Soybean cultivars with high phenotypic plasticity can increase seed yield under elevated CO2 and show higher biomass and pod number per plant at low plant density.
Abstract
Selection for cultivars with superior responsiveness to elevated atmospheric CO2 concentrations (eCO2) is a powerful option for boosting crop productivity under future eCO2. However, neither criteria for eCO2 responsiveness nor prescreening methods have been established. The purpose of this study was to identify traits responsible for eCO2 responsiveness of soybean (Glycine max). We grew 12 Japanese and U.S. soybean cultivars that differed in their maturity group and determinacy under ambient CO2 and eCO2 for 2 years in temperature gradient chambers. CO2 elevation significantly increased seed yield per plant, and the magnitude varied widely among the cultivars (from 0% to 62%). The yield increase was best explained by increased aboveground biomass and pod number per plant. These results suggest that the plasticity of pod production under eCO2 results from biomass enhancement, and would therefore be a key factor in the yield response to eCO2, a resource-rich environment. To test this hypothesis, we grew the same cultivars at low planting density, a resource-rich environment that improved the light and nutrient supplies by minimizing competition. Low planting density significantly increased seed yield per plant, and the magnitude ranged from 5% to 105% among the cultivars owing to increased biomass and pod number per plant. The yield increase due to low-density planting was significantly positively correlated with the eCO2 response in both years. These results confirm our hypothesis and suggest that high plasticity of biomass and pod production at a low planting density reveals suitable parameters for breeding to maximize soybean yield under eCO2.
The atmospheric concentration of carbon dioxide ([CO2]) increased from the preindustrial level of 271 µmol mol–1 to 391 µmol mol–1 in 2011, owing primarily to emissions from combustion of fossil fuels. [CO2] is predicted to rise from the current level to approximately 600 µmol mol–1 by 2050 (Ciais et al., 2013). Elevated atmospheric CO2 concentration (eCO2) is well known to increase leaf photosynthesis by increasing the availability of CO2 as a substrate for the carboxylation reaction with Rubisco; this can increase crop productivity, a phenomenon known as the CO2 fertilization effect, especially for C3 plants such as rice (Oryza sativa), wheat (Triticum aestivum), and soybean (Glycine max; e.g. Kimball et al., 2002), since [CO2] is a growth-limiting resource for C3 plants. There is a large genotypic variation in the yield response to eCO2, both among cultivars and between species, with responses ranging from –15% to +20% per 100 µmol mol–1 CO2 increase from the current level for rice (Ziska et al., 1996; Moya et al., 1998; Baker, 2004; Shimono et al., 2009; Hasegawa et al., 2013), –6% to +35% for wheat (Manderscheid and Weigel, 1997; Ziska et al., 2004; Ziska, 2008; Tausz-Posch et al., 2015), –5% to +55% for soybean (Ziska and Bunce, 2000; Ziska et al., 2001; Bishop et al., 2015; Bunce, 2015), and –6% to +21% for field bean (Phaseolus vulgaris; Bunce, 2008). These large differences in eCO2 responsiveness within crop species suggest that active selection and breeding for genotypes that respond strongly to gradual but steadily increasing [CO2] may ensure sustained productivity and improve food security in a future eCO2 world (Ainsworth et al., 2008; Ziska et al., 2012; Tausz et al., 2013).
Several hypotheses have been proposed about which traits should be targeted by breeders because they are related to intraspecific variation in the responsiveness of seed yield to eCO2. For example, a cultivar’s maturity group is an important growth trait for determining crop productivity. Late-maturing rice cultivars as a result of the longer period in which they can grow could increase grain yield relatively by eCO2 and may therefore benefit more from eCO2 than early-maturing cultivars (Hasegawa et al., 2013). Also, phenological changes by eCO2 could be another good indicator of genotypic variation in eCO2 responsiveness. Recently, Bunce (2015) showed that extension of the duration of vegetative growth until flowering at the apical node of the main stem caused by eCO2 was correlated with an increase in seed yield among some soybean genotypes.
The source-sink relationship is another important aspect of genotypic variation in the responsiveness of a plant to eCO2. CO2 enrichment can increase photosynthesis, especially during the early growth stage of leaves, and the magnitude of the increase of photosynthesis decreases with increasing growth stage because leaf senescence accelerates under eCO2, which has been referred to as acclimation (for review, see Moore et al., 1999). Genotypes with slower acclimation to eCO2 had a greater response of seed yield (Zhu et al., 2014, for rice; Hao et al., 2012, for soybean). Determinacy is strongly related to the source-sink relationship, especially for legume species. Indeterminate soybean cultivars that have more sinks are likely to be superior in responsiveness to eCO2, compared with determinate cultivars (Ainsworth et al., 2004). Aspinwall et al. (2015) emphasized the importance of phenotypic plasticity (the ability of a genotype to alter its phenotype in response to environmental changes) under eCO2 as a key trait for eCO2 responsiveness. Many researchers have suggested that a higher sink plasticity under eCO2 would lead to greater plasticity of tillering, branching, and biomass production, and could therefore be more important than photosynthesis per unit leaf area for adaptation to eCO2 by rice (Shimono et al., 2009; Zhu et al., 2014), soybean (Ziska and Bunce, 2000; Ziska et al., 2001), wheat (Manderscheid and Weigel, 1997; Ziska et al., 2004; Ziska, 2008), and field bean (Bunce, 2008); an exception would occur when other resources are limited, such as during a drought (Tausz-Posch et al., 2015). It is difficult to categorize biomass per se as a function of sink or source factors, but a plant’s final biomass results from efficient formation of sinks in vegetative and reproductive organs, and from efficient filling of these sinks with the products of photosynthesis. This would be a promising hypothesis, and if the hypothesis is confirmed, the phenotypic plasticity would become a useful criterion for identifying eCO2-responsive cultivars.
The first objective of the current study was to examine the genotypic variation of yield enhancement caused by eCO2 in diverse soybean cultivars, as soybean is a major source of plant protein and oil and a major contributor to the world’s food supply. In addition to characterizing the variation, we attempted to identify the factors responsible for it. The soybean genotypes that we chose covered a wide range of maturity groups and determinacy, including near-isogenic lines. We concluded that soybean cultivars varied widely in their responsiveness to eCO2, and that variation in the yield enhancement by eCO2 was determined primarily by the plasticity of biomass and pod production. This suggests that both are suitable parameters for screening cultivars with a strong response to eCO2. However, it is not easy to characterize plasticity of biomass and pod production under eCO2 of each cultivar since CO2 enhancement facilities such as controlled enclosed chambers are extremely expensive and not easily accessible.
