyama S, Billich A. Steroid sulfatase in the human hair follicle concentrates in the dermal papilla. J Invest Dermatol. 2001;117:1342–8. doi: 10.1046/j.0022-202x.2001.01547.x. [DOI] [PubMed] [Google Scholar]
- 161.Toda K, Simpson ER, Mendelson CR, Shizuta Y, Kilgore MW. Expression of the gene encoding aromatase cytochrome P450 (CYP19) in fetal tissues. Mol Endocrinol. 1994;8:210–7. doi: 10.1210/mend.8.2.8170477. [DOI] [PubMed] [Google Scholar]
- 162.Swanson HI. Cytochrome P450 expression in human keratinocytes: an aryl hydrocarbon receptor perspective. Chem Biol Interact. 2004;149:69–79. doi: 10.1016/j.cbi.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 163.Zouboulis CC, Chen WC, Thornton MJ, Qin K, Rosenfield R. Sexual hormones in human skin. Horm Metab Res. 2007;39:85–95. doi: 10.1055/s-2007-961807. [DOI] [PubMed] [Google Scholar]
- 164.Slominski AT, Manna PR, Tuckey RC. Cutaneous glucocorticosteroidogenesis: securing local homeostasis and the skin integrity. Exp Dermatol. 2014;23:369–74. doi: 10.1111/exd.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Manna PR, Dyson MT, Eubank DW, Clark BJ, Lalli E, Sassone-Corsi P, et al. Regulation of steroidogenesis and the steroidogenic acute regulatory protein by a member of the cAMP responseelement binding protein family. Mol Endocrinol. 2002;16:184–99. doi: 10.1210/mend.16.1.0759. [DOI] [PubMed] [Google Scholar]
- 166.Manna PR, Huhtaniemi IT, Stocco DM. Mechanisms of protein kinase C signaling in the modulation of 3′,5′-cyclic adenosine monophosphate-mediated steroidogenesis in mouse gonadal cells. Endocrinology. 2009;150:3308–17. doi: 10.1210/en.2008-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Manna PR, Dyson MT, Stocco DM. Regulation of the steroidogenic acute regulatory protein gene expression: present and future perspectives. Mol Hum Reprod. 2009;15:321–33. doi: 10.1093/molehr/gap025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32:81–151. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Watari H, Arakane F, Moog-Lutz C, Kallen CB, Tomasetto C, Gerton GL, et al. MLN64 contains a domain with homology to the steroidogenic acute regulatory protein (StAR) that stimulates steroidogenesis. Proc Natl Acad Sci U S A. 1997;94:8462–7. doi: 10.1073/pnas.94.16.8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Brown RC, Papadopoulos V. Role of the peripheral-type benzodiazepine receptor in adrenal and brain steroidogenesis. Int Rev Neurobiol. 2001;46:117–43. doi: 10.1016/s0074-7742(01)46061-2. [DOI] [PubMed] [Google Scholar]
- 171.Tuckey RC, Bose HS, Czerwionka I, Miller WL. Molten globule structure and steroidogenic activity of N-218 MLN64 in human placental mitochondria. Endocrinology. 2004;145:1700–7. doi: 10.1210/en.2003-1034. [DOI] [PubMed] [Google Scholar]
- 172.Liu J, Rone MB, Papadopoulos V. Protein-protein interactions mediate mitochondrial cholesterol transport and steroid biosynthesis. J Biol Chem. 2006;281:38879–93. doi: 10.1074/jbc.M608820200. [DOI] [PubMed] [Google Scholar]
- 173.Chen W, Tsai SJ, Liao CY, Tsai RY, Chen YJ, Pan BJ, et al. Higher levels of steroidogenic acute regulatory protein and type I 3beta-hydroxysteroid dehydrogenase in the scalp of men with androgenetic alopecia. J Invest Dermatol. 2006;126:2332–5. doi: 10.1038/sj.jid.5700442. [DOI] [PubMed] [Google Scholar]
- 174.Tiala I, Suomela S, Huuhtanen J, Wakkinen J, Holtta-Vuori M, Kainu K, et al. The CCHCR1 (HCR) gene is relevant for skin steroidogenesis and downregulated in cultured psoriatic keratinocytes. J Mol Med (Berl) 2007;85:589–601. doi: 10.1007/s00109-006-0155-0. [DOI] [PubMed] [Google Scholar]
- 175.Elias PM, Wakefield JS. Therapeutic implications of a barrier-based pathogenesis of atopic dermatitis. Clin Rev Allergy Immunol. 2011;41:282–95. doi: 10.1007/s12016-010-8231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bikle DD. Vitamin D and the skin: Physiology and pathophysiology. Rev Endocr Metab Disord. 2012;13:3–19. doi: 10.1007/s11154-011-9194-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Suomela S, Elomaa O, Skoog T, Ala-aho R, Jeskanen L, Parssinen J, et al. CCHCR1 is up-regulated in skin cancer and associated with EGFR expression. PLoS One. 2009;4:e6030. doi: 10.1371/journal.pone.0006030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zouboulis CC, Makrantonaki E. Clinical aspects and molecular diagnostics of skin aging. Clin Dermatol. 2011;29:3–14. doi: 10.1016/j.clindermatol.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 179.Gruenewald DA, Matsumoto AM. Testosterone supplementation therapy for older men: potential benefits and risks. J Am Geriatr Soc. 2003;51:101–15. doi: 10.1034/j.1601-5215.2002.51018.x. discussion 15. [DOI] [PubMed] [Google Scholar]
