Summary
Social defeat occurs when an animal is attacked and subjugated by an aggressive conspecific. Following social defeat, male Syrian hamsters fail to display species-typical territorial aggression and instead exhibit submissive or defensive behaviors even when in the presence of a non-aggressive intruder. We have termed this phenomenon conditioned defeat (CD). The mechanisms underlying CD are not fully understood, but data from our lab suggest that at least some of the mechanisms are similar to those that mediate classical fear conditioning. The goal of the present experiment was to test the hypothesis that noradrenergic signaling promotes the consolidation of CD, as in classical fear conditioning, by determining whether CD is disrupted by post-training blockade of noradrenergic activity. In Experiment 1, we determined whether systemic infusions of the noradrenergic receptor antagonist propranolol (0, 1.0, 10, or 20 mg/kg) given immediately after a 15 min defeat by a resident aggressor would impair CD tested 48h later. Hamsters that were given immediate post-training infusions of propranolol (1.0, but not 10 or 20 mg/kg) showed significantly less submissive behavior than those given vehicle infusions supporting the hypothesis that there is noradrenergic modulation of social defeat consolidation. In Experiment 2, we demonstrated that propranolol (1.0 mg/kg) given immediately, but not 4 or 24h, after defeat impaired CD tested 48h after defeat indicating that the window within which the memory for social defeat is susceptible to beta-adrenergic modulation is temporary. In Experiment 3, we examined whether central blockade of noradrenergic receptors could recapitulate the effect of systemic injections by giving an intracerebroventricular infusion of propranolol immediately after defeat and examining the effect on CD 24 h later. Centrally administered propranolol (20 μg/3μl but not 2 μg/3μl) was also effective in dose-dependently reducing consolidation of CD. Collectively, the present results indicate that noradrenergic activity promotes the consolidation of CD and suggest that CD is a valuable model to study the processes by which emotion and stress modulate memory in an ethologically relevant context. These data also suggest that the popular conception in the clinical literature that the anxiolytic effect of propranolol is primarily due to the drug’s peripheral effects may need to be reconsidered.
Keywords: Memory consolidation, Beta-blockers, Beta-adrenergic receptors, Social Defeat, Fear conditioning, Norepinephrine
1. Introduction
Social defeat is a potent stressor that occurs when an animal is attacked and subjugated by an aggressive conspecific. Syrian hamsters are solitary animals that display territorial aggression against intruding conspecifics when singly housed under laboratory conditions (Albers et al., 2006; Nowack and Paradiso, 1983; Takahashi and Miczek, 2014). Following social defeat, however, Syrian hamsters fail to display species-typical territorial aggression and instead exhibit submissive or defensive behaviors even when in the presence of a non-aggressive intruder (Huhman et al., 2003; Murphy, 1976; Potegal et al., 1993). This phenomenon is termed conditioned defeat (CD). Social defeat is considered a potent stressor because the effects of an initial defeat are profound and long lasting, and defeated hamsters exhibit activation of the hypothalamic-pituitary-adrenal (HPA) axis (Huhman et al., 2003). Specifically, exposure to agonistic encounters produces increases in plasma adrenocorticotropin (ACTH) and glucocorticoids in defeated but not in dominant hamsters (Huhman et al., 1990; Huhman et al., 1991; Huhman et al., 1992). Furthermore, defeated animals exhibit increased blood pressure and heart rate and compromised immune function in comparison to dominant animals (Blanchard et al., 1995; Bohus et al., 1983; Jasnow et al., 2001). CD is long-lasting; following social defeat, 100% of defeated hamsters exhibit a total absence of territorial aggression and increased submissive/defensive behavior in the presence of smaller, non-aggressive intruders. This response lasts for at least 10 days without further social defeat (Huhman et al., 2003). In fact, for a majority of the defeated animals, CD lasts at least 33 days even without a further social defeat experience (Huhman et al., 2003).
Noradrenergic activity plays a role in anxiety-like processes and is important for stress-related changes in behavior (Bremner et al., 1996; Chen et al., 2012; Morilak et al., 2005). Beta-adrenergic antagonists are widely prescribed, albeit “off-label”, in anxiety disorders such as social phobia (Brunello et al., 2000; Davidson, 2006), posttraumatic stress disorder (Giles, 2005; Kent et al., 2002; Vaiva et al., 2003), and panic disorder (Hirschmann et al., 2000; Sullivan et al., 1999). In addition, beta-blockers reduce acute stage fright (Brantigan et al., 1982; Davidson, 2006), test anxiety (Faigel, 1991), and contextual fear (Grillon et al., 2004) in humans. In rodents, beta-adrenergic antagonists also decrease anxiety (Angrini et al., 1998; Audi et al., 1991; Gorman and Dunn, 1993; Stern et al., 2008; Walker and Davis, 2002), reduce fear conditioning (Chou et al., 2014) and prevent behavioral changes caused by repeated stress (Camp et al., 2012).
Extensive evidence from both human and animal studies indicate that catecholamines released peripherally and centrally during emotional arousal play a role in the consolidation of emotional experiences (Cahill and McGaugh, 1998; McGaugh, 2013). For example, post-training infusions of the stress hormone epinephrine, which is released by the adrenal medulla, enhance memory in a time- and dose-dependent manner in a variety of learning and memory tasks (Ferry and McGaugh, 2000; Gold et al., 2013; McGaugh and Roozendaal, 2002; Roozendaal et al., 2006; Roozendaal et al., 2008). Interestingly, epinephrine-induced memory enhancement is reversed or impaired by removal of the adrenal medulla or by beta-adrenergic receptor antagonists in rodents (McGaugh, 1989; Quirarte et al., 1997; Roozendaal et al., 2006). Similarly, beta-adrenergic receptor antagonists prevent both the memory-enhancing effect of arousal in humans and rodents (Cahill et al., 1994; Kindt et al., 2014; Nielson and Jensen, 1994; van Stegeren et al., 1998) as well as stress-induced impairments in extinction learning (Fitzgerald et al., 2015).
Despite the importance of catecholamines in stress responses and emotional memory consolidation, there is limited research examining the putative roles of noradrenergic transmission in conditioned responses to natural threats such as social defeat. The goal of the present set of experiments was to test the hypothesis that noradrenergic transmission is involved in the consolidation of CD by determining whether CD is susceptible to post-training manipulations of noradrenergic systems. Specifically, Experiment 1 determined whether immediate post-defeat, systemic infusions of the beta-adrenergic antagonist propranolol would dose-dependently impair CD tested 48h after the defeat. If noradrenergic activity is involved in the consolidation of CD, then its effects should be restricted to the time period immediately following the social defeat. To test this, Experiment 2 examined the time-dependence of this post-training effect. Because propranolol effectively crosses the blood brain barrier (Botterblom et al., 1993; Neil-Dwyer et al., 1981), Experiment 3 was designed to determine whether the effect of propranolol observed in Experiment 1 could be due, at least in part, to an action of the drug in the central nervous system. In this experiment, we microinjected propranolol into the lateral ventricle immediately after defeat.