Our second objective was to develop a methodology to characterize intraspecific variation in plasticity. Shimono (2011) proposed a simple and novel idea: to use planting density for prescreening to identify eCO2-responsive cultivars. Solar radiation is the driving force for photosynthesis and plant growth, and crops are grown as a population (not as individual plants) to maximize productivity per unit area rather than per plant. Individual plant growth is usually restricted by interplant competition for solar radiation, soil nutrients, and water, so a lower planting density could potentially increase the source strength for individual plants by increasing the availability of resources. Thus, lower plant density can imitate the greater resource availability that would occur under eCO2. This approach is potentially useful but is insufficient, because Shimono et al. (2014) applied it only to rice, measured only panicle number (not yield) as the phenotype, and combined independent experimental data from different locations and years. Here, we tested the hypothesis using a diverse range of soybean cultivars at a single site and for 2 years, and found a good relationship between the responsiveness to eCO2 and the responsiveness to low planting density (LD) in terms of the seed yield per plant.
RESULTS
Genotypic Variation of Yield Responses to eCO2
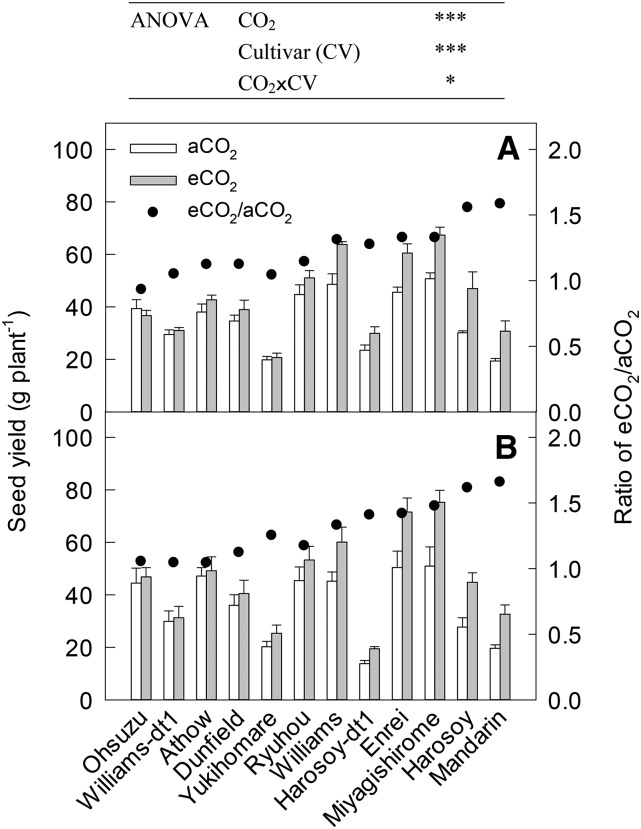
CO2 elevation significantly stimulated seed yield per plant by an average of 25% across the 12 cultivars in both years (P < 0.001; Fig. 1). The magnitude of the seed yield enhancement due to eCO2 differed significantly among the cultivars, with a significant CO2 × cultivar interaction (P < 0.05). The rank of these seed yield enhancements in 2013 was consistent with that in 2014 (Spearman's rank correlation coefficient, rs = 0.874, P < 0.001). When averaged between the two seasons, the relative yield increase of cv Mandarin (62%) and cv Harosoy (58%) was greatest, followed by cv Miyagishirome (40%) and cv Enrei (38%), but cv Ohsuzu had no increase in seed yield.
Figure 1.
Seed yield per plant of several soybean cultivars grown under two [CO2] levels in 2013 (A) and 2014 (B). aCO2, Ambient [CO2]; eCO2, elevated [CO2]. Data are means ± se (n = 4 or 5 plants). From left to right, cultivars are ranked in order of increasing eCO2 responsiveness of seed yield (the ratio of seed yield at eCO2 to that at aCO2), averaged over the 2 years; the rightmost cultivar was the most responsive. ***, P < 0.001; *, P < 0.05.
In terms of growth determinacy, when we compared the results for near-isogenic lines of cv Williams and cv Harosoy (Fig. 1), in both cases, the indeterminate line had superior seed yield, with increases of 32% for cv Williams versus 5% for cv Williams-dt1, and of 58% for cv Harosoy versus 32% for Harosoy-dt1, based on the average for the 2 years. This difference resulted from the different responsiveness of aboveground biomass and pod number per plant (Supplemental Table S1). However, when we pooled all 12 cultivars, the two-way ANOVA showed no significant interaction between CO2 and growth determinacy (P = 0.781), indicating that growth determinacy is not the dominant factor responsible for genotypic variations across a wide range of genotypes.
In terms of phenology, the number of days from date of emergence (VE) to beginning of flowering (R1) was not significantly affected by eCO2, but the number of days from R1 to the date of the beginning of maturity (R7) increased significantly for most cultivars under eCO2 (P < 0.01; Supplemental Table S3). No significant CO2 × cultivar interaction was detected in either phenological phase. Further, there were no significant correlations between the absolute values of these numbers at aCO2 and relative increase of seed yield by CO2, and between the differences in relative changes by eCO2 of these numbers and relative increase of seed yield (Table I), denying the phenology hypothesis (Hasegawa et al., 2013; Bunce, 2015).
Table I. Correlations (Pearson’s r) and their statistical significance for the relationships between the relative increase in seed yield at elevated CO2 (i.e. value at eCO2/value at aCO2) and the absolute and relative values of other parameters measured at aCO2 or eCO2 for the 12 soybean cultivars.
ns, Not significant; ***, P < 0.001; **, P < 0.01; *, P < 0.05; +, P < 0.1; †, Data for photosynthetic rate and NSC content, n = 9.
| Year/Parameters | 2013 |
2014 |
||||||
|---|---|---|---|---|---|---|---|---|
| Absolute Value at aCO2 | Relative Change by eCO2 | Absolute Value at aCO2 | Relative Change by eCO2 | |||||
| Aboveground biomass | −0.011 | ns | 0.903 | *** | −0.291 | ns | 0.980 | *** |
| Branch number per plant | 0.380 | ns | −0.173 | ns | −0.044 | ns | 0.388 | ns |
| Node number of main stem | 0.280 | ns | 0.852 | *** | 0.220 | ns | 0.774 | ** |
| Seed yield per plant | −0.107 | ns | — | — | −0.318 | ns | — | — |
| Harvest index | −0.197 | ns | 0.498 | + | 0.264 | ns | 0.637 | * |
| Pod number per plant | 0.032 | ns | 0.809 | ** | −0.419 | ns | 0.934 | *** |
| Pod number per aboveground biomass | 0.256 | ns | −0.014 | ns | 0.277 | ns | −0.346 | ns |
| Seed number per pod | 0.186 | ns | 0.199 | ns | −0.022 | ns | 0.419 | ns |
| Single seed weight | −0.308 | ns | 0.143 | ns | −0.010 | ns | 0.106 | ns |
| Days from VE to R1 | −0.148 | ns | 0.021 | ns | −0.052 | ns | −0.307 | ns |
| Days from R1 to R7 | −0.092 | ns | 0.161 | ns | −0.236 | ns | −0.252 | ns |
| Photosynthetic rate (flowering)† | — | — | — | — | 0.266 | ns | 0.007 | ns |
| Photosynthetic rate (seed filling)† | — | — | — | — | −0.113 | ns | 0.072 | ns |
| NSC content (flowering)† | — | — | — | — | 0.239 | ns | −0.500 | ns |
| NSC content (seed filling)† | — | — | — | — | 0.270 | ns | −0.495 | ns |
Leaf photosynthetic rate was increased by eCO2 with an average of 14% for nine soybean cultivars, except cv Ohsuzu, cv Athow, and cv Yukihomare, at the flowering stage in 2014 (Supplemental Fig. S1). The degree of photosynthetic stimulation due to eCO2 became smaller at the seed-filling stage (Supplemental Fig. S1), indicating that photosynthetic acclimation occurred in most cultivars. When averaged across the cultivars, eCO2 increased leaf nonstructural carbohydrate (NSC; sum of starch, Suc, Glc, and Fru) content by 46% and 19% at the flowering and seed-filling stages, respectively (Supplemental Fig. S1). However, we did not observed significant correlations between seed yield response to eCO2 and the absolute values at aCO2 and relative changes to eCO2 of photosynthetic rate and NSC content measured at the both stages in 2014 (Table I).
