Abstract
β-Arrestins and β-arrestin2 are important adaptor proteins and signal transduction proteins that are mainly involved in the desensitization and internalization of G-protein-coupled receptors. Fibrosis is characterized by accumulation of excess extracellular matrix (ECM) molecules caused by chronic tissue injury. If highly progressive, the fibrotic process leads to organ malfunction and, eventually, death. The incurable lung fibrosis, renal fibrosis and liver fibrosis are among the most common fibrotic diseases. Recent studies show that β-arrestins can activate signaling cascades independent of G-protein activation and scaffold many intracellular signaling networks by diverse types of signaling pathways, including the Hedgehog, Wnt, Notch and transforming growth factor-β pathways, as well as downstream kinases such as MAPK and PI3K. These signaling pathways are involved in the pathological process of fibrosis and fibrotic diseases. This β-arrestin-mediated regulation not only affects cell growth and apoptosis, but also the deposition of ECM, activation of inflammatory response and development of fibrotic diseases. In this review, we survey the involvement of β-arrestins in various signaling pathways and highlight different aspects of their regulation of fibrosis. We also discuss the important roles of β-arrestins in the process of fibrotic diseases by regulating the inflammation and deposit of ECM. It is becoming more evident that targeting β-arrestins may offer therapeutic potential for the treatment of fibrotic diseases.
Keywords: β-arrestins, fibrotic diseases, lung fibrosis, renal fibrosis, liver fibrosis, transforming growth factor-β, Wnt, MAPK, NF-κB, PI3K/Akt
β-Arrestins are adaptor proteins and signal transduction proteins that play an important role in molecular regulation. Previously, several studies have suggested that β-arrestins are well known for negatively regulating G-protein-coupled receptor (GPCRs) signaling and that they participate in receptor desensitization and internalization. With detailed studies, researchers have determined that β-arrestins not only desensitize GPCR transduction pathways but also activate a second signaling pathway downstream of the GPCR transduction pathways. Further, β-arrestins can form complexes with several signaling proteins, including the receptor tyrosine kinase (RTK) and the mitogen-activated protein kinase (MAPK), and regulate the recruitment, proliferation, apoptosis and activation of cells.
Fibrosis is defined as the accumulation of excess extracellular matrix (ECM) components. If highly progressive, the fibrotic process leads to organ malfunction and, eventually, death. To date, fibrotic diseases have been largely overlooked, despite contributing to as many as 45% of deaths in the industrialized world1. In recent decades, our understanding of the pathogenesis of fibrosis has coalesced into a coherent view of how tissues accumulate in collagen-rich ECM in response to tissue injury. The vibrancy of a recent Keystone Symposium on Tissue Fibrosis demonstrates that fibrotic diseases are becoming therapeutically tractable2. Recent studies have shown that abnormal expression of β-arrestins is closely associated with fibrotic diseases. Thus, in this review, we convey our understanding of how β-arrestins contact the fibrosis signaling pathway, highlighting the emerging consensus regarding the function of β-arrestins in fibrotic diseases and the possibility of therapy by targeting β-arrestins.
Structure and functions of β-arrestins
Arrestins constitute a six-member family. β-Arrestin1 and β-arrestin2 are extensively expressed in mammals. Arrestin1 (visual arrestin) and arrestin4 (cone arrestin) have restricted expression patterns and localized primarily in visual sensory tissue3 but D- and E-arrestins remains to be characterized4. The discovery of arrestins in the late 1980s resulted from the observation that increasingly pure preparations of GPCR kinase2 (GRK2) progressively lost the ability to desensitize G-protein activation in a reconstituted β2-adrenergic receptor (β2-AR) system5, and molecular cloning confirmed and revealed its isoforms. Subsequently, β-arrestin1 was cloned from a bovine brain cDNA library5, and β-arrestin2 was cloned from a rat brain cDNA library6.
The amino acid sequences of the two β-arrestin isoforms are 78% identical. Structurally, these sequences comprise two seven-stranded β sandwiches, termed the N-terminal and C-terminal domains, connected by a 12-residue linker (hinge region)7. The N-terminal and C-terminal domains that may undergo substantial conformational rearrangement after receptor binding are residues 282–309 of β-arrestin1 and the C-tail (which is connected to the C-domain with a residue linker)8. The hinge does not participate in stable molecular interactions, but its main effect is to make the structures of the C- and N-termini move freely and transform β-arrestins into an active conformation9. The N-terminus of the β-arrestins contains Src-SH3 binding sites10, and the C-terminus includes JNK3 binding sites11. The C-terminal end also contains the grid, which is an AP2 binding site12 involved in receptor internalization. The serine 412 of β-arrestin1 is the site of extracellular signal regulated kinase1/2 (ERK1/2)-mediated phosphorylation13. From a structure/function perspective, the phosphorylation and ubiquitination of β-arrestins are important to the interaction of β-arrestins and the receptor. For β-arrestin2, which is phosphorylated by casein kinase II (CK2), threonine 383 is the primary phosphorylation site, and serine 361 represents a secondary site14,15. S412D β-arrestin1, which mimics the phosphorylated form of β-arrestin1, acts as a dominant negative mutant with respect to receptor internalization16. The N-terminal half of β-arrestin2 interacts with residues 383 to 410 of Mdm2, the E3 ligase responsible for β-arrestin2 ubiquitination17, determining the stability of the interactions of β-arrestins with the receptor.
Using mass spectrometry-based proteomics approaches, 337 proteins have been identified that interact with β-arrestins. Seventy-one proteins interact with β-arrestin1, 164 interact with β-arrestin2, and 102 interact with both β-arrestins18. These proteins were ubiquitously distributed in the cell and have numerous functions ranging from receptor desensitization, endocytosis, and signal transduction to the regulation of gene expression, protein synthesis, cellular reorganization, chemotaxis, apoptosis, and many more. In addition, knockout studies have shown that β-arrestin1 knockout mice develop normally but that β-Arrestin2 is required for normal adrenergic response19. β-Arrestin2 knockout mice develop normally as well, but they display increased analgesia in response to morphine20. Even with impaired signaling in numerous pathways, mice with ablation of either β-arrestin1 or β-arrestin2 appear healthy and function normally unless challenged. However, the double-knockout phenotype is embryonically lethal, implying that each β-arrestin can substitute for the other isoform to a certain extent21.
β-Arrestin-mediated signaling pathways involved in fibrotic diseases
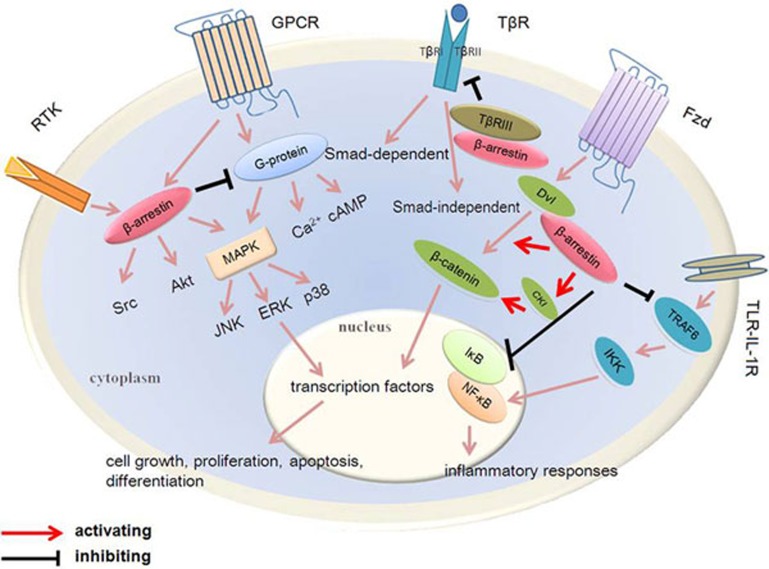
Upon their discovery, β-arrestin1 and β-arrestin2 were named for their capacity to sterically hinder the G-protein coupling of agonist-activated GPCRs, ultimately resulting in receptor desensitization and internalization. In recent years, β-arrestins have been shown to activate signaling cascades independent of G-protein activation and scaffold many intracellular signaling networks by diverse types of signaling pathways, including the Hedgehog, Wnt, Notch, and transforming growth factor-beta (TGF-β) pathways, and downstream kinases such as MAPK and phosphatidylinositol-3 kinase (PI3K, Figure 1). These signaling pathways are involved in the pathological process of fibrosis and fibrotic diseases. This β-arrestin-mediated regulation appears to play important roles in cell growth and apoptosis and affects the deposition of ECM, the activation of the inflammatory response22,23,24,25, and the development of fibrotic diseases.