2. Materials and Methods
2. 1 Subjects
Adult male Syrian hamsters (Mesocricetus auratus; Charles River, Wilmington, MA) weighing 120-130g (63-70 days) upon arrival were used in this study (Experiment 1 n=80; Experiment 2 n=50; Experiment 3 n=32; individual group n’s are indicated in the figures). Animals were housed in the animal facility for one week before the beginning of any manipulation (surgery and/or single housing, as indicated below). Thus, behavioral testing began a minimum of two weeks after arrival. Additional hamsters weighing 180g on average were used as resident aggressors (RA) for CD training, and hamsters weighing 90-100g on arrival were used as nonaggressive intruder stimulus animals during behavioral testing. All hamsters were housed in polycarbonate cages (20 × 40 × 20 cm) with wire mesh tops in a climate-controlled room (70-74 °F), and food and water was available ad libitum. Subjects and resident aggressors were housed individually, whereas nonaggressive intruders were group housed (five hamsters/cage) to minimize aggressiveness. The hamsters were maintained on a 14:10 h light:dark cycle with light off at 1100h, and all training and testing occurred during the first 3h of the dark phase of the daily light:dark cycle. All procedures and protocols involving hamsters were approved by the Georgia State University Institutional Animal Care and Use Committee and were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, revised 1978).
2.2 Conditioned Defeat (CD)
All subjects were single-housed for 7-10 days before the beginning of CD training during which time they were handled four to five times. Hamsters were matched by weight and randomly assigned to experimental or control groups. On the day of CD training, all hamsters were transported from the colony room to the behavioral testing room and were allowed to acclimate to the testing room for at least 30 min. CD training/acquisition consisted of a single resident/intruder pairing in which a subject was placed in a resident aggressor’s home cage for 15 min. During the 15 min defeat session, experienced observers ensured that subjects were routinely attacked by the resident aggressor and that they displayed submissive and defensive behaviors towards this opponent. In the few cases wherein the resident aggressors did not attack within the first 2 min of the defeat session (n = 4, Experiment 1; n = 5, Experiment 2), the subject was immediately moved into the cage of another resident aggressor so that all animals experienced a social defeat. Resident aggressors were used a maximum of two times during any particular day to minimize variability in their behavior due to repeated testing.
Testing for CD began 48h (+/− 1h) after training for Experiments 1 and 2, and 24h (+/− 1 hr) after training for Experiment 3. The extra time was allotted in the first two experiments to ensure that the peripherally administered drug would have ample chance to be metabolized fully before CD testing (Liu et al., 2005). During testing, a non-aggressive intruder was placed into the home cage of the defeated subject for 5 min. All testing sessions were recorded and scored by observers blind to experimental condition using Noldus Observer (version 4; Noldus Information Technology, Wageningen, Netherlands). The following classes of behaviors were recorded as total duration in seconds during the 5 min testing session: (1) Non-social: locomotor/exploratory, self-groom, nesting, feeding, sleeping, (2) Social: attend, approach, investigate, sniff, touching nose, (3) Submissive/defensive: upright/side defense, tail lift, teeth chatter, flee, full submissive posture, and (4) Aggressive: upright/side offense, chase, bite, attack.
2.3 Surgery (Experiment 3)
One week after arrival, animals were initially anesthetized with a 5% concentration of isoflurane to oxygen. Maintenance of the surgical plane of anesthesia occurred at a 2.5-3% concentration of isoflurane, and this maintenance was verified by the lack of a withdrawal of the paw in response to toe-pinch. Animals were placed into the stereotaxic apparatus and the skull was exposed. Bregma and lambda were leveled and a unilateral cannula guide was placed into either the left or right side, aimed at the lateral ventricle with the following coordinates: 0.5 mm A/P, ± 1.4 mm M/L, and 2.0 mm D/V (measuring from dura). A wound clip was attached to the skull posterior to the cannula guide to stabilize the mount and then dental cement was used to anchor the guide in place. During the surgery, animals received an s.c. injection of 1.0 ml of 0.9% saline and 5 mg/kg ketoprofen to restore hydration and to provide pain relief. An obturator was placed into the cannula guide after surgery to maintain patency. After at least 2 days of recovery, animals were then handled each day for 5 days by gently holding the animal in the experimenter’s hand and unscrewing the obdurator, moving it up and down, and screwing the obdurator back onto the cannula guide. Animals were weighed on the last day of handling and assigned to one of the three weight-matched groups.
2.4 Drug Injections
2.4.1 Experiment 1: effects of immediate post-defeat injections of propranolol on CD
Immediately after CD training, hamsters were given systemic infusions of propranolol (0.0, 1.0, 10, or 20 mg/kg IP in sterile 0.9% saline). The doses of propranolol were selected based on previous studies investigating the effects of systemic injections of propranolol in preventing stress-induced death in hamsters (Matsuoka et al., 1998) and inhibiting fear learning and memory in rats (Cain et al., 2004; Do Monte et al., 2008; Introini-Collison et al., 1994; Przybyslawski et al., 1999; Rodriguez-Romaguera et al., 2009; Schneider et al., 2000; Simson et al., 2001; Walker and Davis, 2002). In an effort to minimize the number of animals used in the present study, we did not include a group of animals given propranolol but not defeated. Given the dose and time specificity (see below) of the propranolol effect obtained, as well as the fact that it was given after defeat training, we felt that propranolol was highly unlikely to have an effect on submissive behavior that is independent of social defeat.
2.4.2 Experiment 2: time course of propranolol effect on CD
The same procedures were used as in Experiment 1 with the exception that after CD training, hamsters were given either an immediate or a delayed (4 or 24h) systemic injection of vehicle (0.9 % sterile saline, IP) or the effective dose of propranolol (1.0 mg/kg) as established in Experiment 1.