The aboveground biomass (sum of the weights of stems, pod shells, and seeds) at maturity was significantly increased by eCO2 (P < 0.001), and there was a significant CO2 × cultivar interaction (P < 0.01; Supplemental Table S1). The relative response of biomass was strongly and significantly correlated with the yield response to eCO2 in both years (P < 0.001; Table I; Supplemental Fig. S2). It should be noted that the absolute biomass and seed yield at aCO2 were not significantly correlated with the seed yield enhancement due to eCO2 in either year (Table I), indicating that the cultivars with high biomass and yield at aCO2 may not always have high biomass and yield under eCO2. On the other hand, the harvest index, which we expressed as the ratio of seed yield to aboveground biomass, of all cultivars decreased slightly but significantly under eCO2 (P < 0.01), without a significant CO2 × cultivar interaction (Supplemental Table S3). The correlation between harvest index at aCO2 and yield enhancement due to eCO2 was not significant, but the relative change of harvest index was marginally significantly (in year 2013) and significantly positively (in year 2014) correlated with the relative yield response; the genotypes that maintained a high harvest index showed a higher responsiveness of seed yield to eCO2 (Table I; Supplemental Fig. S2). However, the correlation coefficient was smaller than that for the biomass response. Biomass response to eCO2 therefore appears to be the dominant factor that determines yield responsiveness.
Of the yield components, eCO2 had significant positive effects on node number on the main stem (a change of –2% to +15%) and pod number per plant (–14% to +54%), but not on branch number per plant, seed number per pod, or single seed weight (Supplemental Table S1). Only pod number per plant showed a significant CO2 × cultivar interaction, and it was only marginally significant (P < 0.1). Consequently, there was a strong positive correlation between increased seed yield and increased pod number per plant in both years (Table I; Supplemental Fig. S2; r = 0.809 in 2013, P < 0.01, and r = 0.934 in 2014, P < 0.001). On the other hand, the efficiency of pod production, which we expressed as the ratio of pod number to aboveground biomass, decreased significantly under eCO2, and there was no significant CO2 × cultivar interaction (Supplemental Table S1) and no significant correlation with the genotypic variation in yield enhancement (Table I). These results indicate that genotypic variation of the eCO2 responsiveness within our 12 cultivars could be explained primarily by the variation of biomass plasticity, which led to higher pod production. Although no parameters at aCO2 were significantly correlated with eCO2 yield responsiveness, the plasticity in biomass and pod production in response to eCO2 can play a pivotal role in seed yield responsiveness. We tested this hypothesis under the light- and nutrient-rich environments provided by a LD in the field experiment in the next section.
Methodology for Characterizing eCO2 Responsiveness by Using LD to Simulate a Resource-Rich Environment
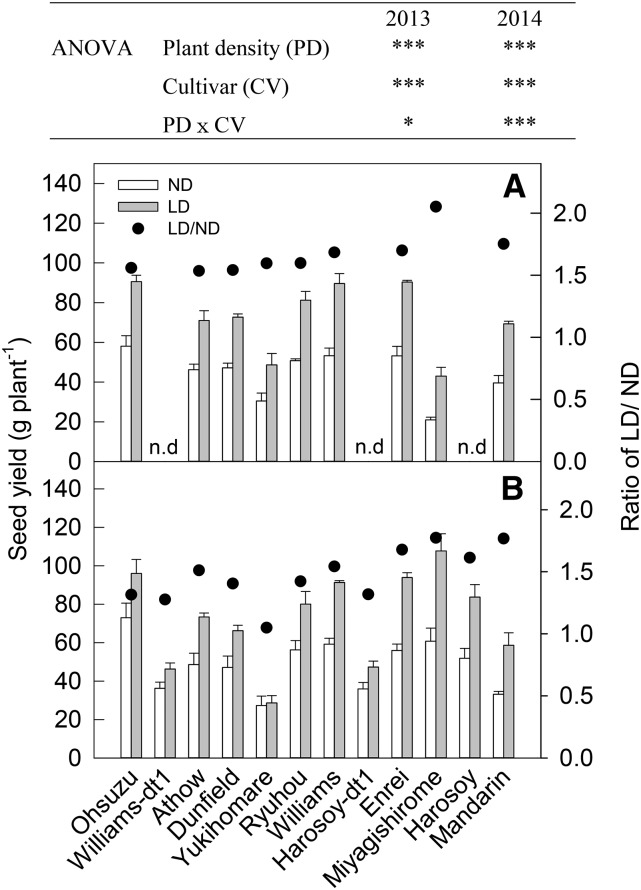
LD significantly increased seed yield per plant by averages of 66% in 2013 and 47% in 2014 (P < 0.001), with significant planting density × cultivar interactions (P < 0.05 in 2013, P < 0.001 in 2014; Fig. 2). cv Miyagishirome (105% and 77%), cv Mandarin (75% and 76%), and cv Enrei (69% and 67%) showed the greatest enhancement of seed yield by LD in 2013 and 2014, respectively. On the other hand, cv Yukihomare showed the smallest relative changes (5%) in 2014. Both aboveground biomass and pod number per plant increased significantly at reduced planting density (P < 0.001), and there were significant density × cultivar interactions in both years (P < 0.1 for biomass, P < 0.05 for pod number in 2013; P < 0.001 for biomass, P < 0.01 for pod number in 2014; Supplemental Table S2). LD also significantly increased the branch number per plant and node number on the main stem in both years (P < 0.001 and P < 0.01, respectively; Supplemental Table S2). On the other hand, planting density had no observed effect on harvest index, the efficiency of pod production, seed number per pod, single seed weight, or the number of days from VE to R1 and from R1 to R7 in either year (Supplemental Table S2). Aboveground biomass and pod number per plant were therefore the main factors responsible for the genotypic variation in the yield response to LD (Table II; Supplemental Fig. S3). These results agree with the results for the eCO2 response (Table I).