- 180.Hull KL, Harvey S. Growth hormone therapy and Quality of Life: possibilities, pitfalls and mechanisms. J Endocrinol. 2003;179:311–33. doi: 10.1677/joe.0.1790311. [DOI] [PubMed] [Google Scholar]
- 181.Rosano GM, Maffei S, Andreassi MG, Vitale C, Vassalle C, Gambacciani M, et al. Hormone replacement therapy and cardioprotection: a new dawn? A statement of the Study Group on Cardiovascular Disease in Women of the Italian Society of Cardiology on hormone replacement therapy in postmenopausal women. J Cardiovasc Med (Hagerstown) 2009;10:85–92. doi: 10.2459/JCM.0b013e328313e979. [DOI] [PubMed] [Google Scholar]
- 182.Maki PM, Henderson VW. Hormone therapy, dementia, and cognition: the Women’s Health Initiative 10 years on. Climacteric. 2012;15:256–62. doi: 10.3109/13697137.2012.660613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Frick KM. Building a better hormone therapy? How understanding the rapid effects of sex steroid hormones could lead to new therapeutics for age-related memory decline. Behav Neurosci. 2012;126:29–53. doi: 10.1037/a0026660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Giannoulis MG, Martin FC, Nair KS, Umpleby AM, Sonksen P. Hormone replacement therapy and physical function in healthy older men. Time to talk hormones? Endocr Rev. 2012;33:314–77. doi: 10.1210/er.2012-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Osterberg EC, Bernie AM, Ramasamy R. Risks of testosterone replacement therapy in men. Indian J Urol. 2014;30:2–7. doi: 10.4103/0970-1591.124197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Osuga J, Ishibashi S, Oka T, Yagyu H, Tozawa R, Fujimoto A, et al. Targeted disruption of hormonesensitive lipase results in male sterility and adipocyte hypertrophy, but not in obesity. Proc Natl Acad Sci U S A. 2000;97:787–92. doi: 10.1073/pnas.97.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Holm C. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem Soc Trans. 2003;31:1120–4. doi: 10.1042/bst0311120. [DOI] [PubMed] [Google Scholar]
- 188.Kraemer FB, Shen WJ, Harada K, Patel S, Osuga J, Ishibashi S, et al. Hormone-sensitive lipase is required for high-density lipoprotein cholesteryl ester-supported adrenal steroidogenesis. Mol Endocrinol. 2004;18:549–57. doi: 10.1210/me.2003-0179. [DOI] [PubMed] [Google Scholar]
- 189.Manna PR, Cohen-Tannoudji J, Counis R, Garner CW, Huhtaniemi I, Kraemer FB, et al. Mechanisms of action of hormone-sensitive lipase in mouse Leydig cells: its role in the regulation of the steroidogenic acute regulatory protein. J Biol Chem. 2013;288:8505–18. doi: 10.1074/jbc.M112.417873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yeaman SJ. Hormone-sensitive lipase--new roles for an old enzyme. Biochem J. 2004;379:11–22. doi: 10.1042/BJ20031811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Krintel C, Morgelin M, Logan DT, Holm C. Phosphorylation of hormone-sensitive lipase by protein kinase A in vitro promotes an increase in its hydrophobic surface area. FEBS J. 2009;276:4752–62. doi: 10.1111/j.1742-4658.2009.07172.x. [DOI] [PubMed] [Google Scholar]
- 192.Strom K, Hansson O, Lucas S, Nevsten P, Fernandez C, Klint C, et al. Attainment of brown adipocyte features in white adipocytes of hormone-sensitive lipase null mice. PLoS One. 2008;3:e1793. doi: 10.1371/journal.pone.0001793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Strom K, Gundersen TE, Hansson O, Lucas S, Fernandez C, Blomhoff R, et al. Hormone-sensitive lipase (HSL) is also a retinyl ester hydrolase: evidence from mice lacking HSL. FASEB J. 2009;23:2307–16. doi: 10.1096/fj.08-120923. [DOI] [PubMed] [Google Scholar]
- 194.Repa JJ, Berge KE, Pomajzl C, Richardson JA, Hobbs H, Mangelsdorf DJ. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. J Biol Chem. 2002;277:18793–800. doi: 10.1074/jbc.M109927200. [DOI] [PubMed] [Google Scholar]
- 195.Rigamonti E, Helin L, Lestavel S, Mutka AL, Lepore M, Fontaine C, et al. Liver X receptor activation controls intracellular cholesterol trafficking and esterification in human macrophages. Circ Res. 2005;97:682–9. doi: 10.1161/01.RES.0000184678.43488.9f. [DOI] [PubMed] [Google Scholar]
- 196.Cummins CL, Volle DH, Zhang Y, McDonald JG, Sion B, Lefrancois-Martinez AM, et al. Liver X receptors regulate adrenal cholesterol balance. J Clin Invest. 2006;116:1902–12. doi: 10.1172/JCI28400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Volle DH, Lobaccaro JM. Role of the nuclear receptors for oxysterols LXRs in steroidogenic tissues: beyond the “foie gras”, the steroids and sex? Mol Cell Endocrinol. 2007;265–266:183–9. doi: 10.1016/j.mce.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 198.Manna PR, Slominski AT, King SR, Stetson CL, Stocco DM. Synergistic activation of steroidogenic acute regulatory protein expression and steroid biosynthesis by retinoids: involvement of cAMP/PKA signaling. Endocrinology. 2014;155:576–91. doi: 10.1210/en.2013-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hanley K, Ng DC, He SS, Lau P, Min K, Elias PM, et al. Oxysterols induce differentiation in human keratinocytes and increase Ap-1-dependent involucrin transcription. J Invest Dermatol. 2000;114:545–53. doi: 10.1046/j.1523-1747.2000.00895.x. [DOI] [PubMed] [Google Scholar]
