Unlike Arabidopsis, ectopic expression of the transcription factor WRINKLED1 involved in oil accumulation causes cell death in Brachypodium distachyon due to the distinct fatty acid metabolism of this grass.
Abstract
Triacylglycerol (TAG) is a storage lipid used for food purposes and as a renewable feedstock for biodiesel production. WRINKLED1 (WRI1) is a transcription factor that governs fatty acid (FA) synthesis and, indirectly, TAG accumulation in oil-storing plant tissues, and its ectopic expression has led to TAG accumulation in vegetative tissues of different dicotyledonous plants. The ectopic expression of BdWRI1 in the grass Brachypodium distachyon induced the transcription of predicted genes involved in glycolysis and FA biosynthesis, and TAG content was increased up to 32.5-fold in 8-week-old leaf blades. However, the ectopic expression of BdWRI1 also caused cell death in leaves, which has not been observed previously in dicotyledonous plants such as Arabidopsis (Arabidopsis thaliana). Lipid analysis indicated that the free FA content was 2-fold elevated in BdWRI1-expressing leaf blades of B. distachyon. The transcription of predicted genes involved in β-oxidation was induced. In addition, linoleic FA treatment caused cell death in B. distachyon leaf blades, an effect that was reversed by the addition of the FA biosynthesis inhibitor cerulenin. Taken together, ectopic expression of BdWRI1 in B. distachyon enhances FA biosynthesis and TAG accumulation in leaves, as expected, but also leads to increased free FA content, which has cytotoxic effects leading to cell death. Thus, while WRI appears to ubiquitously affect FA biosynthesis and TAG accumulation in diverse plants, its ectopic expression can lead to undesired side effects depending on the context of the specific lipid metabolism of the respective plant species.
Triacylglycerol (TAG) derived from seed oil is used as a vegetable oil but can also serve as a renewable source for biofuels and chemicals with industrial applications (Durrett et al., 2008). An extensive knowledge of TAG biosynthesis pathways and its molecular regulation has been developed as reviewed previously (Santos-Mendoza et al., 2008; Baud and Lepiniec, 2010; Bates et al., 2013). Arabidopsis (Arabidopsis thaliana) WRINKLED1 (AtWRI1; At3g54230) encodes a transcription factor with two APETALA2 (AP2) DNA-binding domains and promotes the conversion of sugars to oil by directly activating genes encoding proteins involved in plastid glycolysis and fatty acid (FA) synthesis (Baud et al., 2009; Maeo et al., 2009; Fukuda et al., 2013). The Arabidopsis wri1 mutant has an 80% reduction of seed oil content (Focks and Benning, 1998), whereas overexpression of WRI1 increases seed oil content by 10% to 20% (Cernac and Benning, 2004). WRI1 orthologs from maize (Zea mays), rape (Brassica napus), and oil palm (Elaeis guineensis) have been shown to be involved in TAG production in embryos or fruit mesocarps, and their overexpression leads to increased seed oil content (Liu et al., 2010; Shen et al., 2010; Pouvreau et al., 2011; Ma et al., 2013; van Erp et al., 2014). Ectopic expression of WRI1 has also been used to stimulate oil production in nonseed tissues in both Arabidopsis and tobacco (Nicotiana tabacum; Cernac and Benning, 2004; Sanjaya et al., 2011; Dussert et al., 2013; Kelly et al., 2013; Vanhercke et al., 2014; Grimberg et al., 2015; Ma et al., 2015).
During seed germination, the oxidation of FAs derived from TAGs provides energy for early seedling development (Graham, 2008). In addition to essential structural and nutritional functions, FAs and its derivatives also have many signaling functions. For instance, FAs are important signals in defense responses (Farmer et al., 1998; Kachroo et al., 2003; Chandra-Shekara et al., 2007). Very-long-chain FAs play a role in cell differentiation in Arabidopsis with their effect on polar auxin distribution (Roudier et al., 2010). Thus, overaccumulation of free FAs is expected to be detrimental to tissues. For example, exogenous and endogenous FAs are known to inhibit coleoptile elongation of oat (Avena fatua; Ando and Tsukamotoa, 1981; Ohkawa and Nishikawa, 1987), longitudinal cell growth of Arabidopsis (Li et al., 2011), axillary bud growth of tobacco (Tso, 1964), seedling growth of rice (Oryza sativa; Tsuzuki et al., 1987), and germination of lettuce (Lactuca sativa), oat, and mustard (Sinapsis alba; Le Poidevin, 1965; Berrie, 1979; Stewart and Berrie, 1979; Metzger and Sebesta, 1982). Furthermore, the accumulation of unsaturated FAs has been linked to cell death. Palmitoleic acid is reported to be an inducer of programmed cell death in tobacco and eggplant (Solanum melongena) protoplasts (Peters and Chin, 2005, 2007). Palmitoleic acid and linolenic acid cause the inhibition of photosynthetic electron transport, loss of thylakoid proteins, and release of cytochrome f in isolated chloroplasts from spinach (Spinacia oleracea) and eggplant (Golbeck and Warden, 1984; Warden and Csatorday, 1987; Peters and Chin, 2003). Increase of free FAs is the primary cause of chloroplast membrane damage and chlorophyll degradation and leads to necrosis when the Arabidopsis β-oxidation mutant peroxisomal ABC-transporter1 is subjected to prolonged darkness (Kunz et al., 2009; Slocombe et al., 2009). Additionally, a recent study suggests that the proteins LIPIN, PHOSPHOLIPID:DIACYLGLYCEROL ACYLTRANSFERASE1 (PDAT1), and SUGAR-DEPENDENT1 (SDP1) function synergistically in Arabidopsis to protect plants against FA-induced cell death (Fan et al., 2013, 2014).
Brachypodium distachyon is an experimental model for temperate grasses and belongs to the family Poaceae (Draper et al., 2001; Opanowicz et al., 2008; Brkljacic et al., 2011). Recently, engineering the accumulation of TAG in vegetative tissues has been proposed for increasing the energy density of biomass crops (Ohlrogge and Chapman, 2011). Although significant investments have been made in developing and using B. distachyon as a model for biofuel crops (Brkljacic et al., 2011), little is known about its lipid metabolism pathways. In this study, we identified a WRI1 ortholog of B. distachyon. Its role in FA biosynthesis and TAG accumulation in both storage and vegetative tissues was investigated. Furthermore, a cell death phenotype was observed in transgenic B. distachyon following ectopic expression of WRI1, suggesting that cytotoxic effects of free FAs might be the cause.
RESULTS
Overexpression of BdWRI1 Increases Grain TAG Content in B. distachyon
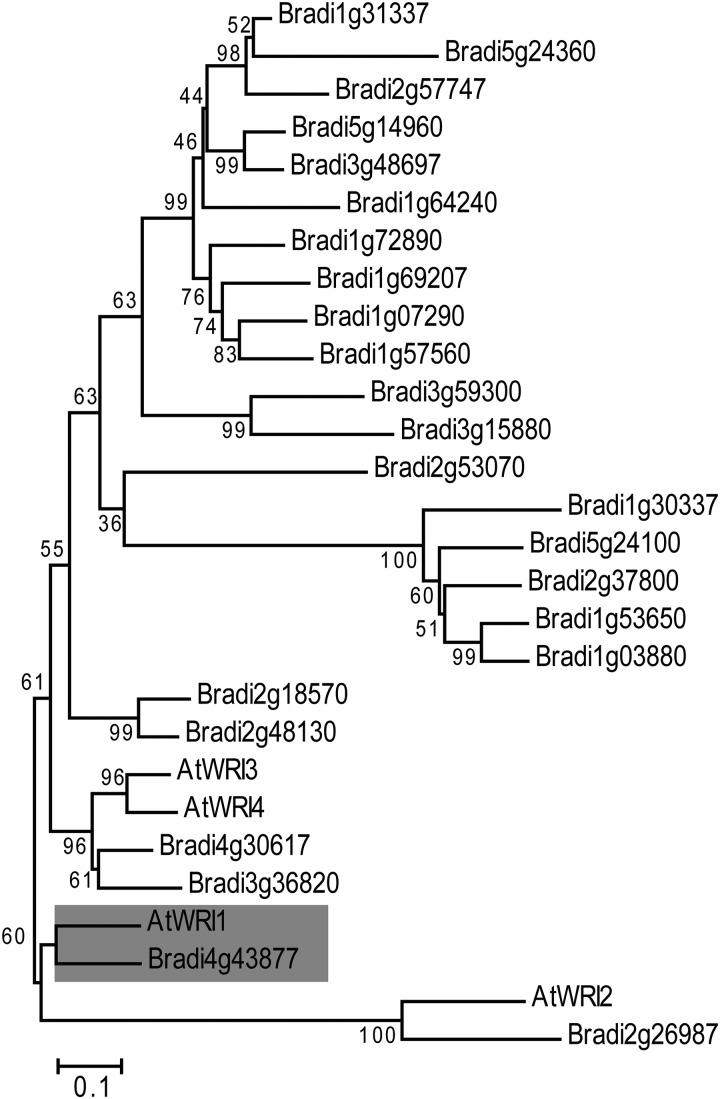
To identify possible WRI1 orthologs in B. distachyon, a BLASTP analysis was performed with an e-value threshold of 1.0E-10 in Phytozome (http://www.phytozome.net/). The Arabidopsis WRI1 sequence was used in a query against the B. distachyon protein database, and 24 AP2 domain-containing proteins were identified. Phylogenetic analysis by MEGA6 (Tamura et al., 2013) showed that the protein encoded by Bradi4g43877 was the closest ortholog to AtWRI1 (Fig. 1). We tentatively designated Bradi4g43877 as BdWRI1. Further alignment of AtWRI1 and BdWRI1 revealed that these two proteins share 76% sequence identity over 420 amino acids covering the full length of BdWRI1. In addition, the sequence VYL present in the first AP2 domain of AtWRI1, which is essential for its function (Ma et al., 2013), was also conserved in BdWRI1 (Supplemental Fig. S1).
Figure 1.
Phylogenetic tree of Arabidopsis WRI1 homologs and presumed B. distachyon AP2 domain-containing WRI1 orthologs. A neighbor-joining tree was generated using all full-length B. distachyon AP2 domain-containing protein sequences that are presumed orthologs of the WRI1 proteins from Arabidopsis. The sequences were aligned with ClustalW. An unrooted phylogenetic tree was constructed with MEGA6 using the neighbor-joining method, and the bootstrap values were derived from 1,000 replicates. The gray box indicates AtWRI1 and its presumed ortholog in B. distachyon.
To obtain in planta experimental verification that BdWRI1 is a true ortholog, we overexpressed BdWRI1 in B. distachyon under the control of the maize ubiquitin promoter (ZmUBI1). Ten independent transgenic B. distachyon lines were generated that harbored the BdWRI1 overexpression construct. Two lines (2A and 5A; Fig. 2A) were found to have high ectopic WRI1 expression relative to wild-type Bd21-3 and were selected for further analysis.
