Abstract
Background
Semen represents a reservoir for human papillomavirus (HPV), rising concern in couples eligible for assisted reproduction techniques (ART). Humoral immunity against HPV is considered to protect from reinfection. We investigated the impact of vaccination on virus clearance in a cohort of infertile male patients showing HPV semen infection.
Methods
179 out of 619 infertile patients, showing HPV-DNA detection in semen by FISH analysis, were enrolled. Subjects were split into 91 vaccine-sensitive (VSPs) and 88 nonvaccine-sensitive patients (NVSPs) by INNO-LiPA. 19 VSPs showed vaccine-type specific seroconvesion at recruitment. All patients underwent specific counselling. 42 seronegative VSPs were randomly assigned to receive quadrivalent vaccination in 6 months, whilst 49 VSPs, 19 seroconverted and 30 seronegative, served as controls. The prevalence of HPV-DNA semen infection and serology was studied in a follow-up of 24 months.
Results
Compared to seronegative patients, VSP seroconverted at recruitment showed absence of multiple infections and reduced prevalence of HPV semen infection at 12 (P = 0.039), 18 (P = 0.034) and 24 months (P = 0.034) of follow-up. Vaccinated VSP showed improved healing (P = 0.001 at 6 months and P < 0.001 at 12 months vs seroconverted VSP), achieving clearance in 12 months.
Discussion
Humoral immunity has a major role in healing from HPV infection. Elder ART patients with HPV semen infection may benefit by the union of both specific counselling and available prophylactic vaccination.
Highlights
-
•
We evaluated whether seroconversion in males influences HPV semen infection.
-
•
Naturally seroconverted patients showed reduced prevalence of HPV semen infection.
-
•
Naturally seroconverted patients also showed virtual absence of HPV multiple infection.
-
•
Prophylactic HPV vaccination induced clearance within 12 months from recruitment.
-
•
Seroconversion represents a key process involved in the clearance of the HPV.
Available prophylactic vaccinations are considered of protective value for genital condyloma and precancerous lesions in female, but cost-effectiveness of the use of HPV vaccine in males is largely underinvestigated. HPV detection in semen is also an emerging problem in couples eligible for assisted reproduction techniques, since persistent infections are not compatible with repeated 6-months counselling-cycles to allow any spontaneous clearance of the virus in older infertile couples.
In this study, we provide evidence that the development of seroconversion in human males affected by HPV infection in the genito-urinary tract, detected by HPV-DNA presence in the semen, has beneficial effects on the clearance of a viral load. Moreover, administration of prophylactic vaccination to HPV infected-seronegative patients induced seroconversion within 6 months from the first dosage administration, achieving 10 folds-higher antibody titre compared to natural seroconversion. If vaccine administration ameliorates the clearance of HPV semen infection, this could be a potential benefit to overcome fertility problems related to persistent HPV infections in males, after an obvious cost-effectiveness analysis.
1. Introduction
Human papillomavirus (HPV) is one of the most diffuse sexually-transmitted diseases, with 60% of sexually active people being infected with at least one high-risk HPV type during their lifetime (Schiffman et al., 2007). 90% of these infections are generally cleared by the immune system within 2 years from primary exposure (Woodman et al., 2007). However, in the remaining cases, infection persists for a long time causing lesions that can progress into cancer (Castle et al., 2011). In particular, a reduced number of HPV types infecting the ano-genital area are actually oncogenic viruses that lead to cervical carcinoma (Tornesello et al., 2014), the seventh most common cancer in the world (Forman et al., 2012) and the second most common cancer among women (Arbyn et al., 2011). High-risk HPVs are also responsible for other types of cancer common to both genders. In fact, around 80% of tumours of the anus, 60% of the vagina, and 40% of vulva and penis are induced by HPV, mainly the HPV 16 type (Parkin and Bray, 2006). Moreover, nearly 90% of oropharynx, 50% of nasopharynx, and 26% of oral cavity tumours show positive detection for high risk-HPV types, highlighting the causative role of this virus in head and neck cancers (Walline et al., 2013).
Current therapies for the treatment of HPV infection of the ano-genital tract are mainly ablative and devoted to the removal of damaged HPV-infected lesions, such as warts and squamous intraepithelial lesions (Krogh von et al., 2000). However, most patients are not featured by macroscopic lesions. In particular, a variable percentage (13 to 88%) of asymptomatic men shows a reservoir for HPV in the penile foreskin (Giuliano et al., 2010). In this regard, semen represents a sampling site of high diagnostic value for the assessment of HPV infection in asymptomatic males (Laprise et al., 2014).
A growing number of evidence concurred to identify the impairment of human reproduction as a consequence of HPV infection (Foresta et al., 2015). In fact, a higher prevalence of HPV infection has been reported in semen from infertile patients, independently of the presence of risk factors for HPV (Garolla et al., 2011, Garolla et al., 2013a, Cai et al., 2014). Detection of HPV in semen has also been associated with an impairment of sperm quality, particularly dealing with cell motility (Foresta et al., 2010), reinforcing the possible involvement of this virus in male infertility (Garolla et al., 2011, Garolla et al., 2013b). In this context, a possible role of sperm as a carrier of HPV has been suggested by the associations between reduced pregnancy rate and detection of HPV-DNA in spermatozoa (Hermonat et al., 1997), as well as HPV detection in recurrent miscarriage (Matovina et al., 2004, Conde-Ferráez et al., 2013). Thus, HPV infection appears as an emergent problem in couples eligible for assisted reproduction techniques (ART) (Garolla et al., 2012). On this basis, healing from virus infection represents a key prerequisite for accession to assisted fertilization techniques, but the achievement of natural clearance could not be compatible with ART schedule, in particular for couples of older age (Foresta et al., 2015, Garolla et al., 2013b, Stoop et al., 2014).
Prophylactic vaccination relies on the development of humoral immune response to viral capsid proteins. This process is assumed to prevent virus infection of basal epithelial cells, through recognition of viral surface by specific neutralizing antibodies (Stanley et al., 2012). Moreover, HPV vaccines offer cross-protection against few specific non-vaccine HPV types for individuals without previous infection, with the quadrivalent vaccine showing some degree of protection against HPV 31, 33, and 45, for persistent infection and CIN2 + disease, and little evidence of cross-protection against HPV 52 and 58 (Malagón et al., 2012). It is generally accepted that this approach is not effective in treating existing infections or established HPV-related diseases. However, in both females and males with HPV-related disease, it has been demonstrated that vaccination with the quadrivalent HPV vaccine reduces the risk of subsequent disease even after surgical treatment (Joura et al., 2012, Swedish et al., 2012), suggesting a possible major role of humoral immunity in the healing process from viral infection. In this regard, we observed that patients featured by HPV detection in semen, together with natural seroconversion, showed faster semen clearance compared to non-seroconverted patients. This observation prompted us to evaluate the effect of seroconversion induced by prophylactic vaccination on clearance in males showing HPV-DNA detection in semen.