2.4.3. Experiment 3: effect of immediate post-defeat injection of propranolol given intracerebroventricularly on consolidation of CD
Hamsters received microinjections of 0.0, 2.0, or 20 μg propranolol in 3 μl 0.9% sterile saline into the lateral ventricle immediately following defeat. These doses were selected based on previous studies where i.c.v. administration of 2 μg propranolol decreased LTP after high-frequency tetanization in rats (Seidenbecher et al., 1997) and 20 μg propranolol blocked the increase in corticosterone caused by i.c.v. norepinephrine (Bugajski et al., 1991). To administer an intracerebroventricular dose after defeat, animals were gently restrained in the experimenter’s hand, the obturator was removed, and a 1.2 mm projection needle was inserted into the cannula guide. This needle was attached to PE-50 tubing filled with water. A 0.2 ul air bubble separated the water and the drug or saline, and the tubing was attached to a 5-ul Hamilton syringe. The animal was then placed into a small cage where it could move freely during injection. A Harvard apparatus infusion pump was used to slowly administer 3 ul of solution over a 1-minute period. The injection needle was left in place for an additional 1 minute for diffusion of the drug, after which the needle was removed from the guide cannula, the obturator was replaced, and the animal was returned to its own cage. A successful injection was verified by movement of the air bubble down the tubing during infusion. Animals were tested for CD 24 hours after defeat/injection as described above. Following the completion of the study, animals were euthanized with sodium pentobarbital and microinjected with 3 ul India ink into the cannula guide to verify successful cannula placement in the lateral ventricle.
2.5 Statistical Analyses
The behavioral data were expressed as means and standard error of the means (S.E.M.). The aggression, submission, and some social data were not normally distributed; therefore, the non-parametric Kruskal-Wallis and Mann-Whitney U tests were used to detect differences between groups. The non-social behavioral data were normally distributed and were analyzed using One-Way Analysis of Variance (ANOVA). Significance was ascribed as p<0.05.
3. Results
3.1 Experiment 1: immediate post-defeat injections of propranolol impair the consolidation of CD in a dose-dependent manner
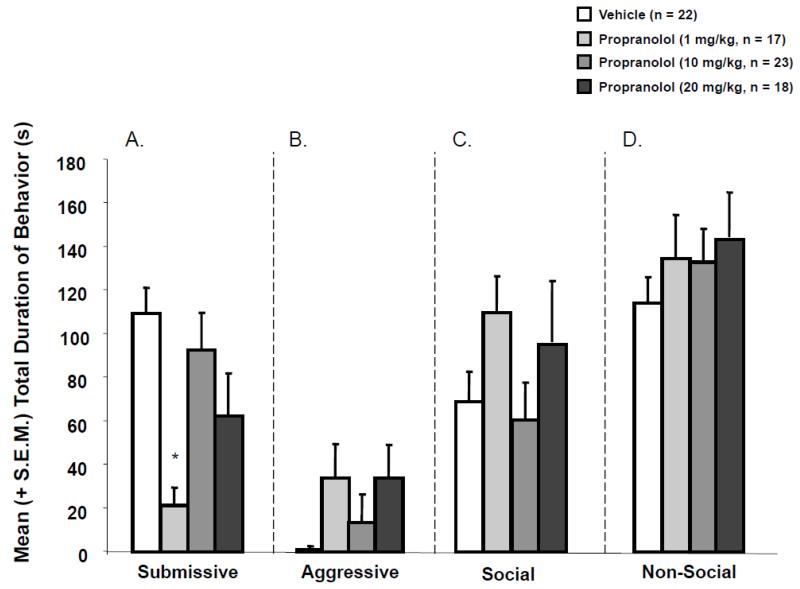
Propranolol infusion given IP immediately following defeat training significantly decreased submissive behavior of defeated animals [H (3) = 16.70; p <.05] (see Figure 1). The total duration of submissive behavior of hamsters given 1.0 mg/kg of propranolol was significantly lower than that of hamsters given systemic infusions of vehicle (U = 50.50, p < .01). There was no significant difference in the total duration of submissive behavior in hamsters given 10 or 20 mg/kg of propranolol and that of hamsters given infusions of vehicle (U = 172.5, p >.05 and U = 105, p>.05, respectively). Additionally, immediate post-defeat injections did not significantly effect aggressive [H (3) = 4.64; p >.05], social [H (3) =2.09; p >.05], or non-social [F (3,76) =.22; p >.05] behaviors (see Figure 1).
Figure 1.
Mean (+/−S.E.M.) total duration of (A) submissive, (B) aggressive, (C)social, and (D) non-social behaviors exhibited by defeated animals during the 5 min test with a non-aggressive intruder. Immediate post-defeat infusions of propranolol (1.0 mg/kg) significantly decreased the mean duration of submissive behavior (*p < .05, vs. saline controls) but did not affect aggressive, social, or non-social behavior.
3.2 Experiment 2: post-defeat injections of propranolol impair the consolidation of CD in a time-dependent manner
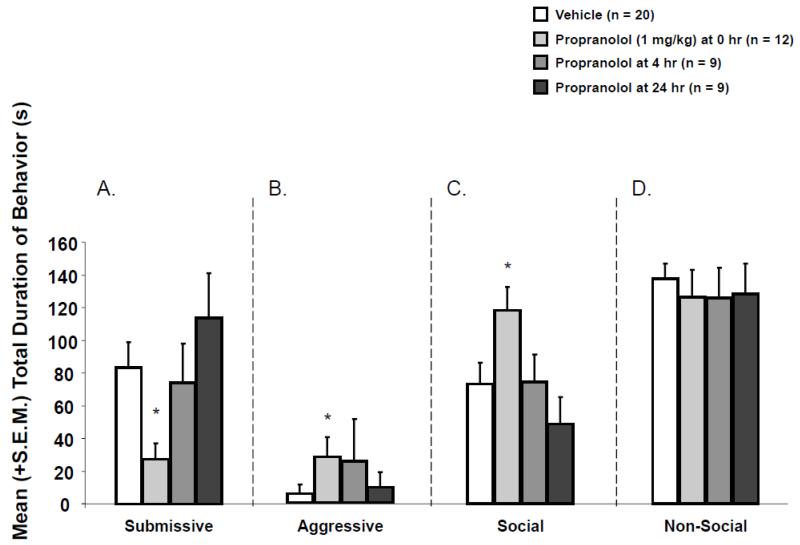
Infusions of vehicle given 0, 4, or 24h post-defeat did not significantly affect aggression [H (2) = 1.71, p > .05], submission [H (2) = 1.37, p > .05], social [H (2) = 2.11, p > .05], or non-social [F (2,17) = .20, p > .05] (data not shown). Therefore, these vehicle control groups were collapsed into one control group in order to increase statistical power. Post-defeat drug infusions significantly altered aggression [H (3) = 10.09; p < .05], submission [H (3) = 13.96; p < .05], and social behavior [H (3) = 9.89; p < .05] (see Figure 2) but did not significantly affect non-social behavior [F (3,46) = 1.15; p > .05] (see Figure 2). Immediate post-defeat injections of propranolol significantly decreased submissive behavior (U = 35, p < .05), but increased aggressive [U = 70, p < .05] and social [U = 57, p < .05] behavior in defeated hamsters. Injections of propranolol 4h post-defeat did not affect aggression [U = 72; p > .05], submission [U = 80; p >.05], or social [U = 87.5; p > .05] behavior in defeated hamsters. Similarly, injections of propranolol 24h post-defeat did not affect aggression [U = 80; p > .05], submission [U = 71; p > .05], or social [U = 69.5; p > .05] behaviors in defeated hamsters.
