Abstract
Background
The overall immunopathology of the T-helper cell (Th)-17 immune response has been implicated in various inflammatory diseases including pulmonary inflammation; however its potential role in acute respiratory distress syndrome (ARDS) is not defined. This study aimed to evaluate the Th-17 response in bronchoalveolar lavage fluid (BALF) and blood and from trauma patients with pulmonary complications.
Methods
A total of 21 severely injured intensive care unit (ICU) subjects, who were mechanically ventilated and undergoing bronchoscopy, were enrolled. BALF and blood were collected and analyzed for Th-1 (interferon [IFN]γ), Th-2 (interleukin [IL]-4, -10), Th-17 (IL-17A, -17F, -22, 23) and pro-inflammatory (IL-1β, IL-6, tumor necrosis factor [TNF]α) cytokine levels.
Results
Significant levels of the Th-17 cytokines IL-17A, -17F and -21 and IL-6 (which can be classified as a Th-17 cytokine) were observed in the BALF of all subjects. There were no significant differences in Th-17 cytokines between those subjects with ARDS and those without, with the exception of plasma and BALF IL-6, which was markedly greater in ARDS subjects, as compared with controls and non-ARDS subjects.
Conclusions
Trauma patients with pulmonary complications exhibited a significant Th-17 response in the lung and blood, suggesting that this pro-inflammatory milieu may be a contributing factor to such complications.
Keywords: Acute Lung Injury, IL-17, Acute Respiratory Distress Syndrome, Inflammation, Injury
1. Introduction
Traumatic injury is a leading cause of hospitalization and mortality and the clinical course for severely injured patients is often complicated [1–3]. Acute respiratory distress syndrome (ARDS) is a pulmonary disease state associated with capillary leak, widespread pulmonary edema, and poor oxygenation and has been reported in up to 23% of mechanically ventilated patients and 40% of burn patients admitted to trauma intensive care units (ICUs) [4, 5]. Although recent literature has suggested that the incidence of ARDS has declined over the years, the mortality in patients that do develop this complication remains high [6]. In this regard, the overall mortality in ICU patients has been shown to be 3-fold higher when ARDS was present (62%) as compared with patients that did not develop ARDS [7]. Moreover, this difference in mortality was particularly striking in trauma ICU patients who had a 56% mortality rate when ARDS was present as compared with a 13% mortality rate in the absence of ARDS [8]. While a relationship between trauma and pulmonary dysfunction has been recognized, both clinically and experimentally, the pathogenesis of trauma-induced lung injury is only partially understood.
Evidence suggests that the activation of a pro-inflammatory cascade plays an important role in this pathogenic process; however, the identification of specific mediators has been elusive. The T helper cell (Th)-17 response and its main effector cytokine, interleukin (IL)-17 have been shown to be intricately involved in non-traumatic lung diseases as well as multiple other inflammatory diseases and is linked in particular to neutrophil activation and tissue infiltration in the lung [9–11]. IL-17 also appears to be central in the regulation of the pulmonary immunoinflammatory response, where excessive IL-17 production plays a role in autoimmune disease and a lack of IL-17 leads to susceptibility to bacterial pathogens [12, 13]. Recent animal studies from our laboratory have demonstrated that lung cells after burn injury have enhanced IL-17 production, suggesting a role in the concurrent development of acute lung injury (ALI) [14]. Moreover, the T-cell subset, γδ T-cells, which are present in high numbers in epithelial rich tissue ( such as the lung) have been shown to be a source of IL-17 [15, 16]. These findings support the concept that tissue injury induces a Th-17 immunoinflammatory response that may potentially contribute to systemic complications at distal organs.
Materials and methods
2.1. Subjects and Sample Collection
A prospective observational trial was designed to enroll subjects from the surgical and trauma ICU that had severe traumatic injury and potential pulmonary complications. This study was conducted over a 1-year period and informed consent was obtained from patients and/or their legally authorized representative. Approval for this project was obtained from the University Hospital and The University of Texas Health Science Center at San Antonio Institutional Review Boards. A total of 21 severely injured and mechanically ventilated subjects were enrolled and compared to six healthy volunteers. Severe traumatic injury for this study was defined as at least one body region injury defined with an Abbreviated Injury Score (AIS) of 3 or greater. Traumatic brain injury (TBI) is known to cause immune dysregulation independent of other injury types; therefore TBI was not an inclusion criterion alone [17]. Inclusion injuries are shown in Table 1. Exclusion criteria included only those patients who fell within protected populations: those that were pregnant, less than 18 years of age, or prisoners. The subjects were enrolled and informed consent was obtained at the time of bronchoscopy. Bronchoalveolar fluid (BALF) samples were obtained by the surgical and trauma ICU team for clinical care. A minimum of 2 mL of normally discarded BALF was collected for the research team. Samples were aliquoted for storage at −70° C until analysis. A blood sample of approximately 6 mL was collected in an ethylenediaminetetraacetic acid (EDTA) tube and processed within 1 hour. Other patient information was captured in the following categories: demographics, injury severity scoring (ISS), injury mechanism, length of stay (LOS), mortality and outcomes. For comparison, 6 normal volunteers also provided a blood sample for analysis of their systemic cytokine levels.