2. Methods
2.1. Participants
The study was conducted at the Unit of Andrology and Reproductive Medicine of the University Hospital of Padova, according to the principles of the Declaration of Helsinki, and approved by the Institutional Ethics Committee of the Padua General Hospital by Protocol No. 2336 and subsequent amendments.
619 male patients attending the unit for assisted reproduction from January 2013 to January 2015 underwent outpatient evaluation and were consecutively recruited, after acceptance of written informed consent. Inclusion criteria were: diagnosis of male infertility defined as alteration of sperm parameters and/or at least 2 years of unprotected sexual intercourse without conception with female partners without main genital diseases (tubal factor, uterine factor, cervical abnormalities, and ovarian disorders were excluded). Exclusion criteria were: history of cryptorchidism, testicular trauma, post-mumps orchitis, prior knowledge of HPV infection, previous or ongoing vaccination at the time of enrolment. Varicocele and bacterial seminal infections were excluded, respectively, by testicular Doppler ultrasound and microbiological sperm culture.
Semen analysis was performed according to the World Health Organization (World Health Organization, 2010). The presence of anti-sperm antibodies by SperMAr test, FISH analysis of whole semen for HPV-DNA, and genotyping at recruitment by the INNO-LiPA Genotyping Extra assay on whole semen were performed as previously described (Garolla et al., 2013a). Multiple infections at recruitment were defined as the detection of ≥ 2 different HPV types in whole semen by a genotyping assay.
2.2. Anti HPV-Antibodies
The presence and titre of serum anti-HPV IgG were assessed on peripheral blood samples throughout an overall follow-up period of 24 months, at recruitment (t0), 6 months (t6), 12 months (t12), 18 months (t18) and 24 (months). Patients of the vaccine arm were assessed also at 3, 6 and 9 and months (t3, t6 and t9 respectively).
Total anti-HPV immunoglobulin G (IgG) antibody detection was performed by an enzyme linked immuno-sorbent assay ELISA using a commercial kit supplied by DRG Diagnostic GmbH (Germany). The assay relies on a micro-well plate coated with virus-like particles derived from L1 protein of viral types 6, 11, 16 and 18 expressed in Saccharomyces cerevisiae. All the assays were performed with the same lot of the ELISA kit within its declared shelf-life period. According to manufacturer's instructions, each sample was diluted 1:100 and analysed in duplicate. The cut-off value was defined as optical density at 450 nm (OD) of negative control plus 0.250 and seropositive were samples having OD higher than the cut-off value. Antibody titre was assessed, with slight modifications, in both clearly-seropositive samples and in samples showing an OD included in the range: (cut-off value) ± 2 × (standard deviation of negative control). In these cases, sera were serially diluted (1:10–1:100–1:1000, respectively) and assessed as described above. Antibody titre was defined as the lowest dilution displaying an OD ≤ (cut-off value) + 2 × (standard deviation of negative control) (Zhao et al., 2014).
2.3. Definition of Treatment Arms
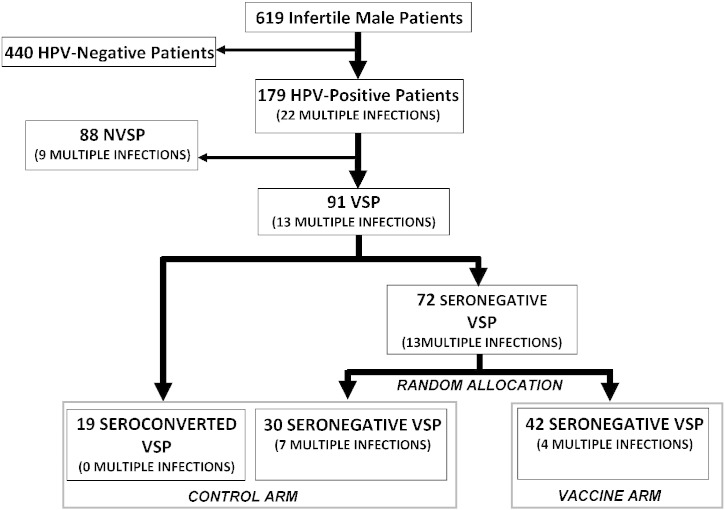
Fig. 1 shows the flow diagram of the study. FISH analysis allowed detection of HPV-DNA in the semen in 179 out of 619 infertile patients (28.9%), further confirmation by INNO-LiPA genotyping allowed us to identify the following HPV types: 6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 43, 44, 45, 51, 52, 53, 54, 56, 58, 59, 66, 68, 69, 71, 70, 73, 74, and 82. According to cross-protection provided by quadrivalent vaccine (Malagón et al., 2012, Draper et al., 2013), we identified 91 out of 179 patients (50.8%) featured by infection of at least one of the HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58, which were defined as vaccine-sensitive patients (VSPs). The remaining 88 patients (49.2%), infected by at least one of the HPV types 26, 35, 39, 40, 42, 43, 44, 51, 53, 54, 56, 59, 66, 68, 69, 71, 70, 73, 74, and 82 were called nonvaccine-sensitive patients (NVSPs). Nineteen out of 91 VSPs (20.9%) showed seroconversion for vaccine-type HPV at recruitment and were specifically considered in the control arm as seroconverted patients. 72 seronegative VSPs at recruitment were then randomly allocated to the control arm, as seronegative patients, or to a vaccine arm in a blinded fashion by third-party true randomization in a 1:1 ratio with a random number generator. Therefore, 49 men (19 seroconverted and 30 seronegative) were allocated to the control arm and 42 to the vaccine arm. Regardless to the assignment to any treatment arm, all HPV-positive patients underwent specific counselling as previously described (Garolla et al., 2014), being advised to have protected intercourse, to avoid smoking, and to refer to our unit in case of wart development.
Fig. 1.
Definition, size and prevalence of multiple infections within the treatment arms of the study cohort.
Abbreviations: HPV: human papillomavirus; VSP: vaccine sensitive-patients; NVSP: nonvaccine-sensitive patients.
The prevalence of HPV-DNA detection by FISH analysis in the semen of patients from the control arm, the vaccine arm and NVSP, was evaluated throughout an overall follow-up period of 24 months, at recruitment (t0), 6 months (t6), 12 months (t12), 18 months (t18) and 24 (months). Patients of the vaccine arm were assessed also at 3 and 6 months (t3 and t6, respectively), after each vaccine dose administration and at 9 months (t9).
