Elevated carbon dioxide reduces the effects of drought on grain filling.
Abstract
Projections indicate an elevation of the atmospheric CO2 concentration ([CO2]) concomitant with an intensification of drought for this century, increasing the challenges to food security. On the one hand, drought is a main environmental factor responsible for decreasing crop productivity and grain quality, especially when occurring during the grain-filling stage. On the other hand, elevated [CO2] is predicted to mitigate some of the negative effects of drought. Sorghum (Sorghum bicolor) is a C4 grass that has important economical and nutritional values in many parts of the world. Although the impact of elevated [CO2] and drought in photosynthesis and growth has been well documented for sorghum, the effects of the combination of these two environmental factors on plant metabolism have yet to be determined. To address this question, sorghum plants (cv BRS 330) were grown and monitored at ambient (400 µmol mol−1) or elevated (800 µmol mol−1) [CO2] for 120 d and subjected to drought during the grain-filling stage. Leaf photosynthesis, respiration, and stomatal conductance were measured at 90 and 120 d after planting, and plant organs (leaves, culm, roots, prop roots, and grains) were harvested. Finally, biochemical composition and intracellular metabolites were assessed for each organ. As expected, elevated [CO2] reduced the stomatal conductance, which preserved soil moisture and plant fitness under drought. Interestingly, the whole-plant metabolism was adjusted and protein content in grains was improved by 60% in sorghum grown under elevated [CO2].
Global food demand is projected to increase up to 110% by the middle of this century (Tilman et al., 2011; Alexandratos and Bruinsma, 2012), particularly due to a rise in world population that is likely to plateau at about 9 billion people (Godfray et al., 2010). Additionally, the average concentration of atmospheric CO2 ([CO2]) has increased 1.75 µmol mol−1 per year between 1975 and today, reaching 400 µmol mol−1 in April 2015 (NOAA, 2015). According to the A2 emission scenario from the U.S. Environmental Protection Agency, in the absence of explicit climate change policy, atmospheric CO2 concentrations will reach 800 µmol mol−1 by the end of this century. The increasing atmospheric [CO2] is resulting in global climate changes, such as reduction in water availability and elevation in temperature. These factors are expected to heavily influence food production in the next years (Godfray and Garnett, 2014; Magrin et al., 2014).
Sorghum (Sorghum bicolor) is a C4 grass, considered a staple food grain for millions of the poorest and most food-insecure people in the semiarid tropics of Africa, Asia, and Central America, serving as an important source of energy, proteins, vitamins, and minerals (Taylor et al., 2006). Moreover, this crop is used for animal feed and as industrial raw material in developed countries such as the United States, which is the main world producer (FAO, 2015). With a fully sequenced genome (Paterson et al., 2009) and over 45,000 accessions representing a large geographic and genetic diversity, sorghum is a good model system in which to study the impact of global climate changes in C4 grasses.
The increase in [CO2] in the atmosphere, which is the main driver of global climate changes (Meehl et al., 2007), is predicted to boost photosynthesis rates and productivity in a series of C3 legumes and cereals, mainly due to a decrease in the photorespiration process (Grashoff et al., 1995; Long et al., 2006). On the contrary, due to their capacity to concentrate CO2 in bundle sheath cells and reduce photorespiration to virtually zero, C4 plants are unlikely to respond to the elevation of atmospheric [CO2] (Leakey, 2009). However, even for C4 plants, elevated [CO2] can ameliorate the effects caused by drought, maintaining higher photosynthetic rates. This is due to an improvement in the efficiency of water use that is achieved by the reduction in stomatal conductance (Leakey et al., 2004; Markelz et al., 2011).
The rate of photosynthesis as well as the redistribution of photoassimilates accumulated in different plant tissues during the day and/or during vegetative growth are crucial to grain development, and later, to its filling (Schnyder, 1993). Due to this relationship, any environmental stress such as drought occurring during the reproductive phase has the potential to result in poor grain filling and losses in yield (Blum et al., 1997). For instance, postanthesis drought can cause up to 30% decrease in yield (Borrell et al., 2000). It is also known that elevated [CO2], drought, high temperature, and any combinations of these stresses can lead to significant changes in grain composition (Taub et al., 2008; Da Matta et al., 2010; Uprety et al., 2010; Madan et al., 2012), suggesting diverse metabolic alterations and/or adaptations that occur in the plant when it is cultivated in such conditions.
Although the impacts of elevated [CO2] and drought on photosynthesis and the growth of sorghum have been well documented (Conley et al., 2001; Ottman et al., 2001; Wall et al., 2001), no attention has been given to the impact of the combination of these two environmental changes on plant metabolism and composition. Regarding physiology, studies on the growth of sorghum under elevated [CO2] and drought showed an increase of the net assimilation rate of 23% due to a decrease of 32% in stomatal conductance (Wall et al., 2001). This resulted in sorghum’s ability to use water 17% more efficiently (Conley et al., 2001). An improvement in the final overall biomass under elevated [CO2] and drought has also been described (Ottman et al., 2001), but without a significant effect in grain yield (Wall et al., 2001).
Few studies have been monitoring metabolic pathways in plants under elevated [CO2] (Li et al., 2008; Aranjuelo et al., 2013) and drought (Silvente et al., 2012; Nam et al., 2015; Wenzel et al., 2015). Furthermore, to our knowledge, there are only two reports in which metabolite profiles or metabolic pathways were investigated under the combination of these two environmental conditions (Sicher and Barnaby, 2012; Zinta et al., 2014). Although it is widely accepted that whole-plant metabolism and composition can impact grain filling and yield, metabolic studies conducted so far have focused on a specific plant organ. For instance, Sicher and Barnaby (2012) analyzed the metabolite profile of leaves from maize (Zea mays) plants that were grown under elevated [CO2] and drought, but they did not show how those environmental changes could have affected the metabolism of other tissues (e.g. culm and roots) or how they might have influenced the biomass or grain composition.
In order to address how the combination of elevated [CO2] and drought can modify whole-plant metabolism as well as biomass composition in sorghum, this study aimed to (1) evaluate photosynthesis, growth, and yield; (2) underline the differences in biomass composition and primary metabolite profiles among leaves, culm, roots, prop roots, and grains; and (3) determine the effect of elevated [CO2] and drought on the primary metabolism of each organ.
RESULTS
Elevated [CO2] Did Not Change the Rate of Photosynthesis in Sorghum Plants Subjected to Drought But Modified Panicle Size and the Timing of Initiation
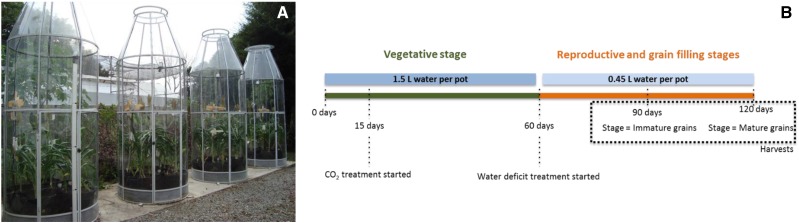
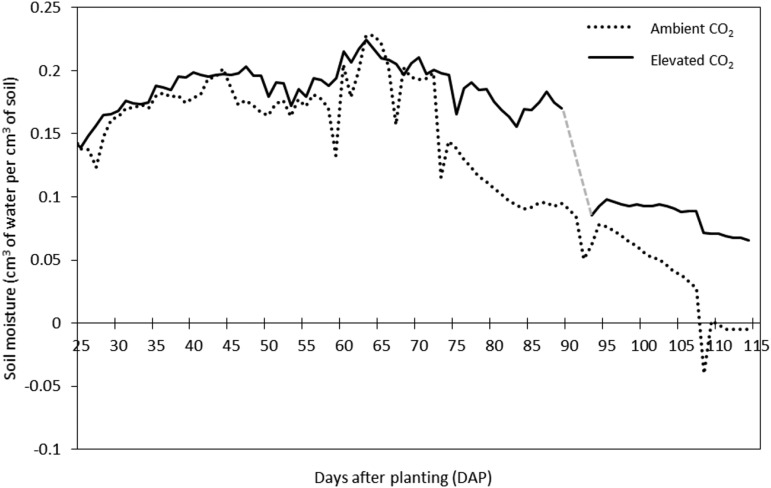
Sorghum plants were grown in open-top chambers (OTCs) under ambient or elevated [CO2] and subjected to drought during grain formation and filling (Fig. 1). In both treatments, the availability of water in the soil was similar during the entire vegetative phase (Fig. 2). At 60 DAP, when the drought treatment started, the soil moisture in pots at ambient [CO2] decreased faster than those at elevated [CO2]. At 90 and 115 DAP, soil moisture in pots at elevated [CO2] was approximately 40% and 1,200% higher than in pots at ambient [CO2], respectively.