Table 1.
Injuries for inclusion in the studya
| Region | Inclusion Injuries | AIS |
|---|---|---|
| Neck | Cervical spine fracture with persistent neurologic deficit | 4+ |
| Face | Lefort III facial fracture | 4+ |
| Lung | Pulmonary contusion in any location | 3+ |
| Chest | Rib fracture > 3 in any location | 3+ |
| Heart | Blunt cardiac injury with cardiac failure/valve involvement/septal rupture | 4+ |
| Vascular | Aortic laceration/intimal tear | 4+ |
| Abdomen | Grade 3 or greater liver, spleen, kidney, pancreas injury | 3+ |
| Pelvis | Open or displaced pelvic fracture | 3+ |
| Extremity | Open or displaced long bone fracture any location | 3+ |
| Extremity | Amputation above fingers/toes | 3+ |
Injury enrollment criteria consisted of Abbreviated Injury Scores (AISs) of 3 or greater, representing severe injury. Individuals with head injuries were enrolled, but head injury alone (in the absence of another severe injury) was not sufficient for enrollment.
2.2. Processing of BALF
Large particulate matter was removed from the BALF by passage through a 100 μm filter. The cellular and fluid fractions were separated by centrifugation (400 × g for 10 min at 4°C). Analysis of cytokine content was performed on the fluid fraction by Bioplex™ assay (Bio-Rad Laboratories, Hercules, CA).
2.3. Processing Blood Sample
Blood samples (6 mL) were collected from an existing central line into an EDTA tube. Samples were centrifuged (400 × g for 10 min at 4°C) in order to separate the plasma component, which was aliquoted and stored at −70°C until analysis for cytokine content.
2.4. Cytokine Analysis
Cytokines in plasma and BALF were analyzed using the Bio-Plex™ Pro human cytokine panel 15-plex immunoassay. Sample preparation, assay procedure, and analysis were performed according to the manufacturer’s recommendations. Cytokines analyzed were: IL-1β, -4, -6, -10, -17A, -17F, -21, -22, -23, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α. The BALF was collected during clinical diagnostic tests; therefore the amount of saline infused and collected was not standardized. For analysis the BALF cytokines were normalized against total protein content of the BALF as determined by bicinchoninic acid (BCA) assay.
2.5. Data and Statistical Analysis
All data were handled confidentially and results were stored in a secure database. The data were maintained according to institutional policy. Access to study data was limited to the research team. Protected health information was deleted from the database prior to final study analysis. All data were tested for normality as well as outliers. Demographic information was analyzed using a student’s two tailed t-test when normally distributed and via a Mann-Whitney U test when not normally distributed. All cytokine profiles failed the normality test and were thus analyzed via the Mann-Whitney U test and are represented graphically as median with first and third quartiles. Data is presented in the text as mean ± SD unless otherwise indicated.
3. Results
3.1. Subject demographics
The basic demographics of the 21 enrolled subjects are shown in Table 2. The mean age was 40.7 ± 19.7 years with a mean ISS of 31 ± 11. Eighteen of the 21 subjects (86%) were male and consisted of 43% non-Hispanic whites and 57% Hispanics or Latinos. Seventy-one percent had a chest AIS score equal to or greater than 3 and 43% had a TBI along with another injury. The subjects were enrolled in the study after the ICU team deemed a bronchoscopy was indicated for clinical reasons. In most cases this was for suspected pneumonia, therefore the hospital day in which the patient underwent bronchoscopy varied. The median hospital day when the subjects were enrolled was 5 days after admission (range 2–19).
Table 2.