2.4. HPV Vaccination
HPV vaccination was administered as off-label prescription of the quadrivalent vaccine Gardasil (Merck Serono S.p.A., Milan, Italy). Vaccine was administered as 3 injections over 6 months, the second dose received 2 months after the first dose, and the third dose received 6 months after the first dose respectively. The procedure was performed at the Unit of Hygiene and Public Health of Padova Hospital after acceptance of written informed consent. Patients receiving HPV vaccination were further assessed for the presence of HPV-DNA in whole semen by FISH analysis at 3 months, t3, 6 months, t6 and 9 months, t9, respectively.
2.5. Statistical Analysis
Data analysis was performed using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). The results were expressed as means ± standard error means (SEM). The Levene's test was used to test the homogeneity of variance among groups prior to data analysis. If homogeneity of variance assumption was violated, Welch test was performed and the respective P value was reported. Differences between two groups were analysed using Student's t-test. Differences between three or more groups were analysed using ANOVA and Fisher's least significant difference (LSD) adjustment for multiple comparisons of groups was applied to the pairwise comparisons of groups. Multivariate logistic regression was performed to adjust for potential confounders, the final model included variables significant at P < 0.10 on univariate analysis. Proportions and discrete variables were compared using the chi-square test or Fisher's exact test, when expected cell sizes were smaller than five. Statistical significance was defined at the P < 0.05 level using 2-sided tests; highly statistical significance was defined for values of P < 0.01.
3. Results
3.1. Patient's Characteristics
Clinical characteristics of the 619 male patients eligible for recruitment, further divided into 440 HPV-negative subjects and 179 patients showing HPV-DNA in whole semen, are summarized on Table 1.
Table 1.
Clinical characteristics of the cohort of 619 infertile patients.
| HPV + patients |
||||
|---|---|---|---|---|
| Parameters | HPV − patients (N = 440) (Mean ± SD) |
HPV + patients (N = 179) (Mean ± SD) |
Seroconverted (N = 19/179) (Mean ± SD) |
Seronegative (N = 160/179) (Mean ± SD) |
| Age (years) | 38.2 ± 8.1 | 37.1 ± 7.4 | 40.3 ± 7.9 | 39.0 ± 9.1 |
| HPV + Female partner (N) | 174 (39.5%) | 150 (83.8%)a | 17 (89.5%)a | 133 (83.1%)a |
| Sperm concentration (× 106/mL) | 35.9 ± 8.4 | 30.4 ± 13.1 | 29.9 ± 7.9 | 33.4 ± 15.2 |
| Total sperm count (× 106) | 112.5 ± 38.7 | 92.9 ± 40.3 | 90.7 ± 42.6 | 93.4 ± 39.8 |
| Progressive motility (%) | 39.3 ± 12.1 | 22.7 ± 13.4a | 15.4 ± 9.5a,b | 23.1 ± 14.2a |
| Normal morphology (%) | 17.4 ± 5.3 | 14.9 ± 8.7 | 14.8 ± 10.0 | 15.1 ± 8.9 |
| Viability (%) | 85.1 ± 6.4 | 74.9 ± 9.8 | 75.2 ± 7.7 | 74.4 ± 10.1 |
| Positive SpermMar test (N) | 32 (18.0%) | 88 (49.2%)a | 18 (94.7%)a,b | 70 (43.8%)a |
Abbreviations: HPV +: patients with positive detection of HPV-DNA in semen; HPV −: patients with negative detection of HPV-DNA in semen; SD: standard deviation of the mean. Significance: a = P < 0.05 vs HPV-patients; b = P < 0.05 vs HPV + seronegative patients.
Compared to negative subjects, HPV-positive patients were featured by higher prevalence of HPV-positive partner (150/179 HPV-positive vs 174/440 HPV-negative, P < 0.001), reduced progressive motility (22.7 ± 13.4% HPV + vs 39.3 ± 12.1 HPV −, P = 0.042) and higher prevalence of positive SperMar test (88/179 HPV + vs 32/440 HPV −, P < 0.001). By the use of the anti-HPV IgG ELISA Kit, among 179 infected patients, we identified 19 subjects showing natural seroconversion at recruitment for at least one of the HPV types 6, 11, 16 and 18 (HPV + seroconverted patients). This sub-population showed further decrease of progressive motility (15.4 ± 9.5% seroconverted vs 23.1 ± 14.2% non-seroconverted P = 0.039) and increased prevalence of positive SperMar test, compared to HPV-positive seronegative patients (18/19 seroconverted vs 70/160 nonseroconverted, P < 0.001).
In the whole group of HPV-positive infertile patients, INNO-LiPA analysis showed that HPV6 was the most represented low-risk HPV type (Schiffman et al., 2009), being detected in 57/179 patients (31.8%). On the other hand, HPV52 was detected in 32/179 (17.9%), representing the most prevalent high risk-type (Schiffman et al., 2009), followed by HPV18 (12/179; 6.7%) and HPV16 (9/179; 5.02%). Multiple infections at recruitment were detected in 22/179 patients (12.3%, Fig. 1), in agreement with previous reports (Rositch et al., 2012, Álvarez-Argüelles et al., 2013), with no differential distribution between VSP and NVSP (13/79 vs 9/88, P = 0.497). In particular, simultaneous detection of two, three and four HPV types was found in 9/22 patients (40.9%) and 8/22 patients (36.4%) and 5/22 (22.7%) respectively. The most frequent association was represented by HPV6 and HPV52, detected in 4/22 patients (18.2%).
After assignment to different arms, no significant segregation of multiple infections was observed between patients of the control arm and the vaccine arm (7/49 vs 4/42 respectively, P = 0.537). Among the 19 seroconverted patients of the control arm, no multiple HPV infections were observed, whilst 13 out of 72 seronegative VSPs were featured multiple infections at recruitment, with a difference close to statistical significance (P = 0.0625, Fig. 1).
3.2. Detection of HPV-DNA in Semen and Serology of Patients of the Control Arm and NVSP
The prevalence of infecting HPV-DNA, detected by FISH analysis, in the semen of patients of both the control arm and NVSPs throughout an overall observation of 24 months, is summarized on Table 2.
Table 2.
Prevalence of HPV-DNA detection in semen of both the control arm and non-vaccine sensitive patients.
| Time-point | Seronegative VSP (N = 30) |
NVSP (N = 88) |
P value (NVSP vs seronegative VSP) |
Seroconverted VSP (N = 19) |
P value (Seroconverted VSP vs seronegative VSP) |
P value (Seroconverted VSP vs NVSP) |
|---|---|---|---|---|---|---|
| t0 | 30 (100%) | 88 (100%) | 1 | 19 (100%) | 1 | 1 |
| t6 | 26 (87%) | 79 (90%) | 0.74 | 16 (84%) | 0.14 | 0.44 |
| t12 | 22 (73%) | 61 (69%) | 0.82 | 8 (42%) | 0.039 | 0.034 |
| t18 | 10 (33%) | 30 (34%) | 0.99 | 1 (5%) | 0.034 | 0.011 |
| t24 | 7 (23%) | 17 (19%) | 0.61 | 0 (0%) | 0.034 | 0.034 |
Abbreviations: t0: recruitment; t6, t12, t18, t24: 6, 12, 18 and 24 months from recruitment respectively; VSP: vaccine sensitive patients; NVSP: nonvaccine-sensitive patients. Significance: bold values = P < 0·05.
