Abstract
Background
Survivors of pediatric cancer have elevated risks of mortality and morbidity. Many adverse late effects associated with cancer treatment (e.g. second cancers, cardiac and pulmonary disease) are also associated with cigarette smoking, suggesting survivors who smoke may be at high risk for these conditions.
Methods
We examined self-reported smoking status in 9,397 adult survivors of childhood cancer across 3 questionnaires (median time interval 13 years). Smoking prevalence among survivors was compared to siblings and expected prevalence based on age-, sex-, race-, and calendar time-specific U.S. population rates. Multivariable regression models examined characteristics associated with longitudinal smoking patterns across all three questionnaires.
Results
At baseline, 19% of survivors were current smokers, compared with 24% of siblings and 29% expected based on U.S. rates. Current smoking among survivors dropped to 16% and 14% on follow-up questionnaires, with similar decreases in siblings and expected prevalence. Characteristics associated with consistent never smoking included higher household income (relative risk 1.16, 95% confidence interval 1.08–1.25), higher education (1.32, 1.22–1.43), and receipt of cranial radiation therapy (1.08, 1.03–1.14). Psychological distress (0.86, 0.80–0.92) and heavy alcohol drinking (0.64, 0.58–0.71) were inversely associated. Among ever smokers, higher income (1.17, 1.04–1.32) and education (1.23, 1.10–1.38) were associated with quitting, whereas cranial radiation (0.86, 0.76–0.97) and psychological distress (0.80, 0.72–0.90) were associated with not having quit (0.85, 0.76–0.96). Development of adverse health conditions was not associated with smoking patterns.
Conclusion
Despite modest declines in smoking prevalence, the substantial number of consistent current smokers reinforces the need for continued development of effective smoking interventions for survivors.
Keywords: childhood cancer survivors, smoking, prevalence, smoking patterns, cancer treatment, longitudinal studies
Introduction
Progress in biology and therapy have dramatically improved outcomes for pediatric cancers, with overall five-year survival rates now exceeding 80%.1 This improvement in survival prompted increased emphasis on the long-term health of survivors, who maintain an elevated risk of mortality and morbidity compared to the general population many decades after their initial cancer treatment.2–4 Childhood cancer survivors have an increased risk of second cancers, pulmonary complications, cardiac disease, fertility impairments, and many other adverse late effects.4–9 Smoking is an established risk factor for many of these outcomes in the general population, suggesting that childhood cancer survivors who smoke may have particularly elevated risk that may be partially preventable. Therefore, it is essential to characterize smoking behaviors among childhood cancer survivors across their lifespan, and to identify opportunities for promotion of smoking prevention and cessation in this high risk group.
Numerous studies have examined smoking prevalence in childhood cancer survivors, and some have identified risk factors for smoking initiation or cessation.10–19 However, these studies have typically included data from only a single questionnaire, precluding them from detailing changes in smoking behaviors over time. The prevalence of smoking in the general population has decreased in recent decades in the United States,20 but it is unclear how this temporal trend has impacted smoking in childhood cancer survivors. At the individual level, identification of characteristics associated with long-term smoking patterns could inform future prevention and control efforts.
A previous analysis by Emmons et al. examined smoking behaviors in survivors of childhood cancer using data from the baseline questionnaire of the Childhood Cancer Survivor Study (CCSS), which was administered starting in 1994.12 The goal of the present study was to extend that work by examining smoking status reported in two subsequent CCSS follow-up questionnaires completed on average 8 and 13 years later, comparing the prevalence of smoking among survivors, siblings, and the comparable U.S. general population. Additionally, we examined long-term participants in CCSS to identify characteristics associated with patterns of consistent never smoking status or consistent current smoking status.
Materials and Methods
Population
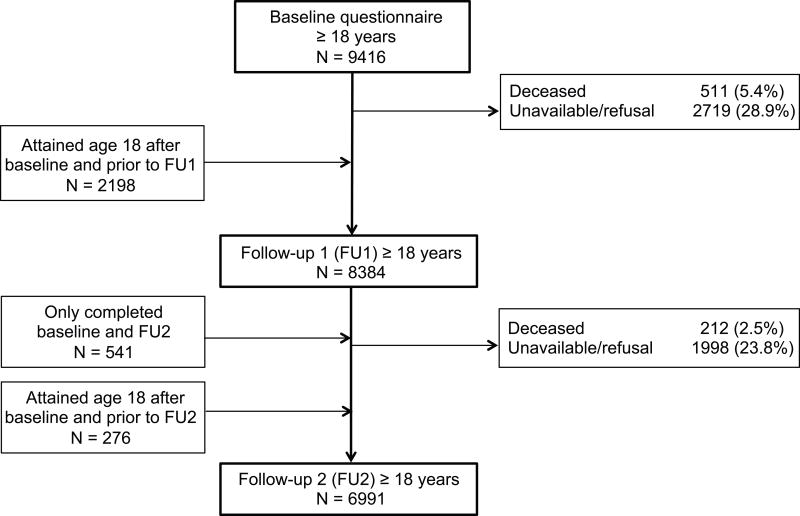
The CCSS, described in detail previously,21–23 is a retrospective cohort study of five year survivors following diagnosis prior to age 21 years with selected cancers (leukemia, central nervous system tumors, Hodgkin lymphoma, non-Hodgkin lymphoma, kidney tumors, neuroblastoma, soft tissue sarcoma, and bone tumors) at one of 26 collaborating institutions between January 1, 1970 and December 31, 1986. The study was approved by the Institutional Review Board at each institution, and participants provided informed consent. A total of 14,358 returned the baseline questionnaire, and participants were very similar to non-participants with regard to gender, cancer diagnosis, age at diagnosis, age at contact, and type of cancer treatment. Race/ethnicity could not be compared because these data were not available for non-participants.21, 22 Follow-up questionnaires that included smoking questions were administered beginning in 2003 (referred to here as follow-up 1 or FU1) and 2007 (referred to here as follow-up 2 or FU2) to participants who were alive and had not refused further participation in the study. The mean time intervals from baseline (administered 1994–1999) to FU1 and FU2 were 7.6 and 12.5 years, respectively. Participants less than 18 years of age at the time of the questionnaire were excluded from the current analysis due to the use of parental report data, as were those who completed the questionnaire but failed to provide data on smoking status. Figure 1 describes participation flow for the cohort included in this study from baseline to FU2.
