Abstract
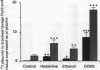
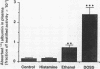
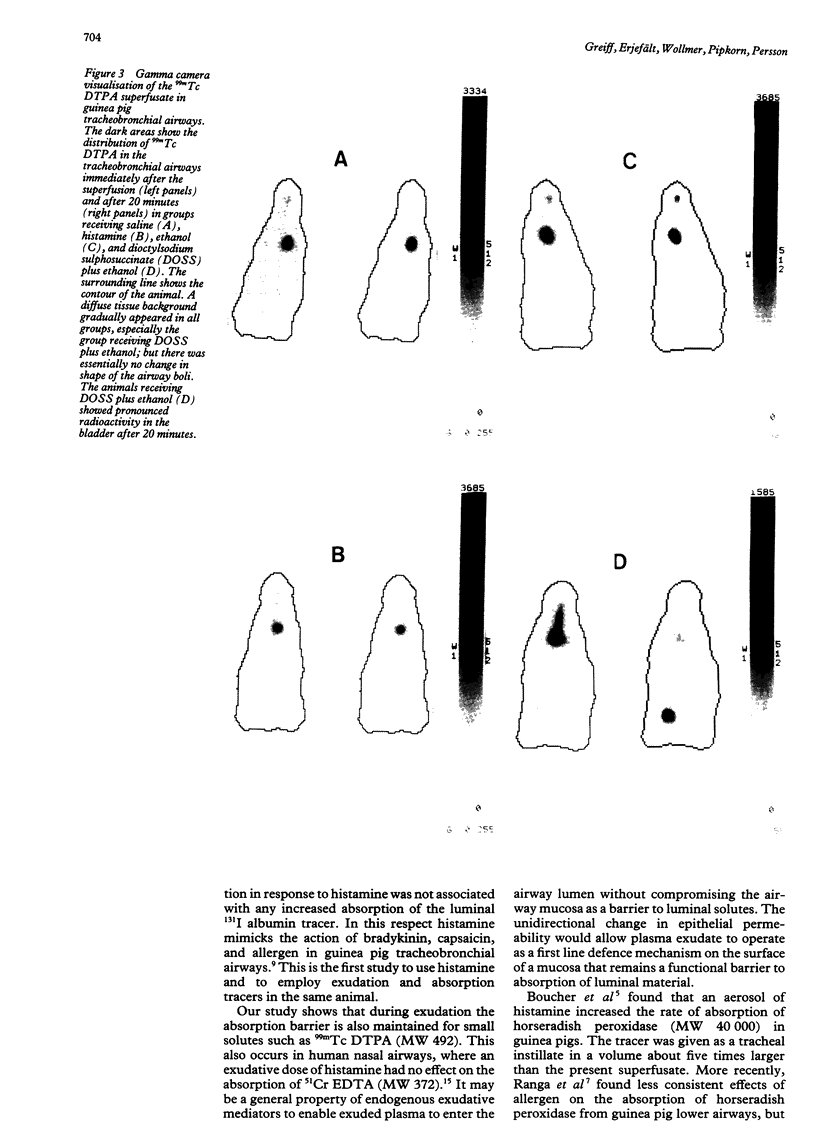
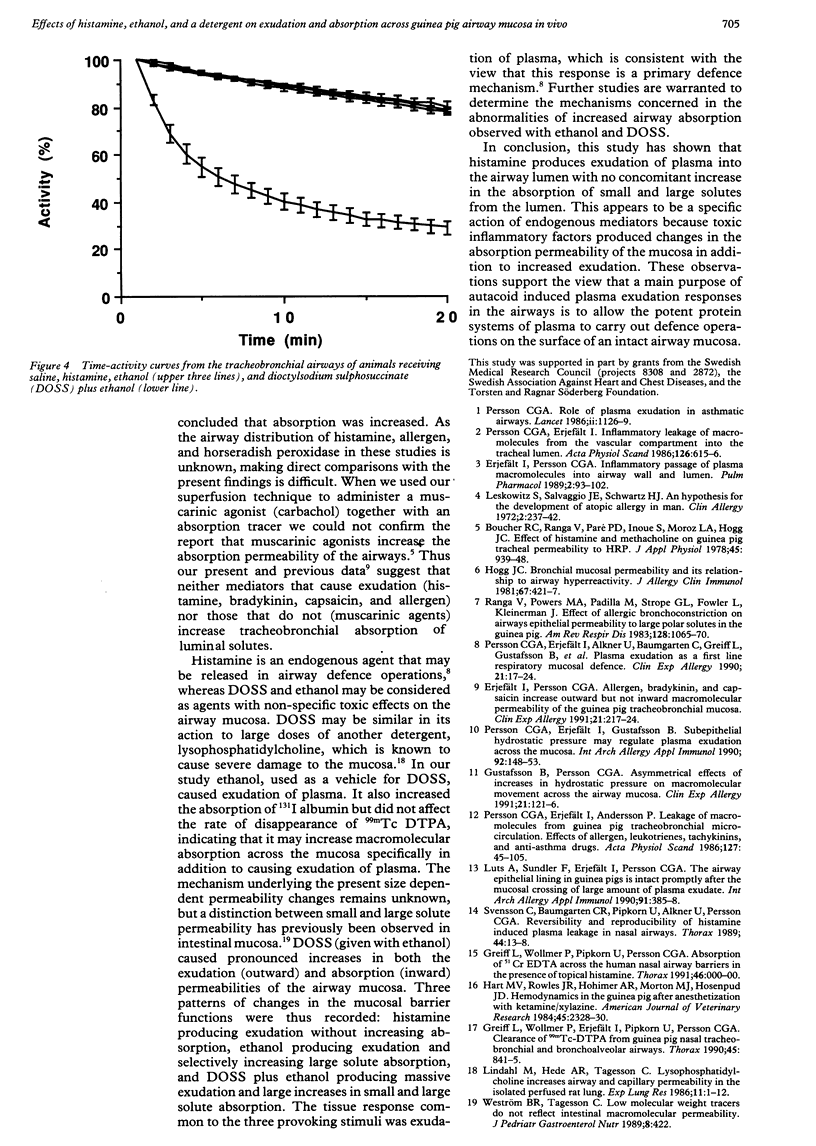
This study examined effects of three substances that cause mucosal provocation (histamine, ethanol, and the detergent dioctylsodium sulphosuccinate (DOSS] on the flux of solutes across airway vascular mucosal barriers in anaesthetised guinea pigs. The inward flux was assessed as absorption of iodine-131 labelled albumin (MW 69,000) from the tracheobronchial surface into the circulation and the outward flux as the exudation of two intravenously administered plasma tracers--125I albumin (MW 69,000) and fluorescein isothiocyanate conjugated (FITC) dextran (MW 70,000)--into the airway. The absorption of technetium-99m labelled DTPA (MW 492) from the tracheobronchial airways was determined in separate experiments. Histamine (5.0 nmol) dissolved in 40 microliters saline and superfused on the tracheobronchial mucosal surface caused significant and similar entry of 125I albumin and FITC dextran into the airway lumen. This dose of histamine did not, however, alter the absorption of small (99mTc DTPA) or large (131I albumin) solutes across the airway mucosa. Ethanol (0.17 mumol), superfused in the same way, also caused significant exudation of the plasma tracers into the airway lumen. In addition, ethanol increased the absorption of 131I albumin without causing change in the disappearance rate of 99mTc DTPA. The detergent, DOSS (0.28 nmol), dissolved in ethanol (0.17 mumol), caused a pronounced increase in exudation and much increased absorption of small and large tracer solutes. Thus three patterns of change in airway mucosal barriers were found. The agents that are toxic to membranes, ethanol and DOSS, caused a bidirectional increase in permeability across the mucosa, whereas histamine caused only an outward exudative flux. The results obtained with histamine are similar to those seen previously with bradykinin, capsaicin, and allergen, suggesting that endogenous inflammatory mediators have a role in mucosal defence, producing entry of plasma exudates into the airway lumen without increasing the mucosal absorption of luminal material.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boucher R. C., Ranga V., Paré P. D., Inoue S., Moroz L. A., Hogg J. C. Effect of histamine and methacholine on guinea pig tracheal permeability to HRP. J Appl Physiol Respir Environ Exerc Physiol. 1978 Dec;45(6):939–948. doi: 10.1152/jappl.1978.45.6.939. [DOI] [PubMed] [Google Scholar]
