Summary
Males often face a trade-off between investments in precopulatory and postcopulatory traits [1], particularly when male-male contest competition determines access to mates [2]. To date, studies of precopulatory strategies have largely focused on visual ornaments (e.g., coloration) or weapon morphology (e.g., antlers, horns, and canines). However, vocalizations can also play an important role in both male competition and female choice [3, 4, 5]. We investigated variation in vocal tract dimensions among male howler monkeys (Alouatta spp.), which produce loud roars using a highly specialized and greatly enlarged hyoid bone and larynx [6]. We examined the relative male investment in hyoids and testes among howler monkey species in relation to the level of male-male competition and analyzed the acoustic consequences of variation in hyoid morphology. Species characterized by single-male groups have large hyoids and small testes, suggesting high levels of vocally mediated competition. Larger hyoids lower formant frequencies, probably increasing the acoustic impression of male body size and playing a role analogous to investment in large body size or weaponry. Across species, as the number of males per group increases, testes volume also increases, indicating higher levels of postcopulatory sperm competition, while hyoid volume decreases. These results provide the first evidence of an evolutionary trade-off between investment in precopulatory vocal characteristics and postcopulatory sperm production.
Highlights
-
•
Howler monkey hyoid volume varies significantly between sexes and among species
-
•
Hyoid volume negatively correlates with number of males per group and testes volume
-
•
Larger hyoids lower formant spacing, increasing the acoustic impression of body size
-
•
Results provide the first evidence of a trade-off between vocal investment and testes
Males often face a trade-off between pre- and postcopulatory investments for reproduction. Dunn et al. report the first evidence for a trade-off between vocal investment and sperm production—howler monkey species with harem groups have large vocal tracts and small testes, whereas those in multimale groups have small vocal tracts and large testes.
Results and Discussion
Large body size, weaponry, and/or ornaments can confer an advantage to males during reproductive competition, allowing them to better dominate precopulatory contests and increase the number of offspring they sire [3]. However, when multiple males copulate with the same female, postcopulatory sperm competition occurs. This favors adaptations in male reproductive physiology, such as the production of more numerous and larger ejaculates (facilitated by larger testes) or faster and more enduring spermatozoa, which increase the likelihood of fertilization by a given male over competitors [7]. Vocalizations are also an important component of sexual selection in many animal species, often playing a crucial role in determining the outcome of agonistic contests and/or female choice [3, 4, 5]. However, despite considerable interest in the idea of vocal trade-offs [8], little is known about the evolutionary dynamics favoring investment in vocal characteristics versus sperm production.
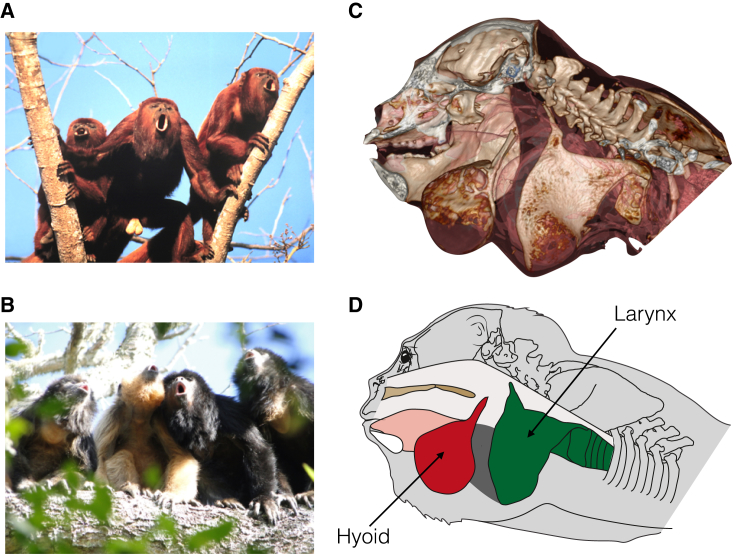
The powerful and characteristic roars of howler monkeys (genus Alouatta) are among the loudest vocalizations produced by any terrestrial animal (Figures 1A and 1B and Movie S1). All howler monkey species have a highly modified larynx with a greatly enlarged cup-shaped hyoid bone containing an air sac, which is thought to function as a resonating chamber for their calls [6, 9] (Figures 1C and 1D and Movie S2). The highly specialized anatomy of the vocal apparatus, coupled with the time and energy invested in vocalizing [10, 11], suggests an important role for roaring in howler monkey fitness—particularly given their energy-minimizing lifestyle [12, 13, 14]. Multiple studies suggest that howler monkey roars function in male-male competition as territorial displays, regulating the use of space by groups [10, 15, 16, 17], although their precise functional significance and evolution is debated [18].