In general, progressive reduction of the prevalence of HPV-semen infection was noticed across the period of observation, with no significant difference between respectively seronegative VSP at recruitment and NVSP at all time points (respectively P = 0.74 at 6 months; P = 0.82 at 12 months; P = 0.99 at 18 months and P = 0.81 at 24 months). In VSP of the control arm showing natural seroconversion at recruitment, the prevalence of HPV-semen infection was lower at 12, 18 and 24 months compared to both seronegative VSP (P = 0.034 at 12 months; P = 0.034 at 18 months and P = 0.034 at 24 months respectively) and NVSP (P = 0.034 at 12 months; P = 0.011 at 18 months and P = 0.034 at 24 months respectively).
The mean percentage of sperm showing positive staining for HPV-DNA is reported on Table 3. Assuming seronegative VSP as reference, seroconverted VSPs showed a reduced percentage of HPV positive sperm cells at recruitment (11.6 ± 3.2% seroconverted VSP vs 22.1 ± 5.6% seronegative VSP, P = 0.026), at 6 months (8.0 ± 1.7% seroconverted VSP vs 14.5 ± 4.2% seronegative VSP, P = 0.031) and at 12 months of follow-up (3.3 ± 2.1% seroconverted VSP vs 16.1 ± 8.1% seronegative VSP, P = 0.014). No statistical inference was allowed at 18 and 24 because of incompatible numerosity of data. Anti-HPV IgG titre in seroconverted patients ranged from 1:58 ± 34 at recruitment to 1:98 ± 71 at 24 months, with no significant difference among time points. At 12 and 18 months of follow-up we observed incident natural seroconversion in respectively 1 and 2 patients within the group of seronegative VSP with an overall development of natural sero-conversion of 3/30 (10%) patients in a 24 months follow up period. However, no significant difference in terms of anti-HPV IgG titre, was observed between these patients and seroconverted VSP at recruitment.
Table 3.
Detection of HPV-positive cells in semen, seroconversion and antibody titre of both patients of the control arm and non-vaccine sensitive patients.
| Seronegative VSP (N = 30) |
NVSP (N = 88) |
Seroconverted VSP (N = 19) |
||||
|---|---|---|---|---|---|---|
| Time-point | % HPV + cells (mean ± SD) |
Seroconverted patients (gm titre ± SD) |
% HPV + cells (mean ± SD) |
Seroconverted patients (gm titre ± SD) |
% HPV + cells (mean ± SD) |
Seroconverted patients (gm mean titre ± SD) |
| t0 | 22.1 ± 5.6 | 0 | 27 ± 6.4 | 2/43 (1:65 ± 58) |
11.6 ± 3.2%* | 19 (1:58 ± 34n.a.) |
| t6 | 14.5 ± 4.2 | 0 | 11 ± 7.2 | 3/43 (1:42 ± 73) |
8.0 ± 1.7%* | 19 (1:66 ± 56n.a.) |
| t12 | 16.1 ± 8.1 | 1 (1:72) |
10 ± 9.3 | 1/43 (1:126) |
3.3 ± 2.1%* | 19 (1:85 ± 43n.a.) |
| t18 | 8.1 ± 3.8 | 3 (1:62 ± 37) |
6.2 ± 5.6 | 2/43 (1:55 ± 41) |
5%n.a. | 19 (1:92 ± 67n.s.) |
| t24 | 4.8 ± 3.4 | 3 (1:94 ± 21) |
4.1 ± 3.4 | 3/43 (1:94 ± 21) |
0%n.a. | 19 (1:98 ± 71n.s.) |
Abbreviations: t0: recruitment; t6, t12, t18, t24: 6, 12, 18 and 24 months from recruitment respectively; % HPV + cells: percentage of HPV-DNA positive-spermatozoa; VSP: vaccine sensitive patients; NVSP: nonvaccine-sensitive patients; gm titre: geometric mean of the antibody titre; SD: standard deviation of the mean.
Significance: n.a. = not available; * = P < 0·05 vs seronegative VSP; n.s. = P ≥ 0·05 vs seronegative VSP.
Contingent seroconversion to HPV-vaccine types in NVSP was assessed in a subgroup of patients (43 out of 88 NVSPs, Table 3), in order to identify any non-specific reactivity of the assay. The highest non-specific prevalence of seroconversion in this group of patients was 3/43 (0.9%).
3.3. Detection of HPV-DNA in Semen and Serology of Patients of the Vaccine Arm
The prevalence of HPV-DNA detection in the semen of patients of the vaccine arm, throughout an overall follow-up period of 24 months, is summarized on Table 4. All patients complied vaccination treatment.
Table 4.
Prevalence of HPV-DNA detection in semen of seronegative vaccine-sensitive patients (Seronegative VSP), seroconverted vaccine-sensitive patients (Seroconverted VSP) and patients of the vaccine arm.
| Seronegative VSP (N = 30) |
Seroconverted VSP (N = 19) |
Patients of the vaccine arm (N = 42) |
|
|---|---|---|---|
| Time-point | Prevalence of HPV-DNA detection in semen (%) | Prevalence of HPV-DNA detection in semen (%) | Prevalence of HPV-DNA detection in semen (%) |
| t0 | 30 (100%) | 19 (100%) | 42 (100%) |
| t3 | N.P. | N.P. | 31 (74%) |
| t6 | 26 (87%) | 16 (84%) | 17 (40%)a,c |
| t9 | N.P. | N.P. | 3 (7%) |
| t12 | 22 (73%) | 8 (42%)a | 0b,d |
| t18 | 10 (33%) | 1 (5%)a | 0 |
| t24 | 7 (23%) | 0 (0%)a | 0 |
Abbreviations: t0: recruitment; t3, t6, t9, t12, t18, t24: 3, 6, 9, 12, 18 and 24 months from recruitment respectively; N.P.: not performed.
Significance: a = P < 0.05 vs seronegative VSP; b = P < 0.001 vs seronegative VSP; c = P < 0.05 vs seroconverted VSP; d = P < 0.001 vs seroconverted VSP.
