Abstract
Industrials interest in fats as raw material, resides in their exceptional quality and potentialities of exploitation in several fields. This study aimed to exalt the optimized shea butter quality and present its wide potentialities of utilization. Hence, the characteristics of beige and yellow optimized shea butters were determined. Both samples recorded very weak acid (0.280 ± 0.001 and 0.140 ± 0.001 mgKOH/g) and peroxide (0.960 ± 0.001 and 1.010 ± 0.001 mEgO2/kg) indexes, when the iodine indexes (52.64 ± 0.20 and 53.06 ± 0.20 gI2/100 g) and the unsaponifiable matters (17.61 ± 0.01 and 17.27 ± 0.01 %) were considerable. The refractive indexes (1.454 ± 0.00 and 1.453 ± 0.00) and the pH (6.50 ± 0.30 and 6.78 ± 0.30) were statistically similar; but the specific gravity (0.915 ± 0.01–0.79 ± 0.01 and 0.94 ± 0.01–0.83 ± 0.01) and the viscosity (90.41 ± 0.20–20.02 ± 0.20 and 125.37 ± 0.20–23.55 ± 0.20 MPas) differed and decreased exponentially with the temperature increasing (35–65 °C), except for the specific gravity of the yellow butter which decreased linearly. The UV–Vis spectrum showed a high peak at 300 nm and a rapid decrease from 300 to 500 nm when the near infra-red one, revealed peaks at 450, 1200, 1400, 1725 and 2150 nm for all the samples. The chromatographic profile identified palmitic (16.42 and 26.36 %), stearic (32.39 and 36.36 %), oleic (38.12 and 29.09 %), linoleic (9.72 and 5.92 %) and arachidic (1.84 and 1.59 %) acids, and also exaltolide compound (1.51 and 0.68 %). The samples also contained essential minerals (Calcium, magnesium, zinc, iron, etc.) carotene (550 ± 50 and 544 ± 50 ppm), vitamins A (0.065 ± 0.001 and 0.032 ± 0.001 µg/g) and E (2992.09 ± 1.90 and 3788.44 ± 1.90 ppm) in relatively important amounts; neither microbiological germs nor heavy were detected. All these valorizing characteristics would confer to the optimized shea butters good aptitude for exportation and exploitation in food, cosmetic and pharmaceutical industries.
Keywords: Shea butters, Optimized, Physicochemical and nutritive characteristics, Potentiality of exploitation, Industrial
Background
Industrials interest in fats as raw material, resides in their exceptional quality and potentialities of exploitation in several fields. Hence cocoa butter, palm, palmist and coco oils are so widely exploited that the Codex Stan 210 (1999) established standards in order to guide traders/industrials. Many other fats like avocado, olive, illipe and jojoba are also involved in cosmetic or pharmaceutical products on the basis of their interesting characteristics (Pontillon 1996; Joanny 2005; AAK Global 2012) Shea (Vitellaria paradoxa, syn. Butyrospermum parkii, B. paradoxa) butter which represents an important socio-economical agro-resource for its producing countries, is more and more being exploited in several industrial fields (Hall et al. 1996, Schreckenberg 2004; PNUD 2010). This increasing importance is mainly linked to its properties which would justify the wide range of its empiric uses (Hall et al. 1996). Indeed, according to these authors, shea butter has long been used in sub-Saharan Africa and elsewhere for medicinal, culinary, and other applications (Tomaszkiewicz-Potepa 2012; Tomaszkiewicz-Potepa and Sliwa 2012). Nowadays, shea buter, mainly the traditional one (called BIO-shea butter) interests cosmetic and pharmaceutical firms (Pontillon 1996; Elias and Carney 2004; Nahm 2011; AAK Global 2012). Nevertheless these firms are highly exigent about shea butter quality. Whereas, the traditional processes often lead to shea butter non-conform to recommended standards (Hall et al. 1996; Mégnanou and Diopoh 2008). Several studies have been undertaken to improve shea butter properties; most of these studies just focused on one or two effecting factors. Those of Mégnanou and Niamke (2013a) took into account many factors like nuts drying mode and time, kernels quality and roasting time, so did shea butter wrapper hygienic quality. The results drew the effects of such factors on shea butter physicochemical and microbiological characteristics, and consequently induced the optimized traditional process (Bup et al. 2012; Pouliot and Elias 2013; Nsogning et al. 2014). The resulted shea butter was in conformity with the unrefined shea butter standards for trade as proposed by UEMOA (2011). Nevertheless, because of the exigencies of industrials which take into account many other properties, shea butter utilization as raw material (Cosmetic and pharmaceutical) might be linked to its exceptional quality and high potential of exploitation (Honfo et al. 2013, 2014).
The present study focused on the optimized shea butters (which process was improved) typical quality and demonstrated its high potential of exploitation in several industries. Therefore, in addition to their ordinary physicochemical characteristics like moisture content, acid, iodine, peroxide indexes and unsaponifiable matter, other characteristics have been taken into account. Hence, physical parameters such as specific gravity, viscosity and UV–Visible/infrared spectra were determined. Samples content in nutritive compound as carotene, fatty acids, minerals, vitamins A and E was also evaluated; so were the microbiological characteristics. Above all, the different aptitudes and fields of exploitation of the beige and yellow optimized shea butters have been underlined.
Results and discussion
Sensorial characteristics of the optimized shea butters
Both beige and yellow shea butters prepared according to the improved Megnanou et al. (2007) method, presented the same fondant (soft) texture and rancidity-less and moderate shea characteristic odor. Such characteristics are conformed to usual consumers’ criteria about shea butter (Carette et al. 2009; Mégnanou and Niamké 2013b). It is important précising about the studied samples that beige shea butter would be a natural (original) fat because of its water-exclusive extraction (Elias 2015, Jasaw et al. 2015). The yellow one, despite of the natural statute of Cochlospermum tinctorium dye, could be considered as an adulterate shea butter. Indeed, Cochlospermum tinctorium is exploited as medicinal plant; its roots aqueous extract would be drink in the treatment of many diseases (Diaw 1982). However, both beige and yellow shea butters of the present study with conform sensorial characteristics would constitute natural (BIO) available cheap and accessible edible fats. Moreover, the present optimized shea butters also exhale a slight sweety fragrance similar to that of the sweet almond oil. Such fragrance was not noticed by Megnanou et al. (2007) about the optimized shea butter, and could be justify by the improvement of the optimized method by avoiding the step of shea oil heating (for dehydration) which could destroy the volatile compounds responsible of the fragrance.
