Abstract
The conserved Hedgehog (HH) signals control animal development, adult stem cell maintenance and oncogenesis. In Drosophila, the HH co-receptor Patched (PTC) controls both HH gradient formation and signalling. PTC is post-translationally downregulated by HH, which promotes its endocytosis and destabilization, but the mechanisms of PTC trafficking and its importance in the control of PTC remain to be understood. PTC interacts with E3 Ubiquitin (UB)-ligases of the C2-WW-HECT family; two of them—SMURF and NEDD4—are known to regulate its levels. We demonstrate that mutation of the PTC PY motif, which mediates binding of C2-WW-HECT family members, inhibits its internalization but not its autonomous and non-autonomous signalling activities. In addition, we show that the two related UB-C2-WW-HECT ligases NEDD4 and SU(DX) regulate PTC trafficking and finely tune its accumulation through partially redundant but distinct functions. While both NEDD4 and SU(DX) promote PTC endocytosis, only SU(DX) is able to induce its lysosomal targeting and degradation. In conclusion, PTC trafficking and homeostasis are tightly regulated by a family of UB-ligases.
Keywords: Drosophila, signal transduction, intracellular protein trafficking, Hedgehog, Patched, C2-WW-HECT Ubiquitin ligase
1. Introduction
Hedgehog (HH) proteins play major roles in the development of many animals [1,2]. In Drosophila, HH controls the antero-posterior (A/P) patterning of the segments, and morphogenesis of numerous structures including the wing, eye and leg. This pathway is widely conserved in metazoans and its deregulation is involved in numerous pathologies in humans. Controlling HH signalling has therefore become a target for the treatment of cancers and cardiovascular diseases, for the repair of ischaemic tissues and more recently to improve cognitive function in Down syndrome patients [3–5].
HH reception relies on co-receptors, including the 12 transmembrane domain protein Patched (PTC). These proteins control, via the serpentine receptor Smoothened (SMO), a large cytoplasmic transduction complex that includes and regulates the transcription factor Cubitus interruptus (CI/GLI) [6]. In the absence of HH, PTC inhibits SMO, leading to the proteasome-dependent truncation of CI/GLI into a repressor form. HH binding to PTC and its co-receptors alleviates these negative effects on SMO, leading to SMO hyperphosphorylation, the inhibition of CI/GLI cleavage, the activation of full-length CI (CIFL) and the upregulation of HH target genes. The ptc gene is one of these targets, thus forming part of a negative feedback loop that controls the spreading of HH within responsive tissues [7,8].
Endocytosis of receptors is classically considered to be a means by which cells finely control signalling [9–11]. This process is used to tune the levels of the receptor accessible for the signal. By promoting the co-internalization and degradation of the bound ligand, it can also lead to signal termination or attenuation and shape the spread of morphogens. In some cases, receptor endocytosis was also reported to have positive effects on signalling [9–11]. The trafficking of transmembrane proteins often relies on their ubiquitination [12]. These post-translational modifications act as molecular signals that allow recognition by proteins of the endocytic or multi-vesicular body sorting machineries (see reviews in [13,14]). Therefore, the identification of the enzymes, Ubiquitin (UB) ligases and deubiquitinases that control receptor ubiquitination and the motifs that they recognize, along with understanding their regulation, is central to decipher the connection between receptor trafficking and activity.
HH signalling controls the dynamics of PTC and SMO, which shuttle from the plasma membrane and intracellular vesicles in flies, and in and out of the primary cilium in mammals. Indeed, experiments in Drosophila cells have shown that in the absence of HH, PTC is present both at the plasma membrane and in endosomes, while SMO is intracellular. By contrast, PTC bound to HH is endocytosed in a dynamin-dependent manner and targeted to the lysosome, while HH signalling leads to the stabilization of SMO at the cell surface due to a reduction of its endocytosis and/or an increase in its recycling back to the cell surface after internalization [7,15–17]. In mammals, HH controls the localization of SMO and PTC in and out of the primary cilium. Thus, in the absence of signal, PTC is localized at the cilium base where it prevents the accumulation of SMO into the cilium. HH reception leads to the movement of PTC out of the cilia and to the concentration of SMO into this structure.
Recent studies in flies have highlighted the importance of PTC and SMO post-translational modifications in the control of their trafficking. SMO accumulation at the plasma membrane requires its hyperphosphorylation by multiple kinases including the PKA and CKI. This is associated with a reduction of its ubiquitination, via the action of the deubiquitinase UBPY/UPS8 [18,19]. Moreover, several E3-UB-ligases of the C2-WW-HECT family proteins seem to bind and control PTC both in fly and in human cells [20]. The SMURF UB-ligase controls PTC ubiquitination and accumulation in Drosophila while its mammalian orthologues Smurf 1 and 2 regulate Ptc1 endocytic turnover and clearance from the primary cilium in response to HH [21,22].
We have previously identified, by yeast two-hybrid screens, several UB-ligases able to interact with the intracellular domains of Drosophila PTC [23]. Among these, NEDD4 (neural precursor cell expressed, developmentally downregulated 4), another C2-WW-HECT UB-ligase, interacts with the C-terminal tail of PTC. This interaction was confirmed by Lu et al. [24], who moreover showed that it requires a conserved L/PPXY (PY) motif known to promote interaction with E3 UB-ligase of the C2-WW-NEDD4 family [25,26]. The PY motif has also been implicated in PTC auto-regulating its own levels [27].
Here, using the Drosophila wing imaginal disc and fly-tissue-cultured cells as models, we further analysed the role of PTC trafficking and its control. Our study demonstrates that the PY motif of PTC is necessary for its internalization but is not absolutely required for its signalling functions. Moreover, we show that NEDD4 acts as a positive regulator of HH signalling by downregulating PTC levels and promoting its endocytosis. Finally, we uncover a role in HH signalling for a third fly C2-WW-HECT UB-ligase, Suppressor of deltex (SU(DX)), in the control of PTC endocytosis and its targeting to the lysosome. Altogether, our data support the conclusion that PTC trafficking is regulated by multiple UB-ligases, which finely tune its levels but are probably not essential for its signalling functions.
