Abstract
Obesity is a significant problem in the United States, with roughly one third of adults having a body mass index (BMI) over thirty. Recent evidence from human studies suggests that pre-existing differences in the function of mesolimbic circuits that mediate motivational processes may promote obesity and hamper weight loss. However, few preclinical studies have examined pre-existing neurobehavioral differences related to the function of mesolimbic systems in models of individual susceptibility to obesity. Here, we used selectively bred obesity-prone and obesity-resistant rats to examine 1) the effect of a novel “junk-food” diet on the development of obesity and metabolic dysfunction, 2) over-consumption of “junk-food” in a free access procedure, 3) motivation for food using instrumental procedures, and 4) cocaine-induced locomotor activity as an index of general mesolimbic function. As expected, eating a sugary, fatty, “junk-food” diet exacerbated weight gain and increased fasted insulin levels only in obesity-prone rats. In addition, obesity-prone rats continued to over-consume junk-food during discrete access testing, even when this same food was freely available in the home cage. Furthermore, when asked to press a lever to obtain food in an instrumental task, rates of responding were enhanced in obesity-prone versus obesity-resistant rats. Finally, obesity-prone rats showed a stronger locomotor response to 15 mg/kg cocaine compared to obesity-resistant rats prior to any diet manipulation. This enhanced sensitivity to this dose of cocaine is indicative of basal differences in the function of mesolimbic circuits in obesity-prone rats. We speculate that pre-existing differences in motivational systems may contribute to over-consumption and enhanced motivation in susceptible individuals.
Keywords: Obesity, cocaine, junk-food, nucleus accumbens, feeding, striatum, reward
1. Introduction
Obesity is the leading cause of type 2 diabetes, and increases the risk of developing cardiovascular disease and some cancers (Luchsinger and Gustafson, 2009, Farag and Gaballa, 2011, Ogden et al., 2014). While metabolic dysfunction and deregulation of hypothalamic circuits that regulate hunger and satiety play critical roles (Levin and Routh, 1996, Williams, 2012, Keen-Rhinehart et al., 2013), numerous studies have highlighted the importance of neural circuits that mediate reward and motivation in over-consumption of calorie-dense, palatable foods (Rothemund et al., 2007, Volkow et al., 2008, Johnson and Kenny, 2010, Volkow et al., 2011, Dagher, 2012, Stice et al., 2012). These motivational circuits include convergent dopamine inputs from the ventral tegmental area (VTA) and glutamatergic inputs from the prefrontal cortex and amygdala to GABAergic medium spiny neurons in striatal regions including the nucleus accumbens (NAc) and dorsal striatum (DS).
In humans, activation of striatal regions in response to stimuli associated with food (food cues) is enhanced in obese individuals (Rothemund et al., 2007, Stoeckel et al., 2008), even before the onset of obesity (Stice et al., 2010, Demos et al., 2012, Murdaugh et al., 2012). Thus, it has been proposed that enhanced striatal reactivity to food-cues may hamper weight loss and promote weight gain in susceptible people (Kramer et al., 1989, Jeffery et al., 2000, Carnell and Wardle, 2008, Stice et al., 2012, Burger and Stice, 2014). Furthermore, PET studies have found lower striatal D2-dopamine receptor binding in obese individuals (Wang et al., 2001), and genetic variation in D2-dopamine receptor and dopamine transporter alleles has been linked to obesity in humans (Stice et al., 2008, Stice and Dagher, 2010, Carpenter et al., 2013), though see also (Steele et al., 2010). These data suggest that basal differences in mesolimbic circuits, and striatal function in particular, may contribute to over-eating in people (Vucetic and Reyes, 2010, Stice et al., 2012, Albuquerque et al., 2015). However, very few studies have examined basal differences in motivation and mesolimbic function in models that capture individual susceptibility to obesity.
Here, we used selectively bred obesity-prone and obesity-resistant rats to examine basal differences in motivation for food using instrumental procedures, and sensitivity to cocaine-induced locomotion as a read out of mesolimbic function. It is well established that the locomotor activating effects of stimulant drugs like amphetamine and cocaine rely in large part on striatal activation, particularly of dopaminergic projections from the VTA to the NAc (Robinson and Becker, 1986, Vezina, 2004), as well as glutamatergic and peptidergic transmission within the striatum (Wolf, 1998, Rebec, 2006, Hubert et al., 2008, Erreger et al., 2012). Thus, differences in sensitivity to the locomotor activating effects of cocaine are indicative of alterations in striatal and mesolimbic function (Meyer et al., 2009, Vezina and Leyton, 2009). Although previous studies have examined the effects of high fat/high sugar diets on mesolimbic systems, no previous studies have examined basal differences in cocaine-induced locomotor activity in obesity-susceptible populations.
The selectively bred rats used here were originally developed by Barry Levin and colleagues (originally referred to as DIO and DR respectively; Levin et al., 1997). Previous work has shown that basal insulin and leptin levels are disrupted in these obesity-prone rats prior to metabolic dysfunction and overt obesity (Levin and Dunn-Meynell, 2002). These peripheral metabolic signals can directly and indirectly affect the firing of VTA neurons, and modulate dopamine- and glutamate-mediated transmission (Perry et al., 2010, Bruijnzeel et al., 2011). In addition, previous work has shown that basal and evoked DA release from brain slices is lower in the NAc shell of female obesity-prone rats prior to diet manipulation (Geiger et al., 2008). However, no studies have examined basal differences in the motivation to obtain food or sensitivity to the locomotor activating effects of cocaine prior to diet manipulation. Examination of basal differences are important because studies in people suggest that differences in the function of striatal circuits may drive the development of obesity (Jansen, 1998, Jansen et al., 2008, Stoeckel et al., 2008, Dagher, 2009, Tetley et al., 2009, Stice et al., 2010, Demos et al., 2012, Vainik et al., 2013, Burger and Stice, 2014)
2. Methods
2.1. Subjects
Twelve obesity-prone (OP) and obesity-resistant (OR) non-sibling breeding pairs were purchased from Taconic Laboratories (Hudson, NY). These rat lines were originally established through selective breeding of Sprague Dawley rats (Levin et al., 1997) and are being maintained by the University of Michigan Breeding Core using an outbred rotational system within closed populations. For all studies, male rats were 70 days old at the start of experiments and were pair-housed on a reverse 12-hour light/dark cycle. All rats had free access to food and water throughout and all measures were made in age-matched rats. Procedures were approved by The University of Michigan Committee on the Use and Care of Animals.
2.2. “Junk-food”
The “junk-food” diet is composed of a mash of: Ruffles original potato chips (40g), Chips Ahoy original chocolate chip cookies (130g), Jiff creamy peanut butter (130g), Nesquik powdered chocolate flavoring (130g), powdered Lab Diet 5001 (200 g) and water (180 ml), giving a diet composed of 19.6% fat, 14% protein, and 58% carbohydrates (4.5 kcal/g). Ingredients were combined in a food processor. These foods contain a rich mix of sugars, salt, and fats, and were chosen as representatives of “junk foods” implicated in human obesity. Diet composition was based on previous studies establishing individual differences in susceptibility to weight gain due to over consumption (Levin et al., 1989, Levin et al., 1997) and was closely matched to kcal/g of standard lab chow (Lab Diet 5001: 4 kcal/g; 4.5% fat, 23% protein, 48.7% carbohydrates; % of caloric content).
2.3. Confirmation of phenotypic differences between obesity-prone and obesity-resistant rats
2.3.1. Weight Gain, Home Cage Food Intake, and Body Composition
Selectively bred obesity-prone and obesity-resistant rats were given free access to either standard lab chow or the junk-food mash described above (OP-Junk-food N=6, OR-Junk-food N=8; OP-Chow N=6, OR-Chow N=6) and were weighed once per week for 4 weeks. After this initial 4-week period, daily home cage consumption was measured in the chow-fed groups (4 consecutive days). Next, these same chow-fed rats were given free access to both junk-food and chow in their home cages and consumption of each diet was measured for an additional 4 days. Body composition measures were made in the chow-fed rats prior to junk-food diet exposure (late adulthood, ~120 days old) and in a separate cohort of chow-fed rats during early adulthood (~70 days old; OP N=10, OR N=12). Body composition (fat, lean, and free fluid mass) was measured using an NMR-based analyzer (Minispec LF90II, Bruker Optics) by the University of Michigan Animal Phenotyping Core. Conscious rats were placed in an oblong measuring tube during the 2-minute scan.