- 200.Kielar D, Kaminski WE, Liebisch G, Piehler A, Wenzel JJ, Mohle C, et al. Adenosine triphosphate binding cassette (ABC) transporters are expressed and regulated during terminal keratinocyte differentiation: a potential role for ABCA7 in epidermal lipid reorganization. J Invest Dermatol. 2003;121:465–74. doi: 10.1046/j.1523-1747.2003.12404.x. [DOI] [PubMed] [Google Scholar]
- 201.Shulman AI, Mangelsdorf DJ. Retinoid x receptor heterodimers in the metabolic syndrome. N Engl J Med. 2005;353:604–15. doi: 10.1056/NEJMra043590. [DOI] [PubMed] [Google Scholar]
- 202.Choudhury RP, Lee JM, Greaves DR. Mechanisms of disease: macrophage-derived foam cells emerging as therapeutic targets in atherosclerosis. Nat Clin Pract Cardiovasc Med. 2005;2:309–15. doi: 10.1038/ncpcardio0195. [DOI] [PubMed] [Google Scholar]
- 203.Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 2008;454:470–7. doi: 10.1038/nature07202. [DOI] [PubMed] [Google Scholar]
- 204.Nohara A, Kobayashi J, Mabuchi H. Retinoid X receptor heterodimer variants and cardiovascular risk factors. J Atheroscler Thromb. 2009;16:303–18. doi: 10.5551/jat.no786. [DOI] [PubMed] [Google Scholar]
- 205.Chang KC, Shen Q, Oh IG, Jelinsky SA, Jenkins SF, Wang W, et al. Liver X receptor is a therapeutic target for photoaging and chronological skin aging. Mol Endocrinol. 2008;22:2407–19. doi: 10.1210/me.2008-0232. [DOI] [PubMed] [Google Scholar]
- 206.Hong I, Rho HS, Kim DH, Lee MO. Activation of LXRalpha induces lipogenesis in HaCaT cells. Arch Pharm Res. 2010;33:1443–9. doi: 10.1007/s12272-010-0919-5. [DOI] [PubMed] [Google Scholar]
- 207.Komuves LG, Schmuth M, Fowler AJ, Elias PM, Hanley K, Man MQ, et al. Oxysterol stimulation of epidermal differentiation is mediated by liver X receptor-beta in murine epidermis. J Invest Dermatol. 2002;118:25–34. doi: 10.1046/j.0022-202x.2001.01628.x. [DOI] [PubMed] [Google Scholar]
- 208.Fowler AJ, Sheu MY, Schmuth M, Kao J, Fluhr JW, Rhein L, et al. Liver X receptor activators display anti-inflammatory activity in irritant and allergic contact dermatitis models: liver-X-receptor-specific inhibition of inflammation and primary cytokine production. J Invest Dermatol. 2003;120:246–55. doi: 10.1046/j.1523-1747.2003.12033.x. [DOI] [PubMed] [Google Scholar]
- 209.Mukherjee S, Date A, Patravale V, Korting HC, Roeder A, Weindl G. Retinoids in the treatment of skin aging: an overview of clinical efficacy and safety. Clin Interv Aging. 2006;1:327–48. doi: 10.2147/ciia.2006.1.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ortonne JP. Retinoid therapy of pigmentary disorders. Dermatol Ther. 2006;19:280–8. doi: 10.1111/j.1529-8019.2006.00085.x. [DOI] [PubMed] [Google Scholar]
- 211.Memezawa A, Takada I, Takeyama K, Igarashi M, Ito S, Aiba S, et al. Id2 gene-targeted crosstalk between Wnt and retinoid signaling regulates proliferation in human keratinocytes. Oncogene. 2007;26:5038–45. doi: 10.1038/sj.onc.1210320. [DOI] [PubMed] [Google Scholar]
- 212.So PL, Fujimoto MA, Epstein EH., Jr Pharmacologic retinoid signaling and physiologic retinoic acid receptor signaling inhibit basal cell carcinoma tumorigenesis. Mol Cancer Ther. 2008;7:1275–84. doi: 10.1158/1535-7163.MCT-07-2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Bushue N, Wan YJ. Retinoid pathway and cancer therapeutics. Adv Drug Deliv Rev. 2010;62:1285–98. doi: 10.1016/j.addr.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Esteban-Pretel G, Marin MP, Cabezuelo F, Moreno V, Renau-Piqueras J, Timoneda J, et al. Vitamin A deficiency increases protein catabolism and induces urea cycle enzymes in rats. J Nutr. 2010;140:792–8. doi: 10.3945/jn.109.119388. [DOI] [PubMed] [Google Scholar]
- 215.Slominski AT, Zmijewski MA, Semak I, Zbytek B, Pisarchik A, Li W, et al. Cytochromes P450 and Skin Cancer: Role of Local Endocrine Pathways. Anticancer Agents Med Chem. 2013 doi: 10.2174/18715206113139990308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Chambon P. The nuclear receptor superfamily: a personal retrospect on the first two decades. Mol Endocrinol. 2005;19:1418–28. doi: 10.1210/me.2005-0125. [DOI] [PubMed] [Google Scholar]
- 217.Germain P, Chambon P, Eichele G, Evans RM, Lazar MA, Leid M, et al. International Union of Pharmacology. LX. Retinoic acid receptors. Pharmacol Rev. 2006;58:712–25. doi: 10.1124/pr.58.4.4. [DOI] [PubMed] [Google Scholar]
