Abstract
The purpose of the current study was to assess social interaction (SI) following acute and repeated methamphetamine (MA) administration.
Rats were injected with 5.0 mg/kg of MA and SI was tested 30 minutes or 24 hours later. In another group of animals, MA sensitization was induced using 5.0 mg/kg of MA, and SI was assessed after one day or thirty days of abstinence.
SI was reduced in rats injected with MA 30 minutes, but not 24 hours, prior to testing, compared with saline controls. Impaired SI was observed in combination with active avoidance of the conspecific animal. Repeated injections of MA progressively reduced locomotor activity and increased stereotypy, indicating that animals were sensitized. However, no differences in SI were observed 24 hours or 30 days following the induction of sensitization.
The absence of detectable differences in SI following MA sensitization may be attributable to the relatively short regimen used to induce sensitization. However, the current series of experiments provides evidence that an acute injection of MA decreases SI and simultaneously increases avoidance behavior, which supports a link between psychostimulant use and impaired social functioning. These data suggest that the acute injection model may provide a useful model to explore the neural basis of impaired social functioning and antisocial behavior.
Keywords: Methamphetamine, psychostimulant, sensitization, abstinence, social interaction, rat, addiction
Introduction
Methamphetamine (MA) is a widely abused and addictive psychostimulant. Psychostimulant abuse can elicit antisocial behaviors such as aggression, violence, and in some cases, complete withdrawal from social situations (Homer et al., 2008; Miczek and Tidey, 1989). Psychostimulant-induced changes in SI model behavioral impairments observed in several psychological disorders (Tordjman et al., 2007), including autism (Scheeren et al., 2012), depression (Steger and Kashdan, 2009), as well as anxiety and phobias (Brown et al. 1997). More specifically, a parametric assessment of how SI is altered following MA use is necessary to identify which behavioral measures are altered as drug use transitions from recreational to habitual use. Towards this goal, the current study examined the time course of impairments in SI following acute and repeated MA administration.
Individuals who chronically abuse MA exhibit cognitive and behavioral alterations, such as increased anxiety (Rawson, 2013) and altered executive function, including impaired decision-making (Paulus et al., 2003) and attentional deficits (Salo et al., 2009). Similarly, rodents exposed to repeated MA show impairments in cognitive flexibility (Kosheleff et al., 2012), in addition to impaired temporal and episodic memory (Janetsian et al., 2015; Belcher et al., 2006). Some deficits in cognition induced by MA use persist after abstinence (Salo and Fassbender 2011; Salo et al. 2009; Janetsian et al. 2015), and may increase the probability of relapse (Tapert et al., 2004; Reichel et al., 2011). However, it is not known if SI is altered after acute or repeated MA use.
Impairments in social functioning are greater in individuals who have high levels of anxiety (i.e. general anxiety disorder) (Henning et al., 2007), which suggests that there may be a link between the two behaviors. Various clinical disorders and animal models also point to a relationship between the two behaviors (for review see Allsop et al. 2014). Animal models also support a role between social functioning and anxiety-like behavior during a SI task (File and Seth, 2003; Lungwitz et al., 2014). Assessment of SI and anxiety-like behaviors in rodent models facilitates the exploration of the neural systems that mediate each behavior and how they might be altered in neuropsychiatric disorders such as addiction. Furthermore, this link between social functioning and anxiety in both humans and animal models suggest a common neural substrate for both behaviors (Allsop et al., 2014).
In addicted individuals, the most common pattern of MA use is using between 1–6 times a day (Homer et al., 2008). Since this pattern is most common in humans, repeated intermittent MA use, such as the regimen used in these experiments, might precede the transition to the stage of addiction where drug use is florid. This makes examination of both repeated and single use very important in order to understand the time course in which cognitive and behavioral changes may occur. Animal models provide a powerful and highly controlled tool to assess the specific behavioral changes that are associated with each stage of addiction, which may not be accessible in clinical populations. For example, one can separate the changes in behavior and neural function that are observed after a single use versus those that require repeated administration. Towards this goal, behavioral sensitization protocols are particularly useful. Sensitization is defined as an augmented psychomotor response with repeated administration of a pharmacological agent (Pierce and Kalivas, 1997) and is paralleled by a number of protracted changes in neurobiology and physiology (Lapish et al., 2012; Lodge and Grace, 2008; Bastia et al., 2005; Janetsian et al., 2015). Importantly, a number of the behavioral and cognitive abnormalities found in addicted individuals are also observed in sensitized animals, thus lending translational validity to this model (Fletcher et al., 2007). Therefore, this study used sensitization as a method to contrast the behavioral effects evoked by acute MA compared with those following repeated MA administration.
The primary goal of the current study was to assess the persistent effects of MA on SI. Towards this goal, SI was first examined in a group of animals either 30 minutes or 24 h after a single injection of MA. MA has been previously shown to impair SI 30 minutes after injection (Slamberova et al., 2010, 2011) and thus provides an important control condition indicating that deficits are observable with the experimental methods used here. Secondly, a behavioral sensitization protocol that was previously shown to induce persistent deficits in recognition memory (Janetsian et al., 2015) was used to examine the persistent effects of repeated MA on SI. Currently there are no published studies on the effects of repeated MA on SI after abstinence. This missing aspect of the literature is particularly salient as a repeated pattern of MA use is common amongst those who abuse MA (Homer et al., 2008). Therefore there is a critical and unmet need to investigate the consequences of repeated MA administration on SI after abstinence.
Methods
Subjects
Adult male Sprague-Dawley rats (n = 96) (Harlan, Indianapolis, IN) that weighed 250–300g were individually housed and maintained on a reverse light/dark schedule, with lights on from 20:00 – 08:00 h (light phase) and lights off from 08:00 – 20:00 h (dark phase). The experiments were tested in the dark phase of the light/dark cycle under the same lighting conditions as in the colony room. All animals had free access to food and water and were handled for 10 minutes a day for one week prior to experimentation. Each experiment was conducted in a separate group of animals. All procedures were approved by the Purdue School of Science Animal Care and Use Committee and conformed to the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Academic Press, 2003).
Locomotor Activity and Stereotypy
Locomotor activity and stereotypy were recorded in ANY-maze (Wood Dale, IL) and acquired with a video camera mounted above the locomotor chamber. For locomotor activity, distance travelled (m) was recorded and included all horizontal movement, whether it was during SI or social avoidance. For stereotypy, behavior was scored manually by an observed blind to experimental conditions (Ellinwood and Balster, 1974).
Social Interaction
All animals were individually habituated in an open field chamber (86.36 × 93.98 × 31.24 cm) for 10 minutes for two consecutive days. The chamber was illuminated with a red light. Conspecific partner rats that were age, gender, and weight matched were used in no more than three test sessions. In line with other labs that use the SI task (e.g. Lungwitz et al., 2014) the partner rat was used more than once in this set of experiments and sessions were separated by at least 30 minutes. Partner rats were handled one-week prior to experimentation and individually habituated under the exact same procedures as the experimental animals. On the testing day, one partner rat was placed in the open field chamber (with no exposure to drug) followed by the experimental rat. The two rats were left in the chamber for 5 minutes and activity was recorded using ANY-maze via a video camera mounted above the chamber. Animals were then placed back into their home cages. Each video was manually scored for interaction, by an observer blind to the conditions, using the methods of Truitt et al. (2007). Interaction was considered as sniffing of and physical contact (e.g. crawling on or grooming) with the partner rat. All SI experiments took place in the same open field to eliminate the possibility of contextual differences.