Figure 1.
Examples of Howler Monkey Vocalizations and Vocal Apparatus
(A and B) Group chorus of (A) unimale Venezuelan red howler monkeys, Alouatta seniculus (copyright Carolyn M. Crockett), and (B) multimale-multifemale black and gold howler monkeys, Alouatta caraya (black, males; gold, females; copyright Mariana Raño).
(C) Computed tomography (CT) surface model of adult male Alouatta sara showing highly modified vocal tract (left mandible removed from image).
(D) Schematic representation of the CT model. Red, hyoid; green, larynx; pink, tongue; dark gray, air sacs; brown, palate.
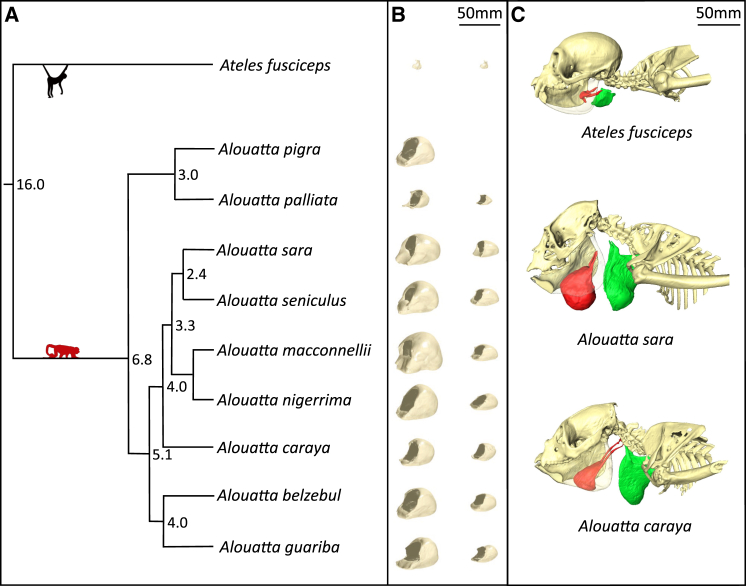
The howler monkey hyoid bone differs considerably in size between the sexes and among species [19, 20], but the full extent of this variation and the selection pressures underlying variability have not been investigated quantitatively. For our core analyses, we collected comparative data on nine of the ten classically recognized Alouatta species [21], using laser surface scanning to produce virtual 3D models of 255 hyoids. We then used phylogenetic methods and average species level data on body weight, skull length, canine length, testes volume, and number of males per group (data from five to nine species, depending on the dataset) to examine whether differences in male hyoid volume were related to variation in male competition among species—the “vocal competition” hypothesis. We also tested an alternative “environmental adaptation” hypothesis, that howler monkey hyoids are adapted to produce different frequency vocalizations in different habitats [22], by analyzing data on net primary productivity. Finally, we used bioacoustic methods to analyze recordings of male roars and examined the acoustic consequences of variation in male hyoid morphology among species, hypothesizing that a more voluminous hyoid bone reduces formant spacing (ΔF) and increases the acoustic impression of body size conveyed by roars [23, 24, 25] (i.e., the “size exaggeration” hypothesis [26, 27]). In order to provide broader comparative context to the core analyses described above, we performed CT and MRI on the cadavers of two adult male howler monkeys (Alouatta sara and A. caraya) and one adult male spider monkey (Ateles fusciceps). This allowed us to visualize the howler monkey vocal tract and measure vocal fold length and vocal tract length (VTL) for comparison with other mammals.
We found that hyoid volume is highly sexually dimorphic (F(1,255) = 497.6, p < 0.001) and varies significantly among species (F(7,255) = 52.4, p < 0.001). We also found a significant interaction between sex and species (F(7,255) = 30.1, p < 0.001), with greater sexual dimorphism in species with larger hyoids (Figure 2 and Table S1). Log10 male hyoid volume was significantly correlated with log10 female hyoid volume (phylogenetic generalized least squares [PGLS]: R2 = 0.89, λ = 0.00, F(1,7) = 54.05, p < 0.0005).
Figure 2.
Variation in Hyoid Morphology among Howler Monkey Species
(A) Phylogeny of the howler monkey (Alouatta) species studied, with Ateles fusciceps as an outgroup. Numbers at the nodes indicate the estimated dates for splitting events (Ma), where known (data from [21]).
(B) 3D models showing the variation in size and shape of average hyoids in males (left) and females (right), corresponding to the species in (A) (hyoids were not available for Alouatta pigra females).
