Abstract
Bone marrow stroma can protect acute myeloid leukemia (AML) cells against chemotherapeutic agents and provide anti-apoptosis and chemoresistance signals through secreting chemokine CXCL12 to activate its receptor CXCR4 on AML cells, resulting in minimal residual leukemia and relapse. Therefore disrupting the CXCR4/CXCL12 axis with antagonists is of great significance for improving chemosensitivity and decreasing relapse rate. In a previous study, we reported a novel synthetic peptide E5 with its remarkable effect on inhibiting CXCR4/CXCL12-mediated adhesion and migration of AML cells. Here we presented E5’s capacity of enhancing the therapeutic efficiency of various chemotherapeutics on AML in vitro and in vivo. Results showed that E5 can diminish bone marrow stromal cell-provided protection to leukemia cells, significantly increasing the apoptosis induced by various chemotherapeutics in multiple AML cell lines. In an AML mouse xenograft model, E5 induced 1.84-fold increase of circulating AML cells out of protective stroma niche. Combined with vincristine or cyclophosphamide, E5 inhibited infiltration of AML cells into bone marrow, liver and spleen, as well as prolonged the lifespan of AML mice compared with mice treated with chemotherapy alone. In addition, E5 presented no toxicity in vivo according to the histological analysis and routine clinical parameters of serum analysis.
Poor prognosis of acute myeloid leukemia (AML) with current treatments is mainly due to the relapse of the disease post chemotherapy1,2. In the last decade, an emerging concept has proposed that interactions between leukemia cells and the bone marrow microenvironment are the major cause of the leukemia relapse3,4,5,6. In the cross-talking between them, leukemia cells highly expressing the chemokine receptor 4 (CXCR4) are recruited into the bone marrow by stromal cells constantly secreting the chemokine ligand 12 (CXCL12)2,7,8. Leukemia cells that adhere to the stromal cells can reside in the bone marrow to acquire anti-apoptosis signals and favorable conditions for survival and growth9,10,11,12. Thus through CXCR4/CXCL12 axis leukemia cells are protected by the stromal cells from cytotoxic chemotherapeutics and represent a reservoir for minimal residual disease and relapses2,13,14.
Given the central role of the CXCR4/CXCL12 axis in mediating leukemia cell-stroma interactions, multiple antagonists targeting CXCR4 have been developed for use in leukemia treatments15,16. For example, a small chemical molecule competitive antagonist of CXCR4, AMD3100, was reported for enhanced effect of cytarabine, decreased tumor burden and improved overall survival of AML mice through mobilizing leukemia cells from bone marrow into the peripheral blood17. In a phase 1/2 study of relapsed or refractory AML, AMD3100 induced a 2-fold mobilization of leukemic blasts into the peripheral circulation and an overall complete remission of patients when combined with chemotherapeutic drugs mitoxantrone, etoposide or cytarabine18. Its analog AMD3465 demonstrated remarkable activity in antagonizing CXCL12-induced CXCR4 signaling pathways, mobilizing AML cells into circulation and enhancing anti-leukemic effects of chemotherapy in vitro and in vivo19,20. AMD11070, another derivative of AMD3100, could effectively block stroma-induced migration and adhesion of leukemia cells21. When combined with cytotoxic drugs, it could prolong the survival of ALL mice and reduce leukemia cell infiltration into organs. The peptide T140 and its analogs TC14012 and TN14003 derived from horseshoe crabs were reported with effective inhibitory roles mainly in lymphocytic leukemia cells by blocking CXCR4/CXCL12 axis-mediated events and enhancing effect of chemotherapy in vitro16,22,23. RCP168 derived from viral macrophage inflammatory protein II is another kind of CXCR4 peptide antagonist that inhibits CXCL12- or MS-5-induced migration of Jurkat or primary CLL cells and increases the drug-induced apoptosis of leukemia cells from patients in vitro20. However, it is notable that until now, peptide antagonists for AML in vivo have been rarely reported. Therefore, it is of great significance to develop novel peptides targeting CXCR4 for providing more therapeutic options in AML treatments.
We have reported a novel peptide E5 by cell-based selection from the de novo designed peptides and demonstrated its effect on interfering CXCR4/CXCL12 axis in vitro24. E5 significantly inhibited migration and adhesion of AML cells to bone marrow stromal cells through inhibiting leukemia cell response to CXCL12- or MS-5-induced activation. Hence we hypothesize that E5 disrupts the interaction of leukemia cells with the bone marrow stromal microenvironment, sensitizing these leukemia cells to toxic drug stresses. In this study, our findings indicate that E5 treatment can diminish marrow stromal protection and confer chemosensitivity to AML cells. By mobilizing leukemia cells from the protective stromal microenvironment, E5 significantly increased the efficiency of chemotherapeutic drugs 10-hydroxy camptothecin, cisplatin or vincristine in vitro, leading to an improved overall survival in the mouse model of AML in combination with vincristine or cyclophosphamide.
Results
E5 reduces marrow stroma-mediated resistance of leukemia cells to various chemotherapies
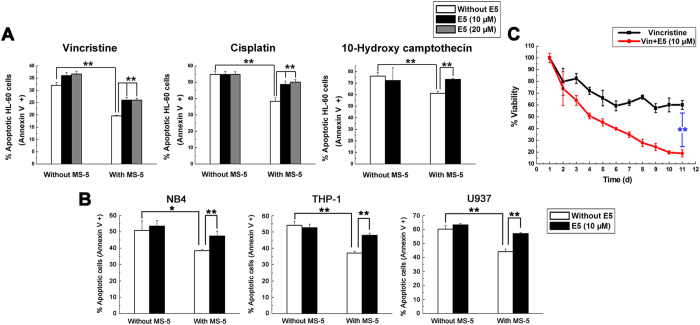
In the first set of experiments, we explored whether E5 can overcome stroma-induced drug resistance and sensitize leukemia cells to chemotherapies. The effect of vincristine, cisplatin or 10-hydroxy camptothecin on inducing apoptosis of HL-60 cells co-cultured with the MS-5 cell layer within 48 h was tested with/without E5. It was found that the stromal cell MS-5 dramatically decreased HL-60 cell apoptosis induced by the chemotherapeutic drugs, suggesting protection from drug-induced apoptosis (Fig. 1A), while addition of E5 to the co-culture system removed the protection of MS-5 and sensitized HL-60 cells to the drugs. In the cultivation of the other AML cells (NB4, THP-1, U937), E5 was also found to increase 10-hydroxy camptothecin-induced apoptosis in these cells when MS-5 cells were present (Fig. 1B). It was found that E5 did not enhance the drug-induced apoptosis in culturing systems without MS-5, indicating that the sensitization effect of E5 can be specifically attributed to interfering with the interaction between leukemic cells and stromal cells.