Avoidance Behavior
Avoidance was assessed as a second behavioral measure to characterize whether the acute effect of MA was associated with an overall decrease in movement (causing decreases in both SI and avoidance) or whether it was specific to one behavior. Avoidance was measured in the first two experiments during the 5 minute SI test, and scored by an observer blind to the experimental conditions. Avoidance consisted of the experimental rat avoiding contact with the partner rat by moving away when the partner rat sniffed or initiated physical contact with the experimental rat. A ratio of this behavior was made using the following data: total time experimental rat avoided contact/total time partner rat initiated and continued making contact with experimental rat (referred to as 'social avoidance (%)' in the figures). More specifically, avoidance behavior consisted of measuring the time when the experimental rat moved away from the conspecific rat following the initiation of contact via sniffing or physical contact by the conspecific. Avoidance was quantified from the moment the experimental animal turned away from the conspecific animal. As long as the experimental rat was actively moving away from the conspecific rat, this was considered avoidance.
Experiments
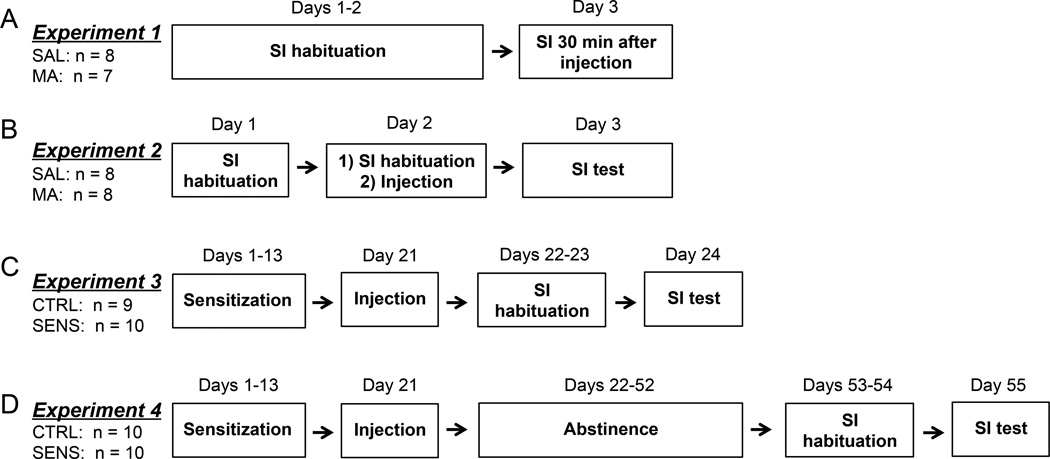
The timeline of experiments and numbers of experimental animals per experiment are summarized in Fig. 1.
Fig. 1.
A timeline of experiments following (A) acute MA injection while MA is 'on-board' (B) single MA injection (C) repeated MA injection after 1 day of abstinence (D) repeated MA injection after 30 days of abstinence.
Experiment 1: The effects of acute MA on SI
To assess the acute 'on-board' effects of MA on SI, rats were first individually habituated for two consecutive days in the SI chamber as described above and no MA was administered. On the testing day, experimental rats received a single injection of MA (n=7) or SAL (n=8). Thirty-minutes after the injection, SI, avoidance behavior, locomotor activity, and stereotypy were assessed in the SI chamber for 5 minutes (i.e. 30–35 min post-injection). In total, six rats were used as partner animals.
Experiment 2: The effects of a single exposure of MA on SI following short-term (24h) abstinence
Experiment 2 was conducted to assess whether a single injection of MA had a residual effect on SI the following day (24 hours following a single MA injection). Rats were first habituated for two days in the SI chamber as in experiment 1. One-hour following the second SI habituation on day 2, rats were placed in the locomotor chamber for 60 min, removed briefly for administration of MA (n=8) or SAL (n=8), and then placed back into the locomotor chamber for an additional 60 min to assess locomotor activity and stereotypy. On the third day, locomotor activity, stereotypy, SI and avoidance behavior were assessed in the SI chamber as in experiment 1. Six partner rats were used in this experiment, which were different from the rats used previously.
Experiment 3: The effects of repeated MA on SI following short-term (24h) abstinence
Animals received repeated injections of MA or SAL (MA: n=10, SAL: n=9). Before injections, animals were acclimated for 60 min to locomotor chambers (54.61-cm diameter × 41.91-cm height). Then, animals were injected with a single injection of 5.0 mg/kg of MA (SENS) or SAL (CTRL) and placed back into the chamber for another 60 min. Animals received a single injection every other day for 13 days (one injection on Day 1, 3, 5, 7, 9, 11, and 13: 7 total injections; induction phase). On days that rats did not receive an injection (Days 2, 4, 6, 8, 10, and 12), they were left undisturbed in their home cages. Following Day 13, rats were then left undisturbed for one week in their home cage (Days 14–20). An identical treatment was administered on the 'Sensitization Test' (Day 21) as during the induction phase (i.e. SENS rats received an injection of 5.0 mg/kg of MA and CTRL rats received an injection of SAL). To assess sensitization, distance travelled (m) and stereotypy (Ellinwood and Balster, 1974) were the dependent variables. Following the last injection on Day 21, all rats were then habituated to SI chambers for two days then tested for SI with a partner rat on the third day to evaluate the consequences of repeated MA administration on SI following short-term abstinence. Seven novel partner rats were used in this experiment.
Experiment 4: The effects of repeated MA on SI following extended (30 day) abstinence
In Experiment 4, a separate group of animals went through the same sensitization induction regimen as in experiment 3 (MA: n=10, SAL: n=10). However, after the final MA injection (Day 21), animals were left in their home cages for thirty days and then assessed on SI after 30 days of abstinence. Seven novel partner rats were used in experiment 4.
Drug Treatments
On injection days, rats were administered either 0.9% saline (SAL) or Methamphetamine Hydrochloride (MA; Sigma Chemical Co., St Louis, Mo., USA) via i.p. injection. MA was injected at a dose of 5.0 mg/kg in a final volume of 1.0 ml/kg after being dissolved in sterile SAL.
Data Analysis
For experiments 1 and 2, a two-tailed unpaired t-test was used to examine differences in locomotor activity between groups injected with SAL or MA. Stereotypy was scored using a 1–9 rating scale (Ellinwood and Balster, 1974) and group differences were evaluated via a two-tailed Mann-Whitney U-test. For each of experiments 3 and 4, locomotor activity was analyzed using repeated-measures analysis of variance (ANOVA) with treatment as a between-subjects factor and days as a within-subjects factor. Using separate Kruskal-Wallis analyses, stereotypy was analyzed comparing Days 1–8 in SENS animals in the short-term abstinence group, CTRL animals in the short-term abstinence group, SENS animals in the extended abstinence group, and CTRL animals in the extended abstinence group. Bonferroni post-hoc and Mann-Whitney U tests were used for between-subjects testing for locomotor activity and stereotypy, respectively. Dunnett's and Dunn's multiple comparisons were used for within-group testing locomotor activity and stereotypy, respectively. To assess the relationship between locomotor activity and stereotypy in animals injected with repeated MA from experiment 3 and 4 (collapsed), a Spearman correlation was conducted. Each value consisted of the rat's mean locomotor activity or stereotypy score over 60 min of recording for that day (12 total bins).