(C) Computed tomography surface models, showing the hyoid (red) and thyroid cartilage (green). The left side of the mandible has been made transparent to make the hyoid bone fully visible.
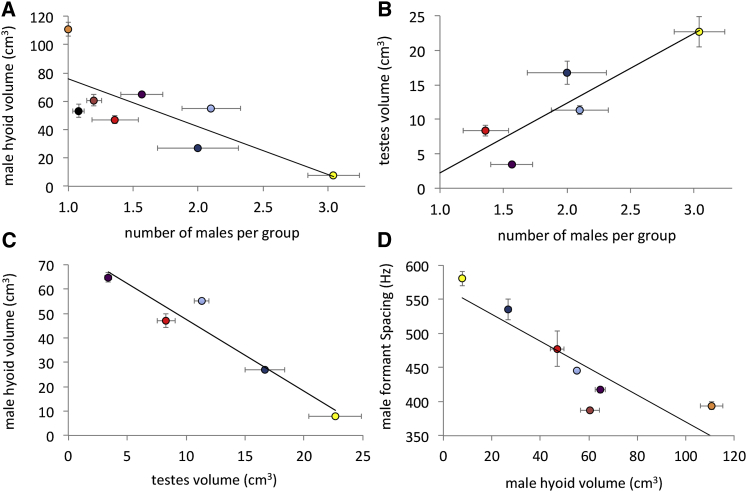
The average number of adult males per social group varied from 1.0 ± 0.0 to 3.0 ± 0.5 across species (Table S1), and log10 male hyoid volume correlates negatively with the number of males per group (PGLS: R2 = 0.83, λ = 0.00, F(1,6) = 29.61, p < 0.005; Figure 3A), consistent with precopulatory sexual selection of this trait. Testes volume also varied significantly among species (F(4,86) = 19.1, p < 0.001) and correlated significantly and positively with the number of males per group (PGLS: R2 = 0.78, λ = 0.00, F(1,3) = 10.45, p < 0.05; Figure 3B), consistent with the hypothesized role for testes volume in postcopulatory sperm competition. Crucially, there was a significant negative correlation between male hyoid volume and testes volume (PGLS: R2 = 0.94, λ = 0.00, F(1,3) = 43.84, p < 0.01; Figure 3C). Canine length was sexually dimorphic (F(1,107) = 148.89, p < 0.001) but did not vary across species (F(8,107) = 1.16, p = 0.33), and there was no interaction between sex and species (F(8,107) = 1.38, p = 0.22). At the species level, canine length was not correlated with body weight, number of males per group, hyoid volume, or testes volume (see the Supplemental Experimental Procedures), suggesting that sexual selection on canine weaponry does not vary across species in this taxon.
Figure 3.
Relationship between Key Variables in Pre- and Postcopulatory Male Strategies across Howler Monkey Species
Regression plots showing (A) log10 mean male hyoid volume versus mean number of males, (B) mean testes volume versus mean number of males per species, (C) log10 mean male hyoid volume versus mean testes volume, and (D) log10 mean male hyoid volume versus ΔF. Each point represents the mean value for a distinct howler monkey species: Alouatta macconnellii (orange), A. belzebul (black), A. sara (pink), A. guariba (red), A. seniculus (purple), A. caraya (dark blue), A. palliata (yellow), and A. pigra (light blue). The slopes and intercepts of the regression lines of the linear model and PGLS model were identical in all cases, so only one line is visible in each figure. Mean values ±SE are shown. Sample sizes are given in Table S1. See also Figure S2 and Tables S1 and S2.
We found no support for the “environmental adaptation” hypothesis: hyoid volume was not predicted by net primary productivity (PGLS: R2 = 0.19, λ = 1.00, F(1,6) = 1.44, p = 0.27; general linear mixed model [GLMM] males: Akaike information criterion [AIC] model = 613.9, AIC null = 611.9, χ2(1) = 0.01, p = 0.90, n = 144; GLMM females: AIC model = 351.7, AIC null = 349.9, χ2(1) = 0.19, p = 0.67, n = 111).