2. Results
2.1. The PY motif of Patched is required for its endocytosis
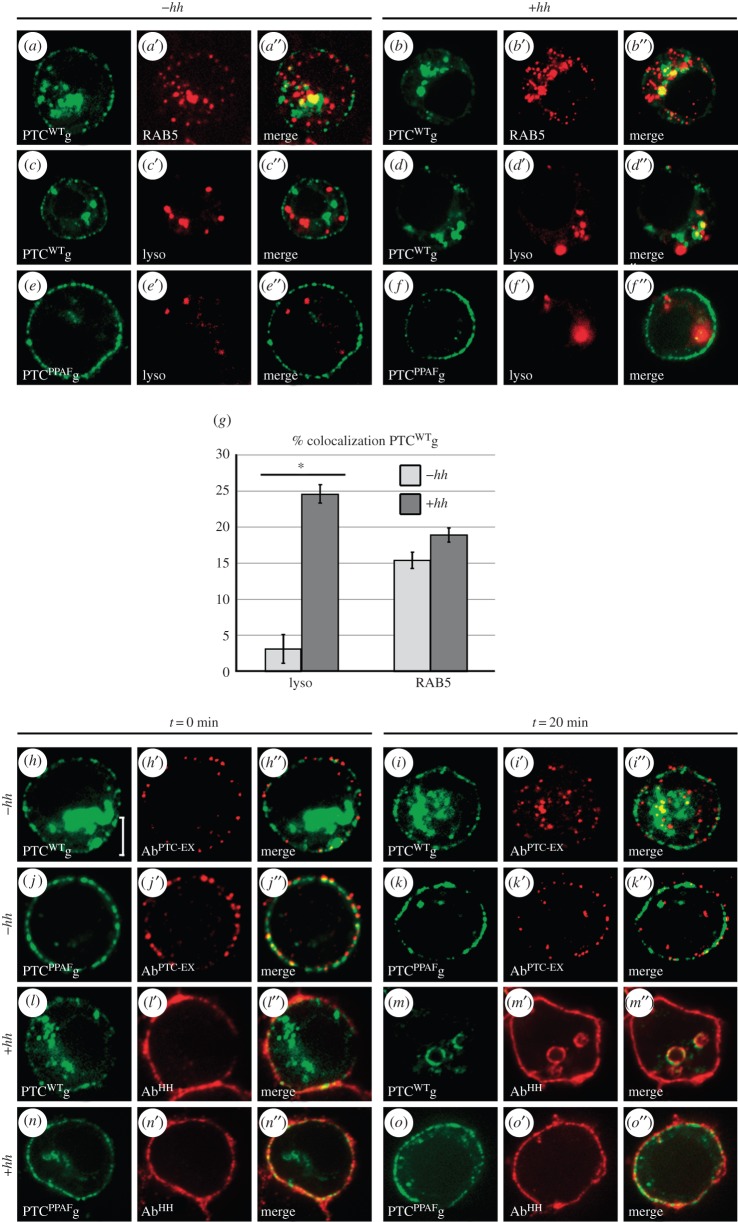
The PY motif of PTC has been shown to control its stability and to interact with the C2-WW-HECT UB-ligases NEDD4 and SMURF [21,24]. We therefore assayed the role of the PY motif in the control of PTC subcellular localization. For that purpose, we mutated the PPAY sequence (aa 1205–08) into PPAF and we compared the subcellular localization of the wild-type (PTCWT) and mutant (PTCPPAF) forms of PTC in transfected S2 cells (figure 1a–g; electronic supplementary material, table S1). As expected, PTCWT fused to GFP (PTCWTg) was at the plasma membrane and in early endosomes (EE): 15% of the PTCWTg was colabelled with RAB5 (figure 1a–a″,g) and only 3% with a lysosome dye that labels the acidic compartments (lysotracker, lyso; figure 1c–c″,g). HH induced PTCWTg targeting to the lysosome, as in this case almost 25% of PTCWTg was lyso+ (figure 1d–d″,g). Notably, the fraction of PTCWTg that was RAB5+ was not significantly affected (figure 1b–b″,g). In contrast, most of the PTCPPAFg was present at the plasma membrane even in the presence of HH (figure 1e–e″,f–f″). An endocytosis assay based on the uptake of an antibody directed against the PTC extracellular domain (AbPTC-EX; electronic supplementary material, figure S1A) shows that this was due to reduced internalization of PTCPPAFg as opposed to increased recycling (figure 1h–k″; electronic supplementary material, figure S1B). A similar test with an anti-HH antibody also shows that HH was endocytosed in the presence of PTCWTg but not in the presence of PTCPPAFg (figure 1l–o″; electronic supplementary material, figure S1B).
Figure 1.
The PTC PY motif controls the accumulation and subcellular localization of PTC. (a–f) Confocal images showing the localization of PTCWTg or PTCPPAFg expressed in transfected S2 cells, in the presence (+) or absence (−) of HH. EE were immunolabelled for RAB5 (red, a′ and b′) and lysosomes were marked with a Lysotracker (lyso) (red, c′–f′). Note that the green fluorescence colocalizing with the Lysotracker may, at least in part, correspond to GFP remaining after PTC degradation in the lysosome. (g) Fraction of GFP+ (PTCWTg) vesicles colabelled for RAB5 or lyso. In all graphs, error bars correspond to standard deviation. HH promotes the targeting of PTCWTg to the lysosome but has no significant effect on PTC colocalization with Rab5. Data were from 250 images from 10 cells taken (with 25 non-overlapping images/cell). See also electronic supplementary material, table S1. See ‘Material and methods’ for further information on images treatment and quantification. Asterisk (*) indicates a statistically significant difference (p < 0.025). (h–o″) Internalization assay of PTCWTg or PTCPPAFg. Briefly, transfected S2 cells were incubated with a surface-bound antibody (AbPTC−EX) directed against an extracellular epitope of PTC after endocytosis was transiently blocked by exposure to 4°C. AbPTC−EX was revealed either immediately after the cold block (t = 0) or 20 min after its release (by returning cells to 25°C) using fluorescent secondary antibodies (in red). See also electronic supplementary material, figure S1A,B. We noted that HH was still present at the plasma membrane even after PTC-WT endocytosis (m), possibly reflecting its anchoring to other transmembrane receptors such as Ihog and Boi. Ring-shaped internal structures were often seen in the presence of HH; some have vesicular structures and some seem to correspond to sections of tubes of the membrane after internalization. Their origin, nature and significance are unclear. In all cell images, scale bars are 5 µm.
Together these data show that PTC PY motif is required for its endocytosis in both absence and presence of HH.