For experiments 1 and 2, avoidance behavior was assessed between SAL and MA via a two-tailed unpaired t-test. For all experiments, SI was assessed between SAL and MA using a two-tailed unpaired t-test. To further assess if there was an interaction between abstinence period and treatment, a two-way ANOVA was used to assess SI data between CTRL and SENS from experiments 3 and 4. The between-subject factors were time (one-day or 30-day abstinence) and treatment (CTRL or SENS). P-values for Bonferroni, Dunnett's, and Dunn's tests are reported as already corrected for number of comparisons by this statistic. Significance thresholds for Mann-Whitney U tests (alpha) were adjusted for the number of comparisons and are stated in the results. Unless otherwise stated, p < 0.05 was used for all statistical analyses.
Results
Experiment 1: The effects of acute MA on SI
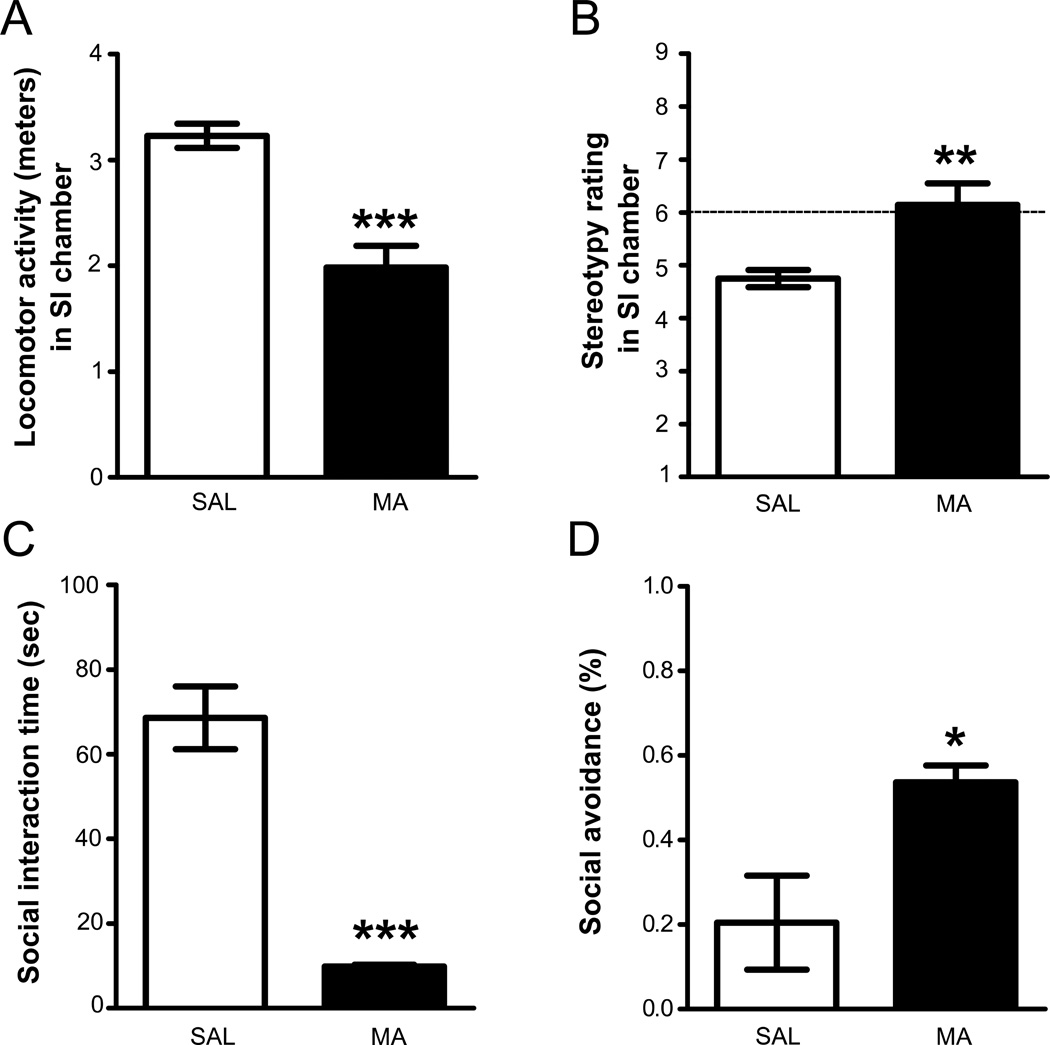
Analysis of locomotor activity revealed significantly lower locomotor activity in animals injected with 5.0 mg/kg MA compared to animals injected with SAL (t(13) = 5.49, p < 0.001) during the 30–35 minute time period following injection (Fig. 2A). Furthermore, animals injected with MA had higher stereotypy scores compared to animals injected with SAL (Mann-Whitney U = 6.00, p < 0.01, Fig. 2B). Animals injected with MA interacted with partner rats significantly less than animals injected with SAL (unpaired t-test, t(13) = 7.37, p < 0.001; Fig. 2C). To further investigate these effects, and because it was possible that reductions in locomotor activity and increased stereotypy could interfere with measurements of SI, the time spent by the experimental rat actively avoiding the partner rat was quantified (see methods). Animals injected with MA spent significantly more time avoiding partner-initiated interactions compared to animals injected with SAL (unpaired t-test, t(13) = 2.66, p < 0.05, Fig. 2D).
Fig. 2.
