Abstract
Signalling networks that control the life or death of a cell are of central interest in modern biology. While the defined roles of the c-Jun N-terminal kinase (JNK) pathway in regulating cell death have been well-established, additional factors that modulate JNK-mediated cell death have yet to be fully elucidated. To identify novel regulators of JNK-dependent cell death, we performed a dominant-modifier screen in Drosophila and found that the Toll pathway participates in JNK-mediated cell death. Loss of Toll signalling suppresses ectopically and physiologically activated JNK signalling-induced cell death. Our epistasis analysis suggests that the Toll pathway acts as a downstream modulator for JNK-dependent cell death. In addition, gain of JNK signalling results in Toll pathway activation, revealed by stimulated transcription of Drosomycin (Drs) and increased cytoplasm-to-nucleus translocation of Dorsal. Furthermore, the Spätzle (Spz) family ligands for the Toll receptor are transcriptionally upregulated by activated JNK signalling in a non-cell-autonomous manner, providing a molecular mechanism for JNK-induced Toll pathway activation. Finally, gain of Toll signalling exacerbates JNK-mediated cell death and promotes cell death independent of caspases. Thus, we have identified another important function for the evolutionarily conserved Toll pathway, in addition to its well-studied roles in embryonic dorso-ventral patterning and innate immunity.
Keywords: cell death, Drosophila, Eiger, c-Jun N-terminal kinase, Toll
1. Background
The excellent work for studying the pivotal functions of the Toll/IL-1R pathway in innate immunity in Drosophila and mammals earned Jules A. Hoffmann and Bruce A. Beutler the Nobel Prize in Medicine in 2011. Toll was initially identified in Drosophila as a type Ι trans-membrane receptor required for establishing the dorsal–ventral polarity during embryonic development [1,2]. Other components of the Toll pathway, including Spätzle, Tube, Pelle, Cactus and Dorsal, were also characterized as crucial regulators of dorsal–ventral patterning [3–9]. Subsequently, the Toll signalling pathway was implicated in host resistance against fungal and Gram-positive bacterial infections [10–12], which triggers in the fat body the production of antimicrobial peptides (AMPs), among which the antifungal peptide Drosomycin (Drs) appears to be the principal target of the Toll pathway [10,13]. To activate the Toll pathway in development or immunity, the first step is to cleave the inactive precursor of the Toll receptor ligand Spätzle (Spz) [8,9]. Upon binding to the active Spz ligand, Toll receptor recruits Tube and the kinase Pelle through the adaptor protein myeloid differentiation primary response protein 88 (MyD88), to assemble a receptor-proximal oligomeric complex [14,15]. Activation of Pelle triggers the phosphorylation and degradation of Drosophila IκB factor Cactus, which sequesters Dorsal and Dif (Dorsal-related immunity factor), the NF-κB factors in Drosophila, in the cytoplasm [16]. Once Cactus is degraded in response to the signal, Dorsal and Dif translocate to the nucleus and activate the transcription of target genes [17]. The innate immune system, which serves as the first-line defence against pathogen infection, appeared early in evolution and is highly conserved in metazoans [18,19]. To date, there are 10 Toll-like receptors (TLRs) in humans and 12 TLRs in mice, which all activate NF-κB factors in a MyD88-dependent manner to induce a set of immune responses, such as inflammation [20,21]. Although the Toll/TLR pathway has been conserved in evolution, mammalian TLR signalling is not involved in development, whereas the Drosophila Toll pathway plays pivotal roles in both development and immunity [10,22,23].
The c-Jun N-terminal kinase (JNK) represents one sub-group of the MAP kinase (MAPK) family [24,25]. The JNK pathway has been evolutionarily conserved from fly to human, and is involved in the regulation of a wide range of cellular activities including proliferation, differentiation, migration and apoptosis [26,27]. In Drosophila, the tumour necrosis factor (TNF) orthologue Eiger (Egr) triggers cell death through its receptor Grindelwald (Grnd) [28] and the TNF receptor-associated factor 2 (dTRAF2), which in turn activates the conserved JNK cascade including the JNKK kinase dTAK1 (MAP3K), the JNK kinase hemipterous (Hep, MAP2K) and Basket (Bsk) that encodes the Drosophila JNK (MAPK) [29–35]. The activated JNK phosphorylates and activates transcription factors including the AP-1 family members Jun and Fos, which are encoded by the jra and kayak genes in Drosophila, respectively [36–39]. JNK signalling also activates another transcription factor forkhead box O (FoxO), which promotes cell death by upregulating the transcription of the pro-apoptotic gene head involution defective (hid) [36–39]. Although recent studies have identified additional components in this pathway [40–44], the regulating network centred on JNK in modulating cell death, as well as the underlying mechanisms, have not been fully elucidated.
Associations among the TNF/JNK pathway, immune signalling and cell death have been reported in Drosophila [45–48], yet the conclusions are controversial, and the underlying mechanisms remain elusive. Seong et al. [46] found low-dose radiation (LDR) induces both Toll signalling-mediated innate immunity and activation of the JNK pathway, yet a potential interaction between the Toll and JNK pathways was not investigated. In contrast to the Toll signalling that is activated by fungi and Gram-positive bacteria, the Immune Deficiency (Imd) pathway is predominantly activated by Gram-negative bacteria [49,50]. Bangi et al. [45] suggested that an Imd–dTab2-dTAK1-JNK signalling is involved in bacterial-induced invasion and dissemination of oncogenic hindgut cells. However, the Vidal laboratory reported that tumours trigger a systemic immune response through the Egr pathway, which upregulates Toll signalling in the fat body. This activation of Toll, in turn, is required to induce tumour cell death through haemocyte-derived Egr, whereas the Imd pathway is not implicated in this crosstalk [47]. Together, these studies suggest that the Toll pathway may interact with Egr–JNK signalling in regulating cell death during tumour development, yet the modulation mechanisms and a direct role of Toll signalling in cell death have not been documented.
In this study, we have characterized the Toll pathway as an essential modulator of Egr-induced JNK-mediated cell death in Drosophila. First, loss of Toll signalling blocks Egr-induced JNK-mediated cell death in eyes and wings. Second, the Toll pathway acts downstream of FoxO to modulate Egr-triggered JNK-mediated cell death. Third, gain of JNK signalling induces Toll pathway activation, indicated by nuclear accumulation of Dorsal and transcriptional activation of Drosomycin. Furthermore, JNK signalling activates the Toll pathway through transcriptional upregulation of the Spz family ligands in a non-cell-autonomous manner. Finally, gain of Toll signalling promotes cell death and synergistically enhances Egr-triggered cell death. In conclusion, we have identified a previous unknown function of the Toll pathway in modulating TNF-induced JNK-dependent cell death, in addition to its well-established roles in dorsal–ventral patterning and immunity.
2. Results
2.1. Depletion of Toll signalling suppresses Egr-induced cell death in eye development
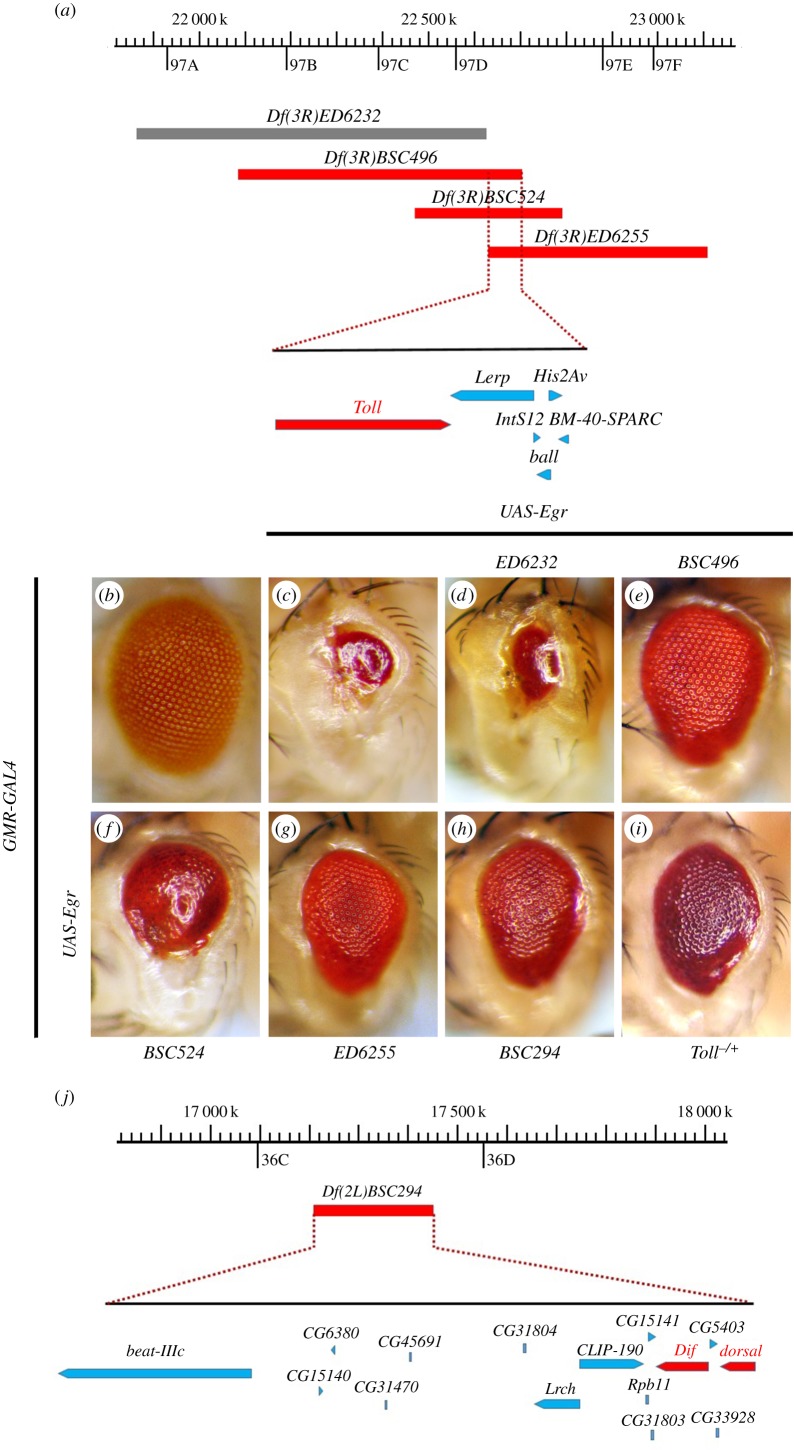
Ectopic expression of Egr in Drosophila eyes driven by GMR-GAL4 leads to vastly reduced adult eye size (figure 1b,c and 2c,d) [29,30], resulting from extensive cell death posterior to the morphogenetic furrow (MF) in third-instar eye discs (figure 2c′,d′), visualized by acridine orange (AO) staining that detects dying cells [51]. To identify additional regulators of Egr-triggered cell death, we performed a genetic screen for dominant modifiers of the GMR>Egr small-eye phenotype using the Bloomington Drosophila Stock Center Deficiency kit that covers more than 95% of the genome. Once a deficiency was found to enhance or suppress the GMR>Egr eye-ablation phenotype, additional overlapping deficiencies were used for fine-mapping of the candidate gene [40,42,52]. One of the suppressors was mapped cytologically within 97D2–97D4, a region uncovered by three overlapping deficiencies: Df(3R)BSC496, Df(3R)BSC524 and Df(3R)ED6255 (figure 1a). The GMR>Egr eye phenotype is partially suppressed by the three deficiencies (figure 1e–g), but not by the adjacent Df(3R)ED6232 (figure 1d). The region of 97D2–97D4 contains six genes including Toll (figure 1a), the Drosophila homologue of TLRs, that encodes a type Ι trans-membrane receptor involved in the activation of NF-κB in dorsal–ventral patterning and immunity [3,10,21]. Accordingly, the GMR>Egr eye phenotype is suppressed to a similar extent by deleting half of the dosage of Toll (figure 1i) or expressing a Toll RNAi (figure 2h). In addition, loss of Toll significantly suppressed GMR>Egr-triggered cell death in eye imaginal discs (figure 2h′,m). Thus, the Toll receptor is required for Egr-triggered cell death in eye development.
