Abstract
Liver cancer is the 5th most common cancer, but the 2nd leading cause of cancer death, in the world, with more than 700,000 fatalities annually. The major etiology of liver cancer is infection with an hepatotropic virus such as hepatitis B virus (HBV) or hepatitis C virus infection (HCV). While chronic viral infection remains the main cause of liver disease and risk of HCC, rates of non –viral associated HCC are occurring at an alarmingly increasing rate. Like many cancers, survival rates are closely associated with time of detection. If HCC is caught early, survival rates can be as high as 50%. Regrettably, most cases of HCC are caught late where survival rates can be as low as 2–7%. Thus, there has been great interest in discovering serum biomarkers that could be used to identify those with HCC. To this end, many groups have examined the N-linked glycans to identify changes that occur with HCC. As the liver secretes the vast majority of proteins into the serum, this has often been a starting point for study. In serum, alterations in core fucosylation, outer-arm fucosylation, increased sialylation and glycan branching have been observed in patients with HCC. Similar findings have been found directly in HCC tissue suggesting that these glycan changes may play a role in tumor formation and development.
Keywords: Fucosylation, glycosylation, hepatocellular carcinoma, hepatitis B virus, hepatitis C virus, NASH, glycomics, glycoproteomics, liver cancer
Hepatocellular carcinoma
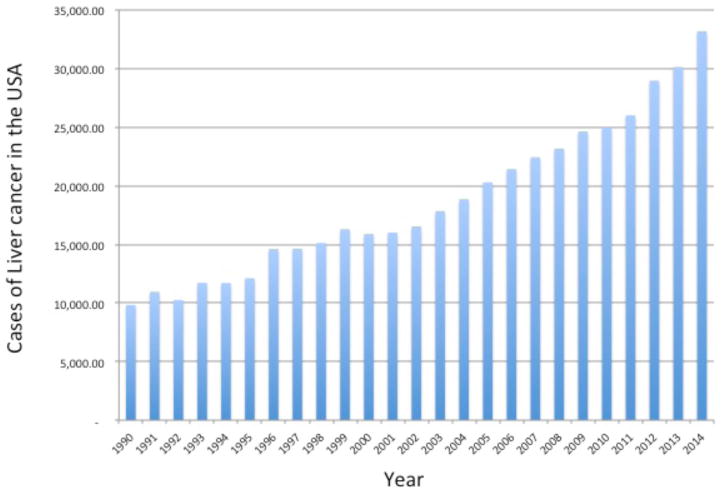
Hepatocellular carcinoma (HCC) is a malignancy of hepatocytes that arises within the liver. This cancer occurs in the background of patients with underlying liver disease such as liver fibrosis and liver cirrhosis. Approximately 80% of all liver cancers are hepatocellular carcinoma (HCC) and it is one of the most common malignancies worldwide (Block et al., 2003). The survival rate of people with primary cancers of the liver and intra-hepatic bile ducts is very low, usually less 2–7%% [7]. The low survival rates have been attributed to the late diagnosis (Di Bisceglie, 1998) and, although liver transplantation is the preferred option for surgical treatment of HCC (Kim, 1998), the paucity of organ donors means that common (worldwide) treatment is partial hepatic resection. Unfortunately, even with the advances in surgery and patient care, reported 5-year survival rates are between 40% and 50% (Poon, 2000). This low rate of survival is made even more problematic when only about 10% of HCC patients are acceptable for resection (Paterlini-Brechot, 2000). In addition, the cumulative 5-year recurrence rate is between 75% and 100% (Poon, 2000) and HCC is consequently responsible for over 700,000 deaths annually (a conservative estimate) and ranks as the 2nd leading cause of cancer death worldwide (Bosch, 1999). Although HCC in the USA is an uncommon cancer, as figure 1 shows, the rates are increasing dramatically both in the USA (El-Serag, 1999) as well as in Japan and Europe (Deuffic, 1998; Okuda, 1987; Taylor-Robinson, 1997). In 2000, there were an estimated 10,000 cases of liver cancer in the USA. Amazingly, by 2013 that number has more than tripled with over 34,000 cases and approximately 23,000 deaths. Indeed, the occurrence of liver cancer is predicted to continue rising in the United States and is currently among the top 10 causes of cancer death (http://www.cancer.gov/cancertopics/types/liver).
Figure 1.
Rates of liver and intrahepatic bile duct cancer in the United States in from 1990 to 2014. Data is based upon the SEER Cancer Statistics Review (CSR) 1975–2011 and the NCI web-site on liver cancer (http://www.cancer.gov/cancertopics/types/liver).
HCC is characterized by its propensity for vascular invasion and microscopic venous or macroscopic portal vein invasion are recorded as being the most significant risk factors for recurrence. Indeed, both intra-hepatic metastasis and/or multi-centric occurrence contribute to recurrence in the liver remnant as does initial large tumor size (especially >5 cm). Even after liver transplantation, often viewed as a cure for HCC, intra-hepatic tumor recurrence occurs and is especially frequent in tumors >3 cm (Paterlini-Brechot, 2000). Although the cause of the tumor (viral infection, alcohol etc.) does not appear to be a significant risk factor for recurrence, there are reports of lower rates of recurrence in HBV infected individuals compared to HCV patients (Kumada, 1997; Yamanaka, 1997).
Hepatitis- A major risk factor for HCC
Infection with hepatitis B virus (HBV) and/or hepatitis C virus (HCV) is the major etiology of HCC (Benvegnu, 1994; Brechot, 1996; Hoofnagle, 1999). HBV causes both acute and chronic infections of the liver and most chronically infected individuals remain asymptomatic for many years and clinical disease (HCC) is not apparent until decades later. Nearly 25% of all chronic carriers eventually develop untreatable liver cancer and it is estimated that over one million people worldwide die due to HB- associated liver cancer (Parkin, 1999). Indeed, HBV infection is associated with over 80% of all HCC cases worldwide and can be as high as 96% in regions where HBV is endemic (Beasley, 1988). More than 350 million people worldwide are chronically infected with HBV, including 1.25 million in the USA (Hoofnagle, 1997). With 140,000–320,000 new cases of HBV reported in the USA each year the at risk population (for HCC) has been consistently rising. This is unfortunate since an effective vaccine has been available for nearly 20 years. Only recently have universal vaccination programs gotten under way in developed countries. However, the huge pool of infected individuals remains since no effective therapy is available.
The progression of liver disease in asymptomatic chronic carriers of HBV and HCV is monitored by serum liver function tests (LFTs) and ultra sound imaging for detection of small masses in the liver (Hepatitis B Foundation, 1994). Many of the constituents of the LFT panel vary throughout the course of chronic hepatitis and are of limited use in early detection of HCC. Ultra-sound detection requires at least a 2 cm tumor mass to be present, and often occurs at a stage at which the prognosis is very poor (Brechot, 1987; Hoofnagle, 1997). Imaging modalities such as computed axial tomography (CAT scan) or Magnetic resonance imaging (MRI) have great value and are in many ways the “gold standard”. However, even these methods have limitations such as poor sensitivity of a per lesion basis and excessive cost. Thus, as early surgical and chemotherapeutic intervention is the best hope for patient survival (Brechot, 1987; Di Bisceglie, 1998), early detection of HCC though the use of a biomarker is necessary to identify the need for intervention.
Proteomic identification of biomarkers of liver cancer
Over the last 15 years various proteomic methodologies have been proposed to identify proteins that are altered in the serum of those with HCC. Most methods have involved the comparative analysis of several patients groups, most notably, healthy subjects, those infected with a hepatitis virus such as HBV or HCV, those with liver cirrhosis and those with liver cirrhosis and HCC. Early proteomic efforts utilized simple two-dimensional gel electrophoresis (2DE) and looked for spot alterations in the serum proteome(Chignard and Beretta, 2004; Feng et al., 2005; Park et al., 2002a; Poon and Johnson, 2001; Shalhoub et al., 2001; Steel et al., 2001; Takashima et al., 2003). Proteins identified using this approach identified limited changes in the serum proteome with the development of HCC, as compared to those with just cirrhosis. Additionally, 2DE was combined with several groups to identify auto-antibodies as potential biomarkers of liver cancer(Le Naour et al., 2002).
2DE was also used initially to examine proteomic differences between HCC tissue and adjacent tissue in an effort to identify those proteins that are specifically altered in HCC. These studies have identified alterations in several tumor pathways that have previously been identified in other cancers(Chignard and Beretta, 2004; Ding et al., 2004; Higai et al., 2003; Melle et al., 2004; Park et al., 2002b; Yokoo et al., 2004; Zeindl-Eberhart et al., 2004).
Recently, more intensive proteomic methods utilizing more complex analysis of been utilized to identify potential biomarkers of HCC. Most of these methods forgo the use of gel based imaging and use mechanisms to deplete serum samples of the major acute phase proteins. By utilizing more sensitive machines and methods, low abundance proteins that change with cancer development can be found and may be more directly related to the cancer. Using such methods, proteins such as peroxiredoxin 3 (Ai et al., 2006; Chen et al., 2010b; Guo et al., 2007; Li et al., 2008; Lu et al., 2010; Qiao et al., 2012; Song et al., 2009; Wang, 2007; Yue et al., 2007) and osteopontin have been identified as potential markers of HCC (Abu El Makarem et al., 2011; Chen et al., 2010a; Chen et al., 2011; Kim et al., 2006; Lin et al., 2011; Phillips et al., 2012; Qin and Tang, 2004; Shang et al., 2012; Tang et al., 2004; Ye et al., 2003; Zekri et al., 2011). While these markers have been shown to be elevated in other cancers, they may still have great value in the early detection of liver cancer (Chahed et al., 2008; Lehtonen et al., 2004; McAllister et al., 2008; Mirza et al., 2008; Park et al., 2008; Park et al., 2007; Reiniger et al., 2007; Schremmer et al., 2007; Tigrani and Weydert, 2007; Tuck et al., 2007; Whiteaker et al., 2007). However, large multi-center studies using peroxiredoxin 3 and osteopontin have either not been done or have not been successful enough at detecting HCC in the background of cirrhosis to alter clinical care. Thus, in many ways, proteomics has failed to discover changes in the serum proteome that could be used to detect cancer in the background of liver cirrhosis.