Demographicsa
| All Subjects (n = 21) | Subjects without ARDS (n = 14) | Subjects with ARDS (n = 7) | |
|---|---|---|---|
| Age (years) | 40.7 ± 19.7 | 41.6 ± 20.6 | 39.0 ± 19.3 |
| Gender (% male) | 86 | 86 | 86 |
| ISS | 31 ± 11 | 33 ± 11 | 27 ± 10 |
| Hospital LOS (days) | 37.6 ± 18.8 | 28.8 ± 10.3 | 39.6 ± 26.7 |
| ICU LOS (days) | 20.6 ± 9.8 | 18 ± 7.0 | 20 ± 8.5 |
| Admission until bronchoscopy (days) | 6.6 ± 4.2 | 6.7 ± 3.3 | 6.4 ± 5.9 |
| Pa02/Fi02 (P/F) Ratio | 241 ± 94 | 278 ± 88 | 167 ± 55* |
| Previous transfusion (days prior) | 3.3 ± 2.7 | 3.3 ± 2.7 | 3.2 ± 2.7 |
| Transfusion amount (units) | 2.3 ± 0.9 | 2.2 ± 0.8 | 2.6 ± 1.1 |
| Total In (liters) | 27.1 ± 17.5 | 29.0 ± 15.8 | 23.3 ± 21.4 |
| Fluid intake/outtake (I/O) balance (liters) | 6.0 ± 5.3 | 6.0 ± 5.2 | 6.0 ± 6.0 |
The left-most column shows the demographics for all 21 enrolled subjects. Of these subjects, 14 did not have ARDS while 7 did have a diagnosis of ARDS. Data are expressed as mean ± SD.
p < 0.05 as w=compared with Subjects without ARDS.
There were 7 subjects classified as having ARDS at the time of enrollment (2 had mild ARDS, 4 had moderate ARDS, and 1 had severe ARDS, as defined by the Berlin criteria [18]) and 14 subjects did not meet criteria for an ARDS diagnosis. The average Pa02/Fi02 (P/F) ratio for those with ARDS was 167 ± 55 and the P/F ratio of those without was 278 ± 88. The P/F ratios were significantly different between the two groups, as expected, since these measurements are part of the clinical ARDS criteria [19]. Of those with ARDS, 5 subjects (71%) also had pneumonia at the time of bronchoscopy. Fourteen of the subjects did not meet ARDS criteria and 8 (57%) had pneumonia at the time of bronchoscopy.
When examining factors related to resuscitation in the subject population that developed ARDS and those that did not, there were no statistically significant differences. Both groups of subjects had the similar amounts of total crystalloid infused for resuscitation and maintenance prior to bronchoscopy (23.3L vs. 29L); both groups had similar volumes of blood transfused (2.6U vs. 2.2U) in a similar time period leading up to bronchoscopy (3.2 days prior vs. 3.3 days prior). The overall fluid balance was also identical between those with ARDS and those without (6L).
3.2. BALF cytokines
Significant levels of the Th-17 cytokines IL-17A, -17F, -21 and -22 were observed in the BALF (Fig 1A). Of these cytokines IL-21 was the highest, being approximately 5-fold greater than that of IL-17A, -17F and -22. Significant levels of the Th-1 cytokine, IFNγ and Th-2 cytokine, IL-10 were also observed in the subject’s BALF (Fig 1B); however their levels were markedly lower than that of the Th-17 cytokines. Not surprisingly, pro-inflammatory cytokines (IL-1β, IL-6, TNFα) were also present in the BALF, with IL-6 (which is also considered part of the Th-17 classification) being the greatest (Fig. 1C).
Fig. 1.
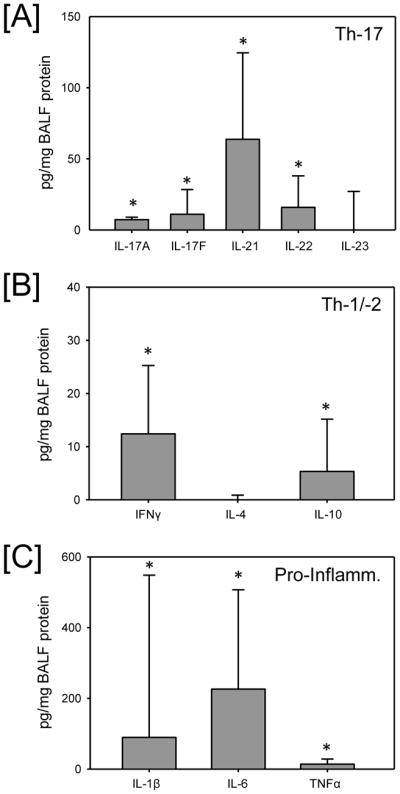
BALF cytokine levels in all subjects. BALF was collected and analyzed for cytokine content as described in the materials and methods and categorized into [A] Th-17 cytokines, [B] Th-1/Th-2 cytokines and [C] pro-inflammatory cytokines. Data are expressed as median and the 75th percentile (n=21/group). * p<0.05 as compared zero.
BALF cytokine levels between subjects without ARDS (n=14) and with ARDS (n=7) were compared (Table 3). No significant differences in cytokine levels between those subjects with ARDS and those without was observed with the exception of IL-6, which was 4-fold greater in the ARDS subjects as compared to those without ARDS (p=0.003).
Table 3.
