Abstract
Evolutionary analyses of population translocations (experimental or accidental) have been important in demonstrating speed of evolution because they subject organisms to abrupt environmental changes that create an episode of selection. However, the strength of selection in such studies is rarely measured, limiting our understanding of the evolutionary process. This contrasts with long-term, mark–recapture studies of unmanipulated populations that measure selection directly, yet rarely reveal evolutionary change. Here, we present a study of experimental evolution of male colour in Trinidadian guppies where we tracked both evolutionary change and individual-based measures of selection. Guppies were translocated from a predator-rich to a low-predation environment within the same stream system. We used a combination of common garden experiments and monthly sampling of individuals to measure the phenotypic and genetic divergence of male coloration between ancestral and derived fish. Results show rapid evolutionary increases in orange coloration in both populations (1 year or three generations), replicating the results of previous studies. Unlike previous studies, we linked this evolution to an individual-based analysis of selection. By quantifying individual reproductive success and survival, we show, for the first time, that males with more orange and black pigment have higher reproductive success, but males with more black pigment also have higher risk of mortality. The net effect of selection is thus an advantage of orange but not black coloration, as reflected in the evolutionary response. This highlights the importance of considering all components of fitness when understanding the evolution of sexually selected traits in the wild.
Keywords: rapid evolution, sexual selection, fitness, mark–recapture, common garden experiments
1. Introduction
Darwin envisioned evolution as a process too slow to be observed within the human lifespan. The last few decades of evolutionary research, however, show that organisms have the potential to adapt to environmental changes in intervals of time sufficiently brief to be observable. This phenomenon is often referred to as contemporary or rapid evolution [1–4]. For example, Grant & Grant [5] studied the adaptation of medium ground finches (Geospiza fortis) to environmental changes in their habitat brought on by El Niño events. Rapid evolution of life history and morphology have also been demonstrated in introduced species such as the mosquitofish Gambusia affinis in Hawaii [6], soapberry bugs Jadera haematoloma in North America [7] and rabbits Oryctolagus cuniculus in Australia [8].
Despite clear examples documenting how ecological change can trigger rapid evolution, our understanding of their dynamics is limited. This is because studies that characterize selection in the wild have done so in the absence of evolution, while those that characterize rapid evolution using common garden or genetic tools have done so without direct measurement of selection. More specifically, long-term individual-based studies of unmanipulated natural populations provide clear evidence for strong selection and genetic variation, but consistently fail to reveal an evolutionary (i.e. genetically based) response to selection [9,10]. For example, Kruuk et al. [9] found heritable variation for antler size in red deer Cervus elaphus and that larger antlers enhanced breeding success, yet no evolution. The reason is that both antler size and breeding success were correlated with nutritional state, thus nullifying the selection for larger antlers. On the other hand, studies that unequivocally demonstrate rapid evolution using genetics or common garden experiments have lacked direct measurement of the strength of selection (e.g. [5,11]). Evolution is instead inferred from changes in the population mean phenotype over time rather than from variation in individual reproductive success [12]. The goal of this article is to combine these two different ways of characterizing evolution using a translocation experiment in wild Trinidadian guppies.
In a classic example of experimental evolution, Endler [13] translocated Trinidadian guppies (Poecilia reticulata) from a stretch of the Aripo River rich in predators, to an upstream reach separated by a waterfall that excluded guppies and most predators. When he resampled the streams 2 years later, he found a significant increase in carotenoid, structural and melanistic coloration in the transplanted guppy populations, which matched the increased colourfulness of natural low-predation populations. This pattern is thought to reflect a release from selection by predators against bright coloration coupled with female guppy preferences for increased coloration [14]. Indeed, laboratory trials showing that female preference for bright males is stronger in low-predation localities [15]. In addition, the difference in male colour between high- and low-predation environments is repeatable among different streams, and the evolution of male coloration in response to experimental introductions has been repeatable in new experiments [13,16,17]. The aggregate of these comparative studies and experiments makes a compelling case for the importance of both predation and sexual selection in male colour evolution. What is missing from these studies is a characterization of how selection produced this result. Specifically, we would like to know the extent to which evolution is shaped by survival versus reproductive selection. Knowing their relative importance will enable us to assess the relative contributions of predation versus female choice in shaping the evolution of male colour patterns.
In this study, we investigate the selective forces behind the rapid evolution of male guppy coloration by replicating translocation experiments from high- to low-predation environments and following individual fitness throughout their evolution. To accomplish this, we first characterize phenotypic change by quantifying the population changes in mean male coloration one year after translocation. Second, we ascertain whether these changes have a genetic basis by comparing the second generation of fish from ancestral and derived populations reared in a common laboratory environment. This was done to confirm that our studies replicate the results of previous translocation studies in guppies [14,17,18]. Third, we measured selection directly using individual-based data on survival and parentage by testing for correlations between colour and fitness components (survival and reproductive success). If the evolution of male coloration is, as hypothesized, due to a relaxation of negative survival selection against coloration and an increase in sexual selection for increased coloration, we predict a strong association between coloration and male reproductive success, but no association between colour and survival.
2. Material and methods
(a). Study system
Guppies (P. reticulata) are small live-bearing fish native to northeastern South America, Trinidad and other continental islands. In the wild, guppies have relatively short generation times (110–210 days [19]) and small body sizes, typically ranging from 15 to 17 mm for male adults and 15 to 30 mm for female adults [20]. They are easy to capture and whole populations can be collected, which facilitates studies of survival in nature [21]. They are also relatively easy to rear and breed in captivity. Male guppies are significantly smaller and exhibit numerous ornamental body and tail colour patterns. Females lack ornamental coloration [15]. Male guppies are colourless as juveniles and do not begin to attain full coloration until approximately 50–80 days of age [15]. Females remain colourless throughout their lives [15]. Female preference causes the evolution of enhanced male secondary sexual characteristics including coloration, enlarged caudal and dorsal fins, and courtship displays [15]. Females also prefer males with unusual colour patterns, causing negative frequency-dependent selection, which enhances the diversity of male coloration [22,23].