Figure 2.
Seed yield per plant of the 12 soybean cultivars grown at two levels of planting density in 2013 (A) and 2014 (B). ND, Normal plant density (= 9.52 plants per m2); LD = 4.76 plants per m2. Data are means ± se (n = 3). ***, P < 0.001; *, P < 0.05.
Table II. Correlations and their statistical significance for the relationships between the relative increase in seed yield (the value at LD/the value at ND) and the absolute values and relative changes of other parameters for the 12 soybean cultivars.
Data for 2013 only, n = 9. ns, Not significant; ***, P < 0.001; **, P < 0.01; *, P < 0.05; +, P < 0.1.
| Year/Parameters | 2013 |
2014 |
||||||
|---|---|---|---|---|---|---|---|---|
| Absolute Value at ND | Relative Change by LD | Absolute Value at ND | Relative Change by LD | |||||
| Aboveground biomass | −0.163 | ns | 0.858 | ** | 0.466 | ns | 0.957 | *** |
| Branch number per plant | 0.253 | ns | 0.523 | ns | 0.433 | ns | 0.498 | + |
| Node number of main stem | 0.176 | ns | 0.440 | ns | 0.679 | * | −0.381 | ns |
| Seed yield per plant | −0.672 | * | — | — | 0.346 | ns | — | — |
| Harvest index | −0.781 | * | −0.183 | ns | −0.483 | ns | 0.357 | ns |
| Pod number per plant | −0.362 | ns | 0.913 | *** | 0.427 | ns | 0.920 | *** |
| Pod number per aboveground biomass | −0.391 | ns | −0.058 | ns | −0.266 | ns | 0.238 | ns |
| Seed number per pod | −0.748 | * | 0.122 | ns | 0.067 | ns | −0.318 | ns |
| Single seed weight | 0.424 | ns | 0.125 | ns | −0.066 | ns | 0.201 | ns |
| Days from VE to R1 | 0.558 | ns | 0.022 | ns | 0.254 | ns | 0.358 | ns |
| Days from R1 to R7 | 0.395 | ns | 0.289 | ns | 0.496 | ns | 0.243 | ns |
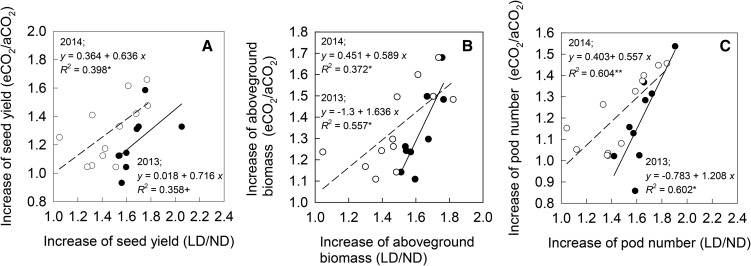
In terms of seed yield per plant, aboveground biomass, and pod number per plant, there were significant positive linear relationships between the relative CO2 responsiveness (the ratio of the value at eCO2 to that at aCO2) and the relative responses to LD (the ratio of the value at LD to that at ND) in both years (Fig. 3), except that the relationship for seed yield was marginally significant in 2013 (P < 0.1). In both years, the rank of the cultivars in terms of their seed yield enhancements under eCO2 was consistent with that due to LD (rs = 0.817, P < 0.01, in 2013; rs = 0.618, P < 0.05, in 2014).
Figure 3.
Linear regressions for the relationships between the ratio of the values at eCO2 to that at aCO2, and the ratio of the value at LD to that at ND for seed yield (A), aboveground biomass (B), and pod number (C) in 2013 (black circles) and 2014 (white circles). **, P < 0.01; *, P < 0.05; +, P < 0.1.
DISCUSSION
What Traits Should Be Targeted to Maximize the CO2 Responsiveness of Seed Yield?
The current study demonstrated that the seed yield enhancement under eCO2 differed substantially among the 12 cultivars, ranging from 0% to 62% (Fig. 1). This suggests that there is considerable potential for genetic improvement. We found that the response of pod number per plant to eCO2 was a useful indicator of the genotypic variation in the yield enhancement under eCO2 (Table I; Supplemental Fig. S2). Since no significant CO2 × cultivar interaction in the efficiency in pod production among the cultivars was observed in this study (Supplemental Table S1), the cultivars with the strongest responsiveness of biomass to eCO2 produced more pods and a greater seed yield, in agreement with Bishop et al. (2015), who compared 18 cultivars over two seasons in a free-air carbon dioxide enrichment study. It is interesting that, although we observed a difference in the photosynthetic stimulation due to eCO2 among the nine cultivars, the degree of photosynthetic stimulation was not related to that of seed yield (Table I), which is in agreement with the previous findings (Ziska et al., 2001; Shimono et al., 2009; Bishop et al., 2015). Thus, there was no evidence for the contribution of leaf photosynthesis and NSC accumulation (Supplemental Fig. S1) in the differences in yield response across nine cultivars. We concluded that a genotype with higher plasticity in sink formation due to eCO2 would be a promising trait for higher yield response to eCO2.
Highlighting the Importance of Plasticity of Biomass and Pod Formation under eCO2 Using LD
LD significantly increased seed yield per plant (by averages of 66% in 2013 and 47% in 2014; Fig. 2). The yield enhancement at LD was largely attributable to the increased pod number per plant (Supplemental Table S2), and the genotypic variation was largely dependent on the pod number per plant (Supplemental Fig. S3), which resulted in a significant cultivar × planting density interaction in both years (Supplemental Table S2). A unique finding was that there were strong positive correlations between the cultivar’s responsiveness for seed yield and the pod number per plant under eCO2 and at LD (Fig. 3), which supported our hypothesis. Thus, our manipulation of planting density demonstrates that the plasticity of pod formation can be used as a target trait in selection to increase seed yield under future eCO2.
