Abstract
Tight junctions of the blood-brain barrier are composed of transmembrane and associated cytoplasmic proteins. The transmembrane claudin proteins form the primary seal between endothelial cells and junctional adhesion molecules (JAMs) regulate tight junction formation. We have previously shown that claudin-1, claudin-5, ZO-1, and ZO-2 exhibit differential developmental regulation from 60% of gestation up to maturity in adult sheep. The purpose of the current study was to examine developmental changes in claudin-3, -12, and JAM-A protein expression in cerebral cortices of fetuses at 60%, 80%, and 90% gestation, and in newborn and adult sheep. We also examined correlations between changes in endogenous cortisol levels and tight junction protein expression in cerebral cortices of the fetuses. Claudin-3, -12 and JAM-A expressions were determined by Western immunoblot. Claudin-3 and -12 were lower (P<0.01) at 60%, 80%, 90% and in newborns than in adults, and JAM-A was lower in adults than in fetuses at 80% and 90% gestation. Claudin-3 expression demonstrated a direct correlation with increasing plasma cortisol levels (r=0.60, n=15, P<0.02) in the fetuses. We conclude that: claudin-3, -12 and JAM-A are expressed as early as 60% of gestation in ovine cerebral cortices, exhibit differential developmental regulation, and that increasing endogenous glucocorticoids modulate claudin-3 expression in the fetus.
Keywords: Blood-brain barrier, claudin, development, sheep, tight junction proteins
INTRODUCTION
The blood-brain barrier is a regulated interface between the peripheral circulation and the central nervous system (CNS) (Hawkins and Davis, 2005). The blood-brain barrier develops during fetal life (Stonestreet et al., 1996, Nico et al., 1999, Virgintino et al., 2004, Daneman, 2012) and consists of a complex cellular system of highly specialized endothelial cells connected by intercellular tight junctions (Brightman and Reese, 1969, Engelhardt, 2003). The endothelial cells of the blood-brain barrier are supported and regulated by pericytes embedded in the vascular basement membrane, perivascular microglial cells, astrocytes and neurons, which together form the neurovascular unit (Virgintino et al., 2004, Abbott et al., 2010, Luissint et al., 2012). In addition to the blood-brain barrier, there are three other main barriers in the brain: blood-cerebral spinal fluid (CSF) barrier, meningeal barrier, and fetal CSF-brain barrier (Ek et al., 2012). Similar to the cellular structure of the cerebrovascular endothelial cells of the blood-brain barrier, the ependymal cells of the choroid plexus of the blood-CSF barrier and the outer cells of the arachnoid membrane of the meningeal barrier are connected by tight junction proteins. The blood-brain barrier along with the blood-cerebral spinal fluid (CSF) barrier, meningeal barrier, and fetal CSF-brain barrier maintains the homeostasis of the central nervous system by limiting the passive diffusion of polar substances from the blood-to-brain and maintaining optimal ionic composition necessary for synaptic signaling function (Betz and Goldstein, 1986, Betz, 1992, Begley and Brightman, 2003, Wolburg et al., 2009, Abbott et al., 2010, Ek et al., 2012, Luissint et al., 2012).
Tight junctions mediate the adhesion between adjacent cells and limit the free passage of molecules through the paracellular pathway (Mullier et al., 2010, Abbott and Friedman, 2012). Previous studies in humans and other species demonstrate that an adult-like pattern of the endothelial tight junctions is present by midgestation, and the paracellular pathway is already limited early in brain development (Stonestreet et al., 1996, Virgintino et al., 2004, Ek et al., 2006).
Tight junctions are complex molecular structures composed of transmembrane and associated cytoplasmic proteins. The transmembrane proteins including occludins, the claudin family, and junctional adhesion molecules (JAMs) are connected to the associated cytoplasmic proteins, zonula occludens (ZO)-1, ZO-2 and ZO-3, etc., which stabilize the tight junctions by connecting them to the cell structure and actin (Hawkins and Davis, 2005, Abbott et al., 2006). More recently, claudin-3 and -12 have been identified to contribute to the integrity of the blood-brain barrier (Wolburg et al., 2003, Neuhaus et al., 2008, Shin et al., 2008, Milatz et al., 2010, Haseloff et al., 2015). They have been shown to participate in tight junction formation, to establish a primary seal between the brain microvascular endothelial cells, and to contribute to the high transendothelial electrical resistance (Abbott et al., 2006, Schrade et al., 2012, Haseloff et al., 2015). In addition, JAM-A is involved in the formation and maintenance of tight junctions (Abbott et al., 2006) and modulates monocyte transmigration through the blood-brain barrier (Aurrand-Lions et al., 2002).
The ovine fetus has been widely used to investigate the development of the brain (Gunn et al., 1997, Stonestreet et al., 2000, Back et al., 2006) because the neurodevelopment of the immature ovine brain is similar to that of premature infants with respect to the time course of neurogenesis, onset of cerebral sulcation, and detection of cortical auditory evoked potentials (Barlow, 1969, Cook et al., 1987, Back et al., 2006). The sheep brain at 80% to 85% gestation is generally thought to be similar to that of a near term newborn infant (Barlow, 1969, Back et al., 2006).
We have previously shown ontogenic decreases in blood-brain barrier permeability with development from 60% of gestation up to maturity in adult sheep, and that endogenous increases in cortisol concentrations are associated with decreases in blood-brain barrier permeability during normal fetal development (Stonestreet et al., 2000). We also previously reported differential developmental regulation of occludin, claudin-1 and -5 and zonula occludens (ZO)-1 and ZO-2 in sheep cerebral cortex (Duncan et al., 2009).
The hypothalamic-pituitary-adrenocortical matures during fetal development and endogenous cortisol concentrations increase markedly in the latter part of part of gestation (Wintour, 1984). Glucocorticoids have been shown to have an important role in modulating blood-brain barrier function during development and affecting the expression of some tight junction proteins during normal and pathological conditions (Stonestreet et al., 2000, Stonestreet et al., 2003, Malaeb et al., 2007, Duncan et al., 2009).
The purpose of the current study was to extend our previous work to examine ontogenic changes in some of the more recently identified tight junction proteins including claudin-3, claudin-12, and the tight junction associated protein, JAM-A, in the cerebral cortices of fetuses at 60%, 80%, and 90% gestation and in newborn and adult sheep. We also examined associations between endogenous increases in plasma cortisol concentrations during gestation and changes in the expression of these tight junction proteins during fetal development.