Physicochemical characteristics of the optimized shea butters
The physicochemical characteristics presented significant difference (p < 0.05) between beige and yellow optimized shea butters, except for peroxide and refractive indexes (Table 1). Acid (0.280 ± 0.001 and 0.140 ± 0.001mgKOH/g, respectively) and peroxide (0.960 ± 0.001 and 1.01 ± 0.001mEqO2/kg, respectively) indexes which are considered as fat quality characteristics were very weak. Indeed, their values were at far slighter than those (4 mgKOH/g and 15 mEqO2/kg) recommended by Codex Stan 210 (1999) about vegetable fats. These values were also weaker than those concerning Megnanou et al. (2007) optimized shea butters shea. This situation could certainly be linked to the improved optimized method which deleted the shea oil heating step. In fact according to Dieffenbacher et al. (2000), fat heating would induce glycerids hydrolysis and unsaturated fatty acids oxidation, and consequently a high amount of free fatty acids and peroxide compounds.
Table 1.
Physicochemical characteristics of the optimized shea butters
| Parameters | Beige shea butter | Yellow shea butter |
|---|---|---|
| Specific gravity (40 °C) | 0.87 ± 0.01b | 0.92 ± 0.01a |
| Refractive index (40 °C) | 1.454 ± 0.00a | 1.453 ± 0.00a |
| Viscosity (mPa.s) (40 °C) | 73.66 ± 0.20b | 96.00 ± 0.20a |
| Activation energy* (kJ/mol) | 46.81 | 50.43 |
| Colour (Ly) | 3.40 ± 0.01b | 3.90 ± 0.01a |
| pH (25 °C) | 06.50 ± 0.30b | 06.78 ± 0.30a |
| Acid index (mgKOH/g) | 0.280 ± 0.001a | 0.140 ± 0.001b |
| Peroxide index (mEqO2/kg) | 0.960 ± 0.001a | 1.010 ± 0.001a |
| Iodine index (gI2/100 g) | 52.64 ± 0.20b | 53.06 ± 0.20a |
Values given in table consist in means ± the standard deviation. Letters a, b resulted from ANOVA test; they must be considered line by line. Different letters (a, b) underline significant difference while the same letter (a, a) indicate no significant difference
Ea as activation energy, R as gaze constant R = 8314 × 10−3 kJ/mol/K. T represents the different temperatures at which viscosity was obtained; it varied from 308 to 338 °K
* Activation energy consists in the potential energy contained in one mole of shea butter. Here, it was determined graphically by drawing the function ln (Viscosity) = Ea/RT + Constante (y = Eax + constante) with
With such acid and peroxide indexes, the studied shea butter would present good aptitude for exportation/international trade though (moreover) the fat just contained 0.2 % of moisture. Additionally, values of specific gravity at 40 °C were 0.87 ± 0.00 and 0.92 ± 0.00 for the beige and the yellow shea butter, respectively. Such values added to those of the refractive index (1.454 and 1.453 for beige and yellow, respectively) and the viscosity (73.66 ± 0.20 and 96.00 ± 0.20 mPa s for beige and yellow, respectively) would confirm the quality of conventional edible vegetable oils to the shea butter samples (Codex-Alimentarius 1993; Besbes et al. 2004).
Furthermore, all studied shea butters could be classified as non-drying fats in view to their refractive index value (Rossell 1991). This property would disqualify them for varnish manufacturing in chemical industry, despite of their relatively high iodine value (52.64 ± 0.20 and 53.06 ± 0.20 gI2/100 g for beige and yellow shea butter, respectively) compared to that of marked shea butters reported by Megnanou et al. (2007). This iodine index value would suggest an interesting amount of unsaturated fatty acids and would confirm the very weak peroxide index.
About the viscosity, those of liquids as vegetable oil are commonly perceived as thickness, or resistance to pouring (Ndangui et al. 2010). Beige shea butter was less viscous than the yellow one, probably due to the presence of mucilage contained in Chochlospermum tinctorium dye (Jensen 2005). In addition, yellow shea butter viscosity was higher than the mean value (75 mPa.s) of most vegetable oils (Besbes et al. 2004). This physical property linked to the solid state of the studied shea butters could be used in food and cosmetic industry to confer an adequate texture to final fat products (Dubois et al. 2007).
The profile of the temperature effect on viscosity and specific gravity are depicted on Figs. 1 and 2, respectively. Each sample observed a typical variation of viscosity (y = 0.0627x2 − 8.6818x + 318.74 and y = 0.106x2 − 13.99x + 485.19, for beige and yellow, respectively) and specific gravity (y = 0.0002x2 − 0.0205x + 1.4299 and y = −0.0037x + 1.0684, for beige and yellow, respectively) which were materialized by the mathematical equations on the figures. These equations (relations) could be exploited by any factory, in order to obtain precisely the viscosity/specific gravity in conformity with any utilization. In summary, the value of viscosity decreases (90.41–20.01 and 125.37–23.55 mPa.s, for beige and yellow shea butters, respectively) continuously when the temperature increases from 35 to 65 °C and would confirm the Arrhenius law (Nzikou et al. 2007). The low indicates that the viscosity of fats decreases exponentially with increasing of temperature. It was also observed a relative similitude in the evolution of the viscosity for both samples; this could suggest similarity in fatty acids composition. However, such rheological property (viscosity/temperature and specific gravity/temperature) linked to the relatively important energy of activation (46.81 and 50.43 kJ/mol for beige and yellow, respectively) of the studied shea butters could be exploited in cosmetic industry for emulsions making (Lefur and Arnaud 2004). Above all, the pH of the studied shea butters would be indicated for such industry, mainly for body care because of its values (06.50 ± 0.30 and 06.78 ± 0.30 for beige and yellow, respectively) which around the human body proteases pH (Forestier 1992).
Fig. 1.
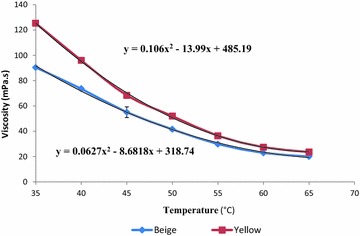
Optimized shea butters viscosity variation as function to the heating temperature. Mean values are plotted as points and standard deviations, as vertical error bars
Fig. 2.