2.2. Patched mutated for its PY motif over accumulates but is still active
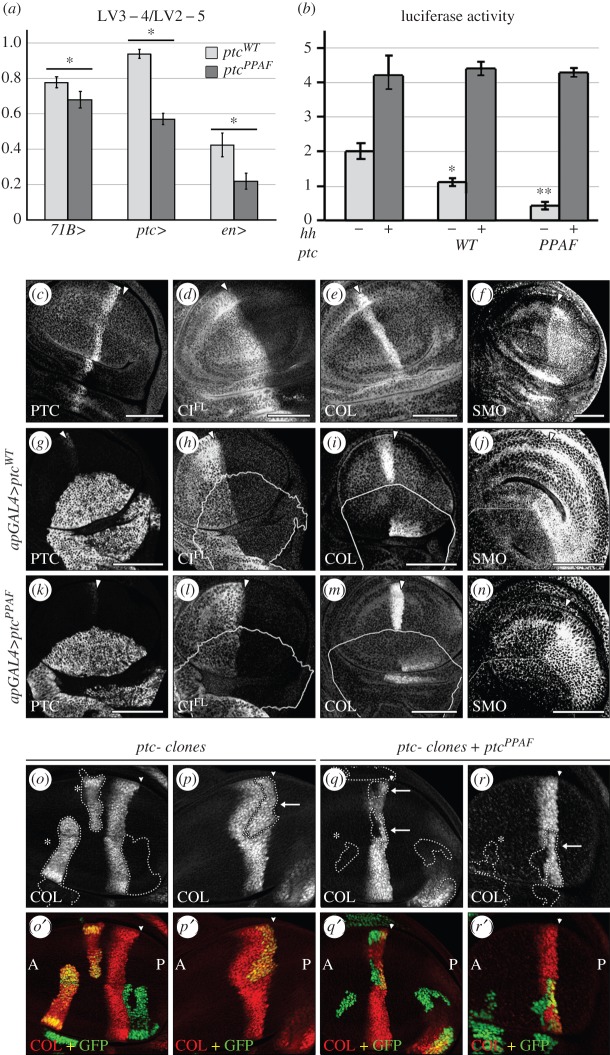
PTC endocytosis may play a role in the inhibition of SMO by PTC, the inhibition of PTC by HH and/or the range of HH diffusion and action. Previous findings suggested that the localization of PTC and SMO in the same endosomal compartment is necessary for the inhibition of SMO by PTC and that HH may antagonize the inhibitory effects of PTC by promoting its degradation [15,28]. However, previous analysis of PTC mutants with altered localization suggests that PTC at the plasma membrane could properly control HH signalling [7], but whether this mutant has increased recycling efficiency or a block in endocytosis is unknown. We took advantage of the fact that PTC endocytosis depends on its PY motif to assay whether it could influence its signalling properties. We compared the effects of overexpressing ptcPPAF or ptcWT in the Drosophila wing imaginal disc, the development of which is controlled by HH [29]. In this structure, HH produced by the cells of the posterior (P) compartment forms a gradient in the anterior (A) compartment, triggering the upregulation of target genes, such as collier (col), decapentaplegic (dpp) and ptc itself. Expression of either ptcWT or ptcPPAF throughout the wing imaginal disc (71BGAL4 driver) or in HH-responsive cells along the A/P boundary (ptcGAL4 driver) reduced spacing between the longitudinal veins (LV) 3 and 4, a mark of lower levels of HH signalling (figure 2a; electronic supplementary material, figure S2A). Accordingly, overexpression of either transgene in the dorsal (D) compartment of the wing imaginal disc (apGAL4 driver) reduced the accumulation of CIFL (figure 2d,h,l) as well as SMO (figure 2f,j,n), which are both normally stabilized by HH (figure 2d,f). It also reduced the expression of col, a gene whose expression is upregulated by high levels of HH signal (figure 2e,i,m). These negative effects of PTCPPAF on HH signalling did not depend on the presence of the endogenous PTC protein. Indeed, as shown in figure 2o–r′, PTCPPAF expression prevented the ectopic col expression that is normally induced in anterior clones mutant for ptc and downregulated the endogeneous expression of col at the A/P boundary. These results extend Casali's previous report, which showed that a PTC mutant with its PPAY motif replaced by AAAA did not have altered expression of dpp, a gene activated by low levels of HH signalling [27]. Together these data show that PTCPPAF has kept the ability to reduce HH signalling.
Figure 2.
The PY motif controls PTC levels but is not essential for its function in HH signalling. (a) Relative LV3–4/LV2–5 spacing (normalized to MS1096 control wings) in wings of flies expressing ptcWT or ptcPPAF in the wing disc under the control of various drivers (as indicated). Expression of either construct led to a reduced LV3–4 spacing. The pattern of expression of the drivers and images of representative wings are shown in electronic supplementary material, figure S2A. (b) Expression of a ptc-luc reporter in transfected S2 cells is reduced by ptcWT or ptcPPAF expression. In the absence of HH, luciferase levels in the presence of ptcWT expression were statistically lower than in untransfected cells (*) but statistically higher than in cells transfected with ptcPPAF (**). These effects of ptcWT or ptcPPAF were suppressed in presence of HH. (c–n) Confocal images of third instar larvae wing discs expressing ptcWT or ptcPPAF with the apGAL4 driver (active exclusively in the dorsal compartment). CIFL, COL or SMO detected by immunofluorescence (IF) were all downregulated, indicating that HH signalling is reduced. To ensure comparable expression levels, the ptcWT and ptcPPAF transgenes were inserted at the same locus. The accumulation of COL seen in the most ventral region, outside the wing pouch in (i) and (m), is reproducible but unexplained. The white line indicates the ventral limit of apGAL4 expression (determined by PTC or GAL4 IF). Full arrowheads indicate the A/P boundary. In all figures, discs are oriented with A to the left and D to the bottom. (o–r′) Images of ptc16/ptc+ disc with clones of ptc16 mutant cells (marked by the GFP, green) expressing (q,r) or not (o,p) ptcPPAF. COL is labelled in red. Arrows: anterior clones near the boundary. Asterisk (*): clones further in the anterior compartment.
Within the ptc− clones expressing PTCPPAF, col expression remained in the cells nearest to the A/P border where the highest levels of HH are present (arrows in figure 2q–r). Additionally, in S2 cells, the negative effects of either PTCPPAF or PTCWT on a HH-responsive reporter (ptc-luc [30]) were both suppressed by HH (figure 2b). Thus, PTCPPAF has kept the ability to be regulated by HH, indicating that HH can antagonize a form of PTC that cannot be endocytosed.
Another aspect of PTC function is to shape the HH gradient by promoting its co-internalization [8]. Thus, ectopic expression of col occurs in cells that anteriorly abut ptc− clones along the A/P compartment boundary (figure 2p) due to increased HH spreading across the clone [8]. This non-autonomous effect of ptc− loss of function is however suppressed when PTCPPAF is expressed within the clone (figure 2q,r), indicating that it has kept the ability to restrain HH spreading. This was confirmed by the fact that PTCPPAF overexpression in the P compartment of the wing imaginal disc (using enGAL4 driver) reduced HH signalling (figure 2a; electronic supplementary material, figure S2A).
All these data indicate that PTC can both restrain HH diffusion and be inhibited by HH independently of its PY motif.
Despite the fact that ptcWT and ptcPPAF were expressed under the same conditions from the same locus, we noted that ptcPPAF had a consistently stronger effect than ptcWT in many of the above in vivo experiments (figure 2a; electronic supplementary material, figure S2A). This difference in effect was confirmed in S2 cells, using the HH-inducible luciferase reporter (figure 2b). Notably, the levels of PTCPPAF fused to a HA tag (PTCPPAF-HA) were five times higher than those of PTCWT-HA (electronic supplementary material, figure S2B). This is in agreement with what was observed by Lu et al. [24] for another mutant of the PY motif (see also electronic supplementary material, figure S2C, for a further comparison) and may be sufficient to account for the stronger effects of PTCPPAF.