The genotypic variation in yield enhancement at LD was more closely related to the aboveground biomass response than to the harvest index. Aboveground biomass was strongly enhanced by a LD (by 5%–82%; Supplemental Table S2). The biomass increase in response to eCO2 has been attributed to increased leaf photosynthesis and radiation use efficiency (Long et al., 2004), whereas the increase due to reduced planting density has been attributed mainly to increasing leaf area and radiation capture (Carpenter and Board, 1997; Frederick et al., 2001, Suhre et al., 2014). In general, soybean plants grown at LD partition a greater proportion of their total vegetative dry matter in branches than plants grown in denser populations. Increased branch number under reduced plant density could lead to higher whole-plant photosynthesis due to the greater leaf area and accelerated light interception, resulting in an increased pod number per plant relative to that in denser populations (Egli, 1988). In our study, LD significantly increased the branch number per plant in both years (Supplemental Table S2), presumably resulting in greater leaf areas per plant at LD (Supplemental Table S2). The magnitude of the increase in branch number caused by reduced planting density was weakly but significantly (P = 0.098) correlated with increased seed yield among the cultivars in 2014, whereas there was no significant correlation between them in 2013 (Supplemental Fig. S2). This result indicates that the increases of biomass and pod number, not the branch number per se, were directly associated with the increase of seed yield per plant at LD, which is similar to the results under eCO2. In summary, the genotypic variation in the response to eCO2 could be simulated by LD, and higher plasticity of biomass and pod production was the key trait responsible for the eCO2 responsiveness.
A Strategy for Screening Cultivars with a Strong Response to eCO2
To identify cultivars with superior responsiveness to eCO2, it is necessary to test a large number of accessions and their progeny over several generations to confirm that the desirable genes or loci have been transferred into the progeny. However, since CO2 enhancement facilities such as controlled enclosed chambers and free-air carbon dioxide enrichment systems are extremely expensive and not easily accessible, it is difficult to screen large numbers of candidate accessions. Thus, an alternative and inexpensive screening method is essential to support evaluations of the yield response to eCO2. Shimono (2011) initially proposed the use of LD as a prescreening method to detect high responsiveness to eCO2 using rice. The present study confirms that the methodology of Shimono (2011) can be used in prescreening to select eCO2-responsive soybean cultivars. Some researchers have expressed concerns about the heritability of the CO2 response in soybean (Bishop et al., 2015) and in other crops. Our experiment raised the possibility that CO2 responsiveness is heritable, since the most responsive cultivar (cv Mandarin) is a parent of the second most responsive cultivar (cv Harosoy). The method using a LD may therefore be a promising tool for genetic analysis in other types of study, such as quantitative trait locus mapping, that needs a large space to test many lines (often >100) to elucidate the heritability of CO2 responsiveness.
Despite our promising results of biomass and yield enhancement by both treatments, the responses of soybean to eCO2 and to LD differed somewhat. For instance, the number of days from R1 to R7 increased significantly under eCO2 (Supplemental Table S1), whereas we observed no significant effect of LD on phenology (Supplemental Table S2). Another notable difference relates to the response of harvest index. eCO2 caused a significant reduction of harvest index and of the efficiency of pod production (Supplemental Table S1), but planting density did not influence either (Supplemental Table S2), indicating the underlying physiological mechanism for yield enhancement differed between eCO2 and LD. However, it is noteworthy that a genotype with higher responsiveness in biomass and yield to eCO2 generally has higher responsiveness to LD, which indicates that there would be a sophisticated mechanism for plant plasticity response to resource-rich environments.
Abiotic stress can affect the potential responses of seed yield to both eCO2 and LD. Our study showed that the degree of the increase in seed yield per plant at reduced planting density was greater in 2013 than in 2014, whereas there was no difference in the response of seed yield to eCO2 (under controlled environmental conditions) between the two years. Drought reduces soybean yield primarily by reducing branch growth and seed yield per branch (Frederick et al., 2001). In both years, the total precipitation during the growing season was more than 750 mm, so it seems that there was no drought stress that would have affected our results. Excessive soil water also limits the response to planting density (Linkemer et al., 1998), but we observed no signs of flooding or flooding damage in the 2014 field study. The difference in the response to LD presumably resulted from differences in precipitation between the two years (Supplemental Table S2). However, the magnitude of the yield response in both years agreed with that (approximately 30%–70%) under similar density conditions of a previous study (De Bruin and Pedersen, 2009), and was likely related to the good environmental conditions. In soybean, lodging resistance is an important trait that affects the response to planting density (Boquet, 1990; Cober et al., 2005). The lodging score for cv Miyagishirome was more severe than that of the other cultivars in 2013 (data not shown). We attributed this to the presence of many immature seeds and thereby a lower seed number per pod and a lower harvest index and presumably failed to estimate the responsiveness to low planting in this cultivar (Supplemental Table S2). In terms of seed yield, the strength of the relationships between the relative CO2 responsiveness and the relative responses to LD increased in 2013 (P < 0.01, n = 8) when we excluded the data from cv Miyagishirome. Thus, prescreening at a LD should be conducted under optimal environmental conditions to remove the effects of other factors that might influence the potential performance of the cultivars.
CONCLUSION
By performing our study in 2 years with a diverse range of soybean cultivars, we found that plasticity of biomass and pod production is a suitable phenotype for predicting the responsiveness to future eCO2, and that this responsiveness could be determined from the response to LD. In other words, manipulating planting density provides a useful alternative method for screening to identify eCO2-responsive cultivars.
MATERIALS AND METHODS
Experimental Site and Cultivars
The experiments were conducted in both naturally sunlit temperature gradient chambers (TGCs) and experimental fields of the National Agriculture and Food Research Organization (NARO) Tohoku Agricultural Research Center (TARC) in Morioka, Japan (39° 44′ N, 141° 7′ E), from June to October in 2013 and 2014. We chose 12 soybean (Glycine max) cultivars from maturity groups I to IV and VII (Supplemental Table S3). Five were from Japan and seven from the United States. Seven were determinate (cv Yukihomare, cv Ohsuzu, cv Ryuhou, cv Enrei, cv Miyagishirome, cv Harosoy-dt1, and cv Williams-dt1’) and five were indeterminate (cv Mandarin, cv Dunfield, cv Athow, cv Harosoy, and cv Williams,). Indeterminate and determinate types are controlled by alleles at the Dt1 locus (Bernard, 1971). cv Williams-dt1 and cv Harosoy-dt1 are backcross-derived, determinate isolines of two indeterminate (Dt1) cultivars, cv Williams and cv Harosoy, respectively. cv Mandarin, cv Williams, and cv Dunfield were previously characterized as having respectively high, moderate, and low responsiveness to eCO2 (Ziska et al., 2001).
