Abstract
Overwhelming evidence supports the amyloid hypothesis of Alzheimer’s disease that stipulates that the relative level of the 42 amino acid β-amyloid peptide (Aβ42) in relationship to Aβ40 is critical to the pathogenesis of the disease. While it is clear that the multi-subunit gamma secretase is responsible for cleavage of the amyloid precursor protein (APP) into Aβ42 and Aβ40, the exact molecular mechanisms regulating the production of the various Aβ species remain elusive. To elucidate the underlying mechanisms, we replaced individual amino acid residues from positions 43 to 52 of Aβ with phenylalanine to examine the effects on the production of Aβ40 and Aβ42. All mutants, except for V50F, resulted in a decrease in total Aβ with a more prominent reduction in Aβ for residues 45, 48, and 51, following an every three residue repetition pattern. In addition, the mutations with the strongest reductions in total Aβ had the largest increases in the ratio of Aβ42/Aβ40. Curiously, the T43F, V44F, and T48F mutations caused a striking decrease in the accumulation of membrane bound Aβ46, albeit by a different mechanism. Our data suggest that initial cleavage of APP at the ε site is crucial in the generation of Aβ. The implicated sequential cleavage and an α-helical model may lead to a better understanding of the γ-secretase-mediated APP processing and may also provide useful information for therapy and drug design aimed at altering Aβ production.
Keywords: Alzheimer’s disease, amyloid, amyloid precursor protein processing
Abnormal generation and accumulation of the β-amyloid peptide (Aβ) is thought to be the causative event in Alzheimer’s disease (AD) pathogenesis (Hardy and Selkoe 2002). This amyloid hypothesis is specifically supported by the observations that all the mutations identified in the three AD genes [amyloid precursor protein (APP), presenilin-1 (PS1), and (PS2)] were found to cause abnormal Aβ formation, either by increasing total Aβ or increasing the ratio of Aβ42/Aβ40. Aβ is proteolytically derived from APP, which can be processed in two different pathways: the amyloidogenic pathway and the non-amyloidogenic pathway. In the amyloidogenic pathway, APP is first processed by β-secretase to produce a large, soluble ectodomain sAPPβ and a membrane-anchored C-terminal fragment (CTFβ). This CTFβ is subsequently processed by γ-secretase, resulting in the Aβ peptide and an APP intracellular domain (which is also known as CTFε as it is generated by the ε-cleavage of γ-secretase). In the non-amyloidogenic pathway, APP is first processed by α-secretase within the Aβ sequence to produce sAPPα and a membrane-associated CTFα, which undergoes further γ-secretase processing to result in the formation of p3 and CTFε. As γ-secretase cleavages generate the C-termini of Aβ and determine the ratio of Aβ42/Aβ40, the mechanism by which γ-secretase mediates processing of APP has been one of the focuses of AD research. Studying genetic, biological, and environmental factors that affect the γ-secretase-mediated cleavages of APP would provide important information on the development of treatment and prevention of the disease. In an effort to understand the molecular events of γ-secretase-mediated processing of APP, we recently identified a new ζ-cleavage site at Aβ46 between the known γ-cleavage site at Aβ40/42 and the ε-cleavage site at Aβ49 (Zhao et al. 2004). More importantly, our finding that these cleavages can be differentially inhibited by different γ-secretase inhibitors made it possible to elucidate a sequential relationship of the cleavage cascade, i.e. ε-cleavage occurs first, followed by ζ-cleavage and γ-cleavage commencing at the site closest to the membrane boundary and proceeding toward the site in the middle of the transmembrane domain of APP (Zhao et al. 2005). These findings prompted us to determine how mutations around these cleavage sites, specifically those at upstream cleavage sites, affect the formation of Aβ. It should be pointed out that, before the discovery of the new ζ-cleavage site at Aβ46, in most previous studies, the effects of genetic factors and pharmaceutical treatment on the specificity or preference of γ-secretase were determined by the formation of the final product, secreted Aβ40/42, without considering the formation of the intermediate product Aβ46. In this regard, it is especially important to determine the effects of APP mutations, which have been shown to alter the generation of secreted Aβ produced by γ-cleavage, on the upstream ε- and ζ-cleavages. In addition, precisely determining the levels of Aβ40, Aβ42 and other Aβ species produced from these interesting APP mutants is a key factor in determining the effects of APP mutations on the formation of Aβ. For this purpose, in the current study, we employed APP knockout cells to eliminate the interference of endogenous Aβ. To this end, we examined the effects of systematically engineered phenylalanine (F) mutations within the intramembrane region of multiple γ-secretase sites, which have been reported to have a pronounced effect on the generation of secreted Aβ without affecting the α-helical structure of the APP transmembrane domain (Lichtenthaler et al. 1999), on the upstream ε- and ζ-cleavages, and the formation of the intermediate long Aβ46. We found that mutations that slow down ε-cleavage, the initial cleavage of the ε-, ζ-, and γ-cleavages, result in a decrease in Aβ formation. Our data also demonstrated that F-mutations caused a progressive, stepwise reduction in total Aβ formation, and this reduction in total Aβ was observed every three residues within a region from Aβ45 to Aβ50. Furthermore, the F-mutations that caused a striking decrease in Aβ40/Aβtotal also caused a remarkable increase in the ratio of Aβ42/Aβ40 and Aβ42/Aβtotal.
Materials and methods
General reagents
γ-Secretase inhibitors, N-[N-(3,5-difluorophenacetyl)-L-alanyl]-(S)-phenylglycine methyl ester (DAPM), compound E, and L-685, 458 were from Calbiochem (San Diego, CA, USA) and were dissolved in dimethylsulfoxide (Me2SO). Aβ40 and Aβ42 were purchased from American Peptide (Sunnyvale, CA, USA). Aβ46 is a customized peptide. Monoclonal antibody 6E10, which recognizes residues 1–17 of the Aβ sequence, and polyclonal antibody anti-Aph-1α, which is specific to the C-terminal of Aph-1αL, were from COVANCE (Dedham, MA, USA). Polyclonal antibody anti-nicastrin (NCT) was developed against a peptide corresponding to the C-terminal of NCT, which was purchased from Sigma (St Louis, MO, USA). APP N-terminal-specific antibody 22C11 was from Boehringer (Mannheim, Indianapolis, IN, USA). Polyclonal antibody C15 raised against the C-terminal 15 residues of human APP has been described previously (Zhao et al. 2004). Horseradish peroxidase conjugated anti-rabbit and anti-mouse antibodies, Protein-A agarose beads, and ECL-Plus western blotting reagents were all purchased from Amersham Biosciences (Piscataway, NJ, USA).
Plasmid construction and mutagenesis
The plasmid APPsw, which expresses a C-terminal truncated Swedish mutant APP (APPsw) (Thinakaran et al. 1996), was kindly provided by Dr Gopal Thinakaran (University of Chicago). All other site-directed mutations were made using APPsw as a template and using the Site-Directed mutaGenesis Kit (Stratagene, La Jolla, CA, USA).
Establishment of APP-knockout cell line
To establish APP−/−−1 neuronal cell lines, the cerebellum was aseptically dissected from a 1-day-old APP−/−−1 mouse and placed in cold phosphate-buffered saline containing sodium pyruvate, penicillin, and streptomycin. The tissues were minced and washed before being dissociated with trypsin–EDTA. After stopping the reaction with Dulbecco’s modified Eagle’s medium (DMEM), the tissues were sheared, centrifuged at 300 g, and resuspended in DMEM. Cell viability was determined using trypan blue before seeding at 1 × 105 cells/mL in growth medium (DMEM supplemented with HEPES, glucose, L-glutamine, sodium pyruvate, 10% fetal calf serum, penicillin, streptomycin, amphotericin B, insulin, selenium, and transferrin) in tissue culture flasks pre-coated with poly-L-lysine solution. Cells were cultured for 3 days in an incubator at 37°C with 5% CO2. Cytosine arabinoside (10 μM) was then added to the culture for an additional 24 h to eliminate the dividing cells. Cells were then washed with medium and replenished with fresh medium. Visible colonies began to appear within 10–14 days after selection with cytosine arabinoside. We then immortalized this cell line by co-transfecting a plasmid (pSV40) that encodes the large T antigen alongside a second plasmid (pcDNA3.1) that encodes the hygromycin selection gene using FUGENE 6 reagent (Roche, Indianapolis, IN, USA). The transfected clones were gradually selected with increasing amounts of hygromycin. We established one long-term culture line, APP−/−−1. This cell line expresses high levels of microtubule associated protein-2 (MAP-2) but lacks nestin and β-3 tubulin. The microtubule associated protein-2 (MAP-2c, 70 kDa) isoform is expressed only in the newborn brain, and its level of expression is reduced by at least 10-fold in a 10- to 20-day-old brain. These results suggest that APP−/−−1 is likely to be derived from an early neuronal progenitor (Kang, Lamb, Wong and Sy, unpublished results).