In summary, PTC PY motif is not required for PTC signalling activity, but is important for its homeostasis by controlling its total levels and its levels at the cell surface, where it can receive HH.
2.3. The E3 Ubiquitin-ligase NEDD4 controls Patched internalization and accumulation levels
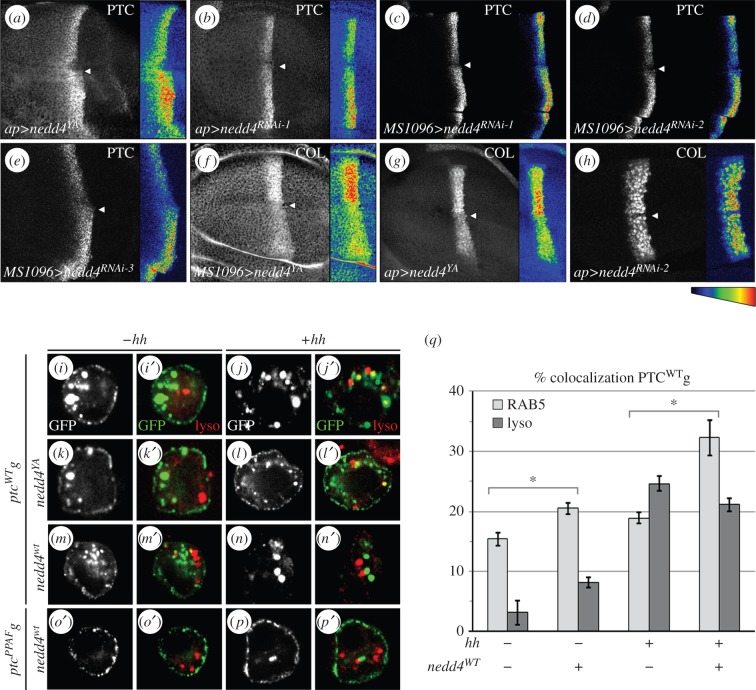
NEDD4 is widely expressed in Drosophila, including the wing imaginal disc, and in S2 cells [31–33]. In cotransfected S2 cells, NEDD4 is present both at the cell membrane and in large vesicular structures, many of them colabelled with PTCWTg (electronic supplementary material, figure S3A). We decreased NEDD4 activity throughout the wing disc either by expression of a catalytically inactive form (nedd4YA) known to act in a dominant-negative fashion or by RNA interference. Both methods have previously been shown to downregulate endogenous NEDD4 activity [31–33]. Moreover, we also used two other independent RNAi constructs directed against different regions of nedd4 mRNA. Downregulation of NEDD4 activity by expression of any of the four transgenes led to the overaccumulation of the endogenous PTC protein (figure 3a–e). This was probably due to a post-translational regulation of PTC levels, rather than an upregulation of its transcription resulting from increased HH signalling. Indeed, the LV3–4 spacing was reduced (electronic supplementary material, figure S3B,C) and the expression of col was attenuated (figure 3f–h), both of which indicate that HH signalling was reduced. This conclusion was further supported by the fact that expression of nedd4YA also led to increased accumulation of PTC in S2 cells in which ptc transcription cannot be upregulated, due to the lack of CI expression (electronic supplementary material, figure S3E).
Figure 3.
NEDD4 downregulates PTC and promotes its internalization. (a–h) Confocal images of wing disc immunolabelled for (a–e) PTC or (f–h) COL and expressing a dominant-negative form of nedd4 (nedd4YA) (a) or three different nedd4RNAi (1, 2, 3; see ‘Material and methods’) with MS1096GAL4 (which is more expressed in the dorsal compartment than in the ventral one), or apGAL4 (exclusively expressed in the dorsal compartment). Staining intensities in pseudocolour are shown on the right. In all cases, downregulation of NEDD4 activity led to an upregulation of PTC accumulation and a reduction in col expression. The effect of nedd4RNAi2 on col was visible in the cells near the D/V boundary, in a region known to be sensitive to small changes in HH signalling. Arrowhead: D/V boundary. (i–p′) Confocal images of S2 cells expressing ptcWTg or ptcPPAFg with nedd4YA (k–l′) or nedd4WT (m–p′) and colabelled with the Lysotracker (lyso). (i–j′) are control cells with ptcWTg alone. (q) Quantification of the effect of nedd4WT on the fraction of PTCWT that colabelled with RAB5 (light grey) or the Lysotracker (lyso, dark grey).
We next tested whether NEDD4 could regulate PTC trafficking using S2 cells. Expression of nedd4YA led to the accumulation of PTCWTg at the plasma membrane, in both the presence and absence of HH (figure 3k–l′, compare to i–j′). Internalization of the AbPTC−EX–PTCWTg complex was also inhibited in these cells, confirming that PTC endocytosis was blocked (electronic supplementary material, figures S1B and S4c–d″). Conversely, cells overexpressing nedd4WT showed a reduced accumulation of PTCWTg at the cell surface (figure 3m–n′) as a result of increased PTC internalization (electronic supplementary material, figures S1B and S4a–b″). This effect was dependent on the PY motif of PTC, even in the presence of HH (figure 3o–p′; electronic supplementary material, figures S1B and S4i–j″). Moreover, nedd4WT overexpression increased PTCWTg localization to the EE (20% of PTCWTg vesicles were RAB5+) but had a weak effect on the targeting to the lysosome (8% of PTCWTg vesicles were lyso+; figure 3q; electronic supplementary material, table S1).
In conclusion, our data indicate that NEDD4 is a positive regulator of HH signalling that directly controls the level of PTC accumulation at the plasma membrane by promoting its endocytosis.