Figure 2.
Mean (+/−S.E.M.) total duration of (A) submissive, (B) aggressive, (C) social, and (D) non-social behaviors exhibited by defeated animals during the 5 min test with a non-aggressive intruder. Immediate post-defeat infusions of propranolol (1.0 mg/kg) significantly decreased the mean duration of submissive behavior (*p < .05, vs. saline controls) and significantly increased aggressive (*p < .05, vs. saline controls) and social (*p < .05, vs. saline controls) behavior. Infusions of propranolol 4 or 24h after CD training did not significantly affect the mean total duration of submissive, aggressive, social, or non-social behaviors (p > .05, vs. saline controls).
3.3 Experiment 3: immediate post-defeat, intracerebroventricular infusion propranolol impairs consolidation of CD in a dose-dependent manner
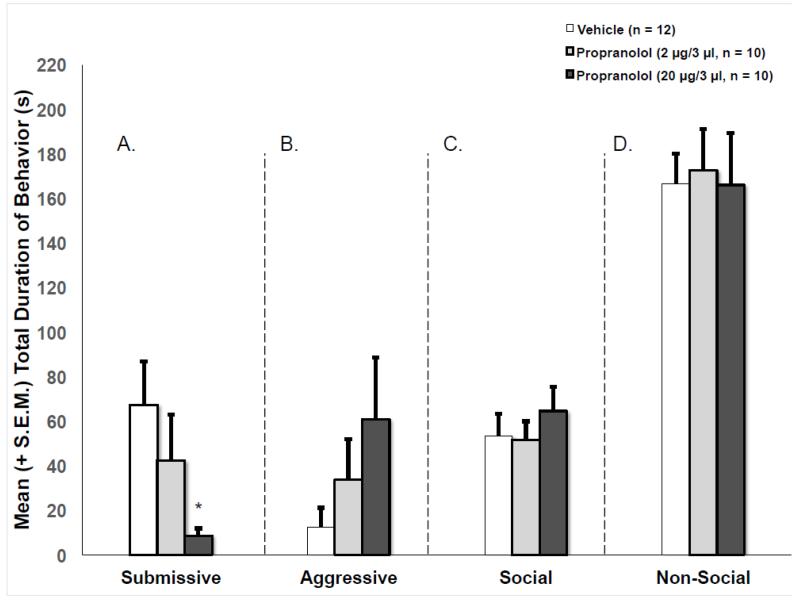
Post-defeat, intracerebroventricular infusion of 20 μg propranolol had a significant effect on submission [H (2) = 7.14; p < .05] but did not affect aggression [H (2) = 3.027; p > .05], social [F (2,29) = 0.488; p > .05] or non-social [F (2,29) = .036; p > .05] behavior. Immediate post-defeat injection of 20 μg of propranolol significantly reduced the duration of submission [U = 20.5; p < .01] exhibited by defeated hamsters during testing (see Figure 3). There was no effect of the lower dose (2 μg) of propranolol on any behavior exhibited during testing as compared to vehicle control.
Figure 3.
Mean (+/−S.E.M.) total duration of (A) submissive, (B) aggressive, (C) social, and (D) non-social behaviors exhibited by defeated animals during the 5 min test with a non-aggressive intruder. Immediate post-defeat infusions of 20 μg, but not 2 μg, propranolol significantly reduced submissive behavior (*p < .05, vs. saline controls).
4. Discussion
These experiments demonstrate that systemic post-defeat infusions of the beta-blocker propranolol impair the consolidation of CD in Syrian hamsters in a dose- and time-dependent manner and that this impairment can be mimicked by delivery of propranolol directly into the central nervous system. Specifically, the present results show that a post-defeat infusion of propranolol impairs CD and that this impairment of CD is observed only when propranolol is given immediately after the defeat, but not when it is given 4 or 24h post-defeat. This result indicates that the window within which the memory for social defeat is susceptible to beta-adrenergic modulation is less than 4h under these conditions. In the final experiment, we determined that propranolol infused into the lateral ventricle after defeat also significantly reduced CD, demonstrating that the effect of systemically administered propranolol on stress- or fear-related memory may have been due, at least in part, to an effect of propranolol within the brain.
Several aspects of the propranolol effect are potentially interesting. First, each time we repeated the systemic treatment, it appeared that there would be an inverted u-shaped dose-response curve for the effect of propranolol on submissive/defensive behavior. This apparent effect never reached significance, however, despite the fact that the manipulation was repeated several times, the same pattern emerged each time, and the group n’s, particularly in Experiment 1, were very high. It is possible that we would have obtained a significant effect if an additional, higher dose of propranolol were included, but given the fact that the receptor specificity of the drug at high doses would have been in question, it is not clear what would have been gained by this addition. Another interesting observation is that doses of propranolol that appeared to increase aggression also stimulated social behavior. This is not surprising in that we have consistently observed across many years of work that social behavior increases as social avoidance decreases and that a return to species typical territorial aggression closely follows an increase in social behavior.
CD is a profound behavioral change in defeated hamsters that is characterized by a total absence of species typical territorial aggression accompanied by a pronounced increase in submissive and defensive behavior (Potegal et al., 1993). In our past work, we have often found that pharmacological manipulations that are effective in altering the amount of submission do not concomitantly alter aggression, indicating that separate circuits may regulate particular aspects of the behavioral profile that we call CD. It also suggests and that we know less about how defeat inhibits aggression than we do about how it stimulates submission. Interestingly, propranolol treatment appeared to reliably increase aggressive behavior similarly in all three experiments (although only significantly in Experiment 2) suggesting that this treatment stimulates a more complete reinstatement of species-typical territorial behavior. It would be interesting to determine if the aggression-stimulating effect of systemic propranolol is recapitulated following systemic administration a beta-adrenergic drug that does not cross the blood-brain barrier. Such a manipulation would be needed to establish definitively that the change in behavior is dependent on central nervous system blockade of beta-adrenergic receptors. Noradrenergic receptor antagonists have previously been shown to induce maternal aggression when given in the lateral septum (Scotti et al., 2011) as well as to block the aggression-reducing effect of chronic variable stress (Zebrowska-Lupina et al., 1997). In the latter study, peripherally administered noradrenergic drugs that crossed the blood-brain barrier (e.g., propranolol) were shown to be more effective than were drugs that did not cross the blood-brain barrier (e.g., acebutolol). Together, these findings strongly suggest that the effect of noradrenergic manipulations on aggression is mediated centrally, although more research is needed to determine where centrally the effects of social defeat on aggression are mediated. Previous research from our lab demonstrated that infusion of a GABAA receptor agonist (Luckett et al., 2012) or a dopamine receptor antagonist (Gray et al., 2015) into the nucleus accumbens before testing also restored aggression in previously defeated hamsters, so it is possible that the nucleus accumbens in a component of this circuit. Finally, we have demonstrated that the pharmacological inactivation of the lateral septum using muscimol also increases aggression in previously defeated hamsters, but it is important to note that this effect was limited to expression and not acquisition of CD and muscimol in the lateral septum increased aggression independent of whether the animals had been previously defeated or not (McDonald et al., 2012).