EXPERIMENTAL PROCEDURES
Animal Preparation and Experimental Design
This study was conducted after approval of the Institutional Animal Care and Use Committees of The Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island, and in accordance with the National Institutes of Health Guidelines for the use of experimental animals.
The plasma and cerebral cortical tissue samples for the present study were obtained from placebo treated control subjects in our former studies (Stonestreet et al., 2000, Sysyn et al., 2001, Ron et al., 2005, Sadowska et al., 2006). The surgical procedures and physiological measures were performed as previously described in detail (Stonestreet et al., 2000, Sysyn et al., 2001, Sadowska et al., 2006). Briefly, under 1–2% halothane anesthesia, polyvinyl catheters were placed into a brachial vein of the fetuses and newborns for isotope administration and the thoracic aorta via the brachial artery for blood sample withdrawal for the previous studies, which were designed to quantify blood-brain barrier permeability (Stonestreet et al., 2000, Sysyn et al., 2001, Sadowska et al., 2006). The fetal sheep at 60% were 87–90 days (n=4–7), 80% were 118–120 days (n=3–5), and 90% were 135–138 days of gestation (n=5), newborn lambs were 4–6 days (n=6) and adult sheep were 3 years (n=3) of age at time of study. Full term gestation in fetal sheep is 147 days.
Western Immunoblot Detection and Quantification of Claudin-3, -12, and JAM-A
Samples from the cerebral cortex were extracted in Triton/Deoxycholate/SDS (100 mM NaCl, 1% Triton X, 0.5 Sodium Deoxycholate, 0.2% SDS, 2 mM EDTA, 1 mM benzamidine) with 1% of complete protease inhibitor cocktail (Sigma, St. Louis, MO). The total protein concentrations of the homogenates were determined with a bicinchoninic acid protein assay (BCA, Pierce, Rockford, IL).
Fifty micrograms of total protein per well were fractionated by SDS-PAGE and transferred onto polyvinylidene diflouride membranes (0.2 μm, Bio-Rad Laboratories, Hercules, CA) using a semi-dry technique. Membranes were blocked with 10% nonfat milk (Bio-Rad) for 1 h at room temperature and then washed in Tris-buffered saline with 0.1% Tween 20 (TBST) three times for 10 min per wash. Membranes were probed with primary rabbit polyclonal antibodies for claudin-3 (Zymed, San Francisco, CA), claudin-12 (Bios, Woburn, MA) and for JAM-A (Zymed) at the dilution of 1:5000. Vinculin was probed with primary mouse monoclonal antibody (Thermo Scientific™ Pierce, Waltham, MA) at a dilution of 1:10,000. Membranes were incubated with primary antibodies overnight in 4°C. After three washes in TBST, the membranes were incubated for 1 h at room temperature with goat anti-rabbit (San Antonio, TX) for claudin -3, -12 and JAM-A or goat anti-mouse Life Technologies, Grand Island, NY) for vinculin at the dilution 1:10000 for all of the examined proteins. After four additional washes, binding of the secondary antibody was detected with enhanced chemiluminescence (ECL Prime, Western Blotting Detection reagents, GE Healthcare Bio-Sciences, Pittsburgh, PA) before an exposure to autoradiography film (Phenix, Candler, NC).
Rat lung lysate served as a positive control for claudin-3, rat brain for claudin-12 and human colorectal adenocarcinoma cells (Caco-2, ProSci, Poway, CA) for JAM-A. Detection of the claudin-3, claudin-12, and JAM-A was dependent on incubation with the primary antibody, the omission of which eliminated the signal (data not shown).
All experimental samples were normalized to an internal control standard protein sample obtained from a single adult sheep brain. For the purpose of this report, we refer to these samples as internal control samples. As previously described, (Duncan et al., 2009, Malaeb et al., 2009, Sadowska et al., 2009, Sadowska et al., 2010, Chen et al., 2012, Sadowska et al., 2012, Spasova et al., 2014) the internal control samples were included in three lanes on each immunoblot and served as a control for the quality of loading, transfer of samples, normalization of densitometric values, and to permit accurate comparisons among the different immunoblots. The experimental protein autoradiographic integrated optical density (IOD) values were expressed as a ratio to the internal control protein values, thus facilitating normalized comparisons among different groups and immunoblots. The use of internal control protein sample is unique to our laboratory and was developed to allow for comparisons among large numbers of study subjects and immunoblots (Spasova et al., 2014). This procedure was necessary because initial studies in our laboratory showed that most of the traditionally used housekeeping protein standards varied during development in sheep. Therefore, they could not be used as control protein standards during ovine development. The experimental values in this study represented the average values from 2–8 different immunoblots. Vinculin was also used as a loading control to ensure that equal amounts of protein were applied to each lane.
The plasma cortisol samples were collected immediately before sacrifice and obtaining the cerebral cortical tissue samples. Plasma cortisol values were determined in the fetal sheep at 60%, 80%, and 90% of gestation as previously described.(Sadowska et al., 2006) The total cortisol concentrations were measured in duplicate using the Clinical Assays™ GammaCoat™ Cortisol 125I-radioimmunoassay (DiaSorin, Stillwater, MN). The GammaCoat™ antiserum exhibits 100% cross reactivity with cortisol (Stonestreet et al., 1999, Stonestreet et al., 2000, Sadowska et al., 2006). The observed coefficient of variation for inter- and intra-assay precision was 10.1 and 7.9%, respectively (Stonestreet et al., 1999, Stonestreet et al., 2000, Sadowska et al., 2006).
Densitometric Analysis
Band intensities were analyzed with a Gel-Pro Analyzer (Media Cybernetics, Silver Spring, MD). As we formerly described, we used the average of the three internal control values on each immunoblot to normalize the experimental samples densitometry readings (Duncan et al., 2009, Sadowska et al., 2009, Sadowska et al., 2010, Chen et al., 2012). The final values represented an average of the IOD values obtained from the different immunoblots and are presented as a ratio to the internal control sample.
Statistical Analysis
All results are expressed as means ± standard deviation (M±SD). One-way ANOVA was used to compare values among different age groups. If a significant difference was found by ANOVA, the Fischer’s protected least-significant difference (LSD) post hoc test was used to detect specific differences among the study groups.