BALF Cytokine Levelsa
| Cytokine Class | Cytokine | Non-ARDS | ARDS |
|---|---|---|---|
| Th-17 | IL-17A | 8 (6,19) | 7 (4,7) |
| IL-17F | 23 (0,67) | 11 (7,26) | |
| IL-21 | 105 (35,191) | 41 (25,86) | |
| IL-22 | 30 (13,62) | 12 (5,20) | |
| IL-23 | 0 (0,30) | 0 (0,10) | |
|
| |||
| Th-1/Th-2 | IFNγ | 22 (2,122) | 7 (7,14) |
| IL-4 | 0 (0,0) | 0 (0,2) | |
| IL-10 | 10 (4,20) | 5 (5,13) | |
|
| |||
| Pro-Inflammatory | IL-1β | 114 (18,688) | 192 (67,323) |
| IL-6 | 102 (27,404) | 469* (375,1099) | |
| TNFα | 16 (2,44) | 15 (13,31) | |
Cytokine levels were assessed in BALF of all subjects and classified into those without ARDS (non-ARDS) and those with ARDS. Cytokine levels are depicted as median with the 25 and 75 percentile (in parentheses).
p<0.05 as compared with the respective non-ARDS group.
The amount of total protein in the BALF samples was inversely correlated with the P/F ratio for the given subject, r2= 0.2, p=0.04 (Fig 2A). This correlation was markedly greater in ARDS subjects (Fig 2C; r2 = 0.327) than non-ARDS subjects (Fig. 2B; r2 = 0.044). Moreover, ARDS subjects had greater BALF total protein levels than those subjects without ARDS (median 2.0 mg/mL and 0.6 mg/mL respectively) (p=0.002).
Fig. 2.
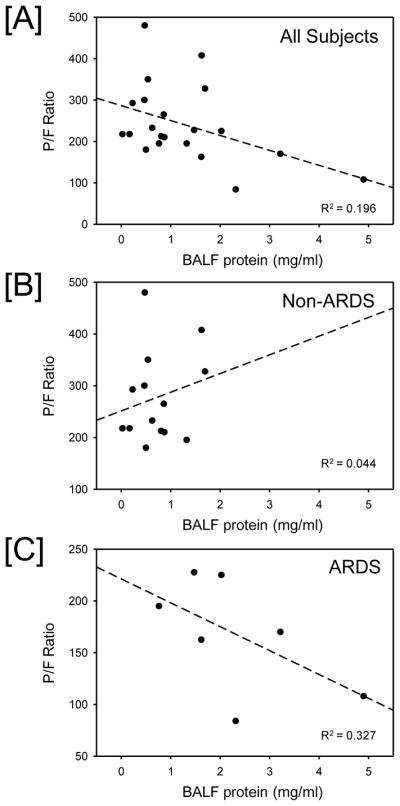
P/F ratio versus bronchoalveolar lavage fluid (BALF) protein. The relationship between P/F ratio and BALF protein content was evaluated in [A] all subjects (n = 21), [B] non-ARDS subjects (n = 14) and [C] ARDS subjects (n = 7). Significant negative correlation between P/F ratio and BALF protein was observed in all subjects and ARDS subjects.
3.3. Plasma cytokines
Cytokine levels of IL-17A, -17F, -21, and -6 were all measurable in the plasma of intubated trauma patients. IL-17A was found at the lowest detectable levels of these cytokines (median = 2 pg/mL), followed by IL-21 (median = 4 pg/mL) and IL-17F (median = 12 pg/mL). The most abundant cytokine was IL-6, which was levels of magnitude higher than the Th-17 specific cytokines, (median = 51 pg/mL). IL-22, -23 were not detected in plasma, while IFN-γ, TNF-α, IL-1β, IL-4, and IL-10 were present only at very low levels.