Guppies in Trinidad inhabit small freshwater rivers. The rivers that drain the Northern Range mountains have been described as a ‘natural experiment’ [24] because the same types of fish community are replicated in a number of streams. Streams are often punctuated with waterfalls, with high-predation environments downstream and low-predation environments (where larger predatory fish are mainly absent) upstream. Adaptation to differences in predation regime has driven the parallel evolution of differences in morphology, behaviour and life history between guppies on either side of the waterfalls, leading to distinct eco-types of guppies in both environments [25]. For example, low-predation guppies are older and larger at maturity, and produce fewer but larger offspring per litter than their counterparts from high-predation environments [21]. Phylogenetic relationships of fish populations between rivers suggest that the adaptive divergence between high- and low-predation guppy populations has occurred independently in many rivers [26], providing evidence that these streams represent independent replicates in which guppies have adapted to life with and without predators. This genetic divergence has been shown to be extremely rapid when guppies are artificially transplanted from high-predation to low-predation locations [11,13,16,19,27].
(b). Introduction experiment
In March 2008, 150 guppies were collected as juveniles from a region of the Guanapo River where guppies co-occur with a diversity of predators (Guanapo high-predation; GHP) and reared to maturity in single-sex groups to keep them virgin until mating. We mated them in tanks of five males and five females, then introduced them into the streams three weeks after mating. March is the early dry season. Guppy populations typically have high reproductive success during the dry season, so the introduction timing was done to maximize their chance of reproductive success. Introductions were made into two tributaries in the same river, each of which was guppy-free and contained only a single species of fish: Rivulus hartii. This is the only species typically found with guppies in low-predation environments. The introduction sites were Upper La Laja (UPL: 43.062° N, 16.047° W) and Lower La Laja (LOL: 42.969° N, 16.000° W). Approximately 75 individuals were introduced into each predation tributary (further details of the introduction provided in [28]). Because the founders were collected as juveniles, we knew every founder. Prior investigators collected mixtures of adult and juvenile fish, then introduced them soon after capture. Doing so means that adult females were carrying stored sperm from unknown males, so one could not know all founders of the introduced population. Both introduction sites were sections of stream bordered on either side by barrier waterfalls. The downstream waterfalls had previously excluded all fish save the killifish (R. hartii). The upstream waterfall limits the introduced population of guppies from moving further upstream. The introduction sites had lengths of 105 m (LOL) and 145 m (UPL).
Before introduction, every fish was anaesthetized using MS-222 (tricaine methylsulfonate), digitally photographed and individually marked by subcutaneously injecting an elastomer dye (Northwest Marine Technologies) following methods explained in [16]. Each individual was given two colour marks (out of 12 possible colours) in two of eight possible body positions. Three scales were collected from each individual to provide a source of DNA for building the genetic pedigree of all individuals in the experiment. The pedigree was reconstructed by genotyping all individuals at 12 microsattellite loci that had an average of 20 alleles each at the beginning of the introduction. Details on the microsatellite properties and pedigree reconstruction methods can be found in electronic supplementary material, appendix S2 and table S1.
Every month we thoroughly censused guppies from both streams and brought them back to the field laboratory, where we identified and photographed all marked individuals, and marked and photographed all new recruits. New recruits were unmarked fish greater than 14 mm standard length. Fish greater than 14 mm standard length can be safely handled and marked without risk of mortality and are close to the size at which the process of maturation begins. A year after introduction, the dataset contained 1467 individually marked fish (664 in the LOL treatment and 803 in the UPL), of which 682 were males (306 and 376, respectively). Due to logistic limitations, we analysed male colour patterns roughly bimonthly from the photographs (on months March 2008, May, July, October, January, March 2009). Mean attributes from each time interval were analysed for phenotypic divergence in male coloration between the ancestral and derived populations.
In March 2009, a subset of juvenile guppies from both introduction sites and the source Guanapo high-predation site were collected. These fish would have reached maturity in the wild in March–April 2009, and hence are comparable to the wild data on adults one year after the introduction. They were reared for two generations under common garden conditions following methods in [11,29] (described in detail in the electronic supplementary material, appendix S1). Second generation offspring were then analysed for genetic divergence in coloration between ancestral and derived populations once they became adults. The final sample contained 18 males from GHP, 47 from LOL and 37 from UPL.
(c). Colour measurement
We used ImageJ to measure coloration on all digital photographs for both wild- and laboratory-reared male guppies. We did so by quantifying the area of each coloured spot in every fish after delineating it manually in ImageJ. All images were measured by a single observer (A.R.) who was blind to the origin of the fish. Colours were categorized as melanistic (black, fuzzy black) and orange. In accordance with previous studies [17,18,30], we measured both black and fuzzy black, then combined them as a single measure of melanistic coloration. The two are often hard to distinguish, and thus the sum showed less variation over several independent measurements of the same picture. The total area for each colour on the fish was summed. We adjusted for the effects of body size (e.g. so spots are not larger simply because male size is larger) by dividing the total area of each colour group by body area. Analyses using relative area of spots and absolute area using body area as a covariate yielded similar results, so only relative area differences are illustrated in the figures. Structural coloration (blue, violet, silver and green) was not included in the analyses because they are not reliably represented in photographs [17,31]. Coloration was measured only on adult males. Because adult males were caught several times and thus have repeated measures, individual coloration changed slightly across months. ANOVA-based individual repeatability was high (0.79 for orange, 0.78 for black) in spite of the combined effects of temporal variation and measurement error.
(d). Statistical analyses
Male capture probabilities were estimated using an open population capture–mark–recapture model that included month-specific recapture probabilities [32]. The model was fitted by maximum likelihood using package Rcapture in program R. A different model was fit to each stream. Recapture probabilities varied among months and streams but were on average high (averaging 0.84; electronic supplementary material, table S2); consequently, the probability of missing an individual for its entire lifetime, after accounting for mortality, was very low (approx. 4%).