TGC Experiments
We grew the 12 soybean cultivars to maturity (from June to October) in TGCs at NARO/TARC. Each TGC was a greenhouse (6 m wide, 30 m long, and 3 m high) with an air inlet at one end and exhaust fans at the other. The air in the TGC flowed continuously from the inlet to the exhaust fans. A temperature gradient was continuously maintained along the longitudinal axis by cooling the air with an air conditioner at the inlet end, by warming the air with solar radiation or a supplemental heat input (an air heater and ducts) at the outlet end, or by both methods. The two TGCs were designed to have the same microclimate except for [CO2] (Okada et al., 2000). One TGC was kept at aCO2, and the other was kept at 200 µmol mol–1 above ambient (eCO2) by injecting pure CO2 at the air inlet between 03:30 and 07:30. The CO2 gas emission rate was proportional to the exhaust rate. We used the same spots in each TGC, where the air temperature was 2.8°C and 2.3°C higher than the outside temperature in 2013 and 2014, respectively, as experimental plots (aCO2 and eCO2; Supplemental Table S4). We used Pt100 resistance thermometers with an aspirated double-tube radiation shield to monitor the air temperature in each spot. An infrared gas analyzer (LI-820; Li-Cor) was used to monitor [CO2] inside each TGC. Temperature and [CO2] were measured every 5 s, and their averages were recorded every 30 min by a data logger (CR1000; Campbell Scientific Inc.). Daytime [CO2] values in the aCO2 and eCO2 treatments, averaged over the growing season, were 401 ± 17 and 593 ± 36 µmol mol–1 (mean ± sd), respectively, in 2013, and 401 ± 18 and 595 ± 37 µmol mol–1, respectively, in 2014. The CO2 treatments were switched between TGCs in 2014 to account for the possibility of chamber-specific effects. We found no significant differences in the average air temperature between the TGCs during a given year.
Before sowing, the seeds were treated with a combined insecticide and fungicide (CruiserMaxx; Syngenta Co.) and inoculated with Bradyrhizobium japonicum (Mamezou; Tokachi-Nokyouren). Three seeds were sown in a 10-L plastic pot (n = 5 and 4 pots per cultivar per TGC in 2013 and 2014, respectively) containing 8 L of commercial soil on June 7, 2013 and June 4, 2014. The commercial soil was Muhiryoubaido (Wataka Co.) in 2013 and a 1:1 (v/v) mixture of Muhiryoubaido and Nippiryousaibaido Pp (Nipponhiryou Co.) in 2014. Each pot was fertilized with 0.40 g of nitrogen in 2013 and 0.45 g in 2014, 5.25 g of phosphorus (P2O5 equivalent) in 2013 and 5.00 g in 2014, and 1.5 g of potassium (K2O equivalent) in 2013 and 1.4 g in 2014. After seedling emergence, plants were thinned to one per pot. Pots were rotated every 7 d to minimize the effects of any environmental differences within the TGC. Plants were irrigated with tap water once or twice a day to maintain soil water near field capacity.
We surveyed the phenology of four or five plants per cultivar at each spot once a day, and recorded the VE (defined as the date when 50% of the plants had appeared aboveground), R1 (defined as the date when 50% of the plants had at least one flower), and R7 (defined as the date when 50% of the plants had one mature pod), according to the staging system of Fehr and Caviness (1977). At maturity, the aboveground parts of four or five plants per spot were harvested from September 12 to October 29, 2013, and from September 17 to October 20, 2014. We then separated the components (leaves, petioles, stems, pod shells, and seeds). Simultaneously, we recorded the numbers of branches, fertile pods, and seeds per plant. Since most of the leaves and petioles had fallen before harvest, the aboveground biomass equaled the sum of the dry weights of stems, pod shells, and seeds. The dry weights of stems, pod shells, and seeds were measured after oven drying at 80°C for 5 d. Seed yield per plant was adjusted to a 15% moisture content, and the mean single-seed weight was determined by dividing the seed yield per plant (at a 15% moisture content) by the number of seeds. The harvest index was determined by dividing the seed yield by aboveground biomass.
Photosynthesis of the most recently expanded terminal leaflet of four different plants from each spot was measured at both flowering and seed filling stages in 2014. Light-saturated photosynthetic rate was measured between 09:00 and 13:00 using a portable photosynthesis measurement system (LI-6400; Li-Cor Inc.). Air temperature in the chamber was adjusted to 28°C. The relative humidity of air entering the chamber was adjusted to approximately 60%. The photosynthetic photon flux density inside the chamber was set at 1,500 μmol m−2 s−1 by means of an internal light source. The [CO2] in the air entering the leaf chamber was set to that of the TGC (400 and 600 μmol mol−1 in aCO2 and eCO2, respectively). After the measurements, the portions of four leaves from four plants were excised, frozen in N2, and then dried at 80°C for 3 d in the oven for determination of NSC content (starch and soluble sugar content). NSCs were extracted and measured as described previously (Okamura et al., 2013). Soluble sugar content was defined as the sum of Suc, Glc, and Fru contents.
We used a randomized complete block experimental design in a split plot arrangement; the main plot was [CO2], the subplot was the cultivar, and there were two replicates (years). The two TGCs within each CO2 treatment were maintained as a block. In each year, each TGC was randomly assigned to a CO2 treatment, and the positions of the cultivars were randomly assigned within a TGC. The mean value of each parameter related to phenology and yield of the four or five plants per CO2 treatment in a given year was averaged and used as a single replicate.
Field Experiments
In 2013, nine cultivars (excluding cv Williams-dt1, cv Harosoy, and cv Harosoy-dt1), and in 2014, all 12 cultivars were grown from sowing (June) to maturity (September to October) in the experimental fields at NARO/TARC. The soil in each field was an Andosol. We applied 80 g m–2 of fused phosphate fertilizer and 80 g m–2 of magnesium lime 28 d before sowing in 2013 and 36 d before in 2014. Additionally, the fields received 3 g m–2 of nitrogen, 12.5 g m–2 of phosphorus (P2O5 equivalent), and 5 g m–2 of potassium (K2O equivalent) in the form of a compound chemical fertilizer 1 d before sowing in each year.
Seeds treated with CruiserMaxx were sown by hand (at a density of three per hill) on June 5, 2013 and June 3, 2014 at two planting densities: the ND was 9.52 hills m–2, at 0.15 m between hills × 0.7 m between rows, and the LD was 4.76 hills m–2, at 0.3 m between hills × 0.7 m between rows (Supplemental Fig. S4). This compares with recommended local planting densities ranging from 7 to 15 plants m–2 in our study region. Plants were thinned to one per hill after establishment. A preemergence herbicide (Ecotop, Maruwa Biochemical Co.) was applied just after sowing for weed control. Application of insecticides and manual weeding were done as necessary. In both years, we used a randomized complete block experimental design in a split plot arrangement; the main plot was plant density, and the subplot was cultivar, with three replicates. In 2013, the subplot consisted of 4 rows × 1.5 m long in ND and 4 rows × 3 m long in LD. In 2014, the subplot consisted of 4 rows × 1.05 m long in ND and 4 rows × 2.1 m long in LD.
We surveyed the phenology of five plants in the center two rows in each plot at 2- or 3-d intervals and recorded the dates of stages VE, R1, and R7. At maturity, the aboveground parts of seven plants (2013) or six plants (2014) from the center two rows per plot were harvested from September 9 to November 11, 2013 and from September 25 to November 10, 2014. After completely air drying the plants, we counted the branch number and removed the few remaining leaves and petioles from the aboveground parts, and then weighed the remaining parts. Aboveground biomass was the sum of the weights of the stems, pod shells, and seeds. Fertile pods and the number of mature seeds were counted, and the mature seeds were weighed. Seed yield per plant was adjusted to a 15% moisture content. The mean single-seed weight and harvest index were determined as described above for the TGC experiment.
