Abstract
Oncogenic EGFR mutations are found in 10-35% of lung adenocarcinomas. Such mutations, which present most commonly as small in-frame deletions in exon 19 or point mutations in exon 21 (L858R), confer sensitivity to EGFR tyrosine kinase inhibitors (TKIs). In analyzing the tumor from a 33-year-old male never smoker, we identified a novel EGFR alteration in lung cancer: EGFR exon 18-25 kinase domain duplication (EGFR-KDD). Through analysis of a larger cohort of tumor samples, we detected additional cases of EGFR-KDD in lung, brain, and other cancers. In vitro, EGFR-KDD is constitutively active, and computational modeling provides potential mechanistic support for its auto-activation. EGFR-KDD-transformed cells are sensitive to EGFR TKIs and, consistent with these in vitro findings, the index patient had a partial response to the EGFR TKI, afatinib. The patient eventually progressed, at which time, re-sequencing revealed an EGFR-dependent mechanism of acquired resistance to afatinib, thereby validating EGFR-KDD as a driver alteration and therapeutic target.
Keywords: Epidermal growth factor receptor (EGFR), non-small cell lung cancer, glioblastoma, sarcoma, next-generation sequencing, targeted therapy, intragenic, gene rearrangement, kinase domain, tyrosine kinase inhibitor (TKI), erlotinib, afatinib, AZD9291
Introduction
The prospective identification and rational therapeutic targeting of tumor genomic alterations have revolutionized the care of patients with lung cancer and other malignancies. Oncogenic mutations in the epidermal growth factor receptor (EGFR) tyrosine kinase domain are found in an important subset of non-small cell lung cancer (NSCLC), and several large phase III clinical trials have shown that patients with EGFR-mutant lung cancer derive superior clinical responses when treated with EGFR tyrosine kinase inhibitors (TKIs) as compared with standard chemotherapy (1-3). Such mutations, which most commonly occur as either small in-frame deletions in exon 19 or point mutations in exon 21 (L858R), confer constitutive activity to the EGFR tyrosine kinase and sensitivity to EGFR TKIs (4). Other oncogenic alterations, including ALK and ROS1 gene rearrangements, have similarly allowed for the rational treatment of molecular cohorts of NSCLC. Unfortunately, despite these significant advances in defining clinically relevant molecular cohorts of lung cancer, the currently identified genomic alterations account for only 50-60% of all tumors. Additional analyses are necessary to identify therapeutically actionable molecular alterations in these tumors.
Here, we describe the case of a 33-year-old male never smoker with metastatic lung adenocarcinoma whose tumor lacked all previously described actionable genomic alterations in this disease. Targeted next generation sequencing (NGS) based genomic profiling identified a novel in-frame tandem duplication of EGFR exons 18-25, the exons that encode the EGFR tyrosine kinase domain. This EGFR kinase domain duplication (EGFR-KDD) had not previously been reported in lung cancer, and there were no pre-clinical data or clinical evidence to support the use of EGFR inhibitors in patients whose tumors harbor the EGFR-KDD. However, the index patient was treated with the EGFR inhibitor, afatinib, with rapid symptomatic improvement and significant decrease in tumor burden. Notably, upon disease progression, the patient's tumor harbored an increase in the copy number of the EGFR-KDD, solidifying the role of this EGFR alteration as a novel driver in this disease. Through analysis of a large set of annotated tumors, we demonstrate that the EGFR-KDD is recurrent in lung, brain, and soft tissue tumors. Overall, our data show, for the first time, that EGFR-KDD is an oncogenic and therapeutically actionable alteration.
Results
Case Report
A 33-year-old male never smoker was diagnosed with stage IV lung adenocarcinoma after presenting with cough and fatigue. His tumor biopsy was sent for genomic profiling using an extensively validated hybrid-capture-based NGS diagnostic assay (Foundation One™) (5). The patient's tumor was found to be negative for any previously described actionable alterations in lung cancer, including negative for the presence of previously described EGFR alterations such as L858R, G719A/C/S, and L861Q point mutations, exon 19 deletion/insertion, and exon 20 insertion. Interestingly, however, the patient's tumor was found to harbor an intragenic alteration in EGFR resulting in the tandem duplication of exons 18-25 (Fig. S1a). The presence of this alteration was confirmed by direct sequencing (data not shown) and by an independent clinical NGS assay (MSK-IMPACT™)(6) (Fig. S1b). Since exons 18-25 of EGFR encode the entire tyrosine kinase domain, this alteration results in an EGFR protein that has an in-frame kinase domain duplication (EGFR-KDD) (Fig. 1a and Fig. S2). Notably, this EGFR alteration had not previously been reported in lung cancer; the EGFR-KDD (as duplication of exons 18-25 or 18-26) had only been reported in isolated cases of glioma to date (7-11). There were no data regarding the frequency of this alteration in tumor samples, nor were there data regarding the efficacy of EGFR-targeted agents against the EGFR-KDD.
Figure 1. The EGFR-KDD is an oncogenic EGFR alteration.