The effects of MA 30- min after an injection of SAL or 5.0 mg/kg MA. (A) Locomotor activity (during the first 5 min of recording). (B) Stereotypy (during the first five minutes of recording), with a score of 6 indicating stereotypy (dashed line). (C) SI time. (D) Avoidance time, measured as total time the experimental animal avoided contact /total time partner the animal initiated and continued making contact with experimental rat. All data are depicted as mean ± SEM; *p<0.05, **p<0.01, ***p<0.001. (MA: n = 7; SAL: n = 8; Partners: n = 6)
Experiment 2: The effects of a single exposure of MA on SI following short-term (24hr) abstinence
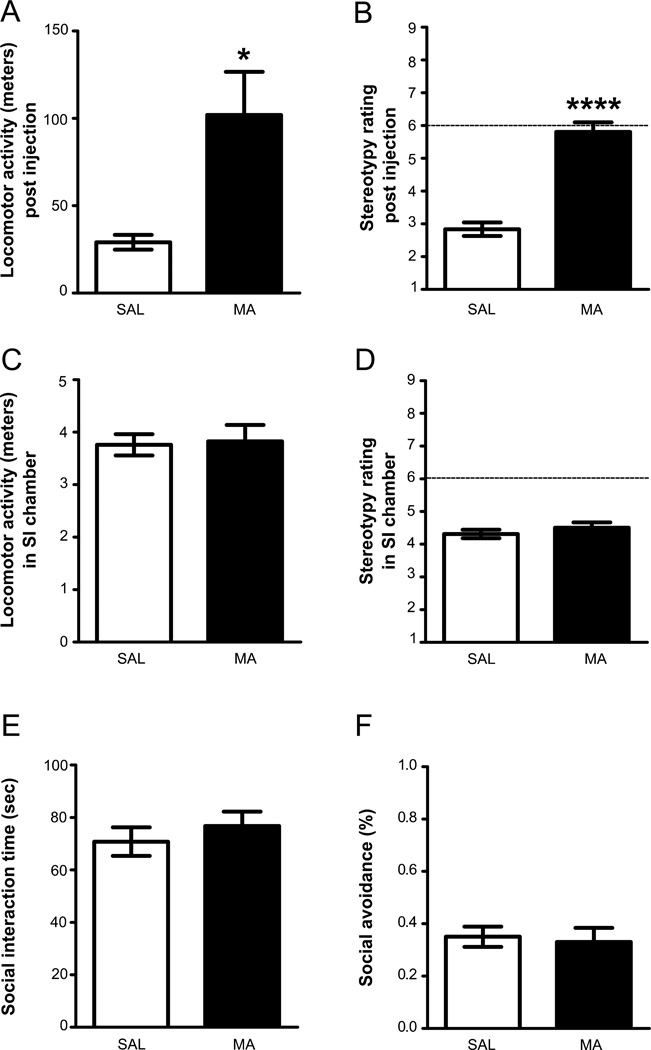
Increased locomotor activity was observed in response to a single injection of 5.0 mg/kg MA 24 h prior to SI testing (unpaired t-test, t(14) = 2.91, p < 0.05; Fig. 3A). Furthermore, animals injected with MA were hyperactive, as indicated by a score between 5 and 6 on the stereotypy scale, and they had significantly higher scores than animals injected with SAL (Mann-Whitney U = 0.0, p < 0.001; Fig. 3B). During SI, there were no significant differences between the two groups in locomotor activity (unpaired t-test, t(10) = 0.17, NS, Fig. 3C) or stereotypy scores (Mann-Whitney U = 24.50, NS; Fig. 3D) in the SI chamber. Furthermore, there was no significant difference between SAL and MA groups in total time interacting during SI (unpaired t-test, t(14) = 0.76, NS; Fig. 3E) or in avoidance time (unpaired t-test, t(14) = 0.30, NS, Fig. 3F), suggesting that there were not residual effects on behavior 24 h after a single MA injection.
Fig. 3.
The effects of a single exposure of MA on SI following short-term (24-h) abstinence. (A) Locomotor activity in the locomotor chamber immediately after a SAL or 5.0 mg/kg MA injection. (B) Stereotypy immediately after a SAL or 5.0 mg/kg MA injection, with a score of 6 or above indicating stereotypy (dashed line). (C) Locomotor activity in the SI chamber 24 h post-injection. (D) Stereotypy score in the SI chamber 24 h post-injection. (E) Mean SI time (throughout five minutes of SI recording) 24 h after a SAL or 5.0 mg/kg MA injection. (F) Mean avoidance time 24 h after a SAL or 5.0 mg/kg MA injection. All data are depicted as mean ± SEM. (MA: n = 8; SAL: n = 8; Partners: n = 6).
Experiments 3 & 4: The effects of repeated MA on SI following short term (one-day) and extended (30-day) abstinence
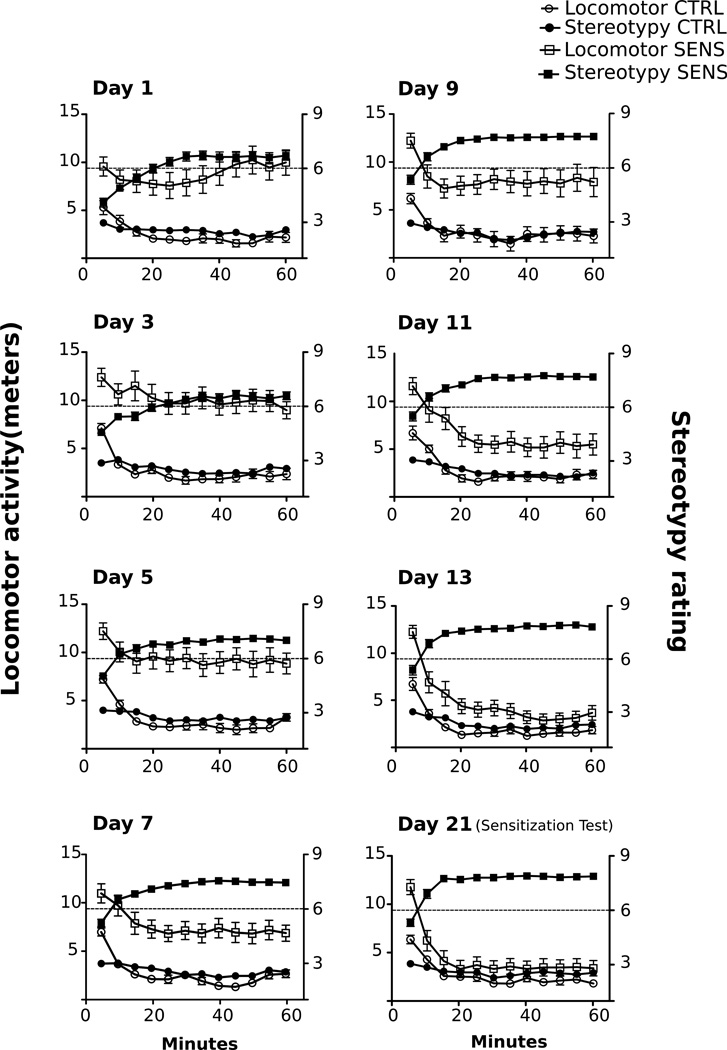
Locomotor activity and stereotypy throughout the injection regimen in animals that received repeated MA or SAL from experiments 3 and 4 is shown in Fig. 4. It is apparent that these two behaviors are inversely related in the SENS group, which became more evident as the injection regimen progressed. When examining the relationship between locomotor activity and stereotypy in SENS animals (Days 1–21), there was an initial nonsignificant negative relationship between locomotor activity and stereotypy on Days 1–9 that reached significance on Days 11–21 (Table 1).
Fig. 4.
Locomotor activity and stereotypy (shown in 5 min bins for a total of 60 min) in animals injected repeatedly with MA (n = 20) or SAL (n = 19) on Days 1–21 (data from experiments 3 & 4). M. Correlation values for the SENS group can be found in Table 1. All data are depicted as mean ± SEM.
Table 1.
The effects of repeated MA on the relationship between locomotor activity and stereotypy across days 1–21
| Day 1 | Day 3 | Day 5 | Day 7 | Day 9 | Day 11 | Day 13 | Day 21 | |
|---|---|---|---|---|---|---|---|---|
| Spearman Rho (ρ) | −0.06 | −0.07 | −0.33 | −0.44 | −0.25 | −0.72** | −0.48* | −0.56* |
(Spearman ρ
p < 0.05,
p < 0.001).
(MA: n = 20; SAL: n = 19).