Figure 1.
A, OTCs used during the experiment to grow sorghum ‘BRS 330’ at ambient and elevated CO2. B, Experimental design.
Figure 2.
Soil moisture (cm3 water cm−3 soil) during the experiment with sorghum ‘BRS 330’ at ambient and elevated CO2. n = 8. Water deficit treatment started at 60 d after planting (DAP). From 90 to 93 DAP, soil moisture sensors from pots at elevated CO2 did not log the data (gray dashed line).
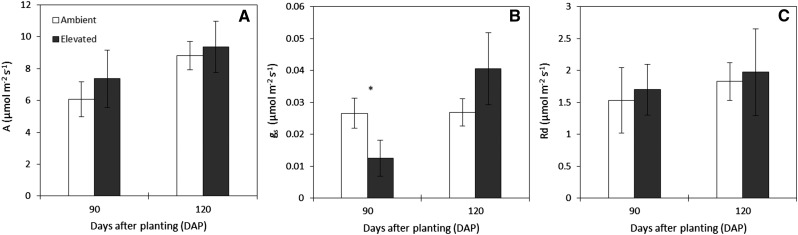
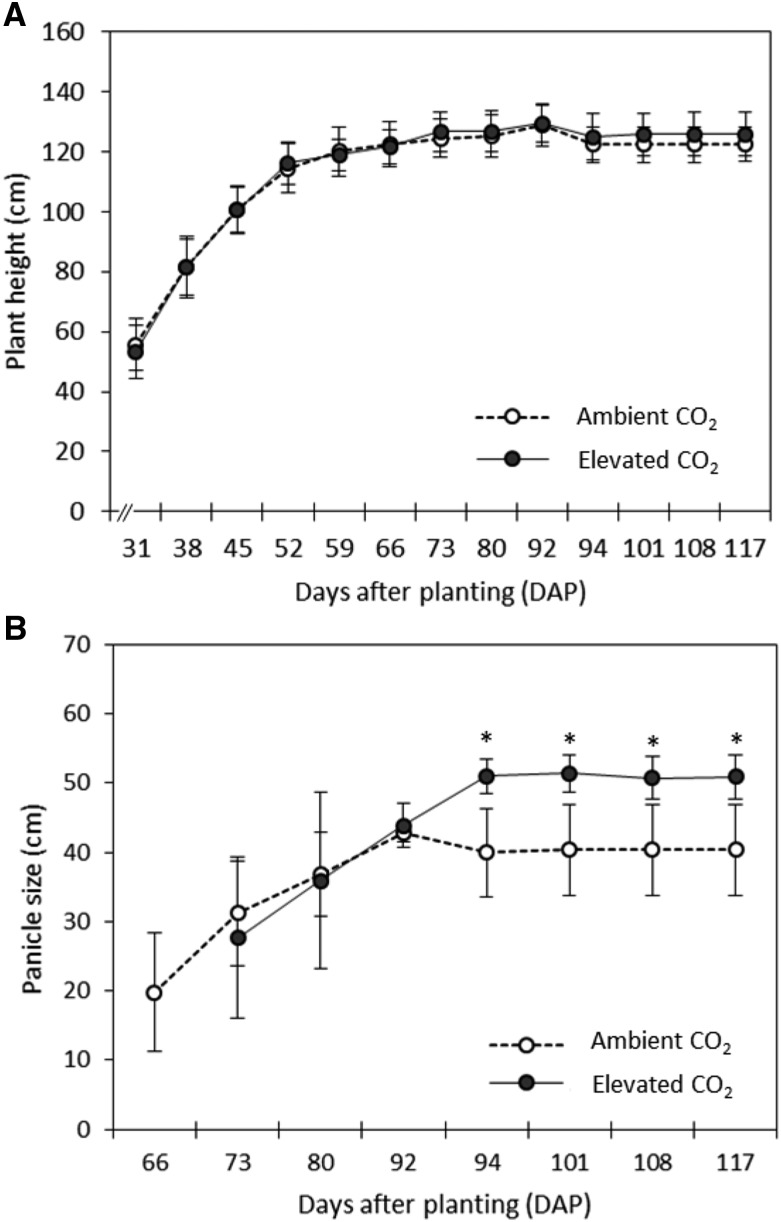
Despite the differences in soil moisture between ambient and elevated [CO2], no significant changes were observed in leaf photosynthesis and leaf respiration (Fig. 3, A and C). However, stomatal conductance in plants grown under elevated [CO2] was approximately 53% lower than ambient [CO2] at 90 DAP (Fig. 3B). Elevated [CO2] treatment did not affect the height of plants but significantly increased their panicle size by 26% to 28% between 94 and 117 DAP (Fig. 4). Furthermore, the higher concentration of CO2 promoted a delay of 1 week in panicle initiation (Fig. 4B).
Figure 3.
Light-saturated rate of leaf photosynthesis (A; A), Stomatal conductance (gs; B), and dark leaf respiration (Rd; C) of the youngest fully expanded leaf of sorghum ‘BRS 330’ at ambient and elevated CO2 at 90 and 120 DAP. Bars represent means ± sd of biological replicates. n = 3. The asterisk indicates significantly statistical differences between treatments (P < 0.05).
Figure 4.
Plant height without panicle (A) and panicle size (B) of sorghum ‘BRS 330’ at ambient and elevated CO2 during the experiment. Data points are means ± sd of biological replicates. n = 3. Asterisks indicate significant statistical differences between treatments (P < 0.05).
Elevated [CO2] Significantly Improved Protein Content in Grains But Did Not Change Yield under Drought
To evaluate whether biomass and its biochemical composition were affected by elevated [CO2] and drought, plants were harvested at 90 and 120 DAP. Only leaf and prop root biomasses increased under elevated [CO2] (Table I). At 120 DAP, leaf biomass was 48% higher in plants grown at elevated [CO2], mostly as a result of a decrease in leaf senescence. Indeed, the biomass of shed leaves under elevated [CO2] was 90% lower than in ambient [CO2] (data not shown). While a significant increase in leaf biomass was observed at the end of the plant cycle, the prop root biomass showed a transitory increase at 90 DAP. At this date, prop roots of plants under elevated [CO2] displayed 94% more biomass than plants under ambient [CO2]. However, this difference did not persist until 120 DAP (Table I). The biomass of grains, which corresponds to yield, did not change significantly between treatments.
Table I. Biomass (g) and starch, fatty acids, and proteins contents (% of dry weight) of leaves, culm, roots, prop roots, and grains of sorghum ‘BRS 330’ at ambient and elevated CO2 at 90 and 120 DAP.