- Erjefält I., Persson C. G. Allergen, bradykinin, and capsaicin increase outward but not inward macromolecular permeability of guinea-pig tracheobronchial mucosa. Clin Exp Allergy. 1991 Mar;21(2):217–224. doi: 10.1111/j.1365-2222.1991.tb00833.x. [DOI] [PubMed] [Google Scholar]
- Erjefält I., Persson C. G. Inflammatory passage of plasma macromolecules into airway wall and lumen. Pulm Pharmacol. 1989;2(2):93–102. doi: 10.1016/0952-0600(89)90030-6. [DOI] [PubMed] [Google Scholar]
- Greiff L., Wollmer P., Erjefält I., Pipkorn U., Persson C. G. Clearance of 99mTc DTPA from guinea pig nasal, tracheobronchial, and bronchoalveolar airways. Thorax. 1990 Nov;45(11):841–845. doi: 10.1136/thx.45.11.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B. G., Persson C. G. Asymmetrical effects of increases in hydrostatic pressure on macromolecular movement across the airway mucosa. A study in guinea-pig tracheal tube preparations. Clin Exp Allergy. 1991 Jan;21(1):121–126. doi: 10.1111/j.1365-2222.1991.tb00813.x. [DOI] [PubMed] [Google Scholar]
- Hart M. V., Rowles J. R., Hohimer A. R., Morton M. J., Hosenpud J. D. Hemodynamics in the guinea pig after anesthetization with ketamine/xylazine. Am J Vet Res. 1984 Nov;45(11):2328–2330. [PubMed] [Google Scholar]
- Hogg J. C. Bronchial mucosal permeability and its relationship to airways hyperreactivity. J Allergy Clin Immunol. 1981 Jun;67(6):421–425. doi: 10.1016/0091-6749(81)90094-4. [DOI] [PubMed] [Google Scholar]
- Kovacs J. A., Gill V., Swan J. C., Ognibene F., Shelhamer J., Parrillo J. E., Masur H. Prospective evaluation of a monoclonal antibody in diagnosis of Pneumocystis carinii pneumonia. Lancet. 1986 Jul 5;2(8497):1–3. doi: 10.1016/s0140-6736(86)92555-9. [DOI] [PubMed] [Google Scholar]
- Leskowitz S., Salvaggio J. E., Schwartz H. J. An hypothesis for the development of atopic allergy in man. Clin Allergy. 1972 Sep;2(3):237–246. doi: 10.1111/j.1365-2222.1972.tb01288.x. [DOI] [PubMed] [Google Scholar]