2.4. The E3 Ubiquitin-ligase SU(DX) regulates Hedgehog signalling and controls Patched sorting to the lysosome
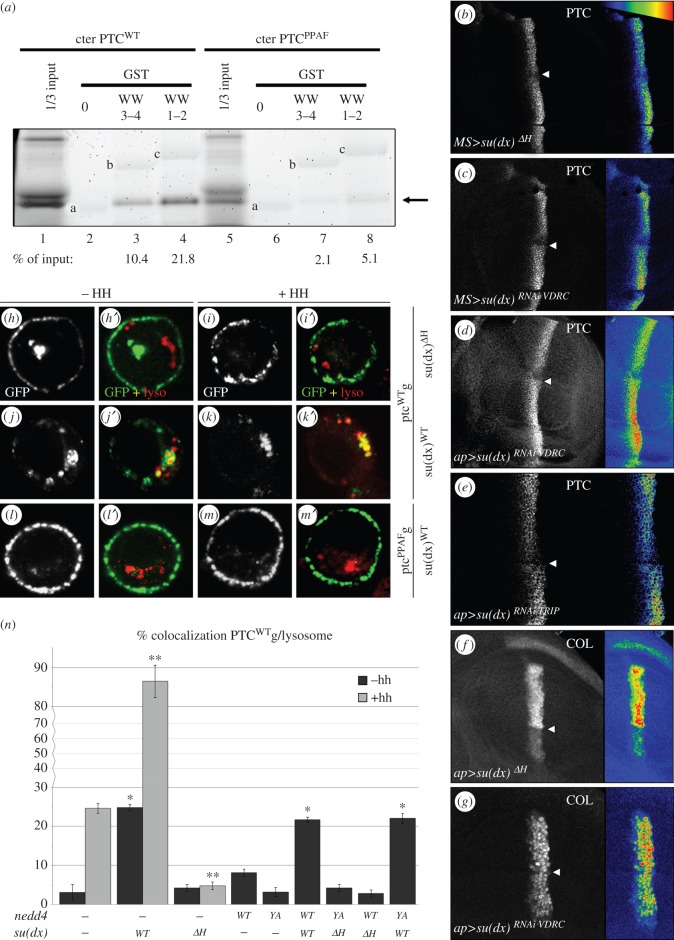
The absence of significant effects of NEDD4 on the lysosomal targeting of PTC suggests that other UB-ligases could control this step. The endocytosis and post-endocytic sorting of transmembrane proteins, such as Notch, have been shown to be regulated, respectively, by NEDD4 and Itch/AIP4/Suppressor of deltex (SU(DX)), a NEDD4-related UB-ligase [32–34], and this could also be the case for PTC. su(dx) is expressed in all cells of the wing imaginal disc and in S2 cells [32], where it colocalizes with PTC in vesicular structures (electronic supplementary material, figure S3A). Moreover, the C-terminal domain of PTC interacts via its PY motif with the WW domains of SU(DX) in vitro (figure 4a). We therefore examined whether SU(DX) could also regulate PTC and HH signalling. Reducing SU(DX) activity by expressing two different RNAi constructs—directed against two different regions of su(dx), (su(dx)RNAiVDRC and su(dx)RNAiTRIP)—or a transgene encoding a dominant-negative HECT domain deletion (su(dx)ΔH) [32,35] led to an increase in PTC protein accumulation at the A/P boundary (figure 4b–e). This effect was associated with a decrease in col expression and a reduction in LV3–4 spacing (figure 4f–g; electronic supplementary material, figure S3B,D), both indicating a reduction in HH signalling.
Figure 4.
SU(DX) downregulates PTC and controls both its endocytosis and sorting to the lysosome. (a) A GST pull-down assay was performed between the in vitro translated, fluorescently labelled C-terminal (C-ter) domain of PTCWT (lanes 1–4) or PTCPPAF (lanes 5–8) and GST alone (lanes 2 and 6), or GST fused to the WW domains (WW1–2 or 3–4) of SU(DX) (lanes 3–4 and 7–8). One-third of the amount used in the pull-down assays was loaded as inputs (lanes 1 and 6). The fraction (%) of PTC C-ter pulled-down is indicated under each corresponding lane. It shows that PTCWT but not PTCPPAF interacts with SU(DX). Arrow: PTC-ter. The bands a, b and c correspond to the GST, GST-WW3–4 and GST-WW1–2, respectively, which emitted low fluorescence under our experimental conditions. (b–g) Confocal images of wing discs immunolabelled for (b–e) PTC or (f–g) COL and expressing (b,f) a dominant-negative form of su(dx) (su(dx)ΔH) or (c–e,g) two different su(dx)RNAi transgenes (VDRC and TRIP; see ‘Material and methods’) through the MS1096GAL4 or apGAL4 drivers. In all cases, downregulation of SU(DX) led to an upregulation of PTC accumulation and a reduction in COL expression. (h–m′) Confocal images of S2 cells expressing (h–k′) ptcWTg or (l–m′) ptcPPAFg (green), along with su(dx)ΔH or su(dx)WT (as indicated) and colabelled with the Lysotracker (red). (n) Quantification of the fraction of PTC colabelled with the Lysotracker dye in S2 cells transfected with ptcWTg alone or in various combinations with su(dx)WT, su(dx)ΔH, nedd4WT or nedd4YA as indicated.
Next, we analysed the effects of su(dx) overexpression on PTC trafficking using S2 cells. PTCWTg accumulation and endocytosis were blocked by su(dx)ΔH expression, with or without HH (figure 4h–i′; electronic supplementary material, figure, S1B and S4 g–h″). By contrast, su(dx)WT overexpression led to a reduced accumulation of PTCWTg at the plasma membrane due to PY-dependent increase in PTC internalization (figure 4j–m′; electronic supplementary material, figures, S1B and S4e–f′,k–l″). Moreover, su(dx))WT expression strongly enhanced PTC targeting to the lysosome as the fraction of GFP+ vesicles that were also lyso+ increased to almost 25% in absence of HH and reached 86% with HH (figure 4n; electronic supplementary material, table S1).
Finally, to better understand the respective role of NEDD4 and SU(DX), we expressed combinations of the wild-type and mutant forms of these two UB-ligases (figure 4n; electronic supplementary material, figures S4 m–t″ and S1B, and table S1). In S2 cells, coexpressing either nedd4WT with su(dx)ΔH or nedd4YA with su(dx)WT, PTCWTg was still internalized, suggesting possible redundant functions for these two proteins in PTC endocytosis in these cell. Co-expression of nedd4YA and su(dx)WT, but not of nedd4WT and su(dx)ΔH, promoted the targeting of PTCWTg to the lysosome at a level similar to that induced by the overexpression of su(dx)WT alone. This further indicates a specific role for SU(DX) in sorting PTC to the lysosome.
3. Discussion
3.1. Multiple Ubiquitin ligases control Patched trafficking
During its journey from the plasma membrane to the lysosome or to a recycling pathway, an integral membrane receptor can undergo several rounds of ubiquitination and deubiquitination, each controlling a particular trafficking step [36,37]. In yeast, these ubiquitination steps are catalysed by the same and specific C2-WW-HECT ligase, Rsp5p [13]. In multicellular eukaryotes, these events may involve multiple UB ligases of the HECT and RING (Really Interesting New Gene) families [38,39]. The accumulation of the HH receptor PTC seems to be regulated by the complex action of the three C2-WW-HECT UB-ligases present in fly. We show that NEDD4 and SU(DX) finely regulate PTC homeostasis both in S2 cultured cells and in vivo, in the wing imaginal disc. We provide evidence that in cultured cells, these two related UB-ligases differentially control PTC internalization and targeting to the lysosome. While NEDD4 and SU(DX) control the internalization of PTC both in the presence and in the absence of HH ligand, only SU(DX) acts on PTC en route to the lysosome, this latter effect being highly enhanced in presence of HH signal. The third UB-ligase of the same family, SMURF, was also reported to induce PTC degradation in a manner that depends on SMO activation, but how it impacts PTC trafficking remains to be determined [21].