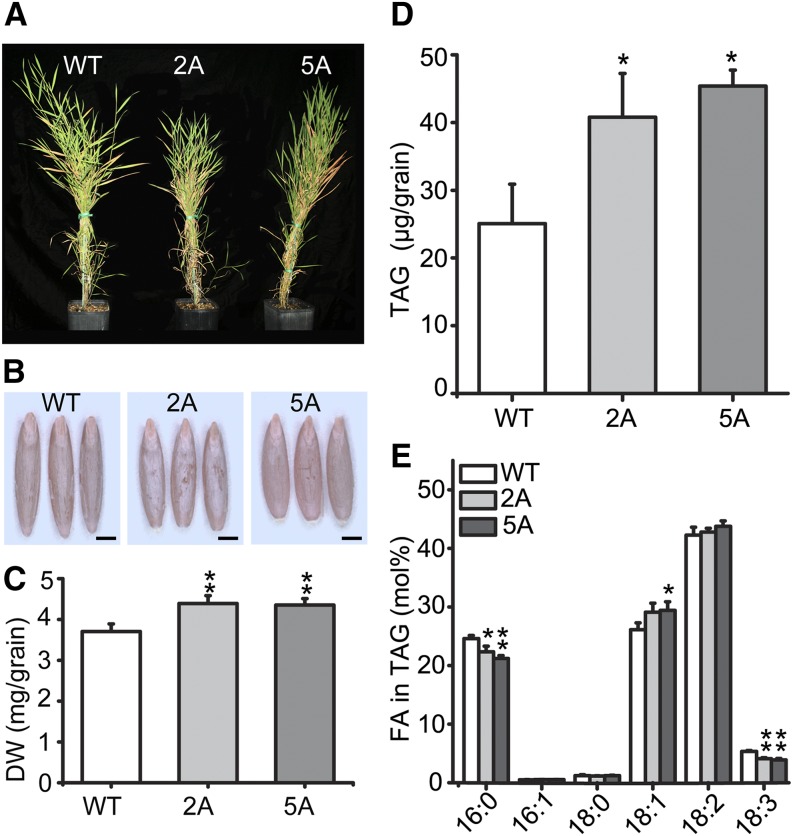
Figure 2.
Overexpression of BdWRI1 increases grain TAG content. A, Phenotypes of 15-week-old UBI::BdWRI1 plants. B, Phenotypes of UBI::BdWRI1 grains without lemma. Bars = 1 mm. C, Dry weights (DW) of UBI::BdWRI1 grains. Each sample contained 15 grains. Data represent three independent measurements, and the error bars represent sd. Asterisks indicate significant differences by Student’s t test: **, P ≤ 0.01. D and E, TAG contents (D) and FA composition of TAG (E) from dry grains of UBI::BdWRI1 and Bd21-3. Total lipids were extracted from the grains. Each sample contained five grains. Three independent measurements were averaged, and the error bars represent sd. Asterisks indicate significant differences by Student’s t test: *, P < 0.05; and **, P ≤ 0.01. WT, Wild type.
Since AtWRI1 overexpression affects seeds of Arabidopsis (Cernac and Benning, 2004), we tested whether the overexpression of BdWRI1 could influence grain development and TAG accumulation in B. distachyon. The grain dry weights were increased approximately 20% compared with Bd21-3 (Fig. 2, B and C). To further determine the effect of BdWRI1 on storage oil accumulation, total lipid was extracted from dry grains and FA methylesters were analyzed by gas chromatography. The UBI::WRI1 lines 2A and 5A showed considerably higher TAG content, 60% and 80% higher (40.8 ± 6.5 and 45.4 ± 2.4 μg grain−1), respectively, compared with wild-type Bd21-3 (25.1 ± 5.8 μg grain−1; Fig. 2D). The acyl group profiles of grain TAGs changed slightly, but statistically significantly, with slight decreases in 16:0 (carbon:double bonds) and 18:3 and an increase in 18:1 (Fig. 2E).
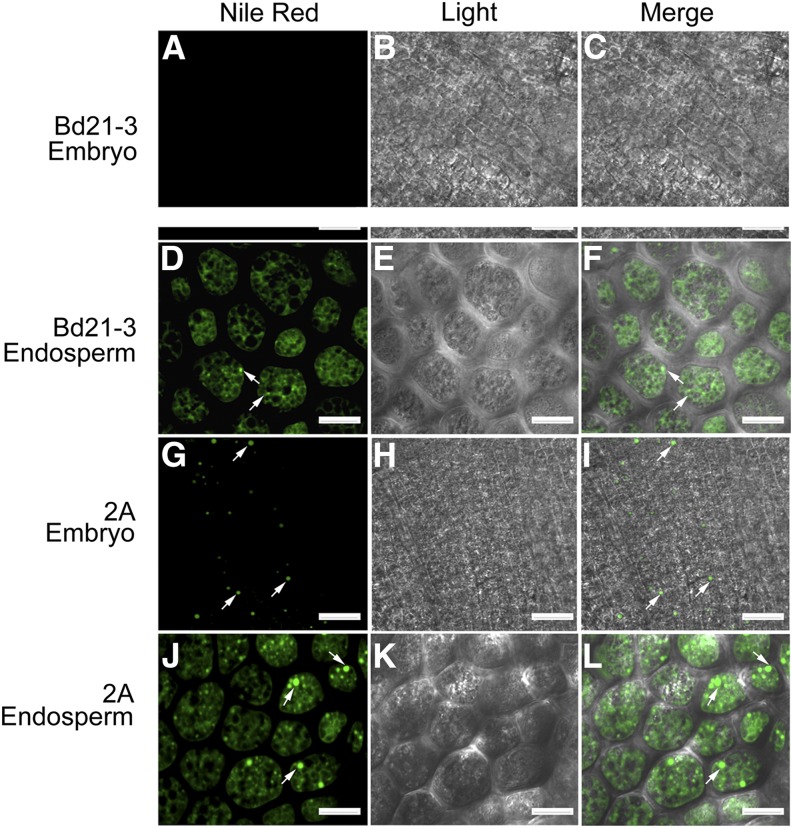
To determine the tissue of grains in which TAG is sequestered into lipid droplets, dry grains of Bd21-3, together with the UBI::BdWRI 2A and 5A lines, were stained with Nile Red and observed by confocal microscopy. In Arabidopsis, most of the storage TAGs accumulate in the embryo, whereas endosperm stores only 10% of the seed TAG (Penfield et al., 2004; Miquel et al., 2014). However, in B. distachyon Bd21-3 grains, most of the storage lipid apparently accumulates in the endosperm, indicated by the presence of lipid droplets (Fig. 3, D–F), while no obvious lipid droplets were observed in the embryo (Fig. 3, A–C). Similar to oat grains (Heneen et al., 2008), most of the lipid droplets in the starchy endosperm were fused with each other, forming a continuous lipid matrix between the protein and starch components. Only a few discrete lipid droplets were observed in the endosperm (Fig. 3, D–F). In contrast, the number of lipid droplets was higher in the endosperm of UBI::BdWRI1 line 2A (Fig. 3, J–L) and line 5A (Supplemental Fig. S2, D–F) compared with the wild type (lipid droplets per cell of 10 cells examined: wild type, 2.5 ± 1; 2A, 4.1 ± 1; and 5A, 7.6 ± 2.8), but lipid droplet size was not statistically different. Taken together, similar to AtWRI1 (Cernac and Benning, 2004; Cernac et al., 2006), BdWRI1 also seems to affect TAG biosynthesis in storage tissues, in the case of B. distachyon in the embryo and endosperm.
Figure 3.
Lipid droplets are abundant in embryo and endosperm of UBI::BdWRI1 dry grains. A to F, Confocal fluorescence images of dry grains of Bd21-3 showing few lipid droplets (arrows) in the endosperm. G to L, Confocal fluorescence micrographs of dry grains of UBI::BdWRI1 line 2A. Lipid droplets were observed in both embryo and endosperm. Bars = 20 μm.
Ectopic Expression of BdWRI1 Leads to TAG Accumulation in Leaf Blades
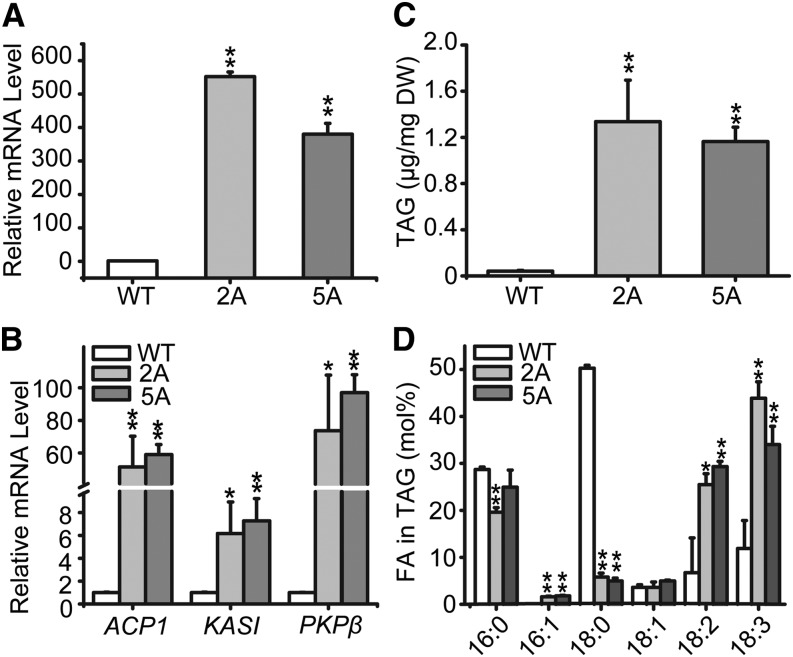
To explore whether the presence of BdWRI1 in vegetative tissues enhances TAG accumulation, the abundance of BdWRI1 mRNA in the transgenic leaf blades was determined by quantitative real-time (RT)-PCR using UBIQUITIN-CONJUGATING ENZYME18 (BdUBC18, Bradi4g00660) as an internal control (Hong et al., 2008). In the UBI::WRI1 lines, transcription levels of WRI1 in 8-week-old plants were 552- and 380-fold higher than in Bd21-3 (Fig. 4A). To test whether BdWRI1 affects the metabolism required for FA biosynthesis in vegetative tissues, presumed orthologs of known WRI1 target genes in dicotyledonous plants (Maeo et al., 2009), such as PLASTIDIC PYRUVATE KINASE β-SUBUNIT1 (PKPβ1), ACYL-CARRIER PROTEIN1 (ACP1), and KETOACYL-ACYL-CARRIER PROTEIN SYNTHASE I (KASI), were identified in the B. distachyon protein database. When normalized to Bd21-3, the mRNA levels of PKPβ1 (Bradi2g45620) and BdACP1 (Bradi1g01000) were enhanced 74- and 51-fold, respectively, whereas BdKASI (Bradi1g46610) expression increased 6.2-fold (Fig. 4B). Taken together, ectopic expression of BdWRI1 led to the induction of presumed orthologs of genes involved in FA biosynthesis in B. distachyon leaf blades.
Figure 4.