Values are means ± sd of biological replicates. n = 3. Boldface values indicate significant statistical differences between treatments (P < 0.05). n.d., Not detected.
| Organ | DAP | Biomass |
Starch |
Fatty Acids |
Proteins |
||||
|---|---|---|---|---|---|---|---|---|---|
| Ambient CO2 | Elevated CO2 | Ambient CO2 | Elevated CO2 | Ambient CO2 | Elevated CO2 | Ambient CO2 | Elevated CO2 | ||
| Leaves | 90 | 23.54 ± 6.84 | 32.63 ± 5.4 | n.d. | n.d. | 1.49 ± 0.09 | 1.42 ± 0.04 | 14.4 ± 1.23 | 16.8 ± 0.45 |
| 120 | 17.75 ± 2.04 | 26.19 ± 3.8 | n.d. | n.d. | 1.09 ± 0.07 | 1.23 ± 0.05 | 16.3 ± 2.01 | 19.1 ± 2.70 | |
| Culm | 90 | 13.25 ± 4.51 | 15.49 ± 2.96 | n.d. | n.d. | 0.29 ± 0.02 | 0.30 ± 0.03 | 2.2 ± 0.26 | 1.9 ± 0.08 |
| 120 | 13.69 ± 4.79 | 10.80 ± 1.96 | n.d. | n.d. | 0.22 ± 0.02 | 0.24 ± 0.00 | 2.0 ± 0.32 | 1.9 ± 0.32 | |
| Roots | 90 | 10.74 ± 3.54 | 9.17 ± 2.03 | n.d. | n.d. | 0.29 ± 0.02 | 0.29 ± 0.01 | 3.9 ± 0.15 | 3.4 ± 0.22 |
| 120 | 10.41 ± 4.34 | 14.25 ± 7.79 | n.d. | n.d. | 0.21 ± 0.00 | 0.21 ± 0.00 | 3.2 ± 0.15 | 3.6 ± 0.42 | |
| Prop roots | 90 | 5.46 ± 1.78 | 10.48 ± 1.2 | n.d. | n.d. | 0.21 ± 0.01 | 0.19 ± 0.02 | 4.1 ± 0.69 | 3.1 ± 0.16 |
| 120 | 2.76 ± 0.16 | 3.5 ± 0.72 | n.d. | n.d. | 0.18 ± 0.01 | 0.17 ± 0.01 | 2.5 ± 0.12 | 2.3 ± 0.28 | |
| Grains | 90 | 34.74 ± 3.14 | 30.08 ± 7.51 | 57.4 ± 4.51 | 51.6 ± 6.06 | 1.91 ± 0.14 | 1.32 ± 0.11 | 7.5 ± 0.53 | 10.1 ± 0.70 |
| 120 | 61.95 ± 16.92 | 46.91 ± 13.56 | 59.9 ± 1.15 | 57.3 ± 2.43 | 2.09 ± 0.03 | 2.10 ± 0.13 | 5.1 ± 0.10 | 8.1 ± 0.68 | |
Although the biomass biochemical composition of different organs was not modified by elevated [CO2], it promoted an increase of 35% and 59% in total protein content of grains at 90 and 120 DAP, respectively (Table I). In contrast, some transitory reduction in total protein content and total fatty acids under elevated [CO2] was observed at 90 DAP in roots and grains, respectively.
Intracellular Metabolism among Organs: The First Step to Assess How Grain Biochemical Composition Is Altered by Elevated [CO2] under Drought
The grain-filling stage in plants is largely dependent on the plant metabolic status (Schnyder, 1993). Nevertheless, there are no studies that describe the primary metabolite profiles of different plant organs and assess the question of how they are interconnected. In order to obtain evidence that supports how each organ can contribute to observed changes in grain biochemical composition under elevated [CO2] and drought, the intracellular amounts of primary metabolites of leaves, culm, roots, prop roots, and grains were quantified by liquid chromatography-tandem mass spectrometry (LC-MS/MS) at 90 and 120 DAP.
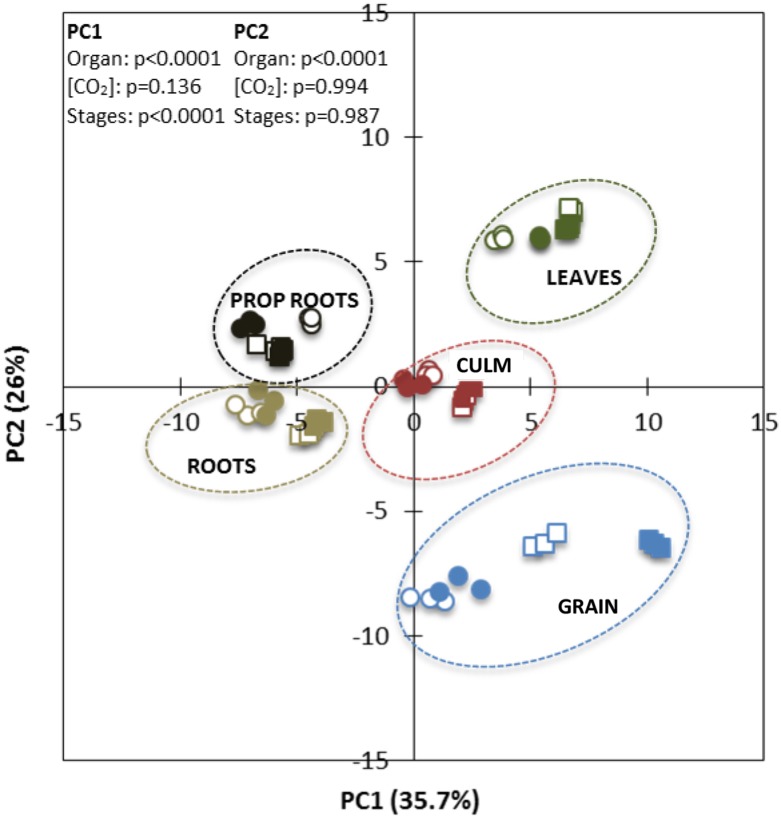
In total, we identified and quantified 76 metabolites in leaves and culm, 77 metabolites in roots and grains, and 75 metabolites in prop roots at 90 and 120 DAP in plants grown under ambient and elevated [CO2] (Supplemental Data Set S1). Principal component analysis (PCA) of the entire data set revealed that differences in the amounts of metabolites were sufficient to distinguish metabolism among the organs, even between prop roots and roots (Fig. 5). Irrespective of the dates and treatments, some metabolites were found to be in higher quantities in specific organs (Supplemental Fig. S1).
Figure 5.
PCA of the metabolites identified by LC-MS/MS of sorghum ‘BRS 330’ at ambient (open symbols) and elevated (closed symbols) CO2 at 90 DAP (squares) and 120 DAP (circles). P values indicate significant statistical differences of the PCs.
Cys levels were highest in grains when compared with other organs. This amino acid is the only metabolite donor of sulfur for the production of other sulfur-containing compounds (Noji and Saito, 2003). Interestingly, γ-aminobutyric acid, a metabolite that has been shown to mitigate drought stress in grasses (Krishnan et al., 2013), was preferentially accumulated in the grains rather than other plant organs such as leaves and roots. Polyols, such as glycerol and sorbitol, were also found to be highest in grains. These have been shown previously to be involved in osmotic stress responses (Bohnert and Shen, 1999). ADP-Glc, a precursor for starch biosynthesis, was only detectable in the grains (Supplemental Fig. S1). The majority of the amino acids were especially accumulated at higher levels in the grains of 90-DAP plants subjected to drought and elevated [CO2].
The plants were harvested between 10 am and 2 pm; therefore, photosynthesis was very active. As anticipated, leaves showed higher amounts of metabolites related to the Calvin cycle (i.e. 2- or 3-phosphoglycerate, ribulose-1,5-bisphosphate, pentose phosphates, sedoheptulose-7-phosphate, and erythrose-4-phosphate) and nucleotide triphosphates. In culm, some metabolites related to the respiration process, such as citrate and isocitrate, were in higher quantities. Trp, a precursor of a large variety of secondary metabolites as well as the precursor of the plant hormone indole-3-acetic acid (Ljung et al., 2002; Kriechbaumer and Glawischnig, 2005), was also accumulated preferentially in the culm. The amount of soluble sugars (Fru, Glc, and Suc) was greater in prop roots, structures described to aid the anchorage of the plant to the soil (Gregory, 2006), than in any other organ.