Figure 1.
A genetic screen for dominant modifiers of GMR>Egr-induced eye-ablation phenotype. (a and j) Schematic of the genomic region surrounding Toll or Dif and dorsal locus. The deleted regions uncovered by deficiencies are indicated in grey (no effect) or red (suppressor). (b–i) Light micrographs of Drosophila adult eyes are shown. Compared with GMR-GAL4 control (b), GMR>Egr-triggered small eye phenotype (c) remains unaffected by Df(3R)ED6232 (d), but is partially inhibited by deficiencies Df(3R)BSC496 (e), Df(3R)BSC524 (f), Df(3R)ED6255 (g), Df(3R)BSC294 (h) or Tollr3mutation (i). See the electronic supplementary material for detailed genotypes.
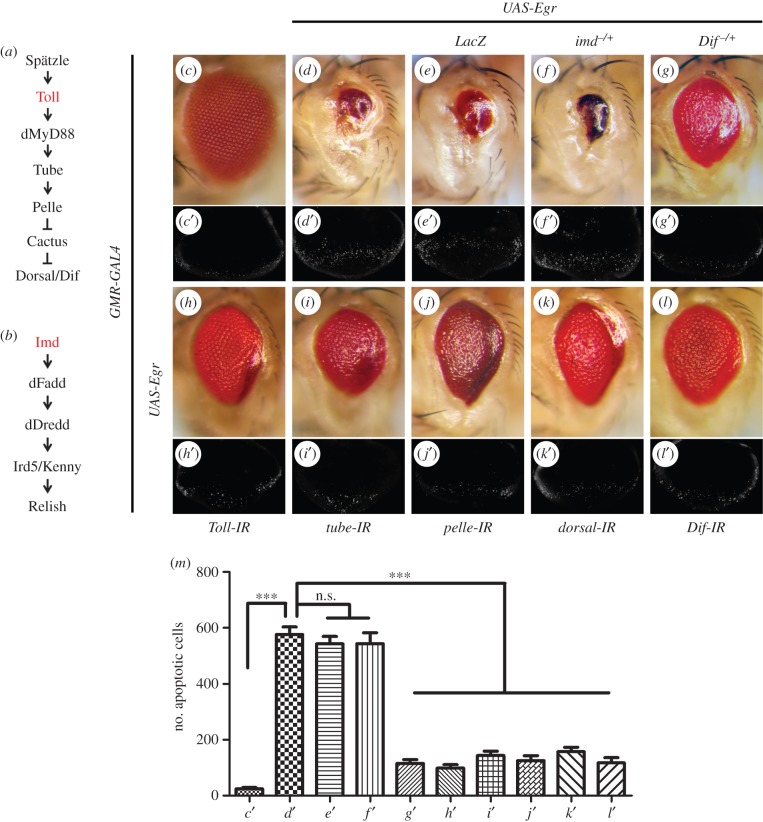
Figure 2.
Loss of Toll signalling inhibits GMR>Egr-triggered cell death. (a,b) Diagrams for the key components of Toll and Imd signalling. Light micrographs of Drosophila adult eyes (c–l) and fluorescence micrographs of third-instar larval eye discs (c′–l′) are shown. Compared with control (c and c′), GMR>Egr-induced small eye phenotype (d) and cell death in eye discs (d′) remain unaffected by expression of LacZ (e and e′) or mutation in imd (f and f′), but are partially suppressed by mutation in Dif (g and g′) or RNAi-mediated downregulation of Toll pathway components: Toll, tube, pelle, dorsal and Dif (h–l and h′–l′). (m) Statistic analysis of cell death in eye discs shown in (c′–l′). Average number of dying cells labelled by AO staining are counted. Error bars indicates standard deviation. One-way ANOVA with Bonferroni multiple comparison test was used to compute p-values, significance was indicated with asterisks (***p < 0.001, n = 10 in each group); n.s., not significant. See the electronic supplementary material for detailed genotypes.
From the same screen, we also identified Df(2L)BSC294 as a suppressor of GMR>Egr-induced eye phenotype (figure 1h). This small deficiency uncovers 36C2–36C9, a region that harbours 15 genes including dorsal and Dif (figure 1j), both of which encode the Drosophila NF-κB factor operating in the Toll pathway (figure 2a). Consistently, GMR>Egr-triggered cell death in eye discs and adult eye phenotype (figure 2d,d′) are partially inhibited by removing one copy of endogenous Dif (figure 2g,g′,m), or RNAi-mediated knocking-down of dorsal or Dif (figure 2k,k′,l,l′,m), but not of LacZ (figure 2e,e′,m), suggesting the involvement of NF-κB in Egr-triggered cell death.
Next, we extended our curiosity to other components of the Toll/NF-κB pathway (figure 2a). We found that both the small eye phenotype and increased cell death induced by GMR>Egr were notably inhibited by RNAi-mediated knocking-down of tube or pelle (figure 2i,i′,j,j′,m). Taken together, these data imply that the Toll pathway plays an essential role in Egr-triggered cell death.
Both Toll and Imd pathways are implicated in Drosophila innate immune response against microbial infection. The Toll pathway is activated primarily by Gram-positive bacteria and fungi while the Imd pathway responds mainly to Gram-negative bacteria infection (figure 2b) [13,53]. As Egr-triggered cell death depends on JNK signalling [29,30], which is also involved in the immune response of the Imd pathway [54,55], we examined a potential role of the Imd pathway in Egr-induced cell death. We found that GMR>Egr-induced cell death phenotypes were not suppressed by knocking-down imd or relish (electronic supplementary material, figure S1a–c), or deleting one or two copies of the endogenous imd (figure 2f,f′,m; electronic supplementary material, figure S1d,f), or homozygous mutation of relish (electronic supplementary material, figure S1g,i), as well as expression of LacZ (electronic supplementary material, figure S1e,h), suggesting the Imd pathway is dispensable for Egr-induced cell death.
2.2. Loss of Toll signalling suppresses JNK-mediated cell death in eye development
Egr-triggered cell death is mainly mediated by JNK signalling [29,30]. To genetically map the epistatic relationship between the Toll pathway and JNK cascade (figure 3p), we first examined the genetic interplays between the Toll pathway and dTAK1 (JNKKK) or Hep (JNKK) in the eye. As expression of dTAK1 driven by GMR-GAL4 (GMR>dTAK1) results in pupa lethality [31], probably caused by the leaky expression of GMR-GAL4 in other tissues including brain, wing and leg discs [56] we used sev-GAL4, another eye specific driver, to express dTAK1 in the eye. Eye specific expression of dTAK1 driven by sevenless (sev)-GAL4 or a constitutive activated form of Hep (HepCA) driven by GMR-GAL4 induces JNK-mediated cell death and generates rough eyes with reduced size (figure 3a,f) [31,42]. Both phenotypes are considerably suppressed by knocking-down pelle or dorsal (figure 3d,e,i,j). In the meantime, expression of LacZ (figure 3b,g) and a bsk RNAi (figure 3c,h) were included as a negative and positive control, respectively. Moreover, ectopic expression of Bsk (Drosophila JNK) under the control of GMR-GAL4 also produces a small and rough eye phenotype (figure 3k), which is clearly suppressed by knockdown Toll signalling components (figure 3m–o), but not the expression of GFP (figure 3l). Taken together, these data suggest that the Toll pathway acts downstream of JNK in modulating cell death.
Figure 3.
Toll signalling acts downstream of JNK in eye development. (a–o) Light micrographs showing Drosophila adult eyes. The small and rough eye phenotype resulting from ectopic expression of dTAK1 (a) or HepCA (f) is suppressed partially by RNAi-mediated knocking-down of bsk (c and h), pelle (d and i) or dorsal (e and j), but not of LacZ (b and g). The rough eye phenotype produced by GMR>Bsk (k) is obviously suppressed by RNAi-mediated inactivation of Toll pathway components: pelle, dorsal and Dif (m–o), but not by the expression of GFP (l). (p) A diagram for the key components of the Egr–JNK pathway. See the electronic supplementary material for detailed genotypes.
