Abstract
Cytotoxic T lymphocytes (CTLs) expressing the CD8 surface marker recognize peptides in association with major histocompatibility complex (MHC) class I molecules. Although most peptides expressed on MHC class I molecules are derived from self- or virally encoded proteins, delivery of exogenous proteins to the cytosol can result in their being processed for presentation to CTLs on MHC class I molecules. We describe two fusion proteins (PEMa and PENP), consisting of the binding and translocating domains of Pseudomonas exotoxin A (PE), fused to peptide epitopes from influenza A matrix protein and nucleoprotein, respectively. These fusion proteins were internalized and processed by MHC class I-positive target cells, resulting in sensitization of target cells for lysis by peptide-specific CTLs. A point mutation known to interfere with intoxication by wild-type PE also reduced the ability of PEMa to sensitize target cells. Fusion of peptide or polypeptide epitopes with PE provides a potential means of eliciting CTLs without the use of self-replicating agents, as well as a useful probe for studying MHC class I-restricted antigen processing.
Full text
PDF
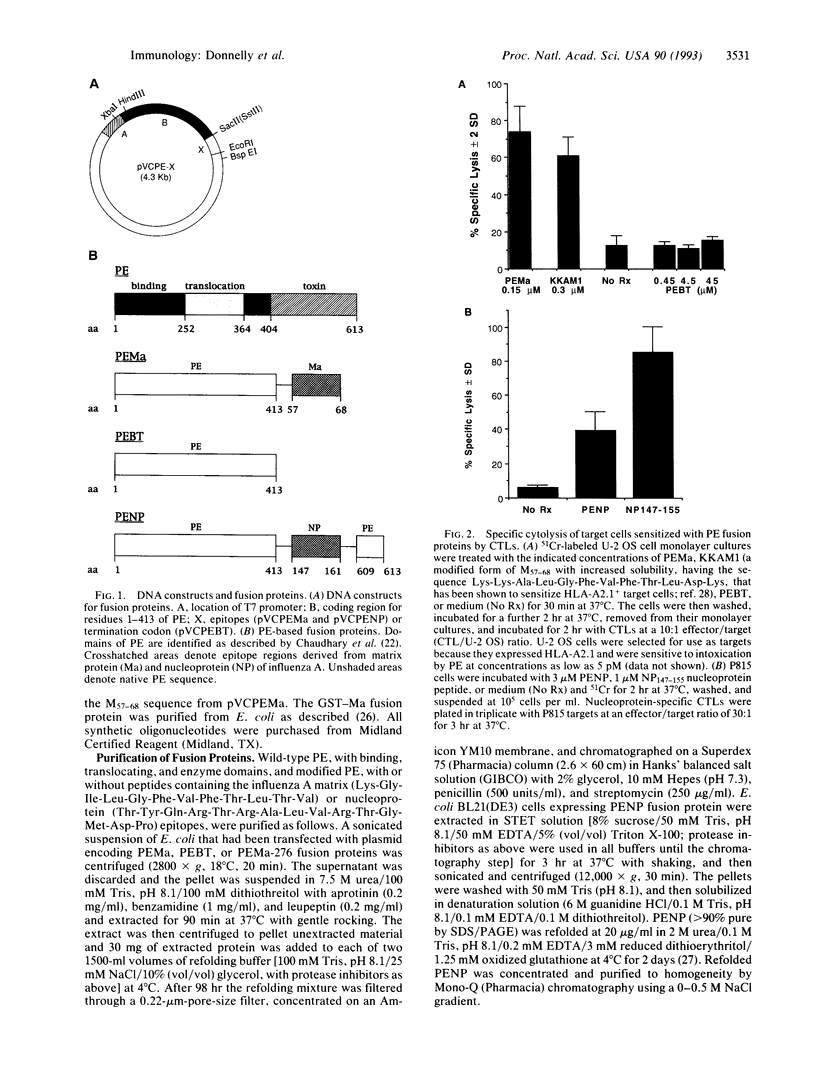
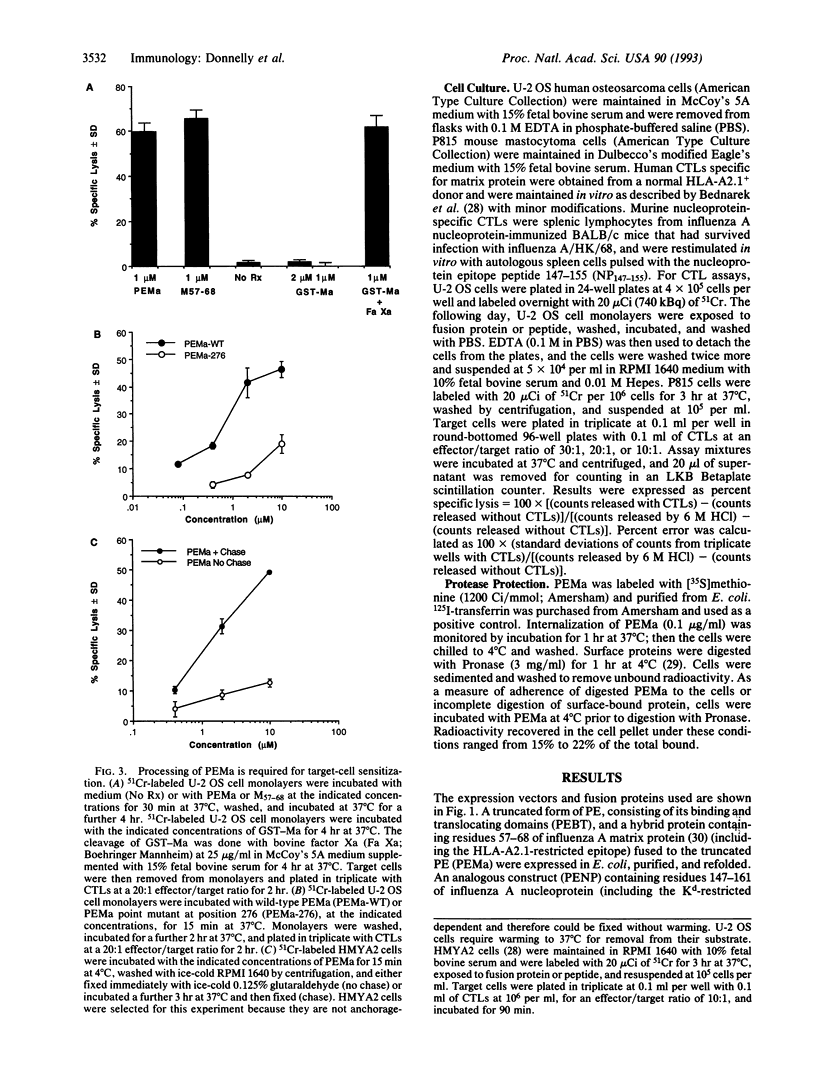


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold D., Driscoll J., Androlewicz M., Hughes E., Cresswell P., Spies T. Proteasome subunits encoded in the MHC are not generally required for the processing of peptides bound by MHC class I molecules. Nature. 1992 Nov 12;360(6400):171–174. doi: 10.1038/360171a0. [DOI] [PubMed] [Google Scholar]
- Bednarek M. A., Engl S. A., Gammon M. C., Lindquist J. A., Porter G., Williamson A. R., Zweerink H. J. Soluble HLA-A2.1 restricted peptides that are recognized by influenza virus specific cytotoxic T lymphocytes. J Immunol Methods. 1991 May 17;139(1):41–47. doi: 10.1016/0022-1759(91)90349-k. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987 Oct 8;329(6139):512–518. doi: 10.1038/329512a0. [DOI] [PubMed] [Google Scholar]
- Buchner J., Rudolph R. Renaturation, purification and characterization of recombinant Fab-fragments produced in Escherichia coli. Biotechnology (N Y) 1991 Feb;9(2):157–162. doi: 10.1038/nbt0291-157. [DOI] [PubMed] [Google Scholar]