Glycomic methodologies for the identification of biomarkers of liver cancer
Glycomics is the analysis of sugars or glycans, either free or attached to larger molecules such as proteins or lipids. In regards to liver cancer, glycomic methodologies have long been used to either improve or discover biomarkers of liver cancer. Initial work showed that AFP with an attached α-1,6 core fucosylated monosaccharide was a better marker of HCC than AFP alone(Aoyagi, 1994; Aoyagi et al., 1998; Aoyagi et al., 2002; Aoyagi et al., 1993a; Aoyagi et al., 1993b; Aoyagi et al., 1988; Buamah et al., 1986; Yamashita et al., 1993). Subsequently, other highly abundant serum proteins such as transferrin and alpha-1-anti-trypsin (A1AT) were found to contain increased levels of fucosylation with HCC(Aoyagi et al., 1993a; Callewaert et al., 2003; Comunale et al., 2006; Goodarzi and Turner, 1995; Miyoshi et al., 1999; Morelle et al., 2006; Naitoh et al., 1999b; Noda et al., 1998; Nuck et al., 1992; Ryden et al., 1999; Thompson et al., 1988; Turner, 1995). Core fucosylated AFP is the success story in the field of glycomics as it has shown great clinical value and is currently the only test approved by the United States Food and Drug Administration (USFDA) for the detection of HCC. This test, known as AFP-L3 has shown much greater specificity in HCC detection as compared to AFP alone. In a recent, well-controlled study of over 800 patients, the core fucosylated form of AFP had a specificity of 94% (92–97 95% CL) while AFP alone had a specificity of only 82% (76–94 95% CL)(Marrero et al., 2009). Unfortunately, the complexity of the assay for the detection of fucosylated forms of AFP lead to a reduction in sensitivity from 70% (56–77 95% CL) to 50% (44–55 95% CL). This result highlights several key points with glycomics markers for HCC. The first is that they can be used to significantly increase the specificity of detection. This implies that the alterations in glycosylation are directly associated with the tumor. The other fact is that the protein to which they are attached limits the information they can provide. For example, total AFP has a sensitivity of ~70%, a value which can not be really improved upon by the examination of the fucosylated glycoforms. That is, the fucosylated glycoform is just a subset of the total AFP protein level, thus the sensitivity will not necessarily be improved. However, as the results with AFP-L3 indicate that fucosylation is a highly specific HCC modification, groups have combined this glycomic information with proteomics to identify other more abundant proteins with glycan changes that could be used as biomarkers of liver cancer(Block et al., 2005a; Comunale, 2006; Comunale et al., 2010; Comunale et al., 2009a; Comunale et al., 2011; Drake et al., 2006; Marrero et al., 2005; Morota et al., 2011b; Wang et al., 2009a; White et al., 2009).
Glycomic analysis of total serum, with limited protein information has been, utilized by several groups to detect HCC. Glycans identified include increases in core α-1,6 linked and α-1,3 linked outer-arm fucosylation as well as increases in branching and sialylation (Block et al., 2005a; Comunale, 2006; Goldman et al., 2009; Liu et al., 2007; Mehta et al., 2012). However, while many of these groups have identified changes in glycosylation, the ability to translate these changes to valid biomarkers of liver cancer has proved challenging.
Some of the initial methods to combine glycomics and proteomics were proposed by Jun Hirabayashi who had developed lectin based systems to analyze specific sets of glycoproteins(Hirabayashi, 2004; Hirabayashi et al., 2002; Hirabayashi and Kasai, 2003). Subsequently, several groups employed these techniques towards the discovery of biomarkers of liver cancer(Block et al., 2005a; Comunale, 2006). Initial work in the woodchuck model of liver cancer identified a number of proteins as being hyperfucosylated with the development of HCC(Block et al., 2005b). One of the proteins identified in the serum of woodchucks with cancer and subsequently in the serum of people with HCC was golgi-proteins 73 (GP73). This protein has subsequently been analyzed in over 10,000 individuals with liver disease(Block et al., 2005b; Gu et al., 2009; Hann et al., 2010; Hu et al., 2010; Iftikhar et al., 2004; Jiang and Zhou, 2012; Liu et al., 2011; Maitra and Thuluvath, 2004; Malaguarnera et al., 2010; Mao et al., 2010; Mao et al., 2008; Marrero et al., 2005; Morota et al., 2011a; Ozkan et al., 2011; Riener, 2011; Riener et al., 2009; Schwegler et al., 2005; Shi et al., 2011; Sun et al., 2011; Tan, 2007; Tan et al., 2009; Tian et al., 2011; Wang et al., 2011a; Wang et al., 2009b; Wang et al., 2011b; Xu et al., 2011; Yamamoto et al., 2010; Zhao et al., 2010; Zhou et al., 2012) and for the most part, shown improved performance in the detection of HCC as compared to AFP. However, in several studies, GP73 either was either not elevated in HCC versus cirrhosis (Morota et al., 2011a; Riener et al., 2009) or was no better than AFP at differentiating HCC from cirrhosis.
Increased levels of fucosylated proteins such as hemopexin(Comunale et al., 2009a; Debruyne et al., 2010; Kobayashi et al., 2012; Matsumoto et al., 1994; Morota et al., 2011a), fetuin A (Ahn et al., 2012b; Comunale et al., 2009b; Matsumoto et al., 1994), A1AT (Ahn et al., 2012a; Ahn et al., 2012b; Block et al., 2005b; Chen et al., 2012; Comunale et al., 2006; Comunale et al., 2010; Naitoh et al., 1999a), ceruloplasmin(Block et al., 2005b; Comunale, 2006; Liu et al., 2010), haptoglobin(Ahn et al., 2012a; Chandler et al., 2013; Pompach et al., 2013; Sanda et al., 2013; Zhu et al., 2014), serum paraoxonase 1 (Ahn et al., 2014; Sun et al., 2012), and histidine-rich glycoprotein (Comunale et al., 2009b; Liu et al., 2010) to name but just a few have been observed in the serum of those with HCC, either by direct glycan sequencing or by lectin based methods. These results strongly suggest that increased fucosylation, both core and outer arm, is observed in liver disease and importantly, occurs on large number of proteins.
Fucosylation is not universally increased in HCC tissue as compared to adjacent or control tissue
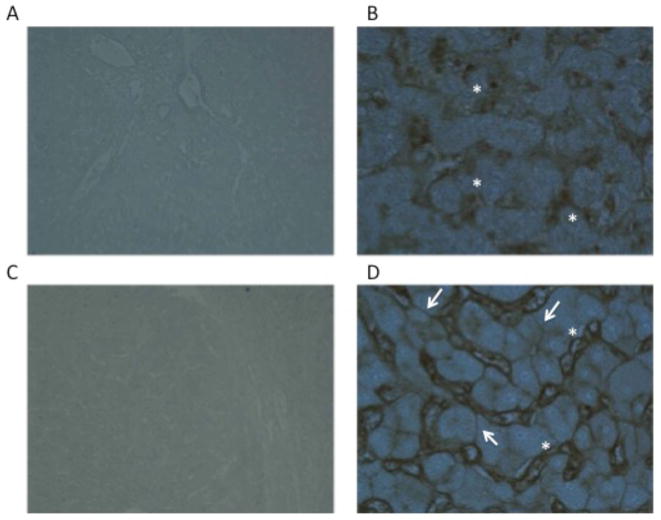
As discussed, increased levels of core and outer-arm fucosylation have been observed in the serum of patients with HCC(Block et al., 2005a; Comunale et al., 2006; Comunale et al., 2009a; Goldman et al., 2009; Miyoshi, 1999; Noda et al., 2003; Noda et al., 2002; Ohno, 1992). The exact reason for this change is not fully understood. The simplest explanation is that there is an increase in the enzymes that are involved in fucosylation that results in increased levels of fucosylated proteins being produced in the liver. This is the case in non-small cell lung carcinoma, where increases in FUT-8 is associated with activation of the β-catenin/Wnt signaling pathway(Chen et al., 2013). Indeed, in liver cancer, in some case of HCC, dramatic increases in fucosylation can be observed in HCC tissue through lectin staining with core fucose binding lectins (Figure 2). In this figure, we stained either HCC tissue or adjacent non malignant tissue with a recombinant Aleuria aurantia lectin that has greater affinity to core fucosylated structures(Romano et al., 2011). As Figure 2 shows, liver tissue from non malignant regions do have areas that stain with fucose binding lectins. These are mostly the liver sinusoid which contain liver endothelial cells that contain large amounts of core fucosylated glycan. This is also observed in the malignant tissue (see figures 2C–D) with additional staining seen on the surface of the hepatocytes. However, such staining is present only on a small proportion of HCC tissue examined (20%) and thus it does not appear that these increases are the result of a universal increase in the level of the enzyme. Indeed, there have been numerous attempts to explain why increased levels of fucosylation are associated with HCC, such as increased levels of enzyme (FUT8) and substrate (UDP-L-fucose) however, none of these have provided a simple answer(Ito et al., 2001; Miyoshi, 1999; Moriwaki et al., 2007; Noda et al., 2003; Noda et al., 2002).
Figure 2.
Lectin staining of HCC or adjacent normal tissue with a recombinant Aleuria aurantia lectin (AAL) that has greater affinity for core fucosylated glycan. Panels A (4X) and B (20x) are from tissue adjacent to the HCC. Areas of staining indicated with the asterisks are the liver sinusoids, which stain with the core fucose binding lectin. Panels C (4X) and D (20x) are from the HCC tissue. In addition to the liver sinusoids, which stain with the core fucose binding lectin as in panels A and B, defined staining of hepatocytes, as indicted by the arrows, can also be seen.
Recently, evidence has been presented that in hepatocytes, fucosylation acts as a sorting signal to secrete proteins into the bile. That is, hepatocytes within the liver are normally organized to be polar, with the basolateral side facing the circulation and the apical side forming the bile canalicular network (Tuma and Hubbard, 2001). This polarity is centrally related to the complementary hepatocyte functions of protein secretion and clearance. There is evidence that N-glycosylation in general (Helenius and Aebi, 2001; Scheiffele et al., 1995), and fucosylation in particular (Nakagawa et al., 2006) plays a role in mediating the sorting and polar secretion of glycoproteins. That is, core fucosylated glycoproteins produced by hepatocytes in the liver are preferentially sorted such that they are directed apically and secreted into the bile. Non-fucosylated glycoforms of the same protein are proposed to be secreted into the circulation. The hypothesis that core fucosylation is related to sorting for apical secretion is supported by evidence from our collaborator, Dr. Miyoshi (Nakagawa et al., 2006).
One explanation that expands on the work of Miyoshi and colleagues as to why liver-derived fucosylated proteins are elevated in the circulation in HCC patients is that cancer cells often loose their polarity and exhibit altered adhesive properties, and hepatocytes are no exception (Cao et al., 2007; Stamataglou and Hughes, 1994). It is further reasoned that if, as the theory suggests, fucosylated proteins are normally not secreted basolaterally into the sinusoids, loss of polarity and/or adhesion will result in their presence in the circulation. It is important to note that the explanation of why increases in fucosylation are observed may not be equal in all cancers. That is, it is understood that HCC, like most cancers, has great heterogeneity in the genetic lesions leading to malignancy.