2.3. Toll signalling modulates JNK-mediated cell death in wing development
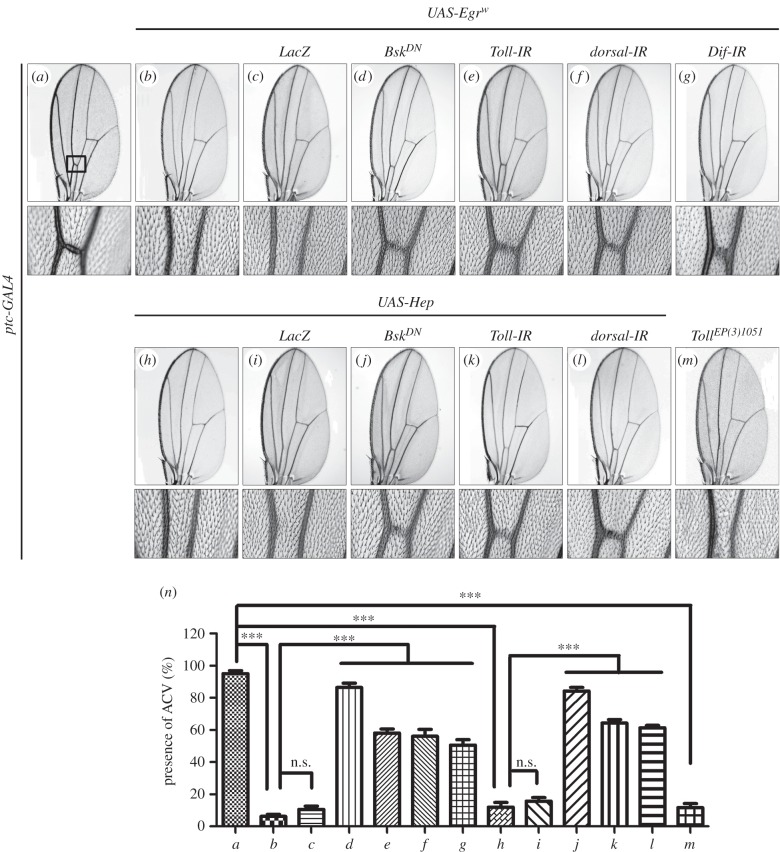
To investigate whether the Toll pathway modulates JNK-mediated cell death in other cellular contexts, we activated JNK signalling in distinct regions of the wing disc. Expression of Egr or Hep along the anterior/posterior (A/P) compartment boundary driven by ptc-GAL4 results in cell death and produces a loss of anterior cross vein (ACV) phenotype (figure 4a,b,h,n) [42,57], which mimics the phenotype generated by expressing the cell death gene grim (ptc>Grim + Tub-GAL80ts; electronic supplementary material, figure S2). This phenotype is recapitulated by Toll expression (figure 4m), and suppressed by expressing a dominant-negative form of Bsk (BskDN) (figure 4d,j) or RNAi-mediated inactivation of Toll pathway components (figure 4e–g,k,l), but not by expressing LacZ (figure 4c,i).
Figure 4.
Toll signalling acts downstream of Hep in wing development. Light micrographs of Drosophila adult wings (a–m). Compared with ptc-GAL4 control (a), ectopic expression of Egrw (b) or Hep (h) or Toll (m) driven by ptc-GAL4 generates loss of ACV phenotype. This phenotype, produced by ptc>EgrW (b) or ptc>Hep (h), is strongly suppressed by a dominant-negative form of Bsk (d and j) or by depletion of Toll signal (e–g, k and l), but not that of LacZ (c and i). The lower panels show high magnification view of the boxed area in upper panels (a–m). (n) Quantification of the ACV phenotype as shown in panels (a–m) (for each genotype, n = 20). Error bars indicates standard deviation. One-way ANOVA with Bonferroni multiple comparison test was used to compute p-values, significance was indicated with asterisks (***p < 0.001); n.s., not significant. See the electronic supplementary material for detailed genotypes.
Furthermore, expression of Hep in the wing pouch driven by Scalloped (Sd)-GAL4 triggers strong cell death (electronic supplementary material, figure S3) that results in severely reduced larval wing disc (electronic supplementary material, figure S4g,h) and adult wing blade (electronic supplementary material, figure S4a,b,f). Both phenotypes are significantly suppressed by expressing BskDN or downregulation of Toll signalling (electronic supplementary material, figure S4d–f,j,k), but not by expressing GFP (electronic supplementary material, figure S4c,f,i), suggesting the Toll pathway modulates JNK-mediated cell death in a non-tissue-specific manner.
2.4. Toll signalling acts downstream of FoxO to modulates JNK-mediated cell death
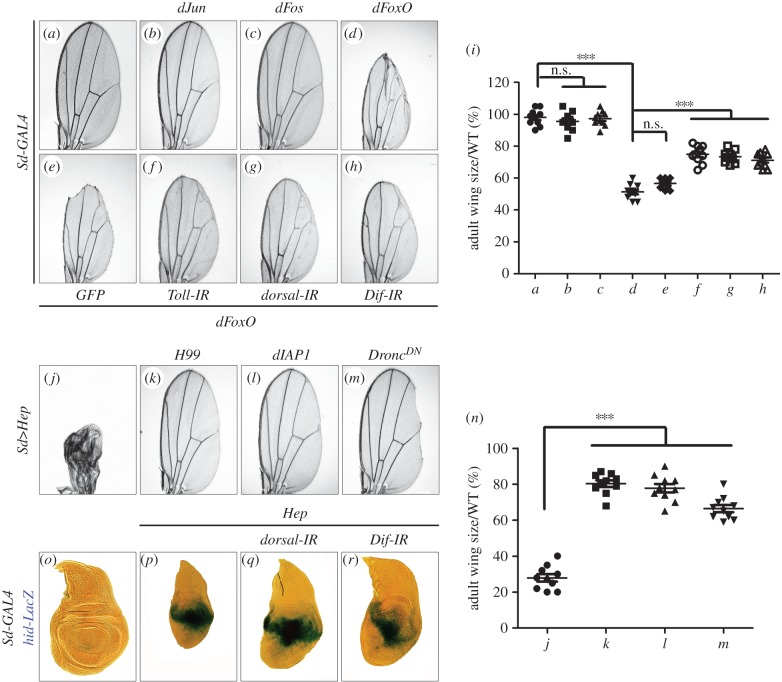
In Drosophila, the AP-1 family members Jun and Fos, and the forkhead box factor FoxO act downstream of JNK as the transcription factors to mediate cell death [36–39]. To further delineate the interaction between JNK and Toll signalling, we ectopically expressed the three transcription factors in the developing wing by Sd-GAL4. Expression of dFoxO (Sd>dFoxO) triggered severe cell death and generated a small wing phenotype (figure 5a,d,i) [58], whereas expression of Jun (Sd>dJun) or Fos (Sd>dFos) did not produce any discernible defects in adult wings (figure 5b,c,i). Furthermore, Sd>dFoxO-induced wing defect could be partially suppressed by knocking-down Toll signalling (figure 5f–i), but remained unaffected by the expression of GFP (figure 5e,i), implying that Toll signalling acts downstream of dFoxO to modulates JNK-mediated cell death in Drosophila.
Figure 5.
Toll signalling acts downstream of FoxO and mediates caspases-independent cell death. (a–h and j–m) Light micrographs of Drosophila adult wings are shown. Compared with Sd-GAL4 control (a), ectopic expression of dFoxO results in an evident reduction in wing size (d), which is suppressed significantly by loss of Toll signalling (f–h), but not that of GFP (e). Expression of dJun or dFos driven by Sd-GAL4 does not produce any visible defects (b and c). The small wing phenotype induced by Sd>Hep (j) is strongly suppressed by Df(3L)H99 that deletes one copy of the apoptotic genes reaper, hid and grim (k), the expression of DIAP1 (l) or DroncDN (m). (i and n) Quantifications of adult wing size/wild-type (WT) ratio shown in figures a–h and j–m, respectively (n = 10). One-way ANOVA with Bonferroni multiple comparison test was used to compute p-values, significance was indicated with asterisks (***p < 0.001); n.s., not significant. (o–r) X-Gal staining of a hid-LacZ reporter in wing discs. Compared with the control (o), expression of Hep in the wing pouch driven by Sd-GAL4 induces hid transcription (p), which cannot be suppressed by knocking-down dorsal or Dif (q and r). See the electronic supplementary material for detailed genotypes.
2.5. Toll pathway is dispensable for caspases-mediated cell death
Previous studies have suggested that Egr–JNK signalling is able to trigger two types of cell death: the apoptotic cell death (caspases-dependent) and the non-apoptotic cell death (caspases-independent) [29,30,44]. In Drosophila, apoptotic cell death is induced by upregulation of three pro-apoptotic genes, rpr, hid and grim, and is mediated by activation of caspases [59]. Consistent with previous study [60], we found that activation of TNF–JNK signalling in the wing pouch by Sd>Hep upregulates the transcription of hid, visualized by X-gal staining of hid-LacZ reporter (figure 5o,p). In addition, Sd>Hep-induced small wing phenotype is partially rescued by the deficiency Df(3L)H99 that deletes rpr, hid and grim, or by expressing the inhibitor of apoptosis protein DIAP1 or a dominant-negative form of Dronc (Drosophila caspase-9) (figure 5j–n) [59,61,62], suggesting TNF–JNK signalling could induce apoptotic cell death in wing development.
To test whether the Toll pathway is involved in JNK-mediated caspases-dependent cell death, we knocked-down Toll signalling and found that loss of the Toll pathway could partially suppress Sd>Hep-induced size reduction, but not the transcriptional upregulation of hid in the wing disc (figure 5o–r). Moreover, ectopic expression of Hid in Drosophila eyes driven by GMR-GAL4 triggers caspases-mediated cell death and produces small adult eye phenotype (electronic supplementary material, figure S5a), which cannot be rescued by depletion of the Toll signalling pathway (electronic supplementary material, figure S5b and c). Hence, we conclude that the Toll pathway is dispensable for caspases-mediated cell death.
2.6. Toll signalling modulates physiological JNK signalling-mediated cell death
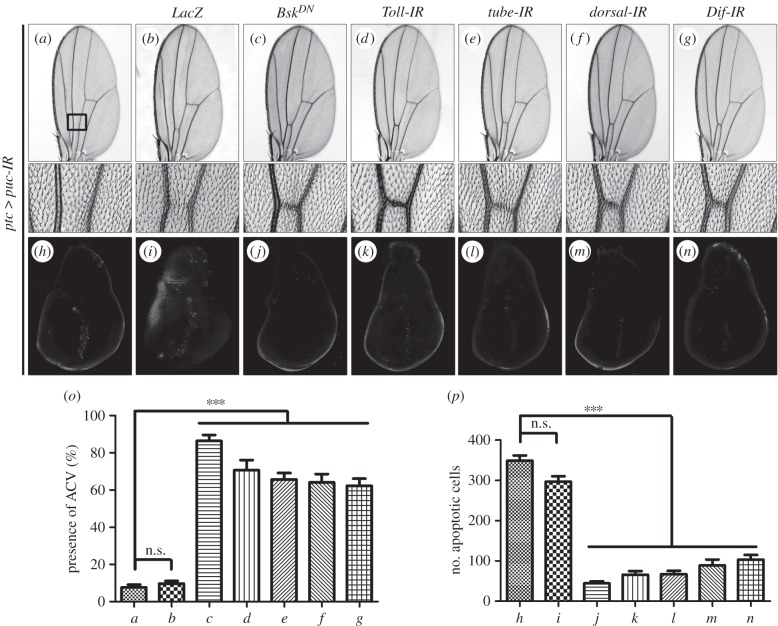
To investigate whether the Toll pathway is involved in the physiological role of JNK signalling in cell death, we knocked-down puc, a negative regulator of JNK activity (figure 3p) [63], along the A/P compartment boundary under the control of ptc-GAL4 (ptc>puc-IR). We observed robust cell death in third-instar larval wing discs and loss of ACV in adult wings (figure 6a,h,o,p). Both phenotypes are fully suppressed by expressing the dominant-negative Bsk (figure 6c,j,o,p), but remain unaffected by expressing LacZ, which serves as a negative control (figure 6b,i,o,p), suggesting loss-of-puc-triggered cell death depends on JNK. Furthermore, ptc>puc-IR-induced cell death and loss-of-ACV phenotypes are suppressed by RNAi-mediated inactivation of Toll pathway components, e.g. Toll, tube, dorsal or Dif (figure 6d–g, k–p), indicating that the Toll pathway is involved in the physiological function of JNK signalling in cell death.