Phenotypic divergence in male coloration between the start of the introduction and 12 months after was analysed by fitting linear models. Melanistic and orange colours were analysed separately. Relative colour area was included as the response variable, and time (at introduction versus 12 months after) as a categorical explanatory variable. Population (LOL, UPL) by time interactions were included to test for population differences in phenotypic trends. As evolution is expected to cause changes in variance, we first tested for unequal variances in the data using a χ2 non-constant variance score test for factor (using function ncvTest in package car). If the test suggested heterogeneity of variances (p < 0.05), we corrected the calculation of p-values using the White method [33]. We used the same approach to test for genetic divergence in male coloration (i.e. a rapid evolutionary response) on the common garden individuals. In this case, the comparison was made between common garden samples of introduced and ancestral population collected the same year.
We next measured the effects of colour phenotype on survival and reproductive success. Because fish can only be individually marked safely at 14 mm, reproductive success refers to the number of 14 mm recruits sired by a given male. To do so, we used generalized linear mixed models GLMM on individual monthly data (function glmer in R package lme4). Survival was modelled as a binomial response variable (1 = alive, 0 = dead). We treated relative reproductive success as a binomial proportion with a logit link (fraction of marked recruits sired by a given male, out of the total number of new recruits in the population per given month). We chose this relative measure because, in non-stationary populations with overlapping generations, lifetime reproductive success is not an appropriate measure of fitness [34,35]. Moreover, this measure is not available for those individuals still alive at the end of the study. However, we also explored monthly absolute reproductive success modelled as a Poisson distribution with log link in order to understand whether monthly fluctuations in the overall level of recruitment affect selection. In all models, month was included as a random effect to account for temporal changes in recruitment and survival. Individual identity was also included as a random effect to account for repeated measurements. Relative orange area, relative melanistic area and their interaction, were included as explanatory variables. Stream was added as a categorical explanatory factor crossed with the phenotypic variables to test for differences in selection between the two streams. We tested for temporal variation in selection by fitting the above full model with alternative monthly random effect structures. We explored the following random effects structures: random monthly intercepts for all measures, stream-specific random intercepts (month by stream interaction) and random slopes for orange and/or melanistic effects. The best random effect structure was tested using likelihood ratio tests. Once that was established, the significance of the fixed effects was tested using χ2 tests. Only interactions with a χ2 higher than one were retained in the model. The full model was fit with each random effects structure using REML.
Finally, for comparability with other studies, we report estimates of directional selection gradient for both survival and reproductive components of selection on colour. We calculated these by fitting binomial GLMMs of survival and reproductive success using standardized measures of relative orange and black area. We standardized the variables by subtracting the monthly averages and dividing by the variance [36]. Additive models were used to measure directional selection gradients, while correlational selection [37] was calculated by incorporating the interaction between the two traits.
3. Results
(a). Phenotypic changes in male colouration
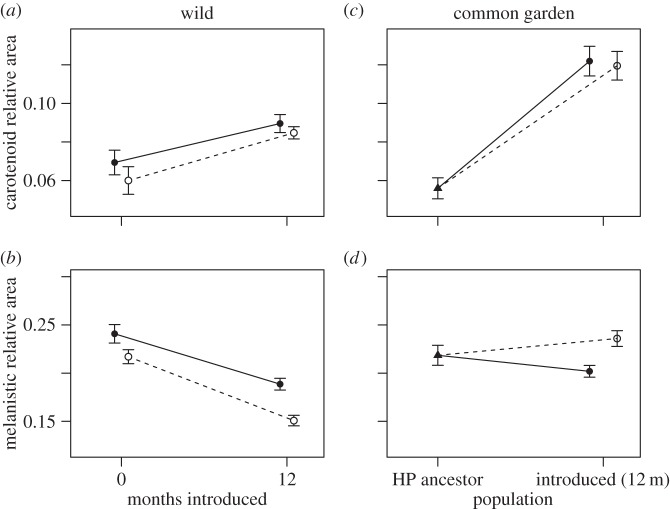
In 12 months, black coloration decreased from 24.08 ± 0.96% to 18.86 ± 0.61% in LOL and from 21.7 ± 0.72% to 15.07 ± 0.54% in UPL, while orange increased from 6.93 ± 0.64 to 8.96 ± 0.61 in LOL and from 6.00 ± 0.72 to 8.47 ± 0.31% in UPL (figure 1a,b). Lateral fish body area increased from 0.47 ± 0.01 cm2 to 0.59 ± 0.01 cm2 in LOL and from 0.46 ± 0.01 cm2 to 0.61 ± 0.01 cm2 in UPL. We examined whether there were significant differences between the ancestral and derived populations. This ‘before’ and ‘after’ analysis is comparable to the analyses reported in earlier studies [17,18], enabling us to assess repeatability of rapid evolution of male coloration to those seen when the same experiment was performed by earlier investigators.
Figure 1.
Changes in coloration one year after introduction. (a,b) Phenotypic changes from wild-caught individuals in orange and melanistic coloration, respectively, 12 months after introduction. (c,d) The common garden differences between the introduced populations (12 months after) and high-predation ancestors for orange and melanistic coloration, respectively. Open circles correspond to Upper La Laja and closed circles to Lower La Laja.
We found a significant increase in the relative area of orange spots after 12 months in both low-predation introduction sites (figure 1a; effect = 0.022 ± 0.005, t176 = 4.161, p < 0.001). According to the non-constant variance score test, the residuals showed evidence of a decrease in variance with time (χ2 = 4.919, p = 0.026). The standard error and p-values presented are corrected for this change in variance. There were no differences between the two introduction sites (UPL effect = −0.006 ± 0.005, t176 = −1.160, p = 0 0.246). By contrast, we found a significant decrease in melanistic coloration after 12 months between the ancestral population and our two introduced populations (figure 1b; effect = −0.052 ± 0.010, t176 = −5.002, p < 0.001). There was no evidence for a change in variance (χ2 = 0.336, p = 0.562). The two populations were again not significantly different from one another (effect = −0.014 ± 0.016, t176 = −0.899, p = 0.369).