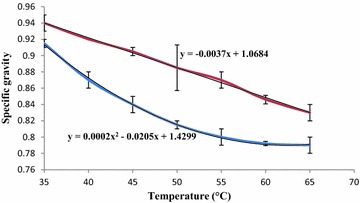
Optimized shea butters specific gravity variation as function to the heating Temperature
The spectra (Near infrared and UV–visible) of both shea butters presented, at the whole, the same profile (Figs. 3, 4); which was in general similar to those of marked and original traditional shea butter reported by Mégnanou and Niamké (2014). This similitude would suggest for both beige and yellow optimized shea butters the content in molecular bearing ethylenic bonds with conjugation and carbonyl compounds (Yadav et al. 2004). The shea butters of these authors would also contain UV-filter compounds (molecular) materialized by the rapid decrease of the absorbance (0.15–0.05 and 0.2–0.075, for yellow and beige shea butters, respectively) from 300 to 400 nm (Besbes et al. 2004). Moreover, another interesting peaks were observed at 400 nm (about the beige shea butter), and at 500 nm (for both butters); they correspond to chlorophyll (A and B) and carorenoïds wavelengths, respectively. The melting of such interesting molecular would justify the great interest of cosmetic and pharmaceutical industry for fats like shea butters, and would then constitute an advantage for using the studied shea butters in cosmetic formulations as UV protectors against carcinogenic UV A and B.
Fig. 3.
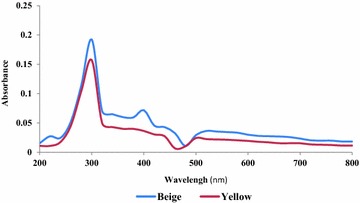
UV-Visible spectrum of optimized beige and yellow shea butters
Fig. 4.
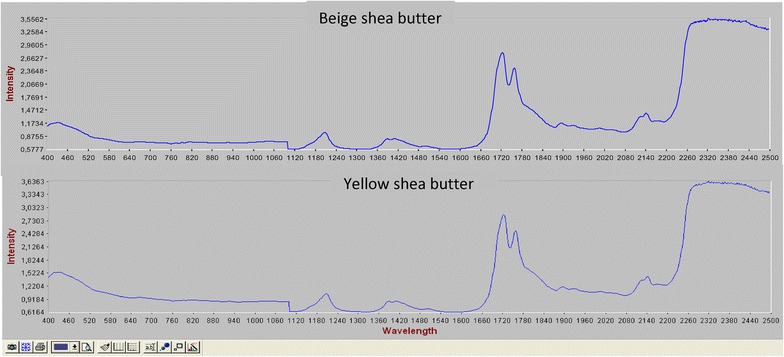
Near infrared spectrum of the optimized beige and yellow shea butters
The information delivered by the optimized shea butters near infrared spectra was identical to that reported by Mégnanou and Niamké (2014) about marked shea butters. Such similitude would recall and confirm the proposition of these authors to adopting the spectra (UV–visible and near infrared) as shea butter essential distinctive characteristics. Hence, any adulteration of shea butter with other fats and/or alteration would be clearly detected through the profile. As precision, the difference noticed about the intensity of peaks at 450–500 nm wavelengths, between the yellow (1.5224) and the beige (1.1734) shea butters, could be correlated to the Lovibond color value in red light (3.9 against 3.4 for the beige one). That dissimilitude would suggest the implication of carotenoids (carotene and other carotenoids) in shea butter color.
In summary, both beige and yellow optimized shea butters would contain the same compounds like hydrocarbon, unsaturated molecular, fatty acids and moleculars protecting against sun rays damages (allergy and cancer), in probably different amounts.
Biochemical characteristics of the optimized shea butters
Biochemical characteristics also varied from a color to another, according to the Duncan’s test (Table 2). At the whole, the characteristics are very interesting and would confer to both butters a wide potential of utilization when added to the upside physicochemical characteristics. In fact, the important amount of unsaponifiable (17.61 ± 0.01 and 17.27 ± 0.01 for beige and yellow shea butters, respectively) was at far higher than those of coconut (0.2–0.4 %) and cocoa (0.5–1 %) oils currently involved in cosmetics (Karleskind 1992). It is worth noting here that the unsaponifiable fraction would contain the main potential of cosmetic and medicinal virtues (Pesquet 1992; Allal et al. 2013).
Table 2.
Biochemical and nutritive properties of the optimized beige and yellow shea butters
| Characteristics | Beige shea butter | Yellow shea butter |
|---|---|---|
| Unsaponifiable (%) | 17.61 ± 0.01a | 17.27 ± 0.01b |
| Vitamin A (µg/g) | 0.065 ± 0.001a | 0.032 ± 0.001b |
| Vitamin E (ppm) | 2992.09 ± 1.90b | 3788.44 ± 1.90a |
| Carotene (ppm) | 550 ± 50.00a | 544 ± 50.00a |
| Free fatty acids (%) | 5.39 ± 0.05a | 5.23 ± 0.05b |
| Ratio oleic/linoleic | 3.92 | 4.91 |
| SFA (%) | 48.81 ± 0.09b | 62.72 ± 0.09a |
| UFA (%) | 51.19 ± 0.19a | 37.28 ± 0.19b |
Values given in table consist in means ± the standard deviation. Letters a, b resulted from ANOVA test; they must be considered line by line. Different letters (a, b) underline significant difference while the same letter (a, a) indicate no significant difference
In addition to their important unsaponifiable matter which would confirm their classification as “variety Mangifolia” according to Mensier (1957), the optimized shea butter contained.
carotene (550 ± 50.00 and 544 ± 50.00 ppm for beige and yellow shea butters, respectively), linoleic acid (9.72 and 5.92 % for beige and yellow shea butters, respectively), vitamins A (0.065 ± 0.001 and 0.032 ± 0.001 µg/g for beige and yellow shea butters, respectively) and E (2992.09 ± 1.90 and 3788.44 ± 1.90 ppm for beige and yellow shea butters, respectively). The presence of linoleic acid could be useful in cosmetic industry to decrease trans-epidermal water loss and to eliminate scaly lesions common in patients with essential fatty acids deficiency (Aburjai and Natsheh 2003). A natural musk fragrance identified as exaltolide was also detected in a relatively interesting amount (1.51 and 0.68 % for beige and yellow shea butters, respectively). All these characteristics would exalt their beneficial utilization in household for body care and their exploitation in cosmetic/pharmaceutical industry (body cream, soap and fragrances/perfumes). The studied samples could easily be involved in cosmetics like cocoa and jojoba fats, without preliminary treatment like deodorization, neutralization and synthetic fragrance (essence) additioning. They present fats would then constitute a valuable cheap, available and accessible material for the cosmetic/pharmaceutical industry.