Figure 6.
Loss of Toll signalling suppresses physiological JNK-induced cell death. Light micrographs of Drosophila adult wings (a–g) and fluorescence micrographs of third-instar larval wing discs (h–n) are shown. RNAi-mediated downregulation of puc along the A/P compartment boundary by ptc-GAL4 produces the loss-of-ACV phenotype in adult wings (a), which results from strong cell death in larval wing discs (h). Both phenotypes depends on endogenous JNK (c and j) and the Toll pathway (d–g and k–n), but not on LacZ (b and i). The bottom panels show high magnification views of the boxed area in upper panels (a–g). (o and p) Statistical analysis of ACV phenotype (n = 20 for each genotype) and cell death in wing discs (n = 10) as shown in figures a–g and h–n respectively. Error bars indicate standard deviation. One-way ANOVA with Bonferroni multiple comparison test was used to compute p-values, significance was indicated with asterisks (***p < 0.001); n.s., not significant. See the electronic supplementary material for detailed genotypes.
2.7. JNK signalling promotes Toll pathway activation
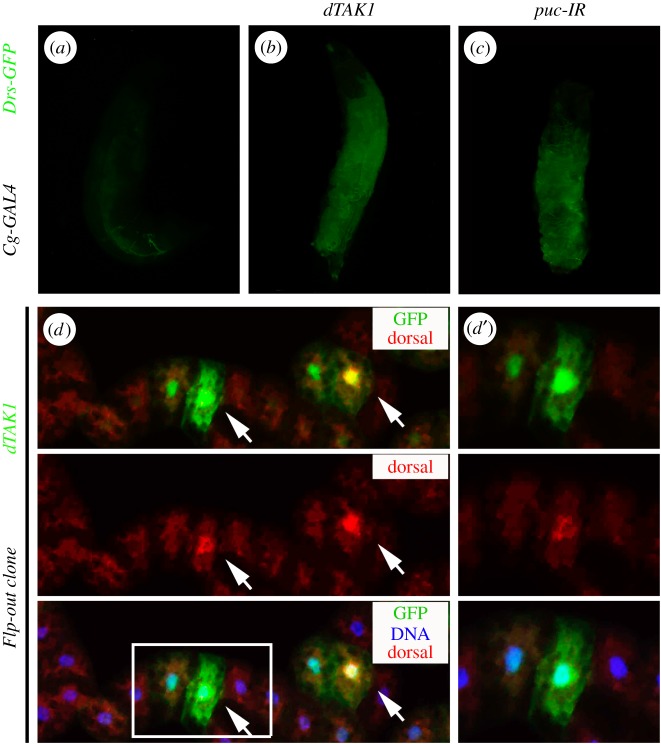
It is known that a fungal infection could trigger the activation of the Toll pathway, which leads to the induction of antifungal peptide Drs in the fat body as its principal target [10,13]. To determine whether JNK signalling is sufficient to elicit Toll pathway activation, we examined the expression of Drs by a previously described Drs-GFP reporter [64], and found that expression of dTAK1 driven by the fat body specific Cg-GAL4 resulted in elevated expression of Drs-GFP (figure 7a,b). Furthermore, knocking-down puc in the fat body also triggers Drs-GFP expression (figure 7c). Hence, both ectopic and physiological JNK activation could induce the transcriptional upregulation of Drs, a primary target gene of the Toll pathway.
Figure 7.
Gain of JNK signalling promotes Drs expression and dorsal nuclear localization. (a–c) Fluorescent microscope images showing third-instar larvae. Compared with control (a), expression of dTAK1 (b) or RNAi inactivation of puc (c) in fat body upregulates Drs-GFP expression. (d) Fluorescent microscope images showing fat body dissected from third-instar larvae stained with anti-dorsal (red). dTAK1-expressing clones were tagged by GFP (green) and induced for 1 h by heat shock at 37°C and recovered for 24 h at 25°C. Nuclei were labelled with DAPI (blue). Endogenous Dorsal protein displays nuclear localization in cells expressing high level of dTAK1 (arrows). (d′) shows high magnification views of the boxed area in (d). DAPI, 4, 6-diamidino-2-phenylindole. See the electronic supplementary material for detailed genotypes.
Our previous data indicated that the Imd pathway is not implicated in TNF/JNK- mediated cell death (figure 2f,f′,m and electronic supplementary material, figure S1). Consistent with these data, activation of JNK signalling did not induce the expression of Diptercicin (Dipt) (electronic supplementary material, figure S6), a reporter of Imd pathway activity [65–68].
In the non-signalling condition, the I-κB orthologue Cactus retains the NF-κB factor Dorsal in the cytoplasm, inhibiting its nuclear localization and transcription factor activity. Upon activation of Toll signalling, Dorsal is released from Cactus and translocates to the nucleus [69]. To monitor the Toll pathway activity directly, we examined the expression level and subcellular localization of Dorsal in vivo by anti-Dorsal staining. Activation of JNK signalling by dTAK1 expression in third-instar eye discs driven by GMR-GAL4 resulted in elevated Dorsal expression posterior to the MF (electronic supplementary material, figure S7). As eye disc cells are too small to distinguish the subcellular distribution of Dorsal protein, we induced ectopic dTAK1-expressing clones in the fat body and examined the nuclear–cytoplasmic shuttling of endogenous Dorsal protein. While Dorsal is mainly localized to the cytoplasm in control cells, it is accumulated in the nuclei and periphery in cells expressing high levels of dTAK1 (figure 7d,d′). Consistently, we observed increased nuclear accumulation of Dorsal in all fat body cells when JNK signalling was activated by Cg-GAL4-driven expression of Egr or puc RNAi (electronic supplementary material, figure S8). These results imply that gain of JNK signalling is sufficient to trigger the activation of the Toll pathway.
Intriguingly, we found that gain of JNK signalling-triggered Toll pathway activation, visualized by stimulated Drs-GFP expression and increased Dorsal nuclear localization, could not be suppressed by expressing caspases inhibitor P35 (electronic supplementary material, figure S9a–c), implying that activation of Toll signalling by activated JNK is caspases independent.
2.8. Gain of JNK signalling upregulates the transcription of Spz family ligands
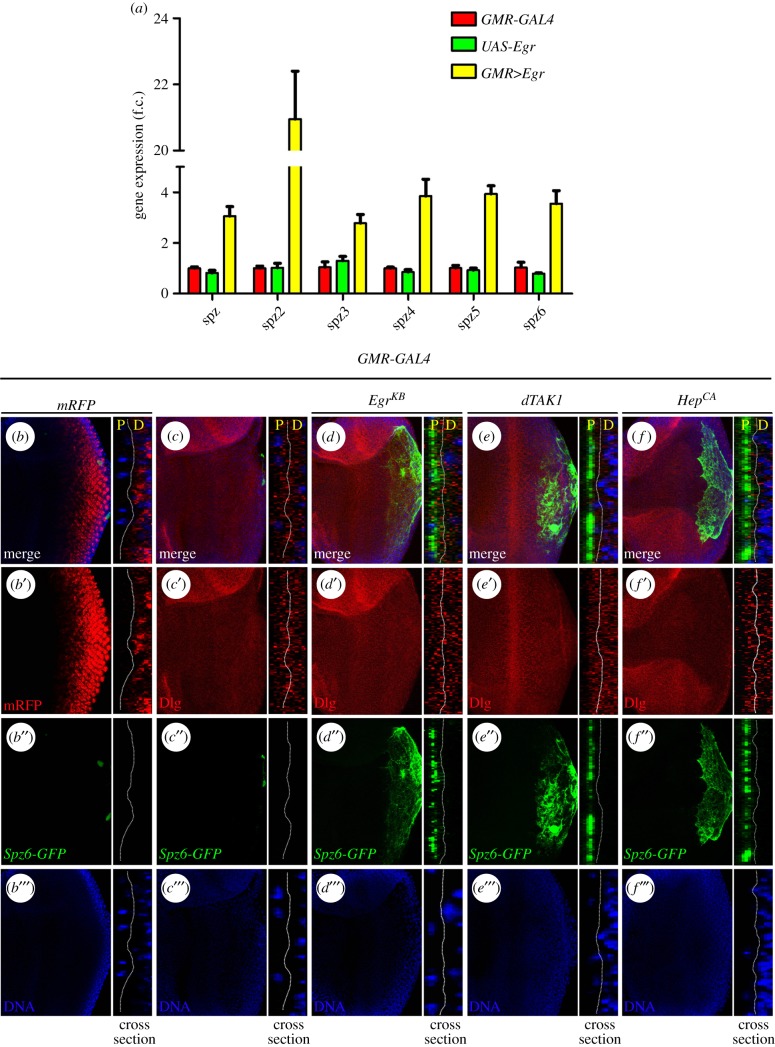
The gene spz encodes the Drosophila ligand for Toll receptor that activates the Toll pathway in embryonic development and the innate immune response [8,9,70]. As JNK signalling triggers Toll pathway activation and depletion of Toll receptor suppresses JNK-induced cell death, we postulated that JNK signalling might operate upstream of Toll receptor, for instance, by regulating the transcription of Spz. To test this hypothesis, we activated JNK signalling in the adult eye (GMR>Egr), extracted total mRNA from the heads and performed a quantitative reverse transcription polymerase chain reaction (qRT-PCR) assay. In support of our assumption, Spz transcription was upregulated more than three-fold upon JNK activation (figure 8a). Spz homologues, referred to as Spz2 to Spz6, have been identified in the Drosophila genome by an iterative searching method [71]. They share a characteristic intron–exon structure with the prototype spz gene, and could execute a similar or redundant function as Spz by binding to the Toll receptors [71]. Intriguingly, activation of JNK signalling is able to upregulate the transcription of all five Spz homologues, with Spz2 level increased by more than 20-fold, as analysed by qRT-PCR assay (figure 8a).