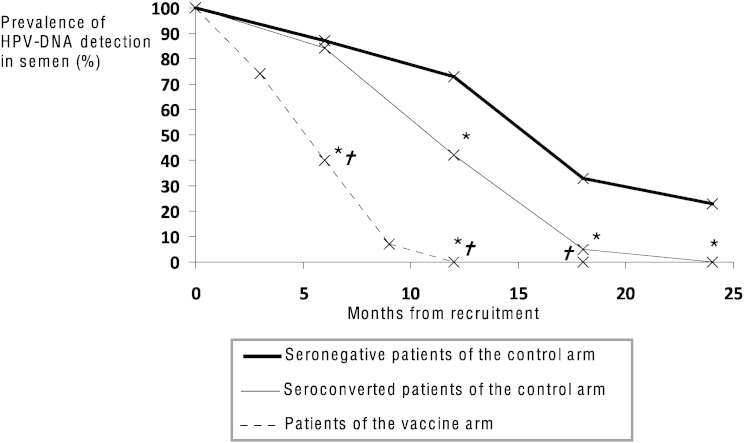
The prevalence of HPV-DNA detection in semen from vaccinated patients underwent a rapid decrease, achieving almost complete abolition within 12 months from recruitment. As shown on Table 4, patients from this arm showed a significantly lower prevalence of HPV-DNA detection in semen, at 6 and 12 months, than both seronegative VSP (P = 0.002 at 6 months and P < 0.001 at 12 months) and seroconverted VSPs (P = 0.001 at 6 months and P < 0.001 at 12 months) of the control arm. Data are graphically summarized in Fig. 2. Also the percentage of HPV-infected spermatozoa decreased progressively from 24.5 ± 7.1% at recruitment to 10.2 ± 3.4%·4.8 ± 5.0%·2.3 ± 2.1% and 0% at respectively 3, 6, 9 and 12 months in course of vaccine treatment (Supplemental Table 1). In addition, all vaccinated patients underwent seroconversion within 6 months from recruitment, showing higher anti-HPV IgG titre than sero-converted patients of the control arm (6 months follow-up 1:882 ± 408 vaccine vs 1:66 ± 56 seroconverted control arm P < 0.001; 12 months follow-up 1:795 ± 320 vaccine vs 1:85 ± 56 seroconverted control arm P < 0.001. Supplemental Table 1).
Fig. 2.
Prevalence of HPV-DNA detection in semen of patients from the vaccine arm (dotted line) and from the control arm. Control arm is further split into patient seronegative at recruitment (thick continuous line) and showing seroconversion at recruitment (thin continuous line).
Significance: * = P < 0.05 vs seronegative patients of the control arm. † = P ≤ 0.001 vs seroconverted patients of the control arm.
4. Discussion
Recent clinical trials demonstrated the high efficacy of prophylactic vaccination for genital condyloma and precancerous lesions, such as cervical and vulvar intraepithelial neoplasia, related to HPV infection in girls and women (Garland et al., 2007, Brisson et al., 2007, Paavonen et al., 2009). However, due to main disparities in the vaccination rate between males and females (Cifu and Davis, 2014), cost-effectiveness of the use of HPV vaccine in males is largely under-investigated. In this study we proof evidence that HPV prophylactic vaccination in adult males significantly improves healing from viral semen infection.
There is a general consensus that immunobiology of HPV clearance in course of natural infection mainly relies on cell-mediated immunity (Stanley, 2006). In this context, the role of natural humoral immunity is believed to exert minor protective effects through the neutralization of functional epitopes on the virus surface, mediating internalization into basal epithelial cells, and preventing the primary entry or re-entry of virions (Carter et al., 1996). However, this model only partially explains major epidemiological and serological differences between genders. In fact, a peak in the prevalence of HPV infection features females at the age of 20–35 years, approximately at the age of sexual debut, followed by a progressive decrease with age. In males, the detection of HPV-DNA in the genital area is observed at a lower prevalence that persists almost unvaried across ages (Giuliano et al., 2010, De Vuyst et al., 2009, Klinglmair et al., 2013). On the other hand, the overall seroprevalence among females appears very low until 15 years and then undergoes a progressive increase to 29.3–32.5% until early 30s and subsequent slight reduction in older ages. In contrast, seroprevalence in males remains below 3% until the age of 19, undergoing a slight increase to 12.2–13.5% until about 20 to 24 years of age, after which it is stabilized (Desai et al., 2011, Markowitz et al., 2009). These data suggest that, despite neutralizing antibodies are not always developed during a natural viral load, they cannot be considered just as a serological marker of an ongoing or previous HPV infection, but a key tool involved in the healing process. This is in agreement with early observations in humans, showing that HPV-type-specific IgG antibodies are detectable in serum during spontaneous regression of warts, presumably resulting from exposure of virions to the immune system at the time of initial infection, or due to re-infection during productive phase of the disease (Greer et al., 1995, Wikström et al., 1995). In our cohort of HPV-positive infertile men, the seroprevalence toward HPV in the group of VSP is 20.8% (19/91), a higher value compared to seroprevalence reported for the general male population (Desai et al., 2011, Markowitz et al., 2009). This is likely to be ascribable to the widely reported higher percentages of semen infection in infertile subjects compared to fertile controls, as reviewed (Foresta et al., 2015). It has to be considered that, at enrolment, patients were counselled for the management of semen infection, posing a forced condition of limited re-infection probability that favours healing (Garolla et al., 2014). In this context, seroconverted patients at recruitment showed a higher prevalence of anti-sperm antibodies compared to the seronegative ones, reflecting an active ongoing immune response in the genito-urinary tract toward virus particles bound on sperm surface (Garolla et al., 2013b). As a counterpart, seroconverted patients showed a further improved clearance and a virtual absence of multiple infections compared to seronegative, even if with low levels of significance probably due to the low sample size of the cohort, reinforcing a role for humoral immunity in healing from viral infection and re-infection.
In agreement with this hypothesis, we evaluated virus clearance in patients undergoing prophylactic treatment with quadrivalent vaccine. In order to exclude any confounding effect of viral re-infection with nonvaccine-sensitive HPV types, we specifically selected patients infected by vaccine-sensitive HPV types (Malagón et al., 2012) in the vaccine arm. Surprisingly, the prevalence of HPV infection underwent a strong reduction at each time point compared to the control arm, achieving almost abrogation within 9 to 12 months. Moreover, within 6 months after the first administration, seroconversion was detected in the whole vaccine arm, displaying an average antibody titre 10 folds higher than patients developing natural seroconversion, coherently with previous reports (Hillman et al., 2012). In this regard, curative effects of neutralizing antibodies against recombinant self-assembled virus-like particles were early suggested in animal models (Ghim et al., 2000, Suzich et al., 1995). These studies showed that protection from oral canine papillomavirus infection was provided by passive transfer of hyperimmune IgG collected from regressor animals, supporting a role for systemic humoral immunity in healing from oral papillomas (Ghim et al., 2000, Suzich et al., 1995). Furthermore recent data from mammalian models, assessing passive immunization with sera from animals immunized with the commercially available vaccines, showed that anti-HPV antibodies are protective even at low titres (Day et al., 2010). This evidence is of particular importance, accounting for the efficiency of cross-protecting anti-HPV antibodies toward non-vaccine types, despite they are generally under-represented compared to vaccine type ones. There is a general debate about the role of HPV on sperm quality (Foresta et al., 2015, Golob et al., 2014). In this regard, a main limitation of the present study is the lack of the investigation on the impact of HPV on sperm quality over the 2 years study. We previously reported that the strict relationship among HPV semen infection, detection of anti-sperm antibodies and impaired sperm motility relies on an actual local immune response toward HPV particles bound to sperm surface (Garolla et al., 2013b, Garolla et al., 2014). Accordingly, healing from viral infection was accompanied by a progressive improvement of both the prevalence of anti-sperm antibodies and sperm motility. If vaccination boosts the clearance of HPV by arousing local immune response in the genito-urinary tract, a temporary increase of the anti-sperm antibodies and consistent reduction of sperm motility should be expected, followed by a global improvement of sperm parameters at the achievement of clearance. However further studies will add further insight on this issue.