To examine relationships between tight junction protein expression and endogenous glucocorticoid concentrations during normal fetal development, tight junction protein expression as a ratio to the internal control samples was compared to plasma cortisol concentrations using the least-squares regression analysis. In this analysis, we examined fetal groups 60%, 80%, and 90% of gestation because of the importance of glucocorticoids during fetal development. Least-squares linear regression analysis was also used to compare our normalization method using the internal control samples with the ratio of study samples to vinculin. A P-value of <0.05 was considered statistically significant.
RESULTS
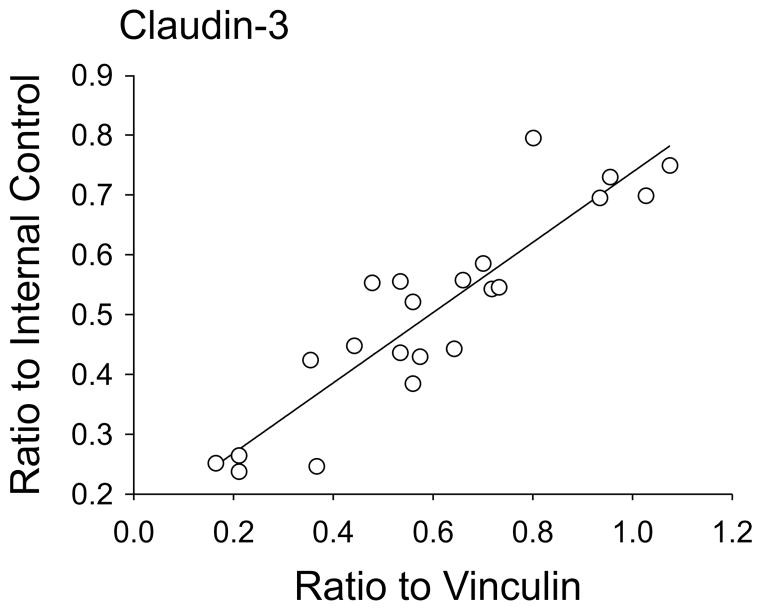
We have previously shown direct linear correlations between IOD values normalized to the internal control samples and those normalized to β-actin in newborn lambs (Kim et al., 2006). In order to validate further the use of the internal control protein standard (Duncan et al., 2009, Malaeb et al., 2009, Sadowska et al., 2009, Sadowska et al., 2010, Chen et al., 2012, Sadowska et al., 2012, Spasova et al., 2014), we compared claudin-3 expression normalized as the ratio to the internal control standard with values normalized as ratios to vinculin, and found that the values demonstrated an excellent correlation (Figure 1, r=0.91, n=22, P<0.001).
Fig. 1.
Claudin-3 protein expression plotted as the ratio to the internal control standard plotted against claudin-3 values normalized as ratios to vinculin, r=0.91, n=22, P<0.05.
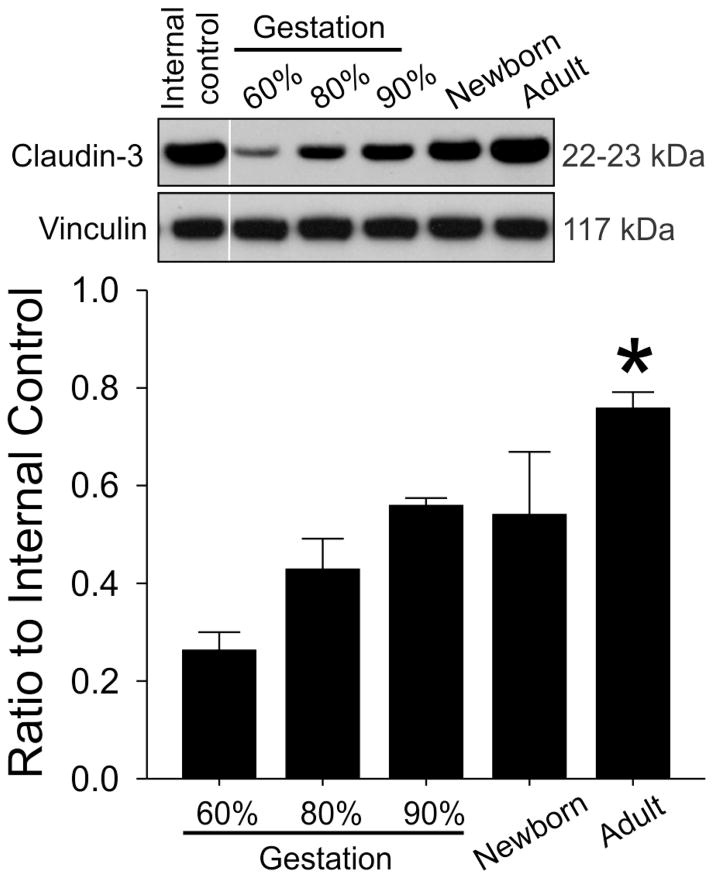
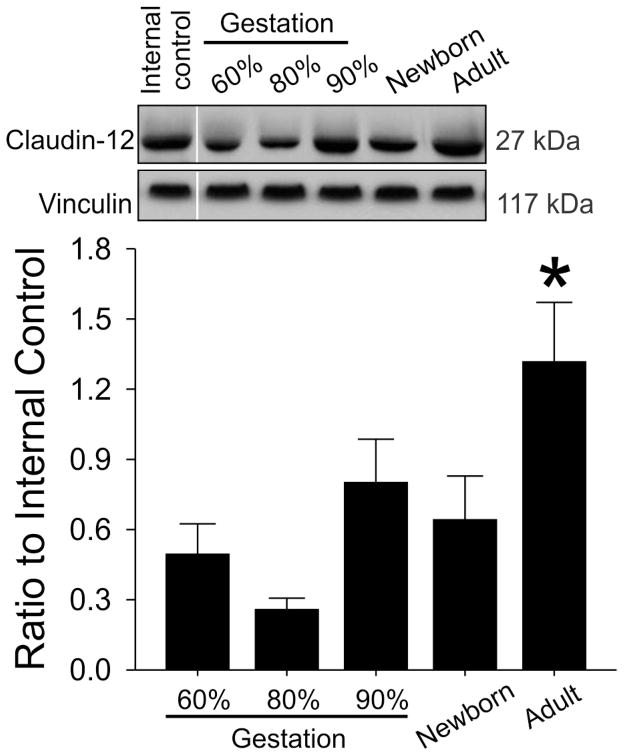
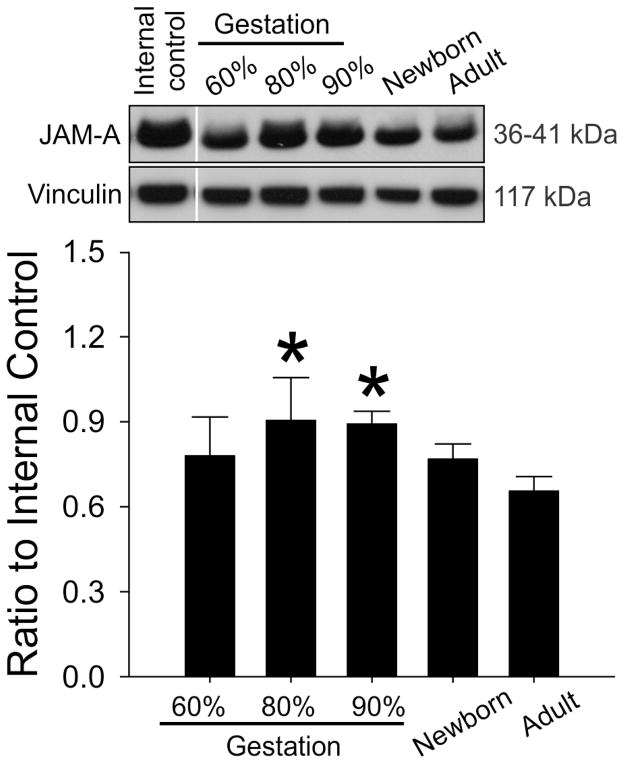
Claudin-3 protein expression demonstrated a gradual steady increase during development (Figure 2, ANOVA, main effects; F=29.7, P<0.001) and was higher in the adult sheep than in the fetuses at 60%, 80%, and 90% of gestation and in the newborn lambs. Claudin-12 protein expression also was higher in the adult sheep than in the fetuses at 60%, 80%, and 90% of gestation and in the newborn lambs (Figure 3, ANOVA, main effects; F=7.46, P<0.001). However, the increases did not appear as gradual during development as those of claudin-3. In contrast, JAM-A protein expression was higher in the fetal sheep at 80% and 90% of gestation compared with the adult sheep (Figure 4, ANOVA, main effects; F=3.7, P<0.03).