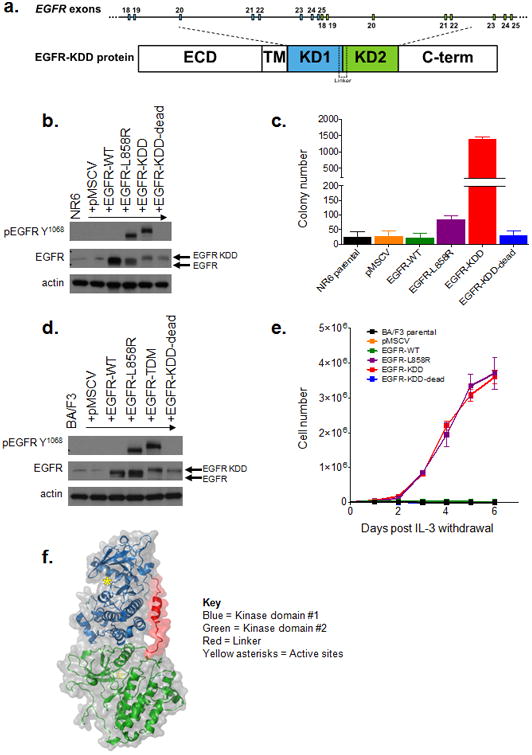
(a) Schematic representation of EGFR-KDD depicting the genetic and protein domain structures. ECD = extracellular domain. TM = transmembrane domain. Blue = EGFR exons 18-25 #1. Green = EGFR exons 18-25 #2. KD1 = first kinase domain. KD2 = second kinase domain. C-term = carboxyl terminus. (b) Representative western blot of NR6 cells stably expressing indicated EGFR constructs. EGFR-KDD-dead is a kinase dead version of EGFR-KDD. (c) NR6 cells stably expressing the indicated constructs (pMSCV = vector only) were plated in triplicate in soft agar, grown for 15 days, and quantified for colony formation. (d) Representative western blot of BA/F3 cells expressing indicated EGFR constructs. (e) BA/F3 cells transfected with indicated constructs (pMSCV = vector only) were grown in the absence of IL-3 and counted every 24 hours. (f) Ribbon diagram and space-filling model of the EGFR-KDD kinase domains (GLY 696 - PRO 1370) illustrating the proposed mechanism of auto-activation. Blue = first kinase domain; green = second kinase domain; red = linker; yellow asterisks = active sites.
Frequency of EGFR-KDD in lung and other cancers
To determine the frequency of the EGFR-KDD in lung cancer and other tumors, we analyzed data from >38,000 clinical cases, each of which had results from Foundation One™ targeted sequencing, analogous to the index patient. The EGFR-KDD was detected in 5 tumors from ∼7,200 total lung cancers tested. In addition, EGFR-KDD was identified in 3 gliomas, 1 sarcoma, 1 peritoneal carcinoma, and 1 Wilms' Tumor (Table 1). Among samples in The Cancer Genome Atlas (TCGA), we found previously unreported cases of the EGFR-KDD in lung adenocarcinoma and glioblastoma multiforme (Table 1 and Fig. S3a-d). Two additional cases were found by MSK-IMPACT™ sequencing (Table 1). Together, these data show that the EGFR-KDD is a recurrent mutation in lung cancer, glioma, and other human malignancies. It is important to note, however, that since most conventional (exomic) sequencing platforms would not routinely detect this particular EGFR alteration (due to its intronic breakpoints), these numbers are likely an underestimate, and the true prevalence of the EGFR-KDD remains unknown.
Table 1. The EGFR-KDD is a recurrent alteration.
| Dataset | Identification # | Age | Gender | Reported diagnosis |
|---|---|---|---|---|
| Foundation Medicine | FM-1 | 52 | Female | Lung adenocarcinoma |
| FM-2* | 33 | Male | Lung adenocarcinoma | |
| FM-3 | 53 | Female | Lung adenocarcinoma | |
| FM-4 | 57 | Female | Lung adenocarcinoma | |
| FM-5 | 29 | Female | Lung non-small cell lung cancer (NOS) | |
| FM-6 | 53 | Female | Brain astrocytoma | |
| FM-7 | 49 | Male | Brain glioblastoma | |
| FM-8 | 54 | Male | Brain glioblastoma | |
| FM-9 | 2 | Female | Kidney Wilms' tumor | |
| FM-10 | 63 | Female | Peritoneal serous carcinoma | |
| FM-11 | 27 | Female | Soft tissue sarcoma (NOS) | |
| TCGA | TCGA-49-4512 | 69 | Male | Lung adenocarcinoma |
| TCGA-12-0821 | 62 | Female | Brain glioblastoma | |
| MSKCC | MSKCC-1* | 33 | Male | Lung adenocarcinoma |
| MSKCC-2 | 67 | Female | Lung adenocarcinoma | |
| MSKCC-3† | 53 | Male | Brain glioblastoma |
Characteristics of EGFR-KDD exons 18–25 patients from Foundation Medicine, TCGA, and Memorial Sloan Kettering Cancer Center datasets. NOS = not otherwise specified.
= index patient.
= this patient's tumor also contained high level amplification of wild-type EGFR and an EGFR G719C mutation. The EGFR-KDD and EGFR G719C alterations were below the level of wild-type EGFR amplification, and presumably reflect sub-clonal populations.