Increased branching is observed in HCC tissue
One specific change that has been associated directly with HCC tissue is the increased presence of tetra-antennary linked glycan. Tetra-antennary N-linked glycans arise from the action of the enzyme UDP-N-acetylglucosamine: α mannoside β 1,6 N-acetylglucosaminyltransferase (MGAT-5) (Srivastava et al., 1988), which catalyzes the addition of β-1,6-GlcNAc to the growing N-linked glycan to form tri- and tetra-antenna-like oligosaccharides. This change has been observed both by N-linked glycan sequencing of excised HCC tissue (Mehta et al., 2012) and through direct N-linked glycan analysis of cancer tissue microarrays(Powers and 2014). In addition, increased levels of MGAT-V have also been observed through immunohistochemical analysis of HCC tissue (Ito et al., 2001). It is noted that MGAT-V has long been associated with cancer development and metastatic potential(Guo et al., 2010; Miyoshi et al., 1993; Srivastava et al., 1988; Yoshimura et al., 1995) and is directly related to alterations in the hexosamine cycle and activation of the AKT pathway (Dennis, 1999; Dennis et al., 1987; Guo et al., 2010; Lau et al., 2007; Mendelsohn et al., 2007). Importantly, increased levels of tetra-antennary glycan have recently also been shown to be found in the circulation through direct glycomics(Kamiyama et al., 2013; Mehta et al., 2012), indicating that this glycan alteration could also be useful in the detection of cancer development.
Effect of glycosylation on hepatocyte growth
What role the specific changes in glycosylation play in either the development or progression of cancer is unknown. Of the major changes observed, increased outer-arm fucosylation has not been shown to be associated with HCC tissue directly, suggesting that this modification may be coming from non cancer tissue, most likely from inflammation. In contrast, increased core fucosylation has been observed in some cases of HCC (see figure 2 and (Mehta et al., 2012)) and in many other cancers as well(Chen et al., 2013; Hu et al., 2008; Ito et al., 2003; Saldova et al., 2011b; Wen et al., 2012). In work involving non small cell carcinoma, it has been shown that regulation of the FUT-8 gene is directly related to the activation of the canonical β-catenin signaling pathway(Chen et al., 2013), suggesting a direct relationship between the alteration in glycosylation with activation of known cancer pathways. Indeed, it has also been shown that alterations in epigenetic control of gene expression can have dramatic impacts upon glycosylation(Saldova et al., 2011a).
Functionally, core fucosylation has shown to increase the activity of the epidermal growth factor receptor (EGF-R). Indeed, core fucosylation of the N-glycans on EGRF may be required for the binding of EGF to the receptor and subsequent signaling(Wang et al., 2006). Thus, as EGFR is often up-regulated in cancer, it is possible that increases in core fucosylation could act as drivers to increase EGR signaling and provide a growth advantage to the transformed cell.
Conclusion
Alterations in glycosylation have long been associated with cancer. In the case of HCC, the first major changes identified included the core fucosylation of AFP, the primary serum biomarker of HCC. Recently, with the advent of modern proteomic and glycomic methodologies, several other alterations have been identified, most notably outer-arm fucosylation, increased branching and increased sialylation. What advantage these changes impart to transformed hepatocyte remains under investigation.
Table 1.
Most commonly identify changes in N-linked glycosylation in HCC.
The identified change in glycosylation.
Protein(s) that have been identified to contain the altered glycan. In some cases, no protein containing the indicated glycan chain have been identified.
References for the indicated glycan change.
References
- Abu El Makarem MA, Abdel-Aleem A, Ali A, Saber R, Shatat M, Rahem DA, Sayed D. Diagnostic significance of plasma osteopontin in hepatitis C virus-related hepatocellular carcinoma. Ann Hepatol. 2011;10:296–305. [PubMed] [Google Scholar]
- Ahn JM, Sung HJ, Yoon YH, Kim BG, Yang WS, Lee C, Park HM, Kim BJ, Lee SY, An HJ, Cho JY. Integrated glycoproteomics demonstrates fucosylated serum paraoxonase 1 alterations in small cell lung cancer. Molecular & cellular proteomics: MCP. 2014;13:30–48. doi: 10.1074/mcp.M113.028621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn YH, Shin PM, Ji ES, Kim H, Yoo JS. A lectin-coupled, multiple reaction monitoring based quantitative analysis of human plasma glycoproteins by mass spectrometry. Anal Bioanal Chem. 2012a;402:2101–2112. doi: 10.1007/s00216-011-5646-3. [DOI] [PubMed] [Google Scholar]
- Ahn YH, Shin PM, Oh NR, Park GW, Kim H, Yoo JS. A lectin-coupled, targeted proteomic mass spectrometry (MRM MS) platform for identification of multiple liver cancer biomarkers in human plasma. J Proteomics. 2012b doi: 10.1016/j.jprot.2012.06.027. [DOI] [PubMed] [Google Scholar]
- Ai J, Tan Y, Ying W, Hong Y, Liu S, Wu M, Qian X, Wang H. Proteome analysis of hepatocellular carcinoma by laser capture microdissection. Proteomics. 2006;6:538–546. doi: 10.1002/pmic.200500257. [DOI] [PubMed] [Google Scholar]
- Aoyagi Y. Molecular discrimination between alpha-fetoprotein from patients with hepatocellular-carcinoma and nonneoplastic liver-diseases by their carbohydrate structures (review) International journal of oncology. 1994;4:369–383. doi: 10.3892/ijo.4.2.369. [DOI] [PubMed] [Google Scholar]
- Aoyagi Y, Isokawa O, Suda T, Watanabe M, Suzuki Y, Asakura H. The fucosylation index of alpha-fetoprotein as a possible prognostic indicator for patients with hepatocellular carcinoma. Cancer. 1998;83:2076–2082. [PubMed] [Google Scholar]
- Aoyagi Y, Mita Y, Suda T, Kawai K, Kuroiwa T, Igarashi M, Kobayashi M, Waguri N, Asakura H. The fucosylation index of serum alpha-fetoprotein as useful prognostic factor in patients with hepatocellular carcinoma in special reference to chronological changes. Hepatol Res. 2002;23:287. doi: 10.1016/s1386-6346(01)00184-x. [DOI] [PubMed] [Google Scholar]
- Aoyagi Y, Suzuki Y, Igarashi K, Saitoh A, Oguro M, Yokota T, Mori S, Suda T, Isemura M, Asakura H. Carbohydrate structures of human alpha-fetoprotein of patients with hepatocellular carcinoma: presence of fucosylated and non-fucosylated triantennary glycans. British journal of cancer. 1993a;67:486–492. doi: 10.1038/bjc.1993.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyagi Y, Suzuki Y, Igarashi K, Yokota T, Mori S, Suda T, Naitoh A, Isemura M, Asakura H. Highly enhanced fucosylation of alpha-fetoprotein in patients with germ cell tumor. Cancer. 1993b;72:615–618. doi: 10.1002/1097-0142(19930715)72:2<615::aid-cncr2820720246>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Aoyagi Y, Suzuki Y, Isemura M, Nomoto M, Sekine C, Igarashi K, Ichida F. The fucosylation index of alpha-fetoprotein and its usefulness in the early diagnosis of hepatocellular carcinoma. Cancer. 1988;61:769–774. doi: 10.1002/1097-0142(19880215)61:4<769::aid-cncr2820610422>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Asazawa H, Kamada Y, Takeda Y, Takamatsu S, Shinzaki S, Kim Y, Nezu R, Kuzushita N, Mita E, Kato M, Miyoshi E. Serum fucosylated haptoglobin in chronic liver diseases as a potential biomarker of hepatocellular carcinoma development. Clinical chemistry and laboratory medicine: CCLM/FESCC. 2014 doi: 10.1515/cclm-2014-0427. [DOI] [PubMed] [Google Scholar]
- Beasley RP. Hepatitis B virus. The major etiology of hepatocellular carcinoma. Cancer. 1988;61:1942–1956. doi: 10.1002/1097-0142(19880515)61:10<1942::aid-cncr2820611003>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Benvegnu L, Fattovich G, Noventa F, Tremolada F, Chemello L, Cecchetto A, Alberti A. Concurrent hepatitis B and C virus infection and risk of hepatocellular carcinoma in cirrhosis. Cancer. 1994;74:2442–2448. doi: 10.1002/1097-0142(19941101)74:9<2442::aid-cncr2820740909>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Block TM, Comunale MA, Lowman M, Steel LF, Romano PR, Fimmel C, Tennant BC, London WT, Evans AA, Blumberg BS, Dwek RA, Mattu TS, Mehta AS. Use of targeted glycoproteomics to identify serum glycoproteins that correlate with liver cancer in woodchucks and humans. Proc Natl Acad Sci U S A. 2005a;102:779–784. doi: 10.1073/pnas.0408928102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block TM, Comunale MA, Lowman M, Steel LF, Romano PR, Fimmel C, Tennant BC, London WT, Evans AA, Blumberg BS, Dwek RA, Mattu TS, Mehta AS. Use of targeted glycoproteomics to identify serum glycoproteins that correlate with liver cancer in woodchucks and humans. Proceedings of the National Academy of Sciences of the United States of America. 2005b;102:779–784. doi: 10.1073/pnas.0408928102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block TM, Mehta AS, Fimmel CJ, Jordan R. Molecular viral oncology of hepatocellular carcinoma. Oncogene. 2003;22:5093–5107. doi: 10.1038/sj.onc.1206557. [DOI] [PubMed] [Google Scholar]
- Bosch FX, Ribes J, Borras J. Epidemiology of primary liver cancer. Semin Liver Dis. 1999;19:271–285. doi: 10.1055/s-2007-1007117. [DOI] [PubMed] [Google Scholar]