Our results assume a particular relevance in the light of the actual lack of an efficient therapy, able to eradicate HPV infection (Rosales and Rosales, 2014). In addition, we recently highlighted HPV infection to be an emergent problem in couples eligible for assisted reproduction techniques (Foresta et al., 2015, Garolla et al., 2013a), in particular when it is not possible to delay fertility seeking due to the age of the female partner. Persistent infection in these cases is not always compatible with repeated 6-months counselling-cycles to allow any spontaneous clearance. Even if the cost-effectiveness analysis of fertility improvement in HPV vaccinated male is a mandatory step, our ancillary results suggest a potential benefit of male vaccination to overcome fertility problems related to persistent HPV infections. In this regard, further investigations are in progress to assess whether HPV clearance in males is relevant for the protection against HPV-related diseases of the female partner.
Persistence of HPV infection depends on both viral and host factors (Veldhuijzen et al., 2010). HPV-type concordance studies (Reiter et al., 2010) are coherent in suggesting penile skin to be more resistant to HPV infection than the cervical epithelium and a considerable contribution of local humoral immunity to this evidence is suggested (Garolla et al., 2013b, Marais et al., 2006). However, specific studies in this field are missing and the lack of a thorough profiling of immune milieu in semen represents a major drawback of the present study. In this regard, data in the HIV model documented mucosal HIV-1-specific IgA, systemic HIV-1-specific CD4 IFNα-producing cells and HIV-1-specific CD8 T lymphocytes in exposed seronegative men, resulting in immune activation in lymphocytes purified from seminal fluid but not in peripheral blood lymphocytes (Lo Caputo et al., 2003). Similarly, serum HIV-specific IgA was observed in exposed seronegative women (Mazzoli et al., 1999), but not detected in exposed seronegative men, supporting a role of mucosal immunity as a more efficient limiting factor for viral infection in males than in females. Thus, in the HPV model, the evaluation of local mucosal and cellular immunity in men could provide a comprehensive explanation of our findings about HPV clearance kinetic in both seropositive and seronegative subjects.
In conclusion, our data suggest that HPV prophylactic vaccination is effective in reducing mean clearance time in patients featured by HPV semen infection. This evidence may have a breakthrough impact on the clinical approach to patients with long-lasting HPV semen infection, and in particular for those infertile patients eligible for ART.
The following is the supplementary data related to this article.
Detection of HPV-positive cells in semen, seroconversion and antibody titre in seronegative vaccine-sensitive patients (Seronegative VSP), seroconverted vaccine sensitive patients (Seroconverted VSP) and patients of the vaccine arm.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2015.09.005.
Author's Contribution
C Foresta, A Garolla, L De Toni: study design; M Ghezzi, A Garolla: patients evaluation, recruitment and randomization; L De Toni, A Di Nisio, S Parisi: literature search; A Bertoldo, L De Toni, A Di Nisio: data collection, data analysis and figures editing; C Foresta, A Garolla, L De Toni, S Parisi: data interpretation and manuscript writing.
Footnotes
Funding: None.
References
- Álvarez-Argüelles M.E., Melón S., Junquera M.L., Boga J.A., Villa L., Pérez-Castro S. Human papillomavirus infection in a male population attending a sexually transmitted infection service. PLoS ONE. 2013;8(1):e54375. doi: 10.1371/journal.pone.0054375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbyn M., Castellsagué X., de Sanjosé S., Bruni L., Saraiya M., Bray F. Worldwide burden of cervical cancer in 2008. Ann. Oncol. 2011;22(12):2675–2686. doi: 10.1093/annonc/mdr015. [DOI] [PubMed] [Google Scholar]
- Brisson M., Van de Velde N., De Wals P., Boily M.C. The potential cost-effectiveness of prophylactic human papillomavirus vaccines in Canada. Vaccine. 2007;25(29):5399–5408. doi: 10.1016/j.vaccine.2007.04.086. [DOI] [PubMed] [Google Scholar]
- Cai T., Wagenlehner F.M., Mondaini N., D'Elia C., Meacci F., Migno S. Effect of human papillomavirus and Chlamydia trachomatis co-infection on sperm quality in young heterosexual men with chronic prostatitis-related symptoms. BJU Int. 2014;113(2):281–287. doi: 10.1111/bju.12244. [DOI] [PubMed] [Google Scholar]
- Carter J.J., Koutsky L.A., Wipf G.C., Christensen N.D., Lee S.K., Kuypers J. The natural history of human papillomavirus type 16 capsid antibodies among a cohort of university women. J. Infect. Dis. 1996;174(5):927–936. doi: 10.1093/infdis/174.5.927. [DOI] [PubMed] [Google Scholar]
- Castle P.E., Rodríguez A.C., Burk R.D., Herrero R., Wacholder S., Hildesheim A. Long-term persistence of prevalently detected human papillomavirus infections in the absence of detectable cervical precancer and cancer. J. Infect. Dis. 2011;203(6):814–822. doi: 10.1093/infdis/jiq116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifu A.S., Davis A.M. Use of HPV vaccine in males and females. J. Am. Med. Assoc. 2014;312(18):1920–1921. doi: 10.1001/jama.2014.12274. [DOI] [PubMed] [Google Scholar]
- Conde-Ferráez L., Chan May A.d.A., Carrillo-Martínez J.R., Ayora-Talavera G., González-Losa M.D.R. Human papillomavirus infection and spontaneous abortion: a case–control study performed in Mexico. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013;170(2):468–473. doi: 10.1016/j.ejogrb.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Day P.M., Kines R.C., Thompson C.D., Jagu S., Roden R.B., Lowy D.R. In vivo mechanisms of vaccine-induced protection against HPV infection. Cell Host Microbe. 2010;8(3):260–270. doi: 10.1016/j.chom.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vuyst H., Clifford G., Li N., Franceschi S. HPV infection in Europe. Eur. J. Cancer. 2009;45(15):2632–2639. doi: 10.1016/j.ejca.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Desai S., Chapman R., Jit M., Nichols T., Borrow R., Wilding M. Prevalence of human papillomavirus antibodies in males and females in England. Sex. Transm. Dis. 2011;38(7):622–629. doi: 10.1097/OLQ.0b013e31820bc880. [DOI] [PubMed] [Google Scholar]