The EGFR-KDD is oncogenic
We expressed EGFR-KDD in NR6 and BA/F3 cells (Figs. 1b-e). We observed expression of EGFR-KDD at the expected molecular weight as compared to EGFR wild-type (WT) and the well-characterized EGFR-L858R mutation (Figs. 1b, d). In contrast to EGFR-WT, both EGFR-L858R and EGFR-KDD displayed high levels of autophosphorylation. Consistent with these data, EGFR-KDD protein is constitutively autophosphorylated in the absence of serum in A1235 cells, a glioma cell line which harbors endogenous EGFR-KDD (Fig. S4) (7). To address whether EGFR-KDD is an oncogenic alteration, we tested its ability to confer anchorage-independent growth to NR6 cells. EGFR-KDD significantly increased colony formation in soft agar as compared to both EGFR-WT and the known oncogenic EGFR-L858R mutation (Figs. 1c and Figs. S5a-d). Expression of a kinase dead version of the EGFR-KDD (called EGFR-KDD-dead) abrogated the growth of NR6 cells in soft agar, consistent with the requirement of kinase activity for anchorage-independent growth. In parallel, we expressed the same EGFR variants in BA/F3 cells (Fig. 1d). EGFR-KDD, but not its kinase dead counterpart, induced IL-3-independent proliferation of BA/F3 cells, an activity phenotype associated with the transforming function of other oncogenic tyrosine kinases (Fig. 1e) (12). As previously reported, EGFR-L858R, but not EGFR-WT, was able to support IL-3 independent growth of BA/F3 cells (12).
Computational modeling demonstrates that EGFR-KDD can form intra-molecular dimers
To provide insight into the mechanism of activation of EGFR-KDD, we examined the structure of the EGF receptor. The EGFR tyrosine kinase is known to be activated either due to increased local concentration of EGFR (e.g., as a result of ligand binding or overexpression), mutations in the activation loop (e.g., L858R), or through formation of asymmetric (N-lobe to C-lobe) inter-molecular dimers between two EGFR proteins (13). Given the presence of two tandem in-frame kinase domains within the EGFR-KDD structure, we hypothesized that EGFR-KDD could form an intra-molecular dimer. To test this hypothesis, we modeled the EGFR-KDD based on the available experimental structure of the active asymmetric EGFR dimer (13). Conformational loop sampling with Rosetta demonstrates that the linker between the tandem tyrosine kinase domains allows for the proper positioning of the two domains necessary for asymmetric dimerization and intra-molecular EGFR activation (Fig. 1f). Therefore, our model suggests that EGFR-KDD is an oncogenic variant of the EGF receptor likely by virtue of its ability to form intra-molecular asymmetric activated dimers. Although modeling demonstrates that the EGFR-KDD is geometrically capable of forming intra-molecular asymmetric dimers, further experimental data would be needed to confirm this mechanism.
The EGFR-KDD can be therapeutically targeted with existing EGFR TKIs
We sought to determine whether EGFR TKIs are an effective therapeutic strategy for tumors harboring the EGFR-KDD. We treated BA/F3 cells expressing EGFR-WT, EGFR-L858R, and EGFR-KDD with erlotinib (1st generation reversible EGFR TKI) (14), afatinib (2nd generation irreversible inhibitor of EGFR/HER2)(15), and AZD9291 (3rd generation mutant specific EGFR TKI) (16) to assess the effects of these inhibitors on the autophosphorylation (and by extension, the kinase activity) and downstream signaling properties of the EGFR kinase. All three EGFR TKIs were able to inhibit EGFR-KDD tyrosine phosphorylation in a dose-dependent manner, albeit to different levels (Fig. 2a). Afatinib was the most potent inhibitor of EGFR-KDD autophosphorylation, at doses similar to those required for EGFR-L858R inhibition. Activation of downstream MAPK signaling was also inhibited in BA/F3 cells expressing EGFR-KDD, as shown by decreased ERK phosphorylation after drug treatment. Similar results were observed in 293T cells transfected with EGFR variants (Fig. S6a) and in A1235 cells which harbor endogenous EGFR-KDD (Fig. S6b). To determine whether inhibition of EGFR autophosphorylation translated to inhibition of cellular proliferation, we treated BA/F3 cells expressing various EGFR constructs with erlotinib, afatinib, and AZD9291. All three distinct EGFR TKIs effectively inhibited the growth of EGFR-KDD BA/F3 cells (Fig. 2b and Table S1). Consistent with our signaling data, the growth inhibition observed was most pronounced with afatinib. Analogous results were seen in A1235 cells (Fig. S6c). Together, these results show that the EGFR-KDD can be potently inhibited by afatinib, leading to decreased cell viability.
Figure 2. The EGFR-KDD can be therapeutically targeted with existing EGFR TKIs.
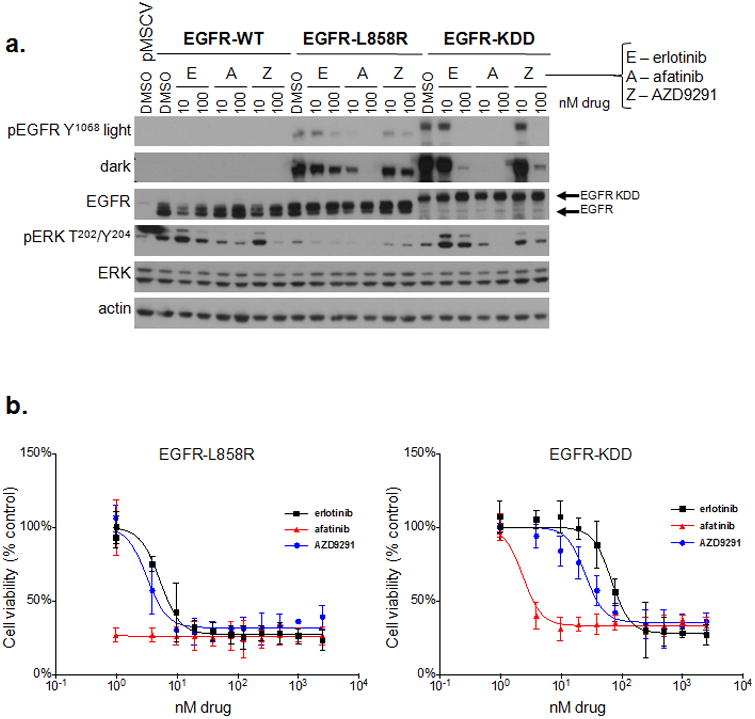
(a) BA/F3 lines stably expressing EGFR-WT, -L858R or -KDD were treated with increasing doses of erlotinib, afatinib, or AZD9291 for 2 hours and lysed for western blot analysis with the indicated antibodies. (b) BA/F3 lines stably expressing EGFR-L858R or EGFR-KDD were treated with increasing doses of erlotinib, afatinib, or AZD9291 for 72 hours. Cell titer blue assays were performed to assess cell viability. Each point represents quadruplicate replicates. Data are presented as the mean percentage of viable cells compared to vehicle control ± s.d.