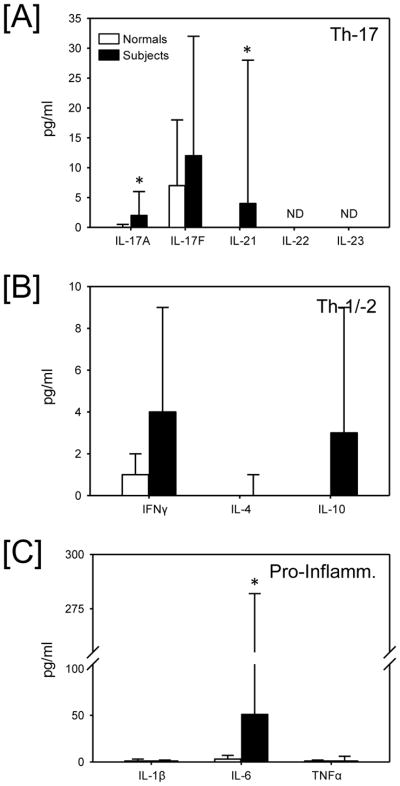
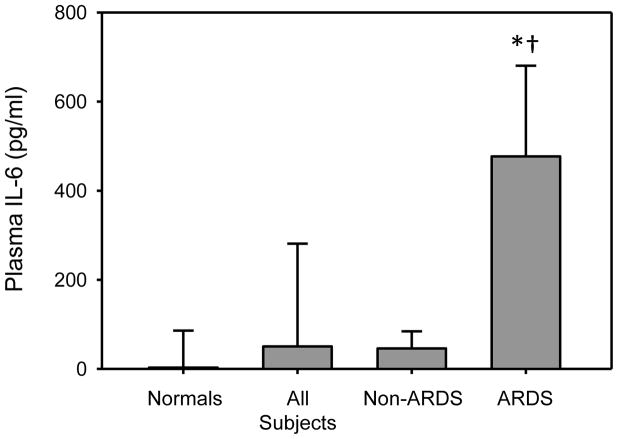
When the entire cohort of intubated trauma patients undergoing bronchoscopy is compared to healthy volunteers (i.e., normals), plasma levels of IL-17A, IL-21 (Fig 3A), and IL-6 (Fig 3C) were all significantly elevated (p<0.05). IFNγ and IL-10 plasma levels did not differ between healthy volunteers and subjects (Fig. 3B). There were no statistically significant differences in plasma cytokine levels between ARDS and non-ARDS patients, with the exception of IL-6 (Table 3). IL-6 was markedly increased in those with ARDS as compared with normals and subjects in the non-ARDS group (Fig. 4). Plasma levels of IL-17A, while low, were significantly elevated in both the non-ARDS (median = 2 pg/mL) and the ARDS (median = 3 pg/mL) subjects as compared to healthy volunteers (median = 0 pg/mL).
Fig. 3.
Plasma cytokine levels in healthy volunteers (normals) and all subjects. Plasma was collected and analyzed for cytokine content as described in the materials and methods and categorized into [A] Th-17 cytokines, Th-1/Th-2 cytokines and [C] pro-inflammatory cytokines. Data are expressed as median and the 75th percentile (n= 21/group). Normals (n = 6). Subjects (n = 21). * p<0.05 as compared with normals. ND = not detectable.
Fig. 4.
Plasma IL-6 BALF cytokine levels in healthy volunteers and subjects without ARDS and those with ARDS. Plasma was collected and analyzed for IL-6 content as described in the materials and methods. *p<0.05 as compared with normal, † p<0.05 as compared with non-ARDS.
4. Discussion
Major injury induces the activation of a complex inflammatory cascade involving pro-inflammatory cytokines (IL-1β, IL-6, TNFα), Th-1 cytokines (IFNγ), Th-2 cytokines (IL-4, IL-10) and potentially Th-17 cytokines (IL-17). In this regard the Th-17 immune response has been implicated in a number of pulmonary disease states involving neutrophil activation in lung infiltration [20–22]. Considering the implication of Th-17 profile in pulmonary disease, the current study was performed to assess the cytokine profile of BALF and blood in trauma ICU patients with suspected pulmonary complications. The findings demonstrate the development of significant pro-inflammatory and Th-17 response in both the BALF and blood of these patients. This cytokine response likely plays an important role in inflammation and the development of ARDS in this critically ill patient population.
With the publication of the Berlin Criteria, the definition of ARDS has undergone further refinement, now allowing for easier comparison between individual studies. Under this definition, ARDS in the critically ill trauma population has been reported between 8% and 82% [23]. Our cohort was defined as patients in the Trauma ICU undergoing bronchoscopy; therefore, this is a sub-population of all critically ill trauma patients. The observed ARDS rate of 33% was not surprising, as these subjects were critically ill trauma patients and mechanically ventilated with a suspected pneumonia. These two clinical insults place them at a higher risk of developing ARDS [24–26].
Somewhat surprisingly, in our study, both the ICU and hospital LOS between the ARDS and non-ARDS populations were not different. This is contradictory to previous studies reporting an increased hospital and ICU LOS in those patients with ARDS [27, 28]. This apparent discrepancy is likely due to previous studies utilizing ALI and ARDS as separate entities (under such a classification, the majority of our ARDS subjects would have been classified as having ALI). In addition, the non-ARDS subjects enrolled in this study were all intubated, resulting in a longer ICU LOS and decreasing the difference between those with ARDS and those without.
Overall, the cohort of 21 patients had a pneumonia rate of 62%. While there was a greater percentage of pneumonia in the ARDS group (71%), there were no significant differences between those with and those without pneumonia as it pertained to immune cell phenotypes or cytokine levels (data not shown). This could be in part due to the heterogeneity of enrollment day (some enrolled within 2 days of injury, while others enrolled weeks after admission). It may also speak to the overall systemic response to the injury overwhelming any noticeable change in response to a subsequent infection. Also, other infections were not obtained during the chart review and it is possible that the patients without pneumonia had another infectious source (urinary tract infection, wound infection, intra-abdominal abscess, etc.).