- 218.Evans RM, Mangelsdorf DJ. Nuclear Receptors, RXR, and the Big Bang. Cell. 2014;157:255–66. doi: 10.1016/j.cell.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Chung SS, Sung W, Wang X, Wolgemuth DJ. Retinoic acid receptor alpha is required for synchronization of spermatogenic cycles and its absence results in progressive breakdown of the spermatogenic process. Dev Dyn. 2004;230:754–66. doi: 10.1002/dvdy.20083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Mark M, Ghyselinck NB, Chambon P. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu Rev Pharmacol Toxicol. 2006;46:451–80. doi: 10.1146/annurev.pharmtox.46.120604.141156. [DOI] [PubMed] [Google Scholar]
- 221.Li H, Clagett-Dame M. Vitamin A deficiency blocks the initiation of meiosis of germ cells in the developing rat ovary in vivo. Biol Reprod. 2009;81:996–1001. doi: 10.1095/biolreprod.109.078808. [DOI] [PubMed] [Google Scholar]
- 222.Clagett-Dame M, Knutson D. Vitamin A in reproduction and development. Nutrients. 2011;3:385–428. doi: 10.3390/nu3040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Olson CR, Mello CV. Significance of vitamin A to brain function, behavior and learning. Mol Nutr Food Res. 2010;54:489–95. doi: 10.1002/mnfr.200900246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Mihaly J, Gamlieli A, Worm M, Ruhl R. Decreased retinoid concentration and retinoid signalling pathways in human atopic dermatitis. Exp Dermatol. 2011;20:326–30. doi: 10.1111/j.1600-0625.2010.01225.x. [DOI] [PubMed] [Google Scholar]
- 225.Ono K, Yamada M. Vitamin A and Alzheimer’s disease. Geriatr Gerontol Int. 2012;12:180–8. doi: 10.1111/j.1447-0594.2011.00786.x. [DOI] [PubMed] [Google Scholar]
- 226.Bonhomme D, Minni AM, Alfos S, Roux P, Richard E, Higueret P, et al. Vitamin A status regulates glucocorticoid availability in Wistar rats: consequences on cognitive functions and hippocampal neurogenesis? Front Behav Neurosci. 2014;8:20. doi: 10.3389/fnbeh.2014.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Chung SS, Wolgemuth DJ. Role of retinoid signaling in the regulation of spermatogenesis. Cytogenet Genome Res. 2004;105:189–202. doi: 10.1159/000078189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hertoghe T. The “multiple hormone deficiency” theory of aging: is human senescence caused mainly by multiple hormone deficiencies? Ann N Y Acad Sci. 2005;1057:448–65. doi: 10.1196/annals.1322.035. [DOI] [PubMed] [Google Scholar]
- 229.Chahal HS, Drake WM. The endocrine system and ageing. J Pathol. 2007;211:173–80. doi: 10.1002/path.2110. [DOI] [PubMed] [Google Scholar]
- 230.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–62. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Manor B, Lipsitz LA. Physiologic complexity and aging: implications for physical function and rehabilitation. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:287–93. doi: 10.1016/j.pnpbp.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Makrantonaki E, Schonknecht P, Hossini AM, Kaiser E, Katsouli MM, Adjaye J, et al. Skin and brain age together: The role of hormones in the ageing process. Exp Gerontol. 2010;45:801–13. doi: 10.1016/j.exger.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 233.Rousseau K, Kauser S, Pritchard LE, Warhurst A, Oliver RL, Slominski A, et al. Proopiomelanocortin (POMC), the ACTH/melanocortin precursor, is secreted by human epidermal keratinocytes and melanocytes and stimulates melanogenesis. FASEB J. 2007;21:1844–56. doi: 10.1096/fj.06-7398com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Pain S, Dezutter C, Reymermier C, Vogelgesang B, Delay E, Andre V. Age-related changes in proopiomelanocortin (POMC) and related receptors in human epidermis. Int J Cosmet Sci. 2010;32:266–75. doi: 10.1111/j.1468-2494.2009.00569.x. [DOI] [PubMed] [Google Scholar]
- 235.Torma H. Regulation of keratin expression by retinoids. Dermatoendocrinol. 2011;3:136–40. doi: 10.4161/derm.3.3.15026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Jang HS, Oh CK, Jo JH, Kim YS, Kwon KS. Detection of telomerase activity in psoriasis lesional skin and correlation with Ki-67 expression and suppression by retinoic acid. J Korean Med Sci. 2001;16:623–9. doi: 10.3346/jkms.2001.16.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Skoog T, Elomaa O, Pasonen-Seppanen SM, Forsberg S, Ahokas K, Jeskanen L, et al. Matrix metalloproteinase-21 expression is associated with keratinocyte differentiation and upregulated by retinoic acid in HaCaT cells. J Invest Dermatol. 2009;129:119–30. doi: 10.1038/jid.2008.206. [DOI] [PubMed] [Google Scholar]
- 238.Lee DD, Stojadinovic O, Krzyzanowska A, Vouthounis C, Blumenberg M, Tomic-Canic M. Retinoidresponsive transcriptional changes in epidermal keratinocytes. J Cell Physiol. 2009;220:427–39. doi: 10.1002/jcp.21784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Gazel A, Nijhawan RI, Walsh R, Blumenberg M. Transcriptional profiling defines the roles of ERK and p38 kinases in epidermal keratinocytes. J Cell Physiol. 2008;215:292–308. doi: 10.1002/jcp.21394. [DOI] [PubMed] [Google Scholar]