Figure 1.
Participants with a completed questionnaire among adult survivors of childhood cancer across 3 questionnaires
A randomly selected population of 4023 siblings of childhood cancer survivors in CCSS was recruited to serve as a comparison group and completed the baseline questionnaire.21 Of these, 73% and 59% completed FU1 and FU2, respectively. Data on the prevalence of smoking status in the general population were obtained from the National Health Interview Survey (NHIS), a cross-sectional household interview survey providing information on the health of the civilian non-institutionalized population of the United States.24
Primary Outcomes
Self-reported smoking status was derived from two items repeated across all questionnaires. Participants were asked whether they had ever smoked at least 100 cigarettes in their lifetime, and then if they answered “yes”, whether they currently smoke cigarettes. Based on answers to these questions participants were categorized as never smokers (<100 cigarettes smoked during lifetime), former smokers (≥100 cigarettes during lifetime, but not smoking currently on some or all days), or current smokers (≥100 cigarettes during lifetime and currently smoking on some or all days) at each questionnaire time point.
Independent Variables
Questionnaires completed by childhood cancer survivors and siblings included items assessing demographics, medical conditions, lifestyle behaviors, and psychosocial variables. Data on radiation and chemotherapy treatments received during the first 5 years after childhood cancer diagnosis were abstracted from medical records. Region-specific dosimetry data were calculated for recipients of radiotherapy, and survivors were categorized by whether they had received cranial or chest radiation of any dose.25 Chemotherapeutic agents were categorized based on existing evidence as toxic to the heart (anthracyclines) or pulmonary system (bleomycin, busulfan, carmustine (BCNU), lomustine (CCNU), or cyclophosphamide). Self-reported medical condition data were used to categorize chronic health conditions using the Common Terminology Criteria for Adverse Events, version 4.0, as previously described.3, 26 Participants completed the Brief Symptom Inventory-18 (BSI-18) as part of the baseline questionnaire, and those with T-scores of 63 or higher on any of the depression, anxiety, or somatization subscales were categorized as having psychological distress.27, 28 Heavy alcohol drinking was defined as >4 drinks per day or >14 per week for men and >3 drinks per day or >7 per week for women.
Statistical Analysis
Descriptive statistics were calculated for participants in each questionnaire, and baseline characteristics were compared between participants in the baseline questionnaire who did and did not provide smoking status information in the FU1 questionnaire. To account for differential attrition by factors potentially related to smoking status (e.g. sex, race, education, household income; see Supplemental Table 1), we used multiple imputation to assign smoking status values to participants who were age ≥18 years at baseline but did not complete the FU1 questionnaire or did not answer the smoking status questions, excluding those who died prior to FU1 (see Supplementary Materials for details).
We examined the cross sectional prevalence of smoking status among childhood cancer survivors and siblings at least 18 years of age based on respondents to each of the baseline, FU1 and FU2 questionnaires. To assess the potential impact of attrition bias, we also carried out a sensitivity analysis comparing prevalence estimates in FU1 with and without inclusion of participants with imputed smoking status. Due to the lengthy time interval since baseline, we did not impute smoking status for non-respondents to the FU2 questionnaire and thus presented only the prevalence among those who responded.
To compare the prevalence of smoking status in CCSS participants with that in a comparable U.S. general population at similar points in time, we used NHIS population age-, sex- and race-specific prevalence rates of smoking from 1997, 2003 and 2007. The baseline CCSS data were collected from 1994–1999; we used NHIS 1997 for comparison because a revised format of the NHIS survey was instituted in that year. The expected prevalence of smoking among CCSS survivors if they had the same smoking prevalence as the U.S. population was calculated by weighting the U.S. age-, sex- and race-specific prevalences by the distribution of these factors in CCSS survivors.