Fig. 2.
Representative Western immunoblots and bar graphs of claudin-3 expression in cerebral cortex of the ovine fetuses at 60% (n=7), 80% (n=5) and 90% of gestation (n=5), newborn lambs (n=6) and adult sheep (n=3). Mean ± SD, *P<0.05 versus 60%, 80% and 90% of gestation and newborn sheep.
Fig. 3.
Representative Western immunoblots and bar graphs of claudin 12 expression in cerebral cortex of the ovine fetuses at 60% (n=7), 80% (n=3) and 90% of gestation (n=5), newborn lambs (n=6) and adult sheep (n=3). Mean ± SD, *P<0.05 versus 60%, 80% and 90% of gestation and newborn sheep.
Fig. 4.
Representative Western immunoblots and bar graphs of JAM-A expression in the cerebral cortex of the ovine fetuses at 60% (n=7), 80% (n=3) and 90% of gestation (n=5), newborn lambs (n=6) and adult sheep (n=3). Mean ± SD, *P<0.05 versus Adult.
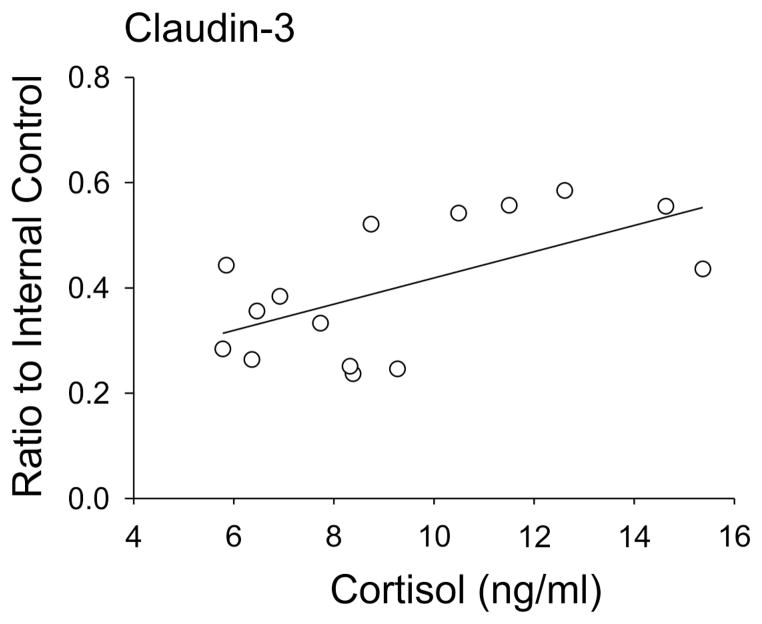
Claudin-3 protein expression exhibited a direct linear correlation with endogenous increases in fetal plasma cortisol concentrations (r=0.60, n=15, P=0.02, Figure 5). However, correlations were not observed between claudin-12 or JAM-A and endogenous changes plasma cortisol concentrations.
Fig. 5.
Claudin-3 protein expression in the cerebral cortex of the ovine fetuses at 60%, 70%, and 90% of gestation plotted against plasma cortisol concentrations, r=0.60, n=15, P<0.05.
DISCUSSION
The purpose of the present study was to examine changes in claudin-3, -12 and JAM-A protein expression in cerebral cortices during development from 60% of gestation up to maturity in adult sheep. Claudin-3, -12 and JAM-A have not been previously examined systematically during development in the brain of a large vertebrate animal species. There are three main findings of our study: 1) claudin-3, -12 and JAM-A are expressed in the cerebral cortex early in fetal life and throughout development; 2) each of the proteins demonstrates a unique pattern of expression during development; 3) increases in endogenous glucocorticoids correlate with increases in claudin-3 protein expression in the fetal cerebral cortex.