The mechanisms underlying CD learning, and indeed even the critical stimuli for this conditioning, are not fully understood. It is possible that the acquisition of CD involves aspects of both Pavlovian and instrumental fear conditioning. Pavlovian fear conditioning entails the contingent pairing of a neutral conditioned stimulus (CS), such as a tone, with an aversive unconditioned stimulus (US), such as a footshock, that elicits a reflexive or unconditioned response (UR), such as freezing. Through multiple CS-US pairings, the CS comes to elicit conditioned fear responses (CR). In contrast, instrumental fear conditioning involves an aversive stimulus, such as social defeat, that is paired contingently with an animal’s response, such as flight. Although CD may mimic some aspects of Pavlovian fear conditioning, it is clear that the potential CSs change fairly dramatically from training to testing in CD. Specifically, the hamster is defeated in the home cage of a larger resident aggressor during CD training, but then it is tested in its own home cage with a smaller, non-aggressive intruder. The present finding that post-training infusions of propranolol impair the consolidation of CD is consistent with the finding that post-training infusions of propranolol impair both Pavlovian contextual fear conditioning (Camp and Johnson, 2015; Grillon et al., 2004; Ji et al., 2003) and instrumental fear conditioning (Gold and van Buskirk, 1978; Lennartz et al., 1996; Schneider et al., 2000). Our present findings are also congruent with evidence indicating that the memory-modulating effects of post-training manipulations on fear conditioning are observed when both instrumentally- and Pavlovian-conditioned responses are involved (Schneider et al., 2011; Vazdarjanova and McGaugh, 1999), but not when Pavlovian-conditioned cued responses are the only option (Lee et al., 2001; Wilensky et al., 1999; Wilensky et al., 2000).
The present study did not reveal the brain regions through which propranolol affects consolidation of CD memory. We did determine in Experiment 3, however, that the effect of propranolol on the consolidation of the memory of social defeat is likely due, at least in part, to its blockade of central beta-adrenergic receptors. There are several brain regions wherein beta-adrenergic receptors may influence the consolidation of social defeat. For instance, post-training, intra-amygdala injections of a beta-adrenergic antagonist produce memory deficits in a shock avoidance (Gallagher et al., 1977; Liang et al., 1995) and a water maze task (Hatfield and McGaugh, 1999). Moreover, intra-amygdala infusions of propranolol, at doses that do not affect memory alone, block the memory-enhancing effects of systemic infusions of epinephrine (McGaugh et al., 1996). Finally, systemic administration of propranolol to mice exposed to chronic social defeat stress reduces the amount of Fos protein within the basolateral amygdala (Chou et al., 2014). Consistent with previous findings showing that the amygdala is important for conditioned fear (Blanchard and Blanchard, 1972; Campeau et al., 1992; Cousens and Otto, 1998; Helmstetter, 1992; LeDoux et al., 1990; LeDoux, 2000; Phillips and LeDoux, 1992; Roozendaal et al., 1991; Sananes and Davis, 1992), temporary inactivation of the amygdala impairs the acquisition and expression of CD (Jasnow and Huhman, 2001) and overexpression of cyclic AMP response element binding protein (CREB) in the basolateral amygdala enhances the acquisition of CD after a sub-optimal defeat (Jasnow et al., 2005). Finally, we have shown that injections of the protein synthesis inhibitor anisomycin into the basolateral amygdala (Markham and Huhman, 2008) or the nucleus accumbens (Thompson et al., in prep.) block the acquisition of social defeat. Together, the data suggest that the basolateral amygdala is a central site wherein noradrenergic modulation of CD consolidation might occur. Additionally, we have shown that the bed nucleus of the stria terminalis and the ventral, but not dorsal, hippocampus are important elements of the neural circuit mediating CD (Markham et al., 2009; Markham et al., 2010). Each of these areas may also contribute to the effects of propranolol, and future studies will explore these possibilities.
In summary, the present results provide convincing evidence that beta-adrenergic receptor activation is involved in the consolidation of the memory for social defeat. Interestingly, these findings are consistent with the notion that CD is an ethologically relevant model of fear conditioning that encompasses elements of both instrumental and Pavlovian fear conditioning, and suggest that CD is a valuable model to study the processes by which emotion and stress modulate memory. Finally, the current data also suggest that the popular conception that the anxiolytic effect of propranolol in humans is primarily due to the drug’s peripheral effects may need to be reconsidered.
Highlights.