Intracellular Metabolism Is Modified by Elevated [CO2] in Plants Grown under Drought
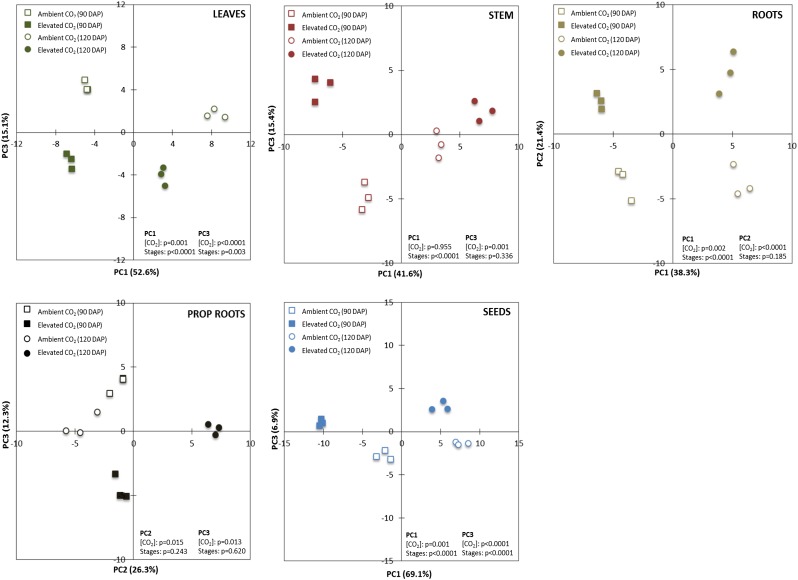
To investigate whether the metabolism of each organ was modified by elevated [CO2] and through different grain-filling stages (90 and 120 DAP), we performed a series of PCAs taking into account all metabolites present in a given organ and tested the significance related to CO2 treatment and the grain developmental stage. All organs showed significant differences between ambient and elevated [CO2]. With the exception of prop roots, they also had significant differences related to the grain developmental stages (Fig. 6).
Figure 6.
PCA of the metabolites identified by LC-MS/MS of leaves, culm, roots, prop roots, and grains of sorghum ‘BRS 330’ at ambient and elevated CO2 at 90 and 120 DAP. P < 0.05 indicates significant statistical differences of the PCs.
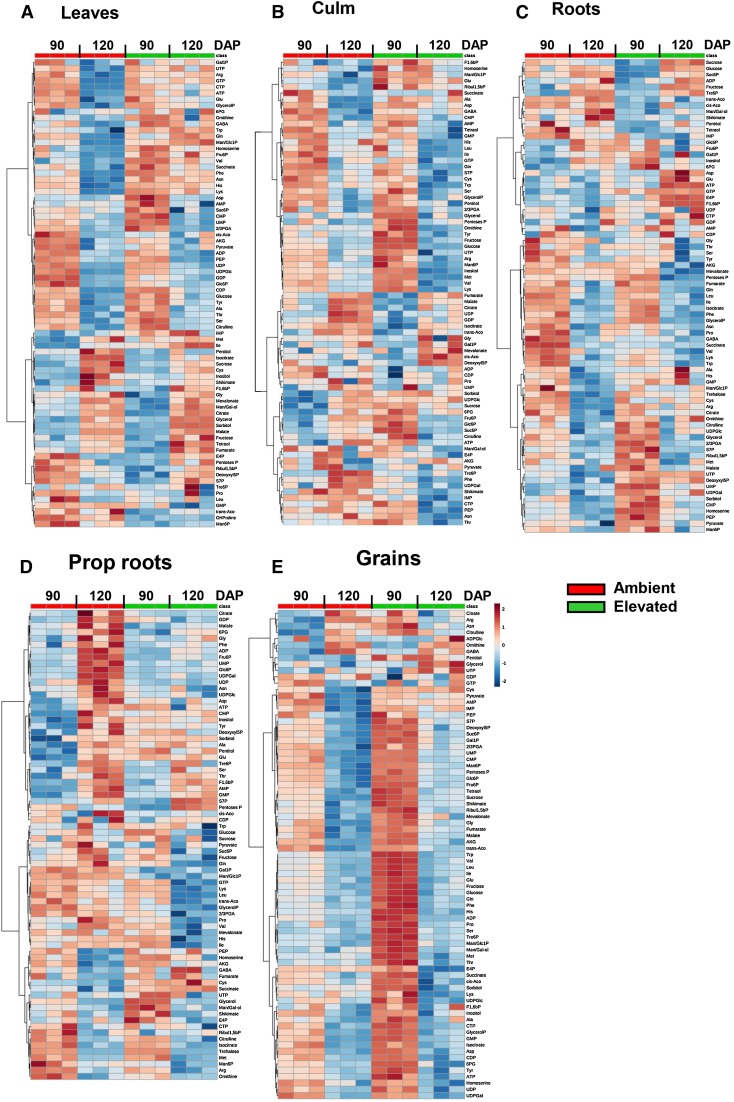
In leaves, most of the amino acids decreased from 90 to 120 DAP at ambient [CO2], whereas they were at similar amounts on both dates under elevated [CO2] (Fig. 7A). Leaves under elevated [CO2] had higher amounts of nucleotide monophosphates, 2- or 3-phosphoglycerate, and Suc-6-P at 90 DAP in comparison with leaves grown under ambient [CO2]. However, no differences between treatments were found at 120 DAP. Both ambient and elevated [CO2] plants showed a reduction in metabolites related to glycolytic processes in leaves at 120 DAP compared with 90 DAP. On the other hand, sugars, sugar alcohols, and intermediaries of the tricarboxylic acid cycle increased in both CO2 treatments between 90 and 120 DAP. Metabolites related to the Calvin cycle were lower under elevated [CO2] at 90 DAP but higher at 120 DAP.
Figure 7.
Heat maps showing the variation in the amount of metabolites in sorghum ‘BRS 330’ grown under ambient and elevated [CO2] at 90 and 120 DAP in leaves (A), culm (B), roots (C), prop roots (D), and grains (E). 2/3PGA, 2- or 3-phosphoglycerate; 6PG, 6-phosphogluconate; AKG, α-ketoglutarate; cis-aco, cis-aconitate; Deoxyxyl5P, deoxyxylulose-5-phosphate; E4P, erythrose-4-phosphate; F1,6bP, Fru-1,6-bisP; Fru6P, Fru-6-P; GABA, γ-aminobutyric acid; Gal1p, Gal-1-P; Glc6P, Glc-6-P; GlycerolP, glycerol-3-phosphate; Man6P, Man-6-P; Man/Gal-ol, mannitol or galactitol; Man/Glc1P, Man- or Glc-1-P; OHPro, Hyp; Pentoses P, pentose-phosphates; PEP, phosphoenolpyruvate; Ribul1,5bP, ribulose-1,5-bisphosphate; S7P, sedoheptulose-7-phosphate; Suc6P, Suc-6-P; trans-aco, trans-aconitate; Tre6P, trehalose-6-phosphate.
Similar to leaves, the majority of the amino acids decreased in culm between 90 and 120 DAP. However, in culm, the reduction was observed in both CO2 treatments. Sugars and sugar alcohols decreased at 120 DAP in both conditions. Also similar to what was observed in leaves, intermediaries of the tricarboxylic acid cycle (fumarate, malate, citrate, isocitrate, trans-aconitate, and cis-aconitate) in the culm of both ambient and elevated [CO2] increased between 90 and 120 DAP. Remarkably, metabolites related to Suc metabolism (UDP-Glc, Suc, Fru-6-P, Glc-6-P, and Suc-6-P) were higher in the culm at 90 DAP under elevated [CO2] and decreased to lower levels than ambient [CO2] at 120 DAP (Fig. 7B).
The amounts of soluble sugars (Suc, Glc, and Fru) and some sugar alcohols in roots were higher under ambient [CO2], especially at 90 DAP. Under elevated [CO2], the levels of these sugars increased only at 120 DAP (Fig. 7C). Roots from plants grown at elevated [CO2] also showed a higher content of hexose phosphates and nucleotide triphosphates at 120 DAP. As observed previously for leaves and culm, the overall metabolism of amino acids was reduced at 120 DAP in roots under both conditions. Overall, the intermediaries of glycolysis and the tricarboxylic acid cycle were reduced at 120 DAP compared with 90 DAP in both CO2 treatments.