Cell culture, transfection, and treatment
Wild type mouse neuroblastoma N2a and the APP-knockout cell lines (APP−/−−1) were grown in DMEM supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin, and 1% L-glutamine. Using the liposome-mediated method, APP−/−−1 cells were transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Six hours after transfection, the medium was replaced, and the cells were cultured for an additional 30 h in the absence or presence of γ-secretase inhibitor as indicated for each specific experiment. Then, the media were collected for analysis of the secreted Aβ and soluble N-terminal fragment of APP (sAPP). The cells were harvested for analyses of the membrane-bound Aβ, full-length APP, APP CTFs, and γ-secretase components.
ELISA, immunoprecipitation, and western blot analysis
ELISA was performed using an Aβ40-specific and Aβ42-specific ELISA kit from Invitrogen following the instructions provided by the manufacturer. Immunoprecipitation and western blot analysis were carried out as described previously (Zhao et al. 2004). Briefly, secreted Aβ was immunoprecipitated from conditioned medium (CM) using a monoclonal Aβ-specific antibody 6E10 (COVANCE). The immunoprecipitates were analyzed by 11% Bicine/urea sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) followed by western blotting. For detecting the membrane bound Aβ46 and other APP derivatives, cells were lysed in western blotting lysis buffer (50 mM Tris–HCl, pH 6.8, 8 M urea, 5% β-mercaptoethanol, 2% SDS, and protease inhibitors) and separated by a 10%–16% two-step Tris–glycine SDS–PAGE system. After the samples were transferred to a polyvinylidene fluoride membrane (Immobilon-P; Millipore, Bedford, MA, USA), the membranes were probed with specific antibodies, and the immunoreactive bands were visualized using ECL-Plus (Amersham Biosciences).
Cell-free assay
The in vitro generation of CTFε (also known as APP intracellular domain) by γ-secretase was assayed in a cell-free assay system established previously (Pinnix et al. 2001), following the procedure described by Sastre et al. (2001) with minor modifications. Briefly, to determine the effect of APPsw and its F-mutations on the generation of CTFε, APP−/−−1 cells were transiently transfected with APPsw and its F-mutations. After culturing 24 h, γ-secretase inhibitor DAPM was added, and cells were cultured for an additional 12 h. The cells were harvested in nine volumes of homogenization buffer [10 mM 4-Morpholinepropanesulfonic acid (MOPS), pH 7.0, and 10 mM KCl] containing protease inhibitors (Complete; Roche, Indianapolis, IN, USA) and homogenized by passing through a 20-gauge needle 30 times. After the removal of unbroken cells and nuclei by centrifugation at 800 g at 4°C for 10 min, membranes were pelleted by centrifugation at 20 000 g at 4°C for 30 min. The membranes were washed once with homogenization buffer and resuspended in assay buffer (150 mM sodium citrate, pH 6.4, protease inhibitor cocktail). Aliquots of membranes were then incubated at 37°C for 2 h. Reactions were stopped by addition of SDS-loading buffer. After boiling for 5 min, the samples were analyzed with a 10–16% two-step Tris–glycine SDS–PAGE system followed by western blotting using C15, an antibody specific for the APP C-terminus.
Results
Characterization of APP knockout cell line
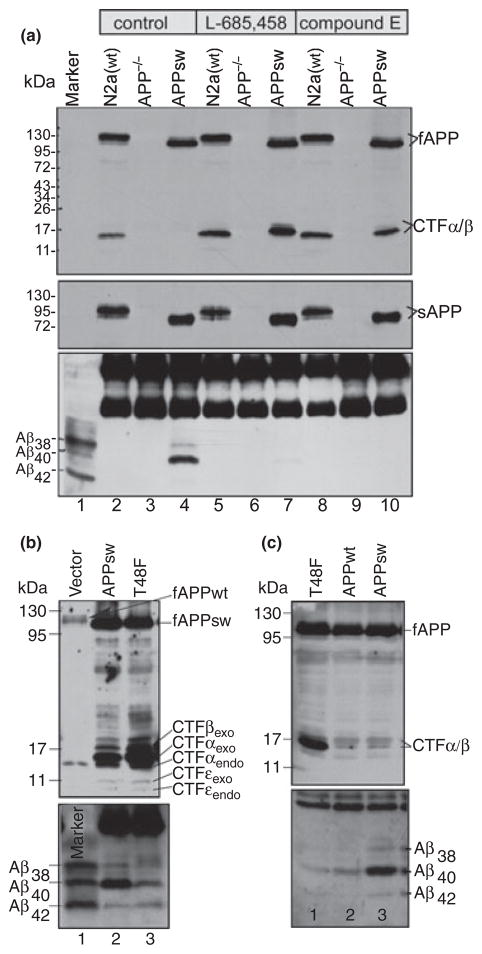
To eliminate the interference of the endogenous mouse App in our assays, we established the APP−/−−1 cell line that does not express APP (see Materials and methods). The absence of endogenous APP in this cell line was first demonstrated by RT-PCR (not shown) and then confirmed by immunoblotting. The neuroblastoma N2a cells from the wild type APP+/+ (APPwt) mouse was used as a control. As shown in the top panel of Fig. 1, when cell lysates from control cells were examined by western blot analysis using an APP C-terminal-specific antibody C15, two isoforms of endogenous APP (mainly 125 kDa and a trace amount of 110 kDa) were detected (lane 2). These proteins were not detected in the mock-transfected APP−/−−1 cells (lane 3). Lane 4 is the lysate from APP−/−−1 cells transfected with APPsw, which is a human APP with 695 amino acids containing the Swedish mutation, and is expressed with a myc tag fused to its C-terminal. As the two endogenous APP isoforms detected in the wild type N2a cells (lane 2) run more slowly than the human APP of 695 amino acids (lane 4), the two endogenous APP isoforms are apparently the APP751 of 751 amino acids and APP695 of 695 amino acids, respectively. As shown in the top panel, the APP CTF (mostly CTFα) was detected in wild type N2a cells (lane 2), but not in the APP−/−−1 cells (lane 3). As expected, when the cells were cultured in the presence of γ-secretase inhibitors, such as L-685,458 or compound E, the CTFs were accumulated to a high level (lanes 5, 7, 8, and 10). Because the CTFs produced from human APPsw contain a myc tag, they have a slower migration rate than endogenous CTFs (compare lane 7 with lane 8, top panel). It was noted that in the absence of inhibitor, a modest level of endogenous CTFα was detected (top panel, lane 2), but almost no CTFs was detected in APP−/−−1 cells transfected with APPsw (lane 4). One possible reason is that as APPsw is mostly processed by β-secretase and CTFβ is apparently a better substrate of γ-secretase, in the absence of inhibitor, almost all of the CTFβ was processed into Aβ, resulting in the undetectable CTFs.
Fig. 1.