Treatment of the index patient with afatinib
Since there were no data regarding the use of EGFR TKIs or monoclonal antibodies in the setting of a tumor harboring the EGFR-KDD, the index patient was initially treated with standard first line chemotherapy for stage IV lung adenocarcinoma (cisplatin/pemetrexed/bevacizumab). However, at the time of disease progression on this treatment regimen, the patient was treated with afatinib. Immediately after beginning afatinib, the patient reported feeling markedly better with improvements in his symptoms of cough and fatigue. After two cycles of afatinib, the patient showed a partial radiographic response (∼50% tumor shrinkage) per RECIST criteria (17) (Fig. 3a). This clinical activity is consistent with our in vitro studies and provides rationale for further clinical investigation.
Figure 3. Serial chest CT scans of 33-year-old male with lung adenocarcinoma harboring EGFR-KDD documenting response to afatinib and subsequent acquired resistance.
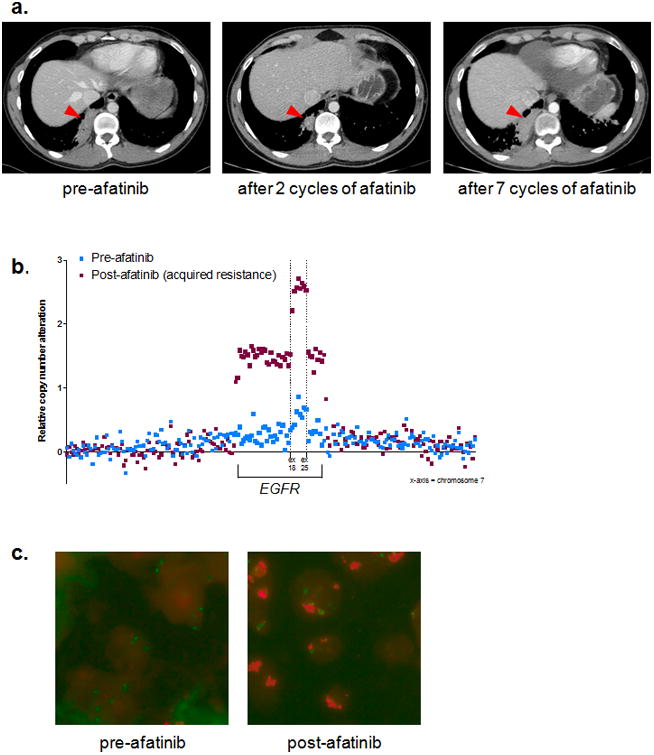
(a) Left image = patient images post six cycles of cisplatin/pemetrexed/bevacizumab (largest mass diameter = 6.62 cm). Middle image = patient images post two cycles of afatinib (largest mass diameter = 2.72 cm). Right image = patient images post seven cycles of afatinib (largest mass diameter = 6.20 cm). The red arrowheads are pointing to the largest mass used for RECIST evaluation. (b) Copy number data from Foundation One™ NGS targets along chromosome 7 demonstrating amplification of the EGFR-KDD allele at the time of acquired resistance to afatinib (maroon squares) compared to the pre-afatinib tumor biopsy sample (blue squares). The x-axis represents chromosome 7. (c) EGFR FISH of pre- (left panel) and post- (right panel) afatinib tumor biopsy samples used for the NGS analysis shown in panel b. Pre-afatinib = 1.6 copies of EGFR per chromosome 7 centromere (1.6 EGFR/CEP7); post afatinib = 4.2 EGFR/CEP7. Green puncta = CEP7; Red puncta = EGFR.
Acquired resistance to afatinib
The index patient developed acquired resistance to afatinib after 7 cycles of therapy (Fig. 3a). This duration of response is in line with the typical responses observed in other EGFR-mutant lung cancers treated with EGFR-TKIs (1-3). Molecular profiling was performed on the afatinib resistant tumor biopsy sample, and this testing uncovered significant amplification of the EGFR-KDD allele as the only genomic alteration that differed from his pre-treatment tumor sample (Fig. 3b). This sequencing result was confirmed via EGFR fluorescence in situ hybridization (FISH, Fig. 3c). Amplification of the mutant EGFR allele has been reported as a mechanism of acquired resistance in the context of canonical EGFR mutations (e.g. exon 19 deletion, L858R) in lung cancer (18). Therefore, amplification of the EGFR-KDD in this post-treatment sample suggests an EGFR-dependent mechanism of resistance, thereby further validating this EGFR alteration as a driver and therapeutic target in patients.