Ectopic expression of BdWRI1 increases TAG content in B. distachyon leaf blades. A and B, Expression levels of BdWRI1 (A) glycolysis and FA biosynthesis orthologs (B) in 8-week-old leaf blades. Total RNA was isolated, and relative mRNA levels were determined by quantitative RT-PCR using BdUBC18 as an internal control. Three independent measurements were averaged, and the error bars represent sd. Asterisks indicate significant differences by Student’s t test: *, P < 0.05; and **, P ≤ 0.01. C and D, TAG contents (C) and FA composition of TAG (D) of 8-week-old leaf blades. Total lipids were extracted, and TAG was separated by thin-layer chromatography (TLC). Three independent measurements were averaged, and the error bars represent sd. Asterisks indicate significant differences by Student’s t test: *, P < 0.05; and **, P ≤ 0.01. DW, Dry weight; WT, wild type.
In addition, TAG content in 8-week-old leaf blades was increased. As shown in Figure 4C, TAG levels in transgenic lines 2A and 5A were 32.5- and 30-fold higher (1.3 ± 0.4 and 1.2 ± 0.1 μg mg−1 dry weight) than in Bd21-3 (0.04 ± 0.01 μg mg−1 dry weight). The ectopic expression of BdWRI1 also resulted in an increase in polyunsaturated FAs at the expense of saturated FAs in TAGs (Fig. 4D), which could be derived from membrane lipids that are being turned over.
It should also be noted that an increased number of lipid droplets was observed in stem internodes of UBI::BdWRI1 transgenic B. distachyon (Supplemental Fig. S3), suggesting that TAG accumulation is stimulated in different vegetative tissues of the transgenic plants.
Ectopic Expression of BdWRI1 in B. distachyon Causes Cell Death in Leaf Blades
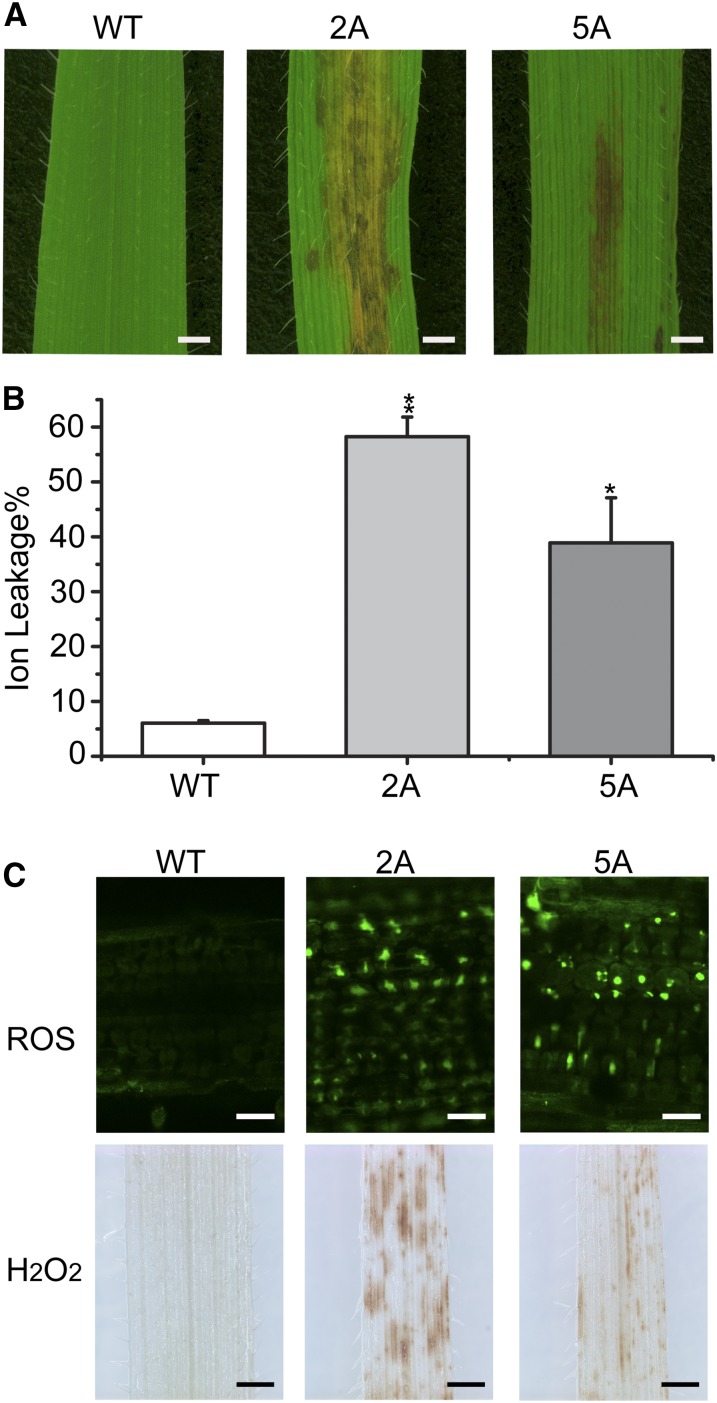
In addition to TAG accumulation, browning of leaf blades due to premature cell death was observed in the vegetative tissues of UBI::WRI1 transgenic plants. Leaf necrotic lesions were first visible in 4-week-old transgenic lines and expanded rapidly during development, whereas Bd21-3 leaves remained green and healthy looking (Fig. 5A). A first sign of the readily ascertainable consequence of cell death in plant tissue is increased membrane permeability measured by increased electrolyte leakage, which can be used to quantify cell death (Baker and Orlandi, 1995; Dellagi et al., 1998; Torres et al., 2002; Hofius et al., 2009). Measurement of percentage of electrolyte leakage in 8-week-old detached leaves was up to 9.3-fold increased in UBI::BdWRI1 leave blades (Fig. 5B). It should be mentioned that constitutive expression of AtWRI1 in B. distachyon under the control of the ZmUBI1 promoter also led to brown lesions and reactive oxygen species (ROS) accumulation (Supplemental Fig. S4), which suggests that cell death in UBI::BdWRI1 plants is caused by the species context rather than by functional differences between AtWRI1 and BdWRI1. However, it should be noted that ectopic expression of AtWRI1 in Arabidopsis did not cause visible cell death or electrolyte leakage under comparable conditions (Supplemental Fig. S5).
Figure 5.
Premature cell death in the leaf blades of UBI::BdWRI1 lines. A, Phenotypes of 8-week-old UBI::BdWRI1 leaf blades. Bars = 1.25 mm. B, Quantification of cell death by electrolyte leakage assay. Eight-week-old detached leaf blades were inoculated with water for 3 h, and then the conductivity of the water was measured. Each value represents the mean and sd of three replicates per experiment. Asterisks indicate significant differences by Student’s t test: *, P < 0.05; and **, P ≤ 0.01. C, ROS accumulation in 8-week-old leaf blades of UBI::BdWRI1 lines and the wild type (WT). General ROS accumulation was detected by H2DCF-DA staining (top; bars = 20 μm), and H2O2 accumulation was detected by DAB staining (bottom; bars = 1 mm).
ROS including hydrogen peroxide (H2O2), superoxide, singlet oxygen, and hydroxyl radicals have been closely associated with plant cell death (del Rio et al., 1998; Apel and Hirt, 2004; Danon et al., 2005; Schmitt et al., 2014). ROS not only cause oxidative damage to cellular constituents, they also act as signals that play a crucial role in the activation of processes leading to cell death (Apel and Hirt, 2004; Baxter et al., 2014; Schmitt et al., 2014). To test if ROS accumulate in UBI::BdWRI1 leaves, 2′,7′-dichlorofluorescein diacetate acetyl ester (H2DCF-DA) was used as an indicator (Schmitt et al., 2014). ROS-dependent fluorescence of H2DCF-DA in leaf tissues was measurably stronger in UBI::BdWRI1 lines (Fig. 5C, top). In addition, 3-diaminobenzidine (DAB) was used as an indicator of H2O2 accumulation (Fryer et al., 2002; Ramel et al., 2009; Schmitt et al., 2014). Widespread DAB staining was seen in UBI::BdWRI1 leaves even in the areas that did not yet have brown lesions (Fig. 5C, bottom). Taken together, the ectopic expression of BdWRI1 causes cell death in the leaf tissues of B. distachyon, which is likely preceded by an increase in ROS.
Free FAs Increased following BdWRI1 Ectopic Expression
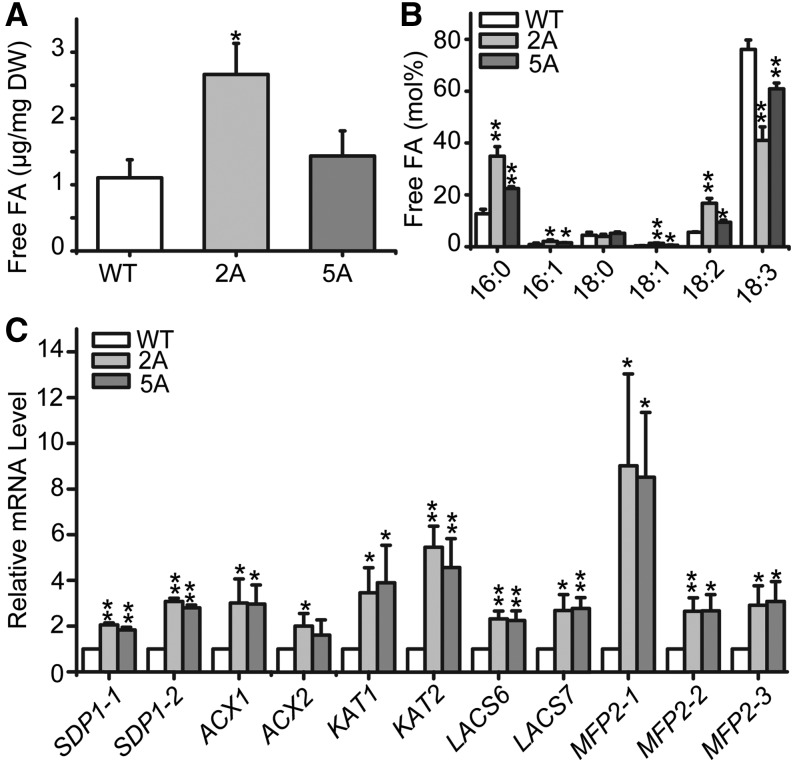
In all previously reported cases of ectopic expression of WRI1 to induce the accumulation of TAG in vegetative tissues (Cernac and Benning, 2004; Sanjaya et al., 2011; Vanhercke et al., 2013, 2014), cell death has never been observed. These studies were all done with dicotyledonous plants. Thus, we focused on determining the possible mechanisms of cell death in UBI::BdWRI1 lines, as this might reveal differences in lipid metabolism and regulation between dicotyledonous and monocotyledonous plants. The accumulation of free FAs is known to have cytotoxic effects not only in yeast and mammalian cells (Garbarino et al., 2009; Kohlwein, 2010; Lee et al., 2010; Fakas et al., 2011) but also in microalgae and plants (Wu et al., 2006; Bosma et al., 2008; Fan et al., 2013, 2014). Since the expression of several presumed orthologs of genes involved in FA biosynthesis was highly induced in UBI::BdWRI1 plants (Fig. 4B), possibly resulting in increased free FA levels, we asked whether the accumulation of free FAs was a factor in the observed induction of cell death. As shown in Figure 6A, free FA contents in 8-week-old leaf blades of UBI::BdWRI1 plants were up to 2-fold increased. In contrast, ectopic expression of AtWRI1 in Arabidopsis did not lead to substantial changes in free FAs (Supplemental Fig. S6B), indicating that free FAs might be the cause of species-specific cell death in B. distachyon ectopically expressing WRI1.