2.3.2. Home Cage Locomotor Activity and Body Temperature
Home cage locomotor activity and body temperature changes were observed over 48 hours. Locomotor activity was measured via radio transmitter telemetry devices (model ER-4000 E-Mitter, Mini Mitter Co., Bend, OR) placed within the abdominal cavity (OP N=6 OR N=6, ~ 90 days old). Animals were housed in a standard 12:12 light:dark cycle. Rats were anesthetized with ketamine (90 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.), the abdomen was cleaned with betadine and alcohol, and then a 1–2 cm rostral-caudal incision was made in the skin and underlying musculature to expose the peritoneal cavity. The telemeter was placed inside the peritoneal cavity and the incision closed with absorbable Ethicon Vicryl 5-0 coated suture (muscle) and non-absorbable Ethicon Nylon 5-0 suture (skin). Rats were allowed to recover for 6–8 days before making measurements. The telemeter transmitted activity and temperature data to a receiver (model ER-4000 Receiver, Mini Mitter Co.) placed directly under the home cage of each rat. Locomotor and body temperature data were collected every 5 minutes over 48 hours and were processed in 1 hour bins using Vital View software (Mini Mitter Co.).
2.3.3. Fasted Plasma Insulin Levels
Fasted (16 hrs) plasma insulin levels were determined after free access to either chow or junk-food in the home cage for 4 weeks (OP-Junk-food N=6, OR-Junk-food N=6; OP-Chow N=4, OR-Chow N=4). Blood samples were collected via tail nick into tubes containing EDTA (1.6 mg/mL, Sarstedt), and plasma was then isolated by centrifugation (1000 x G, 4°C, 10 min) and stored (−20°C) for subsequent analysis. Plasma insulin levels were determined by double-antibody radioimmunoassay using an 125I-Human insulin tracer (Linco Research, St. Charles, MO), a rat insulin standard (Novo Nordisk, Plainsboro, NJ), a guinea pig anti-rat insulin first antibody (Linco Research), and a sheep anti-guinea pig gamma globulin-PEG second antibody (Michigan Diabetes Research Core). The limit of sensitivity for this assay was 1 μU/ml. Inter-assay and intra-assay variability were 11.2% and 3.2%, respectively, at 30.5 μU/ml.
2.4. Over-consumption and instrumental responding for food
2.4.1. Discrete Junk-food Consumption Outside the Home Cage
Above we measured daily home cage food consumption. Here, using another cohort of rats, discrete junk-food consumption outside the home cage was measured prior to, and during home-cage junk-food diet exposure using a within subjects design (OP N=9, OR N=8). Two days prior to the first consumption test rats were given 5 grams of junk-food in their home cages to familiarize them with this new food. Rats were then habituated to the test chamber (20 min/day, 3 days) prior to testing. On each discrete consumption test day, 15 grams of junk-food was placed in a crock in the corner of the testing chamber (operant box, Med Associates; St Albans City, VT) and rats were allowed to eat freely for 20 minutes. The amount consumed was determined by weighing the remaining junk-food, including any spillage. Testing was conducted ~ 3 hours after the onset of the dark cycle. Rats were weighed prior to each test and daily home cage food intake was measured throughout the experiment.
2.4.2. Instrumental Responding for Food
Instrumental training and progressive ratio testing were used to evaluate motivation for food in adult obesity-prone and obesity-resistant rats (OP N=20, OR N=20). Rats were 65 days old at the start of training. First, rats were exposed to food pellets (Bioserv dustless precision pellet, cat. # F0021, 45 mg, 25 pellets) in their home cage and then on a random interval in the food cup of a standard operant box (Med Associates). Next, rats underwent instrumental training in which pressing one lever (active) resulted in the delivery of one food pellet, the illumination of the lever-light, and a tone (3 sec, 4.7 kHz, ~ 60 dB). Pressing on a second, inactive lever earned nothing, but was recorded. Active and inactive levers were counterbalanced for left/right position relative to the food cup. Rats were trained in three sessions in which each response on the active lever resulted in delivery of a food pellet (fixed ratio 1, FR1, 30 min/session) followed by three sessions in which three responses on the active lever were required to receive one food pellet (fixed ratio 3, FR3, 30 min/session) before progressive ratio (PR) testing (3 sessions). During PR testing the number of lever presses required to obtain each subsequent food pellet was gradually increased (5e(delivery# × 0.2)-5; adapted from (Richardson and Roberts, 1996, Naleid et al., 2008). The PR session ended automatically when rats did not meet the next ratio requirement within 30 minutes (i.e., breakpoint). Home cage food intake was measured for 2 days prior to training and body weight was recorded twice per week.
2.5 Assessment of General Mesolimbic Function Prior to Obesity
Cocaine-Induced Locomotor Activity
The acute locomotor response to cocaine was assessed in a separate cohort of rats without diet manipulation (70 days old, OP N=5, OR N=5). Cocaine HCL was provided by the NIDA drug supply program. Locomotor activity was evaluated in testing chambers (41 × 25.4 × 20.3 cm) equipped with an array of photocell beams. Rats were placed in locomotor chambers for a 45-minute habituation period prior to receiving an injection of saline (1 mL/kg, i.p.), followed 1 hour later by a cocaine injection (15 mg/kg, i.p.). Animals were returned to their home cage after an additional hour. Locomotor activity was recorded throughout all testing. In addition, rats were observed for rearing and stereotyped movements of the head and forelimbs. This is important because these behaviors can interfere with locomotor activity measures (see also Ferrario et al., 2005 for discussion).
2.6. Statistical Analysis
Two-tailed t-tests were used for comparisons between two groups unless otherwise stated. For comparison of three or more groups one-way or two-way repeated measures ANOVAs were used, followed by Sidak’s post-hoc multiple comparisons test when appropriate. Statistical analyses were conducted in Prism 6 (GraphPad, San Diego, CA).
3. Results
3.1 Phenotypic differences between obesity-prone and obesity-resistant rats
3.1.1. Spontaneous and junk-food diet-induced differences in weight gain and fat mass in obesity-prone rats
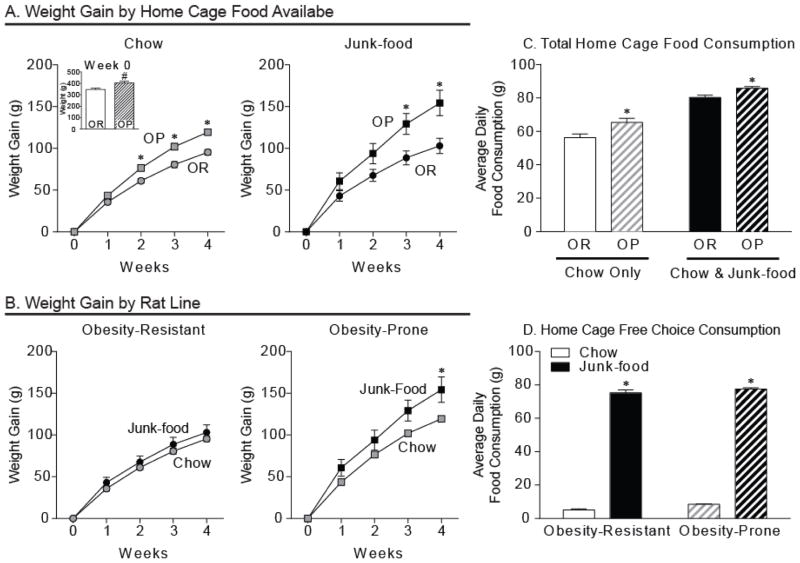
As expected, obesity-prone rats were significantly heavier than obesity-resistant rats prior to any diet manipulation (OP N=12, OR N=14; Fig. 1A inset; t26 = 3.38, p < 0.005), and gained ~25% more weight than obesity-resistant rats even while eating standard lab chow (OP-Chow N=6, OR-Chow N=6; Fig. 1A, left panel; two-way RM ANOVA group x time interaction: F(4, 40) = 12.90, p < 0.0001; Post-hoc: p < 0.01 week 2, p < 0.0001 weeks 3 and 4). This weight gain difference was exacerbated in obesity-prone rats given free home cage access to junk-food, with obesity-prone rats gaining ~50% more than obesity-resistant rats given the same junk-food (OP-Junk-food N=6, OR-Junk-food N=8; Fig. 1A, right panel; two-way RM ANOVA group x time interaction: F(4, 48) = 8.74 p < 0.0001; Post-hoc: p < 0.05 week 3, p < 0.001 week 4). Consistent with the obesity-resistant phenotype, weight gain trajectories of obesity-resistant rats given junk-food did not differ from their chow-fed counterparts (OR-Junk-food N=8, OR-Chow N=6; Fig. 1B, left panel; two-way RM ANOVA: n.s.). In contrast, free access to junk-food in obesity-prone rats resulted in a dramatically increased rate of weight gain compared to obesity-prone rats on chow (OP-Junk-food N=6, OP-Chow N=6; Fig. 1B, right panel; two-way RM ANOVA group x time interaction: F(4, 40) = 4.25, p < 0.01; Post-hoc: p < 0.05 week 4).