The early expression of the tight junction proteins that we observed in the ovine fetal cerebral cortex is consistent with the timing of tight junction development and organization in the human fetal cerebral cortex (Virgintino et al., 2004, Virgintino et al., 2007). Although tight junctions are present from early on in brain development, blood-brain barrier permeability decreases over an extended gestational interval, suggesting progressive maturation of the blood-brain barrier over a prolonged period of development (Kniesel et al., 1996, Stonestreet et al., 1996, Anstrom et al., 2007). In this study, we have shown that the expression levels of claudin-3 protein increase progressively from 60% gestation up to maturity in adult sheep. Our findings concur with previous studies showing that claudin-3 protein expression is associated with blood-brain barrier maturation during development in rodents (Kniesel et al., 1996, Liebner et al., 2008, Kratzer et al., 2012). Although the exact role of claudin-3 at the blood-brain barrier during development is not fully established, claudin-3 induces an increase in tight junction formation at the protoplasmic (P)-face of vascular brain endothelium during maturation (Kniesel et al., 1996, Furuse et al., 1999, Pfeiffer et al., 2011). Claudin-3 may also be important in tightening the paracellular cleft because paracellular permeability decreases and transcellular electrical resistance increases after claudin-3 transfection into renal tubular cells (Milatz et al., 2010). Increases in claudin-3 expression have also been shown to be associated with reductions in tight junction permeability, (Coyne et al., 2003), and claudin-3 expression along with localization at cell-cell contacts increases during development in embryonic and postnatal stages, thus supporting the importance of claudin-3 in blood-brain barrier induction and maintenance throughout development (Wolburg et al., 2003, Liebner et al., 2008). Although tight junctions are most likely enriched in the ependymal lining of the cerebral cortical ventricles (Kratzer et al., 2012, Liddelow et al., 2013), we did not have the ependymal sheet from the cerebral cortical ventricles saved from our previous studies. Therefore, the expression of claudin-3 in ependymal cells during the development cannot be ascertained from our study and requires further investigation. Moreover, the cerebral cortical samples that we used in the current study represented frozen samples primarily from the outer portion of the cerebral cortex and, therefore, were unlikely to contain large contributions from the ependymal lining of the cerebral ventricles.
Claudin-12 remains a less well-described member of the claudin family. Although the amino acid sequences of claudins-1, -3, and -5 are very homologous, suggesting similar properties, the claudin-12 sequence differs (Haseloff et al., 2015). Claudin-12 is mainly observed at tight junctions of brain blood vessels during murine development and appears to contribute to the unique function of the barrier (Nitta et al., 2003, Wolburg et al., 2003, Abbott et al., 2006, Krause et al., 2008, Daneman et al., 2010, Pfeiffer et al., 2011, Schrade et al., 2012). Similar to claudin-3, claudin-12 localizes at cell-cell contacts with immunofluorescence staining of mouse brain capillaries showing expression and co-localization with ZO-1 in brain endothelial cells supporting the presence of well-formed tight junctions at early stages of brain vascularization (Nitta et al., 2003). To the best of our knowledge, our study provides the first evidence to show that claudin-12 expression increases during development in ovine cerebral cortex. Previous work has suggested that claudin-12 may function as a molecular sieve to allow only small molecules (molecular weight cut-off less than 800 dalton) to cross tight junctions in claudin-5 knock-out brain blood vessels (Nitta et al., 2003). However, determination of the precise function of claudin-12 during development in the blood-brain barrier requires further investigation.
Similar to the claudins proteins, JAM-A is expressed at the cell-to-cell junctions of endothelial and epithelial cells. It regulates occlusion of the paracellular space of brain endothelial cells (Fraemohs et al., 2004). JAM-A localizes to the lateral membrane of brain endothelial cells, exhibits continuous staining on the inter-endothelial cell border, and co-localizes with claudin-5, occludin, and ZO-1 (Stamatovic et al., 2012). In the present study, we showed JAM-A expression was present very early in the ovine gestation, which is equivalent to approximately 24 weeks of the human gestation. However, significant differences in JAM-A expression were not observed among fetuses at 60%, 80%, and 90% gestation and newborn lambs in the ovine cerebral cortex. Our findings are consistent with previous work, in which JAM-A expression was observed in human fetuses by 16 weeks of gestation, but significant changes in JAM-A expression were not observed in germinal matrix vasculature compared with cortex and white matter during gestation (Ballabh et al., 2005). In addition, JAM-A expression in fetuses compared with premature infants did not differ significantly (Ballabh et al., 2005). Our study actually showed that JAM-A protein expressions at 80% and 90% of gestation were higher than in the adult ovine cerebral cortex. Our finding that JAM-A protein expression was lower in the adult sheep compared to fetal sheep at mid and late gestation is unique. However, the significance of this finding remains to be determined.
The integrity of tight junctions is the key to maintaining the physiological functioning of the blood-brain barrier during development and in adults. We have previously shown that claudin-1, claudin-5, ZO-1, and ZO-2 expression exhibit differential developmental regulation, exogenous glucocorticoids regulate claudin-5 and ZO-2 in vivo at some, but not all ages in sheep, and that increases in endogenous fetal glucocorticoids are associated with increases ZO-2 expression in ovine cerebral cortices (Duncan et al., 2009). The current study extends our previous work and shows that claudin-3 and claudin-12 also show ontogenic increases from early in fetal life up to maturity in adult sheep, but that, in contrast, JAM-A expressions are lower in adults than in fetuses. The increases in claudin-3 and -12 expression after birth are consistent with the lower barrier permeability that we previously reported in sheep (Stonestreet et al., 1996). In the current study, we were not able to establish correlations between claudin-3, claudin-12 or JAM-A with blood-brain barrier permeability (Ki) values measured with α-aminoisobutyric acid in the fetal, newborn and adult sheep (Stonestreet et al., 2000, Sysyn et al., 2001, Sadowska et al., 2006). This finding could be because 1) the cerebral cortex exhibits relatively small differences in the Ki values between 60% of fetal gestation and adult sheep as a result of its unique cellular composition (Stonestreet et al., 1996, Duncan et al., 2009); 2) in our former work, we observed larger developmental decreases in barrier permeability in the more caudal brain structures (Stonestreet et al., 1996), unfortunately, we did not have caudal brain regional tissue remaining from our prior studies; 3) the amount of protein expression may not be the primary determinant of the barrier permeability properties in vivo (Duncan et al., 2009); 4) more likely, the proteins of the tight junction complex work together to create an effective barrier (Abbott et al., 2006, Abbott et al., 2010).