Figure 6.
Elevated free FA levels in UBI::BdWRI1 leaf blades. A and B, Free FA contents (A) and FA composition (B) in 8-week-old leaf blades. Total lipids were extracted, and free FAs were separated by TLC. Three independent measurements were averaged, and the error bars represent sd. Asterisks indicate significant differences by Student’s t test: *, P < 0.05; and **, P ≤ 0.01. C, Expression of genes predicted to encode proteins involved in TAG degradation in 8-week-old leaf blades. Total RNA was isolated, and relative mRNA levels were determined by quantitative RT-PCR using BdUBC18 as an internal control. Three independent measurements were averaged, and the error bars represent sd. Asterisks indicate significant differences by Student’s t test: *, P < 0.05; and **, P ≤ 0.01. DW, Dry weight; WT, wild type.
The majority of the free FAs in UBI::BdWRI1 leaves were found to be 16:0, 18:2, and 18:3 (Fig. 6B), which corresponded to the FA composition of leaf TAG (Fig. 4D). Therefore, we reasoned that free FAs might be derived from TAG turnover. To examine the origin of free FAs in these lines, the expression of possible orthologs encoding enzymes involved in the degradation of TAGs was analyzed by RT-PCR. Arabidopsis SDP1 encodes a TAG lipase that catalyzes the initial step in TAG breakdown (Eastmond, 2006; Padham et al., 2007), while the β-oxidation pathway subsequently breaks down the released FAs (Goepfert and Poirier, 2007). As shown in Figure 6C, both presumed orthologs of SDP1 (BdSDP1-1 [Bradi2g50610] and BdSDP1-2 [Bradi1g04310]) and presumed β-oxidation pathway genes, such as ACYL-CoA OXIDASE1 (ACX1; Bradi1g52320), ACX2 (Bradi4g14090), KETOACYL-CoA THIOLASE1 (KAT1; Bradi3g27960), KAT2 (Bradi3g55420), LONG-CHAIN ACYL-CoA SYNTHEASE6 (LACS6; Bradi4g26610), LACS7 (Bradi4g42950), and MULTIFUNCTIONAL PROTEIN2 (MFP2; BdMFP2-1 [Bradi2g43020], BdMFP2-2 [Bradi4g28310], and BdMFP2-3 [Bradi4g28310]), were induced, indicating that ectopic expression of BdWRI1 also might cause accelerated TAG turnover. As H2O2 is a by-product of β-oxidation (Graham and Eastmond, 2002; Eastmond, 2007), the observed induction of the β-oxidation pathway raises the possibility that the accumulation of H2O2 in UBI::BdWRI1 plants may partially be caused by increased TAG turnover.
Application of Free FAs to B. distachyon Leaf Blades Leads to Cell Death
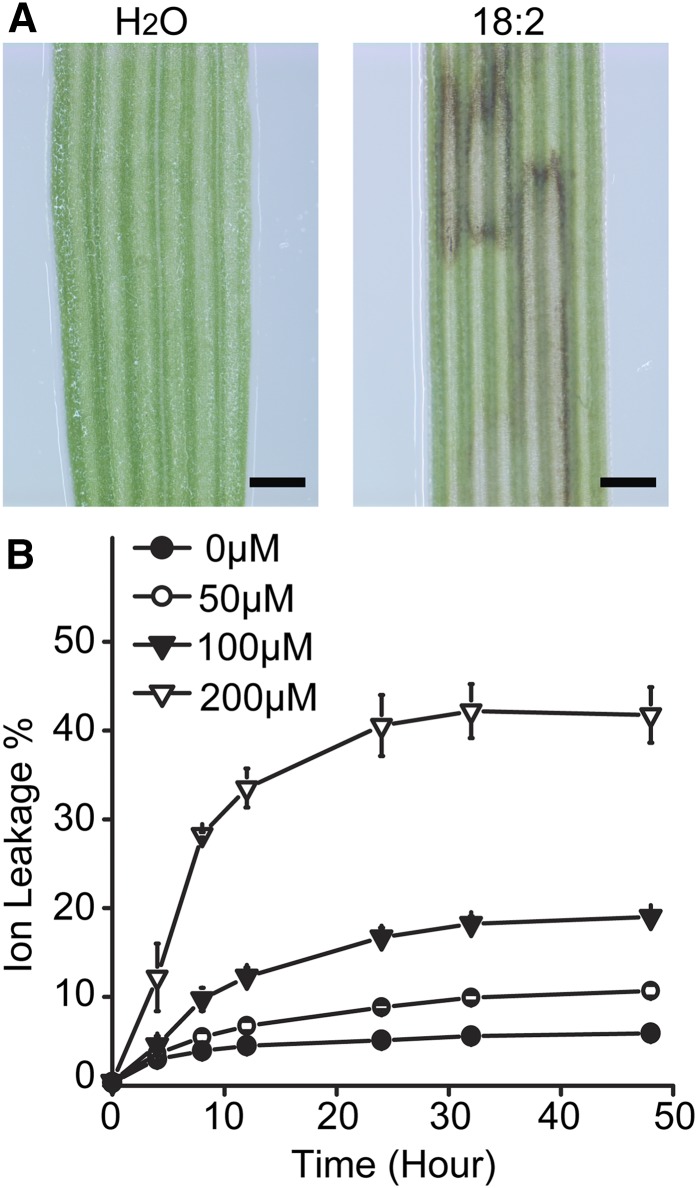
To test whether increased free FAs could be linked to cell death in UBI::BdWRI1 plants, we examined the effects of free FA application to leaves of Bd21-3. Because in UBI::BdWRI1 plants 18:2 and 16:0 were the most increased free FAs (Fig. 6B), 3-week-old detached Bd21-3 leaf blades were inoculated with 200 μm 18:2. After 48 h of treatment, brown lesions were observed (Fig. 7A), which resembled those present in UBI::BdWRI1 plants (Fig. 5A). In addition, externally applied 16:0 also caused brown lesions in Bd21-3 leaves (Supplemental Fig. S7). But because of the low solubility of 16:0, 18:2 was chosen for the following experiments.
Figure 7.
18:2 Treatment causes cell death in B. distachyon leaf blades. A, Phenotypes of wild-type Bd21-3 detached leaf blades treated with 200 μm 18:2 for 48 h. Bars = 0.5 mm. B, Quantification of electrolyte leakage as an indicator for cell death during 18:2 treatment. Three-week-old detached leaf blades were inoculated with water containing different concentrations of 18:2, and conductivity changes were measured at different time points. Each value represents the mean and sd of three replicates per experiment.
To further investigate the influence of free FAs on cell death, 3-week-old Bd2-3 leaves were treated with 0, 50, 100, or 200 μm 18:2. Electrolyte leakage was monitored throughout a 48-h period. As shown in Figure 7B, the 18:2-treated leaves showed increases in electrolyte leakage, which were highest at 200 μm at every time point. Therefore, B. distachyon is sensitive to 18:2 at low concentrations and in a time-dependent manner.
Inhibition of FA Biosynthesis Decreases 18:2-Induced Cell Death in Leaf Blades
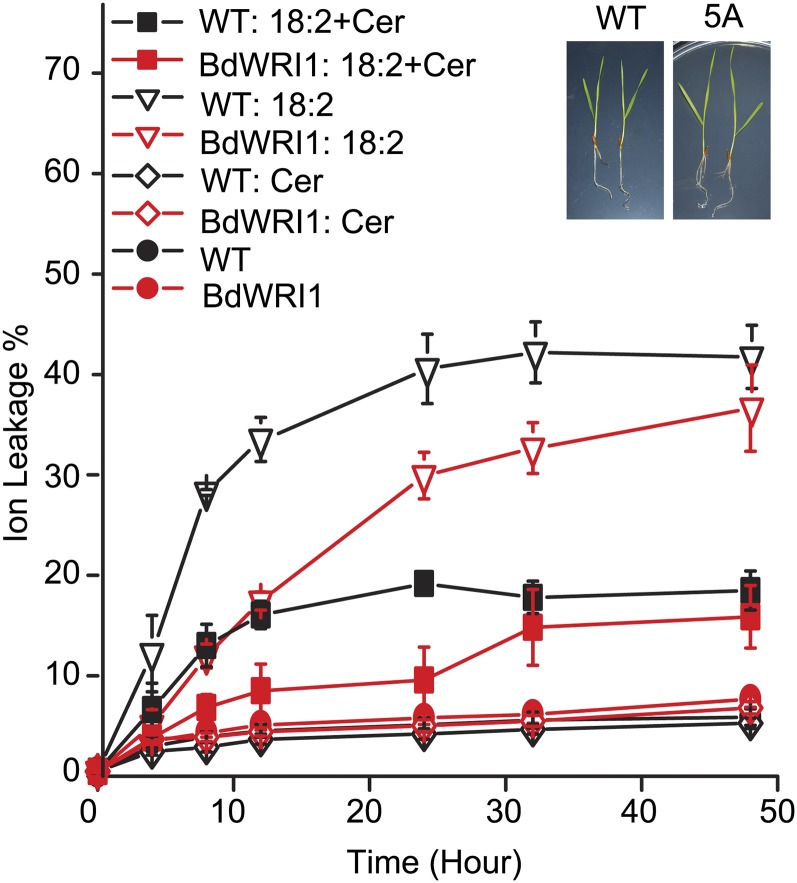
Because WRI1 directly activates genes encoding proteins involved in FA synthesis (Baud et al., 2009; Maeo et al., 2009) and ectopic expression of BdWRI1 induced free FA accumulation, appearing to cause premature cell death in vegetative tissues, we postulated that an inhibition of FA biosynthesis in UBI::BdWRI1 plants might reduce free FA levels in vivo, thus ameliorating the observed cell death. To test this hypothesis, detached 3-week-old leaf blades not yet showing lesions in the transgenic lines were treated with 200 μm 18:2 in the presence or absence of 5 μm cerulenin, a specific inhibitor of KAS I and KAS II, which are key enzymes of de novo FA biosynthesis (Awaya et al., 1975; Packter and Stumpf, 1975; Koo et al., 2005). The extent of cell death was evaluated by electrolyte leakage measurements. During all the treatments without 18:2, ion leakage stayed at a low level during 48 h. When 200 μm 18:2 was added to either UBI::BdWRI1 plants or Bd21-3, the ion leakage increased, whereas the addition of 5 μm cerulenin strongly inhibited 18:2-induced cell death (Fig. 8), which demonstrated that premature cell death in leaf blades of UBI::BdWRI was at least partially caused by increased free FAs.