Figure 1.
Only obesity-prone rats gain substantial weight when given free access to junk-food, but both groups prefer junk-food over chow. A) Average weight (inset) and weight gain (± SEM) between obesity-prone (OP) and obesity-resistant (OR) rats given free home cage access to either standard lab chow (left) or junk-food (right) diet. Obesity-prone rats were heavier at the start of the experiment (inset: # p < 0.01) and consistently gained more weight than obesity-resistant rats. B) Average weight gain (± SEM) within groups. Eating junk-food versus chow produced substantial weight gain in obesity-prone rats, but did not alter weight gain trajectories of obesity-resistant rats. C) Average total home cage consumption (± SEM) in rats given free access to chow or a free choice between chow and junk-food in their home cages. Obesity-prone rats consumed more food than obesity-resistant rats. D) Average consumption (± SEM) of each food type when given a free access to both chow and junk-food in the home cage. Both groups preferred junk-food to chow when given a choice between the two. These data suggest that lower junk-food consumption in obesity-resistant rats is not due to a reduced preference for junk-food.
Consistent with weight gain differences, home cage food intake was greater in obesity-prone versus obesity-resistant rats when either chow or a choice between chow and junk-food diet was available (Fig. 1C; two-way RM ANOVA main effect of strain: F(1, 4) = 14.56, p < 0.05), though food intake did increase in both groups when a choice between the two diets was given (Fig. 1C; two-way RM ANOVA main effect of food type F(1, 4) = 154.1, p < 0.001). Importantly, both groups showed similar preference for junk-food over chow when given a free choice between both in the home cage (Fig. 1D; two-way ANOVA main effect of chow versus junk-food consumed: F(1, 4) = 3281, p < 0.0001), though obesity-prone rats still ate more overall (Fig. 1D; two-way ANOVA main effect of group: F(1, 4) = 12.72, p < 0.04). These free choice data suggest that differences in consumption are likely due to differences in hunger and/or satiety, and not sensory perception, as both groups showed a similar preference for junk-food.
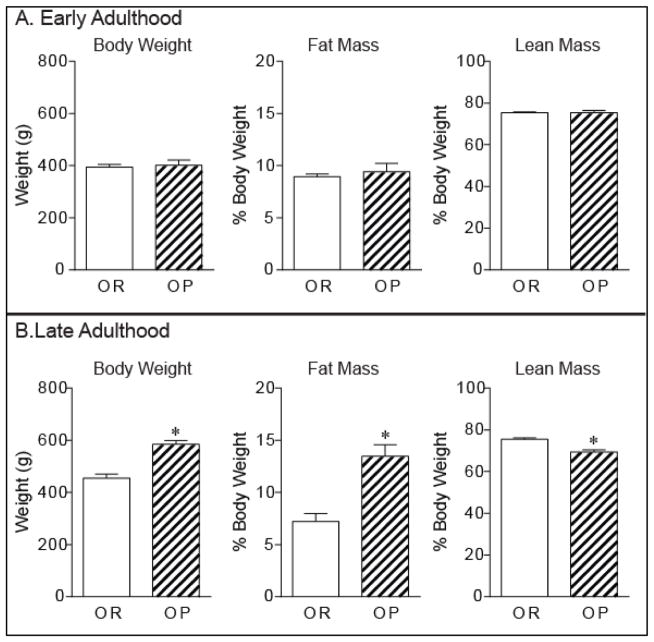
Body composition in chow-fed rats show that fat and lean mass were similar in obesity-prone and obesity-resistant rats during early adulthood (70 days old), a time when weight differences were absent (Fig. 2A). However, in late adulthood (120 days old) weight and fat mass were significantly elevated in obesity-prone versus obesity-resistant rats (Fig. 2;B weight: t10 = 6.25, p < 0.0001 ~25% greater in OP; fat mass: t10 = 4.76, p < 0.001, ~ 2-fold higher in OP). In addition, while fat mass was increased, lean mass was also lower in obesity-prone versus obesity-resistant rats (Fig. 2B; t10 = 4.84, p < 0.001, OP % lean mass = 69.36 ± 1.01%; OR % lean mass = 75.48 ± 0.08%).
Figure 2. Weight gain is accompanied by increased fat mass in obesity-prone rats.
Average (± SEM) weight, fat mass, and lean body mass during early adulthood (A; 70 days old) and late adulthood (B; 120 days old) in obesity-prone and obesity-resistant rats given free access to chow. During early adulthood groups do not differ significantly in weight, fat mass, or lean mass (A). During late adulthood, spontaneous weight gain in obesity-prone rats is accompanied by increased fat mass and reduced lean mass (B; * p < 0.05).
3.1.2. Home cage activity is elevated in obesity-prone rats
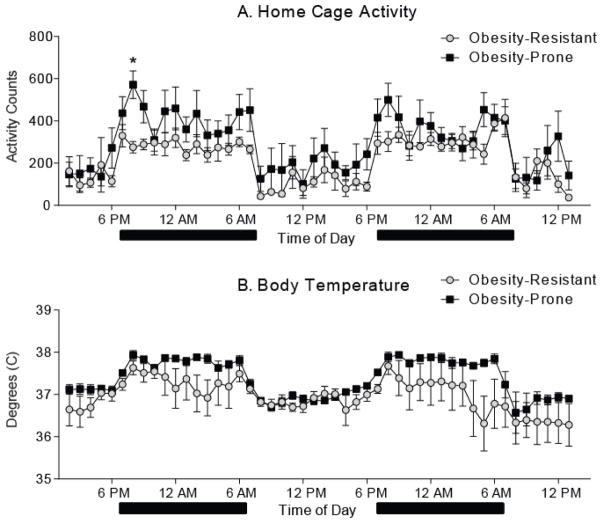
Home cage locomotor activity across the 48 hour recording period was elevated in obesity-prone compared to obesity-resistant rats (Fig. 3A; two-way RM ANOVA: group x time interaction: F(47, 470) = 1.466, p < 0.05). Differences were most pronounced during the first six hours of the dark cycle in the first 24 hour period (Fig. 3A; two-way RM ANOVA, group x time interaction: F(5, 50) = 3.16, p < 0.02; post tests: p < 0.05 at 7 PM). A similar trend was observed during the second 24 hour period, however this did not reach statistical significance. In addition, body temperature across the 48 hour recording period tended to be higher in obesity-prone versus obesity-resistant rats, although this difference did not reach statistical significance (Fig. 3B; two-way RM ANOVA, main effect of group: F(1, 10) = 4.29, p = 0.065).
Figure 3. Locomotor activity during the dark cycle is enhanced in obesity-prone rats.
Home cage activity and body temperature across a 48 hour period were measured via implanted telemeter. Black bars indicate the dark phase of the light/dark cycle. A) Average (± SEM) home cage activity counts. Activity was generally greater in obesity-prone versus resistant rats immediately following dark onset (* p < 0.05). B) Average (± SEM) body temperature. Obesity-prone rats tended to have elevated body temperatures relative to obesity-resistant rats, though this did not reach statistical significance.