Figure 8.
Gain of JNK signalling upregulates the expression of Spz family ligands. (a) Histogram showing the levels of Spz1–6 mRNAs measured by quantitative RT-PCR. Total RNA of Drosophila adult eyes was extracted and normalized for cDNA synthesis. Error bars represent standard deviation from three independent experiments. (b–f) Fluorescence micrographs of Drosophila third-instar larval eye discs are shown. Compared with the GMR-GAL4 control (c), Spz6-GFP expression was evidently increased by expression of Egr (d), dTAK1 (e) or Hep (f), mRFP marking the GMR-GAL4 expression region (b). The right panels show views of vertical cross sections corresponding to the left panels (b–f). Nuclei were labelled with DAPI (blue), cell membranes were stained by anti-Dlg antibody (red). Imaging of prepared samples was conducted by a Leica confocal microscope (Leica SP5). See the electronic supplementary material for detailed genotypes.
To confirm the qRT-PCR data in vivo, we examined the transcription of Spz6 with a Spz6-GFP reporter strain [72]. Elevated Spz6-GFP expression was noted posterior to MF in third-instar larval eye discs when JNK signalling was activated by ectopic expression of Egr, dTAK1 or Hep, driven by GMR-GAL4 whose expression pattern was visualized by mRFP (figure 8b–f), suggesting that gain of JNK signalling could induce Toll pathway activation through transcriptional upregulation of Spz family ligands. Consistent with our observation that induction of the Toll pathway by activated JNK signalling is caspase independent (electronic supplementary material, figure S9a–c), the increased Spz6-GFP expression was not suppressed by expressing the caspases inhibitor P35 (electronic supplementary material, figure S9d,e), but was suppressed by the expression of BskDN that served as a positive control (electronic supplementary material, figure S9f).
While we found that Spz6-GFP expression is upregulated by JNK signalling in third-instar larval eye discs (figure 8b–f), we are not sure this activation is cell autonomous or non-cell autonomous. As previous study has shown that Spz ligands produced by haemocytes could induce the activation of the Toll/NF-κB pathway in the fat body non-cell autonomously [73], we wondered whether the Spz6-GFP expressing cells are haemocytes attached to the eye disc. To test this possibility, we performed antibody staining against the haemocyte marker NimC1 [74]. We observed numerous haemocytes associated with control eye discs (electronic supplementary material, figure S9g), yet the haemocyte number remained unchanged upon activation of JNK signalling by GMR>Egr or GMR>dTAK1 (electronic supplementary material, figure S9h and i). In the meantime, we found that the region attached by haemocytes does not overlap with that of Spz6-GFP expression (figure 8d–f and electronic supplementary material, figure S9g–i). Together, these data suggest that Spz6-GFP expressing cells induced by JNK signalling are not haemocytes.
Anatomically, the eye imaginal disc, with the sac-like two-layered structure, comprises a columnar cell monolayer (named disc proper, DP) covered by a squamous epithelium known as the peripodial membrane (PM) [75]. As the communications between the two distinct cell layers are important for concerted growth and patterning of the disc during development [76,77], we examined the vertical cross section of eye imaginal discs to investigate whether the Spz6-GFP expressing cells are DP or PM cells. Indeed, we observed two opposing cell layers, PM (P) and DP (D), in the control discs (figure 8c), with GMR-GAL4 expression, marked by mFRP, specifically located within the DP cells (figure 8b). When JNK signalling was activated by ectopic expression of Egr, dTAK1 or Hep driven by GMR-GAL4 in the DP cells, Spz6-GFP expression was significantly increased in the PM cells (figure 8d–f). These results suggest that Spz ligands are induced by activated JNK signalling in a non-cell-autonomous manner in eye discs.
2.9. Gain of Toll signalling aggravates Egr-induced cell death in eye development
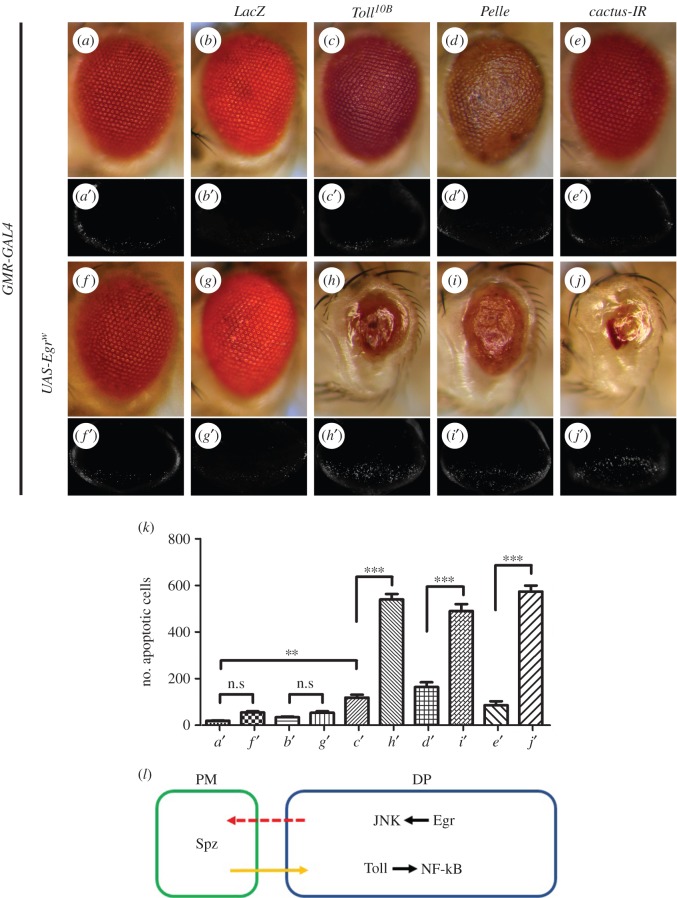
To further characterize the role of the Toll pathway in cell death, we upregulated Toll signalling in the developing eye by expressing Toll, Pelle, Dorsal, Dif, or an RNAi of cactus, the unique Drosophila orthologue of IκB that operates as a negative regulator of the Toll pathway [78]. Compared with the controls or expression of LacZ (figure 9a,a′,b,b′ and k), enhanced Toll signalling results in mild but significant cell death in the eye discs (figure 9c′–e′,k and electronic supplementary material, figure S10a–d) and produces rough eyes in adults (figure 9c–e and electronic supplementary material, figure S10e and i), suggesting that activation of the Toll pathway is sufficient to induce cell death. To confirm the activation of Toll signalling, we expressed Toll, Pelle and cactus-IR in the fat body, and monitored the expression of Drs by Drs-GFP [64], the recognized Toll pathway reporter [10,13]. We found that the activation of Toll signalling induced by Toll10B (an activated form of Toll) or cactus-IR is much stronger than that induced by Pelle (electronic supplementary material, figure S11). We next questioned whether elevated Toll signalling could boost Egr-induced cell death. For this purpose, a weak UAS-Egr line (UAS-Egrw) [29] was expressed in the eye, which caused limited cell death in third-instar eye discs and adult eyes (figure 9f,f′,k). A synergistic enhancement in cell death and eye size reduction was observed when Egrw was co-expressed with Toll, Pelle or the cactus RNAi, but not with LacZ (figure 9g–j,g′–j′,k), suggesting that gain of Toll signalling exacerbates Egr-triggered cell death.
Figure 9.
Gain of Toll signalling enhances GMR>Egr-induced cell death. Light micrographs of Drosophila adult eyes (a–j) and fluorescence micrographs of third-instar larval eye discs (a′–j′) are shown. Compared with control (a and a′), expression of Toll10B, Pelle, a cactus RNAi or a weak Egr line (EgrW) promotes little or mild cell death in eye discs and adult eyes (c–f and c′–f′). Co-expression of EgrW with Toll10B, Pelle or a cactus RNAi displays synergistic enhancement of cell death in eye discs and adult eyes (h–j and h′–j′). As a negative control, expression of LacZ neither triggers cell death by itself (b and b′) nor aggravates EgrW-induced cell death (g and g′). (k) Statistical analysis of cell death in eye discs shown in (a′–j′). Average number of dying cells labelled by AO staining are counted. Error bars indicate standard deviation. One-way ANOVA with Bonferroni multiple comparison test was used to compute p-values, significance was indicated with asterisks (**p < 0.01, ***p < 0.001, n = 10 in each group); n.s., not significant. (l) A model for the role of the Spz/Toll/NF-κB pathway in modulating Egr-triggered JNK-mediated cell death. See the electronic supplementary material for detailed genotypes.
2.10. Toll signalling-triggered cell death is independent of JNK, caspase or necroptosis
While the Toll/NF-κB pathway is able to promote cell death, the underlying mechanisms remains largely unknown. We found that expression of BskDN, or Puckered (Puc) encoding a JNK phosphatase that negatively regulates JNK kinase activity [63], exhibits no suppressive effect on the rough eye phenotypes induced by Dorsal or Dif expression (electronic supplementary material, figure S10e–l), implying that the JNK pathway is not involved in Toll pathway-induced cell death.
Our previous data have implied that the Toll signalling pathway is not required for caspases-mediated cell death (electronic supplementary material, figure S5). To further investigate the relationship between Toll pathway-induced cell death and caspases, we firstly checked antibody staining for the activated form of Caspase-3 (CC-3), a read-out of the initiator caspase Dronc (Drosophila caspase-9) activity [79], and found that gain of Toll signalling by over-expressing Dorsal or Dif did not result in increased caspases activity (electronic supplementary material, figure S12a–d). Consistently, we also observed that the rough and small eye phenotypes induced by Dorsal or Dif expression remain unaffected by blocking caspase signalling (electronic supplementary material, figure S12e–h,k–n). Together, these data indicated that the Toll pathway triggers caspase-independent cell death.
Necroptosis/programmed necrosis, which is a RIP1/RIP3-dependent caspase-independent programmed necrotic cell death, plays essential roles in development [80–84], and has been implicated in a variety of pathological progresses, including tumourigenesis, metastasis, inflammation and liver diseases [85–87]. To investigate whether cell death induced by the Toll/NF-κB pathway is mediated by necroptosis, we depleted dTRAF2, the key component of necroptosis machinery [88,89]. We found that mutation or RNAi-mediated inactivation of dTRAF2 has no effect on the rough eye phenotypes of GMR>Dorsal or GMR>Dif (electronic supplementary material, figure S12i,j,o,p), indicating that Toll/NF-κB pathway-triggered cell death is independent of necroptosis.