Figure 8.
Cerulenin strongly inhibits 18:2-induced electrolyte leakage as an indicator for cell death in B. distachyon leaf blades. Detached 3-week-old leaf blades were inoculated with water containing 200 μm 18:2 and 5 μm cerulenin (Cer) for 48 h. Conductivity changes were measured at different time points. Each value represents the mean and sd of three replicates per experiment. Three-week-old Bd21-3 and UBI::BdWRI1 seedlings not yet showing the cell death phenotype are shown in the insets. WT, Wild type.
DISCUSSION
WRI1 Has a Conserved Function in FA Biosynthesis and TAG Accumulation
In the Arabidopsis wri1 mutant, TAG contents in embryo and endosperm were reduced (Cernac et al., 2006). Conversely, we found that the number of lipid droplets was higher in embryo and endosperm of UBI::BdWRI1 expressing B. distachyon (Fig. 3; Supplemental Fig. S2). Thus, WRI1 has a conserved function during storage TAG accumulation in seed tissues. It should also be noted that the structural features of lipid droplets in the B. distachyon endosperm changed when BdWRI1 was overexpressed. Most of the lipid droplets tended to fuse with each other in Bd21-3 (Fig. 3, D–F). In contrast, more discrete lipid droplets were observed in the UBI::BdWRI1 lines (Fig. 3, J–L; Supplemental Fig. S2, D–F). Coalescence of the lipid droplets has also been observed in oat endosperm (Heneen et al., 2008), probably related to the amount of lipid droplet-associated proteins, such as OLEOSIN, present in the tissue.
Furthermore, in addition to the increased TAG content in leaf blades, ectopic expression of BdWRI1 also increased the number and size of lipid droplets in stem internodes (Supplemental Fig. S3), suggesting that this approach enhances TAG accumulation in all vegetative tissues of B. distachyon. These results are consistent with previous studies conducted in dicotyledonous plants (Cernac and Benning, 2004; Sanjaya et al., 2011; Dussert et al., 2013; Kelly et al., 2013; Vanhercke et al., 2013, 2014; Grimberg et al., 2015) and confirm that BdWRI1 has a conserved function in regulating FA and, indirectly, TAG biosynthesis and that ectopic production of WRI1 serves as a promising tool for enhancing the energy density in vegetative tissues of plants.
Although TAG content increased in both storage and vegetative tissues of UBI::BdWRI1 plants, the FA composition of TAGs in these tissues differed notably. In grain TAGs, approximately 30% of the FAs were de novo synthesized 18:1, whereas 18:3 was less than 6% (Fig. 2E). In striking contrast, the most abundant FA in leaf TAG was 18:3 (nearly 45% of the FAs in TAG), while 18:1 made up only 4% (Fig. 4D). Plants can use alternative routes to produce TAGs: first, TAG assembly from newly synthesized FAs by the Kennedy pathway, which generates TAGs rich in 18:1; and second, from intermediates such as phosphatidylcholine (PtdCho) subjected to acyl-chain editing, which provides polyunsaturated FAs or diacylglycerol rich in polyunsaturated FAs for TAG assembly (Bates and Browse, 2011, 2012; Bates et al., 2012, 2013). Relative fluxes through these pathways vary widely depending on plant species, tissues, and developmental stages (Bates and Browse, 2012), and they are still unclear for B. distachyon. However, the current results provide evidence that, in B. distachyon, TAG synthesis in storage tissues may rely more on the Kennedy pathway, while vegetative tissues may be more dependent on acyl editing and PtdCho-derived precursors to produce polyunsaturated FA-containing TAGs.
Functional Divergence of Lipid Metabolism between Monocots and Dicots
Much of our current understanding of plant gene function is based on the dicotyledonous model plant Arabidopsis. Although many proteins show conserved sequences in different plant species, even highly similar proteins can play distinct, although related, roles in a species-specific context. For instance, while grasses and dicotyledonous plants share many orthologs determining inflorescence development, the corresponding grass orthologs display variations in copy numbers, distinct expression patterns, and functional complexity, which are likely associated with the distinct inflorescence morphogenesis in grasses (Zhang and Yuan, 2014). For example, in the blue light signaling pathway, the interaction between CRYPTOCHROME-INTERACTING basic Helix-Loop-Helix1 (CIB) and CRYPTOCHROME (CRY) is evolutionarily conserved. However, the primary function of the CRY2-CIB complex in Arabidopsis is the regulation of flowering time, whereas the regulation of leaf senescence appears to be the major physiological function in soybean (Glycine max; Meng et al., 2013).
Functional divergence between monocotyledonous and dicotyledonous plants is also prominent in plant storage lipid metabolism. For example, oilseed plants such as Arabidopsis and some Brassica spp. have evolved to accumulate large amounts of TAG as energy stores in embryonic tissues, whereas B. distachyon and other Poaceae accumulate large amounts of polysaccharides in endosperm tissues instead (Penfield et al., 2004; Guillon et al., 2012). Despite the fact that WRI1 shows conserved function in FA biosynthesis and oil production in planta, different expression patterns of WRI1 orthologs or additional phenotypes of the respective overexpression or mutant lines have been reported in many species. AtWRI1 is specifically expressed in siliques, whereas oil palm EgWRI1 is highly expressed in mesocarp (Tranbarger et al., 2011; Dussert et al., 2013). The Arabidopsis wri1 mutant does not show any obvious phenotype in nonseed tissues (Focks and Benning, 1998), whereas silencing of WRI1 expression in cotton (Gossypium spp.) resulted in increased fiber length (Qu et al., 2012). Here, we observed that ectopic expression of BdWRI1 caused cell death in B. distachyon (Fig. 5), which is not observed for Arabidopsis (Supplemental Fig. S5).
Taken together, these findings argue that WRI1-dependent regulation of carbon flow from sugars to FAs is an evolutionarily conserved mechanism in plants, while the specific phenotypes of ectopic expression depend on species context.
TAG Turnover Is Increased in Vegetative Tissues of B. distachyon
During the course of this work, we specifically tried to address the species differences between B. distachyon and Arabidopsis leading to cell death only in B. distachyon when WRI1 is ectopically expressed. Since free FAs could be the causal link to the cell death phenotype, the metabolic origin of the free FAs in UBI::BdWRI1 plants may provide the necessary insight. The free FAs accumulating in B. distachyon transgenic plants may derive from at least three possible sources: (1) de novo synthesized FAs that directly enter the free FA pool; (2) recycled FAs derived from PtdCho acyl editing; and (3) free FAs that arise from the enhanced turnover of leaf TAG.
In UBI::BdWRI1 leaf blades, the FA composition of TAG and free FAs was similar, as both contained more than 60% of polyunsaturated FAs, while 18:1 was present at less than 5% (Figs. 4D and 6B), which indicates that most of the free FAs are unlikely from the de novo synthesized FA pool. PtdCho is a major substrate for FA desaturation, and acyl editing of PtdCho is an integral component of eukaryotic glycerolipid synthesis (Bates et al., 2007). Therefore, increased amounts of polyunsaturated free FAs may indicate that the formation of FAs by PtdCho acyl editing is faster than FA utilization for TAG and membrane lipid synthesis.
In addition, extensive experimental evidence indicates that free FAs supplied to tissues are sequestered into TAGs (Roughan et al., 1987; Koo et al., 2005; Tjellström et al., 2015). These TAGs undergo substantial and rapid turnover (Tjellström et al., 2015). Moreover, the expression of many orthologs presumed to encode enzymes involved in TAG degradation was induced, suggesting that this pathway is highly active (Fig. 6C) in UBI::BdWRI plants. In contrast, the composition of free FAs (Supplemental Fig. S6C) and TAG (Supplemental Fig. S6D) was strikingly different, and the TAG degradation pathway was not induced (Supplemental Fig. S6E) in 35S::AtWRI1 Arabidopsis plants. Based on these data and considerations, leaf TAG turnover appears to occur more rapidly in B. distachyon than in Arabidopsis, resulting in the observed increase in free FAs in UBI::BdWRI leaf blades.
Indirect support for the contribution of TAG turnover to the free FA pool was obtained by a delayed effect of 18:2 application-induced cell death in UBI::BdWRI1-expressing B. distachyon leaves (Fig. 8). Because TAG in vegetative tissues can serve as a buffer for FAs and, thereby, protect against free FA-induced cell death (Kunz et al., 2009; Fan et al., 2013), increased TAG accumulation in UBI::BdWRI1 vegetative tissues may enhance the TAG-buffering capacity, allowing the intermittent sequestration of applied 18:2. Alternatively, we cannot rule out that the delayed cell death in UBI::BdWRI1 lines may be caused by the sequestration of FAs in other structures such as the epidermis or that there may be an adaptive response to sublethal levels of 18:2.
Mammalian cells have at least two mechanisms to prevent free FA-induced cell death: (1) inhibition of FA synthesis; and (2) sequestration of excess free FAs into TAGs in lipid droplets (Lee et al., 2010). Similar mechanisms exist in plants. For instance, feedback inhibition of FA biosynthesis has been demonstrated in various plant systems (Terzaghi, 1986; Shintani and Ohlrogge, 1995; Andre et al., 2012). Additionally, changing the TAG accumulation in plant cells can influence the sensitivity to free FA-induced cell death. PDAT1 is reported to play a role in TAG biosynthesis and lipid homeostasis in Arabidopsis leaves (Fan et al., 2013, 2014); an Arabidopsis pdat1 trigalactosyldiacylglycerol1 (tgd1) double mutant showed reduced leaf TAG content compared with a tgd1 single mutant and displayed a hypersensitive response to free FAs (Fan et al., 2013). Here, we demonstrate an opposite scenario. Although it has been shown that WRI1 is not directly involved in the transcriptional control of genes encoding the final enzymes of TAG synthesis (Baud et al., 2009; Maeo et al., 2009), TAG content increased in UBI::BdWRI1 lines (Fig. 4C). This increased TAG content may have led to an enhanced protective capacity for free FAs applied to the transgenic plants, leading to a delayed cell death (Fig. 8). These results are consistent with the notion that TAG pools play an important role in detoxifying free FAs also in plants.