Figure 1. E5 inhibits MS-5 mediated-protection and sensitizes leukemia cells to different chemotherapies.
(A) E5 enhances vincristine, cisplatin or 10-hydroxy camptothecin-induced apoptosis in HL-60 cells co-cultured with MS-5 cells. HL-60 cells were treated with drugs alone, or drugs in combination with E5 in the absence or presence of MS-5 cells for 48 h. Apoptotic cells were detected by Annexin V flow cytometry. (B) E5 enhances 10-hydroxy camptothecin-induced apoptosis in multiple AML cell lines including NB4, THP-1 and U937 cells co-cultured with MS-5 cells for 48 h. (C) After continuous combination treatment, vincristine drastically reduces HL-60 cell viability. HL-60 cells growing on MS-5 cell layer were treated with vincristine alone or combined with E5 for 10 days and cell viability was detected using trypan blue every day. Data are presented as mean ± SD (n = 3). The * represents significant difference between two groups (*p < 0.05, **p < 0.01).
Then we investigated the effect of E5 on 11-day co-culture of leukemia cells and stromal cells. Using trypan blue exclusion method on a daily basis, we observed that when co-cultured with stromal cells HL-60 remained a constant cell viability level of approximate 60.1% after 5 days of treatment with vincristine, suggesting HL-60 became vincristine-resistant (Fig. 1C). Strikingly, when E5 was added to the co-culture system, no vincristine resistance was observed over the whole incubation period. From day 3 the cell viability level of combination group was significantly lower than the group of vincristine alone. After 10 days the cell viability of HL-60 treated in combination of E5 and vincristine was reduced to 19.0%, indicating the drug resistance developed in the co-culture system was overcome by E5 supplement.
E5-induced leukemia cells mobilization occurs from hematopoietic tissues
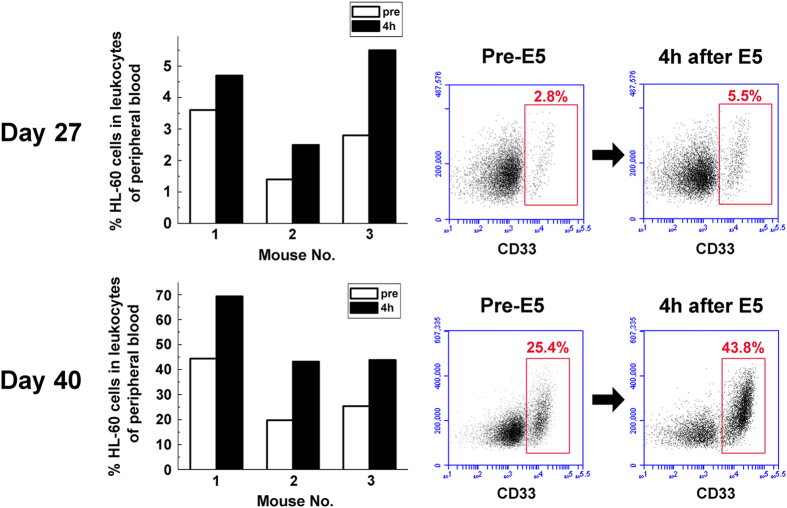
We previously reported that E5 can effectively inhibit leukemia cell adhesion to bone marrow stroma and induce leukemia cell detachment from stromal cell layer in vitro24. Inspired by these results in vitro, we translated our findings into an in vivo system to confirm its primary mechanism of action. To establish the AML mouse model for treatment, HL-60 cells were injected into sublethally irradiated NOD/SCID mice by tail vein, allowing these cells to migrate to bone marrow and form an enlarged leukemia burden. 20 days after the injection, mice showed marked leukemic symptoms including paralysis in the rear limbs, ruffled fur, and remarkably hunched posture in comparison to healthy control mice. On day 20, 34 and 40 after HL-60 transplantation, cells from bone marrow and spleen of the mice were analyzed with flow cytometry (Supplementary Fig. S1A). The HL-60 cell proportion was 6.1%, 40.4%, 91.9% in bone marrow and 3.3%, 10.2%, 65.2% in spleen, demonstrating the successful establishment of leukemia mouse model (Supplementary Fig. S1B). Then we collected peripheral blood samples from E5-monotreated AML mice before and 4 h after administration of E5 and measured the HL-60 proportion. Results showed that E5 induced a significant increase of circulating HL-60 cells in mice (Fig. 2). Taking one set of flow cytometry data for one of the three mice as an example, the circulating HL-60 proportion increased from 2.8% to 5.5% on day 27 of HL-60 implantation and from 25.4% to 43.8% on day 40. Compared with control, the percentage was almost doubled. These data clearly indicate that E5 mobilizes leukemia cells from stromal microenvironment into peripheral circulation.
Figure 2. E5 induces a rapid mobilization of leukemia cells into the peripheral blood.
Peripheral blood samples were collected through tail vein from three E5-monotreated mice before and 4 h after administration of E5. The percentage of CD33 positive cells (HL-60) was detected with flow cytometry.