Many factors are involved in tight junction modulation during CNS development including G-protein signaling, extracellular matrix, and glucocorticoids (Stonestreet et al., 2000, Wolburg and Lippoldt, 2002). In our previous studies, we have demonstrated that endogenous increases in cortisol concentrations are associated with decreases in the permeability of the blood-brain barrier and that glucocorticoids are associated with changes in some but not all tight junction proteins (Stonestreet et al., 2000, Malaeb et al., 2007, Duncan et al., 2009). In the current study, protein expression of claudin-3, but not claudin-12 or JAM-A, demonstrated a significant positive correlation with increasing endogenous cortisol concentrations during development.
There are several limitations to our study. The tight junction proteins are major structures in both endothelial cells of blood-brain barrier and ependymal cells of blood-CSF barriers. The blood-CSF barrier also plays a key role in the CNS development processes and physiology, of which the ependymal cell polarity is maintained by the tight junctions (Jimenez et al., 2014). Due to the limitations in this large animal resource, the ontogenetic changes of tight junction protein in ependymal cells was not examined in this present study. Moreover, the remaining cerebral cortical tissue that we had from our former studies was not appropriately preserved to perform immunohistochemical analysis, and therefore, we could not examine the localization and structural organization of the studied proteins in the blood-brain or brain-CSF barriers. However, these are important considerations for future work. There potentially could be many other contributors to barrier tightening during development that are important components of the neurovascular unit (Abbott et al., 2006, Abbott et al., 2010, Abbott and Friedman, 2012). The developmental trajectory of additional components of the neurovascular unit would also be an important area for future investigation.
CONCLUSIONS
Findings from the present study provide first evidence for unique ontogenic patterns of claudin-3, claudin-12, and JAM-A expression during ovine development. These findings extend our previous work (Duncan et al., 2009) and suggest that the developmental patterns of expression for each tight junction protein are unique to each member of the tight junction protein family. Moreover, consistent with our previous work increases in endogenous glucocorticoids appear to influence the expression of some but not all of the tight junction proteins (Duncan et al., 2009).
Claudin-3, -12 and JAM-A are expressed as very early in ovine gestation.
Claudin-3, -12 and JAM-A each exhibit differential developmental regulation in sheep.
Increasing endogenous glucocorticoids modulate claudin-3 expression in the fetus.
Acknowledgments
GRANTS
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number 1R01-HD-057100, by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20 RR018728 and P20GM103537.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURE
The authors have no duality or conflicts of interests to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott NJ, Friedman A. Overview and introduction: the blood-brain barrier in health and disease. Epilepsia. 2012;53(Suppl 6):1–6. doi: 10.1111/j.1528-1167.2012.03696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Anstrom J, Thore C, Moody D, Brown W. Immunolocalization of tight junction proteins in blood vessels in human germinal matrix and cortex. Histochem Cell Biol. 2007;127:205–213. doi: 10.1007/s00418-006-0232-z. [DOI] [PubMed] [Google Scholar]
- Aurrand-Lions M, Johnson-Leger C, Imhof BA. Role of interendothelial adhesion molecules in the control of vascular functions. Vascul Pharmacol. 2002;39:239–246. doi: 10.1016/s1537-1891(03)00012-0. [DOI] [PubMed] [Google Scholar]
- Back SA, Riddle A, Hohimer AR. Role of instrumented fetal sheep preparations in defining the pathogenesis of human periventricular white-matter injury. J Child Neurol. 2006;21:582–589. doi: 10.1177/08830738060210070101. [DOI] [PubMed] [Google Scholar]
- Ballabh P, Hu F, Kumarasiri M, Braun A, Nedergaard M. Development of tight junction molecules in blood vessels of germinal matrix, cerebral cortex, and white matter. Pediatr Res. 2005;58:791–798. doi: 10.1203/01.PDR.0000180535.14093.FB. [DOI] [PubMed] [Google Scholar]
- Barlow RM. The foetal sheep: morphogenesis of the nervous system and histochemical aspects of myelination. J Comp Neurol. 1969;135:249–262. doi: 10.1002/cne.901350302. [DOI] [PubMed] [Google Scholar]
- Begley DJ, Brightman MW. Structural and functional aspects of the blood-brain barrier. Prog Drug Res. 2003;61:39–78. doi: 10.1007/978-3-0348-8049-7_2. [DOI] [PubMed] [Google Scholar]
- Betz AL. An overview of the multiple functions of the blood-brain barrier. NIDA Res Monogr. 1992;120:54–72. [PubMed] [Google Scholar]
- Betz AL, Goldstein GW. Specialized properties and solute transport in brain capillaries. Annu Rev Physiol. 1986;48:241–250. doi: 10.1146/annurev.ph.48.030186.001325. [DOI] [PubMed] [Google Scholar]
- Brightman MW, Reese TS. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969;40:648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Threlkeld SW, Cummings EE, Juan I, Makeyev O, Besio WG, Gaitanis J, Banks WA, Sadowska GB, Stonestreet BS. Ischemia-reperfusion impairs blood-brain barrier function and alters tight junction protein expression in the ovine fetus. Neuroscience. 2012;226:89–100. doi: 10.1016/j.neuroscience.2012.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook CJ, Williams C, Gluckman PD. Brainstem auditory evoked potentials in the fetal sheep, in utero. J Dev Physiol. 1987;9:429–439. [PubMed] [Google Scholar]
- Coyne CB, Gambling TM, Boucher RC, Carson JL, Johnson LG. Role of claudin interactions in airway tight junctional permeability. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1166–1178. doi: 10.1152/ajplung.00182.2003. [DOI] [PubMed] [Google Scholar]