- Draper E., Bissett S.L., Howell-Jones R., Waight P., Soldan K., Jit M. A randomized, observer-blinded immunogenicity trial of Cervarix(®) and Gardasil(®) human papillomavirus vaccines in 12–15 year old girls. PLoS ONE. 2013;8(5):e61825. doi: 10.1371/journal.pone.0061825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foresta C., Noventa M., De Toni L., Gizzo S., Garolla A. HPV-DNA sperm infection and infertility: from a systematic literature review to a possible clinical management proposal. Andrology. 2015;3(2):163–173. doi: 10.1111/andr.284. [DOI] [PubMed] [Google Scholar]
- Foresta C., Pizzol D., Moretti A., Barzon L., Palù G. Clinical and prognostic significance of human papillomavirus DNA in the sperm or exfoliated cells of infertile patients and subjects with risk factors. Fertil. Steril. 2010;94(5):1723–1727. doi: 10.1016/j.fertnstert.2009.11.012. [DOI] [PubMed] [Google Scholar]
- Forman D., de Martel C., Lacey C.J., Soerjomataram I., Lortet-Tieulent J., Bruni L. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30(Suppl 5):F12–F23. doi: 10.1016/j.vaccine.2012.07.055. [DOI] [PubMed] [Google Scholar]
- Garland S.M., Hernandez-Avila M., Wheeler C.M., Perez G., Harper D.M., Leodolter S. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N. Engl. J. Med. 2007;356(19):1928–1943. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- Garolla A., Pizzol D., Bertoldo A., De Toni L., Barzon L., Foresta C. Association, prevalence, and clearance of human papillomavirus and antisperm antibodies in infected semen samples from infertile patients. Fertil. Steril. 2013;99(1):125–131. doi: 10.1016/j.fertnstert.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Garolla A., Pizzol D., Bertoldo A., Menegazzo M., Barzon L., Foresta C. Sperm viral infection and male infertility: focus on HBV, HCV, HIV, HPV, HSV, HCMV, and AAV. J. Reprod. Immunol. 2013;100(1):20–29. doi: 10.1016/j.jri.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Garolla A., Lenzi A., Palù G., Pizzol D., Bertoldo A., De Toni L. Human papillomavirus sperm infection and assisted reproduction: a dangerous hazard with a possible safe solution. Hum. Reprod. 2012;27(4):967–973. doi: 10.1093/humrep/des009. [DOI] [PubMed] [Google Scholar]
- Garolla A., Pizzol D., Foresta C. The role of human papillomavirus on sperm function. Curr. Opin. Obstet. Gynecol. 2011;23(4):232–237. doi: 10.1097/GCO.0b013e328348a3a4. [DOI] [PubMed] [Google Scholar]
- Garolla A., Pizzol D., Vasoin F., Barzon L., Bertoldo A., Foresta C. Counseling reduces HPV persistence in coinfected couples. J. Sex. Med. 2014;11(1):127–135. doi: 10.1111/jsm.12358. [DOI] [PubMed] [Google Scholar]
- Ghim S., Newsome J., Bell J., Sundberg J.P., Schlegel R., Jenson A.B. Spontaneously regressing oral papillomas induce systemic antibodies that neutralize canine oral papillomavirus. Exp. Mol. Pathol. 2000;68(3):147–151. doi: 10.1006/exmp.1999.2298. [DOI] [PubMed] [Google Scholar]
- Giuliano A.R., Anic G., Nyitray A.G. Epidemiology and pathology of HPV disease in males. Gynecol. Oncol. 2010;117(Suppl. 2):15–19. doi: 10.1016/j.ygyno.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golob B., Poljak M., Verdenik I., Kolbezen Limoniti M., Vrtačnik Bokal E., Zorn B. High HPV infection prevalence in men from infertile couples and lack of relationship between seminal HPV infection and sperm quality. Biomed. Res. Int. 2014;2014:956901. doi: 10.1155/2014/956901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer C.E., Wheeler C.M., Ladner M.B., Beutner K., Coyne M.Y., Liang H. Human papillomavirus (HPV) type distribution and serological response to HPV type 6 virus-like particles in patients with genital warts. J. Clin. Microbiol. 1995;33(8):2058–2063. doi: 10.1128/jcm.33.8.2058-2063.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermonat P.L., Han L., Wendel P.J., Quirk J.G., Stern S., Lowery C.L. Human papillomavirus is more prevalent in first trimester spontaneously aborted products of conception compared to elective specimens. Virus Genes. 1997;14(1):13–17. doi: 10.1023/a:1007975005433. [DOI] [PubMed] [Google Scholar]
- Hillman R.J., Giuliano A.R., Palefsky J.M., Goldstone S., Moreira E.D., Jr., Vardas E. The immunogenicity of quadrivalent Hpv (Types 6/11/16/18) vaccine in males aged 16–26. Clin. Vaccine Immunol. 2012;19(2):261–267. doi: 10.1128/CVI.05208-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joura E.A., Garland S.M., Paavonen J., Ferris D.G., Perez G., Ault K.A. FUTURE I And II Study Group.Effect of the human papillomavirus (HPV) quadrivalent vaccine in a subgroup of women with cervical and vulvar disease: retrospective pooled analysis of trial data. BMJ. 2012;344:e1401. doi: 10.1136/bmj.e1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinglmair G., Pichler R., Zelger B., Dogan H.S., Becker T., Esterbauer J. Prevalence of the human papillomavirus (HPV) expression of the inner prepuce in asymptomatic boys and men. World J. Urol. 2013;31(6):1389–1394. doi: 10.1007/s00345-012-0997-8. [DOI] [PubMed] [Google Scholar]
- Krogh von G., Lacey C.J., Gross G., Barrasso R., Schneider A. European course on HPV associated pathology: guidelines for primary care physicians for the diagnosis and management of anogenital warts. Sex. Transm. Infect. 2000;76(3):162–168. doi: 10.1136/sti.76.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprise C., Trottier H., Monnier P., Coutlée F., Mayrand M.-H. Prevalence of human papillomaviruses in semen: a systematic review and meta-analysis. Hum. Reprod. 2014;29(4):640–651. doi: 10.1093/humrep/det453. [DOI] [PubMed] [Google Scholar]
- Lo Caputo S., Trabattoni D., Vichi F., Piconi S., Lopalco L., Villa M.L. Mucosal and systemic HIV-1-specific immunity in HIV-1-exposed but uninfected heterosexual men. AIDS. 2003;17(4):531–539. doi: 10.1097/00002030-200303070-00008. [DOI] [PubMed] [Google Scholar]