In prop roots, an overall increase of organic acids, hexose phosphates, and sugar alcohols was measured at 120 DAP under ambient [CO2] but not under elevated [CO2] (Fig. 7D). Intriguingly, this response is different from what was found in roots. Additionally, the levels of sugars and metabolites related to Suc metabolism decreased at 120 DAP in prop roots under elevated [CO2], whereas they were maintained at similar levels under ambient [CO2] (Fig. 7D). This response is also opposite to what was observed in roots, where the amounts of sugars increased at 120 DAP under elevated [CO2] (Fig. 7C).
The metabolites in grains were generally lower at 120 DAP than at 90 DAP in both treatments, indicating a reduction in the overall grain metabolism (Fig. 7E). Remarkably, the majority of metabolites related to intermediaries of glycolysis, the tricarboxylic acid cycle, and amino acids were higher under elevated [CO2] at 90 DAP.
DISCUSSION
Elevated [CO2] Changes the Physiological Status and Development of Sorghum under Drought
Losses in grain quality and yield are observed when drought stress occurs, especially during flowering time (Blum et al., 1997). For instance, postanthesis drought can cause up to a 30% decrease in grain yield (Borrell et al., 2000). However, elevated [CO2] commonly ameliorates the effects of environmental stresses (Ottman et al., 2001; Leakey et al., 2006; Geissler et al., 2009; Sicher and Barnaby, 2012; Naudts et al., 2013; Arenque et al., 2014; Zinta et al., 2014), reducing the impact on photosynthesis, biomass production, and loss in productivity.
In C4 plants, the mitigation of the effect of drought stress promoted by elevated [CO2] is thought to be a consequence of the reduction in stomatal conductance, which leads to an increase in water use efficiency (Leakey, 2009). For sorghum, previous studies revealed that the improvement in water use efficiency stimulated by elevated [CO2] in plants cultivated under drought stress increased the rate of photosynthesis and final overall biomass (Conley et al., 2001; Ottman et al., 2001; Wall et al., 2001). As consistently described in the literature, our results show that stomatal conductance under elevated [CO2] was lower than under ambient [CO2] at 90 DAP (Fig. 3B). However, this pattern was reversed at 120 DAP, where plants at elevated [CO2] displayed higher stomatal conductance than at ambient [CO2]. This reversion may reflect the effect of drought on plants at ambient [CO2]. Due to the lower stomatal conductance, soil moisture was conserved during the period of growth under elevated [CO2] (Fig. 2), producing a less severe effect for drought. Nevertheless, it is important to mention that the large difference of up to 1,200% in soil moisture observed between elevated and ambient [CO2] might not reflect values detected under field conditions. These high values may be due to an interference of OTC ventilation on the sensors installed at only 15 to 20 cm from the soil surface.
In spite of the observed effect on stomatal conductance, no significant differences in photosynthesis between ambient and elevated [CO2] were measured (Fig. 3A). Even at the end of the experiment, when plants at ambient [CO2] faced quite a low amount of water in the soil (Fig. 2), both treatments showed similar photosynthetic rates. However, leaf biomasses were different between treatments. Indeed, under elevated [CO2] leaf senescence was reduced, leading to a higher leaf biomass accumulation at 120 DAP (Table I). The reduction in leaf senescence under elevated [CO2] (data not shown) indicates that this treatment alleviates some of the drought effects. In sorghum, drought stress during and after flowering stages (as applied in our experiment) can cause premature leaf senescence (Rosenow and Clark, 1995), and it is well known that the retention of green leaves during the grain-filling stage generally results in higher grain yield (Valdez et al., 2011; Jordan et al., 2012). In our experimental design, the greater amount of green leaves under elevated [CO2] did not have an impact on yield (Table I) but rather can be related to the observed changes in grain quality, as will be discussed further.
Even without a significant impact on yield, plants under elevated [CO2] had their panicle development delayed by 7 d. At the same time, their panicle size was increased significantly (Fig. 4B). The late inflorescence development was already reported in previous experiments with sorghum (Marc and Gifford, 1983). Those authors showed similar 7-d delays in inflorescence development from plants cultivated under 500 µmol CO2 mol−1 air and postulated that CO2 can exert some direct effect on inflorescences, since no other changes in plant development were observed.
Another interesting change related to growth was the transitory increase in biomass accumulation in prop roots at 90 DAP under elevated [CO2] (Table I). Since drought can reduce the growth of prop roots (Nielsen, 2002), the higher biomass found under elevated [CO2] at 90 DAP might also be a consequence of the lower effect of drought under this treatment. At 120 DAP, the biomass of prop roots in both ambient and elevated [CO2] was reduced compared with 90 DAP, and no differences were found between the two treatments (Table I). During the grain-filling stage, there is a mobilization of storage compounds from other organs in order to supply carbon and nitrogen to grain. In sorghum, this storage mobilization can reach up to 40% in optimal conditions and up to 52% under drought stress (Beheshti and Fard, 2010). This is one possible explanation for the observed reductions of 49% and 67% in the biomass of prop roots under ambient and elevated [CO2], respectively.
Elevated [CO2] Preserves the Quality of Grains under Drought through the Maintenance of Whole-Plant Metabolism
Plant responses to environmental factors is not only limited to physiological behavior but also involves a coordinated and complex network of different organizational levels (i.e. physiological, metabolic, and transcriptional) that may take place in different organs. It has been demonstrated previously that plant metabolism can change to maintain whole-plant homeostasis under stress, preventing massive alterations in physiological and growth parameters. For instance, De Souza et al. (2013) showed for Miscanthus × giganteus that even when no significant changes in photosynthesis and dry weight were found, growth under elevated [CO2] promoted a reduction in starch content in leaves, roots, and rhizomes of this C4 plant.
In the experiment reported here, one of the most remarkable differences found between ambient and elevated [CO2] was the one related to the protein content in grains. Even though there were no changes in yield, the protein content per mass in grains was higher at elevated [CO2] (Table I). At 120 DAP, we found averages of 5% and 8% of protein in grains under ambient and elevated [CO2], respectively. According to EMBRAPA Maize and Sorghum (http://www.catalogosnt.cnptia.embrapa.br/catalogo20/catalogo_de_produtos_e_servicos/arvore/CONT000gdhlxxjo02wx5ok0272do2kg8u7h5.html), the cultivar used in this experiment (cv BRS 330) displays around 10% of protein content in its grains under field conditions. Thus, the protein content found in plants under elevated [CO2] was closer to what is usually observed in this cultivar, indicating that elevated [CO2] ameliorates the effects of drought, leading to a preservation of grain quality.
Corroborating the mitigation effects of drought under elevated [CO2], the conservation of water in the soil observed in this treatment most likely extended the activity of metabolites related to the Calvin cycle, Suc, and amino acid metabolisms in leaves (Fig. 7A). This condition also promoted a higher accumulation of metabolites related to Suc metabolism in culm at 90 DAP (Fig. 7B) and the maintenance of metabolites related to energy metabolism such as hexose phosphates and nucleotide triphosphates in roots until 120 DAP (Fig. 7C). Additionally, the appearance of metabolites associated with drought was delayed in roots and prop roots under elevated [CO2]. No responses related to drought were found in prop roots under elevated [CO2] during this experiment. The increase in the levels of sugars and sugar alcohols in roots, which is a consequence of the drought response (Bohnert and Sheveleva, 1998; Pavli et al., 2013), occurred only at 120 DAP under elevated [CO2]. In contrast, high levels of these metabolites were found under ambient [CO2] as soon as 90 DAP (Fig. 7C). Under ambient [CO2], prop roots displayed a later response to drought compared with roots. An increase in the contents of organic acids and sugar alcohols, which are metabolites regarded as characteristic of the drought response in sorghum (Pavli et al., 2013), was observed at 120 DAP in this organ (Fig. 7D). It is also important to note that our metabolomics study revealed a maximal level of all the amino acids in grain under elevated [CO2] at 90 DAP (Fig. 7E; Supplemental Data Set S1). Altogether, these responses accounted for the maintenance of grain metabolism under elevated [CO2] and, consequently, higher protein contents in grains. Moreover, the higher biomass of prop roots and the larger mobilization of sugars in both culm and prop roots from 90 to 120 DAP (Table I; Fig. 7, B and D) at elevated [CO2] also contributed to the higher protein levels in grains. Indeed, the high amounts of Fru, Glc, and Suc found in prop roots (Supplemental Fig. S1) and the decrease of its biomass from 90 to 120 DAP suggest that this organ can serve as a storage organ in addition to its function in plant support.