- Cerundolo V., Lahaye T., Horvath C., Zanovello P., Collavo D., Engers H. D. Functional activity in vivo of effector T cell populations. III. Protection against Moloney murine sarcoma virus (M-MSV)-induced tumors in T cell deficient mice by the adoptive transfer of a M-MSV-specific cytolytic T lymphocyte clone. Eur J Immunol. 1987 Feb;17(2):173–178. doi: 10.1002/eji.1830170204. [DOI] [PubMed] [Google Scholar]
- Chaudhary V. K., Xu Y. H., FitzGerald D., Adhya S., Pastan I. Role of domain II of Pseudomonas exotoxin in the secretion of proteins into the periplasm and medium by Escherichia coli. Proc Natl Acad Sci U S A. 1988 May;85(9):2939–2943. doi: 10.1073/pnas.85.9.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotch F., Rothbard J., Howland K., Townsend A., McMichael A. Cytotoxic T lymphocytes recognize a fragment of influenza virus matrix protein in association with HLA-A2. 1987 Apr 30-May 6Nature. 326(6116):881–882. doi: 10.1038/326881a0. [DOI] [PubMed] [Google Scholar]
- Harding C. V., 3rd Electroporation of exogenous antigen into the cytosol for antigen processing and class I major histocompatibility complex (MHC) presentation: weak base amines and hypothermia (18 degrees C) inhibit the class I MHC processing pathway. Eur J Immunol. 1992 Jul;22(7):1865–1869. doi: 10.1002/eji.1830220728. [DOI] [PubMed] [Google Scholar]
- Jinno Y., Ogata M., Chaudhary V. K., Willingham M. C., Adhya S., FitzGerald D., Pastan I. Domain II mutants of Pseudomonas exotoxin deficient in translocation. J Biol Chem. 1989 Sep 25;264(27):15953–15959. [PubMed] [Google Scholar]
- Kast W. M., Offringa R., Peters P. J., Voordouw A. C., Meloen R. H., van der Eb A. J., Melief C. J. Eradication of adenovirus E1-induced tumors by E1A-specific cytotoxic T lymphocytes. Cell. 1989 Nov 17;59(4):603–614. doi: 10.1016/0092-8674(89)90006-8. [DOI] [PubMed] [Google Scholar]
- Lukacher A. E., Braciale V. L., Braciale T. J. In vivo effector function of influenza virus-specific cytotoxic T lymphocyte clones is highly specific. J Exp Med. 1984 Sep 1;160(3):814–826. doi: 10.1084/jem.160.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden D. R., Gorga J. C., Strominger J. L., Wiley D. C. The structure of HLA-B27 reveals nonamer self-peptides bound in an extended conformation. Nature. 1991 Sep 26;353(6342):321–325. doi: 10.1038/353321a0. [DOI] [PubMed] [Google Scholar]