Concerning the fatty acids composition (Fig. 5), it should underline the high nutritive potential of the optimized shea butters. Indeed, in addition to the carotene, vitamins A and E which would be powerful antioxidant, these butters contain, the fatty acids profile revealed the presence of essential fatty acids (EFA) such as oleic (38.12 and 29.09 % for beige and yellow shea butters, respectively) and linoleic (9.72 and 5.92 % for beige and yellow shea butters, respectively) acids. Their proportions were relatively important, mainly in the beige shea butter and would suggest the possibility of extracting a liquid fraction like “olein de karite” of Burkina Faso. Such fraction could be consumed without heating, preserving hence, vitamins (A and E), carotene and other thermo-sensible moleculars from destruction. It is worth underlining the relatively important amount of oleic acid and oleic/linoleic acids (3.92 and 4.91, for beige and yellow shea butters, respectively) ratio; such disposition would confer to the optimized shea butters the quality of very interesting dietetic fat in cardiovascular, inflammatory, autoimmune diseases and cancer prevention (Harris 2006; Simopoulos 2008). They could them be recommended for usual consumption like colza and sunflower oils which are considered nowadays as the best dietetic oil on markets. The solid fraction of the shea butters which will be constituted by stearic (32.39 and 36.36 % for beige and yellow shea butters, respectively) and palmitic (16.42 and 26.36 % for beige and yellow shea butters, respectively) acids could for it, be involved in margarine and in baking pastes to get leafy-pastes as reported by Pesquet (1992) about shea butter exploitation in bakery.
Fig. 5.
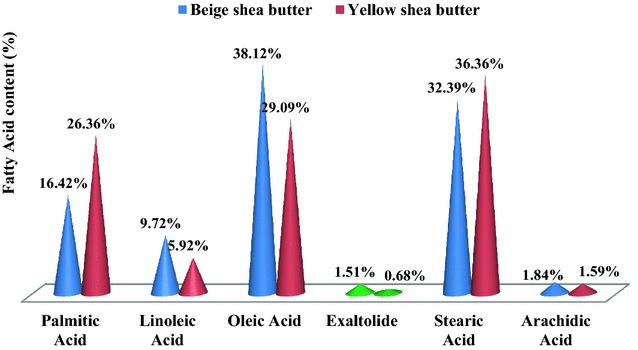
Optimized shea butters fatty acids component and content
Both optimized shea butters had the same fatty acids composition, but a difference in the content was noticed and could difficultly be explained. However, the higher proportion of UFA (51.19 ± 0.19 %) recorded by the beige shea butter was in conformity with Marantz et al. (2004) repartition about west Africa shea butters. The palmitic and linoleic acids proportions were, at far more important than those of Marantz et al. (2004), despite of the absence of adulteration supposed by Mégnanou and Niamké (2014) for Ivorian marked shea butters. This situation far from constituting a scientific problem could be taken into account for a best detail in shea butter characterization as function to the country. More precisely, a correlation might be established between shea trees genetic and agro-morphological characters and their butters physicochemical and biochemical characteristics.
The carotene content was relatively weak in the yellow shea butter (544 ppm) compared with the beige one (550 ppm); this would either confirm the responsibility of Cochlospermum tinctorium dye in yellow butter coloration (Ayeh 1991; Megnanou et al. 2007) or and also suppose eventually the implication of vitamin E or other carotenoids (compounds different from carotene) at regard to the Near infrared observation (peaks at 450 nm).
Microbiological and mineral characteristics of the optimized shea butters
In addition to their high physicochemical and biochemical potentialities as cosmetical/pharmaceutical and nutritive fats, the optimized shea butters contained very interesting minerals for human nutrition as calcium, magnesium, iron and copper, and for body/hair care as for zinc, magnesium and copper (Gueguen 2001). Moreover, neither microbiological germs (Table 3) nor heavy metals (Table 4) were detected. Some authors like Sanou (2002) links shea butters minerals composition to the kernels one; the present results confirmed such approach. Moreover, they suggested the integration of mineral from Cochlospermum tinctorium roots. Indeed, yellow shea butter composition in copper would be relative to the roots content. It is essential recalling here that the yellow optimized shea butter was prepared with the decoction of these roots (Megnanou et al. 2007).
Table 3.
Microbiological characteristics of the optimized shea butters
| Identified germs | Standard | 3 × Standard | Shea butter (yellow and beige) charge |
|---|---|---|---|
| Mesophile bacteria/g | <1 × 10 + 4 | <3 × 10 + 4 | 0 |
| Total Coliforms/g | <25 | <75 | 0 |
| Yeast and moulds/g | <10 | <30 | 0 |
| Salmonella/g | 0 | 0 | 0 |
Table 4.
Optimized shea butters mineral composition and content (mg//kg)
| Samples | Copper | Iron | Lead | Nickel | Zinc | Calcium | Sodium | Magnésium | Potassium |
|---|---|---|---|---|---|---|---|---|---|
| B SB | 0.95 ± 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 212.26 ± 0.05 | 84.57 ± 0.02 | 0.00 | 45.17 ± 0.05 |
| YSB | 7.15 ± 0.01 | 1.67 ± 0.01 | 0.00 | 0.00 | 1.90 ± 0.02 | 238.85 ± 0.05 | 136.62 ± 0.02 | 12.18 ± 0.02 | 36.41 ± 0.05 |
| SK | 3.30 ± 0.01 | 30.65 ± 0.01 | 0.00 | 0.00 | 9.79 ± 0.02 | 2151.80 ± 0.05 | 739.58 ± 0.05 | 1425.60 ± 0.05 | 10488.00 ± 0.05 |
| C.t.r | 12.95 ± 0.01 | 83.89 ± 0.01 | 0.00 | 0.50 ± 0.01 | 11.24 ± 0.02 | 1649.90 ± 0.05 | 696.32 ± 0.05 | 1177.95 ± 0.05 | 3912.75 ± 0.05 |
BSB Beige shea butter, YSB yellow shea butter, SK shea kernels, C. t. r Cochlospermum tinctorium root
The value of zero (0) germs charge recorded by the shea butters, would confirm the deep implication of shea butter conditioning in its microbiological quality (Mégnanou and Diopoh 2008; Mégnanou and Niamké 2013a).