Daily means of temperature, solar radiation, and precipitation during the experiments were recorded at a weather station near the field in the research center (Supplemental Table S4).
Climate Conditions
The 5-month (June to October) mean temperature outside the TGCs (in the field) was 1.1°C higher than the 30-year mean in 2013, and 0.5°C higher in 2014 (Supplemental Table S4). The largest difference was observed in June in both years (1.8°C and 2.4°C above the 30-year mean in 2013 and 2014, respectively). In the TGC experiment, the 5-month mean temperatures in 2013 and 2014 were 2.8°C and 2.3°C higher, respectively, than the outside temperature. The 5-month mean daily solar radiation in 2013 was only 0.1 MJ m–2 d–1 greater than the 30-year mean, although June and July had levels higher and lower than the 30-year mean. The mean daily solar radiation levels in all months of 2014 were 1.5 to 3.5 MJ m–2 d–1 higher than the 30-year mean, except in August, when it was 2.8 MJ m–2 d–1 below the 30-year mean. The 5-month mean precipitation in 2013 was close to the 30-year mean (158.8 versus 146.9 mm) despite reductions of 34% in June and 39% in September compared with the 30-year means for those months. In contrast, precipitation during all of the 2014 growing season, except June, was much higher than the corresponding 30-year mean.
Statistical Analysis
To test for significant differences between [CO2] treatments and among the cultivars, and for significant interactions, we used two-way ANOVA to analyze the data for phenology, yield, and yield components from the two replications (years). Means in each season were analyzed. To test for significant differences between the two planting density levels and among the cultivars, and for significant interactions, we used two-way ANOVA to analyze the data for phenology, seed yield, and yield components from three replicates in each year. We calculated the correlations among the agronomic traits and their response to eCO2 and LD using Pearson’s product-moment correlation (r), and used Spearman’s rank correlation (rs) to compare the cultivar ranks between the eCO2 and planting density experiments. For the relationships between certain pairs of parameters, we performed linear regression. All statistical analyses were performed with version 22.0 of the SPSS statistics software.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Photosynthetic rate and leaf NSC content of several soybean cultivars grown under two [CO2] levels in 2014.
Supplemental Figure S2. Linear regressions between the relative increase in seed yield with elevated [CO2] and the relative changes in some parameters in 2013 and 2014.
Supplemental Figure S3. Linear regressions between the relative increase in seed yield caused by low planting density for some parameters in 2013 and 2014.
Supplemental Figure S4. Pictures of the plant canopy of two plant densities.
Supplemental Table S1. Yield and phenological parameters of 12 soybean cultivars grown under two [CO2] levels in 2013 and 2014.
Supplemental Table S2. Yield and phenological parameters for the 12 soybean cultivars grown under two levels of planting density in 2013 and 2014.
Supplemental Table S3. Lists of 12 soybean cultivars used in this study.
Supplemental Table S4. Meteorological data in 2013 and 2014.
Supplementary Material
Acknowledgments
We thank Masaki Okamura and Yoichi Hashida (University of Tokyo); Akihiko Umihata and Kafumi Segawa (Agro-Environmental Research Division of the NARO TARC); and Eisaku Kumagai, Fumihiko Saitou, and Hisashi Tamura (Field Management Division of the NARO TARC) for technical assistance; and the NIAS Genebank and U.S. Department of Agriculture soybean germplasm collection for providing the seeds.
Glossary
- LD
low planting density
- VE
date of emergence
- NSC
nonstructural carbohydrate
- ND
normal plant density
- TGC
temperature gradient chamber
- NARO
National Agriculture and Food Research Organization
- TARC
Tohoku Agricultural Research Center
Footnotes
This work was supported by the Japan Society for the Promotion of Science KAKENHI (grant no. 25292011 to H.S.).
References
- Ainsworth EA, Beier C, Calfapietra C, Ceulemans R, Durand-Tardif M, Farquhar GD, Godbold DL, Hendrey GR, Hickler T, Kaduk J, et al. (2008) Next generation of elevated [CO2] experiments with crops: a critical investment for feeding the future world. Plant Cell Environ 31: 1317–1324 [DOI] [PubMed] [Google Scholar]
- Ainsworth EA, Rogers A, Nelson R, Long SP (2004) Testing the “source-sink” hypothesis of down-regulation of photosynthesis in elevated [CO2] in the field with single gene substitutions in Glycine max. Agric For Meteorol 122: 85–94 [Google Scholar]
- Aspinwall MJ, Loik ME, Resco de Dios V Tjoelker MG, Payton PR, Tissue DT (2015) Utilizing intraspecific variation in phenotypic plasticity to bolster agricultural and forest productivity under climate change. Plant Cell Environ 38: 1752–1764 [DOI] [PubMed] [Google Scholar]
- Baker JT. (2004) Yield responses of southern US rice cultivars to CO2 and temperature. Agric For Meteorol 122: 129–137 [Google Scholar]
- Bernard RL. (1971) Two genes affecting stem termination in soybeans. Crop Sci 12: 235–239 [Google Scholar]
- Bishop KA, Betzelberger AM, Long SP, Ainsworth EA (2015) Is there potential to adapt soybean (Glycine max Merr.) to future [CO2]? An analysis of the yield response of 18 genotypes in free-air CO2 enrichment. Plant Cell Environ 38: 1765–1774 [DOI] [PubMed] [Google Scholar]
- Boquet DJ. (1990) Plant population density and row spacing effects on soybean at post-optimal planting dates. Agron J 82: 59–64 [Google Scholar]
- Bunce JA. (2008) Contrasting responses of seed yield to elevated carbon dioxide under field conditions within Phaseolus vulgaris. Agric Ecosyst Environ 128: 219–224 [Google Scholar]
- Bunce JA. (2015) Elevated carbon dioxide effects on reproductive phenology and seed yield among soybean cultivars. Crop Sci 55: 339–343 [Google Scholar]
- Carpenter AC, Board JE (1997) Branch yield components controlling soybean yield stability across plant populations. Crop Sci 37: 885–891 [Google Scholar]
- Ciais P, Sabine C, Bala G, Bopp L, Brovkin V, Canadell J, Chhabra A, DeFries R, Galloway J, Heimann M, et al. (2013) Carbon and Other Biogeochemical Cycles. In TF Stocker, D Qin, GK Plattner, M Tignor, SK Allen, J Boschung, A Nauels, Y Xia, V Bex, PM Midgley, eds, Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, United Kingdom and New York. [Google Scholar]