3.1.3. Junk-food produces metabolic dysfunction only in obesity-prone rats
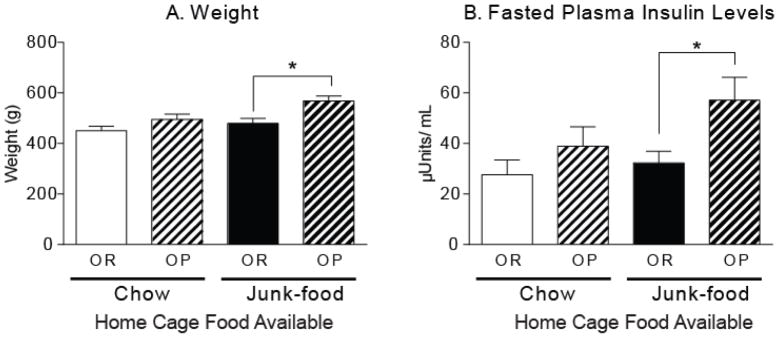
Fasted insulin levels were used to evaluate potential metabolic dysregulation in age-matched obesity-prone and obesity-resistant rats given free access to chow or junk-food diets. Weight did not differ in chow-fed obesity-prone and obesity-resistant rats, though fasted insulin levels tended to be slightly higher in obesity-prone rats (Fig 4). In addition, consistent with food intake data in Figure 1, free access to junk-food in the home cage produced a significant increases in weight of obesity-prone, but not obesity-resistant rats (Fig. 4A; one-way ANOVA, F(3, 16) = 6.78, p < 0.005; Post-hoc: OR-Junk-food versus OP-Junk-food, t16 = 3.48, p < 0.05; OR-Junk-food versus OR-Chow, n.s.). Junk-food induced weight gain was accompanied by elevations in fasted insulin levels only in obesity-prone rats (Fig. 4B; one-way ANOVA F(3, 16) = 3.42, p < 0.05; Post-hoc: OP-Junk-food versus OR-Junk-food, t16 = 2.63, p < 0.05). These data are consistent with the development of metabolic dysregulation in obesity-prone, but not obesity-resistant, rats given free access to junk-food.
Figure 4. Junk-food induced obesity is accompanied by elevated fasted insulin levels only in obesity-prone rats.
A) Average weight (± SEM) of obesity-prone and obesity-resistant rats after 4 weeks of free access to standard lab chow or junk-food in the home cage. As expected, junk-food produced a significant increase in weight only in obesity-prone rats. B) Average fasted plasma insulin levels (± SEM). After 4 weeks of eating junk-food, fasted insulin levels were significantly elevated in obesity-prone versus obesity-resistant rats (* p < 0.05).
3.2. Over-eating of junk-food and motivation for food in obesity-prone rats
3.2.1. Obesity-prone rats over-consume junk-food during discrete access testing
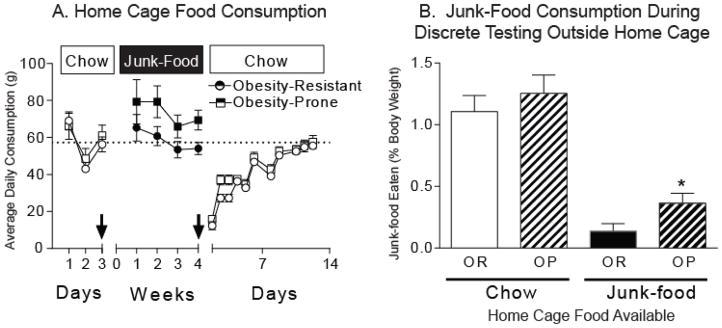
Home cage food consumption and discrete junk-food consumption outside the home cage (20 minutes free access) were measured before and while rats had free access to junk-food in their home cage. When rats had free access to chow in their home cages, consumption of junk-food during the 20 min discrete access test was similar between groups (Fig. 5B light bars; OP = 4.43 ± 0.37 g, OR = 6.27 ± 0.83 g). In addition, chow intake in the home cage did not differ between groups (Fig. 5A). This may be due to age differences between rats used here (~70 days old) and those used in experiments related to Figure 1 (~85 days old) where home cage chow intake was greater in obesity-prone rats. Rats were then given free access to junk-food in their home cage for four weeks and consumption of junk-food during a second 20 min discrete access test was determined. Although home cage access to junk-food generally decreased junk-food consumption outside the home cage (Fig. 5B; two-way RM ANOVA, main effect of home cage diet F(2, 30) = 72.26, p < 0.0001), obesity-prone rats still ate significantly more junk-food during discrete testing than obesity-resistant rats (Fig. 5B: OP = 2.37 ± 0.55 g, OR = 0.73 ± 0.30 g; OP-Junk-food vs. OR-Junk-food, t15 = 2.23, p < 0.05). Thus, in obesity-prone rats free access to junk-food in the home cage did not dampen the motivation to over-consume junk-food as dramatically as in obesity-resistant rats.
Figure 5. Obesity-prone rats over-consume junk-food, even in the face of over-abundance.
A) Average daily home-cage food consumption (± SEM). Boxes show the type of food available. Arrows indicate discrete junk-food consumption tests conducted outside the home cage (20 min/test). Chow intake was similar between groups, and obesity-prone rats tended to eat more junk-food than obesity-resistant rats. In addition, both obesity-prone and obesity-resistant rats dramatically reduce their home cage food intake when returned to standard lab chow, and took nearly two weeks to resume pre-junk-food diet levels of chow intake. B) Average (± SEM) junk-food consumed relative to total body weight during discrete (20 min) testing outside the home cage. When only standard lab chow was available in the home cage, discrete junk-food consumption was similar between groups (light bars). However, after 4 weeks of free access to junk-food in the home cages, obesity-prone rats still consumed significantly more junk-food puring discrete testing than obesity-resistant rats, even when differences in body weight were taken into account (dark bars, * p < 0.05). Thus, although access to junk-food in the home cage decreased discrete consumption in general, obesity-prone rats over-consumed junk-food, despite having continual free access to the same food in their home cages.
3.2.2. Mild enhancement of instrumental responding for food in obesity-prone rats prior to obesity
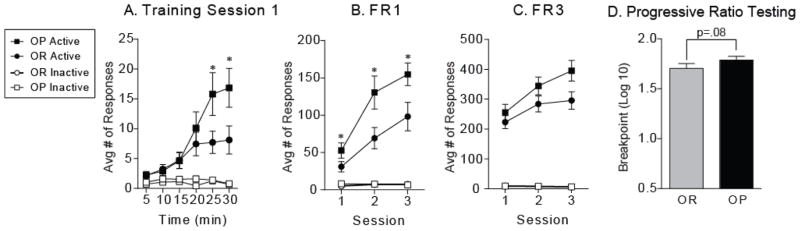
Fixed ratio and progressive ratio testing in an instrumental task were used to evaluate the willingness of obesity-prone and obesity-resistant rats to lever press for a food reward (Fig. 6). Body weight at the start of training was greater in obesity-prone rats (Fig 6A, inset: OP = 388.1 ± 9.7 g, OR = 346.2 ± 6.5 g; t36 = 3.50, p < 0.01). Both groups readily learned to lever press for food during the first training session (Fig. 6A: active versus inactive responding OR: two-way RM ANOVA main effect of lever F(1,17) = 15.5, p < 0.01; active versus inactive responding OP: two-way RM ANOVA main effect of lever F(1,19) = 18.8, p < 0.001), though one obesity-prone rat required an additional FR1 session, and two obesity-resistant rats failed to acquire (i.e., discriminate active from inactive lever responding) and were therefore removed from the study (OP N = 20; OR N =1 8). In addition, responding on the active lever was significantly greater in obesity-prone rats even during the first training session (Fig 6A: two-way RM ANOVA, active responses x time interaction: F(5,180) = 3.72, p < 0.01). This enhanced active lever responding was maintained across additional FR1 training (Fig. 6B; two-way RM ANOVA, Main effect of group: F(1, 38) = 5.83, p < 0.05; Post-hoc: p < 0.05 sessions 2 and 3). In addition, besity-prone rats also received more pellets (data not shown; two-way RM ANOVA, main effect of group: F(1, 36) = 5.19, p < 0.05) and made slightly more food cup entries (data not shown; two-way RM ANOVA, group x session interaction: F(2, 72) = 3.15, p = 0.049; main effect of group: F(1, 36) = 3.55, p = 0.067). A similar pattern of responding was seen when the required number of active lever responses to earn one pellet was increased to 3 (FR3; Fig. 6C; two-way RM ANOVA, main effect of group: F(1, 36) = 3.07, p = 0.089). Willingness to work for food was determined during three PR sessions. Log transformation was performed on breakpoint values to ensure homogeneity of variance for statistical analysis. Consistent with elevated responding during fixed ratio testing, breakpoints were slightly higher in obesity-prone rats, though this did not reach statistical significance (Fig. 6D; one-tailed t test, t36 = 1.41, p = 0.08).
Figure 6. Instrumental responding for food is increased in obesity-prone rats.