The factors surrounding the initial and continued resuscitation of both the ARDS cohort as well as the non-ARDS cohort were examined. The volume of initial crystalloid resuscitation was identical between the two groups. The overall volume overload was increased in the group that was enrolled later in their hospital stay; however when normalized to the length of stay, the degree of overload was similar. In addition, the timing of bronchoscopy in relation to blood product transfusion was no different and the volume of product transfused was no different. These aspects are helpful in determining that any differences, or lack thereof, were not due to differing volume balances or significant blood transfusion causing pulmonary edema.
The Th-17 response involves T-cells that produce IL-17 and has been shown to play a central role in chronic inflammation and autoimmune disorders [29, 30]. Different sources of IL-17 have been identified. These cell types include CD8 T-cells, natural killer (NK) cells, γδ T-cells and neutrophils [31] and it acts upon a wild range of cell types, which includes neutrophils, fibroblasts, epithelial cells and endothelial cells [32, 33]. Based on these observations, an important and unidentified role of Th-17 response in the development of pulmonary complications after injury likely exists. In the current study several plasma Th-17 cytokines (IL-17A, -17F, -21) were measurable in the cohort of intubated trauma patients. Overall, the levels of IL-17A were low, especially when compared with the elevation of IL-6; however they were above the detectable low limit of the assay and greater than the level in normal volunteers. This had previously been poorly characterized in a trauma population. In addition, the plasma IL-17F and -21 were elevated above that of normal volunteers and more importantly, elevated to what has been considered to be clinically relevant levels (> 45 pg/mL) [34, 35]. In contrast, IL-22 and -23 were immeasurable in plasma from either group. Given that several Th-17 cytokines were measurable at random time points during disease progression (at the time of bronchoscopy), a study that obtains longitudinal samples would help to clarify the peak of Th-17 cytokine production and its possible relation to the onset of given pulmonary complications.
While cytokine levels in the peripheral circulation served as markers of systemic illness, cytokine levels in BALF served as a marker for pulmonary-specific disease processes. In the case of BALF Th-17 cytokines, there were higher levels present locally than had been detected systemically. In addition, the two Th-17 cytokines that had not been detected in the systemic circulation (IL-22 and -23) were measurable in BALF. This suggests that the majority of this response is occurring locally at the pulmonary vasculature with only minimal systemic spillover.
The cytokine levels were elevated in the subjects with ARDS, however, so was the amount of lung leak as assessed by total protein in the BAL fluid. As expected, a lower P/F ratio corresponded with an increased amount of total protein in the BAL sample. Data is presented as both before and after normalization in order to show the effect of such a mathematical correction. One cytokine (IL-6) remained elevated in ARDS subjects as compared to non-ARDS subjects both before and after normalization. This is consistent with published literature [36, 37].
A limitation that the study was unable to overcome was the clinical nature of sample collection; the research team received varying concentrations of BALF (whether from the first wash or from subsequent washes). This can be overcome in future studies by normalizing against a pulmonary protein (such as surfactant A) or by obtaining IRB approval for research specific bronchoscopy.
In conclusion, the Th-17 response was measurable both locally (in the BALF) and systemically (in peripheral blood) in a trauma population undergoing bronchoscopy for suspected pneumonia. BALF Th-17 cytokine levels were not significantly different between non-ARDS subjects and with ARDS, however they were elevated compared to normal controls. In addition they were found in higher concentration locally in the lung than systemically. This suggests the importance of the Th-17 cytokines locally in response to a pulmonary insult (ARDS, ventilator associated pneumonia, etc.). More study is needed, with longitudinal samples as well as the addition of intubated trauma patients without pulmonary complications, in order to determine the prognostic or diagnostic values of the Th-17 cytokines in this population. However, it is proven that the response can be measured in this population. Also, in the intubated trauma population with suspected pneumonia, IL-6 was significantly elevated in BAL fluid and peripheral blood samples of ARDS subjects as compared to those without ARDS. IL-6 may play an important marker of not only systemic inflammation in general but pulmonary complication specifically.
Highlights.
Th-17 cytokine levels were significantly elevated in the BALF and blood of trauma patients
IL-6 cytokine levels in blood and BALF correlated with ARDS
Acknowledgments
Sources of funding
TLH was supported under the UTHSCSA Department of Surgery NIH T32 Training Grant NIH Grant (5T32GM079085) and the project was in part supported by NIH/NCRR UL 1RR025767, NIH-NCI P30 CA54174 and UL1RR025767.