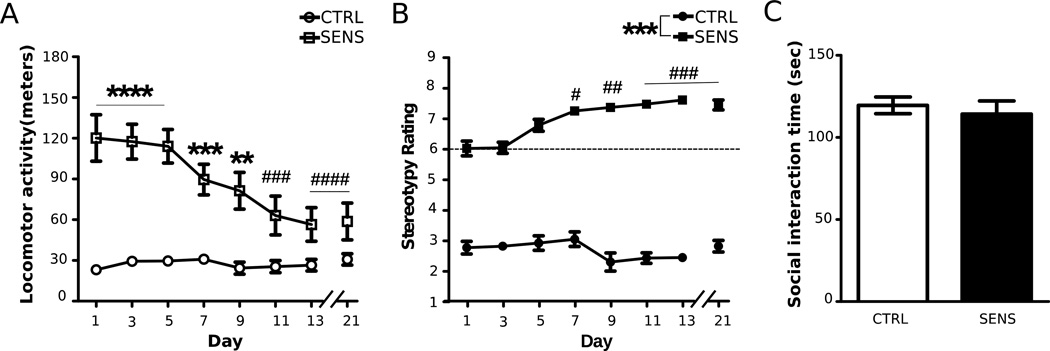
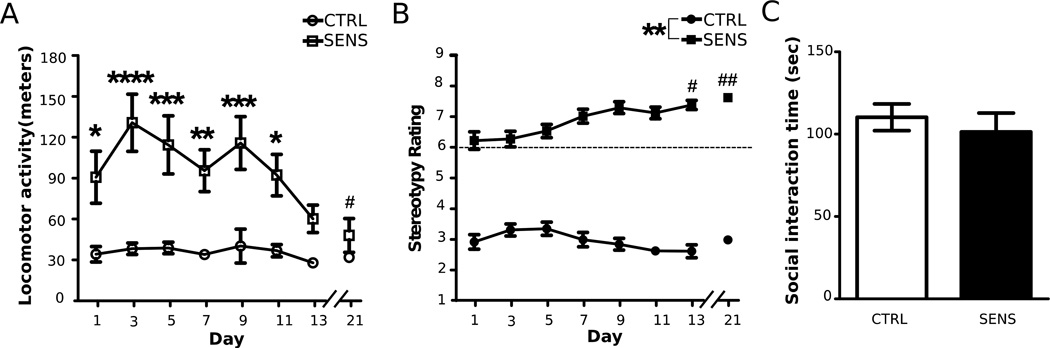
Analysis of locomotor activity from animals in the short-term abstinence group (experiment 3; Days 1–21) revealed an initial increase in the SENS group, indicated by significant main effects of time (F(7,119) = 4.32, p < 0.001) and treatment (F(1,17) = 54.27, p < 0.001), and a significant time by treatment interaction (F(7,119) = 4.36, p < 0.001) (Fig. 5A). When examining within-group differences, Dunnett's multiple comparison indicated that SENS animals had significantly decreased locomotor activity on Days 11 & 13 when compared to Day 1 in the short-term abstinence group. The same pattern followed for animals in the extended abstinence group (experiment 4: time: F(7,126) = 4.96, p < 0.001; treatment: F(1,18) = 22.34, p < 0.001; time by treatment interaction F(7,126) = 3.01, p < 0.01; Fig. 6A). Furthermore, higher locomotor activity was observed in the SENS group compared to the CTRL in both experiments (Figs. 5A & 6A). Lastly, in both experiments, when SENS animals were injected and tested for the 8th time on Day 21, to confirm sensitization, they had significantly decreased locomotor activity compared to Day 1 (Fig,. 5A & Fig,. 6A).
Fig. 5.
The effects of repeated MA on SI after short-term abstinence. (A) Locomotor activity following injections for each day of the induction phase (**p <0.01, ***p <0.001, ****p<0.0001, significantly higher locomotor activity compared to CTRL animals; ###p<0.001, ####p<0.0001, significantly lower within-group locomotor activity compared to Day 1 in SENS animals). (B) Stereotypy following injections for each day of the induction phase (***p< 0.001, significantly higher stereotypy ratings compared to CTRL animals; #p<0.05, ##p<0.01, ###p<0.001, significantly higher within-group stereotypy compared to Day 1 in SENS animals). (C) Interaction time one day after the last SAL or 5.0 mg/kg MA injection. All data are depicted as mean ± SEM. (MA: n = 10; SAL: n = 9; Partners: n = 7).
Fig. 6.
The effects of repeated MA on SI after extended abstinence. (A) Locomotor activity following injections for each day of the induction phase (*p <0.05, **p <0.01, ***p <0.001, ****p<0.0001, significantly higher locomotor activity compared to CTRL animals; #p<0.05, significantly lower within-group locomotor activity compared to Day 1 in SENS animals). (B) Stereotypy following injections for each day of the induction phase (**p< 0.01, significantly higher stereotypy ratings compared to CTRL animals; #p<0.05, ##p<0.01, significantly higher within-group stereotypy compared to Day 1 in SENS animals). (D) Mean interaction time 30 days after the last SAL or 5.0 mg/kg MA injection. All data are depicted as mean ± SEM. (MA: n = 10; SAL: n = 10; Partners: n = 7).
When examining stereotypy on Days 1–21 in animals from experiment 3, an increase in stereotypy was observed in the SENS group only (Kruskal-Wallis, χ2 = 44.71, p < 0.001; Fig. 5B). To assess between-group differences, Mann-Whitney U tests were adjusted to α = 0.00625 for multiple comparisons and a significant difference in stereotypy score between CTRL and SENS groups was observed on all days (Fig. 5B). Lastly, Dunn's multiple comparison revealed that SENS animals had significantly higher stereotypy scores on Days 1–21 compared to Day 1 (Fig. 5B). Similar results were obtained when assessing stereotypy in experiment 4 (Kruskal-Wallis, χ2 = 28.82, p < 0.001; Fig. 6B). Mann-Whitney U tests confirmed differences in stereotypy scores between SENS and CTRL groups on all days (Fig. 6B). Lastly, Dunn's multiple comparisons revealed increased stereotypy scores on Days 13 & 21 when compared to Day 1 in the SENS group (Fig. 6B).
There were no significant differences between CTRL and SENS animals in total time interacting after 24 h (unpaired t-test, t(17) = 0.55, NS; Fig. 5C) or 30 days (unpaired t-test, t(18) = 0.63, NS; Fig. 6C) following the induction of sensitization. There were no differences in SI between SENS versus CTRL following one day or 30 days of abstinence (Figs. 5C and 6C), as indicated by non-significant main effects of time (F(1,35) = 1.63, >NS) and treatment (F(1,35) = 0.68, NS>), and time by treatment interaction (F(1,35) = 0.04, >NS).
Discussion
To our knowledge, this study is the first to directly compare the effects of acute versus repeated, sensitization-inducing regimens, of MA treatment on SI. Our data indicate that SI is reduced when MA is 'on-board', but not 24 h after a single injection or after repeated injections of MA.