Longitudinal data provided by participants who completed all three CCSS questionnaires (baseline, FU1 and FU2) were utilized to evaluate long term smoking patterns. Survivors were categorized first as either consistent never smokers (i.e. those who reported never smoking on all questionnaires) or ever smokers. Ever smokers were then further categorized as either consistent current smokers (i.e. those who reported current smoking on all questionnaires) or quitters (i.e. those who reported former smoking, except those who then reported current smoking on FU2).
Among all participants with three smoking status reports, we identified characteristics associated with consistent never smoking compared to ever smoking using generalized linear models with robust variance estimation to account for within subject correlation. A log-link function was used to directly obtain relative risks (RR) and 95% confidence intervals (95% CI) from multivariable models including age at diagnosis (0–9 years; 10–20 years), age at last questionnaire (<30 years; 30–39 years; ≥40 years), sex, race/ethnicity (non-Hispanic white; other), household income (<$20,000 per year; $20,000–$59,999; ≥$60,000), education (high school or less; some education after high school), presence or new development of severe/disabling/life-threatening chronic conditions (CTCAE grade 3 or 4), psychological distress (yes/no), heavy alcohol drinking (yes/no), and receipt (yes/no) of the following treatments: cranial radiation therapy, chest radiation, cardio-toxic chemotherapy, and pulmonary-toxic chemotherapy. Similar methods were used to identify factors associated with quitting compared to consistent current smoking among all ever smokers.
Results
The 9397 survivors ≥18 years of age at baseline were 53% male and were predominantly less than 35 years of age (87%, mean age 28 years), non-Hispanic white (84%), and had received some education beyond high school (65%). In addition to being older, participants in FU1 and FU2 were more likely to be female, non-Hispanic white, and in higher categories of household income and education compared to baseline (Table 1). They were also younger at childhood cancer diagnosis, more likely to have been diagnosed with leukemia or kidney tumors, and less likely to have been diagnosed with Hodgkin lymphoma or cancers of the central nervous system.
Table 1.
Distribution of demographic and treatment characteristics among survivors of childhood cancer with ongoing follow-up
| Characteristic | Baseline (N=9397)1 N (%) |
Follow-up 1 (FU1) (N=8364) N (%) |
Follow-up 2 (FU2) (N=6951) N (%) |
All 3 questionnaires2 (N=4607) N (%) |
|
|---|---|---|---|---|---|
| Sex | Male | 4997 (53.2) | 4122 (49.3) | 3332 (47.9) | 2201 (47.8) |
| Age at questionnaire | 18–24 years | 3707 (39.4) | 1719 (20.6) | 270 (3.9) | 1725 (37.4) |
| 25–34 years | 4470 (47.6) | 3704 (44.3) | 2700 (38.8) | 2250 (48.8) | |
| 35–44 years | 1196 (12.7) | 2493 (29.8) | 2901 (41.7) | 618 (13.4) | |
| ≥45 years | 24 (0.3) | 448 (5.4) | 1080 (15.5) | 14 (0.3) | |
| Race/Ethnicity | White, not Hispanic | 7870 (84.0) | 7162 (86.0) | 6036 (86.8) | 4023 (87.3) |
| Household income | $60,000+ | 2064 (22.0) | 3146 (37.6) | 3406 (49.0) | 1166 (25.3) |
| $20,000–$59,999 | 4289 (45.6) | 3167 (37.9) | 2266 (32.6) | 2258 (49.0) | |
| < $20,000 | 1857 (19.8) | 887 (10.6) | 635 (9.1) | 758 (16.5) | |
| Not specified | 1187 (12.6) | 1164 (13.9) | 644 (9.3) | 425 (9.2) | |
| Education | College graduate or more | 2792 (29.7) | 3751 (44.8) | 3559 (51.2) | 1796 (39.0) |
| Post high school, some college | 3285 (35.0) | 3099 (37.1) | 1684 (24.2) | 1702 (36.9) | |
| High school graduate or less | 2813 (29.9) | 1460 (17.5) | 759 (10.9) | 926 (20.1) | |
| Not specified | 507 (5.4) | 54 (0.6) | 949 (13.7) | 183 (4.0) | |
| Age at diagnosis | 0–9 years | 4597 (48.9) | 5092 (60.9) | 4160 (59.8) | 2104 (45.7) |
| 10–20 years | 4800 (51.1) | 3272 (39.1) | 2791 (40.2) | 2503 (54.3) | |
| Cancer diagnosis | Leukemia | 2780 (29.6) | 2852 (34.1) | 2396 (34.5) | 1407 (30.5) |
| Central nervous system | 1180 (12.6) | 884 (10.6) | 671 (9.7) | 448 (9.7) | |
| Hodgkin lymphoma | 1670 (17.8) | 1141 (13.6) | 965 (13.9) | 845 (18.3) | |
| NonHodgkin lymphoma | 865 (9.2) | 649 (7.8) | 542 (7.8) | 421 (9.1) | |
| Kidney (Wilms’ tumor) | 611 (6.5) | 790 (9.4) | 647 (9.3) | 290 (6.3) | |
| Neuroblastoma | 372 (4.0) | 549 (6.6) | 478 (6.9) | 174 (3.8) | |
| Soft tissue sarcoma | 902 (9.6) | 762 (9.1) | 637 (9.2) | 479 (10.4) | |
| Bone | 1017 (10.8) | 737 (8.8) | 615 (8.8) | 543 (11.8) | |
| Cranial radiation therapy | Yes | 2559 (27.2) | 2281 (27.3) | 1884 (27.1) | 1283 (27.8) |
| Chest radiation | Yes | 1965 (20.9) | 1581 (18.9) | 1315 (18.9) | 1058 (23.0) |
| Pulmonary-toxic chemo2 | Yes | 3815 (47.1) | 3622 (47.4) | 3072 (47.7) | 2029 (47.6) |
| Anthracyclines | Yes | 3063 (37.8) | 3045 (39.8) | 2590 (40.2) | 1682 (39.4) |
| Number of severe/disabling/life- threatening chronic conditions | 0 | 6514 (69.3) | 5570 (66.6) | 4245 (61.1) | 3144 (68.2) |
| 1 | 2088 (22.2) | 1930 (23.1) | 1708 (24.6) | 1088 (23.6) | |
| 2+ | 795 (8.5) | 864 (10.3) | 998 (14.4) | 375 (8.1) |
Excludes participants eligible in Figure 1 who did not report smoking status on the questionnaire: n=19 at Baseline; n= 20 at FU1; n=40 at FU2.