Characterization of the APP−/−−1 cell line. (a) the APP−/−−1 cells transiently transfected with APPsw and the wild type neuroblastoma N2a cells were cultured in the presence or absence of γ-secretase inhibitor, L685, 458 or compound E (indicated on top of the figure). Total lysates and conditioned media were analyzed by 10–16% (for lysates, top panel) and 10% (for media, middle panel) SDS–PAGE and probed with antibody C15 (for lysate) and 22C11 (for media), respectively. Both endogenous and exogenous full-length APP and CTFs (top panel), and soluble APPα/β (middle panel) were detected by C15 and 22C11, respectively. The secreted Aβs immunoprecipitated from the conditioned media were separated by 11% Bicine/urea SDS–PAGE and probed with 6E10 (bottom panel). Note: a large amount of Aβ was detected only in the conditioned media of the APPsw-transfected APP−/−−1 cells cultured in the absence of any inhibitors (bottom panel, lane 4). Note: the major endogenous APP isoforms is apparently the APP751 of 751 amino acids, which is larger than the recombinant APPsw that is created from APP695 of 695 amino acids. Thus, as shown in the top and middle panels, the endogenous fAPP and sAPP run slower than their counterparts of recombinant APPsw. (b) Wild type N2a cells were transfected with empty vector (lane 1), APPsw (lane 2), and APPsw containing T48F mutation (lane 3). (c) APP−/−−1 cells were transfected with plasmid expressing Swedish mutant APPsw (lane 3), wild type APPwt (lane 2), and APPwt containing T48F mutation (lane 1).
On the other hand, when the CM from these cells were analyzed by western blotting using the APP N-terminal-specific antibody 22C11, which recognizes a region between amino acids 66 and 81 of APP (Weidemann et al. 1989; Hilbich et al. 1993), sAPP was detected in both wild type N2a cells (middle panel, lanes 2, 5, and 8) and the APP−/−−1 cells transfected with APPsw (lanes 4, 7, and 10), but not in the mock-transfected APP−/−−1 cells (lanes 3, 6, and 9). When the CM was immunoprecipitated by 6E10, which is an antibody specific for residues 1–17 of human Aβ (Kim et al. 1990), secreted Aβ38, Aβ40, and a small amount of Aβ42 were detected in cells transfected with APPsw (bottom panel, lane 4), indicating that this APP−/−−1 cell line can process APP and produce Aβ. However, when the cells were cultured in the presence of γ-secretase inhibitors (bottom panel, lanes 7 and 10), secreted Aβ was almost completely abolished. Because of the extremely low affinity of 6E10 for the murine Aβ-containing peptide (Wang et al. 1996), no secreted Aβ was detected in wild type mouse cells, regardless of the presence (bottom panel, lanes 5 and 8) or absence (bottom panel, lane 2) of γ-secretase inhibitors.
As shown in Fig. 1b, when wild type N2a cells were transfected with APPsw, similarly, a high level of secreted Aβ was observed in APPsw-expressing cells (lane 2). However, when N2a cells were transfected with APPsw containing a phenylalanine substitution for threonine at position 48 of the Aβ sequence, APPsw (T48F), a dramatic decrease in Aβ40 and a slight increase in Aβ42 were observed (compare lane 3 with lane 2, bottom panel). As a result, a high level of accumulation of CTFβ/α was observed in T48F mutant-expressing cells in comparison with that in cells expressing APPsw (compare lane 3 with lane 2, top panel). As shown in Fig. 1c, when the APP−/−−1 cells were transfected with APPwt, a much lower level of secreted Aβ was detected in comparison with that produced by APPsw-transfectant (compare lane 2 with lane 3). In addition, when the T48F mutation was introduced into APPwt, the resulting APP(T48F) mutant produced even less secreted Aβ (lane 1). Taken together, these data clearly demonstrate that the APP−/−−1 cell does not express endogenous APP, but is able to normally process exogenously expressed APP by α-, β-, and γ-secretases and produce Aβ and other APP metabolites as the wild type cells do. Therefore, to amplify the readout signal (the secreted Aβ) and to eliminate the interference of the endogenous APP, we used APP−/−−1 cells and APPsw expression constructs to study the effects of mutations within the region of γ-secretase-mediated cleavage sites on Aβ formation and the sequential ε-, ζ-, and γ-cleavage cascade.
Phenylalanine substitutions for residues flanking the ζ- and ε-cleavage site have strong effects on the turnover of the APP CTFs (α and β) and on the formation of secreted Aβ
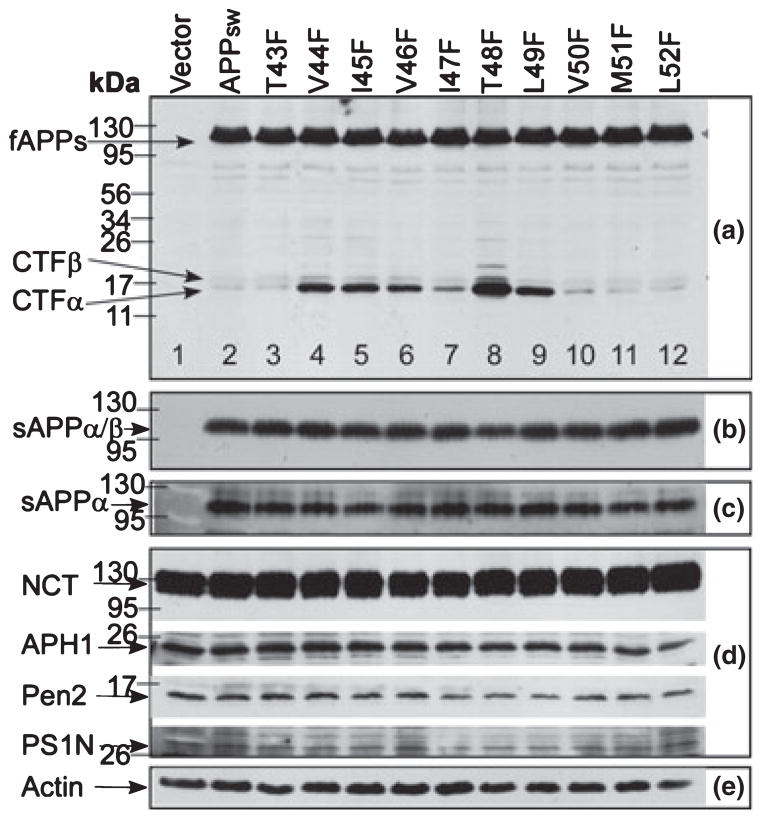
We recently reported that the C-termini of Aβ are generated by a series of sequential γ-secretase-mediated cleavages, i.e. ε-cleavage at Aβ49 followed by ζ-cleavage at Aβ46 and then by γ-cleavage at Aβ40/42 (Zhao et al. 2005). This finding prompted us to determine whether and how the mutations around the two upstream cleavage sites, namely the ζ-cleavage site and the initial ε-cleavage site, influence the sequential ε-, ζ-, and γ-cleavages and the formation of Aβ. For this purpose, a series of site-directed point mutations was created in APPsw by individually substituting F for the residues at positions Aβ43–Aβ52. APP−/−−1 cells were transiently transfected with each of the F-mutants. Immunoblotting of cell lysates of these transfectants with antibody C15 showed that equal levels of the mutant proteins were expressed in APP−/−−1 cells [panel (a) of Fig. 2]. It was noted that some of these mutants [V44F, I45F, V46F, T48F, and L49F (Fig. 2a lanes 4, 5, 6, 8, and 9)] caused an accumulation of CTFα and CTFβ (mostly CTFα). As shown in panels Fig. 2(b, c, and d), no significant difference was observed in the levels of sAPP (sAPPα and sAPPβ) nor in the γ-secretase components, such as NCT, Aph-1α (APH1), Pen-2 (PEN2), and PS-1 (PS1N) among the F-mutants. These results clearly indicate that these F-mutations have no effects on α- and β-cleavage. PS was detected using an antibody specific for the N-terminal of PS-1 (Xu et al. 2002). Actin was used as an internal loading control (panel Fig. 2e).
Fig. 2.
Effects of the substitutions of phenylalanine (F) for residues within a region of the γ-secretase multiple cleavage sites on the turnover of the APP C-terminal fragments (CTFα and CTFβ). The APP−/−−1 cells transiently transfected with APPsw and its F-mutants were cultured for 36 h. Cell lysates (panels a, d, and e) or the conditioned media (panels b and c) were separated by 10–16% or 10% SDS–PAGE, respectively, and probed with the following specific antibodies: C15 (panel a), 22C11(panel b), 6E10 (panel c), antibodies against components of γ-secretase complex (panel d: anti-NCT, anti-APH1α, anti-Pen2, and anti-PS1N), and anti-actin (panel e). 22C11 detects both sAPPα and sAPPβ (panel b); 6E10 detects sAPPα only (panel c). Lane 1 in all panels are samples from the mock-transfected APP−/−−1 cells.