Figure 1.
β-Arrestins are multifunctional signaling regulators that participate in many signaling pathways. There are two ways to regulate GPCR transduction pathways: First, G-proteins combine with agonists, stimulating second-messenger systems, and β-Arrestins bind to GPCRs phosphorylated by GRKs, thus terminating G-protein signaling and initiating a distinct set of signals. Second, they can regulate receptors without stimulating any detectable G-protein signaling. β-Arrestins are also associated with RTK, internalize TβRIII, activate Wnt and decrease TLR-IL-1R signaling pathways in different ways. They affect the growth, proliferation and inflammatory responses of cells. The β-arrestin-mediated regulation found in some fibrotic diseases influences their progress.
GPCR signaling pathway
GPCR is the single largest family of drug targets. Many extracellular stimuli, such as neurotransmitters, hormones, chemokines, inflammatory mediators, and light, are recognized by GPCRs. Advances in the study of GPCR regulation have provided novel insights into the role of β-arrestins in this process.
β-Arrestin binding initially uncouples GPCRs from the G-protein as negative regulatory molecules of the GPCR signaling pathways. Upon ligand binding, GPCRs undergo conformational changes that allow them to be recognized by the family of GRKs that phosphorylate the receptors on their intracellular loops and C-terminal tails26. β-Arrestin binding to the receptors is generally enhanced by GRK-mediated phosphorylation of multiple sites on the inner surface of the receptors. This modification leads to β-arrestin recruitment, which sterically hinders further signaling to G-proteins, thus leading to the classical phenomenon of receptor desensitization. β-Arrestins can also mediate the endocytosis of receptors, leading to numerous physiological outcomes, including receptor degradation, receptor recycling, and the generation of “signalosomes,” in which β-arrestins scaffold various proteins to potentiate distinct downstream signaling events.
β-Arrestin1 and β-arrestin2 can both desensitize GPCRs, but it has been reported that β-arrestin1 is mainly localized in the cytoplasm and nucleus while β-arrestin2 is predominantly distributed in the cytoplasm27,28. The C-terminus of β-arrestin2 facilitates its extranuclear localization and hinders its retention in the nucleus. Thus, the β-arrestin1 subtype might play a more important role in GPCR-mediated nuclear signaling29. In addition, GPCRs can be categorized into one of two classes based on ligand-induced GPCR interaction with β-arrestins. Class A GPCRs, such as β2- and α1-adrenergic, μ-opioid, endothelin ETA, and dopamine D1A receptors30, possess a higher affinity for β-arrestin2 compared to β-arrestin1 and interact with both β-arrestins in a transient manner, leading to transient ubiquitination of β-arrestins. Class B GPCRs, such as neurotensin, thyrotropin-releasing hormone, and angiotensin II type 1 receptors (AT1R)30, exhibit strong and equivalent affinity for both β-arrestin1 and β-arrestin2, resulting in sustained β-arrestin ubiquitination31. The key sequence determinants that distinguish class A and class B receptors, most notably in the C-termini of the receptors32, may result in distinct GPCR signal transduction.
Recent studies have found that β-arrestins can induce G-protein signaling but fail to stimulate G-protein phosphorylation33. G-proteins can stimulate second-messenger systems such as calcium (Ca2+) and cyclic adenosine monophosphate (cAMP). Studies have detected increased phosphorylation of the cAMP response element binding protein (CREB) and elevated β-arrestin2 expression in cystic fibrosis (CF). Eliminating β-arrestin2 expression in a CF mouse model decreased pCREB34. In addition, several ligands, including angiotensin II, antagonists of β2-AR, and chemokines that characterized ligands for a number of receptors, exhibited a reversal of efficacy, where a ligand acting as an antagonist or inverse agonist for G-protein coupling functions as a β-arrestin pathway-selective agonist, or vice versa. These β-arrestin-biased ligands are also involved in fibrotic diseases. Transgenic mice with cardiac-specific overexpression of AT1R second intracellular loop mutant (AT1-i2m), which does not couple to Gαq or Gαi, exhibited increases in p-ERK staining in the cytoplasm (but not in nuclei) of cardiac myocytes but exhibited less apoptosis and fibrosis than did wild type (WT) mice35. The β-arrestin-biased ligand TRV120027, which is improved by angiotensin II combined with AT1R, competitively antagonizes angiotensin II-stimulated G-protein signaling but stimulates β-arrestin recruitment and activates several kinase pathways, including MAPK and Src. Consistent with unbiased antagonists that decreased cardiac performance, TRV120027-activated ERK1/2 increased cardiac performance and preserved cardiac stroke volume36, and may also suppress fibrosis. Several β-AR blockers are known as β-arrestin-biased ligands. They selectively activate a G-protein-independent and GRK5/β-arrestin2-dependent pathway and induce cardiac fibrosis, promoting the expression of fibrotic genes in cardiomyocytes37.
RTK-mediated signaling pathway
RTK is an important signaling protein, as well as a type of receptor regulated by β-arrestins. The RTK/Ras guanosine triphosphatase (GTPase)/MAPK and RTK/PI3K/protein kinase B (PKB/Akt) signaling pathways are repeatedly used during metazoan development to control many different biological processes. Receptors such as the epidermal growth factor (EGF) receptor (EGFR) and the insulin-like growth factor1 (IGF-1) receptor (IGF-1R) belong to RTK. IGF-1 can stimulate collagen synthesis and myoid fibroblast proliferation, and increased IGF-1 expression in multiple mesenchymal cell subtypes and increased numbers of cells with a fibroblast/myofibroblast phenotype are involved in fibrosis associated with Crohn's disease (CD)38. The previous study provides strong evidence that β-arrestins serve as adaptors, bringing the Mdm2 ligase to the IGF-1R for ubiquitination and downregulation and increased IGF-1-induced ERK activation39. Additionally, in response to IGF-1 stimulation, β-arrestin1 mediates the activation of PI3K in a pathway that leads to the subsequent activation of Akt and anti-apoptosis. This process is independent of both G-protein and ERK activity23. EGF can promote a variety of types of cell proliferation. In β1-AR-expressing HEK293 cells, researchers have found that β1-AR transactivation of cardiac EGFR has a cardioprotective role in the face of chronic catecholamine stimulation, and both β-arrestin1 and β-arrestin2 are required for β1-AR transactivation of EGFR. Ligand stimulation of β1-AR leads to GRK5/6-mediated receptor phosphorylation and β-arrestin recruitment. β-Arrestins recruit Src to the activated receptor. This effect leads to matrix metalloproteinase (MMP) activation with the release of heparin-binding EGF, which promotes EGFR dimerization and autophosphorylation and subsequent downstream signaling40.