3. Discussion
The Toll pathway has been implicated in embryonic dorsal–ventral patterning and innate immunity in Drosophila. In this study, we demonstrate that the Toll pathway modulates JNK-mediated cell death in vivo, adding a distinct but vital role to this well-characterized signalling pathway, and further show that JNK signalling triggers Toll pathway activation through transcriptional upregulation of Spz family members encoding the ligands for Toll receptor in a non-cell-autonomous manner (figure 9l). In the light of their conserved function from fly to human, the implication of the Drosophila Toll pathway in JNK signalling-mediated cell death shall provide a novel connection for the crosstalk between IL-1R and JNK signalling in cell death in mammals.
We found that loss of Spz, either by mutation or RNAi inactivation, failed to suppress GMR-Egr-triggered cell death (data not shown), suggest other Spz family ligands may play redundant function in modulating cell death. Consistent with this interpretation, Spz2 was shown to play redundant function with other Spz ligands and regulate cell death in CNS [90]. As there is no mutant allele available for other spz genes at the moment, it is not feasible to determine whether all or a specific set of Spz molecules are required for JNK-induced Toll pathway activation. Further investigation will be required to clarify this matter.
Recent study reported that the Toll pathway could be activated by danger signals released by apoptosis-deficient cells in a non-cell-autonomous manner [91]. Consistently, we found that elevated JNK signalling triggers Toll pathway activation independent of caspase-mediated apoptosis (electronic supplementary material, figure S9a–f). Furthermore, we provide compelling evidence for a molecular mechanism of this activation: activated JNK signalling in the columnar disc (DP) cells induces elevated expression of Spz family ligands in the PM cells (figure 8b–f), which in turn activates the Toll/NF-κB pathway in DP cells (figure 9l). Thus, our data reveal a functional interplay between the DP cells and the PM cells in eye discs. However, the mechanism by which JNK signalling triggers the expression of Spz ligands non-cell autonomously remains elusive.
A recent study suggested that the Toll/NF-κB pathway promotes JNK-independent cell death by upregulating the expression of pro-apoptotic gene rpr in the loser cells of wing discs during cell competition [92]. However, we have provided evidence indicating that Toll pathway-mediated cell death is mostly caspase-independent: (i) loss of Toll signalling does not suppress Sd>Hep induced transcriptional upregulation of hid in the wing disc (figure 5o–r); (ii) loss of Toll signalling does not affect GMR>Hid induced small eye phenotype (electronic supplementary material, figure S5); (iii) GMR>Dorsal or GMR>Dif-induced cell death in third-instar larval eye discs (electronic supplementary material, figure S10a–d) is independent of caspase activation (electronic supplementary material, figure S12a–c) and (iv) GMR>Dorsal and GMR>Dif-induced small eye phenotypes are not suppressed by blocking caspase signalling (electronic supplementary material, figure S12e–h,k–n). In addition, we found that Toll pathway-triggered cell death in the eye is independent of JNK or necroptosis (electronic supplementary material, figure S10e–l; S12i, j, o, p). Thus, activated Toll signalling may promote cell death via distinct mechanisms in a context-dependent manner.
4. Material and methods
4.1. Fly strains
Flies were kept on a cornmeal and agar medium at 25°C according to standard protocols. Drosophila strains used include: Tollr3, Tollr4, Dif1, spz2, spz3, imd1, relishE38, UAS-Toll-IR (31044, 41477 and 35628), UAS-pelle-IR (34733 and 35577), Spz6-GFP (23305), UAS-mGFP (32197), UAS-mRFP (32218), UAS-Dorsal (9319), UAS-Dif (22201), UAS-dJun (7216), UAS-dFos (7213), UAS-dFoxO, UAS-DIAP1, UAS-Bsk, Df(3L)H99, Cg-GAL4 (7011), Tub-GAL80ts (7017), Drs-GFP Dipt-LacZ (55707), yw1118 hs-Flp; act>y + >GAL4 UAS-GFP, these and the deficiency kit were obtained from Bloomington Drosophila stock centre. UAS-puc-IR (3018 and 3019), UAS-dorsal-IR (45996 and 45998), UAS-Dif-IR (30578 and 30579) and UAS-relish-IR (49413 and 49414) were obtained from Vienna Drosophila RNAi centre. UAS-tube-IR (105520R1 and 10520R3), UAS-imd-IR (5576R1 and 5576R2), UAS-cactus-IR (5848R1 and 5848R3) and UAS-bsk-IR (5680R2) were obtained from Fly Stocks of National Institute of Genetics (NIG). GMR-GAL4 [56], Sd-GAL4, ptc-GAL4 [57], UAS-Toll10B [93], TollEP(3)1051 [94], UAS-Egr, UAS-EgrW [29], UAS-EgrKB [30], UAS-BskDN [31], UAS-Puc, sev-GAL4, UAS-dTAK1, UAS-HepCA, UAS-Hep, UAS-GrimM146, UAS-HidM137, UAS-Imd.SNF32, UAS-DroncDN [29], UAS-P35, UAS-GFP [41,42,52], hid-LacZ [40], dTRAF2EX1.1 and UAS-dTRAF2-IR were previously described.
4.2. Acridine orange staining
Eye and wing discs were dissected from third-instar larvae in 0.3% PBST (phosphate-buffered saline (PBS) + 0.3% Triton X-100) and incubated in 1 × 10−5 M AO for 5 min at room temperature prior to imaging as described [40].
4.3. X-gal staining
Wing discs were dissected from third-instar larvae in 0.1% PBST (PBS + 0.1% Triton X-100) and stained for ß-galactosidase activity as described [95].
4.4. Immunohistochemistry
Fat body and eye discs dissected from third-instar larvae were collected in cold PBS, and fixed in 4% paraformaldehyde. After fixation, samples were washed three times in 0.3% PBST, blocked in 10% horse serum and stained with primary antibody overnight at 4°C. Samples were washed as previously described and subjected to secondary antibodies for 2 h. Primary antibodies used included mouse anti-dorsal (1 : 100, Cell Signaling Technology), mouse anti-Dlg (1 : 200, DSHB), mouse anti-NimC1 (1 : 200, kind gift of I. Ando) and rabbit anti-Cleaved Caspase-3 (1 : 200, Cell Signaling Technology). Secondary antibodies used were anti-mouse-Cy3 (1 : 1000, Jackson Immuno Research) and anti-rabbit- Cy3 (1 : 1000, Cell Signaling Technology).
4.5. Quantitative reverse transcription polymerase chain reaction
Thirty adult heads were collected from freshly eclosed flies of indicated genotypes. Total RNA was isolated using TRIzol (Invitrogen), and RT-PCR was performed as previously described [96]. Primers for Rp49 and spz1–6 were kindly provided by Dr Ketu Mishra at Yale University.
5. Statistics
Results are presented as bar graphs or scatter plots created using GraphPad Prism 6.0. A combination of unpaired t-test and one-way ANOVA with Bonferroni's multiple comparison test was used to calculate statistical significance; p-values are included in the relevant figure legends.
Supplementary Material
Acknowledgements
We thank Bloomington, VDRC and NIG stock centres for fly stocks, Dr Ketu Mishra (Yale University) for spz1–6 primers, and members of the Xue laboratory, Margaret Ho and Ying Cao for discussion and critical comments.
Authors' contributions
C.W. and L.X. conceived and designed the experiments. C.W., C.C., J.D., F.Z., Y.C. and W.L. conducted the experiments. C.W. J.C.P.-P. and L.X. analysed the data, C.W. and L.X. wrote the manuscript. All authors gave final approval for publication.
Competing interests
The authors declare no conflict of interest.
Funding
This work was supported by the National Basic Research Program of China (973 Program) (2011CB943903), National Natural Science Foundation of China (31071294, 31171413, 31371490), the Specialized Research Fund for the Doctoral Program of Higher Education of China (20120072110023) and Shanghai Committee of Science and Technology (09DZ2260100, 14JC1406000) to L.X., and National Nature Science Foundation of China (31371459), the Tsinghua Initiative Program (20131089281) and the 1000 Talents award to J.C.P-P.
