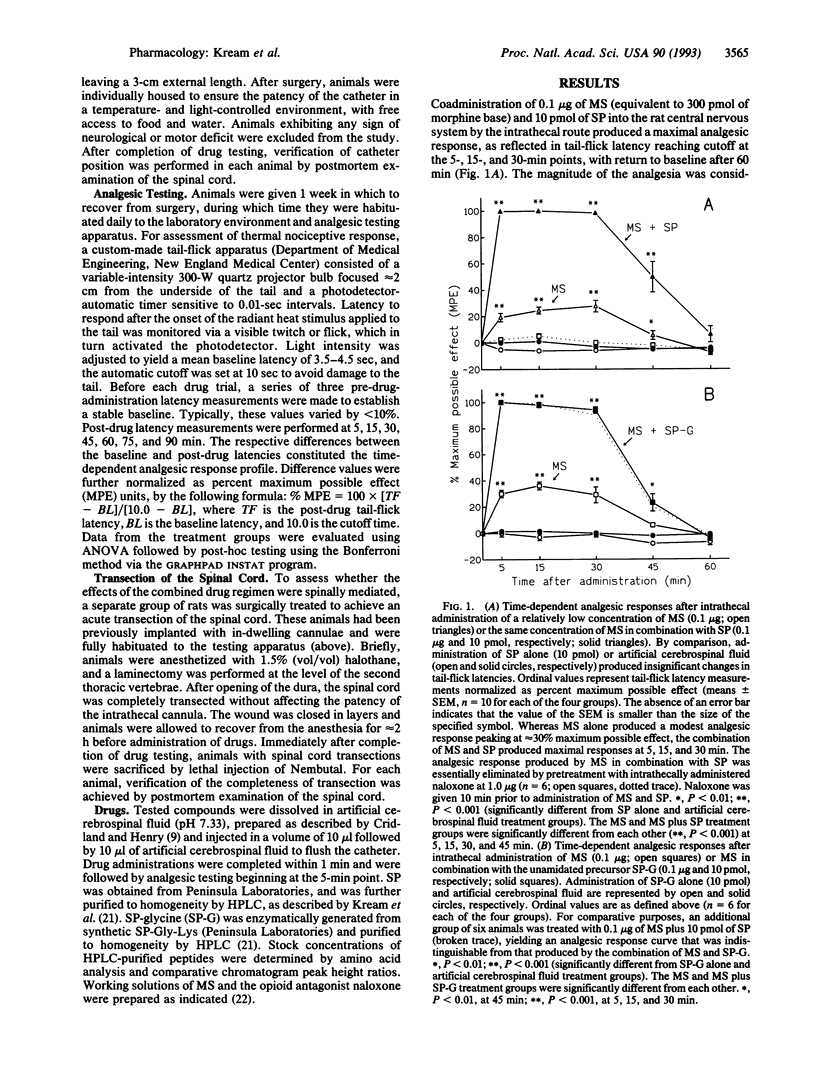
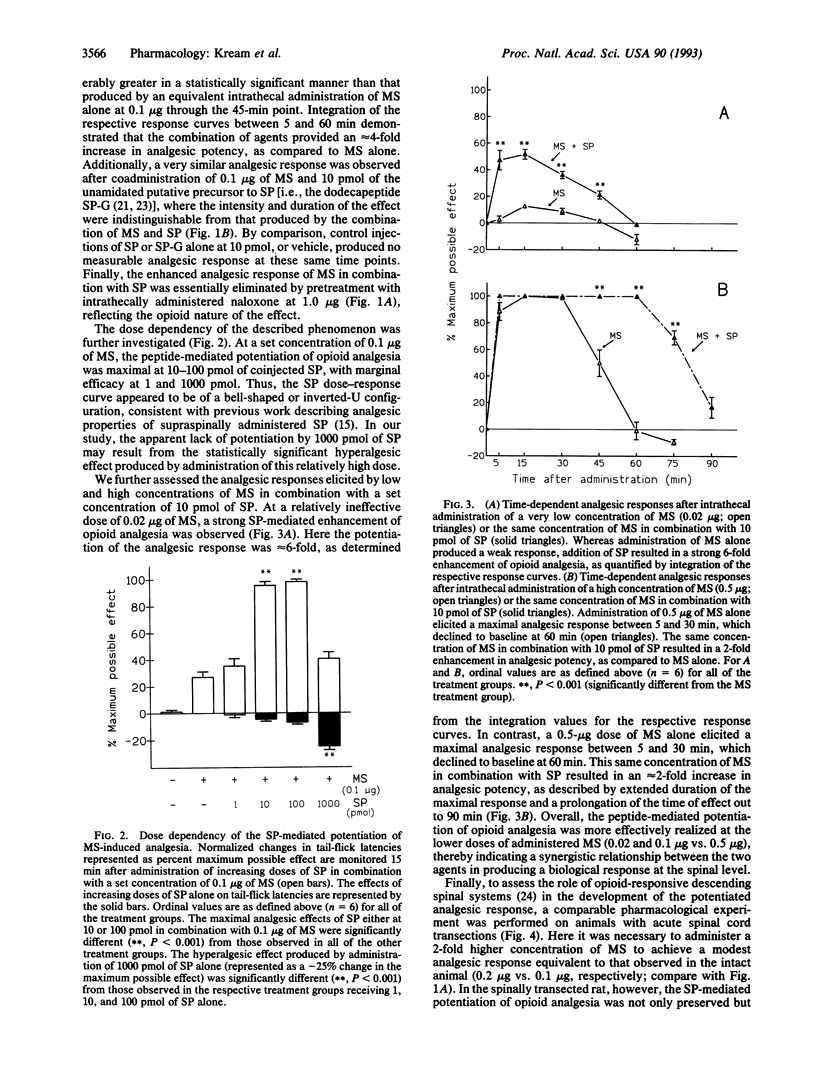
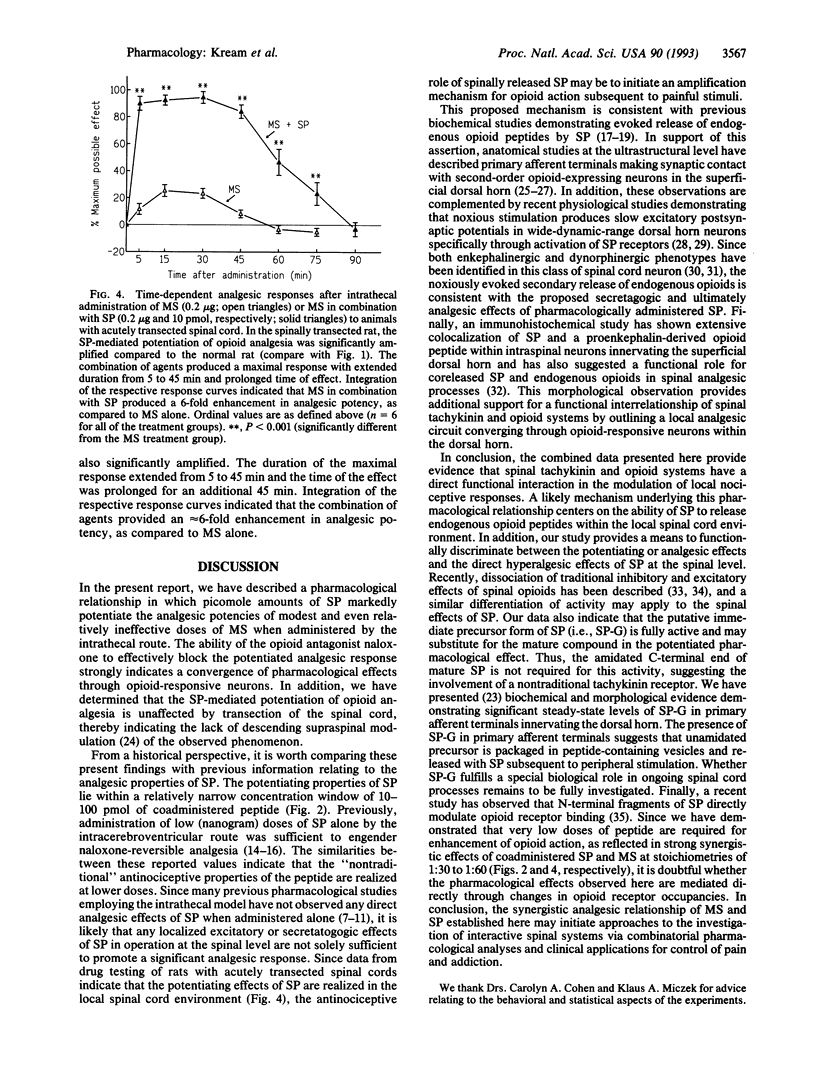
Abstract
The undecapeptide substance P and the alkaloid morphine sulfate are two agents previously thought to have opposite roles in the mediation of spinal nociceptive processes. The present report, however, demonstrates that low doses of substance P when coadministered with marginally effective doses of morphine sulfate into the rat subarachnoid space produce a markedly enhanced analgesic response, as monitored by the tail-flick test. This pharmacological effect is blocked by prior treatment with the opioid antagonist naloxone, indicating that the potentiated analgesic response is mediated by opioid-responsive neurons. In addition, the putative immediate precursor form of substance P (i.e., substance P-glycine) may substitute for the mature compound in the potentiated pharmacological effect. Moreover, the described synergism is unaffected by transection of the spinal cord, demonstrating the lack of supraspinal modulation of the observed phenomenon. Based on these observations, we are now able to dissociate opioid-potentiating and analgesic properties of substance P from traditional hyperalgesic effects realized at significantly higher concentrations. Consistent with previous biochemical data, a likely mechanism underlying the peptide-mediated enhancement of opioid analgesia may center on the ability of substance P to release endogenous opioid peptides within the local spinal cord environment. Finally, the pharmacological relationship of coadministered substance P and morphine sulfate established here supports the hypothesis that spinal tachykinin and opioid systems have a direct functional interaction in the modulation of local nociceptive responses.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basbaum A. I., Fields H. L. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- Chang H. M., Berde C. B., Holz G. G., 4th, Steward G. F., Kream R. M. Sufentanil, morphine, met-enkephalin, and kappa-agonist (U-50,488H) inhibit substance P release from primary sensory neurons: a model for presynaptic spinal opioid actions. Anesthesiology. 1989 Apr;70(4):672–677. doi: 10.1097/00000542-198904000-00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H. J., Basbaum A. I. Ultrastructural analysis of dynorphin B-immunoreactive cells and terminals in the superficial dorsal horn of the deafferented spinal cord of the rat. J Comp Neurol. 1989 Mar 8;281(2):193–205. doi: 10.1002/cne.902810204. [DOI] [PubMed] [Google Scholar]
- Cousins M. J., Mather L. E. Intrathecal and epidural administration of opioids. Anesthesiology. 1984 Sep;61(3):276–310. [PubMed] [Google Scholar]
- Crain S. M., Shen K. F. Opioids can evoke direct receptor-mediated excitatory effects on sensory neurons. Trends Pharmacol Sci. 1990 Feb;11(2):77–81. doi: 10.1016/0165-6147(90)90322-y. [DOI] [PubMed] [Google Scholar]
- Cridland R. A., Henry J. L. Comparison of the effects of substance P, neurokinin A, physalaemin and eledoisin in facilitating a nociceptive reflex in the rat. Brain Res. 1986 Aug 27;381(1):93–99. doi: 10.1016/0006-8993(86)90694-3. [DOI] [PubMed] [Google Scholar]
- De Koninck Y., Henry J. L. Substance P-mediated slow excitatory postsynaptic potential elicited in dorsal horn neurons in vivo by noxious stimulation. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11344–11348. doi: 10.1073/pnas.88.24.11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederickson R. C., Burgis V., Harrell C. E., Edwards J. D. Dual actions of substance P on nociception: possible role of endogenous opioids. Science. 1978 Mar 24;199(4335):1359–1362. doi: 10.1126/science.204012. [DOI] [PubMed] [Google Scholar]
- Gintzler A. R., Xu H. Different G proteins mediate the opioid inhibition or enhancement of evoked [5-methionine]enkephalin release. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4741–4745. doi: 10.1073/pnas.88.11.4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer E. J., Basbaum A. I. Opioid neurons and pain modulation: an ultrastructural analysis of enkephalin in cat superficial dorsal horn. Neuroscience. 1983 Oct;10(2):357–376. doi: 10.1016/0306-4522(83)90139-2. [DOI] [PubMed] [Google Scholar]