(b). Common garden differences in male coloration
Common garden F2 individuals from the source population (GHP) 1 year after introduction showed an average of 5.59 ± 0.54% orange and 21.84 ± 1.04% black. By contrast, the common garden F2 individuals derived from the introduced populations showed 12.19 ± 0.77% (LOL) and 11.95 ± 0.74% (UPL) of orange. Black coloration remained similar in at 20.18 ± 0.61% (LOL) and 23.59 ± 9.81% (UPL; figure 1c,d). While body area diverged upwards in individuals derived from LOL (0.54 ± 0.01 cm2) with respect to GHP (0.49 ± 0.02 cm2), it diverged slightly downwards in UPL (0.46 ± 0.01 cm2). The χ2 non-constant variance score test results show unequal variances for the laboratory-reared fish populations in orange (χ2 = 6.16, p = 0.013), but not for melanistic coloration (χ2 = 1.81, p = 0.178). Therefore, we used the White method to correct for the unequal variances in the analysis of orange [33]. Fish from both introduction sites had significantly more orange coloration 12 months after introduction than the ancestral population (LOL effect = 0.066 ± 0.009, t95 = 7.135, p < 0.001; UPL effect = 0.063 ± 0.009, t95 = 7.032, p < 0.001; figure 1c), indicating rapid evolution of male coloration that matches the phenotypic trends seen in the field. There were no differences between fish from the UPL and LOL introduction sites (effect = −0.002 ± 0.010; t78 = −0.228, p = 0.819). Unlike orange, melanistic coloration did not show significant differences between the ancestral and the two introduction sites in common garden trials (LOL effect = −0.017 ± 0.012, t95 = −1.326, p = 0.188; UPL effect = 0.017 ± 0.013, t95 = 1.359, p = 0.177; figure 1d).
(c). Selection analyses
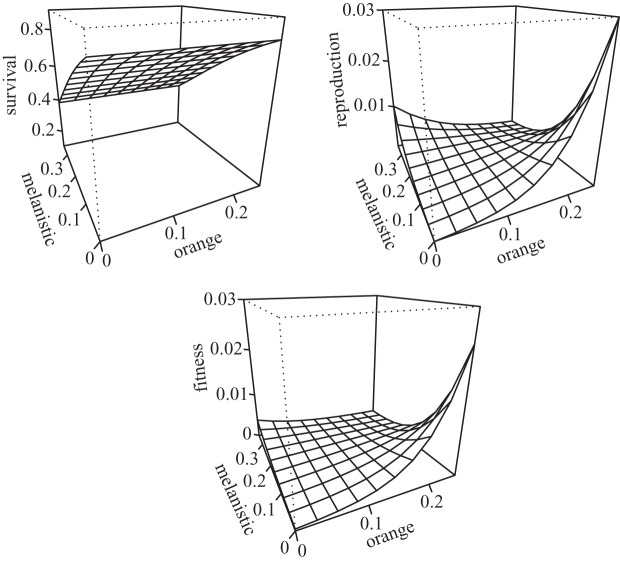
The fish from the UPL stream had higher monthly variation in survival rates than those from the LOL (table 1). However, the analysis of survival and reproduction did not show any significant interaction between population and the effect of colour (all χ2 < 1), indicating that the patterns of selection on pigmentation are the same for both introduction populations. Overall, there was a negative effect of melanin, but not orange, on survival (table 1 and figure 2). There was a negative interaction between the two colours on reproductive success (table 1), such that each colour had a significantly positive effect on reproductive success under low values of the other colour. When both reproduction and survival results are combined into one fitness surface by multiplying survival and reproductive success, the negative reproductive effect on individuals that have more melanization and less orange is muted. The net consequence of this interaction is that individuals with increased orange in the introduced environments have higher fitness. There is no net effect of melanin on fitness.
Table 1.
GLMM estimates of the effects of coloration on fitness components: survival and reproduction.
| survival |
relative reproductive success |
no. offspring |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| estimate | χ2 | p-value | estimate | χ2 | p-value | estimate | χ2 | p-value | |
| random effects (σ2) | |||||||||
| month | 0.007 | 0.794 | 1.653 | ||||||
| month: population (UPL) | 0.317 | 0.915 | 0.452 | ||||||
| individual | <0.001 | 1.722 | 1.652 | ||||||
| fixed effects | |||||||||
| intercept | 1.827 ± 0.462 | −7.790 ± 0.869 | −3.221 ± 0.835 | ||||||
| population (UPL) | −0.692 ± 0.305 | 5.133 | 0.023 | 0.166 ± 0.456 | 0.133 | 0.718 | 0.183 ± 0.438 | 0.174 | 0.677 |
| orange | −0.126 ± 2.802 | 0.002 | 0.964 | 16.929 ± 7.408 | 5.223 | 0.022 | 16.591 ± 7.477 | 4.924 | 0.026 |
| melanistic | −5.582 ± 1.869 | 8.912 | 0.002 | 8.928 ± 3.676 | 5.900 | 0.015 | 8.492 ± 3.678 | 5.329 | 0.021 |
| interaction | — | — | — | −77.609 ± 37.32 | 4.323 | 0.037 | −74.216 ± 37.625 | 3.891 | 0.048 |
Figure 2.
Average predicted survival, reproduction and fitness surfaces for the two streams. Fitness is calculated as the product of the survival and reproduction surfaces.