A) Average number of responses (± SEM) on the active (pellet) and inactive (no pellet) levers during the first training session. Both obesity-prone and obesity-resistant rats readily acquired the lever pressing task, showing strong discrimination between the active versus the inactive lever. In addition, the magnitude of active lever repsonding was greater in obesity-prone versus obesity-resistant rats (* p < 0.05). B) Average number of responses (± SEM) during three FR1 sessions. The magnitude of active lever presses was greater throughout FR1 testing in obesity-prone versus obesity-resistant rats (B, * p < 0.05). C) Average number of responses (± SEM) during three FR3 sessions. D) Average breakpoint (± SEM) across three progressive ratio test sessions was slightly elevated in obesity-prone rats, but did not reach significance (p=0.08).
3.3. Cocaine-induced locomotion
Obesity-prone rats are more sensitive to the locomotor-activating effects of cocaine
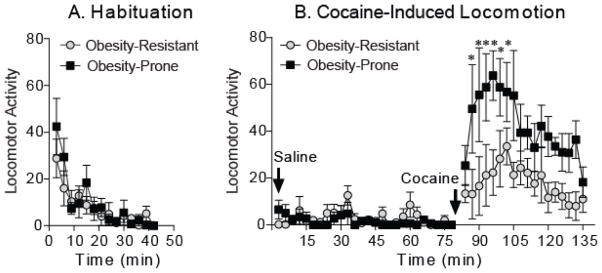
The acute response to a single injection of cocaine (15 mg/kg, i.p.) was used to evaluate general mesolimbic function in obesity-prone and obesity-resistant rats (average weight at time of testing, OP = 411 ± 8.3 g, OR = 365 ± 10.3 g; t8 = 3.51, p <0.01). Cocaine resulted in a significantly stronger locomotor response in obesity-prone versus obesity-resistant rats (Fig. 7; two-way RM ANOVA, group x time interaction: F(58, 464) = 2.60, p < 0.0001). Locomotor activity during habituation and after saline injection were similar between groups, and no rearing or stereotyped head or forelimb movements were observed after cocaine injection (PJV). Importantly, the response to cocaine was greater than the response to saline in obesity-resistant rats (Fig. 7B; two-way RM ANOVA, OR-saline versus OR-cocaine: F(1, 8) = 5.37, p < 0.05). Thus, although cocaine increased locomotor activity in both groups, obesity-prone rats were more sensitive to this effect than obesity-resistant rats.
Figure 7. Obesity-prone rats are more sensitive to the acute locomotor-activating effects of cocaine than obesity-resistant rats.
A) Average locomotor activity (± SEM) during habituation did not differ between groups. B) Average locomotor activity (± SEM) in response to saline and acute cocaine administration (15 mg/kg i.p). The response to saline did not differ between groups. Cocaine produced a stronger locomotor response in obesity-prone versus obesity-resistant rats (* p < 0.05). This enhanced cocaine-induced locomotion is indicative of enhanced responsivity of mesolimbic circuits in obesity-prone versus obesity-resistant rats.
4. Discussion
Recent studies in people suggest that pre-existing differences in striatal function may promote obesity and hamper weight loss (Stoeckel et al., 2008, Dagher, 2009, Tetley et al., 2009, Stice et al., 2010, Demos et al., 2012, Vainik et al., 2013, Burger and Stice, 2014). However, few studies have examined basal differences in mesolimbic or striatal function in models that capture individual susceptibility to obesity (Geiger et al., 2008, Valenza et al., 2015). Here, we first verified obesity-related phenotypes in selectively bred obesity-prone and obesity-resistant rat lines maintained in-house. We then examined basal motivation for, and over-consumption of food, and used cocaine-induced locomotor activity as an index of basal differences in mesolimbic and striatal function.
Consistent with previous results, obesity-prone rats gained significantly more weight than their obesity-resistant counterparts when given free access to standard lab chow (Fig. 1; Levin et al., 1997). However, obesity-prone rats in the current study appear to be slightly heavier than those previously characterized (372 ± 7 g at ~ 75 days old in Levin et al., 1997 compared to 405 ± 13 at ~ 70 days old here). The naturally occurring divergence in weight gain began during early adulthood (~70 to 80 days old) and, similar to findings described by Levin and colleagues, was due at least in part to greater food intake in obesity-prone rats (Fig. 1), though see also (Fig. 5). NMR imaging confirmed that greater body weight in obesity-prone rats was accompanied by an increase in fat mass and concomitant decrease in lean mass (Fig. 2). A previous study of these rats found greater fat mass in young adult rats (2.5 months; Levin, 1997). In contrast, we did not find differences in fat mass until late adulthood (~120 days). This difference may be due to a number of factors including the techniques used to quantify fat mass (NMR here and lipid extraction previously). In addition, we found that obesity-prone rats were more active at the onset of the dark cycle in the home cage than obesity resistant rats (Fig. 3A), while spontaneous locomotor activity in a novel environment outside the home cage did not differ between groups (Fig. 7A). These results differ somewhat from previous reports of lower home cage activity in obesity-prone rats (Levin and Dunn-Meynell, 2004). Again, measured used (wheel-running previously and telemeters here) may contribute to this difference.
When given free-access to junk-food in their home cages, obesity-resistant rats remained on the same weight gain trajectory as their chow-fed counterparts. In contrast, obesity-prone rats given junk-food gained substantially more weight than all other groups (Fig. 1B). These data demonstrate that free-access to junk-food leads to additional weight gain only in obesity-prone rats, and are consistent with previous reports using high energy diet comprised of corn oil and vanilla flavored Ensure (Levin et al., 1997). Furthermore, although food consumption was generally greater in obesity-prone rats, both groups showed a similar preference for junk-food over standard lab chow (Fig. 1D). These data suggest that greater junk-food consumption in obesity-prone rats is not due to differences in sensory perception or hedonic reactivity. Consistent with this interpretation, hedonic responses to sucrose in outbred rats identified as susceptible or resistant to weight gain using this same junk-food diet were similar prior to junk-food exposure (Robinson et al., 2015). In addition, basal intracranial self-stimulation thresholds are similar between obesity-prone and obesity-resistant rats (Valenza et al., 2015). Together, these data suggest that basal hedonic responses to positively reinforcing stimuli do not differ in obesity-prone vs obesity-resistant rats. Finally, as expected, junk-food induced weight gain in obesity-prone rats was accompanied by elevated fasted insulin levels, an indication of metabolic dysfunction (Eckel et al., 2010). Thus, overall obesity-prone and obesity-resistant phenotypes are maintained in our colony and junk-food diet produced obesity and metabolic dysregulation only in obesity-prone rats.
One key aspect of human obesity is continued consumption of palatable, fatty foods in the absence of explicit energy demand (i.e., hedonic consumption). To examine over-consumption, we used a discrete junk-food consumption test in which freely feeding rats were given access to junk-food during two 20-minute sessions outside the home cage. When rats had free access to standard chow in the home cage, obesity-prone and obesity-resistant rats ate equivalent amounts of junk-food relative to their body weights during the discrete test. Thus, when junk-food was not readily available, consumption during the discrete test outside the home cage was balanced in relation to potential energy demand. When rats were given free access to junk-food in the home cage, junk-food intake during discrete testing generally decreased in both groups. However, discrete junk-food consumption was significantly greater in obesity-prone versus obesity-resistant rats, even when differences in body weight were taken into consideration. Thus, obesity-prone rats over-consumed junk-food during discrete access outside the home cage, even when the same junk-food was freely available in the home cage (Fig. 5B). In contrast, obesity-resistant rats appear to better regulate their consumption in the face of over-abundance, eating very little junk-food during the discrete consumption test conducted while junk-food was also freely available in the home cage. Interestingly, this difference seems to be induced by exposure to junk-food (and/or associated weight gain and metabolic dysregulation), as discrete junk-food consumption prior to diet manipulation was similar in obesity-prone and obesity-resistant rats. When rats were given the opportunity to lever press for food, rates of responding were higher in obesity-prone rats when work requirements were relatively low (FR1 and FR3), while break points during progressive ratio testing were only slightly elevated. To our knowledge, no other studies have examined instrumental responding for food in obesity-prone and resistant rats. Taken together with the discrete consumption data discussed above, our results suggest that motivation for food rewards, even in the face of over-abundance, is greater in obesity-prone rats, although obesity-prone rats are not completely incapable of adjusting their behavior to work demands (progressive ratio testing) and food availability.