- Cober ER, Morrison MJ, Ma B, Butler G (2005) Genetic improvement rates of short-season soybean increase with plant population. Crop Sci 45: 1029–1034 [Google Scholar]
- De Bruin JL, Pedersen P (2009) New and old soybean cultivar responses to plant density and intercepted light. Crop Sci 49: 2225–2232 [Google Scholar]
- Egli DB. (1988) Plant density and soybean yield. Crop Sci 28: 977–981 [Google Scholar]
- Fehr WR, Caviness CE (1977) Stages of soybean development. Cooperative Extension Service; Agriculture and Home Economics Experiment Station, Iowa State University of Science and Technology, Ames, IA, 80: 1–12
- Frederick JR, Camp CR, Bauer PJ (2001) Drought-stress effects on branch and mainstem seed yield and yield components of determinate soybean. Crop Sci 41: 759–763 [Google Scholar]
- Hao XY, Han X, Lam XY, Wheeler T, Ju H, Wang HR, Li YC, Lin ED (2012) Effects of fully open-air [CO2] elevation on leaf ultrastructure, photosynthesis, and yield of two soybean cultivars. Photosynthetica 50: 362–370 [Google Scholar]
- Hasegawa T, Sakai H, Tokida T, Nakamura H, Zhu C, Usui Y, Yoshimoto M, Fukuoka M, Wakatsuki H, Katayanagi N, et al. (2013) Rice cultivar responses to elevated CO2 at two free-air CO2 enrichment (FACE) sites in Japan. Funct Plant Biol 40: 148–159 [DOI] [PubMed] [Google Scholar]
- Kimball BA, Zhu J, Cheng L, Kobayashi K, Bindi M (2002) [Responses of agricultural crops of free-air CO2 enrichment]. Ying Yong Sheng Tai Xue Bao 13: 1323–1338 [PubMed] [Google Scholar]
- Linkemer G, Board JE, Musgrave ME (1998) Waterlogging effects on growth and yield components in late-planted soybean. Crop Sci 38: 1576–1584 [DOI] [PubMed] [Google Scholar]
- Long SP, Ainsworth EA, Rogers A, Ort DR (2004) Rising atmospheric carbon dioxide: plants FACE the future. Annu Rev Plant Biol 55: 591–628 [DOI] [PubMed] [Google Scholar]
- Manderscheid R, Weigel HJ (1997) Photosynthetic and growth responses of old and modern spring wheat cultivars to atmospheric CO2 enrichment. Agric Ecosyst Environ 64: 65–73 [Google Scholar]
- Moore BD, Cheng SH, Sims D, Seemann JR (1999) The biochemical and molecular basis for photosynthetic acclimation to elevated atmospheric CO2. Plant Cell Environ 22: 567–582 [Google Scholar]
- Moya TB, Ziska LH, Namuco OS, Olszyk D (1998) Growth dynamics and genotypic variation in tropical, field-grown paddy rice (Oryza sativa L.) in response to increasing carbon dioxide and temperature. Glob Change Biol 4: 645–656 [Google Scholar]
- Okada M, Hamasaki T, Sameshima R (2000) Pre-air-conditioned temperature gradient chambers for research on temperature stress in plants. Biotronics 29: 43–55 [Google Scholar]
- Okamura M, Hirose T, Hashida Y, Yamagishi T, Ohsugi R, Aoki N (2013) Starch reduction in rice stems due to a lack of OsAGPL1 or OsAPL3 decreases grain yield under low irradiance during ripening and modifies plant architecture. Funct Plant Biol 40: 1137–1146 [DOI] [PubMed] [Google Scholar]
- Shimono H. (2011) Rice genotypes that respond strongly to elevated CO2 also respond strongly to low planting density. Agric Ecosyst Environ 141: 240–243 [Google Scholar]
- Shimono H, Ozaki Y, Jagadish KSV, Sakai H, Usui Y, Hasegawa T, Kumagai E, Nakano H, Yoshinaga S (2014) Planting geometry as a pre-screening technique for identifying CO2 responsive rice genotypes: a case study of panicle number. Physiol Plant 152: 520–528 [DOI] [PubMed] [Google Scholar]
- Shimono H, Okada M, Yamakawa Y, Nakamura H, Kobayashi K, Hasegawa T (2009) Genotypic variation in rice yield enhancement by elevated CO2 relates to growth before heading, and not to maturity group. J Exp Bot 60: 523–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhre JJ, Weidenbenner NH, Rowntree SC, Wilson EW, Naeve SL, Conley SP, Casteel SN, Diers BW, Esker PD, Specht JE, et al. (2014) Soybean yield partitioning changes revealed by genetic gain and seeding rate interactions. Agron J 106: 1631–1642 [Google Scholar]
- Tausz M, Tausz-Posch S, Norton RM, Fitzgerald GJ, Nicolas ME, Seneweera S (2013) Understanding crop physiology to select breeding targets and improve crop management under increasing atmospheric CO2 concentrations. Environ Exp Bot 88: 71–80 [Google Scholar]
- Tausz-Posch S, Dempsey RW, Seneweera S, Norton RM, Fitzgerald G, Tausz M (2015) Does a freely tillering wheat cultivar benefit more from elevated CO2 than a restricted tillering cultivar in a water-limited environment? Eur J Agron 64: 21–28 [Google Scholar]
- Zhu C, Zhu J, Cao J, Jiang Q, Liu G, Ziska LH (2014) Biochemical and molecular characteristics of leaf photosynthesis and relative seed yield of two contrasting rice cultivars in response to elevated [CO₂]. CO2. J Exp Bot 65: 6049–6056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziska LH. (2008) Three-year field evaluation of early and late 20th century spring wheat cultivars to projected increases in atmospheric carbon dioxide. Field Crops Res 108: 54–59 [Google Scholar]
- Ziska LH, Bunce JA (2000) Sensitivity of field-grown soybean to future atmospheric CO2: selection for improved productivity in the 21st century. Aust J Plant Physiol 27: 979–984 [Google Scholar]
- Ziska LH, Bunce JA, Caulfield FA (2001) Rising atmospheric carbon dioxide and seed yield of soybean genotypes. Crop Sci 41: 385–391 [Google Scholar]
- Ziska LH, Bunce JA, Shimono H, Gealy DR, Baker JT, Newton PCD, Reynolds MP, Jagadish KSV, Zhu C, Howden M. , et al. (2012) Food security and climate change: on the potential to adapt global crop production by active selection to rising atmospheric carbon dioxide. Proc Biol Sci 279: 4097–4105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziska LH, Manalo PA, Ordonez RA (1996) Intraspecific variation in the response of rice (Oryza sativa L.) to increased CO2 and temperature: growth and yield response of 17 cultivars. J Exp Bot 47: 1353–1359 [Google Scholar]
- Ziska LH, Morris CF, Goins EW (2004) Quantitative and qualitative evaluation of selected wheat varieties released since 1903 to increasing atmospheric carbon dioxide: can yield sensitivity to carbon dioxide be a factor in wheat performance? Glob Change Biol 10: 1810–1819 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.