- 240.Karlsson T, Vahlquist A, Torma H. Keratinocyte differentiation induced by calcium, phorbol ester or interferon-gamma elicits distinct changes in the retinoid signalling pathways. J Dermatol Sci. 2010;57:207–13. doi: 10.1016/j.jdermsci.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 241.Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376:180–8. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Guryev O, Carvalho RA, Usanov S, Gilep A, Estabrook RW. A pathway for the metabolism of vitamin D3: unique hydroxylated metabolites formed during catalysis with cytochrome P450scc (CYP11A1) Proc Natl Acad Sci U S A. 2003;100:14754–9. doi: 10.1073/pnas.2336107100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Slominski AT, Zmijewski MA, Semak I, Sweatman T, Janjetovic Z, Li W, et al. Sequential metabolism of 7-dehydrocholesterol to steroidal 5,7-dienes in adrenal glands and its biological implication in the skin. PLoS One. 2009;4:e4309. doi: 10.1371/journal.pone.0004309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Slominski AT, Kim TK, Chen J, Nguyen MN, Li W, Yates CR, et al. Cytochrome P450scc-dependent metabolism of 7-dehydrocholesterol in placenta and epidermal keratinocytes. Int J Biochem Cell Biol. 2012;44:2003–18. doi: 10.1016/j.biocel.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Slominski AT, Janjetovic Z, Fuller BE, Zmijewski MA, Tuckey RC, Nguyen MN, et al. Products of vitamin D3 or 7-dehydrocholesterol metabolism by cytochrome P450scc show anti-leukemia effects, having low or absent calcemic activity. PLoS One. 2010;5:e9907. doi: 10.1371/journal.pone.0009907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Zmijewski MA, Li W, Zjawiony JK, Sweatman TW, Chen J, Miller DD, et al. Synthesis and photoconversion of androsta- and pregna-5,7-dienes to vitamin D3-like derivatives. Photochem Photobiol Sci. 2008;7:1570–6. doi: 10.1039/b809005j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zmijewski MA, Li W, Zjawiony JK, Sweatman TW, Chen J, Miller DD, et al. Photo-conversion of two epimers (20R and 20S) of pregna-5,7-diene-3 beta, 17 alpha, 20-triol and their bioactivity in melanoma cells. Steroids. 2009;74:218–28. doi: 10.1016/j.steroids.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zmijewski MA, Li W, Chen J, Kim TK, Zjawiony JK, Sweatman TW, et al. Synthesis and photochemical transformation of 3beta,21-dihydroxypregna-5,7-dien-20-one to novel secosteroids that show anti-melanoma activity. Steroids. 2011;76:193–203. doi: 10.1016/j.steroids.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Slominski AT, Li W, Bhattacharya SK, Smith RA, Johnson PL, Chen J, et al. Vitamin D analogs 17,20S(OH)2pD and 17,20R(OH)2pD are noncalcemic and exhibit antifibrotic activity. J Invest Dermatol. 2011;131:1167–9. doi: 10.1038/jid.2010.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Holick MF, MacLaughlin JA, Doppelt SH. Regulation of cutaneous previtamin D3 photosynthesis in man: skin pigment is not an essential regulator. Science. 1981;211:590–3. doi: 10.1126/science.6256855. [DOI] [PubMed] [Google Scholar]
- 251.Tuckey RC, Slominski AT, Cheng CY, Chen J, Kim TK, Xiao M, et al. Lumisterol is metabolized by CYP11A1: Discovery of a new pathway. Int J Biochem Cell Biol. 2014;55C:24–34. doi: 10.1016/j.biocel.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.MacLaughlin JA, Anderson RR, Holick MF. Spectral character of sunlight modulates photosynthesis of previtamin D3 and its photoisomers in human skin. Science. 1982;216:1001–3. doi: 10.1126/science.6281884. [DOI] [PubMed] [Google Scholar]
- 253.Holick MF, Tian XQ, Allen M. Evolutionary importance for the membrane enhancement of the production of vitamin D3 in the skin of poikilothermic animals. Proc Natl Acad Sci U S A. 1995;92:3124–6. doi: 10.1073/pnas.92.8.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Wacker M, Holick MF. Sunlight and Vitamin D: A global perspective for health. Dermatoendocrinol. 2013;5:51–108. doi: 10.4161/derm.24494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Dixon KM, Norman AW, Sequeira VB, Mohan R, Rybchyn MS, Reeve VE, et al. 1alpha,25(OH)(2)- vitamin D and a nongenomic vitamin D analogue inhibit ultraviolet radiation-induced skin carcinogenesis. Cancer Prev Res (Phila) 2011;4:1485–94. doi: 10.1158/1940-6207.CAPR-11-0165. [DOI] [PubMed] [Google Scholar]
- 256.Slominski A, Tuckey RC, Zmijewski MA, Li W, Zjawiony J, Janjetovic Z, et al. Enzymatic production or chemical synthesis and uses for 5,7-dienes and UVB conversion products thereof. PCT/US2009/001324.