- Brechot C. Hepatitis B virus (HBV) and hepatocellular carcinoma. HBV DNA status and its implications. J Hepatol. 1987;4:269–279. doi: 10.1016/s0168-8278(87)80090-9. [DOI] [PubMed] [Google Scholar]
- Brechot C. Hepatitis B and C viruses and primary liver cancer. Baillieres Clin Gastroenterol. 1996;10:335–373. doi: 10.1016/s0950-3528(96)90010-x. [DOI] [PubMed] [Google Scholar]
- Buamah PK, Cornell C, Cassells-Smith AJ, Harris AL. Fucosylation of alpha-fetoprotein in hepatocellular carcinomas. Lancet. 1986;1:922–923. doi: 10.1016/s0140-6736(86)91032-9. [DOI] [PubMed] [Google Scholar]
- Callewaert N, Schollen E, Vanhecke A, Jaeken J, Matthijs G, Contreras R. Increased fucosylation and reduced branching of serum glycoprotein N-glycans in all known subtypes of congenital disorder of glycosylation I. Glycobiology. 2003;13:367–375. doi: 10.1093/glycob/cwg040. [DOI] [PubMed] [Google Scholar]
- Cao Y, Chang H, Cheng RC, Fan XN. Alteration of adhesion molecule expression and cellular polarity in hepatocellular carcinoma. Histopathol. 2007;51:528–538. doi: 10.1111/j.1365-2559.2007.02820.x. [DOI] [PubMed] [Google Scholar]
- Chahed K, Kabbage M, Hamrita B, Guillier CL, Trimeche M, Remadi S, Ehret-Sabatier L, Chouchane L. Detection of protein alterations in male breast cancer using two dimensional gel electrophoresis and mass spectrometry: the involvement of several pathways in tumorigenesis. Clinica chimica acta; international journal of clinical chemistry. 2008;388:106–114. doi: 10.1016/j.cca.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Chandler KB, Pompach P, Goldman R, Edwards N. Exploring site-specific N-glycosylation microheterogeneity of haptoglobin using glycopeptide CID tandem mass spectra and glycan database search. Journal of Proteome Research. 2013;12:3652–3666. doi: 10.1021/pr400196s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Jan YH, Juan YH, Yang CJ, Huang MS, Yu CJ, Yang PC, Hsiao M, Hsu TL, Wong CH. Fucosyltransferase 8 as a functional regulator of nonsmall cell lung cancer. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:630–635. doi: 10.1073/pnas.1220425110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Wang F, Tan Y, Sun Z, Song C, Ye M, Wang H, Zou H. Development of a combined chemical and enzymatic approach for the mass spectrometric identification and quantification of aberrant N-glycosylation. J Proteomics. 2012;75:1666–1674. doi: 10.1016/j.jprot.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Chen RX, Xia YH, Cui JF, Xue TC, Ye SL. Osteopontin, a single marker for predicting the prognosis of patients with tumor-node-metastasis stage I hepatocellular carcinoma after surgical resection. Journal of gastroenterology and hepatology. 2010a;25:1435–1442. doi: 10.1111/j.1440-1746.2010.06277.x. [DOI] [PubMed] [Google Scholar]
- Chen RX, Xia YH, Xue TC, Ye SL. Osteopontin promotes hepatocellular carcinoma invasion by up-regulating MMP-2 and uPA expression. Molecular biology reports. 2011;38:3671–3677. doi: 10.1007/s11033-010-0481-8. [DOI] [PubMed] [Google Scholar]
- Chen XL, Zhou L, Yang J, Shen FK, Zhao SP, Wang YL. Hepatocellular carcinoma-associated protein markers investigated by MALDI-TOF MS. Mol Med Report. 2010b;3:589–596. doi: 10.3892/mmr_00000302. [DOI] [PubMed] [Google Scholar]
- Chignard N, Beretta L. Proteomics for hepatocellular carcinoma marker discovery. Gastroenterology. 2004;127:S120–125. doi: 10.1053/j.gastro.2004.09.025. [DOI] [PubMed] [Google Scholar]
- Comunale MA, Lowman M, Long RE, Krakover J, Philip R, Seeholzer S, Evans AA, Hann HW, Block TM, Mehta AS. Proteomic analysis of serum associated fucosylated glycoproteins in the development of primary hepatocellular carcinoma. J Proteome Res. 2006;5:308–315. doi: 10.1021/pr050328x. [DOI] [PubMed] [Google Scholar]
- Comunale MA, Lowman M, Long RE, Krakover J, Philip R, Seeholzer S, Evans AA, Hann HWL, Block TM, Mehta AS. Proteomic analysis of serum associated fucosylated glycoproteins in the development of primary hepatocellular carcinoma. Journal of Proteome Research. 2006;6:308–315. doi: 10.1021/pr050328x. [DOI] [PubMed] [Google Scholar]
- Comunale MA, Rodemich-Betesh L, Hafner J, Wang M, Norton P, Di Bisceglie AM, Block T, Mehta A. Linkage Specific Fucosylation of Alpha-1-Antitrypsin in Liver Cirrhosis and Cancer Patients: Implications for a Biomarker of Hepatocellular Carcinoma. PLoS ONE. 2010;5:e12419. doi: 10.1371/journal.pone.0012419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comunale MA, Wang M, Hafner J, Krakover J, Rodemich L, Kopenhaver B, Long RE, Junaidi O, Bisceglie AM, Block TM, Mehta AS. Identification and development of fucosylated glycoproteins as biomarkers of primary hepatocellular carcinoma. J Proteome Res. 2009a;8:595–602. doi: 10.1021/pr800752c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comunale MA, Wang M, Hafner J, Krakover J, Rodemich L, Kopenhaver B, Long RE, Junaidi O, Bisceglie AM, Block TM, Mehta AS. Identification and development of fucosylated glycoproteins as biomarkers of primary hepatocellular carcinoma. Journal of Proteome Research. 2009b;8:595–602. doi: 10.1021/pr800752c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comunale MA, Wang M, Rodemich-Betesh L, Hafner J, Lamontagne A, Klein A, Marrero J, Di Bisceglie AM, Gish R, Block T, Mehta A. Novel changes in glycosylation of serum Apo-J in patients with hepatocellular carcinoma. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20:1222–1229. doi: 10.1158/1055-9965.EPI-10-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debruyne EN, Vanderschaeghe D, Van Vlierberghe H, Vanhecke A, Callewaert N, Delanghe JR. Diagnostic value of the hemopexin N-glycan profile in hepatocellular carcinoma patients. Clin Chem. 2010;56:823–831. doi: 10.1373/clinchem.2009.139295. [DOI] [PubMed] [Google Scholar]
- Dennis JW, Laferte S, Waghorne C, Breitman ML, Kerbel RS. Beta 1–6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science. 1987;236:582–585. doi: 10.1126/science.2953071. [DOI] [PubMed] [Google Scholar]
- Dennis JW, Granovsky M, Warren CE. Glycoprotein glycosylation and cancer progression. Biochem Biophys Acta. 1999;1473:21–34. doi: 10.1016/s0304-4165(99)00167-1. [DOI] [PubMed] [Google Scholar]
- Deuffic S, Poynard T, Buffat L, Valleron AJ. Trends in primary liver cancer. Lancet. 1998;351:214–215. doi: 10.1016/S0140-6736(05)78179-4. [DOI] [PubMed] [Google Scholar]
- Di Bisceglie AM, Carithers RL, Jr, Gores GJ. Hepatocellular carcinoma. Hepatology. 1998;28:1161–1165. doi: 10.1002/hep.510280436. [DOI] [PubMed] [Google Scholar]
- Ding SJ, Li Y, Shao XX, Zhou H, Zeng R, Tang ZY, Xia QC. Proteome analysis of hepatocellular carcinoma cell strains, MHCC97-H and MHCC97-L, with different metastasis potentials. Proteomics. 2004;4:982–994. doi: 10.1002/pmic.200300653. [DOI] [PubMed] [Google Scholar]
- Drake RR, Schwegler EE, Malik G, Diaz J, Block T, Mehta A, Semmes OJ. Lectin capture strategies combined with mass spectrometry for the discovery of serum glycoprotein biomarkers. Mol Cell Proteomics. 2006;5:1957–1967. doi: 10.1074/mcp.M600176-MCP200. [DOI] [PubMed] [Google Scholar]
- El-Serag HBaACM. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- Feng JT, Liu YK, Song HY, Dai Z, Qin LX, Almofti MR, Fang CY, Lu HJ, Yang PY, Tang ZY. Heat-shock protein 27: a potential biomarker for hepatocellular carcinoma identified by serum proteome analysis. Proteomics. 2005;5:4581–4588. doi: 10.1002/pmic.200401309. [DOI] [PubMed] [Google Scholar]
- Goldman R, Ressom HW, Varghese RS, Goldman L, Bascug G, Loffredo CA, Abdel-Hamid M, Gouda I, Ezzat S, Kyselova Z, Mechref Y, Novotny MV. Detection of hepatocellular carcinoma using glycomic analysis. Clin Cancer Res. 2009;15:1808–1813. doi: 10.1158/1078-0432.CCR-07-5261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodarzi MT, Turner GA. Decreased branching, increased fucosylation and changed sialylation of alpha-1-proteinase inhibitor in breast and ovarian cancer. Clinica chimica acta; international journal of clinical chemistry. 1995;236:161–171. doi: 10.1016/0009-8981(95)06049-j. [DOI] [PubMed] [Google Scholar]
- Gu Y, Chen W, Zhao Y, Chen L, Peng T. Quantitative analysis of elevated serum Golgi protein-73 expression in patients with liver diseases. Annals of clinical biochemistry. 2009;46:38–43. doi: 10.1258/acb.2008.008088. [DOI] [PubMed] [Google Scholar]
- Guo HB, Johnson H, Randolph M, Nagy T, Blalock R, Pierce M. Specific posttranslational modification regulates early events in mammary carcinoma formation. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21116–21121. doi: 10.1073/pnas.1013405107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo WX, Man XB, Yuan HX, Shi J, Xue J, Wu MC, Cheng SQ. Proteomic analysis on portal vein tumor thrombus-associated proteins for hepatocellular carcinoma. Zhonghua Yi Xue Za Zhi. 2007;87:2094–2097. [PubMed] [Google Scholar]
- Hamazaki K, Yunoki Y, Tagashira H, Mimura T, Mori M, Orita K. Epidermal growth factor receptor in human hepatocellular carcinoma. Cancer detection and prevention. 1997;21:355–360. [PubMed] [Google Scholar]