- Marnell M. H., Shia S. P., Stookey M., Draper R. K. Evidence for penetration of diphtheria toxin to the cytosol through a prelysosomal membrane. Infect Immun. 1984 Apr;44(1):145–150. doi: 10.1128/iai.44.1.145-150.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascola L., Sorvillo F., Neal J., Iwakoshi K., Weaver R. Surveillance of listeriosis in Los Angeles County, 1985-1986. A first year's report. Arch Intern Med. 1989 Jul;149(7):1569–1572. [PubMed] [Google Scholar]
- McMichael A. J., Gotch F. M., Noble G. R., Beare P. A. Cytotoxic T-cell immunity to influenza. N Engl J Med. 1983 Jul 7;309(1):13–17. doi: 10.1056/NEJM198307073090103. [DOI] [PubMed] [Google Scholar]
- Momburg F., Ortiz-Navarrete V., Neefjes J., Goulmy E., van de Wal Y., Spits H., Powis S. J., Butcher G. W., Howard J. C., Walden P. Proteasome subunits encoded by the major histocompatibility complex are not essential for antigen presentation. Nature. 1992 Nov 12;360(6400):174–177. doi: 10.1038/360174a0. [DOI] [PubMed] [Google Scholar]
- Moore M. W., Carbone F. R., Bevan M. J. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988 Sep 9;54(6):777–785. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- Ogata M., Chaudhary V. K., Pastan I., FitzGerald D. J. Processing of Pseudomonas exotoxin by a cellular protease results in the generation of a 37,000-Da toxin fragment that is translocated to the cytosol. J Biol Chem. 1990 Nov 25;265(33):20678–20685. [PubMed] [Google Scholar]
- Prior T. I., FitzGerald D. J., Pastan I. Barnase toxin: a new chimeric toxin composed of pseudomonas exotoxin A and barnase. Cell. 1991 Mar 8;64(5):1017–1023. doi: 10.1016/0092-8674(91)90325-s. [DOI] [PubMed] [Google Scholar]
- Redfield R. R., Wright D. C., James W. D., Jones T. S., Brown C., Burke D. S. Disseminated vaccinia in a military recruit with human immunodeficiency virus (HIV) disease. N Engl J Med. 1987 Mar 12;316(11):673–676. doi: 10.1056/NEJM198703123161106. [DOI] [PubMed] [Google Scholar]