- 257.Slominski A, Semak I, Zjawiony J, Wortsman J, Li W, Szczesniewski A, et al. The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism. FEBS J. 2005;272:4080–90. doi: 10.1111/j.1742-4658.2005.04819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Tuckey RC, Janjetovic Z, Li W, Nguyen MN, Zmijewski MA, Zjawiony J, et al. Metabolism of 1alphahydroxyvitamin D3 by cytochrome P450scc to biologically active 1alpha,20-dihydroxyvitamin D3. J Steroid Biochem Mol Biol. 2008;112:213–9. doi: 10.1016/j.jsbmb.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Tuckey RC, Li W, Zjawiony JK, Zmijewski MA, Nguyen MN, Sweatman T, et al. Pathways and products for the metabolism of vitamin D3 by cytochrome P450scc. FEBS J. 2008;275:2585–96. doi: 10.1111/j.1742-4658.2008.06406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Tuckey RC, Li W, Shehabi HZ, Janjetovic Z, Nguyen MN, Kim TK, et al. Production of 22-hydroxy metabolites of vitamin D3 by cytochrome p450scc (CYP11A1) and analysis of their biological activities on skin cells. Drug Metab Dispos. 2011;39:1577–88. doi: 10.1124/dmd.111.040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Strushkevich N, MacKenzie F, Cherkesova T, Grabovec I, Usanov S, Park HW. Structural basis for pregnenolone biosynthesis by the mitochondrial monooxygenase system. Proc Natl Acad Sci U S A. 2011;108:10139–43. doi: 10.1073/pnas.1019441108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Slominski AT, Kim TK, Shehabi HZ, Tang EK, Benson HA, Semak I, et al. In vivo production of novel vitamin D2 hydroxy-derivatives by human placentas, epidermal keratinocytes, Caco-2 colon cells and the adrenal gland. Mol Cell Endocrinol. 2014;383:181–92. doi: 10.1016/j.mce.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Zbytek B, Janjetovic Z, Tuckey RC, Zmijewski MA, Sweatman TW, Jones E, et al. 20-Hydroxyvitamin D3, a product of vitamin D3 hydroxylation by cytochrome P450scc, stimulates keratinocyte differentiation. J Invest Dermatol. 2008;128:2271–80. doi: 10.1038/jid.2008.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Janjetovic Z, Tuckey RC, Nguyen MN, Thorpe EM, Jr, Slominski AT. 20,23-dihydroxyvitamin D3, novel P450scc product, stimulates differentiation and inhibits proliferation and NF-kappaB activity in human keratinocytes. J Cell Physiol. 2010;223:36–48. doi: 10.1002/jcp.21992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Slominski AT, Janjetovic Z, Kim TK, Wright AC, Grese LN, Riney SJ, et al. Novel vitamin D hydroxyderivatives inhibit melanoma growth and show differential effects on normal melanocytes. Anticancer Res. 2012;32:3733–42. [PMC free article] [PubMed] [Google Scholar]
- 266.Janjetovic Z, Brozyna AA, Tuckey RC, Kim TK, Nguyen MN, Jozwicki W, et al. High basal NF-kappaB activity in nonpigmented melanoma cells is associated with an enhanced sensitivity to vitamin D3 derivatives. Br J Cancer. 2011;105:1874–84. doi: 10.1038/bjc.2011.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Tang EK, Li W, Janjetovic Z, Nguyen MN, Wang Z, Slominski A, et al. Purified mouse CYP27B1 can hydroxylate 20,23-dihydroxyvitamin D3, producing 1alpha,20,23-trihydroxyvitamin D3, which has altered biological activity. Drug Metab Dispos. 2010;38:1553–9. doi: 10.1124/dmd.110.034389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Tieu EW, Tang EK, Chen J, Li W, Nguyen MN, Janjetovic Z, et al. Rat CYP24A1 acts on 20- hydroxyvitamin D(3) producing hydroxylated products with increased biological activity. Biochem Pharmacol. 2012;84:1696–704. doi: 10.1016/j.bcp.2012.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Kim T-K, Wang J, Janjetovic Z, Chen J, Tuckey RC, Nguyen MN, et al. Correlation between secosteroid induced vitamin D receptor activity in melanoma cells and computer-modeled receptor binding strength. Mol Cell Endocrinol. 2012 doi: 10.1016/j.mce.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Slominski AT, Kim TK, Janjetovic Z, Tuckey RC, Bieniek R, Yue J, et al. 20-Hydroxyvitamin D2 is a noncalcemic analog of vitamin D with potent antiproliferative and prodifferentiation activities in normal and malignant cells. Am J Physiol Cell Physiol. 2011;300:C526–41. doi: 10.1152/ajpcell.00203.