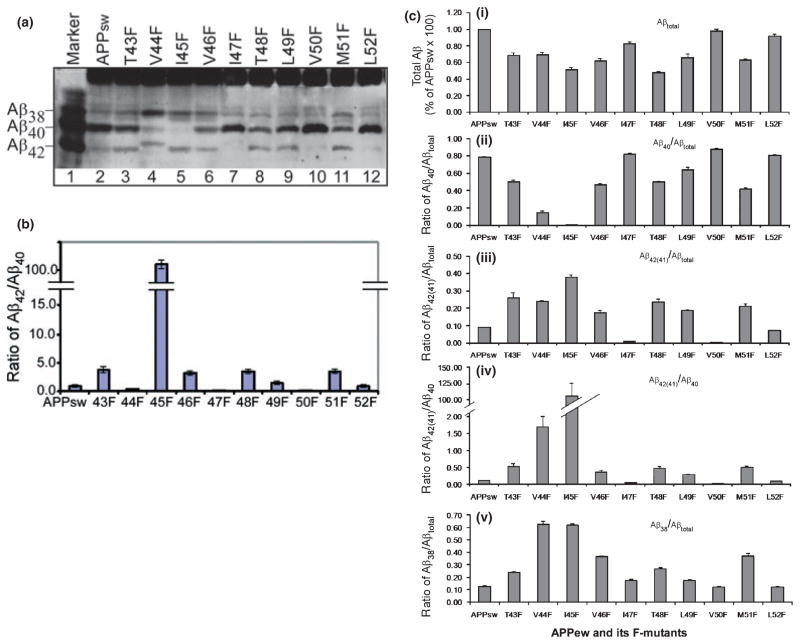
Next, the secreted Aβ produced by these transfected cells was determined by immunoprecipitation and western blot analysis. As shown in Fig. 3a, all F-mutants are processed by γ-secretase to generate secreted Aβ species, albeit with different efficiencies. It was noted that F-mutation V46F at the P1 position of the ζ-cleavage site [P positions on the substrate are counted from the point of cleavage; P1 is the first residue towards the N-terminal, and the P1′ position is the first residue towards the C-terminal side of a cleavage site. For P nomenclature, see (Schechter and Berger 1967)] and F-mutation L49F at the P1 position of the ε-cleavage site caused a significant decrease in Aβ40 and a concomitant increase in Aβ42 in comparison with cells expressing native APPsw (compare lanes 6 and 9 with lane 2). F-mutations at the P2 position of these cleavage sites, I45F and T48F, caused a further decrease in Aβ40 and a concomitant increase in Aβ42 (lanes 5 and 8). When the residues at the P1′ position of these cleavage sites, Aβ47 and Aβ50, were replaced with phenylalanine, the F-mutations I47F and V50F caused a dramatic decrease in Aβ42 (lanes 7 and 10). Interestingly, in the V44F mutant-expressing cells, instead of Aβ40 and Aβ42, significant amounts of Aβ38 and Aβ41 were detected (lane 4). A small amount of Aβ41 was also detected in cells expressing I47F mutant. Aβ38 was detected in all cell lines. However, in cells, which express F-mutants that caused an increase in Aβ42 (or Aβ41), a higher level of Aβ38, sometimes both Aβ38 and Aβ39, was detected. There are also a band likely Aβ37 detected in most of the cells, but a high level of Aβ37 was detected in the F-mutant cells that produced higher level of Aβ40 (such as V50F, lane 10). These observations suggest a product–precursor relationship between Aβ38(39) and Aβ42(41) and between Aβ37 and Aβ40.
Fig. 3.
Effects of the substitutions of phenylalanine (F) for residues within a region of the γ-secretase multiple cleavage sites on the formation of secreted Aβ. (a) The APP−/−−1 cells transiently transfected with APPsw and its F-mutants were cultured for 36 h. The secreted Aβs immunoprecipitated from conditioned media were separated on 11% Bicine/urea SDS–PAGE and probed with 6E10. Lane 1 is the mixture of standard Aβ38, Aβ40, and Aβ42 markers. (b) Ratio of Aβ42/Aβ40 determined by ELISA (representative of three repeated experiments). (c) Graphs represent the average of the results of densitometic analysis of three repeated western blots shown in (a). The density of each band was measured, and the ratios were calculated using the Gel Digitizing Software UN-SCAN-IT (Silk Scientific, Orem, UT, USA). Data from the ratio of total Aβ (panel i); Aβ40/Aβtotal (panel ii); Aβ42(41)/Aβtotal (panel iii); Aβ42(41)/Aβ40 (panel iv); and Aβ38/Aβtotal (panel v) are expressed as the mean ± SEM, n = 3. Note that the V44F mutant produced Aβ41 instead of Aβ42. The amount of Aβ generated was normalized to the expression level of each particular APP variant.
We next carried out a quantitative analysis of the effects of these F-mutations on the generation of the secreted major Aβ species Aβ40 and Aβ42 using commercially available ELISA specific for Aβ40 and Aβ42 (Invitrogen). As shown in Fig. 3b, these F-mutations had different effects on the ratio of Aβ42/Aβ40. The highest ratio of Aβ42/Aβ40 was observed in cells transfected with I45F mutant. As this ELISA kit specifically detects only Aβ40 and Aβ42, the quantitative analysis of total Aβ and also the next abundant species Aβ38 were quantified by densitometric analysis of the western blot results shown in Fig. 3a. The results are summarized in Fig. 3c. First, as shown in panel (iv) of Fig. 3c, except for mutant V44F, the effects of these F-mutants on the ratio of Aβ42/Aβ40 was observed as a pattern exactly the same as the one shown in Fig. 3b, indicating that the densitometric analysis is equally as accurate as ELISA. As shown in Fig. 3a, in some of the mutant-expressing cells (specifically mutants V44F and I47F), instead Aβ42, Aβ41 was detected, we defined the y-axis as Aβ42(41)/Aβ40; this accounts for the only difference between Fig. 3b and panel (iv) of Fig. 3c. As shown in Fig. 3c, most of the F-mutations, except V50F, caused a decrease in the total Aβ [Fig. 3c, panel (i)]. Among them, the mutations that caused a strong decrease in Aβ40 also caused a strong decrease in total Aβ. Furthermore the F-mutations from V50F to T48F caused a progressive stepwise reduction in total Aβ formation. A similar progressive reduction in total Aβ formation was also observed from I47F to I45F and from L52F to M51F. Interestingly, a similar interval repetition (every three residues) of the progressive reduction in the ratio of Aβ40/Aβtotal was also observed from L52F to I45F [Fig. 3c, panel (ii)]. In contrast, as shown in panel Fig. 3c(iii), an opposite interval repetition of the progressive decrease in the ratio of Aβ42(41)/Aβtotal was observed from I45F to L52F [Fig. 3c, panel (iii)]. A similar progressive decrease in the ratio of Aβ42(41)/Aβ40 [Fig. 3c, panel (iv)] and Aβ38/Aβtotal [Fig. 3c, panel (v)] was observed as well. On the other hand, the effects of T43F and V44F mutations did not follow the interval repeating pattern [Fig. 3c, panels (i–v)]. Interestingly, it was noted that substitution of phenylalanine at position Aβ44 resulted in the formation of Aβ41 instead of Aβ42 (Fig. 3a, lane 4). Aβ40 was also dramatically decreased, and Aβ38 became the major species of the secreted Aβ. Moreover, it was noted that Aβ41 is different from Aβ40, which is mainly secreted before being further processed. However, Aβ41 is more like Aβ42 and is further processed into Aβ38 rather than being secreted. Therefore, we grouped Aβ41 with Aβ42 rather than Aβ40 in Fig. 3c. The T43F mutation caused a slight increase in Aβ42, but a decrease in Aβ40 (Fig. 3a, lane 3).