CONCLUSION
Increasing energy density in plant biomass by increasing the TAG content is synergistic with efforts to develop lignocellulosic feedstock for biofuel production. Producing only 10% of TAG (by dry weight) in vegetative tissues will increase the energy yield approximately 40% in biomass crops compared with fermentation to ethanol alone (Ohlrogge and Chapman, 2011). Thus, the accumulation of TAGs in the vegetative tissues of biomass crops is a promising strategy. Although ectopic expression of BdWRI1 induced TAG accumulation in vegetative tissues of B. distachyon, cell death was also observed. Second-generation transgenic B. distachyon plants have to encompass tissue-specific or inducible expression of WRI1 to avoid these deleterious effects. Because TAGs seem to be rapidly turned over in B. distachyon leaf blades, inhibition of TAG degradation along with ectopic expression of WRI1 might provide a more viable strategy to enhance the energy density in monocotyledonous plants.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) Columbia-2 and 35S::AtWRI1 seeds (Sanjaya et al., 2011) were sterilized and plated on Murashige and Skoog medium (Murashige and Skoog, 1962) containing 1% (w/v) Suc. After 7 d, the seedlings were transferred to soil containing 22 μg g−1 nitrate, 3.8 μg g−1 phosphorus, 63 μg g−1 potassium, 75 μg g−1 calcium, 57 μg g−1 magnesium, 34 μg g−1 sodium, and 16 μg g−1 chloride. Seeds were kept at 4°C for 72 h in the dark and then grown in 100 to 200 μE m−2 s−1 with a 16-h-light/8-h-dark cycle and 22°C/18°C (day/night). Brachypodium distachyon Bd21-3 and UBI::BdWRI1 grains were wet briefly with 70% (v/v) ethanol and then surface sterilized with 10% (v/v) bleach containing 0.01% (v/v) SDS for 5 min. Grains were rinsed thoroughly with sterile water five times. The surface-sterilized grains were placed onto Murashige and Skoog medium without Suc. After 7 d, the seedlings were transferred to the same soil mentioned above. Plants were grown under 150 μE m−2 s−1 with a 16-h-light/8-h-dark cycle and 22°C/18°C (day/night).
Plasmid Construction and B. distachyon Transformation
Total RNA was isolated from 44-d-old Bd21-3 flowers using the Plant RNeasy RNA extraction kit (Qiagen), and then 0.5 µg of RNA was reverse transcribed using Moloney murine Leukemia Virus Reverse Transcriptase (Promega) and oligo(dT)18 following the manufacturers’ protocols. A BdWRI1 (Bradi4g43877) overexpression construct was built by amplifying a 1.27-kb full-length complementary DNA sequence using forward 5′-GGGGGTACCCATGAAGAGATCCCCTCCTCAGCCGTC-3′ and reverse 5′-GGGGAATTCTCAATTGCACACAGTGATCATTTTTGG-3′ primers and inserting it using restriction enzymes into pENTR2B (Thermo Fisher). These entry clones are recombined into the plant expression vector pIPKb002 (Himmelbach et al., 2007) using LR clonase (Thermo Fisher). Entry and expression clones were verified by sequencing. Transgenic B. distachyon plants harboring the BdWRI1 overexpression construct were regenerated from Agrobacterium tumefaciens-mediated transformed Bd21-3 embryonic callus tissue (Vogel and Hill, 2008) using A. tumefaciens strain AGL-1 and medium supplemented with 40 units mL−1 hygromycin B (Phytotechnology Laboratories).
Lipid Analysis
TAG and free FA analyses were performed as described previously but with minor modifications (Sanjaya et al., 2013). B. distachyon or Arabidopsis leaf tissues were harvested and freeze dried. Total lipids were extracted from approximately 20 mg of dry tissues or five dry grains with lipid extraction buffer (chloroform:methanol:formic acid, 10:20:1, v/v), with 10 μg of tri-17:0 TAG (Sigma) and 10 μg of 15:0 (Sigma) added as internal standards. Total lipid samples were separated by TLC on silica plates (Si250PA; Mallinckrodt Baker) developed with ether:ethyl ether:acetic acid (80:20:1, v/v). After development, TAG and free FA bands were sprayed with 0.01% (v/v) Primuline in 80% (v/v) acetone and visualized under UV light. TAG or free FA bands were isolated from the TLC plate. FA methyl esters were prepared and quantified as described (Wang and Benning, 2011).
Quantitative Real-Time PCR
Total RNA from 4-week-old Arabidopsis leaves or 8-week-old B. distachyon leaf blades was isolated using an RNeasy Plant Mini Kit (Qiagen). One microgram of total RNA was used to synthesize complementary DNA using SuperScript III Reverse Transcriptase (Invitrogen). Quantitative RT-PCR was performed using the SYBR Green PCR Core Reagents mix (Thermo Fisher) following the manufacturer’s manual. Supplemental Table S1 lists the primers used in this research. The 2-ΔΔCt calculation as described in (Livak and Schmittgen, 2001) was used to determine the relative mRNA levels.
Electrolyte Leakage Assay
Electrolyte leakage assays were performed as described (Gilmour et al., 1988), but with minor modifications. Three to four 3-week-old detached B. distachyon leaf blades were immersed in 5 mL of deionized water, and the samples were gently agitated for 3 h. Conductivity was measured using a conductance meter (YSI model 35). One hundred percent leakage was obtained by placing the leaves in −80°C for 1 h. Electrolyte leakage was expressed as a percentage of the final ion leakage.
Microscopy
Lipid droplets were observed using Nile Red staining. B. distachyon dry grains were first treated with fixation buffer (4% [v/v] paraformaldehyde and 0.25% [v/v] glutaraldehyde in phosphate-buffered saline [PBS], pH 7), then stained with 0.1% (w/v) Nile Red (Molecular Probes) in acetone for 20 min at room temperature, and briefly rinsed with PBS buffer (8.0 g L−1 NaCl, 0.2 g L−1 KCl, 1.44 g L−1 Na2HPO4, and 0.24 g L−1 KH2PO4, pH 7.4). B. distachyon leaf blades and internodes were directly stained with 0.1% (w/v) Nile Red for 20 min at room temperature without fixation and briefly rinsed with PBS buffer. Neutral lipids were observed using an Olympus FluoView 1000 Laser Scanning Confocal Microscope with excitation at 488 nm and emission at 550 to 670 nm. The number and size of lipid droplets in B. distachyon grains were measured using Fluoview Viewer (Olympus).
ROS production was analyzed using H2DCF-DA (Invitrogen) following the manufacturer’s manual. Detached leaf tissues from 8-week-old B. distachyon or 4-week-old Arabidopsis were incubated in H2DCF-DA staining buffer (10 μm H2DCF-DA in PBS buffer). Vacuum was applied for 20 min followed by staining for 20 min at room temperature. After staining, the tissues were briefly washed with PBS buffer. The fluorescence signal was detected using an Olympus FluoView 1000 Laser Scanning Confocal Microscope with excitation at 488 nm and emission at 535 nm.
Production of H2O2 was examined following staining with DAB. B. distachyon leaf blades (8 weeks old) were harvested and immersed in 1 mg mL−1 DAB solution (pH 3.8). Vacuum was applied for 20 min followed by staining for 8 h in the dark at room temperature. After staining, samples were cleared with clearing buffer (ethanol:acetic acid:glycerol, 3:3:1, v/v) at 95°C. Samples were examined using a Leica MZ125 microscope.
FA Treatment
The 18:2 and 16:0 FAs and cerulenin were purchased from Sigma and dissolved in ethanol. FA treatment was performed as described (Fan et al., 2013), with minor modifications. Detached 3-week-old B. distachyon leaf blades were floated in water containing 0.0005% (v/v) Tween 20, 0.25% (v/v) ethanol, and various concentrations of free FAs (0, 50, 100, or 200 μm) in light. The control was treated with water containing 0.0005% (v/v) Tween 20 and 0.25% (v/v) ethanol. Cerulenin was added at a final concentration of 5 μg mL−1.
The Arabidopsis Genome Initiative locus identifiers (https://www.arabidopsis.org/) used in this study are as follows: At3g54230 (AtWRI1), At5g04040 (AtSDP1), At4g16760 (AtACX1), At5g65110 (AtACX2), At3g05970 (AtLACS6), At5g27600 (AtLACS7), At1g04710 (AtKAT1), At2g33150 (AtKAT2), At3g06860 (AtMFP2), and At2g37620 (AtACTIN1). Accession numbers for B. distachyon locus identifiers (http://www.phytozome.net/) are as follows: Brai4g43877 (BdWRI1), Bradi2g45620 (BdPKPβ1), Bradi1g46610 (BdKASІ), Bradi1g01000 (BdACP1), Bradi2g50610 (BdSDP1-1), Bradi1g04310 (BdSDP1-2), Bradi1g52320 (BdACX1), Bradi4g14090 (BdACX2), Bradi4g26610 (BdLACS6), Bradi4g42950 (BdLACS7), Bradi2g43020 (BdMFP2-1), Bradi4g28310 (BdMFP2-2), Bradi4g28310 (BdMFP2-3), Bradi3g27960 (BdKAT1), Bradi3g55420 (BdKAT2), and Bradi4g00660 (BdUBC18). Accession numbers for all the AP2-containing proteins in B. distachyon were obtained from Phytozome (https://www.arabidopsis.org/) and are shown in Figure 1.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Alignment of WRI1 amino acid sequences from B. distachyon and Arabidopsis.
Supplemental Figure S2. Lipid droplets are abundant in embryo and endosperm of UBI::BdWRI1 line 5A dry grains.
Supplemental Figure S3. Lipid droplets in the internodes of UBI::BdWRI1 plants.
Supplemental Figure S4. Ectopic expression of AtWRI1 also led to cell death in B. distachyon.
Supplemental Figure S5. Ectopic expression of AtWRI1 in Arabidopsis did not lead to cell death.
Supplemental Figure S6. No induction of TAG turnover in 35S::AtWRI1 vegetative tissues of Arabidopsis.
Supplemental Figure S7. Palmitic acid (16:0) treatment of B. distachyon leaf blade results in cell death.
Supplemental Table S1. PCR primers used in RT-PCR.
Supplementary Material
Acknowledgments
We thank Dr. Henrik Tjellström (Michigan State University) for advice on lipid analysis and FA treatments, Dr. Sarah Gilmour (Michigan State University) for advice on electrolyte leakage measurement, and Dr. Melinda Frame (Michigan State University Center for Advanced Microscopy) for confocal microscopy experiments.
Glossary
- TAG
triacylglycerol
- FA
fatty acid
- RT
reverse transcription
- ROS
reactive oxygen species
- H2O2
hydrogen peroxide
- H2DCF-DA
2′,7′-dichlorofluorescein diacetate acetyl ester
- DAB
3-diaminobenzidine
- PtdCho
phosphatidylcholine
- TLC
thin-layer chromatography
- PBS
phosphate-buffered saline
- RT
real-time
Footnotes
This work was supported by the U.S. Department of Energy Great Lakes Bioenergy Research Center Cooperative (grant no. DE–FC02–07ER64494) and by Michigan State University AgBioResearch.
Articles can be viewed without a subscription.