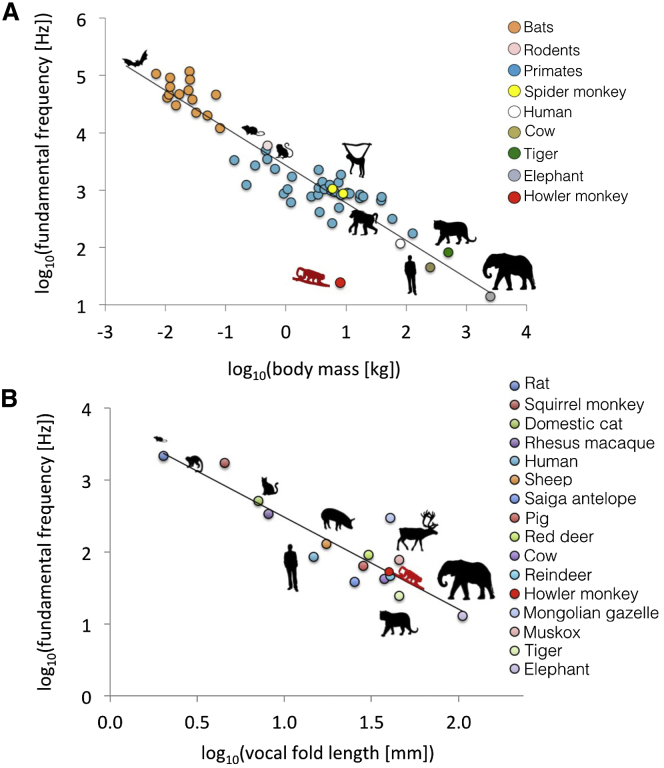
As a result of their anatomical modifications, howler monkeys produce exceptionally low-frequency vocalizations for their body size compared with other mammals (Figure 4A). MRI-based measurements indicated that howler monkey vocal folds are extremely long for an animal of their size (4.08 cm in A. sara and 3.55 cm in A. caraya, Figure S1; human male vocal fold length is ∼1.5 cm [30]). Based on a theoretical string model [30] of the vocal folds, we found that the vocal fold lengths obtained from the MRI-based measurements accurately predict the remarkably low fundamental frequency (F0) of howler monkey vocalizations (see the Supplemental Experimental Procedures). This explains how a howler monkey could produce an F0 similar to that of tigers or reindeer, despite major differences in body size (7 kg versus >100 kg; Figure 4B). However, F0 is not typically measureable in howler monkey roars, which are noisy, broadband sounds presumably generated via deterministic chaos (Figure S2), and in terrestrial mammals, empirical evidence suggests that F0 is not typically a reliable index of body size within age and sex classes [31, 32]. In contrast, numerous studies suggest that formant frequencies can provide reliable information about body size within species [26] and that individuals attend to this information in both inter- and intra-sexual contexts [23, 24, 25]. Male log10 hyoid volume was significantly negatively correlated with ΔF in male roars (R2 = 0.88, λ = 0.00, F(1,5) = 35.14, p < 0.005; Figure 3D). For example, in A. caraya, mean ΔF was 535 Hz, whereas in A. sara mean ΔF was 388 Hz (Table S1). These values predict VTLs of 33 cm and 45 cm, respectively (Table S2), even though total sitting height is only about 40–50 cm in this genus [33]. Although VTL is greater in howler monkeys than other similarly sized primates [26] as a result of their unusual vocal anatomy, these values are inconsistent with our MRI-based VTL measurements of 20.6 cm in A. caraya and 26.3 cm in A. sara (Figure S1). These findings are consistent with the hypothesis that large hyoids may have evolved to enable lower ΔF than expected for body size, thereby increasing the acoustic impression of body size conveyed by howler monkey roars.
Figure 4.
The Exceptionally Low Call Frequency of Howler Monkey Vocalizations
(A) Log-log plot of body weight versus F0 for a range mammals, highlighting the low-frequency vocalizations of howler monkeys (adapted from [28] with permission from AAAS).
(B) Log-log plot of vocal fold length versus F0 for a range mammals (adapted from [29] with permission from Elsevier), showing that the low-frequency vocalizations of howler monkeys are to be expected, given their remarkable vocal fold length.
Data sources are provided in the Supplemental Experimental Procedures. See also Figure S1.
Across species, hyoid volume did not correlate with body weight in either males (PGLS: R2 = 0.06, λ = 1.00, F(1,4) = 0.25, p = 0.65) or females (PGLS: R2 = 0.002, λ = 0.70, F(1,5) = 0.02, p = 0.90), and the PGLS regression at the species level revealed no significant relationship between hyoid volume and skull length (male PGLS: R2 = 0.14, λ = 0.00, F(1,4) = 0.64, p = 0.46; female PGLS: R2 = 0.13, λ = 0.00, F(1,4) = 0.57, p = 0.49). However, hyoid volume was positively correlated with skull length when a larger sample of individual-specific data was used (GLMM: AIC model = 1002.8, AIC null = 2271.6, χ2(1) = 1270.9, p = <0.001, n = 117). This suggests that despite clear differences between species, hyoid volume nonetheless correlates positively with body size within species, and ΔF may thus act as an exaggerated, but honest, signal of body size. This is consistent with studies of other mammal taxa, which have shown that anatomical adaptations of the vocal tract may exaggerate the acoustic impression of body size relative to other species but still convey reliable information about body size relative to conspecifics [34, 35].