TGF-β signaling pathway
The transforming growth factor β (TGF-β) superfamily contains more than 30 secreted proteins and three receptors. These proteins are involved in numerous fibrotic disease development processes, including hepatic fibrosis, pulmonary fibrosis, and cardiac fibrosis. Smad-dependent mechanisms are the most widely studied downstream mediators of TGF-β signaling. Recent studies have also delineated several Smad-independent signaling pathways, including MAPK, PI3K/Akt, and Rho-like GTPases41. As β-arrestins have been reported in the regulation of all of these pathways, it may not be surprising that β-arrestins are also involved in the TGF-β signaling pathway.
The type III TGF-β receptor (TβRIII) is a transmembrane proteoglycan without a functional kinase domain and is considered to be a co-receptor to increase the affinity of ligand binding to TβRII. In interstitial pulmonary fibrosis, TβRIII is significantly downregulated during TGF-β-induced differentiation in fibroblasts. TGF-β-induced α-SMA and procollagen type I expression is markedly inhibited in fibroblasts stably expressing TβRIII. Endogenous TβRIII expression does not alter the TβRI or TβRII levels, but inhibits Smad 2/3, Akt and ERK phosphorylation42. Additionally, P144, a synthetic peptide from TβRIII, inhibits the TGF-β1-dependent signaling pathway and collagen type I synthesis in cardiac fibroblasts43. Studies found that β-arrestin2 binds TβRIII44 and mediates its clathrin-independent/lipid raft pathway-dependent internalization45. The cytoplasmic domain of TβRIII dissociates the type III receptor from the activated signaling complex between TβRII and TβRI. Then, the active signaling complex phosphorylates downstream signaling46, and β-arrestin2 may regulate this process to influence TGF-β signaling in fibrotic diseases.
Wnt signaling pathway
Briefly, Wnt signaling pathways can regulate cell proliferation, differentiation, and polarity at various stages of development47, promote collagen gel contraction, α-SMA expression, and cell migration in human dermal fibroblasts48, and stimulate cell proliferation and collagen expression in skeletal muscle fibrosis49. When Wnt interacts with target cells, it binds a heterodimeric receptor complex consisting of Frizzled (FZD) and a LRP5/6 protein. The cytoplasmic part of FZD interacts with Dishevelled (Dvl)50, and Dvl activation is followed by the inhibition of a destruction complex comprising axin, glycogen synthase kinase 3β (GSK 3β) and adenomatous polyposis coli (APC), which results in the stabilization of β-catenin and the activation of several transcription factors, which can lead to different diseases. β-Arrestin1 has been identified as a positive modulator of the Wnt/β-catenin pathway, and β-arrestin2 has been identified as a mediator for the agonist-induced internalization of FZD451,52. β-Arrestin2 can interact with axin and Dvl after Wnt stimulation of mouse embryo fibroblasts (MEFs)53, resulting in the stabilization of β-catenin. In addition, the absence of β-arrestin2 can reduce Dvl activation in mouse embryonic fibroblasts, inhibiting β-catenin signaling. Furthermore, this protein can regulate transcription factors by indirectly inhibiting C/EBP and PPARγ54, which can suppress fibrosis. Henderson et al55 found that ICG-001, a unique small molecule that selectively inhibits the activation of Wnt/β-catenin signaling induced by bleomycin, in vivo results in the downregulation of a subset of target genes. β-Arrestin1−/− and β-arrestin2−/− mice were remarkably protected from the mortality of bleomycin-induced pulmonary fibrosis24. The loss of β-arrestin2 effectively reduced the activation of β-catenin and Wnt-related targets in chronic myelogenous leukemia (CML) cells56. There is also evidence in other cellular contexts that Wnt ligands can stimulate PI3K-mediated activation of Akt and/or ERK signaling cascades, and these pathways are well known to be regulated by β-arrestins.
MAPK signaling pathway
The MAPK signaling pathway provides cells with the means to interpret external signaling cues or conditions and respond accordingly. This cascade regulates many cell functions such as differentiation, proliferation and migration. Following stimulation, receptors activate MAPK through G-protein- and β-arrestin-dependent mechanisms.
Activated ERK signals are rapid and transient because they are quickly quenched by G-protein. In contrast, β-arrestin-mediated ERK responses are slower and more persistent, generally retaining the activated kinases in the cytosol57,58 and causing a series of cellular responses including cell proliferation, differentiation, and collagen synthesis. β-Arrestin2-mediated activation of ERK stimulates the activity of CREB in CF34. siRNA targeting β-arrestin2 mRNA inhibit the activation of ERK1/2 in hepatic stellate cells (HSCs)22. β-Arrestin2 aggravates atherosclerosis through mechanisms involving SMC proliferation and migration by ERK59. Additionally, β-arrestin1 and β-arrestin2 negatively regulate LPS-induced NF-κB activation, but β-arrestin2-mediated ERK positively regulates LPS-induced IL-6 production and both β-arrestin1 and β-arrestin2 positively regulate LPS-induced IL-8 production60. This signaling pathway may explain the β-arrestin1- and β-arrestin2-promoted inflammation in some fibrotic diseases. JNK3 is another MAPK activated by β-arrestin signaling pathways. β-Arrestin2 acts as a scaffold for JNK3 and its upstream kinases, MAPK kinase 4 (MKK4), and apoptosis signaling kinase1 (ASK1). Cellular transfection of β-arrestin2 causes cytosolic retention of JNK3 and enhances JNK3 phosphorylation stimulated by ASK1. Moreover, the stimulation of the AT1R activates JNK3 and triggers the co-localization of β-arrestin2 and actives JNK3 to intracellular vesicles61, similar to the ERK cascade discussed above. Inhibiting p38 MAPK will control cystic fibrosis and lung fibrosis by reducing inflammation62,63. Studies have found that β-arrestin2 expression enhanced chemokine-induced p38 MAPK activation in HEK293 cells64; similarly, silencing β-arrestin1 expression by siRNA inhibited the early phase activation of p38 MAPK induced by β-2AR65.
NF-κB signaling pathway
The nuclear factor-κB (NF-κB) family is one of the most common transcription factor families, and its members are involved in inflammatory response-related fibrotic diseases such as ulcerative colitis (UC) and cardiac fibrosis. Overexpression of either β-arrestin1 or β-arrestin2 led to marked inhibition of NF-κB activity, as measured by reporter gene activity25. NF-κB activation depends on the CK2 phosphorylation of IκBα at a cluster of C-terminal sites. CK2 phosphorylation of β-arrestin2 blocks its interaction with IκBα and abolishes its suppression of NF-κB activation66. Tumor necrosis receptor-associated factor 6 (TRAF6) is critical for mediating Toll-like receptor (TLR)-interleukin1 receptor (IL-1R) signaling and the subsequent activation of NF-κB.
PI3K/Akt signaling pathway
Signaling via the PI3K/Akt pathway has been a focus in the study of many diseases, including the pathologic conditions of fibrosis. Activated Akt modulates the function of numerous substrates involved in the regulation of cell survival, cell cycle progression and cellular growth. In vitro inhibition of Akt activation in both human and rat HSC can induce HSC apoptosis and suppress collagen synthesis67,68. Additionally, inhibiting Akt signaling protects against pulmonary and kidney fibrosis69,70. This study demonstrated that PI3K/Akt signaling can be adjusted by β-arrestins. In MDA MB-468 and NIH3T3 cells, protease activated receptors 2 (PAR-2) can promote PI3K activity through a Gα-dependent pathway; however, PAR-2 can also inhibit PI3K activity through a β-arrestins-dependent pathway. The PI3K is recruited into a scaffolding complex containing PAR-2 and β-arrestin171. In a study of mouse embryonic fibroblasts, insulin-like growth factor-1 (IGF-1) activated the PI3K/Akt pathway through β-arrestin1, promoting cell proliferation72.