Characteristics from the baseline questionnaire
Pulmonary-toxic chemotherapy included bleomycin, busulfan, carmustine (BCNU), lomustine (CCNU), or cyclophosphamide
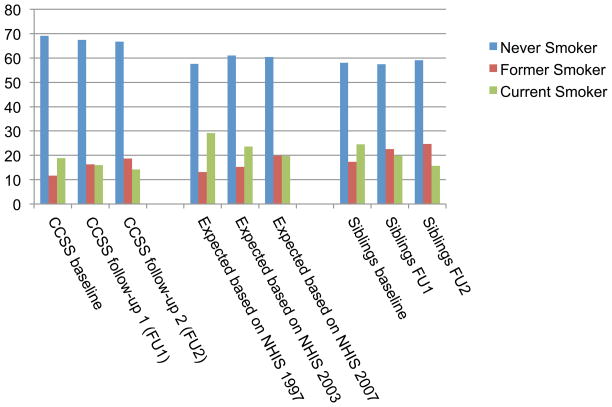
The prevalence of current smoking among participating CCSS survivors at baseline was 19%, compared to the 29% that would have been expected based on prevalence rates in the U.S. population at the time of the survey and the 24% reported among siblings (Figure 2). Current smoking among survivors was less common in the subsequent questionnaires, dropping to 16% at FU1 and 14% at FU2, with corresponding increases in the prevalence of former smoking (12% at baseline; 16% at FU1; 19% at FU2). Similar decreases in the prevalence of current smoking were observed after standardization to U.S. general population rates (24% in 2003; 20% in 2007) and in siblings (20% at FU1; 16% at FU2). After imputation to account for non-respondents in FU1, the prevalence of current smoking in survivors increased from 16% to 17% (Supplemental Table 2).
Figure 2.
Prevalence of smoking among adult survivors of childhood cancer, prevalence expected in CCSS survivor cohort based on sex, race, and age-specific rates in the U.S. population, and prevalence among adult siblings
Among survivors who completed all three questionnaires (mean age 40 years at FU2), 72% maintained never smoking status. In multivariable models (Table 2), consistent never smoking was significantly, although modestly, associated with higher household income (RR=1.16), higher education (RR=1.32), and receipt of cranial radiation as therapy for childhood cancer (RR=1.08). Older age at cancer diagnosis (RR=0.94) and older age at last questionnaire (RR=0.86) were significantly inversely associated with never smoking, as were psychological distress (RR=0.86) and heavy drinking (RR=0.64). Notably, there was no association with receipt of chest radiation or chemotherapy known to be toxic to the lungs or heart nor with the development of severe or disabling chronic health conditions. Univariate associations were not substantively different from the multivariable model results listed in Table 2.
Table 2.