The model selection of random effects showed no evidence for monthly variation in the effect of coloration on fitness. In other words, despite monthly variation in survival and reproduction, the relationship between coloration and fitness was similar across months (survival: ΔlogLik = 2.78, 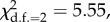 p = 0.06; reproductive success: ΔlogLik = 14.65,
p = 0.06; reproductive success: ΔlogLik = 14.65, 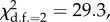 p < 0.001).
p < 0.001).
Note that even though we chose relative reproductive success as a measure of fertility selection, an equivalent Poisson GLMM on the total monthly count of offspring shows qualitatively the same results (table 1), indicating that monthly changes in recruitment are not affecting our estimates of selection.
The directional standardized selection coefficient β for the survival component was −0.270 for black and −0.023 for orange coloration. The selection coefficients for reproductive success were 0.097 for black and 0.130 for orange. The correlational selection coefficient γ for the combination of black and orange was −0.095.
4. Discussion
In this study, we have twice replicated a translocation experiment that manipulates predation pressure in wild guppy populations. Like previous studies [13,17], we detected rapid evolutionary change in male coloration. Unlike previous studies, we combined this assessment of evolution with a selection analysis derived from an individual-based mark–recapture study using a pedigree reconstruction of the evolving populations. Results show that evolutionary change for both introduction populations happened faster than previously reported in this system (1 year or three generations). Introduced fish developed increased orange and decreased melanistic coloration compared with their ancestors, but only the orange increase was genetic. For the first time, we found that this evolutionary change was associated with viability (survival) selection against individuals with more melanin and a reproductive advantage (fertility selection) in favour of higher coloration (either melanistic or orange). When we combine both survival and reproduction into an overall fitness effect, we find that there is no net effect of melanin (due to its detriment to survival), but a fitness advantage for increased orange. These estimates of selection are consistent with the evolutionary outcome shown in the common gardens. While the fertility results nicely match studies showing a mating advantage of orange coloration in male guppies, they also highlight the importance of considering all components of fitness. The evolution of increased orange, but not black, coloration can only be understood as a balance between natural and sexual selection. Black coloration failed to evolve because survival selection against black counters its reproductive advantage.
The negative effect of black coloration on survival is consistent with a previous study [30] that found a survival cost of coloration in a variety of high-predation and low-predation populations. Contrary to Weese et al. [30], we did not find significant evidence for a survival cost of orange coloration. This may be due to local differences in the studied streams. For example, while our study site lies in the southern slope of the mountain range in Trinidad, the majority of sites sampled in [30] are in the northern slope, where predatory prawns are more abundant in both high-predation and low-predation sites, and where the predatory fish species are different from those on the southern slope [38].
Our finding of enhanced reproductive success in individuals with higher orange content is consistent with behavioural studies of mate choice in the laboratory. Endler & Houde [14] found a significant preference for orange in 10 of 11 populations of guppies examined, plus a strong positive correlation between the strength of female preference for orange and the average quantity of orange in mature males from each population. In particular, females from the Guanapo River used in this study preferred more orange, less black and a larger tails [14]. This is consistent with our estimates of selection and phenotypic change (figures 1 and 2). Houde [39] and van Oosterhout et al. [40] also independently report female preference for increased orange coloration, which is linked to male quality [41]. Female preference for conspicuous coloration in males is thought to be risky in high-predation environments because close proximity to a brightly coloured male can increase chance of a predatory attack [42]. These costs of male preference will be reduced when guppies are moved into a predator-free environment and females are open to prefer bright male coloration. These results from prior research combined with our selection analysis in the wild show strong evidence that female preference for orange coloration is the primary driver of the increase in orange coloration in low-predation localities.
High- and low-predation streams typically differ in several environmental variables that correlate to a certain degree with predation [43]. Notably, high-predation streams tend to have significantly less canopy cover, and therefore higher productivity, than low-predation streams. The lack of predation is also typically accompanied by higher guppy population densities [44]. Our two introduction streams also differed in their canopy openness and net primary productivity. UPL had a thinned canopy and higher light levels, and is much more productive [45]. Moreover, guppy population densities were relatively low (ranging between 0.6 and 3.0 ind m−2) and closer to those of a typical high-predation site [43] because they had not yet had time to reach densities typical of low-predation environments. The fact that both streams followed remarkably similar evolutionary patterns despite the differences between them in light and productivity, and despite both having low population densities for the first year, suggests that female preference was likely to be the main factor responsible for our results.
The novelty in our study lies in the individual-based analysis that enabled us to resolve the contributions of survival and reproductive success to the evolution of male coloration and the duplication of the experiment. This is the first direct measure of the strength and direction of selection (both in survival and reproduction) for such a manipulation. We found, as predicted, that there is no increase in mortality but an increase in reproductive success associated with the evolution of increased orange coloration (table 1 and figure 2). Interestingly, a negative interaction with melanin shows that this increase in reproductive success is most obvious under low levels of melanistic coloration, and muted when both survival and reproduction are taken into consideration. As Guanapo high-predation guppies (ancestors of introduced population) prefer less black coloration in mate choice experiments [14], female preference could have driven the decline in melanistic coloration in the introduced populations. In fact, Kemp et al. [17] also found that black (melanin-based) coloration decreased in the El Cedro introduction, which is also a tributary of the Guanapo River and also was an introduction of guppies from a high-predation site downstream into a previously guppy-free low-predation tributary upstream.
The magnitude of survival selection against black coloration (β = −0.270) was high in this study compared with other reported figures in the literature [36], while orange showed a negligible effect on survival. On the other hand, the directional selection gradients for black (β = 0.097) and orange (β = 0.130) were moderate to high.
Interestingly, reproductive success showed an interaction between black and orange such that reproductive success was high only when one of the two colours (but not both) occupied a large portion of the body (table 1 and figure 2). The correlational selection coefficient was therefore negative (γ = −0.095), yet modest compared with previous studies [37]. Correlational selection, where selection acts on trait combinations rather than individual traits, is important to the evolution of complex phenotypes, genetic correlations and phenotypic integration [37]. For example, Svensson et al. [46] found that selection on immune function was contingent on lizard coloration, therefore favouring the maintenance of a genetic correlation between colour morphotype and immune function. It is unclear to us why the reproductive success of individuals with large orange spots is reduced when the amount of black is also large. However, as figure 2 suggests, when put together with the detrimental effect of black on survival, the degree of correlational selection is substantially weaker.