Combination treatment of E5 plus vincristine (Vin) or cyclophosphamide (CTX) prolongs the survival of leukemia mice and reduces leukemia burden
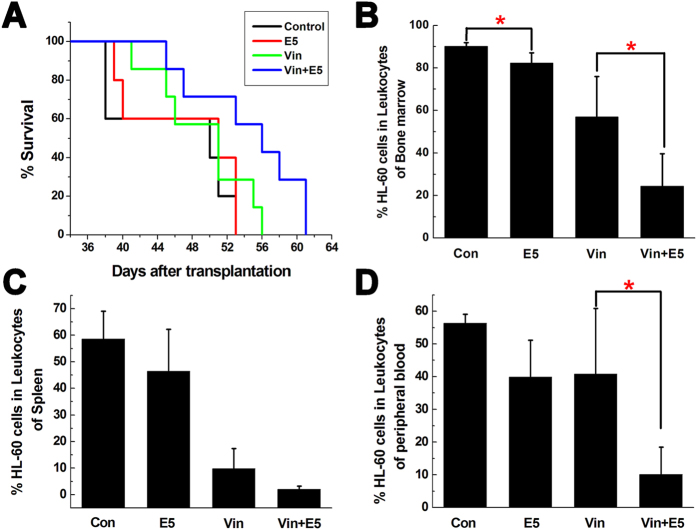
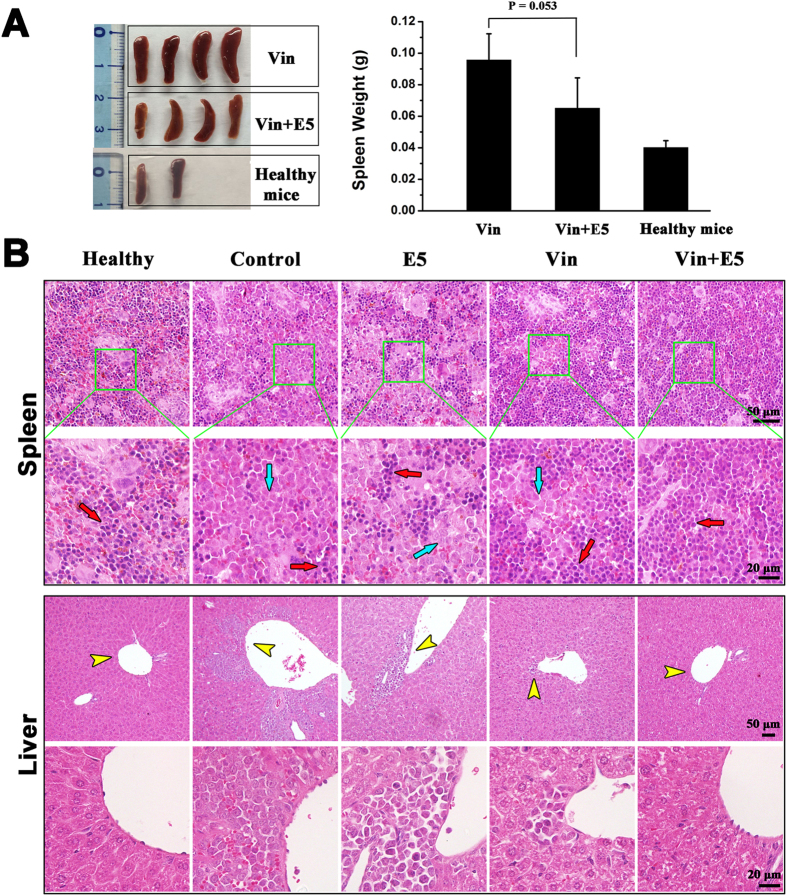
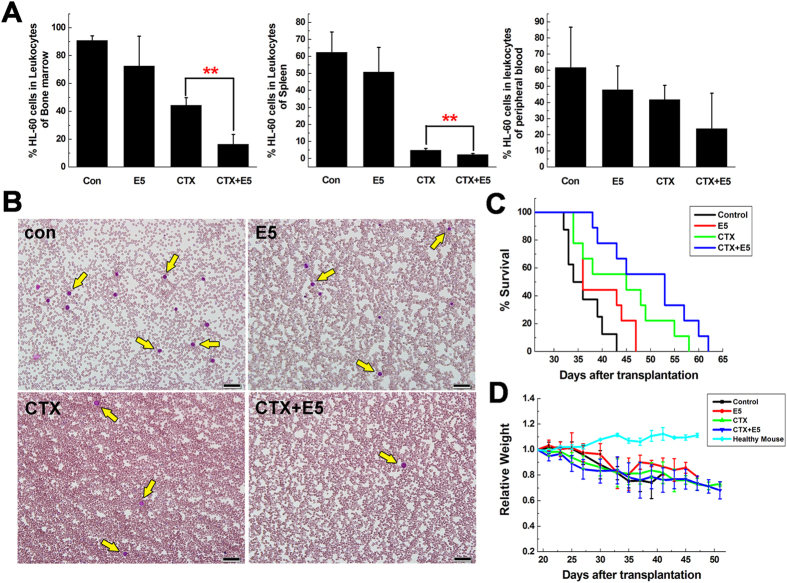
In view of the above results, we combined E5 with chemotherapeutic drugs to examine the elimination effect to the leukemia cells escaped from the protective stromal microenvironment. To ensure that the leukemic cells pushed into the circulation come in contact with the cytotoxic drug, we injected Vin at 4 h after the subcutaneous administration of E5 from day 20 of the HL-60 transplantation. The group receiving combination treatment showed prolonged median survival (56 days) comparing to the group receiving Vin alone (51 days) within the experimental period (p = 0.063, Fig. 3A). Analysis with bone marrow, spleen and peripheral blood showed a clear reduction of AML cells load in the combination treatment group compared with that in the Vin-alone treated group (Fig. 3B–D). E5 alone also slightly decreased the HL-60 percentage in bone marrow, spleen and peripheral blood compared with the control group. Besides bone marrow, leukemia cells tend to infiltrate into spleen and liver. Especially spleens, as the largest lymphoid organ, are usually enlarged in the development of leukemia. As shown in Fig. 4A, the spleens of the combination treatment group were markedly smaller than that of the Vin group. From the representative tissue samples for histopathological analysis (Fig. 4B), reticular cells were regularly distributed in spleen of healthy NOD/SCID mice (the mice without HL-60 transplantation). After transplanted with HL-60 cells, splenic tissues were mainly filled with HL-60 cells and the structure integrity was damaged. In the combination treatment group, less HL-60 cell dissemination was found in the spleen and the histologic structure was basically intact compared with the Vin monotherapy group. In addition, for the histopathological analysis of liver, no obvious infiltration of HL-60 cells around blood vessels was visible in the combination treatment group compared with the marked infiltration in the control, E5 and Vin mono-treatment group. These results clearly indicate that combination therapy with E5 and Vin decreased the AML burden in bone marrow, spleen and liver, and induced leukemia remission.
Figure 3. In vivo combination treatment of AML mice with vincristine (Vin) and E5.
From day 20 of HL-60 transplantation, AML mice received Vin intraperitoneally 4 h after subcutaneous E5 administration twice weekly. (A) Overall survival of AML mice treated with sterile water (n = 5), E5 alone (n = 5), Vin alone (n = 7), or a combination of both (n = 7). (B–D) The percentage of HL-60 cells in the bone marrow, spleen and peripheral blood of AML mice in each group was determined with flow cytometry (n = 4) (*p < 0.05).