References
- 1.Gerttula S, Jin YS, Anderson KV. 1988. Zygotic expression and activity of the Drosophila Toll gene, a gene required maternally for embryonic dorsal–ventral pattern formation. Genetics 119, 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hashimoto C, Hudson KL, Anderson KV. 1988. The Toll gene of Drosophila, required for dorsal–ventral embryonic polarity, appears to encode a transmembrane protein. Cell 52, 269–279. (doi:10.1016/0092-8674(88)90516-8) [DOI] [PubMed] [Google Scholar]
- 3.Steward R. 1987. Dorsal, an embryonic polarity gene in Drosophila, is homologous to the vertebrate proto-oncogene, c-rel. Science 238, 692–694. (doi:10.1126/science.3118464) [DOI] [PubMed] [Google Scholar]
- 4.Roth S, Hiromi Y, Godt D, Nusslein-Volhard C. 1991. cactus, a maternal gene required for proper formation of the dorsoventral morphogen gradient in Drosophila embryos. Development 112, 371–388. [DOI] [PubMed] [Google Scholar]
- 5.Letsou A, Alexander S, Orth K, Wasserman SA. 1991. Genetic and molecular characterization of tube, a Drosophila gene maternally required for embryonic dorsoventral polarity. Proc. Natl Acad. Sci. USA 88, 810–814. (doi:10.1073/pnas.88.3.810) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hecht PM, Anderson KV. 1993. Genetic characterization of tube and pelle, genes required for signaling between Toll and dorsal in the specification of the dorsal-ventral pattern of the Drosophila embryo. Genetics 135, 405–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shelton CA, Wasserman SA. 1993. pelle encodes a protein kinase required to establish dorsoventral polarity in the Drosophila embryo. Cell 72, 515–525. (doi:10.1016/0092-8674(93)90071-W) [DOI] [PubMed] [Google Scholar]
- 8.Morisato D, Anderson KV. 1994. The spatzle gene encodes a component of the extracellular signaling pathway establishing the dorsal–ventral pattern of the Drosophila embryo. Cell 76, 677–688. (doi:10.1016/0092-8674(94)90507-X) [DOI] [PubMed] [Google Scholar]
- 9.Schneider DS, Jin Y, Morisato D, Anderson KV. 1994. A processed form of the Spatzle protein defines dorsal–ventral polarity in the Drosophila embryo. Development 120, 1243–1250. [DOI] [PubMed] [Google Scholar]
- 10.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. 1996. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86, 973–983. (doi:10.1016/S0092-8674(00)80172-5) [DOI] [PubMed] [Google Scholar]
- 11.Hetru C, Hoffmann JA. 2009. NF-κB in the immune response of Drosophila. Cold Spring Harb. Perspect. Biol. 1, a000232 (doi:10.1101/cshperspect.a000232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aggarwal K, Silverman N. 2008. Positive and negative regulation of the Drosophila immune response. BMB Rep. 41, 267–277. (doi:10.5483/BMBRep.2008.41.4.267) [DOI] [PubMed] [Google Scholar]
- 13.De Gregorio E, Spellman PT, Tzou P, Rubin GM, Lemaitre B. 2002. The Toll and Imd pathways are the major regulators of the immune response in Drosophila. EMBO J. 21, 2568–2579. (doi:10.1093/emboj/21.11.2568) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horng T, Medzhitov R. 2001. Drosophila MyD88 is an adapter in the Toll signaling pathway. Proc. Natl Acad. Sci. USA 98, 12 654–12 658. (doi:10.1073/pnas.231471798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun H, Bristow BN, Qu G, Wasserman SA. 2002. A heterotrimeric death domain complex in Toll signaling. Proc. Natl Acad. Sci. USA 99, 12 871–12 876. (doi:10.1073/pnas.202396399) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Towb P, Bergmann A, Wasserman SA. 2001. The protein kinase Pelle mediates feedback regulation in the Drosophila Toll signaling pathway. Development 128, 4729–4736. [DOI] [PubMed] [Google Scholar]
- 17.Wu LP, Anderson KV. 1998. Regulated nuclear import of Rel proteins in the Drosophila immune response. Nature 392, 93–97. (doi:10.1038/32195) [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RA. 1999. Phylogenetic perspectives in innate immunity. Science 284, 1313–1318. (doi:10.1126/science.284.5418.1313) [DOI] [PubMed] [Google Scholar]
- 19.Silverman N, Maniatis T. 2001. NF-κB signaling pathways in mammalian and insect innate immunity. Genes Dev. 15, 2321–2342. (doi:10.1101/gad.909001) [DOI] [PubMed] [Google Scholar]
- 20.Barton GM, Medzhitov R. 2003. Toll-like receptor signaling pathways. Science 300, 1524–1525. (doi:10.1126/science.1085536) [DOI] [PubMed] [Google Scholar]
- 21.Valanne S, Wang JH, Ramet M. 2011. The Drosophila Toll signaling pathway. J. Immunol. 186, 649–656. (doi:10.4049/jimmunol.1002302) [DOI] [PubMed] [Google Scholar]
- 22.Belvin MP, Anderson KV. 1996. A conserved signaling pathway: the Drosophila Toll–Dorsal pathway. Annu. Rev. Cell Dev. Biol. 12, 393–416. (doi:10.1146/annurev.cellbio.12.1.393) [DOI] [PubMed] [Google Scholar]
- 23.Kimbrell DA, Beutler B. 2001. The evolution and genetics of innate immunity. Nat. Rev. Genet. 2, 256–267. (doi:10.1038/35066006) [DOI] [PubMed] [Google Scholar]
- 24.Davis RJ. 2000. Signal transduction by the JNK group of MAP kinases. Cell 103, 239–252. (doi:10.1016/S0092-8674(00)00116-1) [DOI] [PubMed] [Google Scholar]
- 25.Weston CR, Davis RJ. 2002. The JNK signal transduction pathway. Curr. Opin. Genet. Dev. 12, 14–21. (doi:10.1016/S0959-437X(01)00258-1) [DOI] [PubMed] [Google Scholar]
- 26.Weston CR, Davis RJ. 2007. The JNK signal transduction pathway. Curr. Opin. Cell Biol. 19, 142–149. (doi:10.1016/j.ceb.2007.02.001) [DOI] [PubMed] [Google Scholar]
- 27.Dhanasekaran DN, Reddy EP. 2008. JNK signaling in apoptosis. Oncogene 27, 6245–6251. (doi:10.1038/onc.2008.301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andersen DS, et al. 2015. The Drosophila TNF receptor Grindelwald couples loss of cell polarity and neoplastic growth. Nature 522, 482–486. (doi:10.1038/nature14298) [DOI] [PubMed] [Google Scholar]
- 29.Igaki T, et al. 2002. Eiger, a TNF superfamily ligand that triggers the Drosophila JNK pathway. EMBO J. 21, 3009–3018. (doi:10.1093/emboj/cdf306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moreno E, Yan M, Basler K. 2002. Evolution of TNF signaling mechanisms: JNK-dependent apoptosis triggered by Eiger, the Drosophila homolog of the TNF superfamily. Curr. Biol. 12, 1263–1268. (doi:10.1016/S0960-9822(02)00954-5) [DOI] [PubMed] [Google Scholar]
- 31.Xue L, Igaki T, Kuranaga E, Kanda H, Miura M, Xu T. 2007. Tumor suppressor CYLD regulates JNK-induced cell death in Drosophila. Dev. Cell. 13, 446–454. (doi:10.1016/j.devcel.2007.07.012) [DOI] [PubMed] [Google Scholar]
- 32.Takatsu Y, Nakamura M, Stapleton M, Danos MC, Matsumoto K, O'Connor MB, Shibuya H, Ueno N. 2000. TAK1 participates in c-Jun N-terminal kinase signaling during Drosophila development. Mol. Cell Biol. 20, 3015–3026. (doi:10.1128/MCB.20.9.3015-3026.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glise B, Bourbon H, Noselli S. 1995. hemipterous encodes a novel Drosophila MAP kinase kinase, required for epithelial cell sheet movement. Cell 83, 451–461. (doi:10.1016/0092-8674(95)90123-X) [DOI] [PubMed] [Google Scholar]
- 34.Kanda H, Igaki T, Kanuka H, Yagi T, Miura M. 2002. Wengen, a member of the Drosophila tumor necrosis factor receptor superfamily, is required for Eiger signaling. J. Biol. Chem. 277, 28 372–28 735. (doi:10.1074/jbc.C200324200) [DOI] [PubMed] [Google Scholar]
- 35.Kauppila S, et al. 2003. Eiger and its receptor, Wengen, comprise a TNF-like system in Drosophila. Oncogene 22, 4860–4867. (doi:10.1038/sj.onc.1206715) [DOI] [PubMed] [Google Scholar]
- 36.Luo X, Puig O, Hyun J, Bohmann D, Jasper H. 2007. Foxo and Fos regulate the decision between cell death and survival in response to UV irradiation. EMBO J. 26, 380–390. (doi:10.1038/sj.emboj.7601484) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kockel L, Homsy JG, Bohmann D. 2001. Drosophila AP-1: lessons from an invertebrate. Oncogene 20, 2347–2364. (doi:10.1038/sj.onc.1204300) [DOI] [PubMed] [Google Scholar]
- 38.Accili D, Arden KC. 2004. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 117, 421–426. (doi:10.1016/S0092-8674(04)00452-0) [DOI] [PubMed] [Google Scholar]
- 39.Essers MA, Weijzen S, de Vries-Smits AM, Saarloos I, de Ruiter ND, Bos JL, Burgering BMT. 2004. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J. 23, 4802–4812. (doi:10.1038/sj.emboj.7600476) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma X, Huang J, Yang L, Yang Y, Li W, Xue L. 2012. NOPO modulates Egr-induced JNK-independent cell death in Drosophila. Cell Res. 22, 425–431. (doi:10.1038/cr.2011.135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma X, Shao Y, Zheng H, Li M, Li W, Xue L. 2013. Src42A modulates tumor invasion and cell death via Ben/dUev1a-mediated JNK activation in Drosophila. Cell Death Dis. 4, e864 (doi:10.1038/cddis.2013.392) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma X, Yang L, Yang Y, Li M, Li W, Xue L. 2013. dUev1a modulates TNF-JNK mediated tumor progression and cell death in Drosophila. Dev. Biol. 380, 211–221. (doi:10.1016/j.ydbio.2013.05.013) [DOI] [PubMed] [Google Scholar]
- 43.Geuking P, Narasimamurthy R, Basler K. 2005. A genetic screen targeting the tumor necrosis factor/Eiger signaling pathway: identification of Drosophila TAB2 as a functionally conserved component. Genetics 171, 1683–1694. (doi:10.1534/genetics.105.045534) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanda H, Igaki T, Okano H, Miura M. 2011. Conserved metabolic energy production pathways govern Eiger/TNF-induced nonapoptotic cell death. Proc. Natl Acad. Sci. USA 108, 18 977–18 982. (doi:10.1073/pnas.1103242108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bangi E, Pitsouli C, Rahme LG, Cagan R, Apidianakis Y. 2012. Immune response to bacteria induces dissemination of Ras-activated Drosophila hindgut cells. EMBO Rep. 13, 569–576. (doi:10.1038/embor.2012.44) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seong KM, et al. 2012. Low-dose radiation induces Drosophila innate immunity through Toll pathway activation. J. Radiat. Res. 53, 242–249. (doi:10.1269/jrr.11170) [DOI] [PubMed] [Google Scholar]
- 47.Parisi F, Stefanatos RK, Strathdee K, Yu Y, Vidal M. 2014. Transformed epithelia trigger non-tissue-autonomous tumor suppressor response by adipocytes via activation of Toll and Eiger/TNF signaling. Cell Rep. 6, 855–867. (doi:10.1016/j.celrep.2014.01.039) [DOI] [PubMed] [Google Scholar]
- 48.Ganesan S, Aggarwal K, Paquette N, Silverman N. 2011. NF-κB/Rel proteins and the humoral immune responses of Drosophila melanogaster. Curr. Top. Microbiol. Immunol. 349, 25–60. (doi:10.1007/82_2010_107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boutros M, Agaisse H, Perrimon N. 2002. Sequential activation of signaling pathways during innate immune responses in Drosophila. Dev. Cell. 3, 711–722. (doi:10.1016/S1534-5807(02)00325-8) [DOI] [PubMed] [Google Scholar]