Hence, the optimized shea butters might be apt to either exportation or industrial and households’ utilizations.
Conclusion
The present optimized shea butters acid, moisture and peroxide values were very weak; this could be the consequence of the optimized method improvement by deleting the step of shea oil heating at the end of its preparation. Moreover, they were microbiological germs and heavy metal free. These characteristics would suggest them apt to exportation and industrial exploitation. In addition, they contained nutritional elements like essential fatty acids (oleic and linoleic acids), minerals (calcium, iron, copper, magnesium, sodium, potassium and zinc), vitamins (A and E) and carotene, which would present them as available, cheap and accessible nutritive edible fats. Such butters could then be exploited not only in households, but also in food industry, mainly edible oils factories and also in pastries/bakeries because of their high unsaponifiable matter. This latest compounds added to the presence of UV-filter molecules, vitamin E, minerals (zinc, magnesium and copper) and linoleic acid, would present them as useful raw material for cosmetic and pharmaceutical purposes. However, it should be appropriate to conserve them in hygienic conditions in order to preserve such interesting virtues. The optimized shea butters could then be considered as a response to the needs in valuable BIO-products for usual consumers for industrials. They might constitute for industrials a cheaper valuable, available and accessible raw material. Hence, adopting optimized method would guaranty the trading/exportation/industrial exploitation of this fat which is considered as the main source of money for women in shea producing area, and consequently increase monetary currency for shea producing countries.
Methods
Biological material
The biological material was constituted by beige and yellow optimized shea butters prepared at the laboratory following Mégnanou et al. (2007) improved method.
Chemicals
Analytical grade solvents, standards and reagents were used to perform analysis. Solvents (n-hexane, acetic acid, diethyl-ether, ethanol, methanol and n-heptane) were provided from Merck (Germany). Standards such as fatty acids (palmitic acid, stearic acid, oleic acid, and linoleic acid) and erucic acid were purchased from Sigma-Aldrich (Germany). Wijs reagent, β-carotene, retinal palmitate and α-tocopherol were from Prolabo (France).
Microbiological analyses powders: PCA (Plate count agar), YGC (Agar with yeast, glucose Salmonella-Shigella), Mueller-Kauffmann, TS (Trypton salt), Rappaport–Vassiliadis and Kligler-Hajna were from Bio Rad. VRBL (Agar violet red blue and lactose), Hektoen and BPW (Buffered peptone water) powders provided from Diagnostic Pasteur, Scharlau Microbiology and Bio-Mérieux, respectively. The mineral analysis standard solutions (1 g/l) of calcium, copper, iron, lead, magnesium, nickel, potassium, sodium and zinc, used for mineral analysis were from Fisher.
Methods
Shea butter samples preparation
Shea butters were prepared following Mégnanou et al. (2007) method improved by avoiding the step of shea oil dehydratation by heating.
Hence, Shea fruit (10 kg) pulp were revolved and the nuts were dipped in two equivalent volumes of boiling water for 20 min and then put on plates for regular sun drying for one (1) week. The nut kernels were chopped finely with a kitchen chopper and then roasted at 120–150 °C for 5 min (by part of 500 g). The roasted kernels were ground with an electric grinder (moulinex) and the paste of the kernels was boiled for 1 h in 2 equivalent volumes of distilled water for the beige shea butter and 2 equivalent volumes of a decoction of Cochlospermum. tinctorium roots (1 kg of roots boiled for 1 h in 10 L of distilled water), for the yellow shea butter. The floating oil of the boiling solution was delicately collected in a clean glass-pot and stores analyzed. Here, the final dehydratation by heating for 5 min was avoided.
Physicochemical analysis
Chemical indexes, specific gravity, refractive index and viscosity
Chemical indexes such as acid, peroxide and iodine indexes were obtained following AOAC (1997) methods. Specific gravity (35–65 °C) and refractive index (40 °C) of melted butters were determined following IUPAC (1979) method by using a pycnometer and a refractometer (Metller Toledo, Switzerland), respectively. Viscosity was determined at different temperatures (35–65 °C) by using a viscometer apparatus (SVM 3000, Anton Paar GmbH, Austria) equipped with a syringe filled with 1 mL of melted butter sample. Values of viscosities were automatically recorded after temperature programming.
pH, colour and melting point
pH value of melted butter samples was determined at 25 °C according to Afane et al. (1997) by using a pH-meter (Hanna, Hi 8915 ATC, Spain). 2 mL of melted butter sample were dissolved in 15 mL of n-hexane. The pH-meter electrode was standardized with buffer solutions (pH 4.0 and 7.0) and then, immersed into the sample to record pH value.
Colour and melting point were determined according to the MPOB (2005) methods by using a Lovibond colorimeter (Lico, Labomat, France) and a thermometric system (FP900, Metller Toledo, Switzerland), respectively.
UV–Visible spectra
UV–Vis spectra of melted butter samples were determined by measuring absorbance of hexanic melted butter solution (1 %) by using a UV–Visible spectrophotometer (T80+, PG Instruments, England) in the range of 200–600 nm (Besbes et al. 2004).
Near infrared spectrum
Near infrared spectrum (NIR) was determined by reading absorbance of melted butter sample in the range of 400–2500 nm by using an infrared spectrophotometer (Foss Liquid Analyzer, Denmark) equipped with a software (NIR Vision Spectral Analysis, Model 6500) for data acquisition.
Mineral detection
The mineral content of the shea butter ash was determined following the AOAC (1980) method by spectroscopy atomic absorption with a spectrophotometer SpectrAA-5. For the ash, 1 g of shea butter was mineralized in a mineralizing oven (J.P. Selecta, s.a. Ner 0346540) at 550 °C, for 24 h. The mineralization temperature increased progressively (50 °C by 30 min) from 50 to 550 °C, and then the process was stopped 24 h later.
Biochemical and nutritive analysis
Unsaponifiable matter content
Unsaponifiable matter content of oil was determined following the IUPAC (1979) method. Oil sample (5 g) was saponified with 50 mL of 2 N KOH methanolic solution for 1 h. To the resulted mixture, 50 mL of distilled water was added. The unsaponifiable matter was extracted three times with 50 mL of diethyl-ether. Organic fractions were collected, washed three times with 50 mL of distilled water and then dried with sodium sulfate. Diethyl-ether was removed in a rotary evaporator (Heidolph, Hei-Vap, Germany) to recover the unsaponifiable matter which was then weighed.