- Riddell S. R., Rabin M., Geballe A. P., Britt W. J., Greenberg P. D. Class I MHC-restricted cytotoxic T lymphocyte recognition of cells infected with human cytomegalovirus does not require endogenous viral gene expression. J Immunol. 1991 Apr 15;146(8):2795–2804. [PubMed] [Google Scholar]
- Rouse B. T., Norley S., Martin S. Antiviral cytotoxic T lymphocyte induction and vaccination. Rev Infect Dis. 1988 Jan-Feb;10(1):16–33. doi: 10.1093/clinids/10.1.16. [DOI] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Entry of the toxic proteins abrin, modeccin, ricin, and diphtheria toxin into cells. II. Effect of pH, metabolic inhibitors, and ionophores and evidence for toxin penetration from endocytotic vesicles. J Biol Chem. 1982 Jul 10;257(13):7504–7513. [PubMed] [Google Scholar]
- Sandvig K., Olsnes S., Petersen O. W., van Deurs B. Acidification of the cytosol inhibits endocytosis from coated pits. J Cell Biol. 1987 Aug;105(2):679–689. doi: 10.1083/jcb.105.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Stenmark H., Moskaug J. O., Madshus I. H., Sandvig K., Olsnes S. Peptides fused to the amino-terminal end of diphtheria toxin are translocated to the cytosol. J Cell Biol. 1991 Jun;113(5):1025–1032. doi: 10.1083/jcb.113.5.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Gotch F. M., Davey J. Cytotoxic T cells recognize fragments of the influenza nucleoprotein. Cell. 1985 Sep;42(2):457–467. doi: 10.1016/0092-8674(85)90103-5. [DOI] [PubMed] [Google Scholar]
- Townsend A., Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- Wraith D. C., Vessey A. E., Askonas B. A. Purified influenza virus nucleoprotein protects mice from lethal infection. J Gen Virol. 1987 Feb;68(Pt 2):433–440. doi: 10.1099/0022-1317-68-2-433. [DOI] [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R., Hosaka Y. Cells process exogenous proteins for recognition by cytotoxic T lymphocytes. Science. 1988 Feb 5;239(4840):637–640. doi: 10.1126/science.3257585. [DOI] [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R. The binary logic of antigen processing and presentation to T cells. Cell. 1990 Jul 27;62(2):203–206. doi: 10.1016/0092-8674(90)90356-j. [DOI] [PubMed] [Google Scholar]