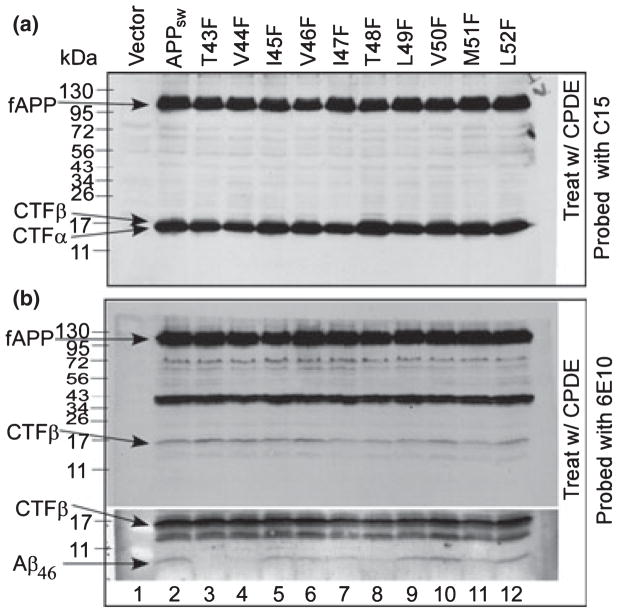
T48F, T43F, and V44F mutations caused a significant decrease in the formation of Aβ46
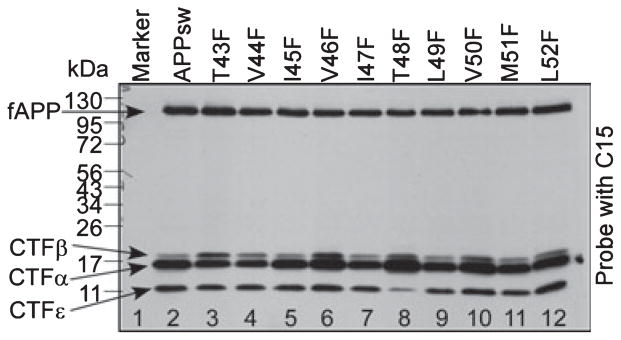
As reported previously, Aβ40 and Aβ42 are produced from CTFβ mediated by the formation of their intermediate precursor Aβ46 (Zhao et al. 2005, 2007); next, we examined the effects of these F-mutants on the formation of Aβ46. To do so, mutants expressing APP−/−−1 cells were treated with γ-secretase inhibitor compound E, which inhibits the turnover of Aβ46 and causes an accumulation of CTFα/β (Zhao et al. 2004). As shown in panel (a) of Fig. 4, in the presence of compound E, accumulation of CTFα/β was observed in all APP variants (lanes 2–12). As shown in the bottom of panel (b) of Fig. 4, when cells were cultured in the presence of compound E, a significant amount of membrane-bound Aβ46 was detected in cells expressing APPsw (lane 2) and in most of the F-mutant-expressing cells, except the cells expressing T43F (lane 3), V44F (lane 4), and T48F (lane 8) mutants, in which Aβ46 level was very low. In addition, a significant decrease in Aβ46 was observed in cells expressing I47F (lane 7) and M51F (lane 11) mutants, and a modest decrease in Aβ46 was observed in cells expressing I45F (lane 5), V46F (lane 6), and L49F (lane 9) mutants. The Aβ46 level remained unchanged in V50F and L52F mutant-expressing cells (lanes 10 and 12).
Fig. 4.
The effects of γ-secretase inhibitor compound E on the formation of Aβ46 from APPsw and its F-mutants. The APP−/−−1 cells transiently transfected with APPsw and its F-mutants were treated with γ-secretase inhibitor compound E for 12 h. Cell lysates were analyzed by 10–16% SDS–PAGE and probed with C15 (panel a) or 6E10 (panel b). Lane 1 includes the lysates of APP−/−−1 cells transfected with pcDNA3.1 vector only. Lanes 2–12 are the lysates of APP−/−−1 cells transfected with APPsw and its F-mutants as labeled on top of the figure. The bottom section in panel (b) is the longer exposure of the lower half of the upper section to visualize the membrane-bound Aβ46.
T48F mutation significantly reduced efficiency of ε-cleavage
We next performed a cell-free assay to determine how these F-mutants affect the upstream ε-cleavage. APP−/−−1 cells transfected with a control APPsw or F-mutants were cultured in the presence of the γ-secretase inhibitor DAPM for 12 h [DAPM has been used for accumulating CTFα/β in previous studies (Zhao et al. 2005, 2007)]. Membranes were prepared, and the cell-free assays were carried out as described in the Materials and methods. As shown in Fig. 5, when these membranes were incubated at 37°C for 2 h, a significant amount of CTFε was detected in the membranes prepared from all cells except cells expressing T48F mutant. In spite of the high level of CTFα/β accumulated, only a very low level of CTFε was detected in the membranes prepared from T48F mutant-expressing cells (lane 8), indicating that this T48F mutant is not efficiently processed by γ-secretase at the ε-cleavage site. This low efficient upstream ε-cleavage may, at least in part, account for the very low level of Aβ46.
Fig. 5.
T48F mutation inhibits ε-cleavage. A cell-free assay was performed as described in the Materials and methods. Reaction mixtures were separated by 10–16% SDS–PAGE followed by western blot analysis.
T43F and V44F mutants are less sensitive to γ-secretase inhibitor compound E
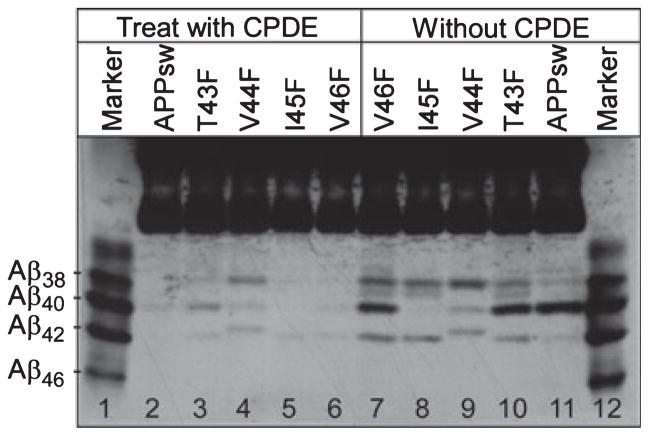
As shown in panel (b) of Fig. 4, almost no detectable Aβ46 was observed in cells expressing T43F (lane 3) or V44F (lane 4) mutants in the presence of compound E, which is known to inhibit the turnover of Aβ46. As these mutations did not cause a reduction of CTFε (Fig. 5), the low level of Aβ46 must not be because of the inhibition of upstream ε- and ζ-cleavages, as seen in the case of the T48F mutant. One possibility is that, when these mutations are present, compound E may be unable to block the γ-cleavage at Aβ40/42, and as a result, the intermediate Aβ46 is further processed into Aβ40/42. To test this possibility, APP−/−−1 cells were transfected with APPsw, T43F, V44F, I45F, and V46F (APPsw, I45F, and V46F were used as controls) and then cultured in the presence or absence of compound E. The secreted Aβ was immunoprecipitated from the CM of each cell line. As shown in Fig. 6, in the absence of an inhibitor, secreted Aβ was detected in all transfectants (lanes 7–11). When the cells were cultured with compound E, almost no secreted Aβ was detected in cells expressing APPsw (lane 2), I45F (lane 5), and V46F (lane 6) mutants. However, a significant amount of each of the secreted Aβ species (Aβ38, Aβ40, Aβ41, and Aβ42) was detected in the media of cells expressing T43F (lane 3) and V44F (lane 4) mutants. These results indicate that the Aβ formation from these mutants is less sensitive to the inhibitory effects of compound E.
Fig. 6.
The T43F and V44F mutants are less sensitive to γ-secretase inhibitor compound E. APP−/−−1 cells transfected with APPsw, T43F, V44F, I45F, or V46F were cultured in the presence (lanes 2–6) or absence (lanes 7–11) of compound E for 24 h. The secreted Aβs were immunoprecipitated with 6E10 from the conditioned media, analyzed by 11% Bicine/urea SDS–PAGE, and probed with 6E10.