Repeated MA-induced sensitization is characterized by a transition from hyperlocomotion to stereotypy
Previous studies show that administering repeated doses of MA induces sensitization in rodents, which is typically measured as progressive increases in locomotor activity or stereotypy to the same dose of drug (Wang et al. 2012). This phenomenon is reported to last as long as a year (Paulson et al., 1991). Furthermore, with repeated treatment, the effect of MA shifts from hyperactivity to stereotypy (Kuczenski et al., 2009; Fujiwara et al., 1987), especially when using higher doses including 5.0 mg/kg and 10.0 mg/kg (Janetsian et al., 2015; McGuire et al., 2011).
It is has been suggested that locomotor activity and stereotypy are competing behavioral states that are mediated by distinct neural circuits (Joyce and Iversen 1984; Segal and Mandell, 1974). Increases in locomotor activity are predominantly due to psychostimulant-induced neuroadaptations in dopamine (DA) neurotransmission within the mesolimbic DA system (Koob and Volkow, 2010). Conversely, it is thought that stereotypic behaviors are driven by neural circuits in the basal ganglia that are distinct from those that mediate hyperlocomotion (Wolgin, 2012). Specifically, gamma-aminobutyric acid (GABA)-ergic inputs from the globus palladus to the thalamus inhibit glutamatergic transmission, causing a decrease in thalamo-cortical output and an inhibition of movement, resulting in stereotypy (Alexander et al., 1986; Parent and Hazrati, 1995; Wolgin, 2012). In the current study, MA administration induced an initial increase in locomotor activity that eventually transitioned into an increase in stereotypy as the injection regimen progressed. Importantly, our data demonstrate that the injection regimen of repeated MA did induce sensitization. Furthermore, the negative correlation between locomotor activity and sensitization (Fig. 4), supports the notion that these behaviors are mutually exclusive and possibly the manifestation of competing neural circuits.
MA acutely inhibits SI
A decrease in SI was observed in animals given MA for the first time 30 minutes prior to behavioral testing. These results are consistent with previous studies that have focused solely on acute treatment and also observed decreases in SI after an injection of 0.5, 1.0, or 1.5 mg/kg of MA 30 minutes prior to SI testing (Šlamberová et al., 2010, 2011). Following acute MA, interpretation of SI can be complicated by changes in locomotor activity and stereotypy that are evoked by the drug (Joyce and Iversen 1984; Segal and Mandell, 1974). It is possible that the decreases in SI were influenced by the emergence of stereotypies. However, an examination of avoidance behavior in animals treated with acute MA showed that suppression of locomotor activity and the emergence of stereotypy were not solely responsible for the changes in behavior, as animals actively avoided contact with the conspecific animal, indicating that they were capable of moving. Furthermore, considering differences were observed in SI in animals that were administered MA in this experiment, this suggests that the scoring methods used are sensitive enough to detect differences in SI. Moreover, our findings fall in line with what has been previously reported with an acute dose of MA (Slamberová et al., 2010, 2011).
Decreases in SI when MA was 'on-board' could also be due to the anxiogenic effects that are brought about by stimulant drugs (Ettenberg and Geist, 1991). Although the current set of experiments did not explicitly measure anxiety, it is has been suggested that a decrease in SI accompanied by an increase in locomotor activity is indicative of a state of high anxiety, whereas an increase in both locomotor activity and SI is indicative of reduced anxiety (File and Seth, 2003). While animals were under the influence of MA, we observed a decrease in locomotor activity and a decrease in SI. Had we observed a decrease in SI but an increase in locomotor activity, a more compelling case could be made that animals were anxious, according to the criteria suggested by File and Seth (2003). However, it is still possible, even likely, that the decrease in locomotor activity was driven by the emergence of behavioral stereotypies, which does not rule out that animals were anxious at this time point. In the current set of experiments, it is possible that animals were avoiding SI because they were anxious. Future studies will be necessary to directly assess the effects of MA on anxiety and how this, in turn, influences SI.
No differences in SI 24 h after a single MA injection or after repeated MA administration
No differences in locomotor activity, stereotypy, SI, or avoidance behavior were observed 24 h after a single injection of MA. These data clearly indicate that the effects of this acute dose of MA on these behavioral measures occur only when the drug is 'on-board' and persist for less than 24 h.
There were also no differences in SI between groups after one or 30 days of abstinence from repeated administration of SAL or MA. Interestingly, rats from experiments that received single injections of MA interacted less than animals that received repeated injections of MA. This difference is possibly attributable to differences in handling across these experiments. In experiments 1 and 2, rats were handled for one week, whereas rats in the repeated-treatment groups were handled for one-week prior to experimentation in addition to handling during the repeated-injection phase for two weeks. Although there are no studies assessing the effects of handling on SI, Andrews and File (1993) demonstrated that handling can reduce behaviors indicative of anxiety on the elevated plus maze. Therefore, animals in experiments 3 and 4 had more handling possibly resulting in more SI compared to animals in experiments 1 and 2.
Another factor contributing to differences in SI could be age. It has been previously demonstrated that older rats typically exhibit less SI compared to younger rats (Garau et al., 2000; O'Shea et al., 2006). However, age was taken into consideration and controlled for as much as possible, such that for experiments 1, 2, and 3, SI was tested on PD 99 or 100. However, SI was assessed on PD 129 in experiment 4. Therefore, age was consistent for the first three experiments. Although there is a 30-day age difference between experiments 3 and 4, it is important to point out that the total interaction time in experiment 3 (on PD 99) and experiment 4 (on PD 129) was not different between groups, which suggests that age did not have an effect on SI. Given that age was controlled for when testing SI, other factors were compromised, such as the amount of time animals were individually housed. Animals from experiments 3 and 4 were singly housed for 22 days and 52 days, respectively, longer than those in experiments 1 and 2, which may have influenced SI times.
When examining SI, SENS animals had very similar SI scores compared to the CTRL group after one day and 30 days of abstinence. This is in contrast to a finding from another lab (Clemens et al., 2004) that employed a 'binge' method, which has been shown to cause neurotoxicity in animals, and to cause decreases in SI in MA-treated rats 4 weeks after the treatment regimen (Cho et al., 2001). In this study, rats were injected with SAL or MA (2.5 mg/kg or 5.0 mg/kg) every two hours for a total of four injections. Four weeks later, rats were assessed on SI while the drug was not 'on-board'. The effect on SI observed in Clemens et al. (2004), in contrast to the lack of effect in this current study, suggests that SI impairments might differ as a function of the regimen used. If this is the case, then the regimen used here was not sufficient in terms of dose or frequency of administration to decrease SI. However, in addition to employing a different MA dosing regimen than the current study, the study by Clemens et al. (2004) assessed SI using a MA-treated partner rat rather than a naive rat with no prior drug treatment, which further complicates direct comparisons between the two studies.
Repeated administration of MA using this dosing regimen was not sufficient to induce deficits in SI, although it does induce deficits in other behaviors such as temporal and recognition memory (Janetsian et al., 2015). MA-induced alterations in behavior may be attributable to adaptations in neural circuits that govern them. For example, transient inactivation of the prefrontal cortex impairs temporal memory (Hannesson et al., 2004), whereas transient inactivation of the hippocampus and perirhinal cortex impair recognition memory (Broadbent et al., 2010; Hannesson et al., 2004). However, neural circuits of the cerebral cortex, limbic system, and cerebellum are all implicated in the control of social behavior (Adolphs, 2002; Bachevalier and Málková, 2006; Baron-Cohen et al., 2000; Truitt et al., 2007), which suggests that the neural circuits that support SI are perhaps more distributed, or redundant, than those necessary for temporal and recognition memory. If the neural circuits that are engaged during SI are indeed distributed and complex, and considering the evolutionary advantage conferred by engaging in social behaviors (Adolphs, 2001), then it follows that this behavior should be particularly robust to insult.
