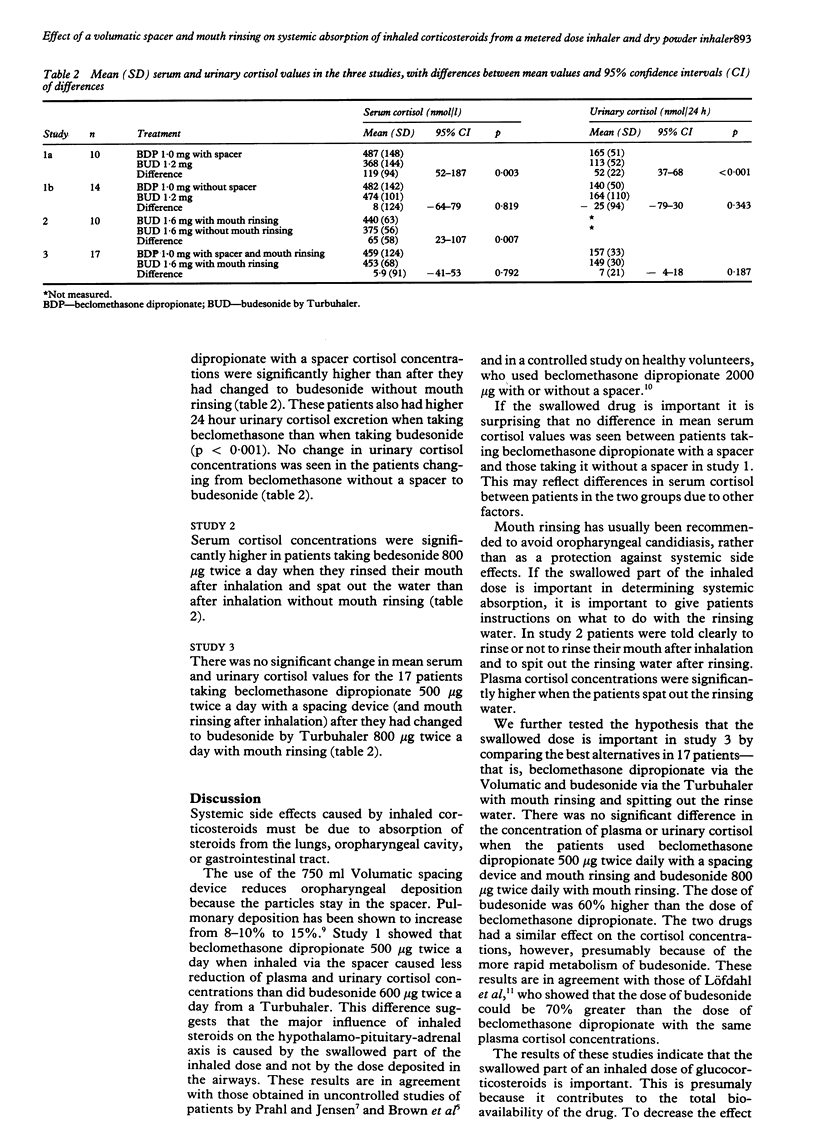
Abstract
BACKGROUND: High doses of inhaled corticosteroids are absorbed systemically and may cause long term side effects. As rinsing the mouth out after use and inhaling through a spacing device may reduce systemic absorption this has been further investigated. METHODS: Three crossover studies were carried out to assess the effect of budesonide given by dry powder inhaler (Turbuhaler) with and without mouth rinsing and beclomethasone dipropionate given by metered dose inhaler with or without a spacing device (Volumatic) on serum cortisol concentrations and urinary cortisol excretion in patients with asthma taking an inhaled corticosteroid. Each treatment period was two weeks with in a two week washout period. Serum cortisol concentrations at 0800 hours on day 14 and the 24 hour urinary excretion of cortisol were measured. In study 1 24 patients taking beclomethasone dipropionate 500 micrograms twice a day inhaled with (n = 10) or without (n = 14) a Volumatic spacing device were switched to a budesonide dry powder inhaler, 600 micrograms to be taken twice a day without mouth rinsing. In study 2 10 patients took budesonide 800 micrograms twice a day with and without mouth rinsing and without swallowing the rinsing water. In study 3 17 patients took budesonide 800 micrograms twice daily with mouth rinsing and beclomethasone dipropionate 500 micrograms twice daily with the spacing device and mouth rinsing. RESULTS: In study 1 no difference was seen between budesonide without mouth rinsing and beclomethasone dipropionate without a spacer: beclomethasone with spacer caused less suppression of cortisol (mean (SD) serum cortisol concentration: beclomethasone and spacer 487(148), budesonide 368(145) nmol/l). In study 2 mouth rinsing caused less suppression of morning serum cortisol concentrations (rinsing 440(63), no rinsing 375(56) nmol/1). In study 3 there was no difference in serum or urinary cortisol concentrations between twice daily beclomethasone dipropionate 500 micrograms inhaled by Volumatic spacer or budesonide by Turbuhaler 800 micrograms twice daily, both with mouth rinsing. Individual serum cortisol values were within the normal range in all patients except one in study 1. CONCLUSION: Systemic absorption of a corticosteroid inhaled from a metered dose inhaler is reduced by using a spacing device and that from a dry powder inhaler by mouth rinsing.