Discussion
Characterization of APP knockout cell line
A key factor in determining the effects of APP mutations on the formation of Aβ or in understanding the preference of γ-secretase cleavage is to precisely determine the levels of Aβ40, Aβ42, and other Aβ species produced from these interesting APP mutants. In most previous studies, these kinds of experiments were carried out in a model using cells that express endogenous APP. However, the most widely used methods, including ELISA and western blotting, are based on the use of specific antibodies that cannot distinguish the Aβ produced from exogenous mutant APP from that of endogenous APPwt. To eliminate the interference of endogenous APP, we established an APP knockout (APP−/−) cell line, APP−/−−1. As shown in Fig. 1, in APP−/−−1 cells neither full-length APP nor its derivatives were detected by APP-specific antibodies. The results presented in Fig. 1 also demonstrate that when the APP−/−−1 cells were transfected with APPsw, the exogenous APP was processed normally by all the α-, β-, and γ-secretases. Specifically, it was clearly demonstrated that secreted Aβ species were produced from the exogenous APPsw and that the production of secreted Aβ and the turnover of CTFα and CTFβ were inhibited by γ-secretase inhibitors. Hence, the APP−/−−1 cell line is a useful tool for studying the mechanism of γ-secretase-mediated Aβ formation. Using this model, we examined the effects of mutations within the region of the γ-secretase cleavage sites on the processing of APP and the generation of Aβ.
Phenylalanine-mutations within the region of multiple γ-secretase sites have different effects on the formation of different Aβ species, but follow an ordered pattern
We and others recently identified long forms of Aβ species Aβ46 (Zhao et al. 2004; Qi-Takahara et al. 2005). The identification of the long Aβ species led to the discovery of a new ζ-cleavage site at Aβ46 between the known γ-cleavage site at Aβ40/42 and ε-cleavage site at Aβ49 (Zhao et al. 2004). Specifically, the finding that some of the known γ-secretase inhibitors can block the formation of short forms of Aβ40/42 and cause a concomitant accumulation of the long form of Aβ46, suggests a possible precursor–product relationship between Aβ46 and Aβ40/42 (Zhao et al. 2004). Indeed, the precursor–product relationship between Aβ46 and Aβ40/42 has been experimentally determined using the differential inhibition strategy (Zhao et al. 2005) and further confirmed by the pulse-chase labeling experiment (Zhao et al. 2007). These observations established a sequential cleavage model, i.e. the C-terminus of secreted Aβ is generated by γ-secretase via a series of sequential cleavages, first by ε-cleavage at Aβ49, followed by ζ-cleavage at Aβ46 and then γ-cleavage at Aβ40/42 (Fig. 7) (Zhao et al. 2005, 2007). These events commence at the site closest to the membrane boundary and proceed toward the site in the middle of the membrane domain of APP (Zhao et al. 2005). Thus, the altered generation of secreted Aβ caused by some factors may be a result of influencing upstream cleavages. However, before the discovery of the new ζ-cleavage site at Aβ46, in most previous studies, the effects of genetic mutations on the formation of Aβ were determined by the detection of the final product, Aβ40/42, without considering the formation of the intermediate product, Aβ46. In this regard, it is especially important to determine the effects of APP mutations within the intramembrane region of multiple γ-secretase sites on the upstream ε- and ζ-cleavages and the formation of the intermediate long Aβ46. For this purpose, we systematically substituted individual F residues within the intramembrane region of multiple γ-secretase sites. The reason for choosing F-mutation is based on the previous observation that F-mutations have a pronounced effect on the generation of secreted Aβwithout affecting the α-helical structure of the APP transmembrane domain (Lichtenthaler et al. 1999).
Fig. 7.
Schematic illustration of APP processing and Aβ formation. The C-termini of secreted Aβ are generated by γ-secretase via a series of sequential cleavages, first by ε-cleavage at Aβ49, followed by ζ-cleavage at Aβ46 to produce the major intermediate Aβ46. Aβ46 is mainly processed at Aβ43 and the resulting Aβ43 is further processed into Aβ40, which can be further processed into Aβ37, but is principally released from the γ-secretase complex and become the major form of secreted Aβ species. Alternatively, Aβ46 can also be processed at Aβ42 at a low efficiency and the resulting Aβ42 can be either further processed into Aβ38(39) or released at a low rate. Amino acids are numbered based on Aβ sequence. The light gray represents the membrane; the dark gray shaded region is the Aβ sequence.
What have we learned from these experiments? First, the pattern of the effects of these F-mutations on the ratio of Aβ42/Aβ40 observed in this study is very similar to that observed in a previous study (Lichtenthaler et al. 1999). In addition, we observed that almost no Aβ40 was detected in I45F mutant-expressing cells and that instead of Aβ42, Aβ41 was detected in V44F mutant-expressing cells, similar to previous observations (Lichtenthaler et al. 1999). However, there are also some significant differences between the current and previous studies. First, our data clearly demonstrated that most of the F-mutations caused a decrease in the levels of total Aβ and some of them, such as I45F and T48F, reduced total Aβ up to 50% (Fig. 3a and c). These observations are different from a previous study, in which no significant differences in the level of total Aβ were observed in these F-mutants (Lichtenthaler et al. 1999). One possibility is that COS7 (African Green Monkey SV40-transf’d kidney fibroblast cell line) cells were used in the previous study, and the endogenous Aβ, which is identical to human Aβ, may have interfered with the detection and quantification of Aβ produced from cells transfected with F-mutant APP. Secondarily, in the previous study, it was reported that a very low amount of Aβ was detected in cells transfected with the L52F mutant (Lichtenthaler et al. 1999), and this has been imputed to the possible rapid degradation of the precursor protein A4CT-L52F, in which the large aromatic phenylalanine at the membrane boundary position might interfere with the correct membrane insertion of A4CT-L52F (Lichtenthaler et al. 1999). However, our data clearly demonstrated that the L52F mutation caused only slight reduction in total Aβ (< 10%, Fig. 3c). These differences might have resulted from different constructs being used in these two studies. In the previous study, the plasmid SPA4CT, which expresses CTFβ with a signal peptide, was used to create the F-mutations and transfect COS7 (African Green Monkey SV40-transf’d kidney fibroblast cell line) cells (Lichtenthaler et al. 1999). In our study, the plasmid APPsw, which expresses full-length APPsw, was used to create the F-mutations and transfect APP−/−−1 cells. However, whether this particular L52F mutation has a different effect on the membrane insertion of these two polypeptides expressed by these two different constructs is currently not clear. Thirdly, in the previous study, a significant amount of Aβ40 was detected in V44F mutant cells (Lichtenthaler et al. 1999). However, in the current study, only a trace amount of Aβ40 was detected in V44F mutant cells (Fig. 3a). This difference may have resulted from the gel system used. The gel system used in the previous study could not separate Aβ40 from Aβ38, and this may account for the overestimation of Aβ40.
Observations from our current study and the previous study revealed some interesting findings: First, a novel finding revealed by our results was that the F-mutations at different positions caused a decrease in total Aβ to a different extent, and the effects of these F-mutations on the decrease in total Aβ followed a well-ordered pattern; i.e. a progressive, stepwise reduction in total Aβ formation was observed every three residues within a region from Aβ45 to Aβ50, and it could possibly extend to Aβ52; Second, the effects of these F-mutations on the ratio of Aβ40/Aβtotal precisely followed the same every three residues interval pattern as the total Aβ. Third, these F-mutations had opposite effects on the ratio of Aβ42/Aβ40, Aβ42/Aβtotal, and Aβ38/Aβtotal; i.e. the mutations, which caused a strong decrease in total Aβ and the ratio of Aβ40/Aβtotal, resulted in a strong increase in the ratio of Aβ42/Aβ40, Aβ42/Aβtotal, and Aβ38/Aβtotal. Fourth, T43F, V44F, and T48F mutations caused a striking decrease in the accumulation of membrane bound Aβ46 using different mechanisms as discussed below.