Even though cultivation under elevated [CO2] diminished the effects of drought, metabolic responses related to this stress can be observed in both treatments at 120 DAP. The contents of sugars and sugar alcohols in leaves and roots increased in both treatments from 90 to 120 DAP (Fig. 7, A and C). Similarly, the levels of some organic acids, primarily the intermediaries of the tricarboxylic acid cycle, were greater in leaves and culm (Fig. 7, A and B). The increase in concentration of these metabolites has been suggested previously to be associated with drought responses in sorghum (Pavli et al., 2013).
During plant development, the overall metabolic response indicated a reduction in plant metabolism from 90 to 120 DAP, with a decrease in amino acid contents in leaves and culm, a concomitant decrease in metabolites related to Suc metabolism in culm and prop roots, and a reduction in intermediaries of glycolysis and the tricarboxylic acid cycle in roots (Fig. 7).
CONCLUSION AND PERSPECTIVES
Our results show that, although few physiological effects were observed, the cultivation of sorghum under elevated atmospheric CO2 alleviated the loss in grain quality caused by drought during the grain-filling stage due to a delay in physiological and metabolic responses to drought. To our knowledge, this is the first study that demonstrates the simultaneous metabolic responses of the different organs of a plant cultivated under elevated atmospheric CO2 and drought at the same time. It also shows, to our knowledge for the first time in sorghum, how changes in each organ can affect grain composition. In this context, in the future, it will be key to evaluate metabolic fluxes within the plant in order to highlight how different organs integrate to produce whole-plant physiological behavior under different environmental conditions. Furthermore, our results set up a map of metabolic events occurring in different organs of sorghum. This information could be used to search candidate genes related to the control of interorgan communication. Thus, they might be targeted in plant engineering to help cope with the impacts of global climate changes on crop grasses.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Seeds of sorghum (Sorghum bicolor ‘BRS 330’) were obtained from EMBRAPA Maize and Sorghum. They were germinated in trays containing Plantmax soil compost (peat, Pinus spp. bark, and vermiculite) in a greenhouse. At 15 DAP, seedlings of similar size were transferred to 40-L pots and randomly distributed through four OTCs of 1.5 × 3.5 m (diameter × height) as described by De Souza et al. (2008; Fig. 1A). Two OTCs were coupled to a CO2 cylinder in order to maintain the chamber internal concentration at approximately 800 µmol mol−1 (elevated [CO2]). The other two chambers were maintained with current atmospheric [CO2] (approximately 400 µmol mol−1; ambient [CO2]).
Every 1 week, pots were rotated inside the OTCs, and every 2 weeks, they were rotated between OTCs to avoid acclimation to the microenvironment. Air temperature, air relative humidity, and [CO2] for each OTC were constantly recorded (Supplemental Fig S2). After 25 d, soil moisture sensors (10HS; ICT International) were installed at 15 to 20 cm from the soil surface in four pots of each OTC, in which the soil moisture values were recorded every 24 h. Once each week, each plant received 400 mL of Hoagland solution (Epstein, 1972) as a source of nutrients until the end of the experiment. During the vegetative phase (from 0 to 60 DAP), each pot received 1.5 L of water per day. At the beginning of the reproductive phase (after 60 DAP, with the initiation of the flag leaf), the amount of water per pot in both treatments was reduced to 0.45 L (reduction of 70%) per day to start the water deficit treatment (Fig. 1B).
Harvests
Five plants from each treatment were harvested at 90 and 120 DAP, corresponding to developmental stages of immature and mature grains, respectively (Fig. 1B). Each harvested plant was separated into leaves, culm, roots, prop roots, and grains, immediately frozen in liquid nitrogen, and freeze dried.
Growth Measurements
Plant height and panicle size were evaluated every 1 week from 31 to 117 DAP and from 66 to 117 DAP, respectively, using a measuring tape. Plant height was considered as being the distance between the root-shoot transition and the tip of the younger leaf, and panicle size as the distance between the peduncle-inflorescence transition and the inflorescence tip. Biomass accumulation in leaves, culm, roots, prop roots, and grains was determined at 90 and 120 DAP in freeze-dried material.
Gas-Exchange Measurements
Before each harvest, leaf CO2 uptake, stomatal conductance, and leaf respiration of the flag leaf were measured with a portable open gas-exchange system (LI-6400 XT; Li-Cor). Leaf temperature was maintained at 26°C, light intensity at 1,000 µmol m−2 s−1 photosynthetically active radiation, and CO2 concentration at 400 or 800 µmol mol−1 depending of the [CO2] treatment. For leaf respiration measurements, the leaf was dark adapted during 30 min. All measurements were made between 10 am and 2 pm.
Fatty Acid, Protein, and Starch Extraction and Quantification
Fatty acids, proteins, and starch were sequentially extracted from 20 mg of freeze-dried ground sorghum tissues as described previously (Cocuron et al., 2014).
Fatty acid content was determined by gas chromatography-mass spectrometry (GC-MS). Fatty acid methyl esters (FAMEs) were analyzed using a Thermo Trace 1310 gas chromatograph coupled to an ISQ single-quadrupole mass spectrometer. FAME derivatives were separated using an Omegawax 250 capillary (30 m × 0.25 mm × 0.25 μm) column from Supelco as described previously (Tsogtbaatar et al., 2015). GC-MS data were acquired and processed using Xcalibur software. FAME derivatives were identified using the National Institute of Standards and Technology 11 library and neat FAME standards purchased from Sigma.
Proteins and starch were quantified following the steps described previously (Cocuron et al., 2014).
Metabolite Extraction
Metabolites were extracted from 20 mg of freeze-dried ground sorghum tissues using boiling water following a protocol described previously (Cocuron and Alonso, 2014). At the time of the extraction, 200, 200, and 50 nmol of [U-13C]Glc, [U-13C]Gly, and [U-13C]fumarate were added, respectively, as internal standards.
LC-MS/MS Quantification of Intracellular Metabolites
After lyophilization, extracts were resuspended in 500 µL of nanopure water and vortexed. Two hundred microliters of sample was loaded onto a 0.2-µm nanosep microfiltration centrifugal device in order to quantify the sugars and sugar alcohols. The remaining 300 µL was transferred to a 3-kD Amicon Ultra 0.5-mL filtering device for the quantification of amino acids, phosphorylated compounds, and organic acids. The samples were centrifuged at 14,000g for 45 min at 4°C. The intracellular metabolites were separated and quantified as described previously (Cocuron et al., 2014) with minor modifications. Liquid chromatography was performed with the UHPLC 1290 device from Agilent Technologies. The tandem mass spectrometry analyses were performed with a hybrid triple-quadrupole/ion-trap mass spectrometer (QTRAP 5500; AB Sciex). LC-MS/MS data were acquired and processed using Analyst 1.6.1 software.
For sugar and sugar alcohol quantification, the extracts from leaves, roots, and grains obtained after centrifugation in the nanosep microfiltration filter were diluted 25 times, and those from culm and prop roots were diluted 50 times, in acetonitrile:water (60:40) solution. For amino acids, the extracts from leaves and roots were diluted 25 times, and those from culm, prop roots, and grains were diluted 50 times, in 1 mm hydrochloric acid. The quantification was made by injecting 5 µL of the diluted sample onto the LC-MS/MS column.
In order to quantify phosphorylated compounds and organic acids, the extracts from leaves, culm, roots, prop roots, and grains were diluted in ultrapure water 25, 25, 20, 20, and 10 times, respectively. Ten microliters was injected onto the column.
Statistical Analysis
Differences between ambient and elevated [CO2] (P < 0.05) were tested by Student’s t test (n = 3) using JMP Statistical Discovery Software, version 5.0.1.
Metabolites were analyzed by PCA using the software Minitab version 14.1. Before PCA, all metabolite data were normalized using log2 function. The significance of each principal component was tested by a two-way ANOVA test, treating grain developmental stages and CO2 concentration as fixed effects.