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown P. H., Blundell G., Greening A. P., Crompton G. K. Do large volume spacer devices reduce the systemic effects of high dose inhaled corticosteroids? Thorax. 1990 Oct;45(10):736–739. doi: 10.1136/thx.45.10.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo-Kang Y. F., Cooper E. J., Tribe A. E., Grant I. W. Beclomethasone dipropionate by inhalation in the treatment of airways obstruction. Br J Dis Chest. 1972 Apr;66(2):101–106. [PubMed] [Google Scholar]
- Farrer M., Francis A. J., Pearce S. J. Morning serum cortisol concentrations after 2 mg inhaled beclomethasone dipropionate in normal subjects: effect of a 750 ml spacing device. Thorax. 1990 Oct;45(10):740–742. doi: 10.1136/thx.45.10.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddie J., Petrie G. R., Reid I. W., Skinner C., Sinclair D. J., Palmer K. N. Aerosol beclomethasone dipropionate: a dose-response study in chronic bronchial asthma. Lancet. 1973 Aug 11;2(7824):280–281. doi: 10.1016/s0140-6736(73)90788-5. [DOI] [PubMed] [Google Scholar]
- Harris D. M., Martin L. E., Harrison C., Jack D. The effect of oral and inhaled beclomethasone dipropionate on adrenal function. Clin Allergy. 1973 Sep;3(3):243–248. doi: 10.1111/j.1365-2222.1973.tb01329.x. [DOI] [PubMed] [Google Scholar]
- Löfdahl C. G., Mellstrand T., Svedmyr N. Glucocorticoids and asthma. Studies of resistance and systemic effects of glucocorticoids. Eur J Respir Dis Suppl. 1984;136:69–79. [PubMed] [Google Scholar]
- Newman S. P., Millar A. B., Lennard-Jones T. R., Morén F., Clarke S. W. Improvement of pressurised aerosol deposition with Nebuhaler spacer device. Thorax. 1984 Dec;39(12):935–941. doi: 10.1136/thx.39.12.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prahl P., Jensen T. Decreased adreno-cortical suppression utilizing the Nebuhaler for inhalation of steroid aerosols. Clin Allergy. 1987 Sep;17(5):393–398. doi: 10.1111/j.1365-2222.1987.tb02031.x. [DOI] [PubMed] [Google Scholar]
- Smith M. J., Hodson M. E. Effects of long term inhaled high dose beclomethasone dipropionate on adrenal function. Thorax. 1983 Sep;38(9):676–681. doi: 10.1136/thx.38.9.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toogood J. H., Jennings B., Baskerville J., Johansson S. A. Clinical use of spacer systems for corticosteroid inhalation therapy: a preliminary analysis. Eur J Respir Dis Suppl. 1982;122:100–107. [PubMed] [Google Scholar]
- Wetterlin K. Turbuhaler: a new powder inhaler for administration of drugs to the airways. Pharm Res. 1988 Aug;5(8):506–508. doi: 10.1023/a:1015969324799. [DOI] [PubMed] [Google Scholar]