Figure 4. In vivo effects of vincristine (Vin) and E5 on infiltration of HL-60 cells into spleen and liver.
(A) The weight and size of spleens in healthy mice (without HL-60 transplantation) and Vin-monotreated or combination treated AML mice. (B) Histologic sections of spleen and liver in each group of mice were stained with H&E. Images of each organ in the second line are the amplification of images in the first line. Red arrows represent normal cells and blue arrows represent HL-60 cells infiltrated in spleens. Yellow arrows represent HL-60 cells infiltrated into livers.
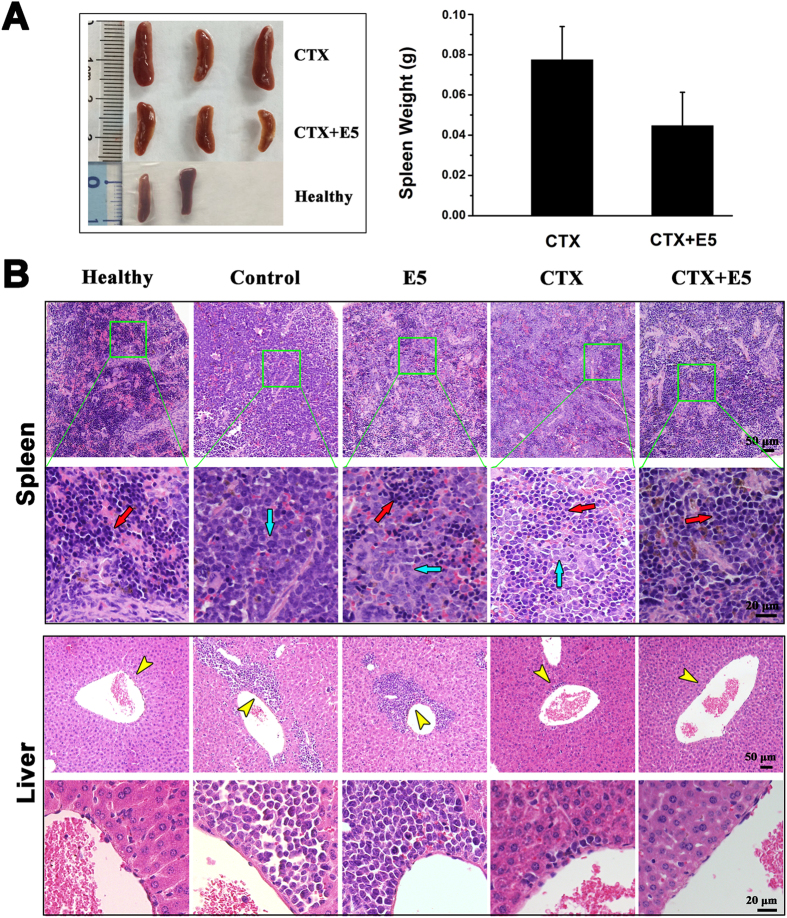
The effect of E5 plus CTX was also tested in the AML mice model. CTX was injected 4 h after the subcutaneous administration of E5 from day 20 after transplantation. Terminal bone marrow, spleen and peripheral blood samples of all AML mice were analyzed for HL-60 cell proportion with flow cytometry. In the combination treatment group, the percentage of HL-60 cells in the leukocytes collected from bone marrow, spleen and peripheral blood respectively was much lower than that of CTX monotherapy group (Fig. 5A). From the peripheral blood smears with Wright’s staining, a clearly reduced amount of HL-60 cells in combination treatment group (Fig. 5B) was also observed. Meanwhile no hyperleukocytosis or leukostasis was observed after long-term E5 treatment. Ultimately a prolonged median survival was observed in those mice that received the combination treatment (53 days) compared with CTX monotherapy (45 days) (p = 0.12, Fig. 5C). E5 alone also slightly extended the survival of leukemia mice. There was no significant effect of drug treatments on the body weights of the mice (Fig. 5D). Moreover, the spleens of the combination treatment group were smaller than that of the CTX group (Fig. 6A). As shown in Fig. 6B, much stronger rescue effect for HL-60 cell dissemination and histologic structural integrity in spleen was observed in combination treatment group. In addition, for the histopathological analysis of liver, no obvious infiltration of HL-60 cells around blood vessels was visible in the combination treatment group compared with the marked infiltration in the control, E5 and CTX mono-treatment group (Fig. 6B). These results clearly indicate E5 treatment significantly enhanced the efficacy of the CTX chemotherapy.
Figure 5. In vivo combination treatment of AML mice with cyclophosphamide (CTX) and E5.
(A) The percentage of CD33 positive cells (HL-60) in the bone marrow, spleen and peripheral blood of AML mice in each group was determined with flow cytometry. Data are presented as mean ± SD (n = 4). The * represents significant difference between two groups (*p < 0.05, **p < 0.01). (B) Wright-stained peripheral blood smears of AML mice in each group. Yellow arrows represent HL-60 cells. Scale bar represents 50 μm. (C) Overall survival of AML mice treated with sterile water, E5 alone, CTX alone, or a combination of both (n = 9). (D) Relative weight of the mice during the period of treatment.
Figure 6. In vivo effects of cyclophosphamide (CTX) and E5 on infiltration of HL-60 cells into spleen and liver.
(A) The weight and size of spleens in healthy mice (without HL-60 transplantation) and CTX-monotreated or combination treated AML mice. (B) Histologic sections of spleen and liver in each group of mice were stained with H&E. Images of each organ in the second line are the amplification of images in the first line. Red arrows represent normal cells and blue arrows represent HL-60 cells infiltrated in spleens. Yellow arrows represent HL-60 cells infiltrated into livers.
Systemic toxicity of E5 in healthy mice
We have tested the cytotoxicity of E5 on two nonmalignant cell lines (human umbilical vein cell ea.hy926 and murine stromal cell MS-5) with low level of CXCR4, and confirmed that E5 did not induce cell apoptosis at concentrations ranging from 1 to 80 μM24. To evaluate systemic toxicity of E5, we gave healthy BALB/c mice a subcutaneous injection of E5 at 40 mg/kg every other day in one month. After the mice were sacrificed, several organs were examined microscopically. Supplementary Fig. S2A shows representative H&E staining of heart, liver, spleen, lung, kidney, brain and lymph node tissues from mice treated either with sterile water or E5 injection. In particular, no central necrosis was observed in heart, liver, spleen, lung, brain or lymph node, and no tubular necrosis in kidney. There was also no significant effect of E5 administration on the organ weights of the mice (Supplementary Fig. S2B). Meanwhile, routine clinical parameters of serum including alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine (Cr(E)) and urea suggest the hepatic and renal function of mice were normal after the long-term E5 treatment (Supplementary Fig. S2C). All these above results demonstrate that there was no damage in these organs of the E5-treated mice and E5 has good security in vivo.