In this study, we have estimated fitness as the proportion of 14 mm recruits sired by individuals. This is unavoidable as offspring cannot be safely handled and genotyped prior to this size. While the number of sired individuals that reach maturity is a standard measure of selection, Wolf & Wade [47] argue that it can be problematic when performing quantitative genetic predictions of evolution. Because a component of offspring fitness (juvenile survival) gets interpreted as parental reproductive output, evolutionary predictions can be wrong if there is a genetic correlation between parental effects and offspring characters on fitness. Given that there is no parental care in guppies, we see no reason to expect such a problem.
Our common garden results suggest that the appearance of reduced melanistic pigmentation may have been caused by plasticity. Moreover, the data on wild individuals showed that reproductive selection for black (under low levels of orange) and survival selection against it balances out, to produce an overall fitness effect of weak to no selection for black coloration (figure 2). Melanin-based coloration has been shown to be labile in fish [48], meaning that fish can readily change their coloration depending on the location of pigment granules within the melanocyte cells (i.e. if dispersed the guppy is quite dark, and if concentrated in one area the guppy is paler). Since guppies can adjust their pigment granules in response to environmental features, such as changes in habitat background coloration, there is likely to be some adaptive significance to plasticity in this trait. Indeed, a recent paper [29] found plasticity in guppy coloration in response to predation. Specifically, guppies reared in laboratory environments with predator cues developed reduced black coloration compared with fish reared without these cues.
The rapid evolution of sexually selected traits is rarely studied in the wild [4]. Introduction experiments give us the opportunity to study how different selective factors interact to shape the evolution of traits [17,19], but are rarely applied to secondary sexual characters. Additionally, even though introduction experiments can provide examples of rapid evolution, more detailed exploration of the selective mechanisms behind such evolution is rarely pursued. Our study shows that male melanistic and orange coloration can rapidly evolve to large-scale changes in predation in less than three generations, even faster than previously reported. Most importantly, we have filled an important gap in this research by linking evolutionary change to the quantified contributions of selection components: survival and reproductive success. Our finding that males with more orange coloration have higher reproductive success bridges laboratory results, which show that females prefer males with more orange coloration, with the repeated observation of the evolution of orange coloration in introduction experiments. This contrasts with a number of studies on non-experimental long-term populations that detect selection and genetic variation in traits, yet fail to detect evolution [49]. By taking the reverse approach of first triggering evolution and then analysing selection, we have found that the direction and strength of selection can explain the evolutionary outcome, but only when both reproductive and survival components are measured. We further show that the primary drive of the evolution of increased orange coloration in our introductions was reproductive success, which is consistent with a host of laboratory studies showing that females prefer males with more orange coloration.
Supplementary Material
Acknowledgements
We are indebted to E. Ruell, C. Cuenca, D. Moore, D. Rumbo, M. Yoakim, and the Reznick and Ghalambor laboratories for help in the maintenance of common garden fish. We thank many students, technicians, interns and volunteers that helped with field collections, and R. Hernandez and I. Ramnarine for logistic support in Trinidad. S. Auer, R. Bassar, A. Furness, M. Torres-Mejıa, Y. Reynoso, M. Turcotte, J. Endler, D. Kemp, D. Fairbairn, B. Rojas and M. Zuk provided insightful discussion and comments on the manuscript. Export permits were granted by the Fisheries Division of Trinidad and Tobago.
Ethics
All research for this specific experiment, including the anaesthesia of the fish, was done in strict accordance with approved animal care and use protocol at the University of California, Riverside (AUP-20080008).
Data accessibility
The raw data used in this study have been deposited in Dryad and are accessible at http://datadryad.org/review?doi=doi:10.5061/dryad.11r1j.
Authors' contributions
S.P.G., D.R., P.B. and A.L-S. conceived the study. D.R. and A.L-S. coordinated the introductions and mark–recapture census. P.B. coordinated the pedigree reconstruction. A.R. measured male coloration. S.P.G., J.D.A. and A.L-S. analysed the data and drafted the manuscript. All authors discussed results, crafted the final document and gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
Funding was provided by a Fonds Québécois de la Recherche sur la Nature et les Technologies (FQRNT) fellowship, a UCR Graduate Dean's Dissertation Research Grant and an Academy of Finland Postdoctoral fellowship (project 2100002744) to S.P.G.; the EvoRange project of the Agence Nationale de Recherche of France and Grup de Calitat de la Generalitat Catalana to A.L-S.; and a National Science Foundation Frontiers for Integrative Biological Research (FIBR) grant (EF 0623632) to D.R.