- Hann HW, Wang M, Hafner J, Long RE, Kim SH, Ahn M, Park S, Comunale MA, Block TM, Mehta A. Analysis of GP73 in patients with HCC as a function of anti-cancer treatment. Cancer biomarkers: section A of Disease markers. 2010;7:269–273. doi: 10.3233/CBM-2010-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A, Aebi M. Intracellular functions of N-linked glycans. Science. 2001;291:2364–2369. doi: 10.1126/science.291.5512.2364. [DOI] [PubMed] [Google Scholar]
- Higai K, Shibukawa K, Muto S, Matsumoto K. Targeted proteo-glycomics analysis of Sialyl Lewis X antigen expressing glycoproteins secreted by human hepatoma cell line. Anal Sci. 2003;19:85–92. doi: 10.2116/analsci.19.85. [DOI] [PubMed] [Google Scholar]
- Hirabayashi J. Lectin-based structural glycomics: glycoproteomics and glycan profiling. Glycoconjugate journal. 2004;21:35–40. doi: 10.1023/B:GLYC.0000043745.18988.a1. [DOI] [PubMed] [Google Scholar]
- Hirabayashi J, Hashidate T, Kasai K. Glyco-catch method: A lectin affinity technique for glycoproteomics. J Biomol Tech. 2002;13:205–218. [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi J, Kasai K. Glycoproteomics of C. elegans. Tanpakushitsu Kakusan Koso. 2003;48:1049–1056. [PubMed] [Google Scholar]
- Hoofnagle JH. Management of hepatitis C: current and future perspectives. Journal of hepatology. 1999;31:264–268. doi: 10.1016/s0168-8278(99)80414-0. [DOI] [PubMed] [Google Scholar]
- Hoofnagle JHaAMdB. The treatment of chronic viral hepatitis. N Engl J Med. 1997;336:347–356. doi: 10.1056/NEJM199701303360507. [DOI] [PubMed] [Google Scholar]
- Hu JS, Wu DW, Liang S, Miao XY. GP73, a resident Golgi glycoprotein, is sensibility and specificity for hepatocellular carcinoma of diagnosis in a hepatitis B-endemic Asian population. Medical oncology. 2010;27:339–345. doi: 10.1007/s12032-009-9215-y. [DOI] [PubMed] [Google Scholar]
- Hu P, Shi B, Geng F, Zhang C, Wu W, Wu XZ. E-cadherin core fucosylation regulates nuclear beta-catenin accumulation in lung cancer cells. Glycoconjugate journal. 2008;25:843–850. doi: 10.1007/s10719-008-9144-6. [DOI] [PubMed] [Google Scholar]
- Iftikhar R, Kladney RD, Havlioglu N, Schmitt-Graff A, Gusmirovic I, Solomon H, Luxon BA, Bacon BR, Fimmel CJ. Disease- and cell-specific expression of GP73 in human liver disease. The American journal of gastroenterology. 2004;99:1087–1095. doi: 10.1111/j.1572-0241.2004.30572.x. [DOI] [PubMed] [Google Scholar]
- Ito Y, Miyauchi A, Yoshida H, Uruno T, Nakano K, Takamura Y, Miya A, Kobayashi K, Yokozawa T, Matsuzuka F, Taniguchi N, Matsuura N, Kuma K, Miyoshi E. Expression of alpha1,6-fucosyltransferase (FUT8) in papillary carcinoma of the thyroid: its linkage to biological aggressiveness and anaplastic transformation. Cancer letters. 2003;200:167–172. doi: 10.1016/s0304-3835(03)00383-5. [DOI] [PubMed] [Google Scholar]
- Ito Y, Miyoshi E, Sakon M, Takeda T, Noda K, Tsujimoto M, Ito S, Honda H, Takemura F, Wakasa K, Monden M, Matsuura N, Taniguchi N. Elevated expression of UDP-N-acetylglucosamine: alphamannoside beta1,6 N-acetylglucosaminyltransferase is an early event in hepatocarcinogenesis. International journal of cancer Journal international du cancer. 2001;91:631–637. [PubMed] [Google Scholar]
- Jiang JC, Zhou LF. Advances on Golgi glycoprotein 73 and its association with diseases. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2012;41:215–221. doi: 10.3785/j.issn.1008-9292.2012.02.017. [DOI] [PubMed] [Google Scholar]
- Kamada Y, Akita M, Takeda Y, Yamada S, Fujii H, Sawai Y, Doi Y, Asazawa H, Nakayama K, Mizutani K, Yakushijin T, Miyazaki M, Ezaki H, Hiramatsu N, Yoshida Y, Kiso S, Imai Y, Kawada N, Takehara T, Miyoshi E. Serum Fucosylated Haptoglobin as a Novel Diagnostic Biomarker for Predicting Hepatocyte Ballooning and Nonalcoholic Steatohepatitis. PLoS ONE. 2013;8:e66328. doi: 10.1371/journal.pone.0066328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiyama T, Yokoo H, Furukawa J, Kurogochi M, Togashi T, Miura N, Nakanishi K, Kamachi H, Kakisaka T, Tsuruga Y, Fujiyoshi M, Taketomi A, Nishimura S, Todo S. Identification of novel serum biomarkers of hepatocellular carcinoma using glycomic analysis. Hepatology (Baltimore, Md. 2013;57:2314–2325. doi: 10.1002/hep.26262. [DOI] [PubMed] [Google Scholar]
- Kim J, Ki SS, Lee SD, Han CJ, Kim YC, Park SH, Cho SY, Hong YJ, Park HY, Lee M, Jung HH, Lee KH, Jeong SH. Elevated plasma osteopontin levels in patients with hepatocellular carcinoma. The American journal of gastroenterology. 2006;101:2051–2059. doi: 10.1111/j.1572-0241.2006.00679.x. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Borsig L, Varki NM, Varki A. P-selectin deficiency attenuates tumor growth and metastasis. Proc Natl Acad Sci U S A. 1998;95:9325–9330. doi: 10.1073/pnas.95.16.9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Nouso K, Kinugasa H, Takeuchi Y, Tomoda T, Miyahara K, Hagihara H, Kuwaki K, Onishi H, Nakamura S, Ikeda F, Miyake Y, Shiraha H, Takaki A, Yamamoto K. Clinical utility of serum fucosylated hemopexin in Japanese patients with hepatocellular carcinoma. Hepatol Res. 2012;42:1187–1195. doi: 10.1111/j.1872-034X.2012.01044.x. [DOI] [PubMed] [Google Scholar]
- Kumada T, Nakano S, Takeda I, Sugiyama K, Osada T, Kiriyama S, Sone Y, Toyoda H, Shimada S, Takahashi M, Sassa T. Patterns of recurrence after initial treatment in patients with small hepatocellular carcinoma. Hepatology. 1997;25:87–92. doi: 10.1053/jhep.1997.v25.pm0008985270. [DOI] [PubMed] [Google Scholar]
- Lau KS, Partridge EA, Grigorian A, Silvescu CI, Reinhold VN, Demetriou M, Dennis JW. Complex N-glycan number and degree of branching cooperate to regulate cell proliferation and differentiation. Cell. 2007;129:123–134. doi: 10.1016/j.cell.2007.01.049. [DOI] [PubMed] [Google Scholar]
- Le Naour F, Brichory F, Misek DE, Brechot C, Hanash SM, Beretta L. A distinct repertoire of autoantibodies in hepatocellular carcinoma identified by proteomic analysis. Molecular & cellular proteomics : MCP. 2002;1:197–203. doi: 10.1074/mcp.m100029-mcp200. [DOI] [PubMed] [Google Scholar]
- Lehtonen ST, Svensk AM, Soini Y, Paakko P, Hirvikoski P, Kang SW, Saily M, Kinnula VL. Peroxiredoxins, a novel protein family in lung cancer. International journal of cancer Journal international du cancer. 2004;111:514–521. doi: 10.1002/ijc.20294. [DOI] [PubMed] [Google Scholar]
- Li Y, Qin X, Cui J, Dai Z, Kang X, Yue H, Zhang Y, Su J, Cao J, Ou C, Yang C, Duan X, Liu Y. Proteome analysis of aflatoxin B1-induced hepatocarcinogenesis in tree shrew (Tupaia belangeri chinensis) and functional identification of candidate protein peroxiredoxin II. Proteomics. 2008;8:1490–1501. doi: 10.1002/pmic.200700229. [DOI] [PubMed] [Google Scholar]
- Lin F, Li Y, Cao J, Fan S, Wen J, Zhu G, Du H, Liang Y. Overexpression of osteopontin in hepatocellular carcinoma and its relationships with metastasis, invasion of tumor cells. Molecular biology reports. 2011;38:5205–5210. doi: 10.1007/s11033-010-0671-4. [DOI] [PubMed] [Google Scholar]
- Liu X, Wan X, Li Z, Lin C, Zhan Y, Lu X. Golgi protein 73(GP73), a useful serum marker in liver diseases. Clinical chemistry and laboratory medicine: CCLM/FESCC. 2011;49:1311–1316. doi: 10.1515/CCLM.2011.640. [DOI] [PubMed] [Google Scholar]
- Liu XE, Desmyter L, Gao CF, Laroy W, Dewaele S, Vanhooren V, Wang L, Zhuang H, Callewaert N, Libert C, Contreras R, Chen C. N-glycomic changes in hepatocellular carcinoma patients with liver cirrhosis induced by hepatitis B virus. Hepatology (Baltimore, Md. 2007;46:1426–1435. doi: 10.1002/hep.21855. [DOI] [PubMed] [Google Scholar]
- Liu Y, He J, Li C, Benitez R, Fu S, Marrero J, Lubman DM. Identification and confirmation of biomarkers using an integrated platform for quantitative analysis of glycoproteins and their glycosylations. J Proteome Res. 2010;9:798–805. doi: 10.1021/pr900715p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Liu J, Lin C, Wang H, Jiang Y, Wang J, Yang P, He F. Peroxiredoxin 2: a potential biomarker for early diagnosis of hepatitis B virus related liver fibrosis identified by proteomic analysis of the plasma. BMC Gastroenterol. 2010;10:115. doi: 10.1186/1471-230X-10-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra A, Thuluvath PJ. GP73 and liver disease: a (Golgi) complex enigma. The American journal of gastroenterology. 2004;99:1096–1098. doi: 10.1111/j.1572-0241.2004.40410.x. [DOI] [PubMed] [Google Scholar]
- Malaguarnera G, Giordano M, Paladina I, Berretta M, Cappellani A, Malaguarnera M. Serum markers of hepatocellular carcinoma. Digestive diseases and sciences. 2010;55:2744–2755. doi: 10.1007/s10620-010-1184-7. [DOI] [PubMed] [Google Scholar]
- Mao Y, Yang H, Xu H, Lu X, Sang X, Du S, Zhao H, Chen W, Xu Y, Chi T, Yang Z, Cai J, Li H, Chen J, Zhong S, Mohanti SR, Lopez-Soler R, Millis JM, Huang J, Zhang H. Golgi protein 73 (GOLPH2) is a valuable serum marker for hepatocellular carcinoma. Gut. 2010;59:1687–1693. doi: 10.1136/gut.2010.214916. [DOI] [PubMed] [Google Scholar]
- Mao YL, Yang HY, Xu HF, Sang XT, Lu X, Yang ZY, Zhang JC, Zhong SX, Huang JF, Zhang HB. Significance of Golgi glycoprotein 73, a new tumor marker in diagnosis of hepatocellular carcinoma: a primary study. Zhonghua Yi Xue Za Zhi. 2008;88:948–951. [PubMed] [Google Scholar]