The current study indicates that a sensitization regimen previously established to impair temporal and recognition memory is insufficient to impair SI. This should be considered in future studies that seek to examine the effects of MA on SI. Moreover, these data indicate that the reductions in SI following acute MA administration are attributable to active avoidance of the conspecific animal, which may reflect an anxiogenic state.
Supplementary Material
Acknowledgements
We would like to thank Maureen M. Timm for technical assistance. CCL is supported by NIAAA grant #AA007611, the ABMRF, and the Indiana Alcohol Research Center P60AA007611. DNL is supported by NIAAA grant # AA022268.
Footnotes
Conflicts of Interest and Source of Funding
None Declared
Contributor Information
Sarine S. Janetsian, Indiana University-Purdue University Indianapolis Department of Psychology, sjanetsi@iupui.edu, 402 N. Blackford, LD 124, Indianapolis, IN 46202, sjanetsi@iupui.edu
Aqilah M. McCane, Indiana University-Purdue University Indianapolis Department of Psychology
David N. Linsenbardt, Indiana University-Purdue University Indianapolis Department of Psychology
Christopher C. Lapish, Indiana University-Purdue University Indianapolis Department of Psychology Indiana University School Of Medicine Stark Neuroscience Institute; Indiana University-Purdue University Indianapolis School of Science Institute for; Mathematical Modeling and Computational Sciences.
References
- Adolphs R. The neurobiology of social cognition. Current opinion in neurobiology. 2001;11(2):231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Neural systems for recognizing emotion. Current opinion in neurobiology. 2002;12(2):169–177. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual review of neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Allsop SA, Vander Weele CM, Wichmann R, Tye KM. Optogenetic insights on the relationship between anxiety-related behaviors and social deficits. Frontiers in behavioral neuroscience. 2014;8:241. doi: 10.3389/fnbeh.2014.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews N, File SE. Handling history of rats modifies behavioural effects of drugs in the elevated plus-maze test of anxiety. European Journal of Pharmacology. 1993;235(1):109–112. doi: 10.1016/0014-2999(93)90827-5. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Málková L. The amygdala and development of social cognition: theoretical comment on Bauman, Toscano, Mason, Lavenex, and Amaral (2006) Behavioral neuroscience. 2006;120(4):989–991. doi: 10.1037/0735-7044.120.4.989. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Bullmore ET, Wheelwright S, Ashwin C, Williams SC. The amygdala theory of autism. Neuroscience and biobehavioral reviews. 2000;24(3):355–364. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- Bastia E, Xu Y-H, Scibelli AC, Day Y-J, Linden J, Chen J-F, Schwarzschild MA. A crucial role for forebrain adenosine A(2A) receptors in amphetamine sensitization. Neuropsychopharmacology?: official publication of the American College of Neuropsychopharmacology. 2005;30(5):891–900. doi: 10.1038/sj.npp.1300630. [DOI] [PubMed] [Google Scholar]
- Belcher AM, O’Dell SJ, Marshall JF. A sensitizing regimen of methamphetamine causes impairments in a novelty preference task of object recognition. Behavioural brain research. 2006;170(1):167–172. doi: 10.1016/j.bbr.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Gaskin S, Squire LR, Clark RE. Learning & memory. 1. Vol. 17. Cold Spring Harbor, N.Y.: 2010. Object recognition memory and the rodent hippocampus; pp. 5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EJ, Turovsky J, Heimberg RG, Juster HR, Brown TA, Barlow DH. Validation of the Social Interaction Anxiety Scale and the Social Phobia Scale across the anxiety disorders [Google Scholar]
- Cho AK, Melega WP, Kuczenski R, Segal DS. Synapse. 2. Vol. 39. New York, N.Y.: 2001. Relevance of pharmacokinetic parameters in animal models of methamphetamine abuse; pp. 161–166. [DOI] [PubMed] [Google Scholar]
- Clemens KJ, Van Nieuwenhuyzen PS, Li KM, Cornish JL, Hunt GE, McGregor IS. MDMA (“ecstasy”), methamphetamine and their combination: long-term changes in social interaction and neurochemistry in the rat. Psychopharmacology. 2004;173(3–4):318–325. doi: 10.1007/s00213-004-1786-x. [DOI] [PubMed] [Google Scholar]
- Ellinwood EH, Balster RL. Rating the behavioral effects of amphetamine. Analysis. 1974;28:35–41. doi: 10.1016/0014-2999(74)90109-5. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Geist TD. Animal model for investigating the anxiogenic effects of self-administered cocaine. Psychopharmacology. 1991;103(4):455–461. doi: 10.1007/BF02244244. [DOI] [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. European journal of pharmacology. 2003;463(1–3):35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y, Kazahaya Y, Nakashima M. Behavioral sensitization to methamphetamine in the rat: an ontogenic study. Psychopharmacology. 1987:316–319. doi: 10.1007/BF00518183. [DOI] [PubMed] [Google Scholar]
- Garau A, Martí MA, Sala J, Balada F. Age effects on the social interaction test in early adulthood male rats. Depression and anxiety. 2000;12(4):226–231. doi: 10.1002/1520-6394(2000)12:4<226::AID-DA6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Hannesson DK, Howland JG, Phillips AG. Interaction between Perirhinal and Medial Prefrontal Cortex Is Required for Temporal Order But Not Recognition Memory for Objects in Rats. The Journal of Neuroscience. 2004;24(19):4596–4604. doi: 10.1523/JNEUROSCI.5517-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning ER, Turk CL, Mennin DS, Fresco DM, Heimberg RG. Impairment and quality of life in individuals with generalized anxiety disorder. Depression and anxiety. 2007;24(5):342–349. doi: 10.1002/da.20249. [DOI] [PubMed] [Google Scholar]
- Homer BD, Solomon TM, Moeller RW, Mascia A, DeRaleau L, Halkitis PN. Methamphetamine abuse and impairment of social functioning: a review of the underlying neurophysiological causes and behavioral implications. Psychological bulletin. 2008;134(2):301–310. doi: 10.1037/0033-2909.134.2.301. [DOI] [PubMed] [Google Scholar]