Discussion
Despite significant improvements in the treatment and steady increase in the survival of other types of leukemia, the 5-year survival of patients with AML remains dissatisfactory and a great number of AML new cases and deaths emerge25. It has been reported that CXCR4 expression is associated with poor prognosis in patients with AML26,27. CXCR4 thus can be incorporated into the risk assessment of AML patients28. Bone marrow is one of the major tissues that produce CXCL12 which activates CXCR429. Through CXCR4/CXCL12 axis, bone marrow stroma creates a protective microenvironment, providing pro-survival and chemotherapy resistance signals for the homing AML cells2. Hence CXCR4 antagonists were developed for leukemia therapy30.
Among developed CXCR4 antagonists, peptides are one attractive kind to block CXCR4/CXCL12 axis and abrogate the stromal cell-mediated leukemia cell drug-resistance. In this work, we demonstrated the strong mobilizing activity of E5 using a mouse model of human AML cells. There is a 1.84-fold increase of circulating HL-60 cells in the peripheral blood of AML mice at 4 h after E5 administration, indicating translocation of leukemia cells by E5 from a protective niche to a location where they are more sensitive to therapeutic drug treatment. The results of combination treatment of cytotoxic drugs and E5 were quite remarkable, leading to reduced leukemia burden and prolonged survival. These results still give strong evidence that E5 plays the mobilizing role in the chemotherapies, and we believe the p value can be improved by optimizing the leukemia animal model and the overall number of animal. It is also noticeable that very similar treatment effects were obtained no matter using E5 plus Vin or plus CTX, though the mode of action and cellular target of CTX are very different from that of Vin. Hence E5 can be expected to combine with more other cytotoxic drugs in the treatment of leukemia. Another point that should be emphasized is that AML is a heterogeneous disease and can be divided into 8 subtypes according to eight major subtypes from M0 to M7 according to its maturation degree in the French-American-British (FAB) classification system31. In the current study, HL-60 and NB4 are defined as subtype M3, THP-1 is defined as M5, and U937 is M6. The experimental results in vitro clearly showed E5 can be effective to the different subtypes of AML. For future studies, it is genuinely very interesting to investigate the effects of E5 obtained from HL-60/NOD-SCID model on the other subtypes of AML primary leukemia cells from patients.
Although various CXCR4 antagonists have the function of mobilizing leukemia cells from bone marrow, E5 has one distinguished advantage over others-good safety. Chemical molecule antagonists such as AMD3100 and AMD11070 mobilize leukemia cells into circulation and improve chemotherapy in vitro and in vivo17,21,32,33,34. However, AMD3100 is reported to display a weak partial agonist activity35,36, inducing CXCL12-like G-protein activation in CXCR4-expression cells upon binding of it, which could be a disadvantage to the treatment of leukemia, because CXCR4 activation provides a survival signal for leukemia cells. Long-term animal studies of AMD11070 revealed histological changes in the liver37 and a phase 1/2 study suggested its potential toxicity in the liver38. Hence, AMD3100 can only be utilized for short-term clinical treatment39. In the previous study24, we have demonstrated E5 induced neither the CXCR4 activation in four AML cell lines nor apoptosis of two nonmalignant cells (ea.hy926 and MS-5) even at a high concentration. In this work, we show that the long-term E5 treatment didn’t affect the major organ functions and no histological changes were observed, demonstrating the safety of E5. Considering that E5 induced mobilization of leukemia cells out of protection into circulation and enhanced chemotherapy sensitivity, this appears to be a remarkably effective method of boosting the cytotoxicity of low-dose chemotherapeutic drugs on leukemia cells, decreasing the risk of side effects caused by high-dose chemotherapies in leukemia treatments40,41,42. Another advantage of E5 is the low cost. Most of currently reported peptide antagonists are derived from cells by bioengineering technology15,43,44. For examples, T140 and its analogs TC14012 and TN14003 are derived from naturally occurring peptides in horseshoe crabs, and RCP168 is derived from viral macrophage inflammatory protein II. Furthermore, concerns of cytotoxicity have been raised for T140 due to its total positive charge45. In contrast, E5 is chemically synthesized according to the sequence of human CXCR4, which makes it more easily, flexibly and economically to be produced.
It should be noted that the relapse of most leukemia cells post chemotherapy is dependent on their intra-and extra-cellular resistances to chemotherapy. The CXCR4/CXCL12 axis mediated drug-resistance of AML cells belongs to the latter. In the current study, the CXCR4/CXCL12 axis is interfered by E5 and lead to the homing AML cells released from bone marrow to blood circulation. As the result, AML cells can be exposed to a higher drug concentration than bone marrow. Nevertheless, intra-cellular drug resistance could be induced for leukemia cells long-term exposed to the drug by expressing extensively drug resistance proteins such as the ATP-binding cassette (ABC) transporter proteins46. ABC proteins that confer drug resistance include P-glycoprotein (genes symbol ABCB1), the multidrug resistance protein 1 (MRP1, gene symbol ABCC1), MRP2 (gene symbol ABCC2) and the breast cancer resistance protein (BCRP, gene symbol ABCG2), induce the drug efflux from tumor cells and decrease cellular drug accumulation47. Several molecules have been developed to inhibit the ABC protein-mediated drug efflux, such as naringenin48, pegylated phosphotidylethanolamine49, apatinib50. The combination therapy of E5 and the ABC protein inhibitors with chemotherapeutic drugs can be expected to overcome the leukemia drug-resistance synergistically.
CXCR4 is also highly expressed by many other malignant cell types, such as breast cancer51, lung cancer52,53, melanoma54, prostate cancer55, and ovarian cancer56, and closely associated with the metastasis, invasion and chemotherapy resistance of these solid tumors. Hence, broader uses of E5 can also be expected in those cancer therapies in combination with chemotherapies.
In conclusion, we here present the evidence that E5 can reduce tumor burden, significantly improve the efficiency of chemotherapies for AML and extend survival in AML mice.