- Marrero JA, Feng Z, Wang Y, Nguyen MH, Befeler AS, Roberts LR, Reddy KR, Harnois D, Llovet JM, Normolle D, Dalhgren J, Chia D, Lok AS, Wagner PD, Srivastava S, Schwartz M. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology. 2009;137:110–118. doi: 10.1053/j.gastro.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrero JA, Romano PR, Nikolaeva O, Steel L, Mehta A, Fimmel CJ, Comunale MA, D’Amelio A, Lok AS, Block TM. GP73, a resident Golgi glycoprotein, is a novel serum marker for hepatocellular carcinoma. Journal of hepatology. 2005;43:1007–1012. doi: 10.1016/j.jhep.2005.05.028. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Shinzaki S, Narisada M, Kawamoto S, Kuwamoto K, Moriwaki K, Kanke F, Satomura S, Kumada T, Miyoshi E. Clinical application of a lectin-antibody ELISA to measure fucosylated haptoglobin in sera of patients with pancreatic cancer. Clinical chemistry and laboratory medicine: CCLM/FESCC. 2010;48:505–512. doi: 10.1515/CCLM.2010.095. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Maeda Y, Kato S, Yuki H. Alteration of asparagine-linked glycosylation in serum transferrin of patients with hepatocellular carcinoma. Clinica chimica acta; international journal of clinical chemistry. 1994;224:1–8. doi: 10.1016/0009-8981(94)90115-5. [DOI] [PubMed] [Google Scholar]
- McAllister SS, Gifford AM, Greiner AL, Kelleher SP, Saelzler MP, Ince TA, Reinhardt F, Harris LN, Hylander BL, Repasky EA, Weinberg RA. Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell. 2008;133:994–1005. doi: 10.1016/j.cell.2008.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta A, Norton P, Liang H, Comunale MA, Wang M, Rodemich-Betesh L, Koszycki A, Noda K, Miyoshi E, Block T. Increased Levels of Tetra-antennary N-Linked Glycan but Not Core Fucosylation Are Associated with Hepatocellular Carcinoma Tissue. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2012;21:925–933. doi: 10.1158/1055-9965.EPI-11-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melle C, Kaufmann R, Hommann M, Bleul A, Driesch D, Ernst G, von Eggeling F. Proteomic profiling in microdissected hepatocellular carcinoma tissue using ProteinChip technology. International journal of oncology. 2004;24:885–891. [PubMed] [Google Scholar]
- Mendelsohn R, Cheung P, Berger L, Partridge E, Lau K, Datti A, Pawling J, Dennis JW. Complex N-glycan and metabolic control in tumor cells. Cancer Res. 2007;67:9771–9780. doi: 10.1158/0008-5472.CAN-06-4580. [DOI] [PubMed] [Google Scholar]
- Mirza M, Shaughnessy E, Hurley JK, Vanpatten KA, Pestano GA, He B, Weber GF. Osteopontin-c is a selective marker of breast cancer. International journal of cancer Journal international du cancer. 2008;122:889–897. doi: 10.1002/ijc.23204. [DOI] [PubMed] [Google Scholar]
- Miyoshi E, Noda K, Yamaguchi Y, Inoue S, Ikeda Y, Wang W, Ko JH, Uozumi N, Li W, Taniguchi N. The alpha1-6-fucosyltransferase gene and its biological significance. Biochem Biophys Acta. 1999;1473:9–20. doi: 10.1016/s0304-4165(99)00166-x. [DOI] [PubMed] [Google Scholar]
- Miyoshi E, Nishikawa A, Ihara Y, Gu J, Sugiyama T, Hayashi N, Fusamoto H, Kamada T, Taniguchi N. N-acetylglucosaminyltransferase III and V messenger RNA levels in LEC rats during hepatocarcinogenesis. Cancer Res. 1993;53:3899–3902. [PubMed] [Google Scholar]
- Miyoshi E, Noda K, Yamaguchi Y, Inoue S, Ikeda Y, Wang W, Ko JH, Uozumi N, Li W, Taniguchi N. The alpha1-6-fucosyltransferase gene and its biological significance. Biochimica et biophysica acta. 1999;1473:9–20. doi: 10.1016/s0304-4165(99)00166-x. [DOI] [PubMed] [Google Scholar]
- Mondal G, Chatterjee U, Chawla YK, Chatterjee BP. Alterations of glycan branching and differential expression of sialic acid on alpha fetoprotein among hepatitis patients. Glycoconjugate journal. 2011;28:1–9. doi: 10.1007/s10719-010-9316-z. [DOI] [PubMed] [Google Scholar]
- Morelle W, Flahaut C, Michalski JC, Louvet A, Mathurin P, Klein A. Mass spectrometric approach for screening modifications of total serum N-glycome in human diseases: application to cirrhosis. Glycobiology. 2006;16:281–293. doi: 10.1093/glycob/cwj067. [DOI] [PubMed] [Google Scholar]
- Moriwaki K, Noda K, Nakagawa T, Asahi M, Yoshihara H, Taniguchi N, Hayashi N, Miyoshi E. A high expression of GDP-fucose transporter in hepatocellular carcinoma is a key factor for increases in fucosylation. Glycobiology. 2007;17:1311–1320. doi: 10.1093/glycob/cwm094. [DOI] [PubMed] [Google Scholar]
- Morota K, Nakagawa M, Sekiya R, Hemken PM, Sokoll LJ, Elliott D, Chan DW, Dowell BL. A comparative evaluation of Golgi protein-73, fucosylated hemopexin, alpha-fetoprotein, and PIVKA-II in the serum of patients with chronic hepatitis, cirrhosis, and hepatocellular carcinoma. Clinical chemistry and laboratory medicine: CCLM/FESCC. 2011a;49:711–718. doi: 10.1515/CCLM.2011.097. [DOI] [PubMed] [Google Scholar]
- Morota K, Nakagawa M, Sekiya R, Hemken PM, Sokoll LJ, Elliott D, Chan DW, Dowell BL. A comparative evaluation of Golgi protein-73, fucosylated hemopexin, alpha-fetoprotein, and PIVKA-II in the serum of patients with chronic hepatitis, cirrhosis, and hepatocellular carcinoma. Clin Chem Lab Med. 2011b doi: 10.1515/CCLM.2011.097. [DOI] [PubMed] [Google Scholar]
- Naitoh A, Aoyagi Y, Asakura H. Highly enhanced fucosylation of serum glycoproteins in patients with hepatocellular carcinoma. Journal of gastroenterology and hepatology. 1999a;14:436–445. doi: 10.1046/j.1440-1746.1999.01882.x. [DOI] [PubMed] [Google Scholar]
- Naitoh A, Aoyagi Y, Asakura H. Highly enhanced fucosylation of serum glycoproteins in patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 1999b;14:436–445. doi: 10.1046/j.1440-1746.1999.01882.x. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Uozumi N, Nakano M, Mizuno-Horikawa Y, Okuyama N, Taguchi T, Gu J, Kondo A, Taniguchi N, Miyoshi E. Fucosylation of N-glycans regulates the secretion of hepatic glycoproteins into bile ducts. J Biol Chem. 2006;281:29797–29806. doi: 10.1074/jbc.M605697200. [DOI] [PubMed] [Google Scholar]
- Noda K, Miyoshi E, Gu J, Gao CX, Nakahara S, Kitada T, Honke K, Suzuki K, Yoshihara H, Yoshikawa K, Kawano K, Tonetti M, Kasahara A, Hori M, Hayashi N, Taniguchi N. Relationship between elevated FX expression and increased production of GDP-L-fucose, a common donor substrate for fucosylation in human hepatocellular carcinoma and hepatoma cell lines. Cancer Res. 2003;63:6282–6289. [PubMed] [Google Scholar]
- Noda K, Miyoshi E, Kitada T, Nakahara S, Gao CX, Honke K, Shiratori Y, Moriwaki H, Sasaki Y, Kasahara A, Hori M, Hayashi N, Taniguchi N. The enzymatic basis for the conversion of nonfucosylated to fucosylated alpha-fetoprotein by acyclic retinoid treatment in human hepatoma cells: activation of alpha1-6 fucosyltransferase. Tumour Biol. 2002;23:202–211. doi: 10.1159/000067253. [DOI] [PubMed] [Google Scholar]
- Noda K, Miyoshi E, Uozumi N, Yanagidani S, Ikeda Y, Gao C, Suzuki K, Yoshihara H, Yoshikawa K, Kawano K, Hayashi N, Hori M, Taniguchi N. Gene expression of alpha1-6 fucosyltransferase in human hepatoma tissues: a possible implication for increased fucosylation of alpha-fetoprotein. Hepatology (Baltimore, Md. 1998;28:944–952. doi: 10.1002/hep.510280408. [DOI] [PubMed] [Google Scholar]
- Nuck R, Orthen B, Reutter W. Occurrence of alpha 1-2-fucosylation in membrane glycoproteins of Morris hepatoma 7777 but not in liver. Aberrant type of fucosylation in a malignant tissue. Eur J Biochem. 1992;208:669–676. doi: 10.1111/j.1432-1033.1992.tb17233.x. [DOI] [PubMed] [Google Scholar]
- Ohno M, Nishikawa A, Koketsu M, Taga H, Endo Y, Hada T, Higashino K, Taniguchi N. Enzymatic basis of sugar structures of alpha-fetoprotein in hepatoma and hepatoblastoma cell lines: correlation with activities of alpha 1-6 fucosyltransferase and N-acetylglucosaminyltransferases III and V. Int J Cancer. 1992;51:315–317. doi: 10.1002/ijc.2910510223. [DOI] [PubMed] [Google Scholar]
- Okuda K, Fujimoto I, Hanai A, Urano Y. Changing incidence of hepatocellular carcinoma in Japan. Cancer Res. 1987;47:4967–4972. [PubMed] [Google Scholar]
- Ozkan H, Erdal H, Tutkak H, Karaeren Z, Yakut M, Yuksel O, Koklu S. Diagnostic and prognostic validity of Golgi protein 73 in hepatocellular carcinoma. Digestion. 2011;83:83–88. doi: 10.1159/000320379. [DOI] [PubMed] [Google Scholar]
- Park HJ, Kim BG, Lee SJ, Heo SH, Kim JY, Kwon TH, Lee EB, Ryoo HM, Cho JY. Proteomic profiling of endothelial cells in human lung cancer. Journal of Proteome Research. 2008;7:1138–1150. doi: 10.1021/pr7007237. [DOI] [PubMed] [Google Scholar]
- Park KS, Cho SY, Kim H, Paik YK. Proteomic alterations of the variants of human aldehyde dehydrogenase isozymes correlate with hepatocellular carcinoma. International journal of cancer Journal international du cancer. 2002a;97:261–265. doi: 10.1002/ijc.1585. [DOI] [PubMed] [Google Scholar]
- Park KS, Kim H, Kim NG, Cho SY, Choi KH, Seong JK, Paik YK. Proteomic analysis and molecular characterization of tissue ferritin light chain in hepatocellular carcinoma. Hepatology (Baltimore, Md. 2002b;35:1459–1466. doi: 10.1053/jhep.2002.33204. [DOI] [PubMed] [Google Scholar]