References
- Ando T, Tsukamotoa Y (1981) Inhibitory action of saturated fatty acids and their derivatives. Phytochemistry 20: 2143–2144 [Google Scholar]
- Andre C, Haslam RP, Shanklin J (2012) Feedback regulation of plastidic acetyl-CoA carboxylase by 18:1-acyl carrier protein in Brassica napus. Proc Natl Acad Sci USA 109: 10107–10112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55: 373–399 [DOI] [PubMed] [Google Scholar]
- Awaya J, Ohno T, Ohno H, Omura S (1975) Substitution of cellular fatty acids in yeast cells by the antibiotic cerulenin and exogenous fatty acids. Biochim Biophys Acta 409: 267–273 [DOI] [PubMed] [Google Scholar]
- Baker CJ, Orlandi EW (1995) Active oxygen in plant pathogenesis. Annu Rev Phytopathol 33: 299–321 [DOI] [PubMed] [Google Scholar]
- Bates PD, Browse J (2011) The pathway of triacylglycerol synthesis through phosphatidylcholine in Arabidopsis produces a bottleneck for the accumulation of unusual fatty acids in transgenic seeds. Plant J 68: 387–399 [DOI] [PubMed] [Google Scholar]
- Bates PD, Browse J (2012) The significance of different diacylgycerol synthesis pathways on plant oil composition and bioengineering. Front Plant Sci 3: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates PD, Fatihi A, Snapp AR, Carlsson AS, Browse J, Lu C (2012) Acyl editing and headgroup exchange are the major mechanisms that direct polyunsaturated fatty acid flux into triacylglycerols. Plant Physiol 160: 1530–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates PD, Ohlrogge JB, Pollard M (2007) Incorporation of newly synthesized fatty acids into cytosolic glycerolipids in pea leaves occurs via acyl editing. J Biol Chem 282: 31206–31216 [DOI] [PubMed] [Google Scholar]
- Bates PD, Stymne S, Ohlrogge J (2013) Biochemical pathways in seed oil synthesis. Curr Opin Plant Biol 16: 358–364 [DOI] [PubMed] [Google Scholar]
- Baud S, Lepiniec L (2010) Physiological and developmental regulation of seed oil production. Prog Lipid Res 49: 235–249 [DOI] [PubMed] [Google Scholar]
- Baud S, Wuillème S, To A, Rochat C, Lepiniec L (2009) Role of WRINKLED1 in the transcriptional regulation of glycolytic and fatty acid biosynthetic genes in Arabidopsis. Plant J 60: 933–947 [DOI] [PubMed] [Google Scholar]
- Baxter A, Mittler R, Suzuki N (2014) ROS as key players in plant stress signalling. J Exp Bot 65: 1229–1240 [DOI] [PubMed] [Google Scholar]
- Berrie AM. (1979) Possible role of volatile fatty acids and abscisic acid in the dormancy of oats. Plant Physiol 63: 758–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma R, Miazek K, Willemsen SM, Vermuë MH, Wijffels RH (2008) Growth inhibition of Monodus subterraneus by free fatty acids. Biotechnol Bioeng 101: 1108–1114 [DOI] [PubMed] [Google Scholar]
- Brkljacic J, Grotewold E, Scholl R, Mockler T, Garvin DF, Vain P, Brutnell T, Sibout R, Bevan M, Budak H, et al. (2011) Brachypodium as a model for the grasses: today and the future. Plant Physiol 157: 3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernac A, Andre C, Hoffmann-Benning S, Benning C (2006) WRI1 is required for seed germination and seedling establishment. Plant Physiol 141: 745–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernac A, Benning C (2004) WRINKLED1 encodes an AP2/EREB domain protein involved in the control of storage compound biosynthesis in Arabidopsis. Plant J 40: 575–585 [DOI] [PubMed] [Google Scholar]
- Chandra-Shekara AC, Venugopal SC, Barman SR, Kachroo A, Kachroo P (2007) Plastidial fatty acid levels regulate resistance gene-dependent defense signaling in Arabidopsis. Proc Natl Acad Sci USA 104: 7277–7282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danon A, Miersch O, Felix G, Camp RG, Apel K (2005) Concurrent activation of cell death-regulating signaling pathways by singlet oxygen in Arabidopsis thaliana. Plant J 41: 68–80 [DOI] [PubMed] [Google Scholar]
- Dellagi A, Brisset MN, Paulin JP, Expert D (1998) Dual role of desferrioxamine in Erwinia amylovora pathogenicity. Mol Plant Microbe Interact 11: 734–742 [DOI] [PubMed] [Google Scholar]
- del Rio LA, Pastori GM, Palma JM, Sandalio LM, Sevilla F, Corpas FJ, Jimenez A, Lopez-Huertas E, Hernandez JA (1998) The activated oxygen role of peroxisomes in senescence. Plant Physiol 116: 1195–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper J, Mur LA, Jenkins G, Ghosh-Biswas GC, Bablak P, Hasterok R, Routledge AP (2001) Brachypodium distachyon: a new model system for functional genomics in grasses. Plant Physiol 127: 1539–1555 [PMC free article] [PubMed] [Google Scholar]
- Durrett TP, Benning C, Ohlrogge J (2008) Plant triacylglycerols as feedstocks for the production of biofuels. Plant J 54: 593–607 [DOI] [PubMed] [Google Scholar]
- Dussert S, Guerin C, Andersson M, Joët T, Tranbarger TJ, Pizot M, Sarah G, Omore A, Durand-Gasselin T, Morcillo F (2013) Comparative transcriptome analysis of three oil palm fruit and seed tissues that differ in oil content and fatty acid composition. Plant Physiol 162: 1337–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastmond PJ. (2006) SUGAR-DEPENDENT1 encodes a patatin domain triacylglycerol lipase that initiates storage oil breakdown in germinating Arabidopsis seeds. Plant Cell 18: 665–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastmond PJ. (2007) MONODEHYROASCORBATE REDUCTASE4 is required for seed storage oil hydrolysis and postgerminative growth in Arabidopsis. Plant Cell 19: 1376–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakas S, Qiu Y, Dixon JL, Han GS, Ruggles KV, Garbarino J, Sturley SL, Carman GM (2011) Phosphatidate phosphatase activity plays key role in protection against fatty acid-induced toxicity in yeast. J Biol Chem 286: 29074–29085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Yan C, Roston R, Shanklin J, Xu C (2014) Arabidopsis lipins, PDAT1 acyltransferase, and SDP1 triacylglycerol lipase synergistically direct fatty acids toward β-oxidation, thereby maintaining membrane lipid homeostasis. Plant Cell 26: 4119–4134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Yan C, Xu C (2013) Phospholipid:diacylglycerol acyltransferase-mediated triacylglycerol biosynthesis is crucial for protection against fatty acid-induced cell death in growing tissues of Arabidopsis. Plant J 76: 930–942 [DOI] [PubMed] [Google Scholar]
- Farmer EE, Weber H, Vollenweider S (1998) Fatty acid signaling in Arabidopsis. Planta 206: 167–174 [DOI] [PubMed] [Google Scholar]
- Focks N, Benning C (1998) wrinkled1: a novel, low-seed-oil mutant of Arabidopsis with a deficiency in the seed-specific regulation of carbohydrate metabolism. Plant Physiol 118: 91–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer MJ, Oxborough K, Mullineaux PM, Baker NR (2002) Imaging of photo-oxidative stress responses in leaves. J Exp Bot 53: 1249–1254 [PubMed] [Google Scholar]
- Fukuda N, Ikawa Y, Aoyagi T, Kozaki A (2013) Expression of the genes coding for plastidic acetyl-CoA carboxylase subunits is regulated by a location-sensitive transcription factor binding site. Plant Mol Biol 82: 473–483 [DOI] [PubMed] [Google Scholar]
- Garbarino J, Padamsee M, Wilcox L, Oelkers PM, D’Ambrosio D, Ruggles KV, Ramsey N, Jabado O, Turkish A, Sturley SL (2009) Sterol and diacylglycerol acyltransferase deficiency triggers fatty acid-mediated cell death. J Biol Chem 284: 30994–31005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour SJ, Hajela RK, Thomashow MF (1988) Cold acclimation in Arabidopsis thaliana. Plant Physiol 87: 745–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goepfert S, Poirier Y (2007) Beta-oxidation in fatty acid degradation and beyond. Curr Opin Plant Biol 10: 245–251 [DOI] [PubMed] [Google Scholar]
- Golbeck JH, Warden JT (1984) Interaction of linolenic acid with bound quinone molecules in photosystem II: time-resolved optical and electron spin resonance studies. Biochim Biophys Acta 767: 263–271 [DOI] [PubMed] [Google Scholar]
- Graham IA. (2008) Seed storage oil mobilization. Annu Rev Plant Biol 59: 115–142 [DOI] [PubMed] [Google Scholar]
- Graham IA, Eastmond PJ (2002) Pathways of straight and branched chain fatty acid catabolism in higher plants. Prog Lipid Res 41: 156–181 [DOI] [PubMed] [Google Scholar]
- Grimberg A, Carlsson AS, Marttila S, Bhalerao R, Hofvander P (2015) Transcriptional transitions in Nicotiana benthamiana leaves upon induction of oil synthesis by WRINKLED1 homologs from diverse species and tissues. BMC Plant Biol 15: 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillon F, Larré C, Petipas F, Berger A, Moussawi J, Rogniaux H, Santoni A, Saulnier L, Jamme F, Miquel M, et al. (2012) A comprehensive overview of grain development in Brachypodium distachyon variety Bd21. J Exp Bot 63: 739–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneen WK, Karlsson G, Brismar K, Gummeson PO, Marttila S, Leonova S, Carlsson AS, Bafor M, Banas A, Mattsson B, et al. (2008) Fusion of oil bodies in endosperm of oat grains. Planta 228: 589–599 [DOI] [PubMed] [Google Scholar]
- Himmelbach A, Zierold U, Hensel G, Riechen J, Douchkov D, Schweizer P, Kumlehn J (2007) A set of modular binary vectors for transformation of cereals. Plant Physiol 145: 1192–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofius D, Schultz-Larsen T, Joensen J, Tsitsigiannis DI, Petersen NH, Mattsson O, Jørgensen LB, Jones JD, Mundy J, Petersen M (2009) Autophagic components contribute to hypersensitive cell death in Arabidopsis. Cell 137: 773–783 [DOI] [PubMed] [Google Scholar]
- Hong SY, Seo PJ, Yang MS, Xiang F, Park CM (2008) Exploring valid reference genes for gene expression studies in Brachypodium distachyon by real-time PCR. BMC Plant Biol 8: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachroo A, Lapchyk L, Fukushige H, Hildebrand D, Klessig D, Kachroo P (2003) Plastidial fatty acid signaling modulates salicylic acid- and jasmonic acid-mediated defense pathways in the Arabidopsis ssi2 mutant. Plant Cell 15: 2952–2965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AA, van Erp H, Quettier AL, Shaw E, Menard G, Kurup S, Eastmond PJ (2013) The SUGAR-DEPENDENT1 lipase limits triacylglycerol accumulation in vegetative tissues of Arabidopsis. Plant Physiol 162: 1282–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlwein SD. (2010) Triacylglycerol homeostasis: insights from yeast. J Biol Chem 285: 15663–15667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo AJ, Fulda M, Browse J, Ohlrogge JB (2005) Identification of a plastid acyl-acyl carrier protein synthetase in Arabidopsis and its role in the activation and elongation of exogenous fatty acids. Plant J 44: 620–632 [DOI] [PubMed] [Google Scholar]