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Janjetovic Z, Zmijewski MA, Tuckey RC, DeLeon DA, Nguyen MN, Pfeffer LM, et al. 20- Hydroxycholecalciferol, product of vitamin D3 hydroxylation by P450scc, decreases NF-kappaB activity by increasing IkappaB alpha levels in human keratinocytes. PLoS One. 2009;4:e5988. doi: 10.1371/journal.pone.0005988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Slominski AT, Kim TK, Takeda Y, Janjetovic Z, Brozyna AA, Skobowiat C, et al. RORalpha and ROR gamma are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20- hydroxy- and 20,23-dihydroxyvitamin D. FASEB J. 2014;28:2775–89. doi: 10.1096/fj.13-242040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Slominski A, Janjetovic Z, Tuckey RC, Nguyen MN, Bhattacharya KG, Wang J, et al. 20SHydroxyvitamin D3, noncalcemic product of CYP11A1 action on vitamin D3, exhibits potent antifibrogenic activity in vivo. J Clin Endocrinol Metab. 2013;98:E298–303. doi: 10.1210/jc.2012-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Wang J, Slominski A, Tuckey RC, Janjetovic Z, Kulkarni A, Chen J, et al. 20-hydroxyvitamin D inhibits proliferation of cancer cells with high efficacy while being non-toxic. Anticancer Res. 2012;32:739–46. [PMC free article] [PubMed] [Google Scholar]
- 275.Chen J, Wang J, Kim T-K, Tieu E, Tang E, Lin Z, et al. Novel Vitamin D Analogs as Potential Therapeutics: Metabolism, Toxicity Profiling, and Antiproliferative Activity. Anticancer Research. 2014;34:2153–63. [PMC free article] [PubMed] [Google Scholar]
- 276.Tongkao-On W, Carter S, Reeve VE, Dixon KM, Gordon-Thomson C, Halliday GM, et al. CYP11A1 in skin: An alternative route to photoprotection by vitamin D compounds. J Steroid Biochem Mol Biol. 2014 doi: 10.1016/j.jsbmb.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 277.Slominski AT, Janjetovic Z, Kim TK, Wasilewski P, Rosas S, Hanna S, et al. Novel non-calcemic secosteroids that are produced by human epidermal keratinocytes protect against solar radiation. J Steroid Biochem Mol Biol. 2015;148:52–63. doi: 10.1016/j.jsbmb.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Slominski A, Semak I, Wortsman J, Zjawiony J, Li W, Zbytek B, et al. An alternative pathway of vitamin D metabolism. Cytochrome P450scc (CYP11A1)-mediated conversion to 20-hydroxyvitamin D2 and 17,20-dihydroxyvitamin D2. FEBS J. 2006;273:2891–901. doi: 10.1111/j.1742-4658.2006.05302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Nguyen MN, Slominski A, Li W, Ng YR, Tuckey RC. Metabolism of vitamin D2 to 17,20,24- trihydroxyvitamin D2 by cytochrome p450scc (CYP11A1) Drug Metab Dispos. 2009;37:761–7. doi: 10.1124/dmd.108.025619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Slominski A, Semak I, Zjawiony J, Wortsman J, Gandy MN, Li J, et al. Enzymatic metabolism of ergosterol by cytochrome p450scc to biologically active 17alpha,24-dihydroxyergosterol. Chem Biol. 2005;12:931–9. doi: 10.1016/j.chembiol.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 281.Tuckey RC, Nguyen MN, Chen J, Slominski AT, Baldisseri DM, Tieu EW, et al. Human cytochrome P450scc (CYP11A1) catalyzes epoxide formation with ergosterol. Drug Metab Dispos. 2012;40:436–44. doi: 10.1124/dmd.111.042515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Bikle DD, Nemanic MK, Gee E, Elias P. 1,25-Dihydroxyvitamin D3 production by human keratinocytes. Kinetics and regulation. J Clin Invest. 1986;78:557–66. doi: 10.1172/JCI112609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Skobowiat C, Dowdy JC, Sayre RM, Tuckey RC, Slominski A. Cutaneous hypothalamic-pituitaryadrenal axis homolog: regulation by ultraviolet radiation. Am J Physiol Endocrinol Metab. 2011;301:E484–93. doi: 10.1152/ajpendo.00217.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Skobowiat C, Slominski AT. UVB Activates Hypothalamic-Pituitary-Adrenal Axis in C57BL/6 Mice. J Invest Dermatol. 2014 doi: 10.1038/jid.2014.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Bolognia J, Murray M, Pawelek J. UVB-induced melanogenesis may be mediated through the MSHreceptor system. J Invest Dermatol. 1989;92:651–6. doi: 10.1111/1523-1747.ep12696836. [DOI] [PubMed] [Google Scholar]
- 286.Pawelek JM, Chakraborty AK, Osber MP, Orlow SJ, Min KK, Rosenzweig KE, et al. Molecular cascades in UV-induced melanogenesis: a central role for melanotropins? Pigment Cell Res. 1992;5:348–56. doi: 10.1111/j.1600-0749.1992.tb00561.x. [DOI] [PubMed] [Google Scholar]