- Park SY, Yu X, Ip C, Mohler JL, Bogner PN, Park YM. Peroxiredoxin 1 interacts with androgen receptor and enhances its transactivation. Cancer research. 2007;67:9294–9303. doi: 10.1158/0008-5472.CAN-07-0651. [DOI] [PubMed] [Google Scholar]
- Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of 25 major cancers in 1990. Int J Cancer. 1999;80:827–841. doi: 10.1002/(sici)1097-0215(19990315)80:6<827::aid-ijc6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Paterlini-Brechot P, Vona G, Brechot C. Circulating tumorous cells in patients with hepatocellular carcinoma. Clinical impact and future directions. Semin Cancer Biol. 2000;10:241–249. doi: 10.1006/scbi.2000.0323. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Helbig KJ, Van der Hoek KH, Seth D, Beard MR. Osteopontin increases hepatocellular carcinoma cell growth in a CD44 dependant manner. World journal of gastroenterology: WJG. 2012;18:3389–3399. doi: 10.3748/wjg.v18.i26.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompach P, Brnakova Z, Sanda M, Wu J, Edwards N, Goldman R. Site-specific glycoforms of haptoglobin in liver cirrhosis and hepatocellular carcinoma. Molecular & cellular proteomics: MCP. 2013;12:1281–1293. doi: 10.1074/mcp.M112.023259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon RT, Fan ST, Ng IO, Won J. Significance of resection margin in hepatectomy for hepatocellular carcinoma: A critical reappraisal. Ann Surg. 2000;231:544–551. doi: 10.1097/00000658-200004000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon TC, Johnson PJ. Proteome analysis and its impact on the discovery of serological tumor markers. Clinica chimica acta; international journal of clinical chemistry. 2001;313:231–239. doi: 10.1016/s0009-8981(01)00677-5. [DOI] [PubMed] [Google Scholar]
- Powers TW, Neely BA, Shao Y, Tang H, Troyer DA, Mehta AS, Haab BB, Drake RR. MALDI Imaging Mass Spectrometry Profiling of N-Glycans in Formalin-Fixed Paraffin Embedded Clinical Tissue Blocks and Tissue Microarrays. PLoS One. 2014 doi: 10.1371/journal.pone.0106255. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao B, Wang J, Xie J, Niu Y, Ye S, Wan Q, Ye Q. Detection and identification of peroxiredoxin 3 as a biomarker in hepatocellular carcinoma by a proteomic approach. Int J Mol Med. 2012;29:832–840. doi: 10.3892/ijmm.2012.916. [DOI] [PubMed] [Google Scholar]
- Qin LX, Tang ZY. Recent progress in predictive biomarkers for metastatic recurrence of human hepatocellular carcinoma: a review of the literature. Journal of cancer research and clinical oncology. 2004;130:497–513. doi: 10.1007/s00432-004-0572-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiniger IW, Wolf A, Welge-Lussen U, Mueller AJ, Kampik A, Schaller UC. Osteopontin as a serologic marker for metastatic uveal melanoma: results of a pilot study. Am J Ophthalmol. 2007;143:705–707. doi: 10.1016/j.ajo.2006.11.040. [DOI] [PubMed] [Google Scholar]
- Riener MO. Diagnosis of tumours of the liver and the biliary tract: new tissue and serum markers. Pathologe. 2011;32(Suppl 2):304–309. doi: 10.1007/s00292-011-1467-6. [DOI] [PubMed] [Google Scholar]
- Riener MO, Stenner F, Liewen H, Soll C, Breitenstein S, Pestalozzi BC, Samaras P, Probst-Hensch N, Hellerbrand C, Mullhaupt B, Clavien PA, Bahra M, Neuhaus P, Wild P, Fritzsche F, Moch H, Jochum W, Kristiansen G. Golgi phosphoprotein 2 (GOLPH2) expression in liver tumors and its value as a serum marker in hepatocellular carcinomas. Hepatology (Baltimore, Md. 2009;49:1602–1609. doi: 10.1002/hep.22843. [DOI] [PubMed] [Google Scholar]
- Romano PR, Mackay A, Vong M, deSa J, Lamontagne A, Comunale MA, Hafner J, Block T, Lec R, Mehta A. Development of recombinant Aleuria aurantia lectins with altered binding specificities to fucosylated glycans. Biochemical and Biophysical Research Communications. 2011 doi: 10.1016/j.bbrc.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryden I, Lundblad A, Pahlsson P. Lectin ELISA for analysis of alpha(1)-acid glycoprotein fucosylation in the acute phase response. Clin Chem. 1999;45:2010–2012. [PubMed] [Google Scholar]
- Saldova R, Dempsey E, Perez-Garay M, Marino K, Watson JA, Blanco-Fernandez A, Struwe WB, Harvey DJ, Madden SF, Peracaula R, McCann A, Rudd PM. 5-AZA-2′-deoxycytidine induced demethylation influences N-glycosylation of secreted glycoproteins in ovarian cancer. Epigenetics. 2011a;6:1362–1372. doi: 10.4161/epi.6.11.17977. [DOI] [PubMed] [Google Scholar]
- Saldova R, Fan Y, Fitzpatrick JM, Watson RW, Rudd PM. Core fucosylation and alpha2-3 sialylation in serum N-glycome is significantly increased in prostate cancer comparing to benign prostate hyperplasia. Glycobiology. 2011b;21:195–205. doi: 10.1093/glycob/cwq147. [DOI] [PubMed] [Google Scholar]
- Sanda M, Pompach P, Brnakova Z, Wu J, Makambi K, Goldman R. Quantitative liquid chromatography-mass spectrometry-multiple reaction monitoring (LC-MS-MRM) analysis of site-specific glycoforms of haptoglobin in liver disease. Molecular & cellular proteomics: MCP. 2013;12:1294–1305. doi: 10.1074/mcp.M112.023325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiffele P, Paranen J, Simons K. N-glycans as apical sorting signals in epithelial cells. Nature. 1995;378:96–98. doi: 10.1038/378096a0. [DOI] [PubMed] [Google Scholar]
- Schremmer B, Manevich Y, Feinstein SI, Fisher AB. Peroxiredoxins in the lung with emphasis on peroxiredoxin VI. Sub-cellular biochemistry. 2007;44:317–344. doi: 10.1007/978-1-4020-6051-9_15. [DOI] [PubMed] [Google Scholar]
- Schwegler EE, Cazares L, Steel LF, Adam BL, Johnson DA, Semmes OJ, Block TM, Marrero JA, Drake RR. SELDI-TOF MS profiling of serum for detection of the progression of chronic hepatitis C to hepatocellular carcinoma. Hepatology (Baltimore, Md. 2005;41:634–642. doi: 10.1002/hep.20577. [DOI] [PubMed] [Google Scholar]
- Shalhoub P, Kern S, Girard S, Beretta L. Proteomic-based approach for the identification of tumor markers associated with hepatocellular carcinoma. Disease markers. 2001;17:217–223. doi: 10.1155/2001/210580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang S, Plymoth A, Ge S, Feng Z, Rosen HR, Sangrajrang S, Hainaut P, Marrero JA, Beretta L. Identification of osteopontin as a novel marker for early hepatocellular carcinoma. Hepatology (Baltimore, Md. 2012;55:483–490. doi: 10.1002/hep.24703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Chen J, Li L, Sun Z, Zen L, Xu S, Zhang Y, Zhang L. A study of diagnostic value of golgi protein GP73 and its genetic assay in primary hepatic carcinoma. Technol Cancer Res Treat. 2011;10:287–294. doi: 10.7785/tcrt.2012.500205. [DOI] [PubMed] [Google Scholar]
- Song P, Bao H, Yu Y, Xue Y, Yun D, Zhang Y, He Y, Liu Y, Liu Q, Lu H, Fan H, Luo J, Yang P, Chen X. Comprehensive profiling of metastasis-related proteins in paired hepatocellular carcinoma cells with different metastasis potentials. Proteomics Clinical applications. 2009;3:841–852. doi: 10.1002/prca.200780131. [DOI] [PubMed] [Google Scholar]
- Srivastava OP, Hindsgaul O, Shoreibah M, Pierce M. Recognition of oligosaccharide substrates by N-acetyl-glucosaminyltransferase-V. Carbohydr Res. 1988;179:137–161. doi: 10.1016/0008-6215(88)84115-6. [DOI] [PubMed] [Google Scholar]
- Stamataglou SC, Hughes RC. Cell adhesion molecules in liver function and pattern formation. FASEB J. 1994;8:420–427. doi: 10.1096/fasebj.8.6.8168692. [DOI] [PubMed] [Google Scholar]
- Steel LF, Mattu TS, Mehta A, Hebestreit H, Dwek R, Evans AA, London WT, Block T. A proteomic approach for the discovery of early detection markers of hepatocellular carcinoma. Disease markers. 2001;17:179–189. doi: 10.1155/2001/963023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Chen P, Chen Q, Sun L, Kang X, Qin X, Liu Y. Serum paraoxonase 1 heteroplasmon, a fucosylated, and sialylated glycoprotein in distinguishing early hepatocellular carcinoma from liver cirrhosis patients. Acta Biochim Biophys Sin (Shanghai) 2012;44:765–773. doi: 10.1093/abbs/gms055. [DOI] [PubMed] [Google Scholar]
- Sun Y, Yang H, Mao Y, Xu H, Zhang J, Li G, Lu X, Sang X, Zhao H, Zhong S, Huang J, Zhang H. Increased Golgi protein 73 expression in hepatocellular carcinoma tissue correlates with tumor aggression but not survival. Journal of gastroenterology and hepatology. 2011;26:1207–1212. doi: 10.1111/j.1440-1746.2011.06733.x. [DOI] [PubMed] [Google Scholar]
- Takashima M, Kuramitsu Y, Yokoyama Y, Iizuka N, Toda T, Sakaida I, Okita K, Oka M, Nakamura K. Proteomic profiling of heat shock protein 70 family members as biomarkers for hepatitis C virus-related hepatocellular carcinoma. Proteomics. 2003;3:2487–2493. doi: 10.1002/pmic.200300621. [DOI] [PubMed] [Google Scholar]
- Takeda Y, Shinzaki S, Okudo K, Moriwaki K, Murata K, Miyoshi E. Fucosylated haptoglobin is a novel type of cancer biomarker linked to the prognosis after an operation in colorectal cancer. Cancer. 2012;118:3036–3043. doi: 10.1002/cncr.26490. [DOI] [PubMed] [Google Scholar]
- Tan LY. Correlation between GP73 protein and human liver disease. Zhonghua gan zang bing za zhi = Zhonghua ganzangbing zazhi = Chinese journal of hepatology. 2007;15:958–959. [PubMed] [Google Scholar]