Propranolol blocked the consolidation of conditioned defeat
Peripheral and central administration significantly reduced submissive behavior
The effect of propranolol administration on consolidation was time-dependent
Acknowledgements
Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number R01MH62044. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albers HE, et al. Role of V1a vasopressin receptors in the control of aggression in Syrian hamsters. Brain Res. 2006;1073-1074:425–30. doi: 10.1016/j.brainres.2005.12.081. [DOI] [PubMed] [Google Scholar]
- Angrini M, Leslie JC, Shephard RA. Effects of propranolol, buspirone, pCPA, reserpine, and chlordiazepoxide on open-field behavior. Pharmacol Biochem Behav. 1998;59:387–97. doi: 10.1016/s0091-3057(97)00457-7. [DOI] [PubMed] [Google Scholar]
- Audi EA, de Oliveira RM, Graeff FG. Microinjection of propranolol into the dorsal periaqueductal gray causes an anxiolytic effect in the elevated plus-maze antagonized by ritanserin. Psychopharmacology (Berl) 1991;105:553–7. doi: 10.1007/BF02244379. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ. Innate and conditioned reactions to threat in rats with amygdaloid lesions. J Comp Physiol Psychol. 1972;81:281–90. doi: 10.1037/h0033521. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, et al. Visible burrow system as a model of chronic social stress: behavioral and neuroendocrine correlates. Psychoneuroendocrinology. 1995;20:117–34. doi: 10.1016/0306-4530(94)e0045-b. [DOI] [PubMed] [Google Scholar]
- Bohus B, et al. Neurohypophysial hormones and central cardiovascular control. Prog Brain Res. 1983;60:445–57. doi: 10.1016/S0079-6123(08)64411-8. [DOI] [PubMed] [Google Scholar]
- Botterblom MH, Feenstra MG, Erdtsieck-Ernste EB. Determination of propranolol, labetalol and clenbuterol in rat brain by high-performance liquid chromatography. J Chromatogr. 1993;613:121–6. doi: 10.1016/0378-4347(93)80204-h. [DOI] [PubMed] [Google Scholar]
- Brantigan CO, Brantigan TA, Joseph N. Effect of beta blockade and beta stimulation on stage fright. Am J Med. 1982;72:88–94. doi: 10.1016/0002-9343(82)90592-7. [DOI] [PubMed] [Google Scholar]
- Bremner JD, et al. Noradrenergic mechanisms in stress and anxiety: I. Preclinical studies. Synapse. 1996;23:28–38. doi: 10.1002/(SICI)1098-2396(199605)23:1<28::AID-SYN4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Brunello N, et al. Social phobia: diagnosis and epidemiology, neurobiology and pharmacology, comorbidity and treatment. J Affect Disord. 2000;60:61–74. doi: 10.1016/s0165-0327(99)00140-8. [DOI] [PubMed] [Google Scholar]
- Bugajski J, et al. Catecholaminergic regulation of the hypothalamic-pituitary-adrenocortical activity. J Physiol Pharmacol. 1991;42:93–103. [PubMed] [Google Scholar]
- Cahill L, et al. Beta-adrenergic activation and memory for emotional events. Nature. 1994;371:702–4. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL. Mechanisms of emotional arousal and lasting declarative memory. Trends Neurosci. 1998;21:294–9. doi: 10.1016/s0166-2236(97)01214-9. [DOI] [PubMed] [Google Scholar]
- Cain CK, Blouin AM, Barad M. Adrenergic transmission facilitates extinction of conditional fear in mice. Learn Mem. 2004;11:179–87. doi: 10.1101/lm.71504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp RM, et al. Fear conditioning can contribute to behavioral changes observed in a repeated stress model. Behav Brain Res. 2012;233:536–44. doi: 10.1016/j.bbr.2012.05.040. [DOI] [PubMed] [Google Scholar]
- Camp RM, Johnson JD. Repeated stressor exposure enhances contextual fear memory in a beta-adrenergic receptor-dependent process and increases impulsivity in a non-beta receptor-dependent fashion. Physiol Behav. 2015 doi: 10.1016/j.physbeh.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau S, Miserendino MJ, Davis M. Intra-amygdala infusion of the N-methyl-D-aspartate receptor antagonist AP5 blocks acquisition but not expression of fear-potentiated startle to an auditory conditioned stimulus. Behav Neurosci. 1992;106:569–574. doi: 10.1037//0735-7044.106.3.569. [DOI] [PubMed] [Google Scholar]
- Chen P, et al. Chronic social defeat up-regulates expression of norepinephrine transporter in rat brains. Neurochem Int. 2012;60:9–20. doi: 10.1016/j.neuint.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou D, Huang CC, Hsu KS. Brain-derived neurotrophic factor in the amygdala mediates susceptibility to fear conditioning. Exp Neurol. 2014;255:19–29. doi: 10.1016/j.expneurol.2014.02.016. [DOI] [PubMed] [Google Scholar]
- Cousens G, Otto T. Both pre- and posttraining excitotoxic lesions of the basolateral amygdala abolish the expression of olfactory and contextual fear conditioning. Behav Neurosci. 1998;112:1092–103. doi: 10.1037//0735-7044.112.5.1092. [DOI] [PubMed] [Google Scholar]
- Davidson JR. Pharmacotherapy of social anxiety disorder: what does the evidence tell us? J Clin Psychiatry. 2006;67(Suppl 12):20–6. [PubMed] [Google Scholar]
- Do Monte FH, et al. New perspectives on beta-adrenergic mediation of innate and learned fear responses to predator odor. J Neurosci. 2008;28:13296–302. doi: 10.1523/JNEUROSCI.2843-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faigel HC. The effect of beta blockade on stress-induced cognitive dysfunction in adolescents. Clin Pediatr (Phila) 1991;30:441–5. doi: 10.1177/000992289103000706. [DOI] [PubMed] [Google Scholar]
- Ferry B, McGaugh JL. Role of amygdala norepinephrine in mediating stress hormone regulation of memory storage. Acta Pharmacol Sin. 2000;21:481–93. [PubMed] [Google Scholar]
- Fitzgerald PJ, et al. Noradrenergic blockade stabilizes prefrontal activity and enables fear extinction under stress. Proc Natl Acad Sci U S A. 2015;112:E3729–37. doi: 10.1073/pnas.1500682112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, et al. Memory formation: evidence for a specific neurochemical system in the amygdala. Science. 1977;198:423–5. doi: 10.1126/science.20664. [DOI] [PubMed] [Google Scholar]
- Giles J. Beta-blockers tackle memories of horror. Nature. 2005;436:448–9. doi: 10.1038/436448a. [DOI] [PubMed] [Google Scholar]
- Gold PE, van Buskirk R. Effects of alpha- and beta-adrenergic receptor antagonists on post-trial epinephrine modulation of memory: relationship to post-training brain norepinephrine concentrations. Behav Biol. 1978;24:168–84. doi: 10.1016/s0091-6773(78)93045-6. [DOI] [PubMed] [Google Scholar]
- Gold PE, et al. Modulation of multiple memory systems: from neurotransmitters to metabolic substrates. Hippocampus. 2013;23:1053–65. doi: 10.1002/hipo.22182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman AL, Dunn AJ. Beta-adrenergic receptors are involved in stress-related behavioral changes. Pharmacol Biochem Behav. 1993;45:1–7. doi: 10.1016/0091-3057(93)90078-8. [DOI] [PubMed] [Google Scholar]