Materials and Methods
Cell lines and maintenance
The acute promyelocytic leukemia cell HL-60 and NB4, acute monocytic leukemia cell THP-1, and myelomonocytic leukemia cell U937 were purchased from the Cell Resource Center of Chinese Academy of Medical Sciences (Beijing, China). Murine stromal cell line MS-5 was kindly provided by Professor Bin Yin, the Cyrus Tang Hematology Center, Soochow University, China. Those cells were cultured in 5% CO2 at 37 °C in RPMI 1640 (Hyclone Thermo Scientific) supplemented with phenol red, 10% fetal bovine serum (FBS; Gibco, Grand Island, NY), 100 U/mL penicillin, 100 U/mL streptomycin.
Peptide and antibody
The peptide E5 (GGRSFFLLRRIQGCRFRNTVDD) used in this study was identified by the cell-based selection24 and purchased from GL Biochem (Shanghai) Ltd. The stock solution of E5 was dissolved in the sterile water. PE anti-human CD33 antibody was purchased from BioLegend (San Diego, CA).
In vitro combination treatments of E5 with chemotherapeutic drugs
Drug combination in short-term
MS-5 cells of 1.5 × 105/well were seeded in 24-well plates in RPMI 1640 containing 10% FBS and incubated overnight to form a stromal cell layer. Leukemia cells of 2.5 × 105 (HL-60, NB4, THP-1 and U937) were treated with E5 at 10 μM or 20 μM in serum-free medium (opti-MEM; Life Technologies, Grand Island, NY) for 1 h, and then cultivated in the presence or absence of MS-5 cell layer for 4 h. After that, chemotherapeutic drugs were added to the culture system (10-hydroxy camptothecin at 40 nM for HL-60, THP-1 and U937, or at 11 nM for NB4; vincristine (Vin; Shenzhen Main Luck Pharmaceuticals, Shenzhen, China) at 2.5 nM and cisplatin (Hospira Australia Pty Ltd, Melbourne, Australia) at 1.2 μM for HL-60) and the cells were cultivated for 48 h. The leukemia cells were then separated from the MS-5 cell layer, washed and stained with FITC-Annexin V (eBioscience, Vienna, Austria) and subjected to C6 Accuri® flow cytometer (Accuri Cytometers, Ann Arbor, MI). In the measurement, 1.5 × 104 cells were collected and the acquired data were analyzed by CFlow Plus software.
Drug combination in long-term
MS-5 cells of 5 × 103/well were seeded in 96-well plates in RPMI 1640 containing 10% FBS and incubated overnight to form a stromal cell layer. HL-60 cells (2 × 104) were treated with E5 (10 μM) in opti-MEM for 1 h, and then inoculated to the MS-5 cell layer. Either vincristine (1 nM) or a combination of vincristine and E5 (10 μM) was added to the culture system. Half of medium was changed every alternate day and replenished with fresh drug. Care was taken not to destroy the culture system. Viability of HL-60 cells was analyzed by the trypan blue exclusion method every day.
Establishment of mouse model for human AML
Five-week old female NOD/SCID mice were maintained in the Experimental Animal Center at the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences (Beijing, China) under specific pathogen-free conditions. All the animal experiments reported herein were carried out in accordance with the approved guideline and approved by the committee on the Animal Care and Use of Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences & Peking Union Medical College. Animals were acclimatized to laboratory conditions for 1 week prior to experiments. HL-60 cells (1 × 106) suspended in 100 μL EDTA/PBS were injected intravenously into the sublethally irradiated (250 cGy) NOD/SCID mice. At about 20 days after transplantation, mice showed leukemia signs. Cells of bone marrow and spleen were collected from the mice and analyzed for HL-60 proportion with flow cytometry on day 20, 34 and 40 after transplantation.
Treatment in leukemia mouse model with E5 and chemotherapeutic drugs
From day 20, the mice were randomly treated with E5 (10 mg/kg), Vin (0.2 mg/kg), cyclophosphamide (CTX, 90 mg/kg) or a combination of E5 and one of the drugs twice a week. For the combination treatment group, Vin or CTX was injected intraperitoneally 4 h after subcutaneous injection of E5. Mice were monitored for the condition and weight loss. On day 40 after transplantation, mice were sacrificed. Cells of bone marrow, spleen and peripheral blood were collected from each group of mice and analyzed for HL-60 proportion with flow cytometry.
Flow cytometry analysis
The bone marrow obtained by femur flushing, spleen and peripheral blood from each group of treated mice were collected to prepare single cell suspension. Erythrocytes were excluded using the blood cells lysis buffer (Beckman Coulter, Krefeld, Germany). The human specific CD33 surface marker was used to identify HL-60 (positive rate was 100% as determined by flow cytometry) with PE anti-human CD33 antibody and 1.5 × 104 cells were analyzed with flow cytometry.
Histological analysis
Livers and spleens from each group of treated mice were fixed in 4% formalin, dehydrated and embedded in paraffin. Tissue sections were prepared and stained with hematoxylin and eosin (H&E). The histology slides were analyzed for HL-60 cells dissemination using an Olympus BX53F microscope equipped with a digital camera (Olympus, Tokyo, Japan) (three representative slides for each group were reviewed).
Wright staining of blood smears
A drop of peripheral blood from each group of treated mice was pulled between two slides at roughly 45° to create a blood film slide which was allowed to air dry. Slides were briefly fixed in methanol prior to staining with Wright’s stain. Slides were then cover slipped prior to scanning using an Olympus BX53F microscope.
In vivo toxicity of E5
Safety experiments were performed on five-week old healthy female BALB/c mice with four mice per group. The mice were randomly divided into two groups and respectively given a subcutaneous injection of E5 at 40 mg/kg (E5-treated group) or sterile water (control group) every other day. After the 30-day treatment, the mice were sacrificed. Their heart, liver, spleen, lung, kidney, brain and lymph node were weighed and the tissue sections were subjected to H&E staining. Blood samples of each group were collected and centrifuged at 3000 rpm for 15 min at 4 °C to obtain serum. The serum samples were used for routine clinical parameters analysis including alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine (Cr(E)) and urea.
Statistical analysis
All experiments were carried out in triplicate and unpaired Student’s t-test was performed to assess statistical significance of the results (*P < 0.05 and **P < 0.01). The pairwise comparisons for the median survival of leukemia mice between Vin (or CTX) alone group and Vin (or CTX) plus E5 were assessed by using the Log-rank test (SAS 8.2 Software, SAS Institute).