- Janetsian SS, Linsenbardt DN, Lapish CC. Memory impairment and alterations in prefrontal cortex gamma band activity following methamphetamine sensitization. Psychopharmacology. 2015 doi: 10.1007/s00213-014-3840-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce EM, Iversen SD. Dissociable effects of 6-OHDA-induced lesions of neostriatum on anorexia, locomotor activity and stereotypy: The role of behavioural competition. Psychopharmacology. 1984;83(4):363–366. doi: 10.1007/BF00428546. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology?: official publication of the American College of Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosheleff AR, Rodriguez D, O’Dell SJ, Marshall JF, Izquierdo A. Comparison of single-dose and extended methamphetamine administration on reversal learning in rats. Psychopharmacology. 2012 doi: 10.1007/s00213-012-2774-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Leith NJ. Chronic amphetamine: is dopamine a link in or a mediator of the development of tolerance and reverse tolerance? Pharmacology, biochemistry, and behavior. 1981;15(3):405–413. doi: 10.1016/0091-3057(81)90270-7. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS, Melega WP, Lacan G, McCunney SJ. Human methamphetamine pharmacokinetics simulated in the rat: behavioral and neurochemical effects of a 72-h binge. Neuropsychopharmacology?: official publication of the American College of Neuropsychopharmacology. 2009;34(11):2430–2441. doi: 10.1038/npp.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapish CC, Chiang J, Wang JZ, Phillips aG. Oscillatory power and synchrony in the rat forebrain are altered by a sensitizing regime of D-amphetamine. Neuroscience. 2012;203:108–121. doi: 10.1016/j.neuroscience.2011.12.020. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Amphetamine activation of hippocampal drive of mesolimbic dopamine neurons: a mechanism of behavioral sensitization. The Journal of neuroscience?: the official journal of the Society for Neuroscience. 2008;28(31):7876–7882. doi: 10.1523/JNEUROSCI.1582-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lungwitz EA, Stuber GD, Johnson PL, Dietrich AD, Schartz N, Hanrahan B, Shekhar A, et al. The role of the medial prefrontal cortex in regulating social familiarity-induced anxiolysis. Neuropsychopharmacology?: official publication of the American College of Neuropsychopharmacology. 2014;39(4):1009–1019. doi: 10.1038/npp.2013.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire BA, Baladi MG, France CP. Eating high-fat chow enhances sensitization to the effects of methamphetamine on locomotion in rats. European journal of pharmacology. 2011;658(2–3):156–159. doi: 10.1016/j.ejphar.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Tidey JW. Amphetamines: aggressive and social behavior. NIDA research monograph. 1989;94:68–100. [PubMed] [Google Scholar]
- Nishikawa T, Mataga N, Takashima M, Toru M. Behavioral sensitization and relative hyperresponsiveness of striatal and limbic dopaminergic neurons after repeated methamphetamine treatment. European journal of pharmacology. 1983;88(2–3):195–203. doi: 10.1016/0014-2999(83)90006-7. [DOI] [PubMed] [Google Scholar]
- O’Shea M, McGregor IS, Mallet PE. Journal of psychopharmacology. 5. Vol. 20. Oxford, England: 2006. Repeated cannabinoid exposure during perinatal, adolescent or early adult ages produces similar longlasting deficits in object recognition and reduced social interaction in rats; pp. 611–621. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain research. Brain research reviews. 1995;20(1):128–154. doi: 10.1016/0165-0173(94)00008-d. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Camp DM, Robinson TE. Time course of transient behavioral depression and persistent behavioral sensitization in relation to regional brain monoamine concentrations during amphetamine withdrawal in rats. Psychopharmacology. 1991;103(4):480–492. doi: 10.1007/BF02244248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Hozack N, Frank L, Brown GG, Schuckit Ma. Decision making by methamphetamine-dependent subjects is associated with error-rate-independent decrease in prefrontal and parietal activation. Biological psychiatry. 2003;53(1):65–74. doi: 10.1016/s0006-3223(02)01442-7. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Research Reviews. 1997;25(2):192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Rawson RA. Current research on the epidemiology, medical and psychiatric effects, and treatment of methamphetamine use. Journal of food and drug analysis. 2013;21(4):S77–S81. doi: 10.1016/j.jfda.2013.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Schwendt M, McGinty JF, Olive MF, See RE. Loss of object recognition memory produced by extended access to methamphetamine self-administration is reversed by positive allosteric modulation of metabotropic glutamate receptor 5. Neuropsychopharmacology?: official publication of the American College of Neuropsychopharmacology. 2011;36(4):782–792. doi: 10.1038/npp.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, Fassbender C. Structural, Functional and Spectroscopic MRI Studies of Methamphetamine Addiction. 2011 doi: 10.1007/7854_2011_172. [DOI] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Galloway GP, Moore CD, Waters C, Leamon MH. Drug abstinence and cognitive control in methamphetamine-dependent individuals. Journal of substance abuse treatment. 2009;37(3):292–297. doi: 10.1016/j.jsat.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheeren AM, Koot HM, Begeer S. Social Interaction Style of Children and Adolescents with High-Functioning Autism Spectrum Disorder. Journal of autism and developmental disorders. 2012 doi: 10.1007/s10803-012-1451-x. [DOI] [PubMed] [Google Scholar]
- Segal DS, Mandell AJ. Long-term administration of d-amphetamine: progressive augmentation of motor activity and stereotypy. Pharmacology, biochemistry, and behavior. 2(2):249–255. doi: 10.1016/0091-3057(74)90060-4. [DOI] [PubMed] [Google Scholar]
- Šlamberová R, Mikulecká A, Pometlová M, Schutová B, Hrubá L, Deykun K. The effect of methamphetamine on social interaction of adult male rats. Behavioural Brain Research. 2010;214(2):423–427. doi: 10.1016/j.bbr.2010.06.019. [DOI] [PubMed] [Google Scholar]
- Slamberová R, Mikulecká A, Pometlová M, Schutová B, Hrubá L, Deykun K. Sex differences in social interaction of methamphetamine-treated rats. Behavioural pharmacology. 2011;22(7):617–623. doi: 10.1097/FBP.0b013e32834afea4. [DOI] [PubMed] [Google Scholar]
- Steger MF, Kashdan TB. Depression and Everyday Social Activity, Belonging, and Well-Being. Journal of counseling psychology. 2009;56(2):289–300. doi: 10.1037/a0015416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapert SF, Ozyurt SS, Myers MG, Brown SA. Neurocognitive ability in adults coping with alcohol and drug relapse temptations. The American journal of drug and alcohol abuse. 2004;30(2):445–460. doi: 10.1081/ada-120037387. [DOI] [PubMed] [Google Scholar]
- Tordjman S, Drapier D, Bonnot O, Graignic R, Fortes S, Cohen D, Millet B, et al. Animal models relevant to schizophrenia and autism: validity and limitations. Behavior genetics. 2007;37(1):61–78. doi: 10.1007/s10519-006-9120-5. [DOI] [PubMed] [Google Scholar]
- Truitt Wa, Sajdyk TJ, Dietrich AD, Oberlin B, McDougle CJ, Shekhar A. From anxiety to autism: spectrum of abnormal social behaviors modeled by progressive disruption of inhibitory neuronal function in the basolateral amygdala in Wistar rats. Psychopharmacology. 2007;191(1):107–118. doi: 10.1007/s00213-006-0674-y. [DOI] [PubMed] [Google Scholar]
- Wang J, Sun L-L, Zhu W-L, Sun Y, Liu J-F, Lu L, Shi J. Role of calcineurin in the VTA in rats behaviorally sensitized to methamphetamine. Psychopharmacology. :1–12. doi: 10.1007/s00213-011-2461-7. [DOI] [PubMed] [Google Scholar]
- Wolgin DL. Amphetamine stereotypy, the basal ganglia, and the “selection problem”. Behavioural brain research. 2012;231(2):297–308. doi: 10.1016/j.bbr.2011.11.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.