Discussion
Although much progress has been made over the past several decades, lung cancer remains the leading cause of cancer deaths worldwide (19). The discovery of oncogenic EGFR mutations that sensitize lung cancers to EGFR TKIs heralded the dawn of molecularly-targeted therapy in this disease (20-22). Indeed, numerous phase III studies have now documented that patients with EGFR-mutant tumors derive significant clinical and radiographic benefit from treatment with EGFR TKIs, such as gefitinib, erlotinib, and afatinib (1-3). The majority of previously described activating mutations in EGFR are a series of small deletions in exon 19 or leucine to arginine substitutions at position 858 (L858R) in exon 21 (23). However, because mutations historically have been interrogated by ‘hot-spot’ PCR-based methods, most EGFR mutations are biased to fall between exons 18 and 21.
Here, we report the EGFR kinase domain duplication (EGFR-KDD) for the first time in lung cancer. This EGFR alteration contains an in-tandem and in-frame duplication of exons 18-25, which encode the entire EGFR kinase domain. We demonstrate that the EGFR-KDD is an oncogenic and constitutively activated form of the EGF receptor. We provide a structural model whereby the EGFR-KDD can be activated by virtue of asymmetric intra-molecular dimerization, as opposed to the typical asymmetric inter-molecular dimerization between adjacent EGFR molecules. Furthermore, we demonstrate that the EGFR-KDD can be therapeutically targeted with EGFR TKIs, many of which are already FDA-approved. Additionally, we establish that the EGFR-KDD alteration is not only recurrent in lung cancer but also in gliomas and other tumor types.
Most importantly, we provide the first documentation of a clinical response to EGFR inhibitor therapy in a lung cancer patient whose tumor harbored the EGFR-KDD alteration. In contrast to lung cancer patients with more common EGFR mutations (e.g., exon 19 deletion and L858R), prior to our study, there was no precedent to support the use of EGFR inhibitors in patients whose lung tumors harbor the EGFR-KDD alteration. Therefore, our patient was not eligible for first-line EGFR TKI therapy and was instead treated with platinum based chemotherapy, the standard of care for metastatic lung adenocarcinoma (24). The index patient was treated with afatinib for second-line therapy because this agent is FDA approved for the treatment of EGFR-mutant NSCLC and because, interestingly, afatinib was consistently the most potent EGFR TKI against the EGFR-KDD across several different assays. This was not unexpected as it has been shown that various EGFR mutations or truncations have differential sensitivity to EGFR TKIs due to nuanced structural differences (25). The marked tumor regression and improved functional status seen with afatinib therapy provides important clinical validation for the EGFR-KDD as an actionable alteration in lung cancer. Overall, the index patient derived a partial response to afatinib for 7 cycles, after which there was progression of disease. His tumor was re-biopsied and found to contain amplification of the EGFR-KDD in this post-treatment sample—suggesting an EGFR-dependent mechanism of resistance and validating this EGFR alteration as a driver and therapeutic target in patients.
This case also reinforces the need to functionally validate and discern the therapeutic ‘actionability’ of genomic alterations as increasingly sophisticated methods of NGS-based assays are being brought to the forefront of clinical diagnostics. Notably, the EGFR-KDD would not have been recognized by the ‘hot-spot’ PCR-based methods for EGFR mutational analysis described above. Therefore, it is not surprising that this EGFR alteration had not previously been detected. In fact, the EGFR-KDD in the index patient's tumor was identified because of a fortuitous intronic breakpoint that lay close to the exonic probes of the NGS diagnostic assay (Fig. S1a). Therefore, we hypothesize that the EGFR-KDD may have gone and may continue to go undetected in other tumors because standard (exomic) sequencing platforms do not target this particular alteration due to its large intragenic repeat and intronic breakpoint. Thus, while our data show that the EGFR-KDD is recurrent in multiple tumor types, this alteration would not be detected with currently approved PCR-based methods and is difficult to detect using standard exomic sequencing, consequently making our reported frequency a likely underestimate. Future design of tumor sequencing platforms should incorporate intronic probes for EGFR in order to more reliably detect the EGFR-KDD.
In summary, we have identified a recurrent, oncogenic, and drug sensitive EGFR-KDD in a subset of patients with lung cancer, glioma, and other cancer types. Our findings provide a rationale for therapeutically targeting this unique subset of EGFR-KDD driven tumors with EGFR tyrosine kinase inhibitors, many of which are already FDA-approved. Therefore, findings from our studies are expected to be rapidly translated into the clinic as they provide a new avenue for precision medicine in these difficult-to-treat malignancies.
Methods
Cell culture
The human lung adenocarcinoma cell line, II-18, has been previously described and was verified to harbor the EGFR-L858R mutation direct by cDNA sequencing (26). A1235 cells were a kind gift from Drs. Fenstermaker and Ciesielski (Roswell Park Cancer Institute, Buffalo, NY) (7). 293T cells were purchased from ATCC. BA/F3 cells were purchased from DSMZ. Plat-GP cells were purchased from CellBioLabs. NR6 cells were a kind gift from Dr. William Pao (27). II-18 and BA/F3 cells were maintained in RPMI 1640 medium (Mediatech, Inc.). A1235, 293T, and NR6 cells were maintained in DMEM (Gibco). Media was supplemented with 10% heat inactivated fetal bovine serum (Atlanta Biologicals) and penicillin-streptomycin (Mediatech, Inc.) to final concentrations of 100 U/ml and 100 μg/ml, respectively. The BA/F3 cell line was supplemented with 1 ng/mL murine IL-3 (Gibco). The Plat-GP cell line was cultured in the presence of 1 μg/mL blasticidin (Gibco). All cell lines were maintained in a humidified incubator with 5% CO2 at 37°C and routinely evaluated for mycoplasma contamination. Besides verifying the status of EGFR mutations in cell lines, no additional cell line identification was performed.