Additional Information
How to cite this article: Li, X. et al. Improving chemotherapeutic efficiency in acute myeloid leukemia treatments by chemically synthesized peptide interfering with CXCR4/CXCL12 axis. Sci. Rep. 5, 16228; doi: 10.1038/srep16228 (2015).
Supplementary Material
Acknowledgments
This work was supported by National Key Basic Research Program of China (2011CB933504) and Key project of the Major Research Plan from National Natural Science Foundation of China (91127043).
Footnotes
Author Contributions C.W. and H.X. designed the peptide and experiments, made data analysis and wrote the main manuscript. X.L. and H.G. conducted main experiments, made data analysis, prepared figures and discussed all sections of the manuscript with the corresponding authors. Y.Y. and H.D. designed the peptide and discussed part sections of the manuscript with the corresponding authors. J.M. and J.L. made data analysis and discussed part sections of the manuscript with the corresponding authors. All authors reviewed the manuscript.
References
- Löwenberg B., Downing J. R. & Burnett A. Acute myeloid leukemia. N Engl J Med. 14, 1051–1062 (1999). [DOI] [PubMed] [Google Scholar]
- Tavor S. & Petit I. Can inhibition of the SDF-1/CXCR4 axis eradicate acute leukemia. Semin Cancer Biol. 20, 178–185 (2010). [DOI] [PubMed] [Google Scholar]
- Li Z. W. & Dalton W. S. Tumor microenvironment and drug resistance in hematologic malignancies. Blood Rev. 20, 333–342 (2006). [DOI] [PubMed] [Google Scholar]
- Ishikawa F. et al. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol. 25, 1315–1321 (2007). [DOI] [PubMed] [Google Scholar]
- Konopleva M. & Andreeff M. Targeting the leukemia microenvironment. Curr Drug Targets. 8, 685–701 (2007). [DOI] [PubMed] [Google Scholar]
- Meads M. B., Hazlehurst L. A. & Dalton W. S. The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clin Cancer Res. 14, 2519–2526 (2008). [DOI] [PubMed] [Google Scholar]
- Domanska U. M. et al. A review on CXCR4/CXCL12 axis in oncology: no place to hide. Eur J Cancer. 49, 219–230 (2013). [DOI] [PubMed] [Google Scholar]
- Burger J. A. & Bürkle A. The CXCR4 chemokine receptor in acute and chronic leukaemia: a marrow homing receptor and potential therapeutic target. Br J Haematol. 137, 288–296 (2007). [DOI] [PubMed] [Google Scholar]
- Konopleva M. et al. Stromal cells prevent apoptosis of AML cells by up-regulation of anti-apoptotic proteins. Leukemia 16, 1713–1724 (2002). [DOI] [PubMed] [Google Scholar]
- Manabe A., Coustan-Smith E., Behm F. G., Raimondi S. C. & Campana D. Bone marrow-derived stromal cells prevent apoptotic cell death in B-lineage acute lymphoblastic leukemia. Blood 79, 2370–2377 (1992). [PubMed] [Google Scholar]
- Panayiotidis P., Jones D., Ganeshaguru K., Foroni L. & Hoffbrand A. V. Human bone marrow stromal cells prevent apoptosis and support the survival of chronic lymphocytic leukaemia cells in vitro. Br J Haematol. 92, 97–103 (1996). [DOI] [PubMed] [Google Scholar]
- Basak P. et al. Leukemic stromal hematopoietic microenvironment negatively regulates the normal hematopoiesis in mouse model of leukemia. Chin J Cancer. 29, 969–979 (2010). [DOI] [PubMed] [Google Scholar]
- Burger J. A. et al. J. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood 96, 2655–2663 (2000). [PubMed] [Google Scholar]
- Burger J. A. & Kipps T. J. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood 107, 1761–1767 (2006). [DOI] [PubMed] [Google Scholar]
- Burger J. A. & Peled A. CXCR4 antagonists: targeting the microenvironment in leukemia and other cancers. Leukemia 23, 43–52 (2009). [DOI] [PubMed] [Google Scholar]
- Juarez J., Bradstock K. F., Gottlieb D. J. & Bendall L. J. Effects of inhibitors of the chemokine receptor CXCR4 on acute lymphoblastic leukemia cells in vitro. Leukemia 17, 1294–300 (2003). [DOI] [PubMed] [Google Scholar]
- Nervi B. et al. Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100. Blood 113, 6206–6214 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uy G. L. et al. A phase 1/2 study of chemosensitization with the CXCR4 antagonist plerixafor in relapsed or refractory acute myeloid leukemia. Blood 119, 3917–3924 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z. et al. Targeting the leukemia microenvironment by CXCR4 inhibition overcomes resistance to kinase inhibitors and chemotherapy in AML. Blood 113, 6215–6224 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z. et al. Inhibition of CXCR4 with the novel RCP168 peptide overcomes stroma-mediated chemoresistance in chronic and acute leukemias. Mol Cancer Ther. 5, 3113–3121 (2006). [DOI] [PubMed] [Google Scholar]
- Parameswaran R., Yu M., Lim M., Groffen J. & Heisterkamp N. Combination of drug therapy in acute lymphoblastic leukemia with a CXCR4 antagonist. Leukemia 25, 1314–1323 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger M. et al. Small peptide inhibitors of the CXCR4 chemokine receptor (CD184) antagonize the activation, migration, and antiapoptotic responses of CXCL12 in chronic lymphocytic leukemia B cells. Blood 106, 1824–1830 (2005). [DOI] [PubMed] [Google Scholar]
- Juarez J. et al. CXCR4 antagonists mobilize childhood acute lymphoblastic leukemia cells into the peripheral blood and inhibit engraftment. Leukemia 21, 1249–1257 (2007). [DOI] [PubMed] [Google Scholar]
- Li X. et al. A designed peptide targeting CXCR4 displays anti-acute myelocytic leukemia activity in vitro and in vivo. Sci Rep. 4, 6610 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R. L., Miller K. D. & Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 65, 5–29 (2015). [DOI] [PubMed] [Google Scholar]
- Spoo A. C., Lübbert M., Wierda W. G. & Burger J. A. CXCR4 is a prognostic marker in acute myelogenous leukemia. Blood 109, 786–791 (2007). [DOI] [PubMed] [Google Scholar]