- Gray CL, et al. Dopamine in the nucleus accumbens modulates the memory of social defeat in Syrian hamsters (Mesocricetus auratus) Behav Brain Res. 2015;286:22–28. doi: 10.1016/j.bbr.2015.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, et al. Effects of the beta-blocker propranolol on cued and contextual fear conditioning in humans. Psychopharmacology (Berl) 2004;175:342–52. doi: 10.1007/s00213-004-1819-5. [DOI] [PubMed] [Google Scholar]
- Hatfield T, McGaugh JL. Norepinephrine infused into the basolateral amygdala posttraining enhances retention in a spatial water maze task. Neurobiol Learn Mem. 1999;71:232–9. doi: 10.1006/nlme.1998.3875. [DOI] [PubMed] [Google Scholar]
- Helmstetter FJ. Contribution of the amygdala to learning and performance of conditional fear. Physiol Behav. 1992;51:1271–6. doi: 10.1016/0031-9384(92)90320-2. [DOI] [PubMed] [Google Scholar]
- Hirschmann S, et al. Pindolol augmentation in patients with treatment-resistant panic disorder: A double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2000;20:556–9. doi: 10.1097/00004714-200010000-00011. [DOI] [PubMed] [Google Scholar]
- Huhman KL, et al. Effects of social conflict on POMC-derived peptides and glucocorticoids in male golden hamsters. Physiol Behav. 1990;47:949–56. doi: 10.1016/0031-9384(90)90023-w. [DOI] [PubMed] [Google Scholar]
- Huhman KL, et al. Acute and repeated exposure to social conflict in male golden hamsters: increases in plasma POMC-peptides and cortisol and decreases in plasma testosterone. Horm Behav. 1991;25:206–16. doi: 10.1016/0018-506x(91)90051-i. [DOI] [PubMed] [Google Scholar]
- Huhman KL, et al. Hormonal responses to fighting in hamsters: separation of physical and psychological causes. Physiol Behav. 1992;51:1083–6. doi: 10.1016/0031-9384(92)90097-l. [DOI] [PubMed] [Google Scholar]
- Huhman KL, et al. Conditioned defeat in male and female Syrian hamsters. Horm Behav. 2003;44:293–9. doi: 10.1016/j.yhbeh.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Introini-Collison IB, Castellano C, McGaugh JL. Interaction of GABAergic and beta-noradrenergic drugs in the regulation of memory storage. Behav Neural Biol. 1994;61:150–5. doi: 10.1016/s0163-1047(05)80068-8. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, et al. Acute and chronic social defeat suppresses humoral immunity of male Syrian hamsters (Mesocricetus auratus) Horm Behav. 2001;40:428–33. doi: 10.1006/hbeh.2001.1708. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Huhman KL. Activation of GABA(A) receptors in the amygdala blocks the acquisition and expression of conditioned defeat in Syrian hamsters. Brain Res. 2001;920:142–50. doi: 10.1016/s0006-8993(01)03054-2. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, et al. Memory of social defeat is facilitated by cAMP response element-binding protein overexpression in the amygdala. Behav Neurosci. 2005;119:1125–30. doi: 10.1037/0735-7044.119.4.1125. [DOI] [PubMed] [Google Scholar]
- Ji JZ, Zhang XH, Li BM. Deficient spatial memory induced by blockade of beta-adrenoceptors in the hippocampal CA1 region. Behav Neurosci. 2003;117:1378–84. doi: 10.1037/0735-7044.117.6.1378. [DOI] [PubMed] [Google Scholar]
- Kent JM, Mathew SJ, Gorman JM. Molecular targets in the treatment of anxiety. Biol Psychiatry. 2002;52:1008–30. doi: 10.1016/s0006-3223(02)01672-4. [DOI] [PubMed] [Google Scholar]
- Kindt M, Soeter M, Sevenster D. Disrupting reconsolidation of fear memory in humans by a noradrenergic beta-blocker. J Vis Exp. 2014 doi: 10.3791/52151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, et al. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci. 1990;10:1062–9. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee HJ, et al. Post-training injections of catecholaminergic drugs do not modulate fear conditioning in rats and mice. Neurosci Lett. 2001;303:123–6. doi: 10.1016/s0304-3940(01)01733-5. [DOI] [PubMed] [Google Scholar]
- Lennartz RC, et al. Inhibitory avoidance impairments induced by intra-amygdala propranolol are reversed by glutamate but not glucose. Behav Neurosci. 1996;110:1033–9. doi: 10.1037//0735-7044.110.5.1033. [DOI] [PubMed] [Google Scholar]
- Liang KC, Chen LL, Huang TE. The role of amygdala norepinephrine in memory formation: involvement in the memory enhancing effect of peripheral epinephrine. Chin J Physiol. 1995;38:81–91. [PubMed] [Google Scholar]
- Liu X, et al. Use of a physiologically based pharmacokinetic model to study the time to reach brain equilibrium: an experimental analysis of the role of blood-brain barrier permeability, plasma protein binding, and brain tissue binding. J Pharmacol Exp Ther. 2005;313:1254–62. doi: 10.1124/jpet.104.079319. [DOI] [PubMed] [Google Scholar]
- Luckett C, Norvelle A, Huhman K. The role of the nucleus accumbens in the acquisition and expression of conditioned defeat. Behav Brain Res. 2012;227:208–14. doi: 10.1016/j.bbr.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham CM, Huhman KL. Is the medial amygdala part of the neural circuit modulating conditioned defeat in Syrian hamsters? Learn Mem. 2008;15:6–12. doi: 10.1101/lm.768208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham CM, Norvelle A, Huhman KL. Role of the bed nucleus of the stria terminalis in the acquisition and expression of conditioned defeat in Syrian hamsters. Behav Brain Res. 2009;198:69–73. doi: 10.1016/j.bbr.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham CM, Taylor SL, Huhman KL. Role of amygdala and hippocampus in the neural circuit subserving conditioned defeat in Syrian hamsters. Learn Mem. 2010;17:109–16. doi: 10.1101/lm.1633710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka N, et al. Characterization of stress-induced sudden death in cardiomyopathic hamsters. J Pharmacol Exp Ther. 1998;284:125–35. [PubMed] [Google Scholar]
- McDonald MM, et al. GABAA receptor activation in the lateral septum reduces the expression of conditioned defeat and increases aggression in Syrian hamsters. Brain Res. 2012;1439:27–33. doi: 10.1016/j.brainres.2011.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. Modulation of memory storage processes. In: Solomon P, Goethels G, Kelley C, Stephens B, editors. Memory: Interdisciplinary approaches. Springer-Verlag; New York: 1989. pp. 33–59. [Google Scholar]