Compounds
Erlotinib, afatinib, and AZD9291 were purchased from Selleck Chemicals.
EGFR plasmid construction
A cDNA encoding the EGFR-KDD (exon 18-25 tandem duplication) was synthesized by Life Technologies based on the consensus coding sequence (Fig. S2). The pMSCV-puro vector backbone (Clontech) was used to construct all retroviruses. Assembly of pMSCV-puro-EGFR-WT and pMSCV-puro-EGFR-L858R was previously described (28). The EGFR-KDD was subcloned from the pMA synthesis vector (Life Technologies) into the HpaI site of pMSCV-puro using blunt end ligation. The pcDNA3.1 vector was used for transient expression experiments in 293T cells. Assembly of pcDNA-EGFR-WT and pcDNA-EGFR-L858R was previously described (22). EGFR-KDD was subcloned from the pMA synthesis vector (Life Technologies) into the pcDNA3.1/V5 vector (Life Technologies) using Gateway cloning (Life Technologies). All plasmids were sequence verified in the forward and reverse directions. EGFR-KDD-dead was constructed using multi-site-directed mutagenesis (Agilent) of the catalytic lysines (K745 and K1096) to methionines using the following primer:
KM-F: 5′–AAAGTTAAAATTCCCGTCGCTATCATGGAATTAAGAGAAGCAAC–3′.
The plasmids were fully re-sequenced in each case to ensure that no additional mutations were introduced.
BA/F3 and NR6 cell line generation
The empty pMSCV-puro retroviral vector or pMSCV-puro vectors encoding EGFR (either EGFR-WT, EGFR-L858R, EGFR-KDD, or EGFR-KDD-dead) were transfected, along with the envelope plasmid pCMV-VSV-G (CellBioLabs), into cells Plat-GP packaging cells (CellBioLabs). Viral media was harvested 48 hours after transfection, spun down to remove debris, and supplemented with 2 μg/mL polybrene (Santa Cruz). 2.5×106 BA/F3 cells (or 1×106 NR6 cells) were re-suspended in 10 mL viral media. Transduced cells were selected for 1 week in 2 μg/mL puromycin (Invitrogen) and BA/F3 cells were selected for an additional week in the absence of IL-3. Stable polyclonal populations were used for experiments and routinely tested for expression of EGFR constructs.
Antibodies and immunoblotting
The following antibodies were obtained from Cell Signaling Technology: phospho-EGFR tyrosine 1068 (#2234, 1:1000 dilution), EGFR (#4267, 1:1250 dilution), phospho-ERK threonine 202/tyrosine 204 (#9101, 1:2000 dilution), ERK (#9102, 1:2000 dilution), HRP-conjugated anti-mouse (#7076, 1:5000 dilution), and HRP-conjugated anti-rabbit (#7074, 1:5000 dilution). The actin antibody (#A2066, 1:5000 dilution) was purchased from Sigma-Aldrich. The EGFR antibody (#610017, 1:2000 dilution) was purchased from BD Pharmingen. For immunoblotting, cells were harvested, washed in PBS, and lysed in RIPA buffer (150 mM NaCl, 1% Triton-X-100, 0.5% Na-deoxycholate, 0.1% SDS, 50 mM Tris·HCl, pH 8.0) with freshly added 40 mM NaF, 1 mM Na-orthovanadate, and protease inhibitor mini tablets (Thermo Scientific). Protein was quantified using protein assay reagent and a SmartSpec plus spectrophotometer (Bio-Rad) per the manufacturer's protocol. Lysates were subjected to SDS-PAGE followed by blotting with the indicated antibodies and detection by Western Lightning ECL reagent (Perkin Elmer).
Cell viability, counting, and clonogenic assays
For viability experiments, cells were seeded at 5,000 cells/well in 96-well plates and exposed to treatment the following day. At 72 hours post drug addition, Cell Titer Blue reagent (Promega) was added, and fluorescence at 570 nm was measured on a Synergy MX microplate reader (Biotek) according to the manufacturer's instructions. For cell counting experiments, cells were seeded at 20,000 cells/well in 24-well plates in the presence or absence of 1 ng/mL IL-3. Every 24 hours, cells were diluted 20 fold and counted using a Z1 Coulter Counter (Danaher). For clonogenic assays, cells were seeded at 5,000 cells/well in 24-well plates and exposed to treatment the following day. Media and inhibitors were refreshed every 72 hours, and cells were grown for 1 week or until confluence in control wells. Cells were fixed with 4% v/v formalin and stained with 0.025% crystal violet. Dye intensity was quantified using an infrared imaging system (LI-COR). Viability assays were set up in quadruplicate, clonogenic assays were set up in triplicate, and cell-counting assays in duplicate. All experiments were performed at least three independent times. Data are presented as the percentage of viable cells compared to control (vehicle only treated) cells. Regressions were generated as sigmoidal dose-response curves using Prism 6 (GraphPad) by normalizing data and constraining the top to 100.
Soft agar assays
1.5 mL of 0.5% agar/DMEM was layered in each well of a 6-well dish. A total of 10,000 NR6 cells in 1.5 mL of 0.33% soft agar/DMEM were seeded on top of the initial agar and allowed to grow for 15 days. Each cell line was plated in triplicate. Colonies were counted using GelCount (Oxford Optronix) with identical acquisition and analysis settings.
Transient transfections
293T cells were transfected using Lipofectamine 2000 (Life Technologies) per the manufacturer's recommendations. 24 hours post-transfection, cells were treated for two hours, gathered for western blot analysis, and prepared as described above.