- Tan LY, Chen J, Wang H, Ye HQ, Li Q, Gu JA, Han LQ. Correlaion between serum Golph2 protein and hepatocellular carcinoma. Zhonghua gan zang bing za zhi = Zhonghua ganzangbing zazhi = Chinese journal of hepatology. 2009;17:288–291. [PubMed] [Google Scholar]
- Tang ZY, Ye SL, Liu YK, Qin LX, Sun HC, Ye QH, Wang L, Zhou J, Qiu SJ, Li Y, Ji XN, Liu H, Xia JL, Wu ZQ, Fan J, Ma ZC, Zhou XD, Lin ZY, Liu KD. A decade’s studies on metastasis of hepatocellular carcinoma. Journal of cancer research and clinical oncology. 2004;130:187–196. doi: 10.1007/s00432-003-0511-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Robinson SD, Foster GR, Arora S, Hargreaves S, Thomas HC. Increase in primary liver cancer in the UK. Lancet. 1997;350:1142–1143. doi: 10.1016/S0140-6736(05)63789-0. [DOI] [PubMed] [Google Scholar]
- Thompson S, Guthrie D, Turner GA. Fucosylated forms of alpha-1-antitrypsin that predict unresponsiveness to chemotherapy in ovarian cancer. British journal of cancer. 1988;58:589–593. doi: 10.1038/bjc.1988.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Wang Y, Xu D, Gui J, Jia X, Tong H, Wen X, Dong Z, Tian Y. Serological AFP/Golgi protein 73 could be a new diagnostic parameter of hepatic diseases. International journal of cancer Journal international du cancer. 2011;129:1923–1931. doi: 10.1002/ijc.25838. [DOI] [PubMed] [Google Scholar]
- Tigrani DY, Weydert JA. Immunohistochemical expression of osteopontin in epithelioid mesotheliomas and reactive mesothelial proliferations. American journal of clinical pathology. 2007;127:580–584. doi: 10.1309/BME4VG11LDX8KMTN. [DOI] [PubMed] [Google Scholar]
- Tuck AB, Chambers AF, Allan AL. Osteopontin overexpression in breast cancer: knowledge gained and possible implications for clinical management. Journal of cellular biochemistry. 2007;102:859–868. doi: 10.1002/jcb.21520. [DOI] [PubMed] [Google Scholar]
- Tuma PL, Hubbard AL. The hepatocyte surface: Dynamic polarity. In: Arias IM, editor. The Liver: Biology and Pathobiology. Lippincott Williams & Wilkins; Philadelphia: 2001. pp. 98–117. [Google Scholar]
- Turner GA. Haptoglobin. A potential reporter molecule for glycosylation changes in disease. Advances in experimental medicine and biology. 1995;376:231–238. [PubMed] [Google Scholar]
- Wang C, Hua-yu Y, Yi-lei M, Jin-chun Z, Hai-feng X, Gui-yong J, Yan-ling J, Xin-xin C, Xin L, Xin-ting S, Hong-bing Z. Value of Golgi protein 73 monoclonal antibody in diagnosis of hepatocellular carcinoma. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2011a;33:39–44. doi: 10.3881/j.issn.1000-503X.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Wang HY. Laser capture microdissection in comparative proteomic analysis of hepatocellular carcinoma. Methods Cell Biol. 2007;82:689–707. doi: 10.1016/S0091-679X(06)82025-X. [DOI] [PubMed] [Google Scholar]
- Wang M, Long RE, Comunale MA, Junaidi O, Marrero J, Di Bisceglie AM, Block TM, Mehta AS. Novel fucosylated biomarkers for the early detection of hepatocellular carcinoma. Cancer Epidemiol Biomarkers Prev. 2009a;18:1914–1921. doi: 10.1158/1055-9965.EPI-08-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Long RE, Comunale MA, Junaidi O, Marrero J, Di Bisceglie AM, Block TM, Mehta AS. Novel fucosylated biomarkers for the early detection of hepatocellular carcinoma. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009b;18:1914–1921. doi: 10.1158/1055-9965.EPI-08-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Zhao LJ, Wang Y, Tao QY, Feitelson MA, Zhao P, Ren H, Qi ZT. Application of HBx-induced anti-URGs as early warning biomarker of cirrhosis and HCC. Cancer biomarkers: section A of Disease markers. 2011b;11:29–39. doi: 10.3233/CBM-2012-0261. [DOI] [PubMed] [Google Scholar]
- Wang X, Gu J, Ihara H, Miyoshi E, Honke K, Taniguchi N. Core fucosylation regulates epidermal growth factor receptor-mediated intracellular signaling. The Journal of biological chemistry. 2006;281:2572–2577. doi: 10.1074/jbc.M510893200. [DOI] [PubMed] [Google Scholar]
- Wei T, Liu Q, He F, Zhu W, Hu L, Guo L, Zhang J. The role of N-acetylglucosaminyltransferases V in the malignancy of human hepatocellular carcinoma. Exp Mol Pathol. 2012;93:8–17. doi: 10.1016/j.yexmp.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Wen CL, Chen KY, Chen CT, Chuang JG, Yang PC, Chow LP. Development of an AlphaLISA assay to quantify serum core-fucosylated E-cadherin as a metastatic lung adenocarcinoma biomarker. J Proteomics. 2012;75:3963–3976. doi: 10.1016/j.jprot.2012.05.015. [DOI] [PubMed] [Google Scholar]
- White KY, Rodemich L, Nyalwidhe JO, Comunale MA, Clements MA, Lance RS, Schellhammer PF, Mehta AS, Semmes OJ, Drake RR. Glycomic characterization of prostate-specific antigen and prostatic acid phosphatase in prostate cancer and benign disease seminal plasma fluids. J Proteome Res. 2009;8:620–630. doi: 10.1021/pr8007545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteaker JR, Zhang H, Zhao L, Wang P, Kelly-Spratt KS, Ivey RG, Piening BD, Feng LC, Kasarda E, Gurley KE, Eng JK, Chodosh LA, Kemp CJ, McIntosh MW, Paulovich AG. Integrated pipeline for mass spectrometry-based discovery and confirmation of biomarkers demonstrated in a mouse model of breast cancer. Journal of Proteome Research. 2007;6:3962–3975. doi: 10.1021/pr070202v. [DOI] [PubMed] [Google Scholar]
- Xu WF, Fei YM, Zhou JK, Shen HJ, Chen XF, Lv QQ, Ding YY. Significance of serum golgi protein 73 (GP73), alpha-fetoprotein (AFP) and lectin-reactive alpha-fetoprotein (AFP-L3) expresssion in primary hepatic carcinoma. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2011;25:286–288. [PubMed] [Google Scholar]
- Yamamoto K, Imamura H, Matsuyama Y, Kume Y, Ikeda H, Norman GL, Shums Z, Aoki T, Hasegawa K, Beck Y, Sugawara Y, Kokudo N. AFP, AFP-L3, DCP, and GP73 as markers for monitoring treatment response and recurrence and as surrogate markers of clinicopathological variables of HCC. Journal of gastroenterology. 2010;45:1272–1282. doi: 10.1007/s00535-010-0278-5. [DOI] [PubMed] [Google Scholar]
- Yamanaka N, Tanaka T, Tanaka W, Yamanaka J, Yasui C, Kuroda N, Takada M, Okamoto E. Correlation of hepatitis virus serologic status with clinicopathologic features in patients undergoing hepatectomy for hepatocellular carcinoma. Cancer. 1997;79:1509–1515. doi: 10.1002/(sici)1097-0142(19970415)79:8<1509::aid-cncr10>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Yamashita K, Taketa K, Nishi S, Fukushima K, Ohkura T. Sugar chains of human cord serum alpha-fetoprotein: characteristics of N-linked sugar chains of glycoproteins produced in human liver and hepatocellular carcinomas. Cancer Res. 1993;53:2970–2975. [PubMed] [Google Scholar]
- Ye QH, Qin LX, Forgues M, He P, Kim JW, Peng AC, Simon R, Li Y, Robles AI, Chen Y, Ma ZC, Wu ZQ, Ye SL, Liu YK, Tang ZY, Wang XW. Predicting hepatitis B virus-positive metastatic hepatocellular carcinomas using gene expression profiling and supervised machine learning. Nature medicine. 2003;9:416–423. doi: 10.1038/nm843. [DOI] [PubMed] [Google Scholar]
- Yokoo H, Kondo T, Fujii K, Yamada T, Todo S, Hirohashi S. Proteomic signature corresponding to alpha fetoprotein expression in liver cancer cells. Hepatology (Baltimore, Md. 2004;40:609–617. doi: 10.1002/hep.20372. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Nishikawa A, Ihara Y, Taniguchi S, Taniguchi N. Suppression of lung metastasis of B16 mouse melanoma by N-acetylglucosaminyltransferase III gene transfection. Proc Natl Acad Sci U S A. 1995;92:8754–8758. doi: 10.1073/pnas.92.19.8754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue HY, Cao J, Cui JF, Dai Z, Su JJ, Duan XX, Yang C, Yue HF, Li Y, Liu YK. The expression of peroxiredoxin II in hepatocellular carcinoma and its significance. Zhonghua gan zang bing za zhi = Zhonghua ganzangbing zazhi = Chinese journal of hepatology. 2007;15:366–369. [PubMed] [Google Scholar]
- Zeindl-Eberhart E, Haraida S, Liebmann S, Jungblut PR, Lamer S, Mayer D, Jager G, Chung S, Rabes HM. Detection and identification of tumor-associated protein variants in human hepatocellular carcinomas. Hepatology (Baltimore, Md. 2004;39:540–549. doi: 10.1002/hep.20060. [DOI] [PubMed] [Google Scholar]
- Zekri AR, Bahnassy AA, Alam El-Din HM, Morsy HM, Shaarawy S, Moharram NZ, Daoud SS. Serum levels of beta-catenin as a potential marker for genotype 4/hepatitis C-associated hepatocellular carcinoma. Oncology reports. 2011;26:825–831. doi: 10.3892/or.2011.1355. [DOI] [PubMed] [Google Scholar]
- Zhao XY, Li N, Ding HG, Jiang FF. Detection and evaluation of serum GP73, a resident Golgi glycoprotein, as a marker in diagnosis of hepatocellular carcinoma. Chinese journal of oncologyZhonghua zhong liu za zhi. 2010;32:943–945. [PubMed] [Google Scholar]
- Zhou Y, Yin X, Ying J, Zhang B. Golgi protein 73 versus alpha-fetoprotein as a biomarker for hepatocellular carcinoma: a diagnostic meta-analysis. BMC Cancer. 2012;12:17. doi: 10.1186/1471-2407-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Lin Z, Wu J, Yin H, Dai J, Feng Z, Marrero J, Lubman DM. Analysis of serum haptoglobin fucosylation in hepatocellular carcinoma and liver cirrhosis of different etiologies. Journal of Proteome Research. 2014;13:2986–2997. doi: 10.1021/pr500128t. [DOI] [PMC free article] [PubMed] [Google Scholar]