- Kunz HH, Scharnewski M, Feussner K, Feussner I, Flügge UI, Fulda M, Gierth M (2009) The ABC transporter PXA1 and peroxisomal β-oxidation are vital for metabolism in mature leaves of Arabidopsis during extended darkness. Plant Cell 21: 2733–2749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JN, Kim H, Yao H, Chen Y, Weng K, Ye J (2010) Identification of Ubxd8 protein as a sensor for unsaturated fatty acids and regulator of triglyceride synthesis. Proc Natl Acad Sci USA 107: 21424–21429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Poidevin N. (1965) Inhibition of the germination of mustard seeds by saturated fatty acids. Phytochemistry 4: 525–526 [Google Scholar]
- Li M, Bahn SC, Guo L, Musgrave W, Berg H, Welti R, Wang X (2011) Patatin-related phospholipase pPLAIIIβ-induced changes in lipid metabolism alter cellulose content and cell elongation in Arabidopsis. Plant Cell 23: 1107–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Hua W, Zhan G, Wei F, Wang X, Liu G, Wang H (2010) Increasing seed mass and oil content in transgenic Arabidopsis by the overexpression of wri1-like gene from Brassica napus. Plant Physiol Biochem 48: 9–15 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−Delta Delta C(T) Method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Ma W, Kong Q, Arondel V, Kilaru A, Bates PD, Thrower NA, Benning C, Ohlrogge JB (2013) Wrinkled1, a ubiquitous regulator in oil accumulating tissues from Arabidopsis embryos to oil palm mesocarp. PLoS One 8: e68887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Kong Q, Grix M, Mantyla JJ, Yang Y, Benning C, Ohlrogge JB (2015) Deletion of a C-terminal intrinsically disordered region of WRINKLED1 affects its stability and enhances oil accumulation in Arabidopsis. Plant J 83: 864–874 [DOI] [PubMed] [Google Scholar]
- Maeo K, Tokuda T, Ayame A, Mitsui N, Kawai T, Tsukagoshi H, Ishiguro S, Nakamura K (2009) An AP2-type transcription factor, WRINKLED1, of Arabidopsis thaliana binds to the AW-box sequence conserved among proximal upstream regions of genes involved in fatty acid synthesis. Plant J 60: 476–487 [DOI] [PubMed] [Google Scholar]
- Meng Y, Li H, Wang Q, Liu B, Lin C (2013) Blue light-dependent interaction between cryptochrome2 and CIB1 regulates transcription and leaf senescence in soybean. Plant Cell 25: 4405–4420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger JD, Sebesta DK (1982) Role of endogenous growth regulators in seed dormancy of Avena fatua. I. Short chain fatty acids. Plant Physiol 70: 1480–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquel M, Trigui G, d’Andréa S, Kelemen Z, Baud S, Berger A, Deruyffelaere C, Trubuil A, Lepiniec L, Dubreucq B (2014) Specialization of oleosins in oil body dynamics during seed development in Arabidopsis seeds. Plant Physiol 164: 1866–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Ohkawa M, Nishikawa Y (1987) Plant growth inhibitory activity of fatty acids and the related compounds by the Avena coleoptile test. Plant Sci 53: 35–38 [Google Scholar]
- Ohlrogge J, Chapman K (2011) The seeds of green energy: expanding the contribution of plant oils as biofuels. Biochemist (Lond) 33: 34–38 [Google Scholar]
- Opanowicz M, Vain P, Draper J, Parker D, Doonan JH (2008) Brachypodium distachyon: making hay with a wild grass. Trends Plant Sci 13: 172–177 [DOI] [PubMed] [Google Scholar]
- Packter NM, Stumpf PK (1975) Fat metabolism in higher plants: the effect of cerulenin on the synthesis of medium- and long-chain acids in leaf tissue. Arch Biochem Biophys 167: 655–667 [DOI] [PubMed] [Google Scholar]
- Padham AK, Hopkins MT, Wang TW, McNamara LM, Lo M, Richardson LG, Smith MD, Taylor CA, Thompson JE (2007) Characterization of a plastid triacylglycerol lipase from Arabidopsis. Plant Physiol 143: 1372–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield S, Rylott EL, Gilday AD, Graham S, Larson TR, Graham IA (2004) Reserve mobilization in the Arabidopsis endosperm fuels hypocotyl elongation in the dark, is independent of abscisic acid, and requires PHOSPHOENOLPYRUVATE CARBOXYKINASE1. Plant Cell 16: 2705–2718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Chin CK (2007) Potassium loss is involved in tobacco cell death induced by palmitoleic acid and ceramide. Arch Biochem Biophys 465: 180–186 [DOI] [PubMed] [Google Scholar]
- Peters JS, Chin C (2005) Evidence for cytochrome f involvement in eggplant cell death induced by palmitoleic acid. Cell Death Differ 12: 405–407 [DOI] [PubMed] [Google Scholar]
- Peters JS, Chin CK (2003) Inhibition of photosynthetic electron transport by palmitoleic acid is partially correlated to loss of thylakoid membrane proteins. Plant Physiol Biochem 41: 117–124 [Google Scholar]
- Pouvreau B, Baud S, Vernoud V, Morin V, Py C, Gendrot G, Pichon JP, Rouster J, Paul W, Rogowsky PM (2011) Duplicate maize Wrinkled1 transcription factors activate target genes involved in seed oil biosynthesis. Plant Physiol 156: 674–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu J, Ye J, Geng YF, Sun YW, Gao SQ, Zhang BP, Chen W, Chua NH (2012) Dissecting functions of KATANIN and WRINKLED1 in cotton fiber development by virus-induced gene silencing. Plant Physiol 160: 738–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel F, Sulmon C, Bogard M, Couée I, Gouesbet G (2009) Differential patterns of reactive oxygen species and antioxidative mechanisms during atrazine injury and sucrose-induced tolerance in Arabidopsis thaliana plantlets. BMC Plant Biol 9: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roudier F, Gissot L, Beaudoin F, Haslam R, Michaelson L, Marion J, Molino D, Lima A, Bach L, Morin H, et al. (2010) Very-long-chain fatty acids are involved in polar auxin transport and developmental patterning in Arabidopsis. Plant Cell 22: 364–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan PG, Thompson GA Jr, Cho SH (1987) Metabolism of exogenous long-chain fatty acids by spinach leaves. Arch Biochem Biophys 259: 481–496 [DOI] [PubMed] [Google Scholar]
- Sanjaya, Durrett TP, Weise SE, Benning C (2011) Increasing the energy density of vegetative tissues by diverting carbon from starch to oil biosynthesis in transgenic Arabidopsis. Plant Biotechnol J 9: 874–883 [DOI] [PubMed] [Google Scholar]
- Sanjaya, Miller R, Durrett TP, Kosma DK, Lydic TA, Muthan B, Koo AJ, Bukhman YV, Reid GE, Howe GA, et al. (2013) Altered lipid composition and enhanced nutritional value of Arabidopsis leaves following introduction of an algal diacylglycerol acyltransferase 2. Plant Cell 25: 677–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Mendoza M, Dubreucq B, Baud S, Parcy F, Caboche M, Lepiniec L (2008) Deciphering gene regulatory networks that control seed development and maturation in Arabidopsis. Plant J 54: 608–620 [DOI] [PubMed] [Google Scholar]
- Schmitt FJ, Renger G, Friedrich T, Kreslavski VD, Zharmukhamedov SK, Los DA, Kuznetsov VV, Allakhverdiev SI (2014) Reactive oxygen species: re-evaluation of generation, monitoring and role in stress-signaling in phototrophic organisms. Biochim Biophys Acta 1837: 835–848 [DOI] [PubMed] [Google Scholar]
- Shen B, Allen WB, Zheng P, Li C, Glassman K, Ranch J, Nubel D, Tarczynski MC (2010) Expression of ZmLEC1 and ZmWRI1 increases seed oil production in maize. Plant Physiol 153: 980–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani DK, Ohlrogge JB (1995) Feedback inhibition of fatty acid synthesis in tobacco suspension cells. Plant J 7: 577–587 [Google Scholar]
- Slocombe SP, Cornah J, Pinfield-Wells H, Soady K, Zhang Q, Gilday A, Dyer JM, Graham IA (2009) Oil accumulation in leaves directed by modification of fatty acid breakdown and lipid synthesis pathways. Plant Biotechnol J 7: 694–703 [DOI] [PubMed] [Google Scholar]
- Stewart RR, Berrie AM (1979) Effect of temperature on the short chain fatty acid-induced inhibition of lettuce seed germination. Plant Physiol 63: 61–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30: 2725–2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzaghi WB. (1986) Metabolism of Tween-fatty acid esters by cultured soybean cells: kinetics of incorporation into lipids, subsequent turnover, and associated changes in endogenous fatty acid synthesis. Plant Physiol 82: 780–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjellström H, Strawsine M, Ohlrogge JB (2015) Tracking synthesis and turnover of triacylglycerol in leaves. J Exp Bot 66: 1453–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Dangl JL, Jones JD (2002) Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA 99: 517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranbarger TJ, Dussert S, Joët T, Argout X, Summo M, Champion A, Cros D, Omore A, Nouy B, Morcillo F (2011) Regulatory mechanisms underlying oil palm fruit mesocarp maturation, ripening, and functional specialization in lipid and carotenoid metabolism. Plant Physiol 156: 564–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso TC. (1964) Plant-growth inhibition by some fatty acids and their analogues. Nature 202: 511–512 [DOI] [PubMed] [Google Scholar]
- Tsuzuki E, Yamamoto Y, Shimizu T (1987) Fatty acids in buckwheat are growth inhibitors. Ann Bot (Lond) 60: 69–70 [Google Scholar]
- van Erp H, Kelly AA, Menard G, Eastmond PJ (2014) Multigene engineering of triacylglycerol metabolism boosts seed oil content in Arabidopsis. Plant Physiol 165: 30–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhercke T, El Tahchy A, Liu Q, Zhou XR, Shrestha P, Divi UK, Ral JP, Mansour MP, Nichols PD, James CN, et al. (2014) Metabolic engineering of biomass for high energy density: oilseed-like triacylglycerol yields from plant leaves. Plant Biotechnol J 12: 231–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhercke T, El Tahchy A, Shrestha P, Zhou XR, Singh SP, Petrie JR (2013) Synergistic effect of WRI1 and DGAT1 coexpression on triacylglycerol biosynthesis in plants. FEBS Lett 587: 364–369 [DOI] [PubMed] [Google Scholar]
- Vogel J, Hill T (2008) High-efficiency Agrobacterium-mediated transformation of Brachypodium distachyon inbred line Bd21-3. Plant Cell Rep 27: 471–478 [DOI] [PubMed] [Google Scholar]
- Wang Z, Benning C (2011) Arabidopsis thaliana polar glycerolipid profiling by thin layer chromatography (TLC) coupled with gas-liquid chromatography (GLC). J Vis Exp 49: 2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden JT, Csatorday K (1987) On the mechanism of linolenic acid inhibition in photosystem II. Biochim Biophys Acta 890: 215–223 [DOI] [PubMed] [Google Scholar]
- Wu JT, Chiang YR, Huang WY, Jane WN (2006) Cytotoxic effects of free fatty acids on phytoplankton algae and cyanobacteria. Aquat Toxicol 80: 338–345 [DOI] [PubMed] [Google Scholar]
- Zhang D, Yuan Z (2014) Molecular control of grass inflorescence development. Annu Rev Plant Biol 65: 553–578 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.