Structural modeling of the EGFR-KDD
The linker residues in the EGFR-KDD protein sequence, FFSSPSTSRTPLLSSLLVEPLTPS, were defined as those between the two kinase domains and not present in the X-ray crystal structure of EGFR in its allosterically activated dimeric form, 2GS6.pdb (13). This linker was manually built and placed into the EGFR crystal structure using PyMOL 1.5.0.3. The conformational space for the linker was then sampled using the loop modeling functionality of Rosetta version 2015.05 (29). 20,000 independent loop modeling runs were performed using kinematic closure. The best model from these runs was still lacking residues 748-750, 992-1004, 1099-1101, and 1343-1355 because these surface-exposed loops were not resolved in the experimental structure. These four loops were reconstructed using Modeller 9.14, and the model with the lowest DOPE score was selected (30). Those loops were then sampled for an additional 20,000 runs using Rosetta to generate the complete energy-minimized model of EGFR-KDD residues GLY 696 - PRO 1370.
Tumor Biopsy Samples
All patient tumor biopsy samples were obtained under Institutional Review Board (IRB) approved protocols (Vanderbilt University IRB# 050644). Written informed consent was obtained from the index patient. All samples were de-identified, protected health information reviewed according to the Health Insurance Portability and Accountability Act (HIPAA) guidelines, and studies conducted in accordance with the Declaration of Helsinki.
Identifying EGFR-KDD in The Cancer Genome Atlas
Copy number data from the Broad Institute TCGA Genome Data Analysis Center 2015-04-02 run were visually inspected to identify samples with focal amplification of the EGFR-KDD region (exons 18-25). RNA-seq (e714b8a4-dd57-4b01-83fd-d3a9fb2d4ad1120 and c552b1e3-9158-4c4d-b02b-b16ff7903552) and whole-genome sequencing (27c2031a-39f1-473c-88a6-9e7a03cedf04) files were inspected to confirm the presence of tandem duplication reads. Raw data available at: doi:10.7908/C1K64H04 and doi:10.7908/C1MP525H.
EGFR fluorescence in situ hybridization (FISH)
EGFR FISH was performed by Integrated Oncology, a LabCorp specialty testing group, using the EGFR-CEP7 Dual Color DNA Probe (Vysis). A trained pathologist quantified the copies of CEP7 and EGFR in 60 nuclei per sample.
Statistics and data presentation
All experiments were performed using at least two technical replicates and at least three independent times (biological replicates). Each figure or panel shows a single representative experiment. Unless indicated otherwise, data is presented as mean ± standard deviation. Western blot autoradiography films were scanned in full color at 600 dpi, desaturated in Adobe Photoshop CC, and cropped in Powerpoint. EGFR-FISH images were normalized using ‘Match Color’ in Adobe Photoshop CC. No other image alterations were made.
Supplementary Material
Statement of significance.
We identified oncogenic and drug sensitive EGFR exon 18-25 kinase domain duplications (EGFR-KDD) that are recurrent in lung, brain, and soft tissue cancers and documented that a patient with metastatic lung adenocarcinoma harboring the EGFR-KDD derived significant anti-tumor response from treatment with the EGFR inhibitor, afatinib. Findings from these studies will be immediately translatable as there are already several approved EGFR inhibitors in clinical use.
Acknowledgments
We first and foremost would like to thank the index patient and his family. This study was supported in part by the National Institutes of Health (NIH) and National Cancer Institute (NCI) R01CA121210 (CML), P01CA129243 (CML, ML, MGK), and P30CA68485. Work in the Meiler laboratory is supported through the NIH (R01GM080403, R01GM099842, R01DK097376, R01HL122010, and R01GM073151) and the NSF (CHE1305874). CML was additionally supported by a Damon Runyon Clinical Investigator Award and a LUNGevity Career Development Award. JNG was supported by MSTP grant T32GM007347. TMS was supported by F31CA183531. Finally, we would like to thank Evelyn Vazquez for her assistance with the EGFR FISH.
Abbreviations
- EGFR
epidermal growth factor receptor
- KDD
kinase domain duplication
- TKI
tyrosine kinase inhibitor
- NSCLC
non-small cell lung cancer
Footnotes
Author disclosures: CML has served as a consultant for Pfizer, Novartis, Genoptix, Sequenom, and Ariad and has been an invited speaker for Abbott and Qiagen. MB, DL, VAM, and SMA are employees of and have equity interest in Foundation Medicine, Inc. JAP has served as a consultant for Roche and is an inventor of IP that Insight Genetics has licensed.
Author contributions: Designed experiments: JNG, JHS, JM, CML; Performed experiments: JNG, JHS, TMS, MB, DL; Generated and analyzed data: JNG, JHS, TMS, MB, DL, JAP, ML, CML; Created figures: JNG, JHS, TMS, MB, DL, RC, CML; Wrote the manuscript: JNG, CML; Provided clinical care of the index patient: SY, MGK; Provided technical support and conceptual advice: MRB; Oversaw research: VAM, SMA; reviewed the data and final manuscript: all authors.