- Go V. L., Yaksh T. L. Release of substance P from the cat spinal cord. J Physiol. 1987 Oct;391:141–167. doi: 10.1113/jphysiol.1987.sp016731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan R. E., Shivers B. D., Romano G. J., Howells R. D., Pfaff D. W. Localization of preproenkephalin mRNA in the rat brain and spinal cord by in situ hybridization. J Comp Neurol. 1987 Apr 8;258(2):159–184. doi: 10.1002/cne.902580202. [DOI] [PubMed] [Google Scholar]
- Helke C. J., Krause J. E., Mantyh P. W., Couture R., Bannon M. J. Diversity in mammalian tachykinin peptidergic neurons: multiple peptides, receptors, and regulatory mechanisms. FASEB J. 1990 Apr 1;4(6):1606–1615. [PubMed] [Google Scholar]
- Hylden J. L., Wilcox G. L. Intrathecal opioids block a spinal action of substance P in mice: functional importance of both mu- and delta-receptors. Eur J Pharmacol. 1982 Dec 17;86(1):95–98. doi: 10.1016/0014-2999(82)90403-4. [DOI] [PubMed] [Google Scholar]
- Hylden J. L., Wilcox G. L. Pharmacological characterization of substance P-induced nociception in mice: modulation by opioid and noradrenergic agonists at the spinal level. J Pharmacol Exp Ther. 1983 Aug;226(2):398–404. [PubMed] [Google Scholar]
- Iadarola M. J., Tang J., Costa E., Yang H. Y. Analgesic activity and release of [MET5]enkephalin-Arg6-Gly7-Leu8 from rat spinal cord in vivo. Eur J Pharmacol. 1986 Feb 11;121(1):39–48. doi: 10.1016/0014-2999(86)90390-0. [DOI] [PubMed] [Google Scholar]
- Kitahata L. M., Collins J. G. Spinal action of narcotic analgesics. Anesthesiology. 1981 Feb;54(2):153–163. doi: 10.1097/00000542-198102000-00010. [DOI] [PubMed] [Google Scholar]