Striatal function is altered in obese people, and can be modulated by hypothalamic inputs and peripheral metabolic signals (Wang et al., 2001, Rothemund et al., 2007, Castellanos et al., 2009, Burger and Stice, 2012). One well accepted way to assess general mesolimbic and striatal function is to measure the locomotor activating effects of stimulant drugs like cocaine (Smith, 1965, Post and Rose, 1976, Delfs et al., 1990). Cocaine blocks the reuptake of dopamine from the synapse via the dopamine transporter (DAT) and thereby enhances postsynaptic activation of dopamine receptors in target regions of the VTA. This increase in dopamine, and particularly activation of dopamine receptors in the NAc, in turn elicits an increase in cocaine-induced locomotor activity. In the current study, we found that without diet manipulation obesity-prone rats showed a stronger locomotor response than obesity-resistant rats to a single cocaine exposure (15 mg/kg). This is consistent with our recent observation that the dose-response function for cocaine-induced locomotion is shifted to the left in obesity-prone versus resistant rats (Oginsky et al., submitted). That is, obesity-prone rats were sensitized compared to obesity-resistant rats prior to any diet manipulation. Importantly, the concentration of cocaine in the brain was similar between groups (unpublished observation, PJV). Although it has been suggested that reduced dopamine levels may produce a reward deficit and thereby promote obesity (Geiger et al., 2008, Geiger et al., 2009), the enhanced sensitivity to the locomotor-activating effects of cocaine at the dose tested here is instead consistent with an overall hyper-responsivity of mesolimbic circuits and/or striatal reactivity in obesity-prone rats prior to diet manipulation. The stronger response to cocaine (15 mg/kg) found here is consistent with a greater change in NAc shell dopamine in obese rats after amphetamine injection (1.5 mg/kg). Specifically, although basal and amphetamine-induced concentrations of dopamine were lower in the NAc shell in obese rats (Geiger et al., 2009; Fig 2B; peak DA concentration post amphetamine: ~0.1 pmol/25 μl in controls, and ~ 0.05 pmol/25 μl in obese rats), the magnitude of this increase relative to baseline was substantially greater in obese rats (Geiger et al., 2009; Fig 2C; ~500% increase in controls, and ~1500% increase in obese rats). Locomotor activity was not measured in the study by Geiger et al. (2009), and obesity-prone and resistant sub-populations were not examined. However, it may be the case that the greater relative change from baseline in obese rats may be sufficient to elicit a stronger locomotor response, even if the absolute concentration of dopamine is reduced. This possibility deserves further study.
Cocaine induced locomotor activity relies heavily on activation of medium spiny neurons within the NAc and dopamine mediated transmission. Thus, the enhanced locomotor response in obesity-prone rats could be due to differences in extracellular dopamine and/or differences in striatal post-synaptic dopamine receptor-mediated transmission. Using in vivo microdialysis with high temporal resolution (3 min/sample) we have found that basal dopamine and cocaine-evoked increases in extracellular dopamine in the NAc core and ventral portion of the dorsal striatum do not differ between obesity-prone and obesity-resistant rats (Vollbrecht et al., in revision). In addition, previous work in ex vivo slices has shown that basal and evoked DA release in the NAc shell and dorsal striatum are lower in obesity-prone compared to obesity-resistant rats prior to diet manipulation (Geiger et al., 2008). Although we did not measure dopamine in the current study, the data described above suggest that enhanced cocaine-induced locomotion found here is not likely mediated by enhanced extracellular striatal dopamine levels.
Regarding dopamine receptor function, we recently found that obesity-prone rats are more sensitive to the D2-receptor mediated effects of quinpirole (Vollbrecht et al., in revision) and activation of D2-receptors mediates locomotor activity (Millan et al., 2004). Thus, although dopamine receptors were not measured here, we speculate that this change in D2-receptor function may result in a shift in the balance of D1 vs D2 receptor mediated transmission that contributes to enhanced locomotor responsivity to cocaine (Kravitz and Kreitzer, 2012, Dreher et al., 1989, Plaznik et al., 1989), although direct tests of this hypothesis are needed. Only two previous studies have examined basal differences in dopamine receptors in obesity-susceptible populations prior to diet manipulation (Geiger et al., 2008; Valenza et al., 2015). Geiger et al. (2008) found lower D2 auto receptor mRNA in VTA cell cultures made from obesity-prone vs. obesity-resistant rats, whereas Valenza et al. (2015) found greater D2 auto receptor mRNA expression in the VTA in obesity-prone rats. In addition, while D1 and D2 mRNA expression in the NAc did not differ (though trends towards increased D2 mRNA were found), both D1 and D2 mRNA expression in the dorsal striatum were greater in obesity-prone rats. The elevation in D2 mRNA in dorsal striatum (Valenza et al., 2015) is consistent with our observation that cocaine induced a stronger locomotor response in obesity-prone rats at the dose tested. Of course, mRNA measures cannot be used to determine receptor expression or function directly, and caution must be used when relating differences in mRNA expression to effects of systemic drug administration. Nevertheless, our data show that obesity-prone rats are more sensitive to locomotion induced by 15 mg/kg of cocaine, and previously observed differences in dopamine mRNA are consistent with this results. Furthermore, it is worthwhile to note that differences in the results between studies described above could be due to sex and/or to the vendor through which obesity-prone and obesity-resistant rats were obtained (females from Charles River in Geiger et al., 2008; males from Taconic in Valenza et al., 2015, and current results obtained from offspring of breeders obtained from Taconic). Finally, stimulant-induced locomotor activity is regulated by several other transmitters (e.g., glutamate, GLP-1 and CART; Wolf 1998; Rebec 2006; Erreger et al., 2012; Hubert et al., 2008). Thus, alterations in systems that modulate dopamine transmission may also contribute to enhance sensitivity to cocaine-induced locomotion found here.
Numerous studies have shown that neuroadaptations accompanying locomotor sensitization enhance the motivational properties of food and stimuli associated with food (i.e., food cues; e.g., Wyvell and Berridge, 2000, 2001). Thus, the current data are consistent with recent work showing that obesity susceptible rats are hyper-responsive to the motivational properties of food cues prior to the development of obesity, and support the idea that basal differences in striatal function may contribute to the development obesity in susceptible populations (Robinson et al., 2015). Interestingly, fMRI studies in people find that while striatal activation in response to food cues is enhanced in susceptible individuals prior to the development of obesity (Stoeckel et al., 2008, Dagher, 2009, Tetley et al., 2009, Stice et al., 2010, Demos et al., 2012, Vainik et al., 2013, Burger and Stice, 2014), striatal activations in response to the consumption of food itself is reduced after obesity develops (Stice et al., 2008, Cosgrove et al., 2015). These data suggest that responsivity of striatal systems may be dynamically and differentially influenced by food cues versus food consumption. Furthermore, these neurobehavioral responses likely differ prior to vs. after the development of obesity in susceptible people (see also (Small, 2009 for review).
Studies examining the effects of diet-induced obesity on mesolimbic function have found evidence for locomotor cross-sensitization between consumption of high-fat diets or sucrose and stimulant drugs (Volkow et al., 2002, Baladi et al., 2012a), though no change or reductions in amphetamine induced locomotion (i.e., cross-tolerance), consistent with reduced dopamine function, have also been reported (Davis et al., 2008, Hryhorczuk et al., 2015). In addition, examination of the effects of high-sugar/high-fat diets on striatal D2 receptor mRNA expression and D2 receptor function in outbred rats have produced mixed results, with both increases and decreases found (Huang et al., 2006, South and Huang, 2008, Johnson and Kenny, 2010), Robinson et al., 2015, Baladi et al., 2012b). Although a number of factors may contribute, it is possible that interactions between predisposition and consumption of sugary, fatty foods may result in different alterations in mesolimbic function in susceptible versus resistant individuals and thus account for the opposing results found in the literature. In addition, it’s important to note that diet-induced reductions in D2 receptor mRNA and protein expression and function have been found in the absence of obesity (Robinson et al., 2015; Hryhorczuk et al., 2015; Baladi et al., 2012a). Although results are varied, it is clear that diet-induced obesity as well as consumption of sugary, fatty foods produce dramatic alterations in the function of mesolimbic circuits that govern motivational processes.
In summary, data presented above show that instrumental responding for food is increased in obesity-prone versus resistant rats, and obesity-prone rats over-consume junk-food even in the face of over-abundance of the same food in their home cages. Furthermore, without diet manipulation obesity-prone rats were more sensitive to the locomotor activating effects of cocaine compared to obesity-resistant rats, at the dose tested. This sensitized response is indicative of enhanced responsivity of mesolimbic systems and is consistent with studies in humans suggesting that pre-existing differences in striatal motivational systems may contribute to obesity and hamper weight loss (Stice and Dagher, 2010, Berthoud et al., 2011, Volkow et al., 2011, Demos et al., 2012, Murdaugh et al., 2012). We speculate that pre-existing hyper-sensitivity of mesolimbic systems may facilitate neuroadaptations induced by eating sugary, fatty foods, and thereby further enhance responsivity to food cues in susceptible individuals (Robinson et al., 2015) and hamper the control of food intake (Wang et al., 2002, Johnson and Kenny, 2010, de Jong et al., 2013). Further studies using models that differentiate pre-existing from diet and/or obesity induced plasticity in motivational systems will continue to add to our understanding of how interactions between these factors contribute to the development and persistence of obesity.