Mechanisms underlying the effects of F-mutations on the formation of Aβ
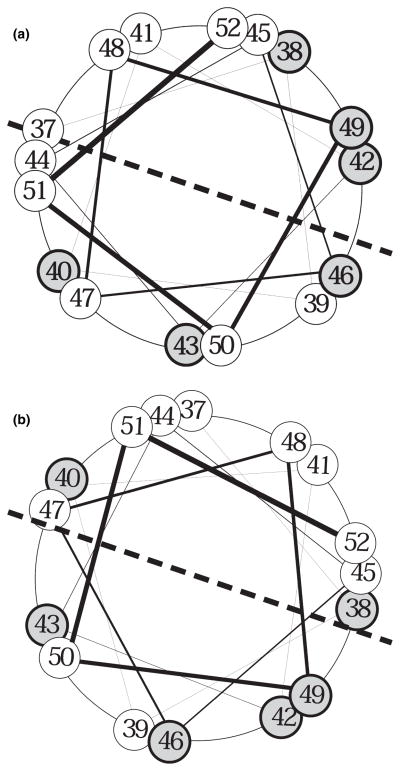
To understand the mechanism by which APP is processed by γ-secretase within its transmembrane domain, an α-helical model of the APP transmembrane domain was proposed in previous studies (Lichtenthaler et al. 1999; Wolfe et al. 1999). Based on recent findings, we adapted the α-helical model to recently suggest that the same γ-secretase is responsible for the multiple intramembrane cleavages of APP (Zhao et al. 2005, 2007). The proposed α-helical structure of the APP transmembrane domain and the single enzyme model may also provide an explanation for the every three residue repetition effect of these F-mutations on Aβ formation observed in this study. First, how do some of the F-mutations cause a strong increase in the ratio of Aβ42/Aβ40? As shown in Fig. 8a, in their native state, the peptide bonds, which are hydrolyzed by γ-secretase to produce the major intermediates Aβ46 and Aβ43, are aligned on the same side of the α-helical wheel, which may be the enzyme targeting side. This may explain the fact that the Aβ46 generated by ζ-cleavage is mostly cleaved at Aβ43 to produce Aβ43 that is further mainly processed at Aβ40 to produce Aβ40, which is the major secreted Aβ species under normal physiological conditions. However, Aβ46 may also be cleaved with lower efficiency at Aβ42. Specifically, under some circumstances, such as unknown environmental factors and mutations in both APP and PS, which may cause conformational changes, altering the relative position of the enzyme attacking site, the efficiency of cleavage at Aβ42 may be increased. For example, as shown in Fig. 8b, if this relative positional change between APP and the γ-secretase complex can be mimicked by rotating the α-helical wheel clockwise to a certain degree, the peptide bond between residues Aβ43 and Aβ42 would move toward the center of the enzyme attacking site and become more susceptible to the γ-secretase cleavage, resulting in an increase in Aβ42. When the F-mutations cause the preference shift in favor of cleavage at Aβ42, the cleavage at Aβ43 would be reduced, resulting in a decrease in Aβ40, which is formed from Aβ43. This model provides a mechanism by which F-mutations cause a striking increase in the ratio of Aβ42/Aβtotal resulting in a drastic concomitant decrease in the ratio of Aβ40/Aβtotal. In this regard, it was noted that F-mutations I45F, T48F, and M51F, which caused stronger increases in Aβ42/Aβtotal, are aligned on the same side of the α-helical wheel, providing a possible explanation for the interval (every three residues) repetition pattern of the effects of these F-mutations on Aβ formation. As the F-mutations that caused a shift in the preference of the γ-secretase cleavage may have altered the native relative position of the substrate to the enzyme, the efficiency of the γ-secretase cleavage may have been reduced. This reduced cleavage efficiency may account for the fact that F-mutations that caused a striking increase in the ratio of Aβ42/Aβ40 also caused a marked reduction in total Aβ.
Fig. 8.
Helical wheel arrangement of amino acids 37–52 of CTFβ, a view from the cytosol side. The C-terminal residues of the major detectable Aβ species produced by γ-secretase-mediated cleavages are shown in gray with a bold outline. (a) Note that, under normal conditions, the peptide bond between residues 46 and 47 and the peptide bond between residues 43 and 44 are aligned to the center of the lower half of the α-helical wheel, which may be the enzyme attacking site. It is noted that the peptide bond between Aβ39 and Aβ40 is also aligned to the center of the lower half of the wheel. However, under normal conditions, the Aβ40 resulting from cleavage at Aβ40 is rapidly released from the γ-secretase complex, the peptide bond between Aβ39 and Aβ40 has less chance to be cleaved. In contrast, under some circumstances, such as environmental or genetic abnormalities, specifically, in the presence of mutations in presenilin or in APP, the relative position between the γ-secretase active site and its substrate may be altered during the assembly of the γ-secretase complex and its substrate. (b) If these changes in the position of CTFβ relative to the γ-secretase active site can be mimicked by rotating the CTFβ α-helical wheel clockwise 60°, the peptide bond between Aβ42 and Aβ43 will move toward the center of the lower half of the wheel and become much more susceptible to γ-secretase activity, resulting in an increase in the formation of Aβ42.
It was noted that the striking increase in the ratio of Aβ42/Aβ40 is mostly due to the marked reduction in Aβ40 rather than an increase in the level of Aβ42. One possibility for the fact that striking decrease in Aβ40 is not accompanied with significant increase in Aβ42 is that as Aβ42 is longer than Aβ40, which is mostly released with limited further processing into Aβ37 (see Fig. 2a, lanes 10 and 12, for example), most of the Aβ42 is not released from the γ-secretase complex immediately after being formed, but is further processed mostly into Aβ38 (possibly also into Aβ39, see Fig. 2a, lanes 5 and 11) as proposed in a previous study (Zhao et al. 2007). This conclusion is also supported by our finding that the modest increase in Aβ42 was always accompanied by an increase in Aβ38 or Aβ39. This may also be explained by the α-helical wheel model, in which the peptide bond between residues Aβ43 and Aβ42 and the peptide bond between residues Aβ39 and Aβ38 are aligned on the same side. If the mutations render the peptide bond between residues Aβ43 and Aβ42 more susceptible to γ-secretase cleavage, the peptide bond between residues Aβ39 and Aβ38 would also become more susceptible to γ-secretase cleavage. Accordingly, the V44F mutation may have caused a further relative conformational change between substrate and enzyme by rotating the α-helical wheel to a position at which the peptide bond between residues Aβ42 and Aβ41 can be cleaved by γ-secretase; accordingly, the peptide bond between residues Aβ39 and Aβ38 would also become more susceptible to γ-secretase cleavage, leading to the formation of Aβ41 and even more Aβ38 (Fig. 2a, lane 4).
In contrast to the V44F, I45F, and T48F mutations, which caused a decrease in the ratio of Aβ40/Aβtotal and in the level of total Aβ, mutations I47F, V50F, and L52F, which had no significant effects in the level of total Aβ and the ratio of Aβ40/Aβtotal but caused a decrease in the ratio of Aβ42/Aβtotal, may just have the opposite effect on the relative conformation between substrate and enzyme, by rotating the α-helical wheel counterclockwise. This change would push the peptide bond between Aβ42 and Aβ43 further away from the center of the enzyme-attacking site, resulting in a decrease in Aβ42 and its derivative Aβ38 or Aβ39.
The other possibility that may account for the effects of certain F-mutations on the cleavage preference shift from Aβ40 to Aβ42 is the model proposed by a recent study that indicates there are two product lines: one is a major line that starts with the formation of Aβ49 with subsequent turnover of Aβ49 into Aβ46 to Aβ43 to Aβ40; the other line is started with the formation of Aβ48 with subsequent turnover of Aβ48 to Aβ45 to Aβ42 to Aβ38 (Qi-Takahara et al. 2005). However, as shown in Fig. 8b, when the peptide bond between Aβ42 and Aβ43 is aligned to the center of the enzyme-attacking site, the peptide bond between Aβ48 and Aβ49 is still not in the favorable position. Furthermore, even in the case of an F-mutation, such as I45F mutation, that caused a striking increase in the Aβ42/Aβ40 ratio, the intermediate detected is Aβ46 rather than Aβ45 (Fig. 4), indicating that the formation of Aβ45 is not necessary for the formation of Aβ42. Therefore, even the two product lines model cannot be ruled out, it may not be the major mechanism that accounts for the increase in Aβ42 caused by the F-mutations tested in this study.