- Kream R. M., Schoenfeld T. A., Mancuso R., Clancy A. N., el-Bermani W., Macrides F. Precursor forms of substance P (SP) in nervous tissue: detection with antisera to SP, SP-Gly, and SP-Gly-Lys. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4832–4836. doi: 10.1073/pnas.82.14.4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMotte C. C., de Lanerolle N. C. Ultrastructure of chemically defined neuron systems in the dorsal horn of the monkey. II. Methionine-enkephalin immunoreactivity. Brain Res. 1983 Sep 5;274(1):51–63. doi: 10.1016/0006-8993(83)90520-6. [DOI] [PubMed] [Google Scholar]
- Malick J. B., Goldstein J. M. Analgesic activity of substance P following intracerebral administration in rats. Life Sci. 1978 Aug 28;23(8):835–844. doi: 10.1016/0024-3205(78)90518-0. [DOI] [PubMed] [Google Scholar]
- Marchand J. E., Shimonaka H., Kream R. M. Biochemical characterization and anatomical distribution of a major form of unamidated precursor of substance P in rat brain. Brain Res. 1991 Dec 20;567(2):290–305. doi: 10.1016/0006-8993(91)90808-9. [DOI] [PubMed] [Google Scholar]
- Naranjo J. R., Arnedo A., De Felipe M. C., Del Rio J. Antinociceptive and Met-enkephalin releasing effects of tachykinins and substance P fragments. Peptides. 1986 May-Jun;7(3):419–423. doi: 10.1016/0196-9781(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Pernow B. Substance P. Pharmacol Rev. 1983 Jun;35(2):85–141. [PubMed] [Google Scholar]
- Radhakrishnan V., Henry J. L. Novel substance P antagonist, CP-96,345, blocks responses of cat spinal dorsal horn neurons to noxious cutaneous stimulation and to substance P. Neurosci Lett. 1991 Oct 28;132(1):39–43. doi: 10.1016/0304-3940(91)90428-v. [DOI] [PubMed] [Google Scholar]
- Sasek C. A., Seybold V. S., Elde R. P. The immunohistochemical localization of nine peptides in the sacral parasympathetic nucleus and the dorsal gray commissure in rat spinal cord. Neuroscience. 1984 Jul;12(3):855–873. doi: 10.1016/0306-4522(84)90175-1. [DOI] [PubMed] [Google Scholar]
- Sawynok J., Moochhala S. M., Pillay D. J. Substance P, injected intrathecally, antagonizes the spinal antinociceptive effect of morphine, baclofen and noradrenaline. Neuropharmacology. 1984 Jul;23(7A):741–747. doi: 10.1016/0028-3908(84)90106-0. [DOI] [PubMed] [Google Scholar]