Heat maps were generated using MetaboAnalyst version 3.0 (Xia et al., 2009, 2012, 2015), a free online software (www.metaboanalyst.ca). Briefly, metabolite levels were first normalized using log2 function, mean centered, and divided by the sd of each variable. Finally, Ward’s hierarchical clustering algorithm was used to group metabolites that have the same pattern of distribution.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Heat map comparing metabolite levels in different organs.
Supplemental Figure S2. Atmospheric [CO2], air temperature, and air relative humidity inside the OTCs.
Supplemental Data Set S1. List of metabolites and their quantification in sorghum tissues under ambient and elevated [CO2].
Supplementary Material
Acknowledgments
We thank the Ohio State University Targeted Metabolomics Laboratory (metabolomics.osu.edu) for access to the GC-MS and LC-MS/MS equipment and Erin Ponting for critical reading of the article.
Glossary
- OTC
open-top chamber
- DAP
days after planting
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- PCA
principal component analysis
- GC-MS
gas chromatography-mass spectrometry
- FAME
fatty acid methyl ester
Footnotes
This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Microsoft Research (grant no. 2011/52065–3) and by the Ohio State University/FAPESP Joint Mobility Grant Program (grant no. 2013/50316–4).
Articles can be viewed without a subscription.
References
- Alexandratos N, Bruinsma J (2012) World Agriculture towards 2030/2050: The 2012 Revision. Working paper No. 12-03. Food and Agriculture Organization of the United Nations, Rome
- Aranjuelo I, Sanz-Sáez Á, Jauregui I, Irigoyen JJ, Araus JL, Sánchez-Díaz M, Erice G (2013) Harvest index, a parameter conditioning responsiveness of wheat plants to elevated CO2. J Exp Bot 64: 1879–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenque BC, Grandis A, Pocius O, De Souza AP, Buckeridge MS (2014) Responses of Senna reticulata, a legume tree from the Amazonian floodplains, to elevated atmospheric CO2 concentration and waterlogging. Trees (Berl) 28: 1021–1034 [Google Scholar]
- Beheshti AR, Fard BB (2010) Dry matter accumulation and remobilization in grain sorghum genotypes (Sorghum bicolor L. Moench) under drought stress. Australian Journal of Crop Science 4: 185–189 [Google Scholar]
- Blum A, Golan G, Mayer J, Sinmena B (1997) The effect of dwarfing genes on sorghum grain filling from remobilized stem reserves, under stress. Field Crops Res 52: 43–54 [Google Scholar]
- Bohnert HJ, Shen B (1999) Transformation and compatible solutes. Sci Hortic (Amsterdam) 78: 237–260 [Google Scholar]
- Bohnert HJ, Sheveleva E (1998) Plant stress adaptations: making metabolism move. Curr Opin Plant Biol 1: 267–274 [DOI] [PubMed] [Google Scholar]
- Borrell AK, Hammer GL, Henzel RG (2000) Does maintaining green leaf area in sorghum improve yield under drought? II. Dry matter production and yield. Crop Sci 40: 1037–1048 [Google Scholar]
- Cocuron JC, Alonso AP (2014) Liquid chromatography tandem mass spectrometry for measuring ¹³C-labeling in intermediates of the glycolysis and pentose phosphate pathway. Methods Mol Biol 1090: 131–142 [DOI] [PubMed] [Google Scholar]
- Cocuron JC, Anderson B, Boyd A, Alonso AP (2014) Targeted metabolomics of Physaria fendleri, an industrial crop producing hydroxy fatty acids. Plant Cell Physiol 55: 620–633 [DOI] [PubMed] [Google Scholar]
- Conley MM, Kimball BA, Brooks TJ, Pinter PJ Jr, Hunsaker HJ, Wall GW, Adam NR, La Morte RL, Matthias AD, Thompson TL, et al. (2001) CO2 enrichment increases water-use efficiency in Sorghum. New Phytol 151: 407–412 [Google Scholar]
- Da Matta FM, Grandis A, Arenque BC, Buckeridge MS (2010) Impacts of climate changes on crop physiology and food quality. Food Res Int 43: 1814–1823 [Google Scholar]
- De Souza AP, Arundale RA, Dohleman FG, Long SP, Buckeridge MS (2013) Will exceptional productivity of Miscanthus × giganteus increase further under rising atmospheric CO2? Agric For Meteorol 171-172: 82–92 [Google Scholar]
- De Souza AP, Gaspar M, Da Silva EA, Ulian EC, Waclawovsky AJ, Nishiyama MY Jr, Dos Santos RV, Teixeira MM, Souza GM, Buckeridge MS (2008) Elevated CO2 increases photosynthesis, biomass and productivity, and modifies gene expression in sugarcane. Plant Cell Environ 31: 1116–1127 [DOI] [PubMed] [Google Scholar]
- Epstein E. (1972) Mineral Nutrition of Plants: Principles and Perspectives. Wiley, New York [Google Scholar]
- FAO (2015) Statistics of Production. Food and Agriculture Organization of the United Nations, Rome, http://faostat3.fao.org/home/E (May 25, 2015)
- Geissler N, Hussin S, Koyro HW (2009) Elevated atmospheric CO2 concentration ameliorates effects of NaCl salinity on photosynthesis and leaf structure of Aster tripolium L. J Exp Bot 60: 137–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfray HCJ, Beddington JR, Crute IR, Haddad L, Lawrence D, Muir JF, Pretty J, Robinson S, Thomas SM, Toulmin C (2010) Food security: the challenge of feeding 9 billion people. Science 327: 812–818 [DOI] [PubMed] [Google Scholar]
- Godfray HCJ, Garnett T (2014) Food security and sustainable intensification. Philos Trans R Soc Lond B Biol Sci 369: 20120273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grashoff C, Dijkstra P, Nonhebel S, Schapendonk AHCM, Van de Geijn SC (1995) Effects of climate change on productivity of cereals and legumes: model evaluation of observed year-to-year variability of the CO2 response. Glob Change Biol 1: 417–428 [Google Scholar]
- Gregory PJ. (2006) Plant Roots: Growth, Activity and Interactions with the Soil. Wiley-Blackwell, Oxford, UK [Google Scholar]
- Jordan DR, Hunt CH, Cruickshank AW, Borrell AK, Henzell RG (2012) The relationship between the stay-green trait and grain yield in elite sorghum hybrids grown in a range of environments. Crop Sci 52: 1153–1161 [Google Scholar]
- Kriechbaumer V, Glawischnig E (2005) Auxin biosynthesis within the network of tryptophan metabolism. Journal of Nano and Bio Tech 2: 53–58 [Google Scholar]
- Krishnan S, Laskowski K, Shula V, Merewitz EB (2013) Mitigation of drought stress damage by exogenous application of a non-protein amino acid γ-aminobutyric acid on perennial ryegrass. J Am Soc Hortic Sci 138: 358–366 [Google Scholar]
- Leakey ADB. (2009) Rising atmospheric carbon dioxide concentration and the future of C4 crops for food and fuel. Proc Biol Sci 276: 2333–2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leakey ADB, Bernacchi CJ, Dohleman FG, Ort DR, Long SP (2004) Will photosynthesis of maize (Zea mays) in the US Corn Belt increase in future [CO2] rich atmospheres? An analyses of diurnal courses of CO2 uptake under free-air concentration enrichment (FACE). Glob Change Biol 10: 951–962 [Google Scholar]
- Leakey ADB, Uribelarrea M, Ainsworth EA, Naidu SL, Rogers A, Ort DR, Long SP (2006) Photosynthesis, productivity, and yield of maize are not affected by open-air elevation of CO2 concentration in the absence of drought. Plant Physiol 140: 779–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Ainsworth EA, Leakey ADB, Ulanov A, Lozovaya V, Ort DR, Bohnert HJ (2008) Arabidopsis transcript and metabolite profiles: ecotype-specific responses to open-air elevated [CO2]. Plant Cell Environ 31: 1673–1687 [DOI] [PubMed] [Google Scholar]