This study was presented in part at the 42nd Annual Congress of the Society of Critical Care Medicine. The authors would like to acknowledge Dr. Linda M. McManus for providing additional supervision over the study progression as well as manuscript preparation and thank the following staff from the Division of Trauma and Emergency Surgery: Mark DeRosa, Rachelle Jonas, Janet McCarthy, Rick Sambucini, Kristin Rocchi and Qiong Zhang. The authors also thank the United States Army Institute of Surgical Research for their assistance with sample preparation and analysis: Robbie K. Montgomery, Teresa Craig and Bijaya K. Parida. The opinions or assertions contained herein are the private views of the author and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense.
Footnotes
Contributions
TLH modified study design, prepared compliance documents, collected and analyzed samples, prepared the manuscript and all statistical analysis. MR assisted in study design, data analysis, and manuscript revision. APC and RMS supervised the sample collection, assisted with data interpretation, and revised the manuscript. MGS was the principal investigator and oversaw all aspects of the study including design, interpretation of results, statistical analysis and manuscript preparation. The authors declare that they have no competing interests. All authors read and approved the final version of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Calfee CS, Eisner MD, Ware LB, Thompson BT, Parsons PE, Wheeler AP, et al. Trauma-associated lung injury differs clinically and biologically from acute lung injury due to other clinical disorders. Crit Care Med. 2007;35:2243–50. doi: 10.1097/01.ccm.0000280434.33451.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shere-Wolfe RF, Galvagno SM, Jr, Grissom TE. Critical care considerations in the management of the trauma patient following initial resuscitation. Scand J Trauma Resusc Emerg Med. 2012;20:68. doi: 10.1186/1757-7241-20-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ang DN, Rivara FP, Nathens A, Jurkovich GJ, Maier RV, Wang J, et al. Complication rates among trauma centers. J Am Coll Surg. 2009;209:595–602. doi: 10.1016/j.jamcollsurg.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trouillet JL, Chastre J, Vuagnat A, Joly-Guillou ML, Combaux D, Dombret MC, et al. Ventilator-associated pneumonia caused by potentially drug-resistant bacteria. Am J Respir Crit Care Med. 1998;157:531–9. doi: 10.1164/ajrccm.157.2.9705064. [DOI] [PubMed] [Google Scholar]
- 5.Dancey DR, Hayes J, Gomez M, Schouten D, Fish J, Peters W, et al. ARDS in patients with thermal injury. Intensive Care Med. 1999;25:1231–6. doi: 10.1007/pl00003763. [DOI] [PubMed] [Google Scholar]
- 6.Erickson SE, Martin GS, Davis JL, Matthay MA, Eisner MD. Recent trends in acute lung injury mortality: 1996–2005. Crit Care Med. 2009;37:1574–9. doi: 10.1097/CCM.0b013e31819fefdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151:293–301. doi: 10.1164/ajrccm.151.2.7842182. [DOI] [PubMed] [Google Scholar]
- 8.Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151:293–301. doi: 10.1164/ajrccm.151.2.7842182. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka S, Yoshimoto T, Naka T, Nakae S, Iwakura Y, Cua D, et al. Natural occurring IL-17 producing T cells regulate the initial phase of neutrophil mediated airway responses. J Immunol. 2009;183:7523–30. doi: 10.4049/jimmunol.0803828. [DOI] [PubMed] [Google Scholar]
- 10.Wilson RH, Whitehead GS, Nakano H, Free ME, Kolls JK, Cook DN. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am J Respir Crit Care Med. 2009;180:720–30. doi: 10.1164/rccm.200904-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.You QH, Zhang D, Niu CC, Zhu ZM, Wang N, Yue Y, et al. Expression of IL-17A and IL-17F in lipopolysaccharide-induced acute lung injury and the counteraction of anisodamine or methylprednisolone. Cytokine. 2014;66:78–86. doi: 10.1016/j.cyto.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 12.Nembrini C, Marsland BJ, Kopf M. IL-17-producing T cells in lung immunity and inflammation. J Allergy Clin Immunol. 2009;123:986–94. doi: 10.1016/j.jaci.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 13.Gu C, Wu L, Li X. IL-17 family: cytokines, receptors and signaling. Cytokine. 2013;64:477–85. doi: 10.1016/j.cyto.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oppeltz RF, Rani M, Zhang Q, Schwacha MG. Burn-induced alterations in toll-like receptor-mediated responses by bronchoalveolar lavage cells. Cytokine. 2011;55:396–401. doi: 10.1016/j.cyto.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwacha MG. Gammadelta T-cells: potential regulators of the post-burn inflammatory response. Burns. 