Fatty acids composition
Gas chromatography (GC) was used to set the fatty acids profile. The compounds were first converted into their methyl esters (FAMEs) as described by the European Communities (1991) methods. Shea butter (0.1 g) was diluted in 2 mL of n-heptane and then 0.2 mL of a methanolic solution of potassium hydroxide (2 N) was added. The whole solution was shaken up for 30 s and stored for 5 min, then 1 mL of the top layer solution (FAMEs) mixed with the internal standard (erucic acid) was injected in the chromatograph (Shimadzu, GC-9A, Japan). The analysis conditions were:
Chromatograph equipped with a mass spectrometer (MS),
RTX5 fused silica capillary column (30 m X 0.32 mm i.d. X 0.25 µm film thickness),
Carrier gas, helium,
Flow rate adjusted to 23 mL/min.
Temperatures of detector and injector, 250 °C; initial column temperature, 100 °C and programmed to increase by 5 °C per min intervals until 220 °C and, kept for 10 min at this temperature.
Vitamins A and E
Vitamins A and E were firstly extracted and then the different fractions were separated by high performance liquid chromatography (ACQUITY WATERS, USA). About the extraction, 200 µL of diluted shea butter (1 g of shea butter in 10 mL of hexan), were mixed with 800 µL of methanol, centrifuged and the supernatant was filtered (0.45 µm pore size). The filtered solution was used for chromatography analysis according to Gimeno et al. (2000) method under these following conditions:
Liquid chromatography system with an optical detector TUV system
BEH C18 column (150 × 0.25 mm i.d., 1.7 µm particle size), kept at 45 °C
Injection volume, 10 µL
Mobile phase, methanol/water (98/2: v/v)
Elution flow, 2 mL/min
Detection wavelength, 325 and 292 nm for vitamins A and E, respectively
Vitamins A and E standards solutions were retinol palmitate and α-tocopherol acetate, respectively.
Microbiological analysis
Microbiological analyses concerned the presence of Salmonella and counting microbial organisms such as aerobic mesophile bacteria (on Plate Count Agar [PCA] for 72 h), total coliforms (on Violet Red Bile Lactose agar [VRBL] at 30 °C for 24 h), thermotolerent coliforms (on VRBL at 44 °C for 24 h), yeast and moulds (on YGC at 25 °C for 72 h). The different methods used for these analyses are described by the French standards (AFNOR) numbered NF V 08-052 (AFNOR 1997), NF V 08-051 (AFNOR 1999b), NF V 08-050 (AFNOR 1999a), NF V 08-060 (AFNOR 1996) and NF V 08-059 (AFNOR 2002), respectively. For the principal suspensions, 10 g of melted shea butter were added to 90 mL of buffered peptone water.
Concerning Salmonella detection (presence), 10 g of shea butter were pre-enriched in a non-selective buffer (buffered peptone water) after incubation at 37 °C for 24 h. Aliquots of the previous solution were inoculated into selective brothes (Rappaport Vasiliadis Soy (RVS) and Salmonella-Shighella) and incubated at 42 °C and 37 °C for 24 h, respectively before being struck into Salmonella-Shighella and Hektoen Enteric agar. Both agars were incubated at 37 °C for 24 h. Several tests of confirmation were performed on typical Salmonella colonies (transparent with a black center and blue-green, respectively). Hence, after culture on Kligler-Hajna agar, urea-tryptophan and glycerol, at 37 °C for 24 h, salmonella colonies were detected by the negative results for lactose, urease, glycerol and indole, and positive result for glucose, gaz and sulfite, they presented.
Statistical analysis
In the present experiment, each test for the sample was analyzed in triplicate. Data were expressed as mean ± standard deviation (SD). Differences between means were analysed by analysis of variance (one way ANOVA) using XLstat 2009 software. Statistical significant difference was stated at p < 0.05.
Authors’ contributions
MR-M have made analyses and redacted the manuscript (Interpretation of data and drafting the manuscript). NS have been involved in revising the manuscript critically for important intellectual content; Both authors (MR-M and NS) have given final approval of the version to be published; Both authors (MR-M and NS) agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Both authors read and approved the final manuscript.
Authors’ information
NS is professor Biochemist (enzymologist).at the University Félix Houphouët-Boigny of Côte d’Ivoire. He holds the position of Vice-Director charged of the research at the UFR Biosciences of the said university. He is the Director of the Laboratory of Biotechnology where he co-directs the program of research on shea butter. He is deeply implicated in both the execution of this program and the publication of the results. Rose-Monde is Lecturer Biochemist at the University Félix Houphouët-Boigny of Côte d’Ivoire Her thesis (For Ph.D) research concerned shea butter quality improvement. Since 2010, she is responsible in charge of the shea butter research program. She has so far published seven research papers on shea butter, locally and in international journals indexed and impacted. The program she executes now aims not only to improve the the traditionally manufactured shea butter quality according to industrials exigencies but also to valorize it as the cheapest and valuable African fat for common and industrial exploitation.
Acknowledgements
Dr. ZOUE LT for the acquisition of some data; he helped in the determination of vitamins contents and the interpretation of the UV–Visible and Infrared spectra. KRA KAS for his critical remarks and suggestions about the manuscript.
Competing interests
About The article titled: “improving the optimized shae butter quality: a great potential of utilization for common consumers and industrials” the authors MEGNANOU Rose-Monde (M R-M) and NIAMKE Sébastien (NS) declare that they have neither financial competing interests nor non-financial competing interests.
Contributor Information
Rose-Monde Megnanou, Phone: + (225) 06 00 67 21, Email: megnanour@yahoo.fr.
Sébastien Niamke, Email: niamkes@yahoo.fr.