Our results provide strong evidence for the vocal competition hypothesis, consistent with Darwin’s suggestion that the vocal organs of male Alouatta have been sexually selected [17]. Females are likely to require large hyoids for some of the same reasons as males, e.g., inter-group resource defense (infants, food, and territory) and predator deterrence [18]. However, it is unclear why female hyoid volume should correlate with male hyoid volume. One reason could be that female hyoid volume is a “correlated response” of selection for large hyoids in males [36]. Another reason could be that there is independent selection for larger hyoids in the females of species in groups with fewer males (in which males also have large hyoids), e.g., as a strategy against male infanticide [37], or owing to variation in female contest competition among species [38]. These phenomena are not mutually exclusive, and further research would be necessary to disentangle this interesting question.
These data provide the first evidence in any species of an evolutionary trade-off between a precopulatory vocal-investment strategy and postcopulatory sperm competition. The phylogenetic correlations we observe are consistent with at least two non-mutually exclusive functional mechanisms, which may work at different phylogenetic levels. The first model, known as the “Y model,” or the acquisition-allocation model, holds that for a given amount of a resource, it is not possible to increase allocation to two traits at once [39]. Traits used in pre- and postcopulatory male-male competition may both be energetically expensive [1, 40], leading to a trade-off in resource allocation. The second mechanism results from trade-offs that occur when evolutionary change in one trait directly decreases the relevance or performance of another [39, 41]. Under this model, the coevolution of intense female monopolization and large hyoids in unimale species limits the opportunity for sperm competition, leading to relaxed selection pressure on testes. In contrast, a failure of precopulatory male-male competition to repel rivals results in increased postcopulatory competition. Matching data on testes and hyoids from the same males across multiple species would be required to fully explore the precise functional nature of trade-offs within and between species, providing an exciting avenue for future research.
Experimental Procedures
Morphological Traits
We analyzed 255 (111 females and 144 males) apparently non-pathological, adult hyoids at a number of museums. Species were identified on the basis of geographic location of the site of provenance of the specimens. Following a standardized protocol, we scanned the bullate basihyoid bone using a 3D laser surface scanner and calculated hyoid volume from the resulting models (see the Supplemental Experimental Procedures). We used both new and published data on testes volume, canine length, and body weight, though the datasets were not matching, i.e., were not from the same individuals (see the Supplemental Experimental Procedures). However, where possible, we collected matching data on skull length for the hyoids analyzed in the dataset (see the Supplemental Experimental Procedures). Data on morphological traits are given in Table S1. In order to analyze VTL and vocal fold length, we also performed CT and MRI on the cadavers of two adult male howler monkeys of different species (Alouatta sara and A. caraya) and one adult male spider monkey (Ateles fusciceps) (see the Supplemental Experimental Procedures).
Group Size and Composition
We compiled data on group size and composition from the literature for each of the howler monkey species studied (see the Supplemental Experimental Procedures and Table S3). Given that local environmental factors, such as variations in climate and vegetation, may affect group size and composition within species, we calculated mean values per study site and then took the average across study sites. We also ran analyses using the mean values for all groups (rather than sites), and the results did not change (Table S4).
Net Primary Productivity
We used 2013 data from the Moderate Resolution Imaging Spectroradiometer (MODIS) on the Terra satellite [42] to calculate the annual NPP for the location of provenance of each hyoid specimen (see the Supplemental Experimental Procedures).
Acoustic Analyses
We searched the Macaulay Library (http://macaulaylibrary.org/) and the British Library Sounds archive (http://sounds.bl.uk/) for high-quality recordings of lone adult male Alouatta roars. We selected the highest-quality recording available of an adult male for each species (see the Supplemental Experimental Procedures). Given the very small level of within species variation in hyoid volume (Table S1), we considered these single recordings to be representative. We extracted three roars per recording for analysis. From these, we calculated ΔF and apparent VTL using published methods (see the Supplemental Experimental Procedures). We could not routinely measure F0 in the roars of the males because of the deterministic chaos typically present. However, we were able to measure F0 in other call types in order to make a general comparison with other mammals (Figure 4). We performed all acoustic analyses in Praat version 5.3.51 [43].