The mechanisms underlying the distinct actions of these related UB-ligases remain to be understood. It may be related to differences in the UB chain formed, as in mammals, Itch/AIP4 has been reported to catalyse Notch polyubiquitination through unusual K29-linked chains [40], indicating a link between this type of chain and lysosomal degradation [41]. Alternatively, these differences could be the consequence of the different subcellular localization of these ligases and/or reflect changes in their interaction with PTC at different stages of the endocytic pathway via the recruitment of specific adaptor proteins or changes in post-translational modification [42–46].
3.2. Patched endocytosis controls its homeostasis
A key issue regarding PTC is the role of trafficking in the control of its activity. To answer such a question requires tools that specifically target PTC trafficking as opposed to blocking the endocytic machinery in general, which would be likely to have pleiotropic effects. Using a PTC mutant specifically impaired for its internalization due to the mutation of its PY motif, we provide further proof that PTC trafficking is not necessary for its intrinsic signalling properties. This implies that PTC colocalization with SMO in endosomes is not required for PTC to inhibit SMO and that HH can inhibit PTC independently of promoting its degradation. This differs with observations made for other receptors such as Frizzled, Notch or EGFR, for which intact trafficking is important in productive signalling [9,10]. Nonetheless, PTC trafficking plays a major role in controlling its levels (in both the presence and absence of HH), which is important for the finely tuned control of both HH pathway activity and HH gradient formation. Noteworthy, three other UB-ligases unrelated to NEDD4, SU(DX) and SMURF were also found to interact with PTC through a high-throughput protein interaction screen approach [23]. The multiplicity of the UB-ligases potentially involved in the control of PTC homeostasis could reflect redundancy and/or differential use in different biological contexts, and highlight the crucial importance of controlling of PTC levels for proper development.
3.3. Broader implications
Strikingly, the hPTC proteins contain two PY motifs that can bind numerous C2-WW-HECT UB-ligases homologous to NEDD4 and SU(DX) [20,22]. It was recently reported that the mutation of these PY sites prevents the HH-induced removal of hPTC from the primary cilia in response to HH [20]. This shows that, despite the apparent divergence of the pathway and the requirement for the primary cilium in mammals, the same processes are used to control the internalization of PTC from the plasma membrane in flies, and its removal from the primary cilium shaft in mammals. Moreover, similar to our observations in flies, these mutations do not prevent PTC from negatively regulating SMO nor responding to HH, confirming that PTC internalization is not crucial for HH signalling. PTC is related to transmembrane transporters and has been proposed to control SMO activity via lipids. The fact that it can act independently of its own trafficking constrains how this might take place and suggests that HH might regulate PTC transport activity by promoting structural changes and/or a so-far-unknown post-translational modification.
It is noteworthy that the Nieman-Pick1 (NPC1) protein, which is involved in NPC disease (a fatal lysosomal storage disorder) and is also related to PTC, contains an intracellular PY motif and has highly dynamic trafficking. Thus, the present characterization of specific proteins and the sequence involved in the regulation of PTC trafficking is very likely to be applicable to the other members of the PTC/NPC family and should have important implications for their regulation.
4. Material and methods
4.1. Fly strains and genetics
Flies were raised at 25°C. pUASt-AttB-ptcWT and pUASt-AttB-ptcPPAF transgenes were inserted by the фC31 method [47] in the Drosophila line 9725 [48], which contains an attP site at position 75A10. Injections were carried out by BestGene Inc. Transgenes of interest were expressed using the UAS-GAL4 system [49]. Fly strains were described previously, as follows: UASt-nedd4WT (chr. II) [33], UASt-nedd4YA (chr. II) [33], UASt-nedd4RNAi1 (chr. II from [31]), UASt-nedd4RNAi2 (chr. III, from Bloomington, stock 31687) UASt-nedd4RNAi3 (chr. III, from Bloomington, stock 34741), UASt-sudxWT (chr. II) [32], UASt-sudxΔH (chr. II) [32], UASt-su(dx)RNAi VDRC (chr. II, from the VDRC, 102798 insertion), UASt-su(dx)RNAi TRIP (chr III, from Bloomington, stock 36836), MS1096GAL4 (chr. X) [50], 71BGAL4 (chr. III) [49], apGAL4 (chr. II) [51], enGAL4 (chr II, gift from P. Thérond). MARCM clones: FRT42D ptc16/CyO; HMC/TM6b or FRT42D ptc16/CyO; UAS ptcPPAF females were crossed to y, w, hsFLP, tub-GAL4 UAS-gfp/Y; tub-GAL80ts FRT42D/CyO males; larvae were incubated 1 h at 37°C for three consecutive days to induce mosaic clones.
4.2. Quantification of PTCg colabelling with vesicular markers
The proportion of green pixels (PTCg) superimposing with pixels labelled in red (RAB5 or Lysotracker) was determined on untreated images. Automated quantification was performed with ImageJ software; vesicle segmentation was carried out using grey level thresholds on wavelet-transformed images. To calculate the percentage of colocalization occurring by chance, PTCg images were rotated by 90° respective to their matching vesicular marker images and re-examined for colocalization with markers. We found that less than 5% of PTCg randomly colocalized with any given marker. For each vesicular marker, we measured 10 cells (with 25 images per stack) selected randomly from three independent transfections. Note that a similar PTCg construct was previously reported to rescue a ptc null mutation [7].
4.3. Endocytosis assay
Forty-eight hours after transfection, S2 cells were placed on ice for 10 min, before being incubated for 20 min with anti-PTC antibody (Apa1 [50], 1/500). After washing the unbound antibody, half of the cells were plated on Concanavalin A (Sigma) coated glass coverslips for 5 min before fixation for 30 min with 4% paraformaldehyde. The other half was further incubated for 20 min at 25°C before plating on Concanavalin A and fixation. The two samples were then permeabilized three times for 5 min in PBS + 0.1%Tween (PBST) and incubated for 1 h with the secondary antibody. This was followed by incubation for 5 min with DAPI and three washes in PBST, before mounting in Citifluor.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank M. Baron, F. Bernard, S. Cohen, M.Crozatier, M. Gonzales-Gaitan, I. Guerrero, S. Hayashi, T. Kornberg, T. Murphy, D. Rotin, T. Tabata and N. Vodovar for flies, plasmids and antibodies. We are grateful to I. Becam, M. Coppey, R. Hagenauer-Tsapis, C. Lamour-Isnard, S. Leon, F. Pichaud, L. Pintard, B. Pourcet, F. Schweisguth, M. Scott and P. Therond for their help and suggestions. Antibodies from the DSHB were developed under the auspices of the NICHD and maintained by the University of Iowa. Drosophila embryo injections were carried out by BestGene Inc.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the CNRS, University Paris Diderot and the ARC (grant no. 5769). We acknowledge the ImagoSeine facility (Jacques Monod Institute, Paris, France) and the France BioImaging infrastructure supported by the ANR (ANR-10-INSB-04) for their precious help for imaging and image quantitative analysis. A.B. was supported by the MENRT and the ARC, L.H. by the ARC and the LIGUE contre le Cancer, C.A. by the ARC.