References
- AAK Global (2012) Lipex® Shea butter family.Technical bulletin AarhusKarlshamn Lipids care. AarhusKarlshamn. Retrieved from http://www.integrityingredientscorp.com/resources/Shea+Butter+family.pdf
- Aburjai T, Natsheh FM. Plants used in cosmetics. Phytother Res. 2003;17:987–1000. doi: 10.1002/ptr.1363. [DOI] [PubMed] [Google Scholar]
- Afane E, lando G, biyiti L, ossima GA, Atchou G (1997) Boiling palm oil vapours, a bronchoannoying acid. Med Afr Noire 44:604–607. http://www.santetropicale.com/resume/114409.pdf
- AFNOR (1996) Microbiologie des aliments—Dénombrement des coliformes thermotolérants à 44 °C. NF V 08-060 Mars 1996
- AFNOR (1997) Microbiologie des aliments—Recherche des Salmonelles. NF V 08-052 Mai 1997
- AFNOR (1999a) Microbiologie des aliments—Dénombrement des coliformes présumés par comptage des colonies obtenues à 30 °C. NF V 08-050 Février1999
- AFNOR (1999b) Microbiologie des aliments—Dénombrement de la flore aérobie mésophile par comptage des colonies obtenues à 30 °C. NF V 08-051 Février 1999
- AFNOR (2002) Microbiologie des aliments—Dénombrement des levures et moisissures par comptage des colonies à 25 °C. NF V 08-059 Avril 2002
- Allal F, Piombo G, Kelly BA, Okullo JBL, Thiam M, Diallo OB, Nyarko G, Davrieux F, Lovett PN, Bouvet JM. Fatty acid and tocopherol patterns of variation within the natural range of the shea tree (Vitellaria paradoxa) Agrofor Syst. 2013;87:1065–1082. doi: 10.1007/s10457-013-9621-1. [DOI] [Google Scholar]
- AOAC (1997) Official Methods of Analysis. Association of Official Analytical Chemists Ed, Washington
- Ayeh FYO (1991) Solar drying of shea nuts. Cocoa Research Institute of Ghana. Annual Report 1988/89
- Besbes S, Blecker C, Deroanne C, Lognay G, Drira NE, Attia H. Quality characteristics and oxidative stability of date seed oil. Food Sci Technol Intern. 2004;10:333–338. doi: 10.1177/1082013204047777. [DOI] [Google Scholar]
- Bup DN, Abi CF, Tenin D, Kapseu C, Tchiegang C. Optimisation of the cooking process of sheanut kernels (Vitellaria paradoxa Gaertn.) using the doehlert experimental design. Food Bioproc Technol. 2012;5:108–117. doi: 10.1007/s11947-009-0274-z. [DOI] [Google Scholar]
- Carette C, Malotaux M, Van Leeuwen M, Tolkamp M (2009) Shea nut and butter in Ghana: Opportunities and constraints for local processing. Report of the project on the opportunities of shea nuts for Northern Ghana. Resilience. Retrieved from http://www.resilience-foundation.nl/docs/shea.pdf
- Codex Stan 201 (1999) Norme pour les huiles végétales portant un nom spécifique
- Codex-Alimentarius (1993) Fats and Vegetable oils. FAO/WHO, Geneva
- Diaw MM (1982) Contribution a l’étude de l’effet hépato-protecteur du Cochlospermum tinctorium a. Rich. (Cochlospermacees). These d’Etat, Faculté de Médecine et de Pharmacie de Dakar
- Dieffenbacher A, Buxtorf P, Derungs R, Friedli R, Zürcher K. Graisses comestibles, huiles comestibles et graisses émulsionnées. In: Neukom Zimmermann., editor. Manuel suisse des denrées alimentaires. Berne (Suisse): Société des chimistes annalistes suisses; 2000. [Google Scholar]
- Dubois V, Breton S, Linder M, Fanni J, Parmentier M. Fatty acid profiles of 80 vegetable oils with regard to their nutritional potential. Eur J Lipid Sci Technol. 2007;109:710–732. doi: 10.1002/ejlt.200700040. [DOI] [Google Scholar]
- Elias M. Gender, knowledge-sharing and management of shea (Vitellaria paradoxa) parklands in central-west Burkina Faso. J Rur Stud. 2015;38:27–38. doi: 10.1016/j.jrurstud.2015.01.006. [DOI] [Google Scholar]
- Elias M, Carney J. La filière féminine du karité: productrice burkinabée, «éco-consommatrices» occidentales et commerce équitable. Cahi géog Québec. 2004;48:1–26. [Google Scholar]
- European Communities (1991) Determination of free fatty acid methyl esters. In Official Journal (Ed.). Characteristics of olive oil and the relevant methods of analysis, Geneva
- Forestier JP. Les enzymes de I’espace extra-cellulaire du stratum corneum. Inter J Cosm Sci. 1992;14:47–63. doi: 10.1111/j.1467-2494.1992.tb00039.x. [DOI] [PubMed] [Google Scholar]
- Gimeno E, Castellote AI, Lamuela-Ravento RM, Lopez-Sabater M. Rapid determination of vitamin E in vegetable oils by reversed phase high-performance liquid chromatography. J Chromatogr. 2000;881:251–254. doi: 10.1016/S0021-9673(00)00219-3. [DOI] [PubMed] [Google Scholar]
- Gueguen L. Apports nutritionnels conseillés pour la population française. Paris: TEC & DOC, Lavoisier; 2001. [Google Scholar]
- Hall JB, Aebischer DP, Tomlinson HF, Osei-Amaning E, Hindle JR (1996) Vitellaria paradoxa. School of Agricultural and Forest Sciences. Publication Number 8, University of Wales
- Harris WS. The omega-6/omega-3 ratio and cardiovascular disease risk: uses and abuses. Curr Atheroscler Rep. 2006;8(6):453–459. doi: 10.1007/s11883-006-0019-7. [DOI] [PubMed] [Google Scholar]
- Honfo FG, Linnemann AR, Akissoe N, Soumanou MM, Van Boekel M. Characteristics of traditionally processed shea kernels and butter. Intern J Food SciTechnol. 2013;48:1714–1721. doi: 10.1111/ijfs.12142. [DOI] [Google Scholar]
- Honfo FG, Akissoe N, Linnemann AR, Soumanou M, Van Boekel M. Nutritional composition of shea products and chemical properties of shea butter: a review. Critic Rev Food Sci Nutr. 2014;54:673–686. doi: 10.1080/10408398.2011.604142. [DOI] [PubMed] [Google Scholar]
- IUPAC . Standard methods for the analysis of oils, fats and derivatives. 6. Oxford: Pergamon; 1979. [Google Scholar]