β-Arrestins in the progression of fibrotic diseases
Excessive tissue scarring (ie, fibrosis) is characterized by the excessive deposition of ECM, including collagen, glycoproteins and fibronectin. In normal situations, remodeling of the ECM ends and the formed scar tissue is partially degraded, resulting in new, functioning tissue73. Therefore, fibrosis can be defined as the net result of the balance between ECM production and degradation, and fibrosis is initially a reversible process74. However, when fibrosis continues uncontrolled, this process can be dangerous and disruptive for the affected tissue.
Although fibrotic diseases have become increasingly recognized as a problem, at present, success in treating fibrosis has been limited, and inhibiting the ECM, accelerating ECM degradation, and fighting inflammation are the major treatment approaches. In recent years, more and more studies have found that β-arrestins play a role in fibrosis and hence affect the process of fibrosis.
Liver fibrosis and cirrhosis
Studies showed that in a porcine serum-induced hepatic fibrosis model, hepatic fibrosis is aggravated by gradually increasing the expression of β-arrestin2 in the hepatic tissues, but not β-arrestin1. The activation of HSCs involves transdifferentiation from a quiescent state to myofibroblast-like cells with the appearance of α-SMA75, playing a significant role in the progression of fibrosis. An immunofluorescence double-labeling assay indicated that the expression of β-arrestin2 has a positive correlation with the expression of α-SMA. In vitro, the expression of β-arrestin2 increased remarkably in platelet derived growth factor (PDGF)-BB-stimulated HSCs. Furthermore, transfection of siRNA targeting β-arrestin2 mRNA into HSCs abolished the effect of PDGF-BB on the proliferation of HSCs and the inhibition of the activation of ERK1/2 in HSCs22. Selective targeting of β-arrestin2 into HSCs might present as a novel strategy for the treatment of hepatic fibrosis.
Studies based on PBC patients have found that in peripheral blood mononuclear cells (PBMCs), β-arrestin1 increased and the activities of NF-κB and AP-1 were downregulated. The overexpression of β-arrestin1 increased T cell proliferation, and β-arrestin1 inhibited AP-1 and NF-κB activation76 and regulated the expression of CD40L, LIGHT, IL-17, IFNγ, and TRAIL. As these factors are important in autoimmune responses, these data suggest that silencing β-arrestin1 might be a strategy for treating PBC. In liver cirrhosis, the binding of AT1R protein to β-arrestin2, was upregulated in aortas from CCl4 rats compared with those of noncirrhotic controls. This usually results in the desensitization of receptor-coupled signaling by G-protein. Stimulation with angiotensin II resulted in Rho kinase activation in aortas from noncirrhotic control rats, and this was not observed in aortas from cirrhotic CCl4 rats. In conclusion, impaired activation of the contraction-mediated Rho kinase may explain the limited contractile response of extrahepatic vessels in CCl4 rats. This is caused by dysregulation of vasoconstrictor receptors by the receptor-modifying protein, which may contribute at least partially to the vasodilation of CCl4 rats77. Chronic hepatitis B and C are major causes of liver cirrhosis. Liver biopsies from HBV and HCV patients showed that based on expression activity, the β-arrestin2 encoding gene ARRB2 exhibited higher activity in the liver78.
β-Arrestin1 and β-arrestin2 both promote liver fibrosis, cirrhosis and chronic hepatitis B and chronic hepatitis C, but their functions are different. The above evidence suggests that β-arrestins are regulators of multiple signaling pathways involved in the formation of liver fibrosis. Further study of the role of β-arrestins in liver fibrotic diseases may focus on a more clear mechanism of how β-arrestin isoforms regulate ECM protein and gene expression.
Lung fibrotic diseases
Lovgren et al24 used mice deficient in either β-arrestin1 or β-arrestin2 in the well-established bleomycin mouse model of lung fibrosis. WT mice had approximately a 50% mortality rate within 21 days after treatment. However, both the β-arrestin1−/− and β-arrestin2−/− mice were remarkably protected from mortality. Knockdown of β-arrestin2 and β-arrestin1 in idiopathic pulmonary fibroblasts resulted in an inhibition of fibroblast invasion in patients and model rats. Loss of β-arrestin1 or β-arrestin2 in primary lung fibroblasts resulted in the altered expression of genes such as Col5a1, MMP1a, and Cdh1, which are involved in matrix production, basement membrane degradation, and cell adhesion. Thus, β-arrestin induced intrinsic defects in fibroblast proliferative pathways, which can promote the progression of pulmonary fibrosis.
Asthma is a chronic inflammatory disorder of the airways, and airway fibrosis is one of the key pathological features of asthma phenotypes throughout the progression of the disease79. β-Arrestin2 promotes IL-4 production of CD4+ T lymphocytes in a murine allergic asthma model partly through mediating β-2AR internalization80. Additionally, β-arrestin2, p-ERK1/2 and IL-17 expression in CD4+T lymphocytes from a murine asthma model increased compared with those from WT mice, and treatment of CD4+T lymphocytes with siRNAs targeting β-arrestin2 significantly downregulated p-ERK1/2 and IL-17 expression81. When treated with OVA, WT mice develop symptoms of allergic asthma; in contrast, the symptoms of allergic asthma, including airway inflammation and airway hyperresponsiveness, do not appear in similarly treated β-arrestin2−/− mice82.
CF is caused by the loss of cystic fibrosis transmembrane conductance regulator (CFTR) function, resulting in the dysregulation of ion transport, abnormal inflammatory response signaling, and altered cholesterol homeostasis both in CF-cell models and in vivo studies. CF cells exhibited an increase in the protein expression of β-arrestin2 coincident with the perinuclear accumulation of free cholesterol and the depletion of β-arrestin2 expression in CF cells with shRNA reduced cholesterol accumulation. In vivo, the CF model reverted a quantifiable measure of cholesterol processing toward WT levels83. As CFTR is a cAMP-dependent negative regulator of Na+ channels, the above-described data demonstrate that elevating the cAMP pathway is sufficient to initiate cholesterol accumulation. CREB can activate cAMP, and increased β-arrestin2 expression in CF cells has a positive correlation with CREB84. Thus, increased β-arrestin2 expression is a key to the development of cholesterol-related phenotypes in CF cells.
β-Arrestin1 and β-arrestin2 contribute to lung fibrotic diseases by promoting ECM deposition; β-arrestins/cAMP mediate cholesterol accumulation, and β-arrestins/ERK-mediated inflammation. It is clear that β-arrestins can regulate certain aspects of fibroblast behavior.
Renal fibrosis
Renal fibrosis is central to the progression of diabetic nephropathy (DN). Nephrin is a novel podocyte-specific protein that localizes at the slit diaphragm. Nephrin has shown low protein and mRNA expression in DN85,86. Co-immunoprecipitation with β-arrestin2 showed a specific β-arrestin2 interaction with nephrin, leading to a decrease of cell surface nephrin with increasing amounts of β-arrestin2. Intensive study has found that the nephrin tyrosine residue is a phosphorylation-dependent switch87. Increasing glucose levels induced a significant dose-dependent increase in β-arrestin2 binding to nephrin. Overexpressing β-arrestin2 and the nephrin C-terminus were treated with phorbol 12-myristate 13-acetate in HEK293K, inducing a marked, significant increase in the β-arrestin2-nephrin interaction after 20 min, and PKCα reduced β-arrestin2 binding after 30 min of treatment. PKC is a promising therapeutic target for diabetic nephropathy, as it decreases the β-arrestin2-nephrin interaction88.