Associations from multivariable models between participant characteristics and a pattern of consistent never smoking among all participants or quitting among ever smokers
| Characteristic | Consistent Never Smoking (N=3304) | Quitting Among Ever Smokers (N=862) | |||
|---|---|---|---|---|---|
| RR (95% CI)1 | P | RR (95% CI)1 | P | ||
| Age at diagnosis | 0–9 years | 1.00 (reference) | 1.00 (reference) | ||
| 10–20 years | 0.94 (0.89–0.99) | 0.02 | 0.93 (0.83–1.03) | 0.17 | |
| Age at last questionnaire | <30 years | 1.00 (reference) | 1.00 (reference) | ||
| 30–39 years | 0.92 (0.75–1.13) | 1.09 (0.65–1.85) | |||
| ≥40 years | 0.86 (0.70–1.06) | 0.03 | 1.09 (0.64–1.86) | 0.94 | |
| Sex | Male | 1.00 (reference) | 1.00 (reference) | ||
| Female | 1.01 (0.96–1.05) | 0.79 | 0.95 (0.88–1.03) | 0.23 | |
| Race/Ethnicity | White, not Hispanic | 1.00 (reference) | 1.00 (reference) | ||
| Others | 1.05 (0.98–1.12) | 0.15 | 1.00 (0.87–1.15) | 0.97 | |
| Household income | <$20,000 | 1.00 (reference) | 1.00 (reference) | ||
| $20,000$59,999 | 1.12 (1.04–1.20) | 1.02 (0.91–1.15) | |||
| $60,000+ | 1.16 (1.08–1.25) | 0.0004 | 1.17 (1.04–1.32) | 0.002 | |
| Education | ≤ High school | 1.00 (reference) | 1.00 (reference) | ||
| > High school | 1.32 (1.22–1.43) | <0.0001 | 1.23 (1.10–1.38) | 0.0002 | |
| Cranial radiation therapy | No | 1.00 (reference) | 1.00 (reference) | ||
| Yes | 1.08 (1.03–1.14) | 0.002 | 0.86 (0.76–0.97) | 0.01 | |
| Chest radiation | No | 1.00 (reference) | 1.00 (reference) | ||
| Yes | 1.02 (0.97–1.08) | 0.46 | 1.07 (0.98–1.17) | 0.11 | |
| Pulmonary-toxic chemotherapy | No | 1.00 (reference) | 1.00 (reference) | ||
| Yes | 1.02 (0.97–1.08) | 0.40 | 0.98 (0.90–1.07) | 0.67 | |
| Cardio-toxic chemotherapy | No | 1.00 (reference) | 1.00 (reference) | ||
| Yes | 1.03 (0.98–1.09) | 0.28 | 1.05 (0.96–1.15) | 0.32 | |
| Number of severe/disabling chronic conditions2 | 0 | 1.00 (reference) | 1.00 (reference) | ||
| 1 | 1.00 (0.94–1.05) | 1.01 (0.92–1.10) | |||
| 2+ | 1.04 (0.96–1.13) | 0.61 | 1.06 (0.92–1.21) | 0.75 | |
| Development of new severe/disabling conditions | No Change | 1.00 (reference) | 1.00 (reference) | ||
| Increased | 0.99 (0.93–1.05) | 0.67 | 0.95 (0.86–1.05) | 0.35 | |
| Psychological distress | No | 1.00 (reference) | 1.00 (reference) | ||
| Yes | 0.86 (0.80–0.92) | <0.0001 | 0.80 (0.72–0.90) | <0.0001 | |
| Heavy drinking | No | 1.00 (reference) | 1.00 (reference) | ||
| Yes | 0.64 (0.58–0.71) | <0.0001 | 0.93 (0.85–1.02) | 0.13 | |
Multivariable models included all variables listed in the table
Conditions reported at the time of the first smoking status report
Of 1292 participants who reported ever smoking at FU2, 63% reported they were not currently smoking, suggesting at least temporary success in quitting. Among participants who initiated smoking at some point in their lives, characteristics significantly associated with quitting (Table 2) included higher household income (RR=1.17) and higher education (RR=1.23). Receipt of cranial radiation was inversely associated with quitting (RR=0.86), indicating that those who received cranial radiation were more likely to remain current smokers over the course of follow-up. Psychological distress was also significantly associated with continued current smoking (RR=0.80).
Discussion
In this study we extended previous analyses of smoking in CCSS participants by incorporating data from two follow-up questionnaires, showing a modest decline in the prevalence of current smoking from 19% at baseline to 17% and 14% among those participating on average 8 and 12 years later. This decline appears to be consistent with broader temporal and age-dependent trends in smoking status in the U.S., as evidenced by similar decreases in current smoking reported for siblings and the comparable general population. The more recent estimates suggest that while survivors continue to smoke at a rate below those of siblings and the general population, the prevalence of current smoking remains high for a group already at risk for poor cardiopulmonary outcomes and second cancers, emphasizing the importance of continued smoking prevention and cessation efforts in this group.
Although smoking has been clearly linked to risk of numerous chronic health conditions,29 neither having one or more severe/disabling/life-threatening chronic health conditions at baseline nor developing such a condition during follow-up were associated with longitudinal smoking status patterns in CCSS participants. This novel result reflects the powerful influence of nicotine addiction in smokers and emphasizes the need for increased efforts to reinforce the substantial negative impact of beginning or continuing to smoke in childhood cancer survivors. There may be opportunities to leverage the fact that many survivors have already experienced adverse effects of their cancer treatment, potentially increasing the saliency of anti-smoking messages. Survivors experiencing chronic health conditions likely have increased interaction with the healthcare system, so increased emphasis on addressing tobacco use in the clinical setting (e.g. as recommended in Public Health Service guidelines30) is particularly important in this group.