- Peled A. & Tavor S. Role of CXCR4 in the pathogenesis of acute myeloid leukemia. Theranostics 3, 34–39 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn J. Y., Seo K., Weinberg O. K. & Arber D. A. The prognostic value of CXCR4 in acute myeloid leukemia. Appl Immunohistochem Mol Morphol. 21, 79–84 (2013). [DOI] [PubMed] [Google Scholar]
- Imai K. et al. Selective secretion of chemoattractants for haemopoietic progenitor cells by bone marrow endothelial cells: a possible role in homing of haemopoietic progenitor cells to bone marrow. Br J Haematol. 106, 905–911 (1999). [DOI] [PubMed] [Google Scholar]
- Juarez J., Bendall L. & Bradstock K. Chemokines and their receptors as therapeutic targets: the role of the SDF-1/CXCR4 axis. Curr Pharm Des. 10, 1245–1259 (2004). [DOI] [PubMed] [Google Scholar]
- Harris N. L. et al. The World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. Report of the Clinical Advisory Committee meeting, Airlie House, Virginia, November, 1997. Ann Oncol. 10, 1419–1432 (1999). [DOI] [PubMed] [Google Scholar]
- Weisberg E. et al. Inhibition of CXCR4 in CML cells disrupts their interaction with the bone marrow microenvironment and sensitizes them to nilotinib. Leukemia 26, 985–990 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavor S. et al. CXCR4 regulates migration and development of human acute myelogenous leukemia stem cells in transplanted NOD/SCID mice. Cancer Res. 64, 2817–2824 (2004). [DOI] [PubMed] [Google Scholar]
- Kawaguchi A. et al. Inhibition of the SDF-1alpha-CXCR4 axis by the CXCR4 antagonist AMD3100 suppresses the migration of cultured cells from ATL patients and murine lymphoblastoid cells from HTLV-I Tax transgenic mice. Blood 114, 2961–2968 (2009). [DOI] [PubMed] [Google Scholar]
- Trent J. O. et al. Lipid bilayer simulations of CXCR4 with inverse agonists and weak partial agonists. J Biol Chem. 278, 47136–47144 (2003). [DOI] [PubMed] [Google Scholar]
- Zhang W. B. et al. A point mutation that confers constitutive activity to CXCR4 reveals that T140 is an inverse agonist and that AMD3100 and ALX40-4C are weak partial agonists. J Biol Chem. 277, 24515–24521 (2002). [DOI] [PubMed] [Google Scholar]
- Moyle G. et al. Proof of activity with AMD11070, an orally bioavailable inhibitor of CXCR4-tropic HIV type 1. Clin Infect Dis. 48, 798–805 (2009). [DOI] [PubMed] [Google Scholar]
- Stone N. D. et al. Multiple-dose escalation study of the safety, pharmacokinetics, and biologic activity of oral AMD070, a selective CXCR4 receptor inhibitor, in human subjects. Antimicrob Agents Chemother. 51, 2351–2358 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S. B. et al. Identification of LY2510924, a novel cyclic peptide CXCR4 antagonist that exhibits antitumor activities in solid tumor and breast cancer metastatic models. Mol Cancer Ther. 14, 480–490 (2015). [DOI] [PubMed] [Google Scholar]
- Ahles T. A. et al. Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J Clin Oncol. 20, 485–493 (2002). [DOI] [PubMed] [Google Scholar]
- Partridge A. H., Burstein H. J. & Winer E. P. Side effects of chemotherapy and combined chemohormonal therapy in women with early-stage breast cancer. J Natl Cancer Inst Monogr. 30, 135–142 (2001). [DOI] [PubMed] [Google Scholar]
- Schwartz C. L. Long-term survivors of childhood cancer: the late effects of therapy. Oncologist 4, 45–54 (1999). [PubMed] [Google Scholar]
- Debnath B., Xu S., Grande F., Garofalo A. & Neamati N. Small molecule inhibitors of CXCR4. Theranostics 3, 47–75 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrançois M. et al. Agonists for the Chemokine Receptor CXCR4. ACS Med Chem Lett. 2, 597–602 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamura H. et al. Synthesis of potent CXCR4 inhibitors possessing low cytotoxicity and improved biostability based on T140 derivatives. Org Biomol Chem. 1, 3656–3662 (2003). [DOI] [PubMed] [Google Scholar]
- Schinkel A. H. & Jonker J. W. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv Drug Deliv Rev. 55, 3–29 (2003). [DOI] [PubMed] [Google Scholar]
- Leslie E. M., Deeley R. G. & Cole S. P. Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol. 204, 216–237 (2005). [DOI] [PubMed] [Google Scholar]
- Zhang F. Y. et al. Naringenin enhances the anti-tumor effect of doxorubicin through selectively inhibiting the activity of multidrug resistance-associated proteins but not P-glycoprotein. Pharm Res. 26, 914–925 (2009). [DOI] [PubMed] [Google Scholar]
- Wang J. et al. Pegylated phosphotidylethanolamine inhibiting P-glycoprotein expression and enhancing retention of doxorubicin in MCF7/ADR cells. J Pharm Sci. 100, 2267–2277 (2011). [DOI] [PubMed] [Google Scholar]
- Mi Y. J. et al. Apatinib (YN968D1) reverses multidrug resistance by inhibiting the efflux function of multiple ATP-binding cassette transporters. Cancer Res. 70, 7981–7991 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller A. et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 410, 50–56 (2001). [DOI] [PubMed] [Google Scholar]
- Burger M. et al. Functional expression of CXCR4 (CD184) on small-cell lung cancer cells mediates migration, integrin activation, and adhesion to stromal cells. Oncogene 22, 8093–8101 (2003). [DOI] [PubMed] [Google Scholar]
- Kijima T. et al. Regulation of cellular proliferation, cytoskeletal function, and signal transduction through CXCR4 and c-Kit in small cell lung cancer cells. Cancer Res. 62, 6304–6311 (2002). [PubMed] [Google Scholar]
- Scala S. et al. Expression of CXCR4 predicts poor prognosis in patients with malignant melanoma. Clin Cancer Res. 11, 1835–1841 (2005). [DOI] [PubMed] [Google Scholar]
- Wang J. et al. Diverse signaling pathways through the SDF-1/CXCR4 chemokine axis in prostate cancer cell lines leads to altered patterns of cytokine secretion and angiogenesis. Cell Signal. 17, 1578–1592 (2005). [DOI] [PubMed] [Google Scholar]
- Scotton C. J. et al. Multiple actions of the chemokine CXCL12 on epithelial tumor cells in human ovarian cancer. Cancer Res. 62, 5930–5938 (2002). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.