Intestinal fibrosis
NF-κB is a crucial factor in chronic inflammation that can be increased in the intestinal mucosa, accentuating inflammation of the intestinal mucosa. In the intestine, chronic inflammation results in impaired healing and fibrosis, and intestinal fibrosis is closely associated with inflammation89. Inflammatory bowel disease (IBD) is a chronic and multifactorial gastrointestinal inflammatory condition that is clinically categorized as ulcerative colitis (UC) or CD, and fibrosis is a common problem in IBD.
Some studies have demonstrated that β-arrestin1 KO mice exhibit attenuated disease pathology in experimental UC induced by trinitrobenzene sulfonic acid (TNBS) or dextran sulfate sodium (DSS). Diminished weight loss and clinical disease severity were observed in β-arrestin1 KO mice compared with DSS- or TNBS-induced UC mice, and β-arrestin1 KO mice exhibited a longer colon length compared with the WT group. Additionally, the activation of ERK and NF-κB pathways is regulated by β-arrestin1 in the colon90. These data support the theory that β-arrestin1 promotes NF-κB, which is different from other studies, and its mechanism remains unclear. In addition, Fan et al91 demonstrated that in the experimental UC rats induced by TNBS, compared with the normal control group, the protein expression of NF-κB p65 was significantly increased in the model group while the expression of β-arrestin2 expression was significantly decreased in the colonic mucosa and in lymphocytes of the spleen. As β-arrestin2 is frequently anti-inflammatory in a variety of diseases, these data suggest that β-arrestin2 may also play a role in the development of ulcerative colitis.
Intriguingly, in UC, β-arrestin1 has been demonstrated to promote the NF-κB signaling pathway, even though previous studies have shown a negative regulatory role for β-arrestin-1 in TLR-IL-1R-NF-κB signaling at the cellular level. Thus, the different functions of β-arrestin1 may be due to the interaction of β-arrestin1 with receptors such as TLRs or GPCRs. Phosphorylated β-arrestin2 exhibited significantly reduced interaction with IκBα, and the dephosphorylated β-arrestin2 displayed much stronger interactions with IκBα than β-arrestin292. The phosphorylation/dephosphorylation of β-arrestin1 may regulate the interaction with IκBα, subsequently affecting the activation of NF-κB. The function of β-arrestin1 in different cell types may also influence distinct cellular responses. The function and prognostic roles of β-arrestins in UC still need further exploration.
Fibrotic cardiovascular diseases
Cardiovascular risk factors can initiate chronic inflammatory responses and the formation of fibrosis, a key process in the initiation and progression of atherosclerosis. The proliferation and migration of smooth muscle cells (SMCs) are important to the formation of atherosclerosis. Transcriptional profiling data in severely atherosclerotic and non-atherosclerotic human coronary arteries showed that β-arrestin2 mRNA levels are two-fold higher in atherosclerotic patients than non-atherosclerotic controls93. In animal models, genetic deletion of the low-density lipoprotein (LDL) receptor (LDLR) causes a moderate increase in plasma LDL cholesterol levels when mice are fed normal chow, and severe elevated plasma LDL cholesterol levels were associated with aortic atherosclerotic lesions in mice that were fed a high-fat diet94. Kim et al59 demonstrated that deficiency of β-arrestin2 in LDLR-knockout mice reduced aortic atherosclerosis by 40% and decreased the prevalence of atheroma SMCs by 35%, suggesting that β-arrestin2 promotes atherosclerosis through effects on SMCs. To test this potential atherogenic mechanism more specifically, one study performed carotid endothelial denudation in congenic wild-type β-arrestin1−/− and β-arrestin2−/− mice. Neointimal hyperplasia was enhanced in β-arrestin1−/− mice and diminished in β-arrestin2−/− mice. After carotid injury, ERK activation and SMC proliferation were increased in β-arrestin1−/− and decreased in β-arrestin2−/− mice. β-Arrestin2 aggravates atherosclerosis through mechanisms involving SMC proliferation and migration. The difference between β-arrestin2 and β-arrestin1 is that ERK activation triggered by the GPCRs for lysophosphatidic acid receptors, thrombin, and sphingosine-1-phosphate was diminished in β-arrestin2−/− SMCs but enhanced in β-arrestin1−/− SMCs.
In cardiac studies, metoprolol, a β1-AR-selective blocker, increased the expression of fibrotic genes responsible for cardiac fibrosis in cardiomyocytes. Furthermore, metoprolol induced the interaction between β1-AR and β-arrestin2, but not between β1-AR and β-arrestin1. The interaction between β1-AR and β-arrestin2 by metoprolol was impaired in the GRK5-knockdown cells37.
These findings identify the inhibition of β-arrestin2 as a novel therapeutic strategy for combating atherosclerosis and cardiac fibrosis, but they also show that β-arrestin1 and β-arrestin2 function in the same pathways redundantly, sometimes opposing one another's function in the pathway.
Myelofibrosis
Chronic myelogenous leukemia (CML) involves the proliferation of tumors in the bone marrow, and the BCR-ABL1 gene is responsible for 95% of all diagnosed chronic myeloid leukemia cases95. In the natural progression of CML, different degrees of myelofibrosis appear. Mice transplanted with BCR-ABL displayed symptoms of CML disease onset. Fereshteh et al found that a loss of β-arrestin2 preferentially leads to a severe impairment in the establishment and propagation of the chronic and blast crisis phases of CML in mice when infected with BCR-ABL. In vitro, the absence of β-arrestin2 preferentially led to significant defects in the function of hematopoietic stem cells. The loss of β-arrestin2 led to a significant inhibition of β-catenin stabilization, and ectopic activation of Wnt signaling reversed the defects observed in the β-arrestin2 mutation cells56. Delivery of the β-arrestin2-targeting aptamer, which inhibits β-arrestin2-mediated signaling, to K562 leukemia cells significantly decreased the β-arrestin2 level, as well as both the β-catenin and Gli levels. Leukemic cells were then harvested from the spleens of these animals after disease onset, and once treated with the β-arrestin2-targeting aptamer, β-arrestin2-targeting chimera were able to inhibit the clonogenicity of these cells. Even more importantly, this inhibitory effect extended to cells from human leukemia patients. The ability to target the scaffolding protein β-arrestin2 with RNA aptamers may prove beneficial as a therapeutic strategy96.
Together, these data show that β-arrestin2 is essential for leukemic cell propagation in CML disease and indicate that β-arrestin2 and the Wnt/β-catenin pathway lie in a signaling hierarchy in the context of CML cancer stem cell maintenance.
Multiple sclerosis
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system that manifests morphologically by inflammation, demyelination, axonal loss and gliosis97. Hypertrophy of astrocytes, which constitute the main cell type in glial scars, was noted as a type of pathology of MS. Most central nervous system injury responses are associated with hypertrophy of resident astrocytes, a process termed reactive gliosis98,99.
Ohguro et al100 discovered that sera from MS patients formed an autoimmune complex with β-arrestin1, while serum auto-Abs were not detected in patients with other neurological diseases. Forooghian et al101 demonstrated that MS patients had a greater prevalence of positive T cell proliferative responses to β-arrestin1 than did healthy controls. Clinical studies have demonstrated that β2-AR is decreased in the plaque and white matter of MS patients in postmortem brain sections compared with non-neurologic disease patients. As β-arrestin1 regulates β2-AR internalization and degradation, increased β-arrestin1 expression may result in decreases of β2-AR, reducing its neuroprotective effect102. Another clinical study demonstrated that β-arrestin1 expression was increased in the brains of MS patients and varied inversely with the A1 adenosine receptor (A1AR). A1AR has an anti-inflammatory effect and protects the brain. Another in vitro study demonstrated that β-arrestin1 overexpression downregulated A1AR expression, and when treated with glucocorticoid, β-arrestin1 decreased and A1AR increased103. β-Arrestin1 may be associated with the promotion of inflammatory reactions involved in MS.