- Malagón T., Drolet M., Boily M.C., Franco E.L., Jit M., Brisson J. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect. Dis. 2012;12(10):781–789. doi: 10.1016/S1473-3099(12)70187-1. [DOI] [PubMed] [Google Scholar]
- Marais D.J., Sampson C., Jeftha A., Dhaya D., Passmore J.A., Denny L. More men than women make mucosal IgA antibodies to human papillomavirus type 16 (HPV-16) and HPV-18: a study of oral HPV and oral HPV antibodies in a normal healthy population. BMC Infect. Dis. 2006;6:95. doi: 10.1186/1471-2334-6-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz L.E., Sternberg M., Dunne E.F., McQuillan G., Unger E.R. Seroprevalence of human papillomavirus types 6, 11, 16, and 18 in the United States: National Health And Nutrition Examination Survey 2003–2004. J. Infect. Dis. 2009;200(7):1059–1067. doi: 10.1086/604729. [DOI] [PubMed] [Google Scholar]
- Matovina M., Husnjak K., Milutin N., Ciglar S., Grce M. Possible role of bacterial and viral infections in miscarriages. Fertil. Steril. 2004;81(3):662–669. doi: 10.1016/j.fertnstert.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Mazzoli S., Lopalco L., Salvi A., Trabattoni D., Lo Caputo S., Semplici F. Human immunodeficiency virus (HIV)-specific IgA and HIV neutralizing activity in the serum of exposed seronegative partners of HIV-seropositive persons. J. Infect. Dis. 1999;180(3):871–875. doi: 10.1086/314934. [DOI] [PubMed] [Google Scholar]
- Paavonen J., Naud P., Salmerón J., Wheeler C.M., Chow S.N., Apter D. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374(9686):301–314. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- Parkin D.M., Bray F. The burden of HPV-related cancers. Vaccine. 2006;24(Suppl. 3):11–25. doi: 10.1016/j.vaccine.2006.05.111. [DOI] [PubMed] [Google Scholar]
- Reiter P.L., Pendergraft W.F., Brewer N.T. Meta-analysis of human papillomavirus infection concordance. Cancer Epidemiol. Biomark. Prev. 2010;19(11):2916–2931. doi: 10.1158/1055-9965.EPI-10-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosales, R., Rosales, C. 2014 Immune therapy for human papillomaviruses-related cancers. World J. Clin. Oncol. 5(5),1002–1119. doi: http://dx.doi.org/10.5306/wjco.v5.i5.1002 [DOI] [PMC free article] [PubMed]
- Rositch A.F., Poole C., Hudgens M.G., Agot K., Nyagaya E., Moses S. Multiple human papillomavirus infections and type competition in men. J. Infect. Dis. 2012;205(1):72–81. doi: 10.1093/infdis/jir709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman M., Castle P.E., Jeronimo J., Rodriguez A.C. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- Schiffman M., Clifford G., Buonaguro F.M. Classification of weakly carcinogenic human papillomavirus types: addressing the limits of epidemiology at the borderline. Infect. Agents Cancer. 2009;4:8. doi: 10.1186/1750-9378-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley M. Immune responses to human papillomavirus. Vaccine. 2006;24(Suppl. 1):S16–S22. doi: 10.1016/j.vaccine.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Stanley M., Pinto L.A., Trimble C. Human papillomavirus vaccines—immune responses. Vaccine. 2012;30(Suppl 5):F83–F87. doi: 10.1016/j.vaccine.2012.04.106. [DOI] [PubMed] [Google Scholar]
- Stoop D., Cobo A., Silber S. Fertility preservation for age-related fertility decline. Lancet. 2014;384(9955):1311–1319. doi: 10.1016/S0140-6736(14)61261-7. [DOI] [PubMed] [Google Scholar]
- Suzich J.A., Ghim S.J., Palmer-Hill F.J., White W.I., Tamura J.K., Bell J.A. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc. Natl. Acad. Sci. USA. 1995;92(25):11553–11557. doi: 10.1073/pnas.92.25.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swedish K.A., Factor S.H., Goldstone S.E. Prevention of recurrent high-grade anal neoplasia with quadrivalent human papillomavirus vaccination of men who have sex with men: a nonconcurrent cohort study. Clin. Infect. Dis. 2012;54(7):891–898. doi: 10.1093/cid/cir1036. [DOI] [PubMed] [Google Scholar]
- Tornesello M.L., Perri F., Buonaguro L., Ionna F., Buonaguro F.M., Caponigro F. HPV-related oropharyngeal cancers: from pathogenesis to new therapeutic rapproaches. Cancer Lett. 2014;351(2):198–205. doi: 10.1016/j.canlet.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Veldhuijzen N.J., Snijders P.J., Reiss P., Meijer C.J., van de Wijgert J.H. Factors affecting transmission of mucosal human papillomavirus. Lancet Infect. Dis. 2010;10(12):862–874. doi: 10.1016/S1473-3099(10)70190-0. [DOI] [PubMed] [Google Scholar]
- Walline H.M., Komarck C., McHugh J.B., Byrd S.A., Spector M.E., Hauff S.J. High-risk human papillomavirus detection in oropharyngeal, nasopharyngeal, and oral cavity cancers: comparison of multiple methods. JAMA Otolaryngol. Head Neck Surg. 2013;139(12):1320–1327. doi: 10.1001/jamaoto.2013.5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikström, A., van Doornum, G.J., Kirnbauer, R., Quint, W.G., Dillner. J. 1995 Prospective study on the development of antibodies against human papillomavirus type 6 among patients with condyloma acuminata or new asymptomatic infection. J. Med. Virol. 46(4),368–374. [DOI] [PubMed]
- Woodman C.B.J., Collins S.I., Young L.S. The natural history of cervical HPV infection: unresolved issues. Na.t Rev. Cancer. 2007;7(1):11–22. doi: 10.1038/nrc2050. [DOI] [PubMed] [Google Scholar]
- World Health Organization . 2010. WHO laboratory manual for the examination and processing of human semen. (ISBN: 978 92 4 154778 9) [Google Scholar]
- Zhao H., Lin Z.J., Huang S.J., Li J., Liu X.H., Guo M. Correlation between ELISA and pseudovirion-based neutralisation assay for detecting antibodies against human papillomavirus acquired by natural infection or by vaccination. Hum. Vaccin. Immunother. 2014;10(3):740–746. doi: 10.4161/hv.27619. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detection of HPV-positive cells in semen, seroconversion and antibody titre in seronegative vaccine-sensitive patients (Seronegative VSP), seroconverted vaccine sensitive patients (Seroconverted VSP) and patients of the vaccine arm.