- Jansen PCM (2005) Cochlospermum tinctorium Perr. ex A.Rich. In: Jansen PCM, Cardon v (Eds.). Ressources végétales de l’Afrique Tropicale 3: Dyes and tannins/Colorants et tannins. PROTA Network Office Europe, Wageningen University, P.O. Box 341, 6700 AH Wageningen, Netherlands
- Jasaw GS, Saito O, Takeuchi K. Shea (Vitellaria paradoxa) butter production and resource use by urban and rural processors in northern Ghana. Sustainability. 2015;7(3592–3614):10. [Google Scholar]
- Joanny MB. Plantes et vieillissement, données actuelles. Phytothérapie. 2005;3:57–71. doi: 10.1007/s10298-005-0070-5. [DOI] [Google Scholar]
- Karleskind A. Manuel des corps gras. Paris: Technique et documentation; 1992. [Google Scholar]
- Lefur A, Arnaud JP (2004) Les lipides polaires : actifs et vecteurs cosmétiques. OCL 11:436–439. http://www.jle.com/e-docs/00/04/0D/06/vers_alt/VersionPDF.pdf
- Maranz S, Wiesman Z, Bisgaard J, Bianchi G. Germoplasm resources of Vitellaria paradoxa based on variations in fats composition across the species distribution range. Agrofor Syst. 2004;60:71–76. doi: 10.1023/B:AGFO.0000009406.19593.90. [DOI] [Google Scholar]
- Mégnanou R-M, Diopoh KJ. Caractérisation sensorielle, physicochimique et microbiologique du beurre de karité (Vitellaria paradoxa) commercialisé en Côte d’Ivoire. Agron Afr. 2008;20:221–231. [Google Scholar]
- Mégnanou RM, Niamké S (2013) Contribution to the constitution of shea butter sensorial standard based on Ivoirian consumers criteria. IJRBS 2(1):13–21. http://www.ijrbs.in/download.php?file=13-21.pd
- Mégnanou R-M, Niamké S. Effect of nut treatments on shea butter physicochemical criteria and wrapper hygienic quality influence on microbiological properties. J Food Res. 2013;2(5):66–76. doi: 10.5539/jfr.v2n5p66. [DOI] [Google Scholar]
- Mégnanou R-M, Niamké S. Marketed and original shea butters of Côte d’Ivoire: physicochemical and biochemical characterization and evaluation of the potential utilizations. SAR. 2014;3(1):50–59. doi: 10.5539/sar.v3n1p50. [DOI] [Google Scholar]
- Megnanou RM, Niamke S, Diopoh J (2007) Physicochemical and microbiological characteristics of optimized and traditional shea butters from Côte d’Ivoire. Afr J Bioch Res 1(4):041–047. http://www.academicjournals.org/article/article1380017959_Megnanou%20et%20al.pd
- Mensier PH (1957) Dictionnaire des huiles végétales. Encyclopédie Biologique
- MPOB (Malaysian Palm Oil Board) (2005) MPOB test methods: A compendium of test on palm oil products, palm kernel products, fatty acids, food related products and other. (Ed) Ministry of Plantation Industries and Commodities Malaysia
- Nahm HS. Quality characteristics of West African shea butter (Vitellaria paradoxa) and approaches to extend shelf-life. USA: Master of Science, State Universty of New Jersey; 2011. [Google Scholar]
- Ndangui CB, Kimbonguila A, Nzikou JM, Matos L, Pambou-Tobi NPG, Abena AA, Desobry S (2010). Nutritive composition and properties physico-chemical of gumbo (Abelmoschus esculentus L.) seed oil. Res J Environ Earth Sci 2(1):49–54. Retrieved from http://maxwellsci.com/print/rjees/v2-49-54.pdf
- Nsogning Dongmo S, Womeni HM, Tchouanguep Mbiapo F, Linder M, Fanni J, Zarnkow M, Becker T (2014) Cooking and drying process optimisation of shea (Butyrospermum parkii) butter extraction. Czech J Food Sci 32:578–584 http://www.agriculturejournals.cz/publicFiles/138035.pdf (30th/à9/2015)
- Nzikou JM, Mvoula-Tsieri M, Matos L, Matouba E, Ngakegni-Limbili AC, Linder M, Desobry S. Solanum nigrum L. seeds as an alternative source of edible lipids and nutriment in Congo Brazzaville. J Appl Sci. 2007;7:1107–1115. doi: 10.3923/jas.2007.1107.1115. [DOI] [Google Scholar]
- Pesquet JJ(1992) Le karité. APROMA
- PNUD (Programme des Nations Unies pour le Développement) (2010) Shea butter scoping paper: Green commodities facility, Internal working document
- Pontillon J. Cocoa, Borneo illipe, Karité. In: Karleskind A, Wolf JP, editors. Oils and fats manual. Paris: Lavoisier; 1996. [Google Scholar]
- Pouliot M, Elias M. To process or not to process? Factors enabling and constraining shea butter production and income in Burkina Faso. Geoforum. 2013;50:211–220. doi: 10.1016/j.geoforum.2013.09.014. [DOI] [Google Scholar]
- Rossel JB (1991) Vegetable oils and fats. In: Rossell JB (ed). Analysis of oilseeds. London
- Sanou H (2002) Espèce ligneuse du Mali: Vitellaria paradoxa (Sapotaceae). In: Matig EO, Gaoué OG, Dossou B (eds). Espèces ligneuses alimentaires. IPGRI Nairobi (Kenya)
- Schreckenberg K (2004) The contribution of shea butter (Vitellaria paradoxa C.F. Gaertner) to local livelihoods in Benin. In: Sunderland T, Ndoye O (eds). Forest products, livelihoohs and conservation. Indonesia Printer, Indonesia
- Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood) 2008;233(6):674–688. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- Tomaszkiewicz-Potepa A. Shea butter (beurre de karite). Part 1 Origin and recovery. Przemysl Chemiczny. 2012;91:2361–2364. [Google Scholar]
- Tomaszkiewicz-Potepa A, Sliwa K. Shea butter (beurre de karite). Part 2. Chemical composition, methods for processing and application. Przem Chem. 2012;91:2365–2369. [Google Scholar]
- UEMOA (Union Economique et Monétaire Ouest Africaine) (2011). Spécification du beurre de karité non raffiné. PN UEMOA ICS–67
- Yadav MK, Chudasama CD, Jasra RV. Isomerisation of α-pinene using modified montmorillonite clays. J Mol Catal Chem. 2004;216:51–59. doi: 10.1016/j.molcata.2004.02.004. [DOI] [Google Scholar]