2009;35:318–26. doi: 10.1016/j.burns.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roark CL, Simonian PL, Fontenot AP, Born WK, O’Brien RL. gammadelta T cells: an important source of IL-17. Curr Opin Immunol. 2008;20:353–7. doi: 10.1016/j.coi.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hellewell SC, Morganti-Kossmann MC. Guilty molecules, guilty minds? The conflicting roles of the innate immune response to traumatic brain injury. Mediators Inflamm. 2012;2012:356494. doi: 10.1155/2012/356494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 19.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka S, Yoshimoto T, Naka T, Nakae S, Iwakura Y, Cua D, et al. Natural occurring IL-17 producing T cells regulate the initial phase of neutrophil mediated airway responses. J Immunol. 2009;183:7523–30. doi: 10.4049/jimmunol.0803828. [DOI] [PubMed] [Google Scholar]
- 21.Wilson RH, Whitehead GS, Nakano H, Free ME, Kolls JK, Cook DN. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am J Respir Crit Care Med. 2009;180:720–30. doi: 10.1164/rccm.200904-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.You QH, Zhang D, Niu CC, Zhu ZM, Wang N, Yue Y, et al. Expression of IL-17A and IL-17F in lipopolysaccharide-induced acute lung injury and the counteraction of anisodamine or methylprednisolone. Cytokine. 2014;66:78–86. doi: 10.1016/j.cyto.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 23.Walkey AJ, Summer R, Ho V, Alkana P. Acute respiratory distress syndrome: epidemiology and management approaches. Clin Epidemiol. 2012;4:159–69. doi: 10.2147/CLEP.S28800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calfee CS, Eisner MD, Ware LB, Thompson BT, Parsons PE, Wheeler AP, et al. Trauma-associated lung injury differs clinically and biologically from acute lung injury due to other clinical disorders. Crit Care Med. 2007;35:2243–50. doi: 10.1097/01.ccm.0000280434.33451.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–93. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 26.Brun-Buisson C, Minelli C, Bertolini G, Brazzi L, Pimentel J, Lewandowski K, et al. Epidemiology and outcome of acute lung injury in European intensive care units. Results from the ALIVE study. Intensive Care Med. 2004;30:51–61. doi: 10.1007/s00134-003-2022-6. [DOI] [PubMed] [Google Scholar]
- 27.Kramer AA, Zimmerman JE. A predictive model for the early identification of patients at risk for a prolonged intensive care unit length of stay. BMC Med Inform Decis Mak. 2010;10:27. doi: 10.1186/1472-6947-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Treggiari MM, Hudson LD, Martin DP, Weiss NS, Caldwell E, Rubenfeld G. Effect of acute lung injury and acute respiratory distress syndrome on outcome in critically ill trauma patients. Crit Care Med. 2004;32:327–31. doi: 10.1097/01.CCM.0000108870.09693.42. [DOI] [PubMed] [Google Scholar]
- 29.Fouser LA, Wright JF, Dunussi-Joannopoulos K, Collins M. Th17 cytokines and their emerging roles in inflammation and autoimmunity. Immunol Rev. 2008;226:87–102. doi: 10.1111/j.1600-065X.2008.00712.x. [DOI] [PubMed] [Google Scholar]
- 30.Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11:763–76. doi: 10.1038/nrd3794. [DOI] [PubMed] [Google Scholar]
- 31.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–52. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 32.Flierl MA, Rittirsch D, Gao H, Hoesel LM, Nadeau BA, Day DE, et al. Adverse functions of IL-17A in experimental sepsis. FASEB J. 2008;22:2198–205. doi: 10.1096/fj.07-105221. [DOI] [PubMed] [Google Scholar]
- 33.Dong C. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nat Rev Immunol. 2006;6:329–33. doi: 10.1038/nri1807. [DOI] [PubMed] [Google Scholar]
- 34.Yiu HH, Graham AL, Stengel RF. Dynamics of a cytokine storm. PLoS One. 2012;7:e45027. doi: 10.1371/journal.pone.0045027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McTiernan CF, Lemster BH, Frye C, Brooks S, Combes A, Feldman AM. Interleukin-1 beta inhibits phospholamban gene expression in cultured cardiomyocytes. Circ Res. 1997;81:493–503. doi: 10.1161/01.res.81.4.493. [DOI] [PubMed] [Google Scholar]
- 36.Agrawal A, Zhuo H, Brady S, Levitt J, Steingrub J, Siegel MD, et al. Pathogenetic and predictive value of biomarkers in patients with ALI and lower severity of illness: results from two clinical trials. Am J Physiol Lung Cell Mol Physiol. 2012;303:L634–L639. doi: 10.1152/ajplung.00195.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parsons PE, Eisner MD, Thompson BT, Matthay MA, Ancukiewicz M, Bernard GR, et al. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med. 2005;33:1–6. doi: 10.1097/01.ccm.0000149854.61192.dc. [DOI] [PubMed] [Google Scholar]