Survivors who may be at risk for pulmonary or cardiac late effects due to their initial cancer therapies had similar patterns of smoking prevalence to those not exposed to agents with known toxicity, suggesting a need for additional education regarding the potential for smoking to exacerbate already elevated risk levels. This finding extends that of Emmons et al. from the CCSS baseline questionnaire alone, where receipt of pulmonary-toxic treatment was inversely associated with ever initiating smoking, but not with overall prevalence of current smoking.12 Similarly, our results confirm that receipt of cranial radiation therapy was associated with a long-term never smoking pattern, but also with not quitting over time among survivors who ever smoked. Cranial radiation has been associated with neuro- and neuropsychological impairments ranging from mild to severe that may leave survivors more dependent on others or living in restricted environments, potentially limiting their opportunities to smoke or exposure to situations that facilitate smoking initiation.12, 13 The mechanism for an inverse association between cranial radiation and quitting is unclear, but may be related to neurocognitive deficits. The inverse association between older age at diagnosis and never smoking may reflect smoking initiation prior to cancer diagnosis in teens.
Our results concur with existing evidence in childhood cancer survivors and the general population that both lower household income and lower education are associated with becoming and remaining a current smoker. 12, 13, 31, 32 Similarly in line with previous research, the presence of psychological distress or heavy alcohol drinking at the baseline questionnaire was inversely associated with never smoking, and ever smokers with psychological distress were less likely to quit.33, 34 Although our study assessed prevalence and thus was not designed to determine temporality of these associations, both alcohol and psychological distress should be considered in design of interventions.
The previous study in CCSS by Emmons et al. reported 17% current smoking at baseline.12 Our analysis differed in that we excluded participants with missing smoking status at baseline, resulting in the 19% current smoking reported here. Our results extend previous work in CCSS and other studies by providing more recent prevalence estimates with data collected from 2003–2005 and 2007–2009. Another large cohort study of childhood cancer survivors in Britain (71% of whom were under age 35) reported a current smoking prevalence of 20%, assessed at one point in time primarily from 2001–2003. British survivors had significantly lower odds of being a current smoker compared to the general population.13 Similarly, in CCSS, the rate of smoking initiation in survivors at baseline was 27% less than expected based on the general population rates, whereas the rate of smoking cessation was 22% higher than expected.12 Our results suggest a modest decline in the prevalence of current smoking over time among survivors, with a similar decline among siblings over the same time period, reflecting smoking trends in the general population.
The relevant literature on smoking prevention and cessation interventions in childhood cancer survivors has been reviewed elsewhere,34, 35 but two cessation interventions are noteworthy for having been conducted within the CCSS cohort. The Partnership for Health Study (PFHS) was a randomized controlled trial in which 796 current smokers from CCSS were randomized to either self-help or a peer-led telephone counseling intervention.36 The self-reported quit rate was modestly but significantly higher in the counseling intervention group compared to the self-help group at 12 months (15% vs. 9%) and remained significant among those followed-up again at 2–6 years post-randomization.37, 38 Only 15% of current smokers in our analysis of longitudinal smoking patterns were participants in the PFHS trial, and among this subgroup there was no difference in smoking status by intervention versus control group. Another randomized controlled trial randomized 519 smokers to two different tobacco quitline interventions that also included nicotine replacement therapy.39 Notably, recruitment for this intervention occurred after completion of the follow-up questionnaires. Although self-reported cessation rates in the trial were comparable to other intervention studies in survivors (7 day point prevalence = 21%), the rates of cotinine-verified smoking cessation in both quitline groups was less than 2% at 12 months. These intervention studies highlight how difficult it is to meaningfully change long-term smoking behavior, but the substantial number of CCSS survivors who self-reported consistent current smoking in our analysis clearly calls for continued work in this area. A recent study demonstrating that even moderate smoking exposure negatively impacted pulmonary function in a relatively young group of childhood cancer survivors (median age 35) emphasizes the importance of early intervention.40
Notable strengths of our analysis included the large cohort of childhood cancer survivors, the wealth of data collected in CCSS on demographic and treatment characteristics, the ability to examine the impact of chronic health conditions, and collection of smoking status data at multiple points in time. A clear limitation is reliance on self-reported smoking status. In their quitline intervention study, Klesges et al. reported that 80% of participants claiming abstinence from smoking who underwent cotinine testing failed biochemical verification.39 Our prevalence estimates for current smoking in CCSS are likely underestimates of the true smoking prevalence, although the magnitude of misclassification may be smaller in a cohort study where participants do not feel pressure to meet the goals of an intervention study.41 Childhood cancer survivors may be less likely to report current smoking compared to siblings, and those participating in CCSS may have higher socioeconomic status than non-participants, both of which may further contribute to underestimation of current smoking. Never smoking was defined as less than 100 cigarettes smoked during the lifetime, which may have misclassified some emergent smokers who had just started smoking as never smokers. Attrition bias is a potential limitation of our analyses of the CCSS follow-up questionnaires, as factors associated with continued study participation were also associated with smoking. We accounted for attrition bias by employing multiple imputation to obtain smoking status for the full cohort at the FU1 questionnaire, finding only a modest impact on the prevalence of current smoking (17% versus 16%). The impact of attrition bias may have been greater for the FU2 questionnaire, and the reported 14% prevalence of current smoking is likely an underestimate. Finally, examination of characteristics associated with long term smoking patterns was limited to the subgroup of survivors who were age 18 years or older at baseline and completed both follow-up questionnaires, resulting in a sample with overrepresentation of non-Hispanic whites and survivors with higher income and education.