Conclusions and perspectives
There are many distinct immunological and molecular mechanisms that can contribute to the progression of fibrotic disease. As adaptor proteins and signal transduction proteins, β-arrestins can enhance or repress fibrotic signaling. However, their exact role still remains to be determined. Although β-arrestin1 and β-arrestin2 exhibit high amino acid homology and share similar functions in the regulation of GPCR signaling, they still have distinct functions in the progression of fibrosis. In most fibrotic diseases, β-arrestin1 and β-arrestin2 regulate GPCR-dependent signaling, inhibit NF-κB in inflammation, and promote α-SMA and collagen, which contribute to deposits of ECM. However, in some diseases, only β-arrestin1 or β-arrestin2 is involved, and sometimes β-arrestin1 and β-arrestin2 have opposite functions. The answer to the disparate roles of β-arrestins in fibrosis will most likely lie in various factors that influence the function of β-arrestin1 and β-arrestin2. The balance between β-arrestin-mediated G-protein signaling and independent G-protein signaling, and the way in which β-arrestin isoforms regulate the distinct spatial and temporal activation of signaling proteins that activate different target proteins, are not yet clear. However, it is certain that the biological functions of β-arrestins are much more important than initially thought. Therefore, regulation by β-arrestins is a key step in signal transduction and could be critical in determining the status of health and fibrosis. Further studies are warranted to open up avenues of research related to novel and previous functions of β-arrestins. Evaluation of the effect of β-arrestin1 and β-arrestin2 in different cells at different stages of fibrotic diseases may generate a clearer picture of how these unique proteins change in fibrosis and promote the development of novel approaches to treat a variety of fibrotic diseases.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No 81300332), the Specialized Research Fund for the Doctoral Program of Higher Education of China (No 20113420120002), and the Natural Science Foundation of the Higher Education Institutions of Anhui Province (No KJ2012A153).
References
- 1Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol 2008; 214: 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2Friedman SL, Sheppard D, Duffield JS, Violette S. Therapy for fibrotic diseases: nearing the starting line. Sci Transl Med 2013; 5: 161s–167s. [DOI] [PubMed] [Google Scholar]
- 3Reiter E, Lefkowitz RJ. GRKs and beta-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrinol Metab 2006; 17: 159–65. [DOI] [PubMed] [Google Scholar]
- 4Craft CM, Whitmore DH, Wiechmann AF. Cone arrestin identified by targeting expression of a functional family. J Biol Chem 1994; 269: 4613–9. [PubMed] [Google Scholar]
- 5Lohse MJ, Benovic JL, Codina J, Caron MG, Lefkowitz RJ. Beta-arrestin: a protein that regulates beta-adrenergic receptor function. Science 1990; 248: 1547–50. [DOI] [PubMed] [Google Scholar]
- 6Attramadal H, Arriza JL, Aoki C, Dawson TM, Codina J, Kwatra MM, et al. Beta-arrestin2, a novel member of the arrestin/beta-arrestin gene family. J Biol Chem 1992; 267: 17882–90. [PubMed] [Google Scholar]
- 7Granzin J, Wilden U, Choe HW, Labahn J, Krafft B, Buldt G. X-ray crystal structure of arrestin from bovine rod outer segments. Nature 1998; 391: 918–21. [DOI] [PubMed] [Google Scholar]
- 8Han M, Gurevich VV, Vishnivetskiy SA, Sigler PB, Schubert C. Crystal structure of beta-arrestin at 1.9 A: possible mechanism of receptor binding and membrane translocation. Structure 2001; 9: 869–80. [DOI] [PubMed] [Google Scholar]
- 9Vishnivetskiy SA, Hirsch JA, Velez MG, Gurevich YV, Gurevich VV. Transition of arrestin into the active receptor-binding state requires an extended interdomain hinge. J Biol Chem 2002; 277: 43961–7. [DOI] [PubMed] [Google Scholar]
- 10Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della RG, et al. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science 1999; 283: 655–61. [DOI] [PubMed] [Google Scholar]
- 11Seo J, Tsakem EL, Breitman M, Gurevich VV. Identification of arrestin-3-specific residues necessary for JNK3 kinase activation. J Biol Chem 2011; 286: 27894–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12Kang DS, Kern RC, Puthenveedu MA, von Zastrow M, Williams JC, Benovic JL. Structure of an arrestin2-clathrin complex reveals a novel clathrin binding domain that modulates receptor trafficking. J Biol Chem 2009; 284: 29860–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13Lin FT, Miller WE, Luttrell LM, Lefkowitz RJ. Feedback regulation of beta-arrestin1 function by extracellular signal-regulated kinases. J Biol Chem 1999; 274: 15971–4. [DOI] [PubMed] [Google Scholar]
- 14Kim YM, Barak LS, Caron MG, Benovic JL. Regulation of arrestin-3 phosphorylation by casein kinase II. J Biol Chem 2002; 277: 16837–46. [DOI] [PubMed] [Google Scholar]
- 15Lin FT, Chen W, Shenoy S, Cong M, Exum ST, Lefkowitz RJ. Phosphorylation of beta-arrestin2 regulates its function in inter-nalization of beta2-adrenergic receptors. Biochemistry-Us 2002; 41: 10692–9. [DOI] [PubMed] [Google Scholar]
- 16Lin FT, Krueger KM, Kendall HE, Daaka Y, Fredericks ZL, Pitcher JA, et al. Clathrin-mediated endocytosis of the beta-adrenergic receptor is regulated by phosphorylation/dephosphorylation of beta-arrestin1. J Biol Chem 1997; 272: 31051–7. [DOI] [PubMed] [Google Scholar]
- 17Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ. Regulation of receptor fate by ubiquitination of activated beta 2-adrenergic receptor and beta-arrestin. Science 2001; 294: 1307–13. [DOI] [PubMed] [Google Scholar]
- 18Xiao K, McClatchy DB, Shukla AK, Zhao Y, Chen M, Shenoy SK, et al. Functional specialization of beta-arrestin interactions revealed by proteomic analysis. Proc Natl Acad Sci U S A 2007; 104: 12011–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Conner DA, Mathier MA, Mortensen RM, Christe M, Vatner SF, Seidman CE, et al. beta-Arrestin1 knockout mice appear normal but demonstrate altered cardiac responses to beta-adrenergic stimulation. Circ Res 1997; 81: 1021–6. [DOI] [PubMed] [Google Scholar]
- 20Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science 1999; 286: 2495–8. [DOI] [PubMed] [Google Scholar]
- 21DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol 2007; 69: 483–510. [DOI] [PubMed] [Google Scholar]
- 22Sun WY, Song Y, Hu SS, Wang QT, Wu HX, Chen JY, et al. Depletion of beta-arrestin2 in hepatic stellate cells reduces cell proliferation via ERK pathway. J Cell Biochem 2013; 114: 1153–62. [DOI] [PubMed] [Google Scholar]
- 23Povsic TJ, Kohout TA, Lefkowitz RJ. Beta-arrestin1 mediates insulin-like growth factor 1 (IGF-1) activation of phosphatidylinositol 3-kinase (PI3K) and anti-apoptosis. J Biol Chem 2003; 278: 51334–9. [DOI] [PubMed] [Google Scholar]