- Daneman R. The blood-brain barrier in health and disease. Ann Neurol. 2012;72:648–672. doi: 10.1002/ana.23648. [DOI] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Agalliu D, Cahoy JD, Kaushal A, Barres BA. The Mouse Blood-Brain Barrier Transcriptome: A New Resource for Understanding the Development and Function of Brain Endothelial Cells. PLoS One. 2010;5:e13741. doi: 10.1371/journal.pone.0013741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan AR, Sadowska GB, Stonestreet BS. Ontogeny and the effects of exogenous and endogenous glucocorticoids on tight junction protein expression in ovine cerebral cortices. Brain Res. 2009;1303:15–25. doi: 10.1016/j.brainres.2009.09.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ek CJ, Dziegielewska KM, Habgood MD, Saunders NR. Barriers in the developing brain and Neurotoxicology. Neurotoxicology. 2012;33:586–604. doi: 10.1016/j.neuro.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Ek CJ, Dziegielewska KM, Stolp H, Saunders NR. Functional effectiveness of the blood-brain barrier to small water-soluble molecules in developing and adult opossum (Monodelphis domestica) J Comp Neurol. 2006;496:13–26. doi: 10.1002/cne.20885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt B. Development of the blood-brain barrier. Cell Tissue Res. 2003;314:119–129. doi: 10.1007/s00441-003-0751-z. [DOI] [PubMed] [Google Scholar]
- Fraemohs L, Koenen RR, Ostermann G, Heinemann B, Weber C. The functional interaction of the beta 2 integrin lymphocyte function-associated antigen-1 with junctional adhesion molecule-A is mediated by the I domain. J Immunol. 2004;173:6259–6264. doi: 10.4049/jimmunol.173.10.6259. [DOI] [PubMed] [Google Scholar]
- Furuse M, Sasaki H, Tsukita S. Manner of interaction of heterogeneous claudin species within and between tight junction strands. J Cell Biol. 1999;147:891–903. doi: 10.1083/jcb.147.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn AJ, Gunn TR, de Haan HH, Williams CE, Gluckman PD. Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. J Clin Invest. 1997;99:248–256. doi: 10.1172/JCI119153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseloff RF, Dithmer S, Winkler L, Wolburg H, Blasig IE. Transmembrane proteins of the tight junctions at the blood-brain barrier: structural and functional aspects. Semin Cell Dev Biol. 2015;38:16–25. doi: 10.1016/j.semcdb.2014.11.004. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Jimenez AJ, Dominguez-Pinos MD, Guerra MM, Fernandez-Llebrez P, Perez-Figares JM. Structure and function of the ependymal barrier and diseases associated with ependyma disruption. Tissue Barriers. 2014;2:e28426. doi: 10.4161/tisb.28426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CR, Sadowska GB, Petersson KH, Merino M, Sysyn GD, Padbury JF, Stonestreet BS. Effects of postnatal steroids on Na+/K+-ATPase activity and alpha1- and beta1-subunit protein expression in the cerebral cortex and renal cortex of newborn lambs. Reprod Fertil Dev. 2006;18:413–423. doi: 10.1071/rd05114. [DOI] [PubMed] [Google Scholar]
- Kniesel U, Risau W, Wolburg H. Development of blood-brain barrier tight junctions in the rat cortex. Brain Res Dev Brain Res. 1996;96:229–240. doi: 10.1016/0165-3806(96)00117-4. [DOI] [PubMed] [Google Scholar]
- Kratzer I, Vasiljevic A, Rey C, Fevre-Montange M, Saunders N, Strazielle N, Ghersi-Egea JF. Complexity and developmental changes in the expression pattern of claudins at the blood-CSF barrier. Histochem Cell Biol. 2012;138:861–879. doi: 10.1007/s00418-012-1001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause G, Winkler L, Mueller SL, Haseloff RF, Piontek J, Blasig IE. Structure and function of claudins. Biochim Biophys Acta. 2008;1778:631–645. doi: 10.1016/j.bbamem.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Liddelow SA, Dziegielewska KM, Ek CJ, Habgood MD, Bauer H, Bauer H-C, Lindsay H, Wakefield MJ, Strazielle N, Kratzer I, Møllgård K, Ghersi-Egea J-F, Saunders NR. Mechanisms That Determine the Internal Environment of the Developing Brain: A Transcriptomic, Functional and Ultrastructural Approach. PLoS One. 2013;8:e65629. doi: 10.1371/journal.pone.0065629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, Taketo MM, von Melchner H, Plate KH, Gerhardt H, Dejana E. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol. 2008;183:409–417. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luissint AC, Artus C, Glacial F, Ganeshamoorthy K, Couraud PO. Tight junctions at the blood brain barrier: physiological architecture and disease-associated dysregulation. Fluids Barriers CNS. 2012;9:23. doi: 10.1186/2045-8118-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaeb SN, Hovanesian V, Sarasin MD, Hartmann SM, Sadowska GB, Stonestreet BS. Effects of maternal antenatal glucocorticoid treatment on apoptosis in the ovine fetal cerebral cortex. J Neurosci Res. 2009;87:179–189. doi: 10.1002/jnr.21825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaeb SN, Sadowska GB, Stonestreet BS. Effects of maternal treatment with corticosteroids on tight junction protein expression in the cerebral cortex of the ovine fetus with and without exposure to in utero brain ischemia. Brain Res. 2007;1160:11–19. doi: 10.1016/j.brainres.2007.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milatz S, Krug SM, Rosenthal R, Gunzel D, Muller D, Schulzke JD, Amasheh S, Fromm M. Claudin-3 acts as a sealing component of the tight junction for ions of either charge and uncharged solutes. Biochim Biophys Acta. 2010;1798:2048–2057. doi: 10.1016/j.bbamem.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Mullier A, Bouret SG, Prevot V, Dehouck B. Differential distribution of tight junction proteins suggests a role for tanycytes in blood-hypothalamus barrier regulation in the adult mouse brain. J Comp Neurol. 2010;518:943–962. doi: 10.1002/cne.22273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus W, Wirth M, Plattner VE, Germann B, Gabor F, Noe CR. Expression of Claudin-1, Claudin-3 and Claudin-5 in human blood-brain barrier mimicking cell line ECV304 is inducible by glioma-conditioned media. Neurosci Lett. 2008;446:59–64. doi: 10.1016/j.neulet.2008.09.025. [DOI] [PubMed] [Google Scholar]