Statistical Methods
We first used a general linear model to examine differences in hyoid volume and canine length between sexes and among species and a one-way ANOVA to examine variation in testes volume among species. Then, to analyze the covariance between variables while accounting for the non-independence of data points due to shared ancestry of species, we used PGLS regressions (see the Supplemental Experimental Procedures). In the analyses that included the mean number of males per species, we used this variable as the independent variable and the morphological traits (i.e., hyoid volume, canine length, and testes volume) as dependent variables. When analyzing the relationship between testes volume and hyoid volume, ΔF and hyoid volume, and skull length and hyoid volume, we assigned hyoid volume as the dependent variable. In order to account for potential error in the branch lengths used, we recalculated all of the PGLS analyses with branch lengths of 1, and the results did not change (Table S4). We present absolute hyoid volume and testes volume in the main text, as there was no correlation between either hyoid volume or testes volume and male body weight in our species-level data and, therefore, no effect of isometric scaling. When added to the models as a covariate, body weight accounted for very little variance and did not change our results (see the Supplemental Experimental Procedures). We log10 transformed variables in those cases where this improved the linearity of the relationships and performed all statistical analyses in R version 2.15.2 [44].
Acknowledgments
We are grateful to Alexander Sliwa, Catalina Gomez, Robert Wallace, Michael Plavkan, Zelinda Braga Hirano, and Julio Cesar de Souza, Jr. for sharing data, Andrew Kitchener (National Museums Scotland) for loaning whole animal specimens, Michaela Gumpenberger and Jaap Saers for support with CT and MRI, Carolyn M. Crockett, Mariana Raño, and La Senda Verde Animal Refuge Bolivia for providing photographs and videos, Nadja Kavcik for help with the figures, and Dieter Lukas for help with statistical analyses. J.C.D. was funded by a Cambridge Humanities Research Grant. W.T.F. acknowledges support of ERC Advanced Grant SOMACCA (#230604) and Austrian Science Fund (FWF) grant W1234-G17.
Published: October 22, 2015
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Supplemental Information includes Supplemental Results, Supplemental Experimental Procedures, two figures, four tables, and two movies and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2015.09.029.
Contributor Information
Jacob C. Dunn, Email: jacobcdunn@gmail.com.
W. Tecumseh Fitch, Email: tecumseh.fitch@univie.ac.at.
Supplemental Information
An adult male Bolivian red howler monkey (Alouatta sara) roaring (copyright La Senda Verde Animal Refuge, Bolivia, used with permission).
A 3D animation of the vocal tract morphology of an adult male black-and-gold howler monkey (Alouatta caraya), an adult male Bolivian red howler monkey (Alouatta sara), and an adult male black-headed spider monkey (Ateles fusciceps). The hyoid bone is shown in red and the thyroid cartilage shown in green.
References
- 1.Parker G.A., Lessells C.M., Simmons L.W. Sperm competition games: a general model for precopulatory male-male competition. Evolution. 2013;67:95–109. doi: 10.1111/j.1558-5646.2012.01741.x. [DOI] [PubMed] [Google Scholar]
- 2.Lüpold S., Tomkins J.L., Simmons L.W., Fitzpatrick J.L. Female monopolization mediates the relationship between pre- and postcopulatory sexual traits. Nat. Commun. 2014;5:3184. doi: 10.1038/ncomms4184. [DOI] [PubMed] [Google Scholar]
- 3.Anderson M. Princeton University Press; 1994. Sexual Selection. [Google Scholar]
- 4.Clutton-Brock T.H., Albon S.D. The roaring of red deer and the evolution of honest advertisement. Behaviour. 1979;69:145–170. [Google Scholar]
- 5.Davies N., Halliday T. Deep croaks and fighting assessment in toads Bufo bufo. Nature. 1978;274:683–685. [Google Scholar]
- 6.Schön M.A. The anatomy of the resonating mechanism in howling monkeys. Folia Primatol. (Basel) 1971;15:117–132. doi: 10.1159/000155371. [DOI] [PubMed] [Google Scholar]