References
- 1.Thompson JN. 1998. Rapid evolution as an ecological process. Trends Ecol. Evol. 13, 329–332. ( 10.1016/S0169-5347(98)01378-0) [DOI] [PubMed] [Google Scholar]
- 2.Stockwell CA, Hendry AP, Kinnison MT. 2003. Contemporary evolution meets conservation biology. Trends Ecol. Evol 18, 94–101. ( 10.1016/S0169-5347(02)00044-7) [DOI] [Google Scholar]
- 3.Hendry AP, Kinnison MT. 1999. The pace of modern life: measuring rates of contemporary microevolution. Evolution 53, 1637–1653. ( 10.2307/2640428) [DOI] [PubMed] [Google Scholar]
- 4.Svensson EI, Gosden TP. 2007. Contemporary evolution of secondary sexual traits in the wild. Func. Ecol. 21, 422–433. ( 10.1111/j.1365-2435.2007.01265.x) [DOI] [Google Scholar]
- 5.Grant PR, Grant RB. 1995. Predicting microevolutionary responses to directional selection on heritable variation. Evolution 49, 241–251. ( 10.2307/2410334) [DOI] [PubMed] [Google Scholar]
- 6.Stearns SC. 1983. The evolution of life-history traits in mosquito fish since their introduction to Hawaii in 1905: rates of evolution, heritabilities, and developmental plasticity. Am. Zool. 23, 65–76. [Google Scholar]
- 7.Carroll SP, Dingle H, Famula TR, Fox CW. 2001. Genetic architecture of adaptive differentiation in evolving host races of the soapberry bug, Jadera haematoloma. Genetica 112–113, 257–271. ( 10.1023/A:1013354830907) [DOI] [PubMed] [Google Scholar]
- 8.Williams CK, Moore RJ. 1989. Phenotypic adaptation and natural selection in the wild rabbit, Oryctolagus cuniculus, in Australia. J. Anim. Ecol. 58, 495–507. ( 10.2307/4844) [DOI] [Google Scholar]
- 9.Kruuk LEB, Slate J, Pemberton JM, Brotherstone S, Guinness FE, Clutton-Brock TH. 2002. Antler size in red deer: heritability and selection but no evolution. Evolution 56, 1683–1695. ( 10.1111/j.0014-3820.2002.tb01480.x) [DOI] [PubMed] [Google Scholar]
- 10.Charmantier A, McCleery RH, Cole LR, Perrins CM, Kruuk LEB, Sheldon BC. 2008. Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science 320, 800–803. ( 10.1126/science.1157174) [DOI] [PubMed] [Google Scholar]
- 11.Reznick DN, Bryga H, Endler JA. 1990. Experimentally induced life-history evolution in a natural population. Nature 346, 357–359. ( 10.1038/346357a0) [DOI] [Google Scholar]
- 12.Lande R, Arnold SJ. 1983. The measurement of selection on correlated characters. Evolution 37, 1210–1226. ( 10.2307/2408842) [DOI] [PubMed] [Google Scholar]
- 13.Endler JA. 1980. Natural selection on colour patterns in Poecilia reticulata. Evolution 34, 76–91. ( 10.2307/2408316) [DOI] [PubMed] [Google Scholar]
- 14.Endler JA, Houde AE. 1995. Geographic variation in female preferences for male traits in Poecilia reticulata. Evolution 49, 456–468. ( 10.2307/2410270) [DOI] [PubMed] [Google Scholar]
- 15.Houde AE. 1997. Sex, colour, and mate choice in guppies. Princeton, NJ: Princeton University Press. [Google Scholar]
- 16.Gordon SP, Reznick DN, Kinnison MT, Bryant MJ, Weese DJ, Räsänen K, Millar NP, Hendry AP. 2009. Adaptive changes in life history and survival following a new guppy introduction. Am. Nat. 174, 34–45. ( 10.1086/599300) [DOI] [PubMed] [Google Scholar]
- 17.Kemp DJ, Reznick DN, Grether GF. 2009. Ornamental evolution in Trinidadian guppies (Poecilia reticulata): insights from sensory processing-based analyses of entire colour patterns. Biol. J. Linn. Soc. 95, 734–747. ( 10.1111/j.1095-8312.2008.01112.x) [DOI] [Google Scholar]
- 18.Karim N, Gordon SP, Schwartz AK, Hendry AP. 2007. This is not déjà vu all over again: male guppy colour in a new experimental introduction. J. Evol. Biol. 20, 1339–1350. ( 10.1111/j.1420-9101.2007.01350.x) [DOI] [PubMed] [Google Scholar]
- 19.Reznick DN, Shaw FH, Rodd FH, Shaw RG. 1997. Evaluation of the rate of evolution in natural populations of guppies (Poecilia reticulata). Science 275, 1934–1937. ( 10.1126/science.275.5308.1934) [DOI] [PubMed] [Google Scholar]
- 20.Magurran AE. 2005. Evolutionary ecology: the Trinidadian guppy. New York, NY: Oxford University Press. [Google Scholar]
- 21.Reznick DN, Butler MJ IV, Rodd FH, Ross P. 1996. Life-history evolution in guppies (Poecilia reticulata). 6. Differential mortality as a mechanism for natural selection. Evolution 50, 1651–1660. ( 10.2307/2410901) [DOI] [PubMed] [Google Scholar]
- 22.Hughes KA, Du L, Rodd FH, Reznick DN. 1999. Familiarity leads to female mate preference for novel males in the guppy, Poecilia reticulata. Anim. Behav. 58, 907–916. ( 10.1006/anbe.1999.1225) [DOI] [PubMed] [Google Scholar]
- 23.Hughes KA, Houde AE, Price AC, Rodd FH. 2013. Mating advantage for rare males in wild guppy populations. Nature 503, 108–110. ( 10.1038/nature12717) [DOI] [PubMed] [Google Scholar]
- 24.Haskins CP, Haskins EF, McLaughlin JJA, Hewitt RE. 1961. Polymorphisms and population structure in Lebistes reticulates, an ecological study. In Vertebrate speciation (ed. Frank Blair W.), pp. 320–395. Austin, TX: University of Texas Press. [Google Scholar]
- 25.Endler JA. 1995. Multiple trait coevolution and environmental gradients in guppies. Trends Ecol. Evol. 10, 22–29. ( 10.1016/S0169-5347(00)88956-9) [DOI] [PubMed] [Google Scholar]