- 50.Lemaitre B, Hoffmann J. 2007. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 25, 697–743. (doi:10.1146/annurev.immunol.25.022106.141615) [DOI] [PubMed] [Google Scholar]
- 51.Abrams JM, White K, Fessler LI, Steller H. 1993. Programmed cell death during Drosophila embryogenesis. Development 117, 29–43. [DOI] [PubMed] [Google Scholar]
- 52.Ma X, Li W, Yu H, Yang Y, Li M, Xue L, Xu T. 2014. Bendless modulates JNK-mediated cell death and migration in Drosophila. Cell Death and Differ. 21, 407–415. (doi:10.1038/cdd.2013.154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoffmann JA, Reichhart JM. 2002. Drosophila innate immunity: an evolutionary perspective. Nat. Immunol. 3, 121–126. (doi:10.1038/ni0202-121) [DOI] [PubMed] [Google Scholar]
- 54.Vidal S, Khush RS, Leulier F, Tzou P, Nakamura M, Lemaitre B. 2001. Mutations in the Drosophila dTAK1 gene reveal a conserved function for MAPKKKs in the control of rel/NF-κB-dependent innate immune responses. Genes Dev. 15, 1900–1912. (doi:10.1101/gad.203301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Delaney JR, Stoven S, Uvell H, Anderson KV, Engstrom Y, Mlodzik M. 2006. Cooperative control of Drosophila immune responses by the JNK and NF-κB signaling pathways. EMBO J. 25, 3068–3077. (doi:10.1038/sj.emboj.7601182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li WZ, Li SL, Zheng HY, Zhang SP, Xue L. 2012. A broad expression profile of the GMR-GAL4 driver in Drosophila melanogaster. Genet Mol Res. 11, 1997–2002. (doi:10.4238/2012.August.6.4) [DOI] [PubMed] [Google Scholar]
- 57.Wang X, Wang Z, Chen Y, Huang X, Hu Y, Zhang R, Ho MS, Xue L. 2014. FoxO mediates APP-induced AICD-dependent cell death. Cell Death Dis. 5, e1233 (doi:10.1038/cddis.2014.196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Junger MA, Rintelen F, Stocker H, Wasserman JD, Vegh M, Radimerski T, Greenberg ME, Hafen E. 2003. The Drosophila forkhead transcription factor FOXO mediates the reduction in cell number associated with reduced insulin signaling. J. Biol. 2, 20 (doi:10.1186/1475-4924-2-20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Steller H. 2008. Regulation of apoptosis in Drosophila. Cell Death Differ. 15, 1132–1138. (doi:10.1038/cdd.2008.50) [DOI] [PubMed] [Google Scholar]
- 60.Shlevkov E, Morata G. 2012. A dp53/JNK-dependant feedback amplification loop is essential for the apoptotic response to stress in Drosophila. Cell Death Differ. 19, 451–460. (doi:10.1038/cdd.2011.113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dick SA, Megeney LA. 2013. Cell death proteins: an evolutionary role in cellular adaptation before the advent of apoptosis. BioEssays 35, 974–983. (doi:10.1002/bies.201300052) [DOI] [PubMed] [Google Scholar]
- 62.Kumar S. 2007. Caspase function in programmed cell death. Cell Death Differ. 14, 32–43. (doi:10.1038/sj.cdd.4402060) [DOI] [PubMed] [Google Scholar]
- 63.Martin-Blanco E, Gampel A, Ring J, Virdee K, Kirov N, Tolkovsky AM, Martinez-Arias A. 1998. puckered encodes a phosphatase that mediates a feedback loop regulating JNK activity during dorsal closure in Drosophila. Genes Dev. 12, 557–570. (doi:10.1101/gad.12.4.557) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ferrandon D, et al. 1998. A drosomycin-GFP reporter transgene reveals a local immune response in Drosophila that is not dependent on the Toll pathway. EMBO J. 17, 1217–1227. (doi:10.1093/emboj/17.5.1217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dijkers PF, O'Farrell PH. 2007. Drosophila calcineurin promotes induction of innate immune responses. Curr. Biol. 17, 2087–2093. (doi:10.1016/j.cub.2007.11.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim T, Kim YJ. 2005. Overview of innate immunity in Drosophila. J. Biochem. Mol. Biol. 38, 121–127. (doi:10.5483/BMBRep.2005.38.2.121) [DOI] [PubMed] [Google Scholar]
- 67.Tanji T, Ip YT. 2005. Regulators of the Toll and Imd pathways in the Drosophila innate immune response. Trends Immunol. 26, 193–198. (doi:10.1016/j.it.2005.02.006) [DOI] [PubMed] [Google Scholar]
- 68.Myllymaki H, Valanne S, Ramet M. 2014. The Drosophila imd signaling pathway. J. Immunol. 192, 3455–3462. (doi:10.4049/jimmunol.1303309) [DOI] [PubMed] [Google Scholar]
- 69.Lemaitre B, et al. 1995. Functional analysis and regulation of nuclear import of dorsal during the immune response in Drosophila. EMBO J. 14, 536–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arnot CJ, Gay NJ, Gangloff M. 2010. Molecular mechanism that induces activation of Spatzle, the ligand for the Drosophila Toll receptor. J. Biol. Chem. 285, 19 502–19 509. (doi:10.1074/jbc.M109.098186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Parker JS, Mizuguchi K, Gay NJ. 2001. A family of proteins related to Spatzle, the toll receptor ligand, are encoded in the Drosophila genome. Proteins 45, 71–80. (doi:10.1002/prot.1125) [DOI] [PubMed] [Google Scholar]
- 72.Bellen HJ, et al. 2004. The BDGP gene disruption project: single transposon insertions associated with 40% of Drosophila genes. Genetics 167, 761–781. (doi:10.1534/genetics.104.026427) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shia AK, Glittenberg M, Thompson G, Weber AN, Reichhart JM, Ligoxygakis P. 2009. Toll-dependent antimicrobial responses in Drosophila larval fat body require Spatzle secreted by haemocytes. J. Cell Sci. 122, 4505–4515. (doi:10.1242/jcs.049155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kurucz E, et al. 2007. Nimrod, a putative phagocytosis receptor with EGF repeats in Drosophila plasmatocytes. Curr. Biol. 17, 649–654. (doi:10.1016/j.cub.2007.02.041) [DOI] [PubMed] [Google Scholar]
- 75.Haynie JL, Bryant PJ. 1986. Development of the eye-antenna imaginal disc and morphogenesis of the adult head in Drosophila melanogaster. J. Exp. Zool. 237, 293–308. (doi:10.1002/jez.1402370302) [DOI] [PubMed] [Google Scholar]
- 76.Gibson MC, Schubiger G. 2000. Peripodial cells regulate proliferation and patterning of Drosophila imaginal discs. Cell 103, 343–350. (doi:10.1016/S0092-8674(00)00125-2) [DOI] [PubMed] [Google Scholar]
- 77.Cho KO, Chern J, Izaddoost S, Choi KW. 2000. Novel signaling from the peripodial membrane is essential for eye disc patterning in Drosophila. Cell 103, 331–342. (doi:10.1016/S0092-8674(00)00124-0) [DOI] [PubMed] [Google Scholar]
- 78.Fernandez NQ, Grosshans J, Goltz JS, Stein D. 2001. Separable and redundant regulatory determinants in Cactus mediate its dorsal group dependent degradation. Development 128, 2963–2974. [DOI] [PubMed] [Google Scholar]
- 79.Rodriguez-Berriguete G, et al. 2013. Clinical significance of both tumor and stromal expression of components of the IL-1 and TNF-α signaling pathways in prostate cancer. Cytokine 64, 555–563. (doi:10.1016/j.cyto.2013.09.003) [DOI] [PubMed] [Google Scholar]
- 80.Linkermann A, Green DR. 2014. Necroptosis. N. Engl. J. Med. 370, 455–465. (doi:10.1056/NEJMra1310050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vanlangenakker N, Bertrand MJ, Bogaert P, Vandenabeele P, Vanden Berghe T. 2011. TNF-induced necroptosis in L929 cells is tightly regulated by multiple TNFR1 complex I and II members. Cell Death Dis. 2, e230 (doi:10.1038/cddis.2011.111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Holler N, et al. 2000. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 1, 489–495. (doi:10.1038/82732) [DOI] [PubMed] [Google Scholar]
- 83.Degterev A, et al. 2008. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat. Chem. Biol. 4, 313–321. (doi:10.1038/nchembio.83) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Christofferson DE, Yuan J. 2010. Necroptosis as an alternative form of programmed cell death. Curr. Opin. Cell Biol. 22, 263–268. (doi:10.1016/j.ceb.2009.12.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Su Z, Yang Z, Xu Y, Chen Y, Yu Q. 2015. Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol. Cancer 14, 48 (doi:10.1186/s12943-015-0321-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saeed WK, Jun DW. 2014. Necroptosis: an emerging type of cell death in liver diseases. World J. Gastroenterol. 20, 12 526–12 532. (doi:10.3748/wjg.v20.i35.12526) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Obata F, et al. 2014. Necrosis-driven systemic immune response alters SAM metabolism through the FOXO-GNMT axis. Cell Rep. 7, 821–833. (doi:10.1016/j.celrep.2014.03.046) [DOI] [PubMed] [Google Scholar]
- 88.Park SM, Yoon JB, Lee TH. 2004. Receptor interacting protein is ubiquitinated by cellular inhibitor of apoptosis proteins (c-IAP1 and c-IAP2) in vitro. FEBS Lett. 566, 51–56. (doi:10.1016/j.febslet.2004.04.021) [DOI] [PubMed] [Google Scholar]
- 89.Haas TL, et al. 2009. Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction. Mol. Cell. 36, 831–844. (doi:10.1016/j.molcel.2009.10.013) [DOI] [PubMed] [Google Scholar]
- 90.Zhu B, et al. 2008. Drosophila neurotrophins reveal a common mechanism for nervous system formation. PLoS Biol. 6, e284 (doi:10.1371/journal.pbio.0060284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ming M, Obata F, Kuranaga E, Miura M. 2014. Persephone/Spatzle pathogen sensors mediate the activation of Toll receptor signaling in response to endogenous danger signals in apoptosis-deficient Drosophila. J. Biol. Chem. 289, 7558–7568. (doi:10.1074/jbc.M113.543884) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Meyer SN, et al. 2014. An ancient defense system eliminates unfit cells from developing tissues during cell competition. Science 346, 1258236 (doi:10.1126/science.1258236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hu X, Yagi Y, Tanji T, Zhou S, Ip YT. 2004. Multimerization and interaction of Toll and Spatzle in Drosophila. Proc. Natl Acad. Sci. USA 101, 9369–9374. (doi:10.1073/pnas.0307062101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pena-Rangel MT, Rodriguez I, Riesgo-Escovar JR. 2002. A misexpression study examining dorsal thorax formation in Drosophila melanogaster. Genetics 160, 1035–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xue L, Noll M. 2000. Drosophila female sexual behavior induced by sterile males showing copulation complementation. Proc. Natl Acad. Sci. USA 97, 3272–3275. (doi:10.1073/pnas.97.7.3272) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang MC, Bohmann D, Jasper H. 2003. JNK signaling confers tolerance to oxidative stress and extends lifespan in Drosophila. Dev. Cell. 5, 811–816. (doi:10.1016/S1534-5807(03)00323-X) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.