As mentioned above, it was found that T43F, V44F, and T48F mutations caused a striking decrease in the accumulation of membrane bound Aβ46 using different mechanisms. The observations that T48F mutation caused an increase in the accumulation of CTFα/β (Fig. 2a) as well as a dramatic decrease in CTFε production (Fig. 5), strongly suggest that this T48F mutation causes reduction in the bound intermediate Aβ46 most likely by inhibiting the ε-cleavage, which is the initial and rate determining step of the γ-secretase-mediated sequential cleavage cascade (Zhao et al. 2005).
On the other hand, T43F and V44F mutations did not inhibit the generation of CTFε, indicating that these mutations have no effect on the upstream ε-cleavage. In contrast, the observation that secreted Aβ was detectable in cells expressing these mutations even in the presence of compound E (Fig. 6) strongly suggests that these mutants are no longer sensitive to compound E, which block the turnover of the intermediate Aβ46 by inhibiting the downstream γ-cleavage at Aβ40/42 (Zhao et al. 2005). One possible reason for this insensitivity is that the introduction of this large amino acid at these positions obstructed compound E from approaching the γ-cleavage site, either by causing a local conformational change between the substrate and enzyme or by functioning as a barrier; as a result, Aβ46 was further processed into secreted Aβ species.
I47F and M51F mutations also caused a modest decrease in the accumulation of membrane-bound Aβ46 (Fig. 4). The fact that no significant reduction of CTFε was observed in a cell-free assay (Fig. 5) suggests the modest reduction in Aβ46 is not due to the inhibition of ε-cleavage as T48F mutation does. In addition, secreted Aβ was not detected in the presence of compound E (data not shown). These observations suggest that the mechanism causing the decrease in accumulation of membrane-bound Aβ46 in I47F and M51F is different from the ones accounting for the effects of T43F, V44F, and T48F mutations. One possible reason is that these mutations, by themselves, may have no inhibitory effect on the ε-cleavage. However, in the presence of these mutations, the inhibitor compound E, which usually does not inhibit the formation of Aβ46, may exhibit an inhibitory effect on the ε-cleavage and, as a result, block the formation of Aβ46.
It has been proposed previously that γ-secretase may contain a PS dimer as the core of the enzyme (Schroeter et al. 2003). Whether this PS dimer may provide more than one catalytic site and account for the multiple cleavages of APP transmembrane domain remain to be determined.
Acknowledgments
This work was supported by Grants from NIH (R01AG026640) and the Alzheimer’s Association (IIRG-05-14584) to XX and Grants from NIH (R01HL074341) to M-ZC.
Abbreviations used
- AD
Alzheimer’s disease
- AICD
APP intracellular domain
- APP
amyloid precursor protein
- APPsw
Swedish mutant APP
- APPwt
wild type APP
- Aβ
β-amyloid peptide
- CM
conditioned media
- CTF
C-terminal fragment
- DMEM
Dulbecco’s modified Eagle’s medium
- NCT
nicastrin
- PS
presenilin
- sAPP
soluble APP
- SDS-PAGE
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
References
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hilbich C, Monning U, Grund C, Masters CL, Beyreuther K. Amyloid-like properties of peptides flanking the epitope of amyloid precursor protein-specific monoclonal antibody 22C11. J Biol Chem. 1993;268:26571–26577. [PubMed] [Google Scholar]
- Kim KS, Wen GY, Bancher C, Chen CMJ, Sapienza VJ, Hong H, Wisniewski HM. Detection and quantitation of amyloid B-peptide with 2 monoclonal antibodies. Neurosci Res Commun. 1990;7:113–122. [Google Scholar]
- Lichtenthaler SF, Wang R, Grimm H, Uljon SN, Masters CL, Beyreuther K. Mechanism of the cleavage specificity of Alzheimer’s disease gamma-secretase identified by phenylalanine-scanning mutagenesis of the transmembrane domain of the amyloid precursor protein. Proc Natl Acad Sci USA. 1999;96:3053–3058. doi: 10.1073/pnas.96.6.3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinnix I, Musunuru U, Tun H, Sridharan A, Golde T, Eckman C, Ziani-Cherif C, Onstead L, Sambamurti K. A novel gamma-secretase assay based on detection of the putative C-terminal fragment-gamma of amyloid beta protein precursor. J Biol Chem. 2001;276:481–487. doi: 10.1074/jbc.M005968200. [DOI] [PubMed] [Google Scholar]
- Qi-Takahara Y, Morishima-Kawashima M, Tanimura Y, et al. Longer forms of amyloid beta protein: implications for the mechanism of intramembrane cleavage by gamma-secretase. J Neurosci. 2005;25:436–445. doi: 10.1523/JNEUROSCI.1575-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre M, Steiner H, Fuchs K, Capell A, Multhaup G, Condron MM, Teplow DB, Haass C. Presenilin-dependent gamma-secretase processing of beta-amyloid precursor protein at a site corresponding to the S3 cleavage of Notch. EMBO Rep. 2001;2:835–841. doi: 10.1093/embo-reports/kve180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter I, Berger A. On the size of the active site in proteases. I Papain. Biochem Biophys Res Commun. 1967;27:157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- Schroeter EH, Ilagan MX, Brunkan AL, et al. A presenilin dimer at the core of the gamma-secretase enzyme: insights from parallel analysis of Notch 1 and APP proteolysis. Proc Natl Acad Sci USA. 2003;100:13075–13080. doi: 10.1073/pnas.1735338100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thinakaran G, Teplow DB, Siman R, Greenberg B, Sisodia SS. Metabolism of the “Swedish” amyloid precursor protein variant in neuro2a (N2a) cells. Evidence that cleavage at the “beta-secretase” site occurs in the Golgi apparatus. J Biol Chem. 1996;271:9390–9397. doi: 10.1074/jbc.271.16.9390. [DOI] [PubMed] [Google Scholar]
- Wang R, Sweeney D, Gandy SE, Sisodia SS. The profile of soluble amyloid beta protein in cultured cell media. Detection and quantification of amyloid beta protein and variants by immunoprecipitation-mass spectrometry. J Biol Chem. 1996;271:31894–31902. doi: 10.1074/jbc.271.50.31894. [DOI] [PubMed] [Google Scholar]
- Weidemann A, Konig G, Bunke D, Fischer P, Salbaum JM, Masters CL, Beyreuther K. Identification, biogenesis, and localization of precursors of Alzheimer’s disease A4 amyloid protein. Cell. 1989;57:115–126. doi: 10.1016/0092-8674(89)90177-3. [DOI] [PubMed] [Google Scholar]
- Wolfe MS, Xia W, Moore CL, Leatherwood DD, Ostaszewski B, Rahmati T, Donkor IO, Selkoe DJ. Peptidomimetic probes and molecular modeling suggest that Alzheimer’s gamma-secretase is an intramembrane-cleaving aspartyl protease. Biochemistry (Mosc) 1999;38:4720–4727. doi: 10.1021/bi982562p. [DOI] [PubMed] [Google Scholar]
- Xu X, Shi YC, Gao W, Mao G, Zhao G, Agrawal S, Chisolm GM, Sui D, Cui MZ. The novel presenilin-1-associated protein is a proapoptotic mitochondrial protein. J Biol Chem. 2002;277:48913–48922. doi: 10.1074/jbc.M209613200. [DOI] [PubMed] [Google Scholar]
- Zhao G, Mao G, Tan J, Dong Y, Cui MZ, Kim SH, Xu X. Identification of a new presenilin-dependent ζ-cleavage site within the transmembrane domain of amyloid precursor protein. J Biol Chem. 2004;279:50647–50650. doi: 10.1074/jbc.C400473200. [DOI] [PubMed] [Google Scholar]
- Zhao G, Cui MZ, Mao G, Dong Y, Tan J, Sun L, Xu X. γ-Cleavage is dependent on ζ-cleavage during the proteolytic processing of amyloid precursor protein within its transmembrane domain. J Biol Chem. 2005;280:37689–37697. doi: 10.1074/jbc.M507993200. [DOI] [PubMed] [Google Scholar]
- Zhao G, Tan J, Mao G, Cui MZ, Xu X. The same gamma-secretase accounts for the multiple intramembrane cleavages of APP. J Neurochem. 2007;100:1234–1246. doi: 10.1111/j.1471-4159.2006.04302.x. [DOI] [PubMed] [Google Scholar]