- McGaugh JL, Cahill L, Roozendaal B. Involvement of the amygdala in memory storage: interaction with other brain systems. Proc Natl Acad Sci U S A. 1996;93:13508–14. doi: 10.1073/pnas.93.24.13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Curr Opin Neurobiol. 2002;12:205–10. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Making lasting memories: remembering the significant. Proc Natl Acad Sci U S A. 2013;110(Suppl 2):10402–7. doi: 10.1073/pnas.1301209110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morilak DA, et al. Role of brain norepinephrine in the behavioral response to stress. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1214–24. doi: 10.1016/j.pnpbp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Murphy MR. Olfactory stimulation and olfactory bulb removal: effects on territorial aggression in male Syrian golden hamsters. Brain Res. 1976;113:95–110. doi: 10.1016/0006-8993(76)90009-3. [DOI] [PubMed] [Google Scholar]
- Neil-Dwyer G, et al. Beta-adrenoceptor blockers and the blood-brian barrier. Br J Clin Pharmacol. 1981;11:549–53. doi: 10.1111/j.1365-2125.1981.tb01169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielson KA, Jensen RA. Beta-adrenergic receptor antagonist antihypertensive medications impair arousal-induced modulation of working memory in elderly humans. Behav Neural Biol. 1994;62:190–200. doi: 10.1016/s0163-1047(05)80017-2. [DOI] [PubMed] [Google Scholar]
- Nowack RM, Paradiso JL. Walker’s Mammals of the World. Johns Hopkins University Press; Baltimore, MD: 1983. [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–85. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Potegal M, et al. Conditioned defeat in the Syrian golden hamster (Mesocricetus auratus) Behav Neural Biol. 1993;60:93–102. doi: 10.1016/0163-1047(93)90159-f. [DOI] [PubMed] [Google Scholar]
- Przybyslawski J, Roullet P, Sara SJ. Attenuation of emotional and nonemotional memories after their reactivation: role of beta adrenergic receptors. J Neurosci. 1999;19:6623–8. doi: 10.1523/JNEUROSCI.19-15-06623.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirarte GL, Roozendaal B, McGaugh JL. Glucocorticoid enhancement of memory storage involves noradrenergic activation in the basolateral amygdala. Proc Natl Acad Sci U S A. 1997;94:14048–53. doi: 10.1073/pnas.94.25.14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Romaguera J, et al. Systemic Propranolol Acts Centrally to Reduce Conditioned Fear in Rats Without Impairing Extinction. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Koolhaas JM, Bohus B. Attenuated cardiovascular, neuroendocrine, and behavioral responses after a single footshock in central amygdaloid lesioned male rats. Physiol Behav. 1991;50:771–5. doi: 10.1016/0031-9384(91)90016-h. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, et al. Glucocorticoids interact with emotion-induced noradrenergic activation in influencing different memory functions. Neuroscience. 2006;138:901–10. doi: 10.1016/j.neuroscience.2005.07.049. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Barsegyan A, Lee S. Adrenal stress hormones, amygdala activation, and memory for emotionally arousing experiences. Prog Brain Res. 2008;167:79–97. doi: 10.1016/S0079-6123(07)67006-X. [DOI] [PubMed] [Google Scholar]
- Sananes CB, Davis M. N-methyl-D-aspartate lesions of the lateral and basolateral nuclei of the amygdala block fear-potentiated startle and shock sensitization of startle. Behav Neurosci. 1992;106:72–80. doi: 10.1037//0735-7044.106.1.72. [DOI] [PubMed] [Google Scholar]
- Schneider AM, et al. Beta-adrenergic receptor blockade by propranolol enhances retention in a multitrial passive-avoidance procedure. Behav Neurosci. 2000;114:1256–60. [PubMed] [Google Scholar]
- Schneider AM, et al. Stress-dependent impairment of passive-avoidance memory by propranolol or naloxone. Pharmacol Biochem Behav. 2011;98:539–43. doi: 10.1016/j.pbb.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotti MA, Lee G, Gammie SC. Maternal defense is modulated by beta adrenergic receptors in lateral septum in mice. Behav Neurosci. 2011;125:434–45. doi: 10.1037/a0023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenbecher T, Reymann KG, Balschun D. A post-tetanic time window for the reinforcement of long-term potentiation by appetitive and aversive stimuli. Proc Natl Acad Sci U S A. 1997;94:1494–9. doi: 10.1073/pnas.94.4.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simson PE, et al. Dose-sensitive excitation and inhibition of spontaneous amygdala activity by propranolol. Pharmacol Biochem Behav. 2001;69:85–92. doi: 10.1016/s0091-3057(01)00503-2. [DOI] [PubMed] [Google Scholar]
- Stern CA, Carobrez AP, Bertoglio LJ. Aversive learning as a mechanism for lack of repeated anxiolytic-like effect in the elevated plus-maze. Pharmacol Biochem Behav. 2008;90:545–50. doi: 10.1016/j.pbb.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, et al. The noradrenergic system in pathological anxiety: a focus on panic with relevance to generalized anxiety and phobias. Biol Psychiatry. 1999;46:1205–18. doi: 10.1016/s0006-3223(99)00246-2. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Miczek KA. Neurogenetics of aggressive behavior: studies in rodents. Curr Top Behav Neurosci. 2014;17:3–44. doi: 10.1007/7854_2013_263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson B, Norvelle A, Huhman K. in prep. [Google Scholar]
- Vaiva G, et al. Immediate treatment with propranolol decreases posttraumatic stress disorder two months after trauma. Biol Psychiatry. 2003;54:947–9. doi: 10.1016/s0006-3223(03)00412-8. [DOI] [PubMed] [Google Scholar]
- van Stegeren AH, et al. Memory for emotional events: differential effects of centrally versus peripherally acting beta-blocking agents. Psychopharmacology (Berl) 1998;138:305–10. doi: 10.1007/s002130050675. [DOI] [PubMed] [Google Scholar]
- Vazdarjanova A, McGaugh JL. Basolateral amygdala is involved in modulating consolidation of memory for classical fear conditioning. J Neurosci. 1999;19:6615–22. doi: 10.1523/JNEUROSCI.19-15-06615.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Davis M. Light-enhanced startle: further pharmacological and behavioral characterization. Psychopharmacology (Berl) 2002;159:304–10. doi: 10.1007/s002130100913. [DOI] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, LeDoux JE. Functional inactivation of the amygdala before but not after auditory fear conditioning prevents memory formation. J Neurosci. 1999;19:RC48. doi: 10.1523/JNEUROSCI.19-24-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, LeDoux JE. The amygdala modulates memory consolidation of fear-motivated inhibitory avoidance learning but not classical fear conditioning. J Neurosci. 2000;20:7059–66. doi: 10.1523/JNEUROSCI.20-18-07059.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebrowska-Lupina I, et al. Prolonged treatment with beta-adrenoceptor antagonists counteracts the aggression deficit induced by chronic stress. Pol J Pharmacol. 1997;49:283–9. [PubMed] [Google Scholar]