Highlights.
Obesity-prone rats show enhanced motivation for food prior to metabolic dysfunction and without diet manipulation.
Obesity-prone rats are hyper-responsive to cocaine prior to the onset of obesity.
Obesity-prone rats over-consume “junk-foods” in a novel testing environment, even in the face of over-abundance of this same food in the home cage.
Acknowledgments
We would like to thank Reed Horwitz and Aman Mandaira for technical assistance, the NIDA drug supply program for providing cocaine for this study, and Dr. Barry E. Levin for helpful conversations.
Funding: This work was supported by R01DK106188 and a Brain and Behavior Research Foundation NARSAD Young Investigator Award and R01DK106188 to CRF, and the Biology of Drug Abuse training grant fellowship (T32DA07268) awarded to PJV. Studies also utilized the Chemistry Core of the Michigan Diabetes Research and Training Center funded by DK020572 awarded by NIDDK, and the University of Michigan Animal Phenotyping Core supported by P30 grants DK020572 (MDRC) and DK089503 (MNORC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albuquerque D, Stice E, Rodriguez-Lopez R, Manco L, Nobrega C. Current review of genetics of human obesity: from molecular mechanisms to an evolutionary perspective. Molecular genetics and genomics: MGG. 2015 doi: 10.1007/s00438-015-1015-9. [DOI] [PubMed] [Google Scholar]
- Baladi MG, Koek W, Aumann M, Velasco F, France CP. Eating high fat chow enhances the locomotor-stimulating effects of cocaine in adolescent and adult female rats. Psychopharmacology. 2012a;222:447–457. doi: 10.1007/s00213-012-2663-7. [DOI] [PubMed] [Google Scholar]
- Baladi MG, Thomas YM, France CP. Sensitivity to apomorphine-induced yawning and hypothermia in rats eating standard or high-fat chow. Psychopharmacology. 2012b;222:27–36. doi: 10.1007/s00213-011-2620-x. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Lenard NR, Shin AC. Food reward, hyperphagia, and obesity. American journal of physiology Regulatory, integrative and comparative physiology. 2011;300:R1266–1277. doi: 10.1152/ajpregu.00028.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Corrie LW, Rogers JA, Yamada H. Effects of insulin and leptin in the ventral tegmental area and arcuate hypothalamic nucleus on food intake and brain reward function in female rats. Behavioural brain research. 2011;219:254–264. doi: 10.1016/j.bbr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger KS, Stice E. Frequent ice cream consumption is associated with reduced striatal response to receipt of an ice cream-based milkshake. The American journal of clinical nutrition. 2012;95:810–817. doi: 10.3945/ajcn.111.027003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger KS, Stice E. Greater striatopallidal adaptive coding during cue-reward learning and food reward habituation predict future weight gain. NeuroImage. 2014;99:122–128. doi: 10.1016/j.neuroimage.2014.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnell S, Wardle J. Appetite and adiposity in children: evidence for a behavioral susceptibility theory of obesity. The American journal of clinical nutrition. 2008;88:22–29. doi: 10.1093/ajcn/88.1.22. [DOI] [PubMed] [Google Scholar]
- Carpenter CL, Wong AM, Li Z, Noble EP, Heber D. Association of dopamine D2 receptor and leptin receptor genes with clinically severe obesity. Obesity (Silver Spring, Md) 2013;21:E467–473. doi: 10.1002/oby.20202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos EH, Charboneau E, Dietrich MS, Park S, Bradley BP, Mogg K, Cowan RL. Obese adults have visual attention bias for food cue images: evidence for altered reward system function. International journal of obesity (2005) 2009;33:1063–1073. doi: 10.1038/ijo.2009.138. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Veldhuizen MG, Sandiego CM, Morris ED, Small DM. Opposing relationships of BMI with BOLD and dopamine D2/3 receptor binding potential in the dorsal striatum. Synapse (New York, NY) 2015;69:195–202. doi: 10.1002/syn.21809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagher A. The neurobiology of appetite: hunger as addiction. International journal of obesity. 2009;33(Suppl 2):S30–33. doi: 10.1038/ijo.2009.69. [DOI] [PubMed] [Google Scholar]
- Dagher A. Functional brain imaging of appetite. Trends in endocrinology and metabolism: TEM. 2012;23:250–260. doi: 10.1016/j.tem.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Davis JF, Tracy AL, Schurdak JD, Tschop MH, Lipton JW, Clegg DJ, Benoit SC. Exposure to elevated levels of dietary fat attenuates psychostimulant reward and mesolimbic dopamine turnover in the rat. Behavioral neuroscience. 2008;122:1257–1263. doi: 10.1037/a0013111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong JW, Meijboom KE, Vanderschuren LJ, Adan RA. Low control over palatable food intake in rats is associated with habitual behavior and relapse vulnerability: individual differences. PloS one. 2013;8:e74645. doi: 10.1371/journal.pone.0074645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfs JM, Schreiber L, Kelley AE. Microinjection of cocaine into the nucleus accumbens elicits locomotor activation in the rat. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1990;10:303–310. doi: 10.1523/JNEUROSCI.10-01-00303.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demos KE, Heatherton TF, Kelley WM. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:5549–5552. doi: 10.1523/JNEUROSCI.5958-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel RH, Alberti KG, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2010;375:181–183. doi: 10.1016/S0140-6736(09)61794-3. [DOI] [PubMed] [Google Scholar]
- Erreger K, Davis AR, Poe AM, Greig NH, Stanwood GD, Galli A. Exendin-4 decreases amphetamine-induced locomotor activity. Physiology & behavior. 2012;106:574–578. doi: 10.1016/j.physbeh.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag YM, Gaballa MR. Diabesity: an overview of a rising epidemic. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2011;26:28–35. doi: 10.1093/ndt/gfq576. [DOI] [PubMed] [Google Scholar]
- Geiger BM, Behr GG, Frank LE, Caldera-Siu AD, Beinfeld MC, Kokkotou EG, Pothos EN. Evidence for defective mesolimbic dopamine exocytosis in obesity-prone rats. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2008;22:2740–2746. doi: 10.1096/fj.08-110759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger BM, Haburcak M, Avena NM, Moyer MC, Hoebel BG, Pothos EN. Deficits of mesolimbic dopamine neurotransmission in rat dietary obesity. Neuroscience. 2009;159:1193–1199. doi: 10.1016/j.neuroscience.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hryhorczuk C, Florea M, Rodaros D, Poirier I, Daneault C, Des Rosiers C, Arvanitogiannis A, Alquier T, Fulton S. Dampened Mesolimbic Dopamine Function and Signaling by Saturated but not Monounsaturated Dietary Lipids. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XF, Zavitsanou K, Huang X, Yu Y, Wang H, Chen F, Lawrence AJ, Deng C. Dopamine transporter and D2 receptor binding densities in mice prone or resistant to chronic high fat diet-induced obesity. Behavioural brain research. 2006;175:415–419. doi: 10.1016/j.bbr.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Hubert GW, Jones DC, Moffett MC, Rogge G, Kuhar MJ. CART peptides as modulators of dopamine and psychostimulants and interactions with the mesolimbic dopaminergic system. Biochemical pharmacology. 2008;75:57–62. doi: 10.1016/j.bcp.2007.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen A. A learning model of binge eating: cue reactivity and cue exposure. Behaviour research and therapy. 1998;36:257–272. doi: 10.1016/s0005-7967(98)00055-2. [DOI] [PubMed] [Google Scholar]
- Jansen A, Vanreyten A, van Balveren T, Roefs A, Nederkoorn C, Havermans R. Negative affect and cue-induced overeating in non-eating disordered obesity. Appetite. 2008;51:556–562. doi: 10.1016/j.appet.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Jeffery RW, Drewnowski A, Epstein LH, Stunkard AJ, Wilson GT, Wing RR, Hill DR. Long-term maintenance of weight loss: current status. Health psychology: official journal of the Division of Health Psychology, American Psychological Association. 2000;19:5–16. doi: 10.1037/0278-6133.19.suppl1.5. [DOI] [PubMed] [Google Scholar]
- Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nature neuroscience. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen-Rhinehart E, Ondek K, Schneider JE. Neuroendocrine regulation of appetitive ingestive behavior. Frontiers in neuroscience. 2013;7:213. doi: 10.3389/fnins.2013.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer FM, Jeffery RW, Forster JL, Snell MK. Long-term follow-up of behavioral treatment for obesity: patterns of weight regain among men and women. International journal of obesity. 1989;13:123–136. [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA. Reduced central leptin sensitivity in rats with diet-induced obesity. American journal of physiology Regulatory, integrative and comparative physiology. 2002;283:R941–948. doi: 10.1152/ajpregu.00245.2002. [DOI] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA. Chronic exercise lowers the defended body weight gain and adiposity in diet-induced obese rats. American journal of physiology Regulatory, integrative and comparative physiology. 2004;286:R771–778. doi: 10.1152/ajpregu.00650.2003. [DOI] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. The American journal of physiology. 1997;273:R725–730. doi: 10.1152/ajpregu.1997.273.2.R725. [DOI] [PubMed] [Google Scholar]
- Levin BE, Hogan S, Sullivan AC. Initiation and perpetuation of obesity and obesity resistance in rats. The American journal of physiology. 1989;256:R766–771. doi: 10.1152/ajpregu.1989.256.3.R766. [DOI] [PubMed] [Google Scholar]
- Levin BE, Routh VH. Role of the brain in energy balance and obesity. The American journal of physiology. 1996;271:R491–500. doi: 10.1152/ajpregu.1996.271.3.R491. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Gustafson DR. Adiposity, type 2 diabetes, and Alzheimer’s disease. Journal of Alzheimer’s disease: JAD. 2009;16:693–704. doi: 10.3233/JAD-2009-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, Meshul CK, Phillips TJ. Ethanol- and cocaine-induced locomotion are genetically related to increases in accumbal dopamine. Genes, brain, and behavior. 2009;8:346–355. doi: 10.1111/j.1601-183X.2009.00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ, Seguin L, Gobert A, Cussac D, Brocco M. The role of dopamine D3 compared with D2 receptors in the control of locomotor activity: a combined behavioural and neurochemical analysis with novel, selective antagonists in rats. Psychopharmacology. 2004;174:341–357. doi: 10.1007/s00213-003-1770-x. [DOI] [PubMed] [Google Scholar]
- Murdaugh DL, Cox JE, Cook EW, 3rd, Weller RE. fMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. NeuroImage. 2012;59:2709–2721. doi: 10.1016/j.neuroimage.2011.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naleid AM, Grimm JW, Kessler DA, Sipols AJ, Aliakbari S, Bennett JL, Wells J, Figlewicz DP. Deconstructing the vanilla milkshake: the dominant effect of sucrose on self-administration of nutrient-flavor mixtures. Appetite. 2008;50:128–138. doi: 10.1016/j.appet.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. Jama. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry ML, Leinninger GM, Chen R, Luderman KD, Yang H, Gnegy ME, Myers MG, Jr, Kennedy RT. Leptin promotes dopamine transporter and tyrosine hydroxylase activity in the nucleus accumbens of Sprague-Dawley rats. Journal of neurochemistry. 2010;114:666–674. doi: 10.1111/j.1471-4159.2010.06757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post RM, Rose H. Increasing effects of repetitive cocaine administration in the rat. Nature. 1976;260:731–732. doi: 10.1038/260731a0. [DOI] [PubMed] [Google Scholar]
- Rebec GV. Behavioral electrophysiology of psychostimulants. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2006;31:2341–2348. doi: 10.1038/sj.npp.1301160. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. Journal of neuroscience methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Burghardt PR, Patterson CM, Nobile CW, Akil H, Watson SJ, Berridge KC, Ferrario CR. Individual Differences in Cue-Induced Motivation and Striatal Systems in Rats Susceptible to Diet-Induced Obesity. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2015;40:2113–2123. doi: 10.1038/npp.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain research. 1986;396:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H, Klapp BF. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. NeuroImage. 2007;37:410–421. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Small DM. Individual differences in the neurophysiology of reward and the obesity epidemic. International journal of obesity 2005. 2009;33(Suppl 2):S44–48. doi: 10.1038/ijo.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CB. EFFECTS OF D-AMPHETAMINE UPON BRAIN AMINE CONTENT AND LOCOMOTOR ACTIVITY OF MICE. The Journal of pharmacology and experimental therapeutics. 1965;147:96–102. [PubMed] [Google Scholar]
- South T, Huang XF. High-fat diet exposure increases dopamine D2 receptor and decreases dopamine transporter receptor binding density in the nucleus accumbens and caudate putamen of mice. Neurochemical research. 2008;33:598–605. doi: 10.1007/s11064-007-9483-x. [DOI] [PubMed] [Google Scholar]
- Steele KE, Prokopowicz GP, Schweitzer MA, Magunsuon TH, Lidor AO, Kuwabawa H, Kumar A, Brasic J, Wong DF. Alterations of central dopamine receptors before and after gastric bypass surgery. Obesity surgery. 2010;20:369–374. doi: 10.1007/s11695-009-0015-4. [DOI] [PubMed] [Google Scholar]
- Stice E, Dagher A. Genetic variation in dopaminergic reward in humans. Forum of nutrition. 2010;63:176–185. doi: 10.1159/000264405. [DOI] [PubMed] [Google Scholar]
- Stice E, Figlewicz DP, Gosnell BA, Levine AS, Pratt WE. The contribution of brain reward circuits to the obesity epidemic. Neuroscience and biobehavioral reviews. 2012 doi: 10.1016/j.neubiorev.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science (New York, NY) 2008;322:449–452. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Bohon C, Marti N, Smolen A. Reward circuitry responsivity to food predicts future increases in body mass: moderating effects of DRD2 and DRD4. NeuroImage. 2010;50:1618–1625. doi: 10.1016/j.neuroimage.2010.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel LE, Weller RE, Cook EW, 3rd, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. NeuroImage. 2008;41:636–647. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Tetley A, Brunstrom J, Griffiths P. Individual differences in food-cue reactivity. The role of BMI and everyday portion-size selections. Appetite. 2009;52:614–620. doi: 10.1016/j.appet.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Vainik U, Dagher A, Dube L, Fellows LK. Neurobehavioural correlates of body mass index and eating behaviours in adults: a systematic review. Neuroscience and biobehavioral reviews. 2013;37:279–299. doi: 10.1016/j.neubiorev.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenza M, Steardo L, Cottone P, Sabino V. Diet-induced obesity and diet-resistant rats: differences in the rewarding and anorectic effects of D-amphetamine. Psychopharmacology. 2015 doi: 10.1007/s00213-015-3981-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neuroscience and biobehavioral reviews. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Vezina P, Leyton M. Conditioned cues and the expression of stimulant sensitization in animals and humans. Neuropharmacology. 2009;56(Suppl 1):160–168. doi: 10.1016/j.neuropharm.2008.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends in cognitive sciences. 2011;15:37–46. doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Jayne M, Franceschi D, Wong C, Gatley SJ, Gifford AN, Ding YS, Pappas N. “Nonhedonic” food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse (New York, NY) 2002;44:175–180. doi: 10.1002/syn.10075. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2008;363:3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucetic Z, Reyes TM. Central dopaminergic circuitry controlling food intake and reward: implications for the regulation of obesity. Wiley interdisciplinary reviews Systems biology and medicine. 2010;2:577–593. doi: 10.1002/wsbm.77. [DOI] [PubMed] [Google Scholar]
- Wang G-J, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusll N, Fowler JS. Brain dopamine and obesity. The Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS. The role of dopamine in motivation for food in humans: implications for obesity. Expert Opin Ther Targets. 2002;6:601–609. doi: 10.1517/14728222.6.5.601. [DOI] [PubMed] [Google Scholar]
- Williams LM. Hypothalamic dysfunction in obesity. The Proceedings of the Nutrition Society. 2012;71:521–533. doi: 10.1017/S002966511200078X. [DOI] [PubMed] [Google Scholar]
- Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Progress in neurobiology. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward “wanting” without enhanced “liking” or response reinforcement. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20:8122–8130. doi: 10.1523/JNEUROSCI.20-21-08122.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Incentive sensitization by previous amphetamine exposure: increased cue-triggered “wanting” for sucrose reward. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2001;21:7831–7840. doi: 10.1523/JNEUROSCI.21-19-07831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]