- Lindahl M., Hede A. R., Tagesson C. Lysophosphatidylcholine increases airway and capillary permeability in the isolated perfused rat lung. Exp Lung Res. 1986;11(1):1–12. doi: 10.3109/01902148609062823. [DOI] [PubMed] [Google Scholar]
- Luts A., Sundler F., Erjefält I., Persson C. G. The airway epithelial lining in guinea pigs is intact promptly after the mucosal crossing of a large amount of plasma exudate. Int Arch Allergy Appl Immunol. 1990;91(4):385–388. doi: 10.1159/000235146. [DOI] [PubMed] [Google Scholar]
- Persson C. G., Erjefält I., Alkner U., Baumgarten C., Greiff L., Gustafsson B., Luts A., Pipkorn U., Sundler F., Svensson C. Plasma exudation as a first line respiratory mucosal defence. Clin Exp Allergy. 1991 Jan;21(1):17–24. doi: 10.1111/j.1365-2222.1991.tb00799.x. [DOI] [PubMed] [Google Scholar]
- Persson C. G., Erjefält I., Andersson P. Leakage of macromolecules from guinea-pig tracheobronchial microcirculation. Effects of allergen, leukotrienes, tachykinins, and anti-asthma drugs. Acta Physiol Scand. 1986 May;127(1):95–105. doi: 10.1111/j.1748-1716.1986.tb07880.x. [DOI] [PubMed] [Google Scholar]
- Persson C. G., Erjefält I., Gustafsson B., Luts A. Subepithelial hydrostatic pressure may regulate plasma exudation across the mucosa. Int Arch Allergy Appl Immunol. 1990;92(2):148–153. doi: 10.1159/000235206. [DOI] [PubMed] [Google Scholar]
- Persson C. G., Erjefält I. Inflammatory leakage of macromolecules from the vascular compartment into the tracheal lumen. Acta Physiol Scand. 1986 Apr;126(4):615–616. doi: 10.1111/j.1748-1716.1986.tb07863.x. [DOI] [PubMed] [Google Scholar]
- Ranga V., Powers M. A., Padilla M., Strope G. L., Fowler L., Kleinerman J. Effect of allergic bronchoconstriction on airways epithelial permeability to large polar solutes in the guinea pig. Am Rev Respir Dis. 1983 Dec;128(6):1065–1070. doi: 10.1164/arrd.1983.128.6.1065. [DOI] [PubMed] [Google Scholar]
- Svensson C., Baumgarten C. R., Pipkorn U., Alkner U., Persson C. G. Reversibility and reproducibility of histamine induced plasma leakage in nasal airways. Thorax. 1989 Jan;44(1):13–18. doi: 10.1136/thx.44.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weström B. R., Tagesson C. Low molecular weight markers do not reflect intestinal macromolecular permeability. J Pediatr Gastroenterol Nutr. 1989 Apr;8(3):422–423. doi: 10.1097/00005176-198904000-00030. [DOI] [PubMed] [Google Scholar]