- 7.Birkhead T.R., Møller A.P. Academic Press; 1998. Sperm Competition and Sexual Selection. [Google Scholar]
- 8.Simmons L.W., Peters M., Rhodes G. Low pitched voices are perceived as masculine and attractive but do they predict semen quality in men? PLoS ONE. 2011;6:e29271. doi: 10.1371/journal.pone.0029271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelemen G., Sade J. The vocal organ of the howling monkey (Alouatta palliata) J. Morphol. 1960;107:123–140. doi: 10.1002/jmor.1051070202. [DOI] [PubMed] [Google Scholar]
- 10.Da Cunha R.G.T., Byrne R. Roars of black howler monkeys (Alouatta caraya): evidence for a function in inter-group spacing. Behaviour. 2006;143:1169–1199. [Google Scholar]
- 11.Van Belle S., Estrada A., Garber P.A. The function of loud calls in black howler monkeys (Alouatta pigra): food, mate, or infant defense? Am. J. Primatol. 2014;76:1196–1206. doi: 10.1002/ajp.22304. [DOI] [PubMed] [Google Scholar]
- 12.Dunn J.C., Cristóbal-Azkarate J., Schulte-Herbrüggen B., Chavira R., Veà J.J., Vea J.J. Travel time predicts fecal glucocorticoid levels in free-ranging howlers (Alouatta palliata) Int. J. Primatol. 2013;34:246–259. [Google Scholar]
- 13.Cristóbal-Azkarate J., Arroyo-Rodríguez V. Diet and activity pattern of howler monkeys (Alouatta palliata) in Los Tuxtlas, Mexico: effects of habitat fragmentation and implications for conservation. Am. J. Primatol. 2007;69:1013–1029. doi: 10.1002/ajp.20420. [DOI] [PubMed] [Google Scholar]
- 14.Milton K. Columbia University Press; 1980. The Foraging Strategy of Howler Monkeys: A Study of Primate Economics; p. 165. [Google Scholar]
- 15.Sekulic R. The function of howling in red howler monkeys (Alouatta seniculus) Behaviour. 1982;81:38–54. [Google Scholar]
- 16.Carpenter C.R. Johns Hopkins Press; 1934. A Field Study of the Behavior and Social Relations of Howling Monkeys (Alouatta palliata) [Google Scholar]
- 17.Darwin C. John Murray; 1871. The descent of man and selection in relation to sex. [Google Scholar]
- 18.Kitchen D.M., Teixeira da Cunha R.G., Holzmann I., de Oliviera D. Function of loud calls in howler monkeys. In: Kowalewski M.M., Garber P.A., Cortés-Ortiz L., Urbani B., Youlatos D., editors. Howler Monkeys: Adaptive Radiation, Systematics, and Morphology. Springer; 2015. pp. 369–399. [Google Scholar]
- 19.Hershkovitz P. Mammals of northern Colombia. Preliminary report no. 4: monkeys (primates), with taxonomic revisions of some forms. Proc. U. S. Natl. Mus. 1949;98:323–427. [Google Scholar]
- 20.Youlatos D., Couette S., Halenar L.B. Morphology of howler monkeys: a review and quantitative analysis. In: Kowalewski M.M., Garber P.A., Cortés-Ortiz L., Urbani B., Youlatos D., editors. Howler Monkeys: Adaptive Radiation, Systematics, and Morphology. Springer; 2015. pp. 133–176. [Google Scholar]
- 21.Cortés-Ortiz L., Bermingham E., Rico C., Rodríguez-Luna E., Sampaio I., Ruiz-García M. Molecular systematics and biogeography of the Neotropical monkey genus, Alouatta. Mol. Phylogenet. Evol. 2003;26:64–81. doi: 10.1016/s1055-7903(02)00308-1. [DOI] [PubMed] [Google Scholar]
- 22.Ey E., Fischer J. The “acoustic adaptation hypothesis” - a review of the evidence from birds, anurans and mammals. Bioacoustics. 2009;19:21–48. [Google Scholar]
- 23.Reby D., McComb K., Cargnelutti B., Darwin C., Fitch W.T., Clutton-Brock T. Red deer stags use formants as assessment cues during intrasexual agonistic interactions. Proc. Biol. Sci. 2005;272:941–947. doi: 10.1098/rspb.2004.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charlton B.D., Zhihe Z., Snyder R.J. Giant pandas perceive and attend to formant frequency variation in male bleats. Anim. Behav. 2010;79:1221–1227. [Google Scholar]
- 25.Charlton B.D., Ellis W.A.H., Brumm J., Nilsson K., Fitch W.T. Female koalas prefer bellows in which lower formants indicate larger males. Anim. Behav. 2012;84:1565–1571. [Google Scholar]
- 26.Fitch W.T. Vocal tract length and formant frequency dispersion correlate with body size in rhesus macaques. J. Acoust. Soc. Am. 1997;102:1213–1222. doi: 10.1121/1.421048. [DOI] [PubMed] [Google Scholar]