- 26.Alexander HJ, Taylor JS, Wu SST, Breden F. 2006. Parallel evolution and vicariance in the guppy (Poecilia reticulata) over multiple spatial and temporal scales. Evolution 60, 2352–2369. ( 10.1111/j.0014-3820.2006.tb01870.x) [DOI] [PubMed] [Google Scholar]
- 27.O'Steen S, Cullum AJ, Bennett AF. 2002. Rapid evolution of escape ability in Trinidadian guppies (Poecilia reticulata). Evolution 56, 776–784. ( 10.1111/j.0014-3820.2002.tb01388.x) [DOI] [PubMed] [Google Scholar]
- 28.Travis J, Reznick DN, Bassar RD, López-Sepulcre A, Ferriére R, Coulson T. 2014. Do eco–evo feedbacks help us understand nature? Answers from studies of the Trinidadian guppy. Adv. Ecol. Res. 50, 1–40. ( 10.1016/B978-0-12-801374-8.00001-3) [DOI] [Google Scholar]
- 29.Ruell EW, Handelsman CA, Hawkins CL, Sofaer HR, Ghalambor CK, Angeloni L. 2013. Fear, food and sexual ornamentation: plasticity of colour development in Trinidadian guppies. Proc. R. Soc. B 280, 20122019 ( 10.1098/rspb.2012.2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weese DJ, Gordon SP, Hendry AP, Kinnison MT. 2010. Spatio-temporal variation in linear natural selection on body color in wild guppies (Poecilia reticulata). Evolution 64, 1802–1815. ( 10.1111/j.1558-5646.2010.00945.x) [DOI] [PubMed] [Google Scholar]
- 31.Endler JA, Mielke PW. 2005. Comparing entire colour patterns as birds see them. Biol. J. Linn. Soc. 86, 405–431. ( 10.1111/j.1095-8312.2005.00540.x) [DOI] [Google Scholar]
- 32.Amstrup SC, McDonald TL, Manly BFJ. 2005. Handbook of capture–recapture analysis. Princeton, NJ: Princeton University Press. [Google Scholar]
- 33.White H. 1980. A heteroscedasticity-consistent covariance matrix estimator and a direct test for heteroscedasticity. Econometrica 48, 817–838. ( 10.2307/1912934) [DOI] [Google Scholar]
- 34.Mylius SD, Diekmann O. 1995. On evolutionarily stable life histories, optimization and the need to be specific about density dependence. Oikos 74, 218–224. ( 10.2307/3545651) [DOI] [Google Scholar]
- 35.Otto SP, Day T. 2007. A biologist's guide to mathematical modeling in ecology and evolution. Princeton, NJ: Princeton University Press. [Google Scholar]
- 36.Kingsolver JG, Hoekstra HE, Hoekstra JM, Berrigan D, Vignieri SN, Hill CE, Hoang A, Gibert P, Beerli P. 2001. The strength of phenotypic selection in natural populations. Am. Nat. 157, 245–261. ( 10.1086/319193) [DOI] [PubMed] [Google Scholar]
- 37.Sinervo B, Svensson E. 2002. Correlational selection and the evolution of genetic architecture. Heredity 89, 329–338. ( 10.1038/sj.hdy.6800148) [DOI] [PubMed] [Google Scholar]
- 38.Rodd FH, Reznick DN. 1991. Life history evolution in guppies: III. The impact of prawn predation on guppy life histories. Oikos 62, 13–19. ( 10.2307/3545440) [DOI] [Google Scholar]
- 39.Houde AE. 1987. Mate choice based upon naturally occurring colour pattern variation in a guppy population. Evolution 41, 1–10. ( 10.2307/2408968) [DOI] [PubMed] [Google Scholar]
- 40.Van Oosterhout C, Trigg RE, Carvalho GR, Magurran AE, Hauser L, Shaw PW. 2003. Inbreeding depression and genetic load of sexually-selected traits: how the guppy lost its spots. J. Evol. Biol. 16, 273–281. ( 10.1046/j.1420-9101.2003.00511.x) [DOI] [PubMed] [Google Scholar]
- 41.Grether GF. 2000. Carotenoid limitation and mate preference evolution: a test of the indicator hypothesis in guppies Poecilia reticulata. Evolution 54, 1712–1724. ( 10.1111/j.0014-3820.2000.tb00715.x) [DOI] [PubMed] [Google Scholar]
- 42.Godin JGJ, Briggs SE. 1996. Female mate choice under predation risk in the guppy. Anim. Behav. 51, 117–130. ( 10.1006/anbe.1996.0010) [DOI] [Google Scholar]
- 43.Reznick D, Butler MJ IV, Rodd H. 2001. Life history evolution in guppies. VII. The comparative ecology of high- and low-predation environments. Am. Nat. 157, 126–140. ( 10.1086/318627) [DOI] [PubMed] [Google Scholar]
- 44.Rodd FH, Reznick DN. 1997. Variation in the demography of guppy populations: the importance of predation on life histories. Ecology 78, 405–418. [Google Scholar]
- 45.Kohler TJ, Heatherly TN, El-Sabaawi RW, Zandona E, Marshall MC, Flecker AS, Pringle CM, Reznick DN, Thomas SA. 2011. Flow, nutrients, and light availability influence Neotropical epilithon biomass and stoichiometry. Freshwater Sci. 31, 1019–1034. ( 10.1899/11-141.1) [DOI] [Google Scholar]
- 46.Svensson E, Sinervo B, Comendant T. 2001. Density-dependent competition and selection on immune function in genetic lizard forms. Proc. Natl Acad. Sci. USA 98, 12 561–12 565. ( 10.1073/pnas.211071298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolf JB, Wade MJ. 2001. On the assignment of fitness to parents and offspring: whose fitness is it and when does it matter? J. Evol. Biol. 14, 347–356. ( 10.1046/j.1420-9101.2001.00277.x) [DOI] [Google Scholar]
- 48.Sumner FB. 1935. Studies on protective colour change. III. Experiments with fishes both as predator and prey. Proc. Natl Acad. Sci. USA 21, 345–353. ( 10.1073/pnas.21.6.345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Merilä J, Sheldon BC, Kruuk LE. 2001. Explaining stasis: microevolutionary studies in natural populations. Genetica 112, 199–222. ( 10.1023/A:1013391806317) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data used in this study have been deposited in Dryad and are accessible at http://datadryad.org/review?doi=doi:10.5061/dryad.11r1j.