- Nico B, Quondamatteo F, Herken R, Marzullo A, Corsi P, Bertossi M, Russo G, Ribatti D, Roncali L. Developmental expression of ZO-1 antigen in the mouse blood-brain barrier. Brain Res Dev Brain Res. 1999;114:161–169. doi: 10.1016/s0165-3806(99)00008-5. [DOI] [PubMed] [Google Scholar]
- Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer F, Schafer J, Lyck R, Makrides V, Brunner S, Schaeren-Wiemers N, Deutsch U, Engelhardt B. Claudin-1 induced sealing of blood-brain barrier tight junctions ameliorates chronic experimental autoimmune encephalomyelitis. Acta Neuropathol. 2011;122:601–614. doi: 10.1007/s00401-011-0883-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron NP, Kazianis JA, Padbury JF, Brown CM, McGonnigal BG, Sysyn GD, Sadowska GB, Stonestreet BS. Ontogeny and the effects of corticosteroid pretreatment on aquaporin water channels in the ovine cerebral cortex. Reprod Fertil Dev. 2005;17:535–542. doi: 10.1071/rd03044. [DOI] [PubMed] [Google Scholar]
- Sadowska GB, Malaeb SN, Stonestreet BS. Maternal glucocorticoid exposure alters tight junction protein expression in the brain of fetal sheep. Am J Physiol Heart Circ Physiol. 2010;298:H179–188. doi: 10.1152/ajpheart.00828.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowska GB, Patlak CS, Petersson KH, Stonestreet BS. Effects of multiple courses of antenatal corticosteroids on blood-brain barrier permeability in the ovine fetus. J Soc Gynecol Investig. 2006;13:248–255. doi: 10.1016/j.jsgi.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Sadowska GB, Stopa EG, Stonestreet BS. Ontogeny of connexin 32 and 43 expression in the cerebral cortices of ovine fetuses, newborns, and adults. Brain Res. 2009;1255:51–56. doi: 10.1016/j.brainres.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowska GB, Threlkeld SW, Flangini A, Sharma S, Stonestreet BS. Ontogeny and the effects of in utero brain ischemia on interleukin-1beta and interleukin-6 protein expression in ovine cerebral cortex and white matter. Int J Dev Neurosci. 2012;30:457–463. doi: 10.1016/j.ijdevneu.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrade A, Sade H, Couraud PO, Romero IA, Weksler BB, Niewoehner J. Expression and localization of claudins-3 and -12 in transformed human brain endothelium. Fluids Barriers CNS. 2012;9:6. doi: 10.1186/2045-8118-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JS, Hyun SY, Kim DH, Lee S, Jung JW, Choi JW, Ko KH, Kim JM, Ryu JH. Chronic hypoperfusion increases claudin-3 immunoreactivity in rat brain. Neurosci Lett. 2008;445:144–148. doi: 10.1016/j.neulet.2008.08.082. [DOI] [PubMed] [Google Scholar]
- Spasova MS, Sadowska GB, Threlkeld SW, Lim YP, Stonestreet BS. Ontogeny of inter-alpha inhibitor proteins in ovine brain and somatic tissues. Exp Biol Med (Maywood) 2014;239:724–736. doi: 10.1177/1535370213519195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatovic SM, Sladojevic N, Keep RF, Andjelkovic AV. Relocalization of junctional adhesion molecule A during inflammatory stimulation of brain endothelial cells. Mol Cell Biol. 2012;32:3414–3427. doi: 10.1128/MCB.06678-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stonestreet BS, Elitt CM, Markowitz J, Petersson KH, Sadowska GB. Effects of antenatal corticosteroids on regional brain and non-neural tissue water content in the ovine fetus. J Soc Gynecol Investig. 2003;10:59–66. doi: 10.1016/s1071-5576(02)00258-7. [DOI] [PubMed] [Google Scholar]
- Stonestreet BS, Patlak CS, Pettigrew KD, Reilly CB, Cserr HF. Ontogeny of blood-brain barrier function in ovine fetuses, lambs, and adults. Am J Physiol. 1996;271:R1594–1601. doi: 10.1152/ajpregu.1996.271.6.R1594. [DOI] [PubMed] [Google Scholar]
- Stonestreet BS, Petersson KH, Sadowska GB, Pettigrew KD, Patlak CS. Antenatal steroids decrease blood-brain barrier permeability in the ovine fetus. Am J Physiol. 1999;276:R283–289. doi: 10.1152/ajpregu.1999.276.2.R283. [DOI] [PubMed] [Google Scholar]
- Stonestreet BS, Sadowska GB, McKnight AJ, Patlak C, Petersson KH. Exogenous and endogenous corticosteroids modulate blood-brain barrier development in the ovine fetus. Am J Physiol Regul Integr Comp Physiol. 2000;279:R468–477. doi: 10.1152/ajpregu.2000.279.2.R468. [DOI] [PubMed] [Google Scholar]
- Sysyn GD, Petersson KH, Patlak CS, Sadowska GB, Stonestreet BS. Effects of postnatal dexamethasone on blood-brain barrier permeability and brain water content in newborn lambs. Am J Physiol Regul Integr Comp Physiol. 2001;280:R547–553. doi: 10.1152/ajpregu.2001.280.2.R547. [DOI] [PubMed] [Google Scholar]
- Virgintino D, Errede M, Robertson D, Capobianco C, Girolamo F, Vimercati A, Bertossi M, Roncali L. Immunolocalization of tight junction proteins in the adult and developing human brain. Histochem Cell Biol. 2004;122:51–59. doi: 10.1007/s00418-004-0665-1. [DOI] [PubMed] [Google Scholar]
- Virgintino D, Girolamo F, Errede M, Capobianco C, Robertson D, Stallcup WB, Perris R, Roncali L. An intimate interplay between precocious, migrating pericytes and endothelial cells governs human fetal brain angiogenesis. Angiogenesis. 2007;10:35–45. doi: 10.1007/s10456-006-9061-x. [DOI] [PubMed] [Google Scholar]
- Wintour EM. Developmental aspects of the hypothalamic-pituitary-adrenal axis. J Dev Physiol. 1984;6:291–299. [PubMed] [Google Scholar]
- Wolburg H, Lippoldt A. Tight junctions of the blood–brain barrier: development, composition and regulation. Vascul Pharmacol. 2002;38:323–337. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- Wolburg H, Noell S, Mack A, Wolburg-Buchholz K, Fallier-Becker P. Brain endothelial cells and the glio-vascular complex. Cell Tissue Res. 2009;335:75–96. doi: 10.1007/s00441-008-0658-9. [DOI] [PubMed] [Google Scholar]
- Wolburg H, Wolburg-Buchholz K, Kraus J, Rascher-Eggstein G, Liebner S, Hamm S, Duffner F, Grote EH, Risau W, Engelhardt B. Localization of claudin-3 in tight junctions of the blood-brain barrier is selectively lost during experimental autoimmune encephalomyelitis and human glioblastoma multiforme. Acta Neuropathol. 2003;105:586–592. doi: 10.1007/s00401-003-0688-z. [DOI] [PubMed] [Google Scholar]