- 27.Ohala J.J. An ethological perspective on common cross-language utilization of F0 of voice. Phonetica. 1984;41:1–16. doi: 10.1159/000261706. [DOI] [PubMed] [Google Scholar]
- 28.Herbst C.T., Stoeger A.S., Frey R., Lohscheller J., Titze I.R., Gumpenberger M., Fitch W.T. How low can you go? Physical production mechanism of elephant infrasonic vocalizations. Science. 2012;337:595–599. doi: 10.1126/science.1219712. [DOI] [PubMed] [Google Scholar]
- 29.Charlton B.D., Frey R., McKinnon A.J., Fritsch G., Fitch W.T., Reby D. Koalas use a novel vocal organ to produce unusually low-pitched mating calls. Curr. Biol. 2013;23:R1035–R1036. doi: 10.1016/j.cub.2013.10.069. [DOI] [PubMed] [Google Scholar]
- 30.Titze I. Prentice Hall; 1994. Principles of Voice Production. [Google Scholar]
- 31.Ey E., Pfefferle D., Fischer J. Do age- and sex-related variations reliably reflect body size in non-human primate vocalizations? A review. Primates. 2007;48:253–267. doi: 10.1007/s10329-006-0033-y. [DOI] [PubMed] [Google Scholar]
- 32.Taylor A.M., Reby D. The contribution of source-filter theory to mammal vocal communication research. J. Zool. (Lond.) 2010;280:221–236. [Google Scholar]
- 33.Kelaita M., Dias P.A.D., Aguilar-Cucurachi Mdel.S., Canales-Espinosa D., Cortés-Ortiz L. Impact of intrasexual selection on sexual dimorphism and testes size in the Mexican howler monkeys Alouatta palliata and A. pigra. Am. J. Phys. Anthropol. 2011;146:179–187. doi: 10.1002/ajpa.21559. [DOI] [PubMed] [Google Scholar]
- 34.Charlton B.D., Ellis W.A., McKinnon A.J., Cowin G.J., Brumm J., Nilsson K., Fitch W.T. Cues to body size in the formant spacing of male koala (Phascolarctos cinereus) bellows: honesty in an exaggerated trait. J. Exp. Biol. 2011;214:3414–3422. doi: 10.1242/jeb.061358. [DOI] [PubMed] [Google Scholar]
- 35.Reby D., McComb K. Anatomical constraints generate honesty: acoustic cues to age and weight in the roars of red deer stags. Anim. Behav. 2003;65:519–530. [Google Scholar]
- 36.Lande R. Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution (N. Y.) 1980;34:292–305. doi: 10.1111/j.1558-5646.1980.tb04817.x. [DOI] [PubMed] [Google Scholar]
- 37.Agoramoorthy G., Rudran R. Infanticide by adult and subadult males in free-ranging red howler monkeys, Alouatta seniculus, in Venezuela. Ethology. 1995;88:75–88. [Google Scholar]
- 38.Amundsen T. Why are female birds ornamented? Trends Ecol. Evol. 2000;15:149–155. doi: 10.1016/s0169-5347(99)01800-5. [DOI] [PubMed] [Google Scholar]
- 39.Garland T., Jr. Trade-offs. Curr. Biol. 2014;24:R60–R61. doi: 10.1016/j.cub.2013.11.036. [DOI] [PubMed] [Google Scholar]
- 40.Fitzpatrick J.L., Almbro M., Gonzalez-Voyer A., Kolm N., Simmons L.W. Male contest competition and the coevolution of weaponry and testes in pinnipeds. Evolution. 2012;66:3595–3604. doi: 10.1111/j.1558-5646.2012.01713.x. [DOI] [PubMed] [Google Scholar]
- 41.Roff D.A., Fairbairn D.J. The evolution of trade-offs: where are we? J. Evol. Biol. 2007;20:433–447. doi: 10.1111/j.1420-9101.2006.01255.x. [DOI] [PubMed] [Google Scholar]
- 42.Zhao M., Heinsch F.A., Nemani R.R., Running S.W. Improvements of the MODIS terrestrial gross and net primary production global data set. Remote Sens. Environ. 2005;95:164–176. [Google Scholar]
- 43.Boersma P. Praat, a system for doing phonetics by computer. Glot. Int. 2001;5:341–345. [Google Scholar]
- 44.R Development Core Team . R Foundation for Statistical Computing; 2008. R: a language and environment for statistical computing. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
An adult male Bolivian red howler monkey (Alouatta sara) roaring (copyright La Senda Verde Animal Refuge, Bolivia, used with permission).
A 3D animation of the vocal tract morphology of an adult male black-and-gold howler monkey (Alouatta caraya), an adult male Bolivian red howler monkey (Alouatta sara), and an adult male black-headed spider monkey (Ateles fusciceps). The hyoid bone is shown in red and the thyroid cartilage shown in green.