- Ljung K, Hull AK, Kowalczyk M, Marchant A, Celenza J, Cohen JD, Sandberg G (2002) Biosynthesis, conjugation, catabolism and homeostasis of indole-3-acetic acid in Arabidopsis thaliana. Plant Mol Biol 49: 249–272 [PubMed] [Google Scholar]
- Long SP, Ainsworth EA, Leakey AD, Nösberger J, Ort DR (2006) Food for thought: lower-than-expected crop yield stimulation with rising CO2 concentrations. Science 312: 1918–1921 [DOI] [PubMed] [Google Scholar]
- Madan P, Jagadish SVK, Craufurd PQ, Fitzgerald M, Lafarge T, Wheeler TR (2012) Effect of elevated CO2 and high temperature on seed-set and grain quality of rice. J Exp Bot 63: 3843–3852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrin GO, Marengo JA, Boulanger JP, Buckeridge MS, Castellanos E, Poveda G, Scarano FR, Vicuña S (2014) Central and South America. In Barros VR, Field CB, Dokken DJ, Mastrandrea MD, Mach KJ, Bilir TE, Chatterjee M, Ebi KL, Estrada YO, Genova RC, Girma B, Kissel ES, Levy AN, MacCracken S, Mastrandrea PR, White LL, eds, Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part B. Regional Aspects. Cambridge University Press, Cambridge, UK, pp 1499–1566 [Google Scholar]
- Marc J, Gifford RM (1983) Floral initiation in wheat, sunflower, and sorghum under carbon dioxide enrichment. Can J Bot 62: 9–14 [Google Scholar]
- Markelz RJC, Strellner RS, Leakey ADB (2011) Impairment of C4 photosynthesis by drought is exacerbated by limiting nitrogen and ameliorated by elevated [CO2] in maize. J Exp Bot 62: 3235–3246 [DOI] [PubMed] [Google Scholar]
- Meehl GA, Stocker TF, Collins WD, Friedlingstein P, Gaye AT, Gregory JM, Kitoh A, Knutti R, Murphy JM, Noda A, et al. (2007) Global climate projections. In Solomon S, Qin D, Manning M, Chen ZC, Marquis M, Averty KB, Tignor M, Miller HL, eds, Climate Change 2007: The Physical Science Basis. Cambridge University Press, Cambridge, UK, pp 747–845 [Google Scholar]
- Nam KH, Shin HJ, Pack IS, Park JH, Kim HB, Kim CG (March 16, 2015) Metabolomic changes in grains of well-watered and drought-stressed transgenic rice. J Sci Food Agric http://dx.doi.org/10.1002/jsfa.7152 [DOI] [PubMed] [Google Scholar]
- Naudts K, Van Den Berge J, Janssens IA, Nijs I, Ceulemans R (2013) Combined effects of warming and elevated CO2 on the impact of drought in grassland species. Plant Soil 369: 497–507 [Google Scholar]
- Nielsen RL. (2002) Root lodging concerns in corn. Purdue University Department of Agronomy, https://www.agry.purdue.edu/ext/corn/news/articles.02/RootLodge-0711.html (June 20, 2015)
- NOAA (2015) Trends in atmospheric carbon dioxide. National Oceanic and Atmospheric Administration, http://www.esrl.noaa.gov/gmd/ccgg/trends/ (June 30, 2015)
- Noji M, Saito K (2003) Sulphur amino acids: biosynthesis of cysteine and methionine. In Abrol YP, Ahmad A, eds, Sulphur in Plants. Springer, Dordrecht, The Netherlands, pp 135–144 [Google Scholar]
- Ottman MJ, Kimball BA, Pinter PJ Jr, Wall GW, Vanderlip RL, Leavitt SW, LaMorte RL, Matthias AD, Brooks TJ (2001) Elevated CO2 increases sorghum biomass under drought conditions. New Phytol 150: 261–273 [Google Scholar]
- Paterson AH, Bowers JE, Bruggmann R, Dubchak I, Grimwood J, Gundlach H, Haberer G, Hellsten U, Mitros T, Poliakov A, et al. (2009) The Sorghum bicolor genome and the diversification of grasses. Nature 457: 551–556 [DOI] [PubMed] [Google Scholar]
- Pavli OI, Vlachos CE, Kalloniati C, Flemetakis E, Skaracis GN (2013) Metabolite profiling reveals the effect of drought on sorghum (Sorghum bicolor L. Moench) metabolism. Plant Omics Journal 6: 371–376 [Google Scholar]
- Rosenow DT, Clark LE (1995) Drought and lodging resistance for quality sorghum crop. In Proceedings of the 50th Annual Corn and Sorghum Industry Research Conference. American Seed Trade Association, Washington, DC, pp 82–97 [Google Scholar]
- Schnyder H. (1993) The role of carbohydrates storage and redistribution in the source-sink relations of wheat and barley during grain filling: a review. New Phytol 123: 233–245 [Google Scholar]
- Sicher RC, Barnaby JY (2012) Impact of carbon dioxide enrichment on the responses of maize leaf transcripts and metabolites to water stress. Physiol Plant 144: 238–253 [DOI] [PubMed] [Google Scholar]
- Silvente S, Sobolev AP, Lara M (2012) Metabolite adjustments in drought tolerant and sensitive soybean genotypes in response to water stress. PLoS One 7: e38554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub DR, Miller B, Allen H (2008) Effects of elevated CO2 on the protein concentration of food crops: a meta-analysis. Glob Change Biol 14: 565–575 [Google Scholar]
- Taylor JRN, Schober TJ, Bean SR (2006) Novel food and non-food uses of sorghum and millets. J Cereal Sci 44: 252–271 [Google Scholar]
- Tilman D, Balzer C, Hill J, Befort BL (2011) Global food demand and the sustainable intensification of agriculture. Proc Natl Acad Sci USA 108: 20260–20264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsogtbaatar E, Cocuron JC, Sonera MC, Alonso AP (2015) Metabolite fingerprinting of pennycress (Thlaspi arvense L.) embryos to assess active pathways during oil synthesis. J Exp Bot 66: 4267–4277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uprety DC, Sen S, Dwivedi N (2010) Rising atmospheric carbon dioxide on grain quality in crop plants. Physiol Mol Biol Plants 16: 215–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez V, Deshpande SP, Kholova J, Hammer GL, Borrell AK, Talwar HS, Hash CT (2011) Stay-green quantitative trait loci’s effects on water extraction, transpiration efficiency and seed yield depend on recipient parent background. Funct Plant Biol 38: 553–566 [DOI] [PubMed] [Google Scholar]
- Wall GW, Brooks TJ, Adam NR, Cousins AB, Kimball BA, Pinter PJ Jr, LaMorte RL, Triggs J, Ottman MJ, Leavitt SW, et al. (2001) Elevated atmospheric CO2 improved Sorghum plant water status by ameliorating the adverse effects of drought. New Phytol 152: 231–248 [Google Scholar]
- Wenzel A, Frank T, Reichenberger G, Herz M, Engel KH (2015) Impact of induced drought stress on the metabolite profiles of barley grain. Metabolomics 11: 454–467 [Google Scholar]
- Xia J, Mandal R, Sinelnikov IV, Broadhurst D, Wishart DS (2012) MetaboAnalyst 2.0: a comprehensive server for metabolomic data analysis. Nucleic Acids Res 40: W127–W133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Psychogios N, Young N, Wishart DS (2009) MetaboAnalyst: a web server for metabolomic data analysis and interpretation. Nucleic Acids Res 37: W652–W660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Sinelnikov IV, Han B, Wishart DS (2015) MetaboAnalyst 3.0: making metabolomics more meaningful. Nucleic Acids Res 43: W251–W257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinta G, AbdElgawad H, Domagalska MA, Vergauwen L, Knapen D, Nijs I, Janssens IA, Beemster GTS, Asard H (2014) Physiological, biochemical, and genome-wide transcriptional analysis reveals that elevated CO2 mitigates the impact of combined heat wave and drought stress in Arabidopsis thaliana at multiple organizational levels. Glob Change Biol 20: 3670–3685 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.