Childhood cancer survivors have elevated risks for a wide range of adverse health outcomes, and those risks continue to increase with advancing age.42, 43 By age 50 years, the majority of survivors have experienced a severe, disabling, or life-threatening event or death. Thus, it is important to monitor the health behaviors of aging survivors and to identify opportunities to prevent further increases in risk. Our results from continued follow-up of a large cohort of childhood cancer survivors indicate that 14% or more of survivors continue to be current smokers, further exacerbating risk of morbidity and mortality. Effective interventions in this population are urgently needed, particularly targeting survivors at the highest risk of adverse late effects. The difficulty of achieving long-term cessation suggests increased emphasis on prevention of smoking initiation.
Supplementary Material
Acknowledgments
Funding: NCI grant U24CA055727; Cancer Center Support (CORE) grant CA21765
Footnotes
Conflict of interest: None
References
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2010. 2013 Apr; http://seer.cancer.gov/csr/1975_2010/, based on November 2012 SEER data submission, posted to the SEER web site.
- 2.Armstrong GT, Liu Q, Yasui Y, et al. Late mortality among 5-year survivors of childhood cancer: a summary from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2328–2338. doi: 10.1200/JCO.2008.21.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 4.Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer. 2014;14:61–70. doi: 10.1038/nrc3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman DL, Whitton J, Leisenring W, et al. Subsequent neoplasms in 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2010;102:1083–1095. doi: 10.1093/jnci/djq238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green DM, Kawashima T, Stovall M, et al. Fertility of female survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2677–2685. doi: 10.1200/JCO.2008.20.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green DM, Kawashima T, Stovall M, et al. Fertility of male survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2010;28:332–339. doi: 10.1200/JCO.2009.24.9037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mertens AC, Yasui Y, Liu Y, et al. Pulmonary complications in survivors of childhood and adolescent cancer. A report from the Childhood Cancer Survivor Study. Cancer. 2002;95:2431–2441. doi: 10.1002/cncr.10978. [DOI] [PubMed] [Google Scholar]
- 9.Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellizzi KM, Rowland JH, Jeffery DD, McNeel T. Health behaviors of cancer survivors: examining opportunities for cancer control intervention. J Clin Oncol. 2005;23:8884–8893. doi: 10.1200/JCO.2005.02.2343. [DOI] [PubMed] [Google Scholar]
- 11.Demark-Wahnefried W, Werner C, Clipp EC, et al. Survivors of childhood cancer and their guardians. Cancer. 2005;103:2171–2180. doi: 10.1002/cncr.21009. [DOI] [PubMed] [Google Scholar]
- 12.Emmons K, Li FP, Whitton J, et al. Predictors of smoking initiation and cessation among childhood cancer survivors: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2002;20:1608–1616. doi: 10.1200/JCO.2002.20.6.1608. [DOI] [PubMed] [Google Scholar]
- 13.Frobisher C, Winter DL, Lancashire ER, et al. Extent of smoking and age at initiation of smoking among adult survivors of childhood cancer in Britain. J Natl Cancer Inst. 2008;100:1068–1081. doi: 10.1093/jnci/djn210. [DOI] [PubMed] [Google Scholar]
- 14.Haupt R, Byrne J, Connelly RR, et al. Smoking habits in survivors of childhood and adolescent cancer. Med Pediatr Oncol. 1992;20:301–306. doi: 10.1002/mpo.2950200406. [DOI] [PubMed] [Google Scholar]
- 15.Larcombe I, Mott M, Hunt L. Lifestyle behaviours of young adult survivors of childhood cancer. Br J Cancer. 2002;87:1204–1209. doi: 10.1038/sj.bjc.6600632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mulhern RK, Tyc VL, Phipps S, et al. Health-related behaviors of survivors of childhood cancer. Med Pediatr Oncol. 1995;25:159–165. doi: 10.1002/mpo.2950250302. [DOI] [PubMed] [Google Scholar]
- 17.Tao ML, Guo MD, Weiss R, et al. Smoking in adult survivors of childhood acute lymphoblastic leukemia. J Natl Cancer Inst. 1998;90:219–225. doi: 10.1093/jnci/90.3.219. [DOI] [PubMed] [Google Scholar]
- 18.Tyc VL, Hadley W, Crockett G. Predictors of intentions to use tobacco among adolescent survivors of cancer. J Pediatr Psychol. 2001;26:117–121. doi: 10.1093/jpepsy/26.2.117. [DOI] [PubMed] [Google Scholar]
- 19.Verrill JR, Schafer J, Vannatta K, Noll RB. Aggression, antisocial behavior, and substance abuse in survivors of pediatric cancer: possible protective effects of cancer and its treatment. J Pediatr Psychol. 2000;25:493–502. doi: 10.1093/jpepsy/25.7.493. [DOI] [PubMed] [Google Scholar]
- 20.Pierce JP, Messer K, White MM, Cowling DW, Thomas DP. Prevalence of heavy smoking in California and the United States, 1965–2007. JAMA. 2011;305:1106–1112. doi: 10.1001/jama.2011.334. [DOI] [PubMed] [Google Scholar]
- 21.Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: experience of the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2319–2327. doi: 10.1200/JCO.2008.21.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project. Med Pediatr Oncol. 2002;38:229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 23.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. [accessed 03/03/2015, 2015];National Health Interview Survey. Available from http://www.cdc.gov/nchs/nhis.htm.