- 24Lovgren AK, Kovacs JJ, Xie T, Potts EN, Li Y, Foster WM, et al. Beta-arrestin deficiency protects against pulmonary fibrosis in mice and prevents fibroblast invasion of extracellular matrix. Sci Transl Med 2011; 3: 23r–74r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25Witherow DS, Garrison TR, Miller WE, Lefkowitz RJ. Beta-arrestin inhibits NF-kappaB activity by means of its interaction with the NF-kappaB inhibitor IkappaBalpha. Proc Natl Acad Sci U S A 2004; 101: 8603–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26Premont RT, Gainetdinov RR. Physiological roles of G protein-coupled receptor kinases and arrestins. Annu Rev Physiol 2007; 69: 511–34. [DOI] [PubMed] [Google Scholar]
- 27Laporte SA, Oakley RH, Holt JA, Barak LS, Caron MG. The interaction of beta-arrestin with the AP-2 adaptor is required for the clustering of beta 2-adrenergic receptor into clathrin-coated pits. J Biol Chem 2000; 275: 23120–6. [DOI] [PubMed] [Google Scholar]
- 28Scott MG, Le Rouzic E, Perianin A, Pierotti V, Enslen H, Benichou S, et al. Differential nucleocytoplasmic shuttling of beta-arrestins. Characterization of a leucine-rich nuclear export signal in beta-arrestin2. J Biol Chem 2002; 277: 37693–701. [DOI] [PubMed] [Google Scholar]
- 29Wang P, Wu Y, Ge X, Ma L, Pei G. Subcellular localization of beta-arrestins is determined by their intact N domain and the nuclear export signal at the C terminus. J Biol Chem 2003; 278: 11648–53. [DOI] [PubMed] [Google Scholar]
- 30Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS. Differential affinities of visual arrestin, beta arrestin1, and beta arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem 2000; 275: 17201–10. [DOI] [PubMed] [Google Scholar]
- 31Leonard AP, Appleton KM, Luttrell LM, Peterson YK. A high-content, live-cell, and real-time approach to the quantitation of ligand-induced beta-arrestin2 and class A/class B GPCR mobilization. Microsc Microanal 2013; 19: 150–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32Lohse MJ, Hoffmann C. Arrestin interactions with G protein-coupled receptors. Handb Exp Pharmacol 2014; 219: 15–56. [DOI] [PubMed] [Google Scholar]
- 33Whistler JL, von Zastrow M. Morphine-activated opioid receptors elude desensitization by beta-arrestin. Proc Natl Acad Sci U S A 1998; 95: 9914–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34Manson ME, Corey DA, Rymut SM, Kelley TJ. Beta-arrestin-2 regulation of the cAMP response element binding protein. Biochemistry-Us 2011; 50: 6022–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Zhai P, Yamamoto M, Galeotti J, Liu J, Masurekar M, Thaisz J, et al. Cardiac-specific overexpression of AT1 receptor mutant lacking G alphaq/Galphai coupling causes hypertrophy and bradycardia in transgenic mice. J Clin Invest 2005; 115: 3045–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36Violin JD, DeWire SM, Yamashita D, Rominger DH, Nguyen L, Schiller K, et al. Selectively engaging beta-arrestins at the angiotensin II type 1 receptor reduces blood pressure and increases cardiac performance. J Pharmacol Exp Ther 2010; 335: 572–9. [DOI] [PubMed] [Google Scholar]
- 37Nakaya M, Chikura S, Watari K, Mizuno N, Mochinaga K, Mangmool S, et al. Induction of cardiac fibrosis by beta-blocker in G protein-independent and G protein-coupled receptor kinase 5/beta-arrestin2-dependent signaling pathways. J Biol Chem 2012; 287: 35669–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38Pucilowska JB, McNaughton KK, Mohapatra NK, Hoyt EC, Zimmermann EM, Sartor RB, et al. IGF-I and procollagen alpha1(I) are coexpressed in a subset of mesenchymal cells in active Crohn's disease. Am J Physiol Gastrointest Liver Physiol 2000; 279: G1307–22. [DOI] [PubMed] [Google Scholar]
- 39Girnita L, Shenoy SK, Sehat B, Vasilcanu R, Girnita A, Lefkowitz RJ, et al. Beta-arrestin is crucial for ubiquitination and down-regulation of the insulin-like growth factor-1 receptor by acting as adaptor for the MDM2 E3 ligase. J Biol Chem 2005; 280: 24412–9. [DOI] [PubMed] [Google Scholar]
- 40Noma T, Lemaire A, Naga PS, Barki-Harrington L, Tilley DG, Chen J, et al. Beta-arrestin-mediated beta1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J Clin Invest 2007; 117: 2445–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41Kovacs JJ, Hara MR, Davenport CL, Kim J, Lefkowitz RJ. Arrestin development: emerging roles for beta-arrestins in developmental signaling pathways. Dev Cell 2009; 17: 443–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42Ahn JY, Park S, Yun YS, Song JY. Inhibition of type III TGF-beta receptor aggravates lung fibrotic process. Biomed Pharmacother 2010; 64: 472–6. [DOI] [PubMed] [Google Scholar]
- 43Hermida N, Lopez B, Gonzalez A, Dotor J, Lasarte JJ, Sarobe P, et al. A synthetic peptide from transforming growth factor-beta1 type III receptor prevents myocardial fibrosis in spontaneously hypertensive rats. Cardiovasc Res 2009; 81: 601–9. [DOI] [PubMed] [Google Scholar]
- 44Chen W, Kirkbride KC, How T, Nelson CD, Mo J, Frederick JP, et al. Beta-arrestin 2 mediates endocytosis of type III TGF-beta receptor and down-regulation of its signaling. Science 2003; 301: 1394–7. [DOI] [PubMed] [Google Scholar]
- 45Finger EC, Lee NY, You HJ, Blobe GC. Endocytosis of the type III transforming growth factor-beta (TGF-beta) receptor through the clathrin-independent/lipid raft pathway regulates TGF-beta signaling and receptor down-regulation. J Biol Chem 2008; 283: 34808–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46Blobe GC, Schiemann WP, Pepin MC, Beauchemin M, Moustakas A, Lodish HF, et al. Functional roles for the cytoplasmic domain of the type III transforming growth factor beta receptor in regulating transforming growth factor beta signaling. J Biol Chem 2001; 276: 24627–37. [DOI] [PubMed] [Google Scholar]
- 47Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet 2004; 5: 691–701. [DOI] [PubMed] [Google Scholar]
- 48Poon R, Nik SA, Ahn J, Slade L, Alman BA. Beta-catenin and transforming growth factor beta have distinct roles regulating fibroblast cell motility and the induction of collagen lattice contraction. BMC Cell Biol 2009; 10: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49Trensz F, Haroun S, Cloutier A, Richter MV, Grenier G. A muscle resident cell population promotes fibrosis in hindlimb skeletal muscles of mdx mice through the Wnt canonical pathway. Am J Physiol Cell Physiol 2010; 299: C939–47. [DOI] [PubMed] [Google Scholar]
- 50Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell 2012; 149: 1192–205. [DOI] [PubMed] [Google Scholar]
- 51Chen W, Hu LA, Semenov MV, Yanagawa S, Kikuchi A, Lefkowitz RJ, et al. beta-Arrestin1 modulates lymphoid enhancer factor transcriptional activity through interaction with phosphorylated dishevelled proteins. Proc Natl Acad Sci U S A 2001; 98: 14889–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52Chen W, Ten BD, Brown J, Ahn S, Hu LA, Miller WE, et al. Dishevelled 2 recruits beta-arrestin 2 to mediate Wnt5A-stimulated endocytosis of Frizzled 4. Science 2003; 301: 1391–4. [DOI] [PubMed] [Google Scholar]