- Senba E., Yanaihara C., Yanaihara N., Tohyama M. Co-localization of substance P and Met-enkephalin-Arg6-Gly7-Leu8 in the intraspinal neurons of the rat, with special reference to the neurons in the substantia gelatinosa. Brain Res. 1988 Jun 21;453(1-2):110–116. doi: 10.1016/0006-8993(88)90148-5. [DOI] [PubMed] [Google Scholar]
- Skilling S. R., Smullin D. H., Larson A. A. Differential effects of C- and N-terminal substance P metabolites on the release of amino acid neurotransmitters from the spinal cord: potential role in nociception. J Neurosci. 1990 Apr;10(4):1309–1318. doi: 10.1523/JNEUROSCI.10-04-01309.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J. M., Getto C. J., Neldner K., Reeve E. B., Krivoy W. A., Zimmermann E. Substance P and analgesia. Nature. 1976 Aug 26;262(5571):784–785. doi: 10.1038/262784a0. [DOI] [PubMed] [Google Scholar]
- Tang J., Chou J., Yang H. Y., Costa E. Substance P stimulates the release of Met5-enkephalin-Arg6-Phe7 and Met5-enkephalin from rat spinal cord. Neuropharmacology. 1983 Sep;22(9):1147–1150. doi: 10.1016/0028-3908(83)90052-7. [DOI] [PubMed] [Google Scholar]
- Yaksh T. L., Jessell T. M., Gamse R., Mudge A. W., Leeman S. E. Intrathecal morphine inhibits substance P release from mammalian spinal cord in vivo. Nature. 1980 Jul 10;286(5769):155–157. doi: 10.1038/286155a0. [DOI] [PubMed] [Google Scholar]
- Yaksh T. L., Rudy T. A. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976 Dec;17(6):1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- Yaksh T. L. Spinal opiate analgesia: characteristics and principles of action. Pain. 1981 Dec;11(3):293–346. doi: 10.1016/0304-3959(81)90633-3. [DOI] [PubMed] [Google Scholar]
- Yasphal K., Wright D. M., Henry J. L. Substance P reduces tail-flick latency: implications for chronic pain syndromes. Pain. 1982 Oct;14(2):155–167. doi: 10.1016/0304-3959(82)90096-3. [DOI] [PubMed] [Google Scholar]