References
- 1.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 2.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 3.Sequist LV, Yang JCH, Yamamoto N, O'Byrne K, Hirsh V, Mok T, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. Journal of Clinical Oncology. 2013;31:3327–34. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 4.Carey KD, Garton AJ, Romero MS, Kahler J, Thomson S, Ross S, et al. Kinetic analysis of epidermal growth factor receptor somatic mutant proteins shows increased sensitivity to the epidermal growth factor receptor tyrosine kinase inhibitor, erlotinib. Cancer Research. 2006;66:8163–71. doi: 10.1158/0008-5472.CAN-06-0453. [DOI] [PubMed] [Google Scholar]
- 5.Frampton GM, Fichtenholtz A, Otto GA, Wang K, Downing SR, He J, et al. Nature Biotechnology. Vol. 31. Nature Publishing Group; 2013. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing; pp. 1023–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J Mol Diagn. 2015;17:251–64. doi: 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fenstermaker RA, Ciesielski MJ, Castiglia GJ. Tandem duplication of the epidermal growth factor receptor tyrosine kinase and calcium internalization domains in A-172 glioma cells. Oncogene. 1998;16:3435–43. doi: 10.1038/sj.onc.1202156. [DOI] [PubMed] [Google Scholar]
- 8.Ciesielski MJ, Fenstermaker RA. Oncogenic epidermal growth factor receptor mutants with tandem duplication: gene structure and effects on receptor function. Oncogene. 2000;19:810–20. doi: 10.1038/sj.onc.1203409. [DOI] [PubMed] [Google Scholar]
- 9.Frederick L, Wang XY, Eley G, James CD. Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Research. 2000;60:1383–7. [PubMed] [Google Scholar]
- 10.Ozer BH, Wiepz GJ, Bertics PJ. Oncogene. Vol. 29. Nature Publishing Group; 2009. Activity and cellular localization of an oncogenic glioblastoma multiforme- associated EGF receptor mutant possessing a duplicated kinase domain; pp. 855–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furgason JM, Li W, Milholland B, Cross E, Li Y, McPherson CM, et al. Whole genome sequencing of glioblastoma multiforme identifies multiple structural variations involved in EGFR activation. Mutagenesis. 2014;29:341–50. doi: 10.1093/mutage/geu026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greulich H, Chen TH, Feng W, Jänne PA, Alvarez JV, Zappaterra M, et al. PLoS Med. Vol. 2. Public Library of Science; 2005. Oncogenic Transformation by Inhibitor-Sensitive and -Resistant EGFR Mutants; p. e313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X, Gureasko J, Shen K, Cole PA, Kuriyan J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell. 2006;125:1137–49. doi: 10.1016/j.cell.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 14.Moyer JD, Barbacci EG, Iwata KK, Arnold L, Boman B, Cunningham A, et al. Induction of apoptosis and cell cycle arrest by CP-358,774, an inhibitor of epidermal growth factor receptor tyrosine kinase. Cancer Research. 1997;57:4838–48. [PubMed] [Google Scholar]
- 15.Li D, Ambrogio L, Shimamura T, Kubo S, Takahashi M, Chirieac LR, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008;27:4702–11. doi: 10.1038/onc.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cross DAE, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, et al. Cancer Discovery. Vol. 4. American Association for Cancer Research; 2014. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer; pp. 1046–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Science Translational Medicine. Vol. 3. American Association for the Advancement of Science; 2011. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors; pp. 75ra26–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 20.Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. Science. Vol. 304. American Association for the Advancement of Science; 2004. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy; pp. 1497–500. [DOI] [PubMed] [Google Scholar]
- 21.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 22.Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, et al. Proc Natl Acad Sci USA. Vol. 101. National Acad Sciences; 2004. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib; pp. 13306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forbes SA, Beare D, Gunasekaran P, Leung K, Bindal N, Boutselakis H, et al. COSMIC: exploring the world's knowledge of somatic mutations in human cancer. Nucleic Acids Research. 2015;43:D805–11. doi: 10.1093/nar/gku1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ettinger DS, Wood DE, Akerley W, Bazhenova LA, Borghaei H, Camidge DR, et al. Non-small cell lung cancer, version 6.2015. J Natl Compr Canc Netw. 2015;13:515–24. doi: 10.6004/jnccn.2015.0071. [DOI] [PubMed] [Google Scholar]
- 25.Vivanco I, Robins HI, Rohle D, Campos C, Grommes C, Nghiemphu PL, et al. Differential Sensitivity of Glioma- versus Lung Cancer-Specific EGFR Mutations to EGFR Kinase Inhibitors. Cancer Discovery. 2012;2:458–71. doi: 10.1158/2159-8290.CD-11-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohashi K, Sequist LV, Arcila ME, Moran T, Chmielecki J, Lin YL, et al. Proceedings of the National Academy of Sciences. Vol. 109. National Acad Sciences; 2012. Lung cancers with acquired resistance to EGFR inhibitors occasionally harbor BRAF gene mutations but lack mutations in KRAS, NRAS, or MEK1; pp. E2127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Regales L, Gong Y, Shen R, de Stanchina E, Vivanco I, Goel A, et al. Dual targeting of EGFR can overcome a major drug resistance mutation in mouse models of EGFR mutant lung cancer. J Clin Invest. 2009;119:3000–10. doi: 10.1172/JCI38746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Red Brewer M, Yun CH, Lai D, Lemmon MA, Eck MJ, Pao W. Proceedings of the National Academy of Sciences. Vol. 110. National Acad Sciences; 2013. Mechanism for activation of mutated epidermal growth factor receptors in lung cancer; pp. E3595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stein A, Kortemme T. Improvements to robotics-inspired conformational sampling in rosetta. PLoS ONE. 2013;8:e63090. doi: 10.1371/journal.pone.0063090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eswar N, Eramian D, Webb B, Shen MY, Sali A. Methods Mol Biol. Vol. 426. Totowa, NJ: Humana Press; 2008. Protein structure modeling with MODELLER; pp. 145–59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