References
- 1.Ingham PW, Nakano Y, Seger C. 2011. Mechanisms and functions of Hedgehog signalling across the metazoa. Nat. Rev. Genet. 12, 393–406. (doi:10.1038/nrg2984) [DOI] [PubMed] [Google Scholar]
- 2.Briscoe J, Therond PP. 2013. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat. Rev. Mol. Cell Biol. 14, 416–429. (doi:10.1038/nrm3598) [DOI] [PubMed] [Google Scholar]
- 3.Das I, Park JM, Shin JH, Jeon SK, Lorenzi H, Linden DJ, Worley PF, Reeves RH. 2013. Hedgehog agonist therapy corrects structural and cognitive deficits in a Down syndrome mouse model. Sci. Transl. Med. 5, 201ra120. (doi:10.1126/scitranslmed.3005983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scales SJ, de Sauvage FJ. 2009. Mechanisms of Hedgehog pathway activation in cancer and implications for therapy. Trends Pharmacol. Sci. 30, 303–312. (doi:10.1016/j.tips.2009.03.007) [DOI] [PubMed] [Google Scholar]
- 5.Lavine KJ, Ornitz DM. 2008. Fibroblast growth factors and Hedgehogs: at the heart of the epicardial signaling center. Trends Genet. 24, 33–40. (doi:10.1016/j.tig.2007.10.007) [DOI] [PubMed] [Google Scholar]
- 6.Robbins DJ, Fei DL, Riobo NA. 2013. The Hedgehog signal transduction network. Sci. Signal. 5, re6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torroja C, Gorfinkiel N, Guerrero I. 2004. Patched controls the Hedgehog gradient by endocytosis in a dynamin-dependent manner, but this internalization does not play a major role in signal transduction. Development 131, 2395–2408. (doi:10.1242/dev.01102) [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Struhl G. 1996. Dual roles for patched in sequestering and transducing Hedgehog. Cell 87, 553–563. (doi:10.1016/S0092-8674(00)81374-4) [DOI] [PubMed] [Google Scholar]
- 9.Piddini E, Vincent JP. 2003. Modulation of developmental signals by endocytosis: different means and many ends. Curr. Opin. Cell Biol. 15, 474–481. (doi:10.1016/S0955-0674(03)00072-3) [DOI] [PubMed] [Google Scholar]
- 10.Gagliardi M, Piddini E, Vincent JP. 2008. Endocytosis: a positive or a negative influence on Wnt signalling? Traffic 9, 1–9. (doi:10.1111/j.1600-0854.2007.00662.x) [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Gaitan M. 2003. Endocytic trafficking during Drosophila development. Mech. Dev. 120, 1265–1282. (doi:10.1016/j.mod.2003.06.002) [DOI] [PubMed] [Google Scholar]
- 12.Hicke L, Dunn R. 2003. Regulation of membrane protein transport by Ubiquitin and Ubiquitin-binding proteins. Annu. Rev. Cell Dev. Biol. 19, 141–172. (doi:10.1146/annurev.cellbio.19.110701.154617) [DOI] [PubMed] [Google Scholar]
- 13.Belgareh-Touze N, Leon S, Erpapazoglou Z, Stawiecka-Mirota M, Urban-Grimal D, Haguenauer-Tsapis R. 2008. Versatile role of the yeast Ubiquitin ligase Rsp5p in intracellular trafficking. Biochem. Soc. Trans. 36, 791–796. (doi:10.1042/BST0360791) [DOI] [PubMed] [Google Scholar]
- 14.Tanno H, Komada M. 2013. The Ubiquitin code and its decoding machinery in the endocytic pathway. J. Biochem. 153, 497–504. (doi:10.1093/jb/mvt028) [DOI] [PubMed] [Google Scholar]
- 15.Incardona JP, Gruenberg J, Roelink H. 2002. Sonic Hedgehog induces the segregation of Patched and Smoothened in endosomes. Curr. Biol. 12, 983–995. (doi:10.1016/S0960-9822(02)00895-3) [DOI] [PubMed] [Google Scholar]
- 16.Gallet A, Therond PP. 2005. Temporal modulation of the Hedgehog morphogen gradient by a patched-dependent targeting to lysosomal compartment. Dev. Biol. 277, 51–62. (doi:10.1016/j.ydbio.2004.09.005) [DOI] [PubMed] [Google Scholar]
- 17.Gallet A, Therond PP. 2007. Confocal analysis of Hedgehog morphogenetic gradient coupled with fluorescent in situ hybridization of Hedgehog target genes. Methods Mol. Biol. 397, 105–114. (doi:10.1007/978-1-59745-516-9_9) [DOI] [PubMed] [Google Scholar]
- 18.Li S, Chen Y, Shi Q, Yue T, Wang B, Jiang J. 2012. Hedgehog-regulated ubiquitination controls Smoothened trafficking and cell surface expression in Drosophila. PLoS Biol. 10, e1001239 (doi:10.1371/journal.pbio.1001239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xia R, Jia H, Fan J, Liu Y, Jia J. 2012. USP8 promotes Smoothened signaling by preventing its ubiquitination and changing its subcellular localization. PLoS Biol. 10, e1001238 (doi:10.1371/journal.pbio.1001238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J, EY CH, Brigui A, Plessis A, Beachy PA, Zheng X. 2015. The role of ciliary trafficking in Hedgehog receptor signaling. Sci Signal. 8, ra55. (doi:10.1126/scisignal.aaa5622) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang S, et al. 2013. Activation of Smurf E3 ligase promoted by Smoothened regulates Hedgehog signaling through targeting patched turnover. PLoS Biol. 11, e1001721 (doi:10.1371/journal.pbio.1001721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yue S, et al. 2014. Requirement of Smurf-mediated endocytosis of Patched1 in sonic Hedgehog signal reception. eLife 3, e02555 (doi:10.7554/eLife.02555) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Formstecher E, et al. 2005. Protein interaction mapping: a Drosophila case study. Genome Res. 15, 376–384. (doi:10.1101/gr.2659105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu X, Liu S, Kornberg TB. 2006. The C-terminal tail of the Hedgehog receptor Patched regulates both localization and turnover. Genes Dev. 20, 2539–2551. (doi:10.1101/gad.1461306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myat A, Henry P, McCabe V, Flintoft L, Rotin D, Tear G. 2002. Drosophila Nedd4, a Ubiquitin ligase, is recruited by Commissureless to control cell surface levels of the roundabout receptor. Neuron. 35, 447–459. (doi:10.1016/S0896-6273(02)00795-X) [DOI] [PubMed] [Google Scholar]
- 26.Staub O, Dho S, Henry P, Correa J, Ishikawa T, McGlade J, Rotin D. 1996. WW domains of Nedd4 bind to the proline-rich PY motifs in the epithelial Na+ channel deleted in Liddle's syndrome. EMBO J. 15, 2371–2380. [PMC free article] [PubMed] [Google Scholar]
- 27.Casali A. 2010. Self-induced patched receptor down-regulation modulates cell sensitivity to the Hedgehog morphogen gradient. Sci. Signal. 3, ra63. (doi:10.1126/scisignal.2001059) [DOI] [PubMed] [Google Scholar]