- 287.Chakraborty AK, Funasaka Y, Slominski A, Bolognia J, Sodi S, Ichihashi M, et al. UV light and MSH receptors. Ann N Y Acad Sci. 1999;885:100–16. doi: 10.1111/j.1749-6632.1999.tb08668.x. [DOI] [PubMed] [Google Scholar]
- 288.Tiganescu A, Walker EA, Hardy RS, Mayes AE, Stewart PM. Localization, age- and site-dependent expression, and regulation of 11beta-hydroxysteroid dehydrogenase type 1 in skin. J Invest Dermatol. 2011;131:30–6. doi: 10.1038/jid.2010.257. [DOI] [PubMed] [Google Scholar]
- 289.Tiganescu A, Tahrani AA, Morgan SA, Otranto M, Desmouliere A, Abrahams L, et al. 11beta- Hydroxysteroid dehydrogenase blockade prevents age-induced skin structure and function defects. J Clin Invest. 2013;123:3051–60. doi: 10.1172/JCI64162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Tiganescu A, Hupe M, Uchida Y, Mauro T, Elias PM, Holleran WM. Increased glucocorticoid activation during mouse skin wound healing. J Endocrinol. 2014;221:51–61. doi: 10.1530/JOE-13-0420. [DOI] [PubMed] [Google Scholar]
- 291.Terao M, Murota H, Kimura A, Kato A, Ishikawa A, Igawa K, et al. 11beta-Hydroxysteroid dehydrogenase-1 is a novel regulator of skin homeostasis and a candidate target for promoting tissue repair. PLoS One. 2011;6:e25039. doi: 10.1371/journal.pone.0025039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Terao M, Itoi S, Murota H, Katayama I. Expression profiles of cortisol-inactivating enzyme, 11betahydroxysteroid dehydrogenase-2, in human epidermal tumors and its role in keratinocyte proliferation. Exp Dermatol. 2013;22:98–101. doi: 10.1111/exd.12075. [DOI] [PubMed] [Google Scholar]
- 293.Skobowiat C, Slominski AT. UVB Activates Hypothalamic-Pituitary-Adrenal Axis in C57BL/6 Mice. J Invest Dermatol. 2014 doi: 10.1038/jid.2014.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Slominski A. Neuroendocrine system of the skin. Dermatology. 2005;211:199–208. doi: 10.1159/000087012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Slominski AT. Ultraviolet radiation (UVR) activates central neuro-endocrine-immune system. Photodermatol Photoimmunol Photomed. 2015 doi: 10.1111/phpp.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Schwarz A, Navid F, Sparwasser T, Clausen BE, Schwarz T. In vivo reprogramming of UV radiationinduced regulatory T-cell migration to inhibit the elicitation of contact hypersensitivity. J Allergy Clin Immunol. 2011;128:826–33. doi: 10.1016/j.jaci.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 297.Kripke ML. Ultraviolet radiation and immunology: something new under the sun--presidential address. Cancer Res. 1994;54:6102–5. [PubMed] [Google Scholar]
- 298.Hiramoto K, Yanagihara N, Sato EF, Inoue M. Ultraviolet B irradiation of the eye activates a nitric oxide-dependent hypothalamopituitary proopiomelanocortin pathway and modulates functions of alpha-melanocyte-stimulating hormone-responsive cells. J Invest Dermatol. 2003;120:123–7. doi: 10.1046/j.1523-1747.2003.12004.x. [DOI] [PubMed] [Google Scholar]
- 299.Hiramoto K. Ultraviolet A irradiation of the eye activates a nitric oxide-dependent hypothalamopituitary pro-opiomelanocortin pathway and modulates the functions of Langerhans cells. J Dermatol. 2009;36:335–45. doi: 10.1111/j.1346-8138.2009.00649.x. [DOI] [PubMed] [Google Scholar]
- 300.Hiramoto K, Mashita Y, Katada T, Konishi H, Sugiura M, Hayakawa R. Immunosuppression by ultraviolet B rays via eyes in mice. Arch Dermatol Res. 1997;289:709–11. doi: 10.1007/s004030050266. [DOI] [PubMed] [Google Scholar]
- 301.Yamate Y, Hiramoto K, Kasahara E, Sato EF. UVA irradiation of the eye modulates the contact hypersensitivity of the skin and intestines by affecting mast cells in mice. Photodermatol Photoimmunol Photomed. 2014 doi: 10.1111/phpp.12157. [DOI] [PubMed] [Google Scholar]
- 302.Nolan BV, Taylor SL, Liguori A, Feldman SR. Tanning as an addictive behavior: a literature review. Photodermatology, Photoimmunology & Photomedicine. 2009;25:12–9. doi: 10.1111/j.1600-0781.2009.00392.x. [DOI] [PubMed] [Google Scholar]
- 303.Kourosh AS, Harrington CR, Adinoff B. Tanning as a behavioral addiction. The American journal of drug and alcohol abuse. 2010;36:284–90. doi: 10.3109/00952990.2010.491883. [DOI] [PubMed] [Google Scholar]