- 25.Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat Res. 2006;166:141–157. doi: 10.1667/RR3525.1. [DOI] [PubMed] [Google Scholar]
- 26.National Cancer Institute. Common Terminology Criteria for Adverse Events v4.0. NCI, NIH, DHHS; 2009. [Google Scholar]
- 27.Derogatis L. Brief symptom inventory (BSI) 18: administration, scoring, and procedures manual. Minneapolis, MN: NCS Pearson; 2000. [Google Scholar]
- 28.Recklitis CJ, Parsons SK, Shih MC, Mertens A, Robison LL, Zeltzer L. Factor structure of the brief symptom inventory--18 in adult survivors of childhood cancer: results from the Childhood Cancer Survivor Study. Psychol Assess. 2006;18:22–32. doi: 10.1037/1040-3590.18.1.22. [DOI] [PubMed] [Google Scholar]
- 29.U.S. Department of Health and Human Services; U.S. Department of Health and Human Services, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, editor. The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: 2014. [Google Scholar]
- 30.Fiore MC, Jaen CR, Baker TB, et al. U.S. Department of Health and Human Services, editor. Treating Tobacco Use and Dependence: 2008 Update. Rockville, MD: Public Health Service; 2008. [Google Scholar]
- 31.Carswell K, Chen Y, Nair RC, et al. Smoking and binge drinking among Canadian survivors of childhood and adolescent cancers: a comparative, population-based study. Pediatr Blood Cancer. 2008;51:280–287. doi: 10.1002/pbc.21568. [DOI] [PubMed] [Google Scholar]
- 32.Graham H, Inskip HM, Francis B, Harman J. Pathways of disadvantage and smoking careers: evidence and policy implications. J Epidemiol Community Health. 2006;60(Suppl 2):7–12. doi: 10.1136/jech.2005.045583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Falk DE, Yi HY, Hiller-Sturmhofel S. An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Res Health. 2006;29:162–171. [PMC free article] [PubMed] [Google Scholar]
- 34.Klosky JL, Tyc VL, Garces-Webb DM, Buscemi J, Klesges RC, Hudson MM. Emerging issues in smoking among adolescent and adult cancer survivors: a comprehensive review. Cancer. 2007;110:2408–2419. doi: 10.1002/cncr.23061. [DOI] [PubMed] [Google Scholar]
- 35.de Moor JS, Elder K, Emmons KM. Smoking prevention and cessation interventions for cancer survivors. Semin Oncol Nurs. 2008;24:180–192. doi: 10.1016/j.soncn.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Emmons KM, Butterfield RM, Puleo E, et al. Smoking among participants in the childhood cancer survivors cohort: the Partnership for Health Study. J Clin Oncol. 2003;21:189–196. doi: 10.1200/JCO.2003.06.130. [DOI] [PubMed] [Google Scholar]
- 37.Emmons KM, Puleo E, Mertens A, Gritz ER, Diller L, Li FP. Long-term smoking cessation outcomes among childhood cancer survivors in the Partnership for Health Study. J Clin Oncol. 2009;27:52–60. doi: 10.1200/JCO.2007.13.0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Emmons KM, Puleo E, Park E, et al. Peer-delivered smoking counseling for childhood cancer survivors increases rate of cessation: the Partnership for Health Study. J Clin Oncol. 2005;23:6516–6523. doi: 10.1200/JCO.2005.07.048. [DOI] [PubMed] [Google Scholar]
- 39.Klesges RC, Krukowski RA, Klosky JL, et al. Efficacy of a Tobacco Quitline Among Adult Survivors of Childhood Cancer. Nicotine Tob Res. 2014 doi: 10.1093/ntr/ntu216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oancea SC, Gurney JG, Ness KK, et al. Cigarette smoking and pulmonary function in adult survivors of childhood cancer exposed to pulmonary-toxic therapy: results from the St. Jude Lifetime Cohort Study. Cancer Epidemiol Biomarkers Prev. 2014;23:1938–1943. doi: 10.1158/1055-9965.EPI-14-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Velicer WF, Prochaska JO, Rossi JS, Snow MG. Assessing outcome in smoking cessation studies. Psychol Bull. 1992;111:23–41. doi: 10.1037/0033-2909.111.1.23. [DOI] [PubMed] [Google Scholar]
- 42.Armstrong GT, Kawashima T, Leisenring W, et al. Aging and risk of severe, disabling, life-threatening, and fatal events in the Childhood Cancer Survivor Study. J Clin Oncol. 2014;32:1218–1227. doi: 10.1200/JCO.2013.51.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hudson MM, Oeffinger KC, Jones K, et al. Age-dependent changes in health status in the childhood cancer survivor cohort. J Clin Oncol. 2015;33:479–491. doi: 10.1200/JCO.2014.57.4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.