- 28.Denef N, Neubuser D, Perez L, Cohen SM. 2000. Hedgehog induces opposite changes in turnover and subcellular localization of Patched and Smoothened. Cell. 102, 521–531. (doi:10.1016/S0092-8674(00)00056-8) [DOI] [PubMed] [Google Scholar]
- 29.Tabata T, Kornberg TB. 1994. Hedgehog is a signaling protein with a key role in patterning Drosophila imaginal discs. Cell 76, 89–102. (doi:10.1016/0092-8674(94)90175-9) [DOI] [PubMed] [Google Scholar]
- 30.Chen Y, Cardinaux JR, Goodman RH, Smolik SM. 1999. Mutants of Cubitus interruptus that are independent of PKA regulation are independent of Hedgehog signaling. Development 126, 3607–3616. [DOI] [PubMed] [Google Scholar]
- 31.Ing B, Shteiman-Kotler A, Castelli M, Henry P, Pak Y, Stewart B, Boulianne GL, Rotin D. 2007. Regulation of Commissureless by the Ubiquitin ligase DNedd4 is required for neuromuscular synaptogenesis in Drosophila melanogaster. Mol. Cell Biol. 27, 481–496. (doi:10.1128/MCB.00463-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilkin MB et al. 2004. Regulation of notch endosomal sorting and signaling by Drosophila Nedd4 family proteins. Curr. Biol. 14, 2237–2244. (doi:10.1016/j.cub.2004.11.030) [DOI] [PubMed] [Google Scholar]
- 33.Sakata T, Sakaguchi H, Tsuda L, Higashitani A, Aigaki T, Matsuno K, Hayashi S. 2004. Drosophila Nedd4 regulates endocytosis of notch and suppresses its ligand-independent activation. Curr. Biol. 14, 2228–2236. (doi:10.1016/j.cub.2004.12.028) [DOI] [PubMed] [Google Scholar]
- 34.Levy F, Muehlethaler K, Salvi S, Peitrequin AL, Lindholm CK, Cerottini JC, Rimoldi D. 2005. Ubiquitylation of a melanosomal protein by HECT-E3 ligases serves as sorting signal for lysosomal degradation. Mol. Biol. Cell 16, 1777–1787. (doi:10.1091/mbc.E04-09-0803) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dalton HE, Denton D, Foot NJ, Ho K, Mills K, Brou C, Kumar S. 2011. Drosophila Ndfip is a novel regulator of Notch signaling. Cell Death Differ. 18, 1150–1160. (doi:10.1038/cdd.2010.130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berthouze M, Venkataramanan V, Li Y, Shenoy SK. 2009. The deubiquitinases USP33 and USP20 coordinate β2 adrenergic receptor recycling and resensitization. EMBO J. 28, 1684–1696. (doi:10.1038/emboj.2009.128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leon S, Haguenauer-Tsapis R. 2009. Ubiquitin ligase adaptors: regulators of ubiquitylation and endocytosis of plasma membrane proteins. Exp. Cell Res. 315, 1574–1583. (doi:10.1016/j.yexcr.2008.11.014) [DOI] [PubMed] [Google Scholar]
- 38.Girnita L, Girnita A, Larsson O. 2003. Mdm2-dependent ubiquitination and degradation of the insulin-like growth factor 1 receptor. Proc. Natl Acad. Sci. USA 100, 8247–8252. (doi:10.1073/pnas.1431613100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sehat B, Andersson S, Girnita L, Larsson O. 2008. Identification of c-Cbl as a new ligase for insulin-like growth factor-I receptor with distinct roles from Mdm2 in receptor ubiquitination and endocytosis. Cancer Res. 68, 5669–5677. (doi:10.1158/0008-5472.CAN-07-6364) [DOI] [PubMed] [Google Scholar]
- 40.Chastagner P, Israel A, Brou C. 2006. Itch/AIP4 mediates Deltex degradation through the formation of K29-linked polyubiquitin chains. EMBO Rep. 7, 1147–1153. (doi:10.1038/sj.embor.7400822) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim HC, Huibregtse JM. 2009. Polyubiquitination by HECT E3 s and the determinants of chain type specificity. Mol. Cell Biol. 29, 3307–3318. (doi:10.1128/MCB.00240-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garrone NF, Blazer-Yost BL, Weiss RB, Lalouel JM, Rohrwasser A. 2009. A human polymorphism affects NEDD4 L subcellular targeting by leading to two isoforms that contain or lack a C2 domain. BMC Cell Biol. 10, 26 (doi:10.1186/1471-2121-10-26) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Plant PJ, Lafont F, Lecat S, Verkade P, Simons K, Rotin D. 2000. Apical membrane targeting of Nedd4 is mediated by an association of its C2 domain with annexin XIIIb. J. Cell Biol. 149, 1473–1484. (doi:10.1083/jcb.149.7.1473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bachhuber T, Almaca J, Aldehni F, Mehta A, Amaral MD, Schreiber R, Kunzelmann K. 2008. Regulation of the epithelial Na+ channel by the protein kinase CK2. J. Biol. Chem. 283, 13 225–13 232. (doi:10.1074/jbc.M704532200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.French ME, Kretzmann BR, Hicke L. 2009. Regulation of the RSP5 Ubiquitin ligase by an intrinsic ubiquitin-binding site. J. Biol. Chem. 284, 12 071–12 079. (doi:10.1074/jbc.M901106200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rotin D, Kumar S. 2009. Physiological functions of the HECT family of Ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 10, 398–409. (doi:10.1038/nrm2690) [DOI] [PubMed] [Google Scholar]
- 47.Bischof J, Maeda RK, Hediger M, Karch F, Basler K. 2007. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc. Natl Acad. Sci. USA 104, 3312–3317. (doi:10.1073/pnas.0611511104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Venken KJ, He Y, Hoskins RA, Bellen HJ. 2006. P[acman]: a BAC transgenic platform for targeted insertion of large DNA fragments in D. melanogaster. Science 314, 1747–1751. (doi:10.1126/science.1134426) [DOI] [PubMed] [Google Scholar]
- 49.Brand AH, Perrimon N. 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415. [DOI] [PubMed] [Google Scholar]
- 50.Capdevila J, Pariente F, Sampedro J, Alonso JL, Guerrero I. 1994. Subcellular localization of the segment polarity protein patched suggests an interaction with the wingless reception complex in Drosophila embryos. Development 120, 987–998. [DOI] [PubMed] [Google Scholar]
- 51.Milan M, Campuzano S, Garcia-Bellido A. 1997. Developmental parameters of cell death in the wing disc of Drosophila. Proc. Natl Acad. Sci. USA 94, 5691–5696. (doi:10.1073/pnas.94.11.5691) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.