- 53Bryja V, Gradl D, Schambony A, Arenas E, Schulte G. Beta-arrestin is a necessary component of Wnt/beta-catenin signaling in vitro and in vivo. Proc Natl Acad Sci U S A 2007; 104: 6690–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54Okamura M, Kudo H, Wakabayashi K, Tanaka T, Nonaka A, Uchida A, et al. COUP-TFII acts downstream of Wnt/beta-catenin signal to silence PPARgamma gene expression and repress adipogenesis. Proc Natl Acad Sci U S A 2009; 106: 5819–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55Henderson WJ, Chi EY, Ye X, Nguyen C, Tien YT, Zhou B, et al. Inhibition of Wnt/beta-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc Natl Acad Sci U S A 2010; 107: 14309–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56Fereshteh M, Ito T, Kovacs JJ, Zhao C, Kwon HY, Tornini V, et al. beta-Arrestin2 mediates the initiation and progression of myeloid leukemia. Proc Natl Acad Sci U S A 2012; 109: 12532–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57Ahn S, Shenoy SK, Wei H, Lefkowitz RJ. Differential kinetic and spatial patterns of beta-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J Biol Chem 2004; 279: 35518–25. [DOI] [PubMed] [Google Scholar]
- 58Ahn S, Nelson CD, Garrison TR, Miller WE, Lefkowitz RJ. Desensitization, internalization, and signaling functions of beta-arrestins demonstrated by RNA interference. Proc Natl Acad Sci U S A 2003; 100: 1740–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59Kim J, Zhang L, Peppel K, Wu JH, Zidar DA, Brian L, et al. Beta-arrestins regulate atherosclerosis and neointimal hyperplasia by controlling smooth muscle cell proliferation and migration. Circ Res 2008; 103: 70–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60Fan H, Luttrell LM, Tempel GE, Senn JJ, Halushka PV, Cook JA. Beta-arrestins 1 and 2 differentially regulate LPS-induced signaling and pro-inflammatory gene expression. Mol Immunol 2007; 44: 3092–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61McDonald PH, Chow CW, Miller WE, Laporte SA, Field ME, Lin FT, et al. Beta-arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science 2000; 290: 1574–7. [DOI] [PubMed] [Google Scholar]
- 62Underwood DC, Osborn RR, Bochnowicz S, Webb EF, Rieman DJ, Lee JC, et al. SB239063, a p38 MAPK inhibitor, reduces neutrophilia, inflammatory cytokines, MMP-9, and fibrosis in lung. Am J Physiol Lung Cell Mol Physiol 2000; 279: L895–902. [DOI] [PubMed] [Google Scholar]
- 63Raia V, Maiuri L, Ciacci C, Ricciardelli I, Vacca L, Auricchio S, et al. Inhibition of p38 mitogen activated protein kinase controls airway inflammation in cystic fibrosis. Thorax 2005; 60: 773–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64Sun Y, Cheng Z, Ma L, Pei G. Beta-arrestin2 is critically involved in CXCR4-mediated chemotaxis, and this is mediated by its enhancement of p38 MAPK activation. J Biol Chem 2002; 277: 49212–9. [DOI] [PubMed] [Google Scholar]
- 65Gong K, Li Z, Xu M, Du J, Lv Z, Zhang Y. A novel protein kinase A-independent, beta-arrestin-1-dependent signaling pathway for p38 mitogen-activated protein kinase activation by beta2-adrenergic receptors. J Biol Chem 2008; 283: 29028–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66Luan B, Zhang Z, Wu Y, Kang J, Pei G. Beta-arrestin2 functions as a phosphorylation-regulated suppressor of UV-induced NF-kappaB activation. Embo J 2005; 24: 4237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67Paik YH, Kim JK, Lee JI, Kang SH, Kim DY, An SH, et al. Celecoxib induces hepatic stellate cell apoptosis through inhibition of Akt activation and suppresses hepatic fibrosis in rats. Gut 2009; 58: 1517–27. [DOI] [PubMed] [Google Scholar]
- 68Son G, Hines IN, Lindquist J, Schrum LW, Rippe RA. Inhibition of phosphatidylinositol 3-kinase signaling in hepatic stellate cells blocks the progression of hepatic fibrosis. Hepatology 2009; 50: 1512–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69Kulkarni AA, Thatcher TH, Olsen KC, Maggirwar SB, Phipps RP, Sime PJ. PPAR-gamma ligands repress TGFbeta-induced myofibroblast differentiation by targeting the PI3K/Akt pathway: implications for therapy of fibrosis. PLoS One 2011; 6: e15909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70Qin J, Xie YY, Huang L, Yuan QJ, Mei WJ, Yuan XN, et al. Fluorofenidone inhibits nicotinamide adeninedinucleotide phosphate oxidase via PI3K/Akt pathway in the pathogenesis of renal interstitial fibrosis. Nephrology (Carlton) 2013; 18: 690–9. [DOI] [PubMed] [Google Scholar]
- 71Wang P, DeFea KA. Protease-activated receptor-2 simultaneously directs beta-arrestin-1-dependent inhibition and Galphaq-dependent activation of phosphatidylinositol 3-kinase. Biochemistry-Us 2006; 45: 9374–85. [DOI] [PubMed] [Google Scholar]
- 72Povsic TJ, Kohout TA, Lefkowitz RJ. Beta-arrestin1 mediates insulin-like growth factor 1 (IGF-1) activation of phosphatidylinositol 3-kinase (PI3K) and anti-apoptosis. J Biol Chem 2003; 278: 51334–9. [DOI] [PubMed] [Google Scholar]
- 73Mutsaers SE, Bishop JE, McGrouther G, Laurent GJ. Mechanisms of tissue repair: from wound healing to fibrosis. Int J Biochem Cell Biol 1997; 29: 5–17. [DOI] [PubMed] [Google Scholar]
- 74Ahmad W, Ijaz B, Gull S, Asad S, Khaliq S, Jahan S, et al. A brief review on molecular, genetic and imaging techniques for HCV fibrosis evaluation. Virol J 2011; 8: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75Macias-Barragan J, Sandoval-Rodriguez A, Navarro-Partida J, Armendariz-Borunda J. The multifaceted role of pirfenidone and its novel targets. Fibrogenesis Tissue Repair 2010; 3: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76Hu Z, Huang Y, Liu Y, Sun Y, Zhou Y, Gu M, et al. beta-Arrestin 1 modulates functions of autoimmune T cells from primary biliary cirrhosis patients. J Clin Immunol 2011; 31: 346–55. [DOI] [PubMed] [Google Scholar]
- 77Hennenberg M, Trebicka J, Kohistani AZ, Heller J, Sauerbruch T. Vascular hyporesponsiveness to angiotensin II in rats with CCl4-induced liver cirrhosis. Eur J Clin Invest 2009; 39: 906–13. [DOI] [PubMed] [Google Scholar]
- 78Ciesla A, Kusmider M, Faron-Gorecka A, Dziedzicka-Wasylewska M, Bociaga-Jasik M, Owczarek D, et al. Intrahepatic expression of genes related to metabotropic receptors in chronic hepatitis. World J Gastroenterol 2012; 18: 4156–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79Royce SG, Cheng V, Samuel CS, Tang ML. The regulation of fibrosis in airway remodeling in asthma. Mol Cell Endocrinol 2012; 351: 167–75. [DOI] [PubMed] [Google Scholar]
- 80Wang G, Liu Y, Yang M, Liu S, Ma L, Gong S, et al. Effects of beta-arrestin 2 on cytokine production of CD4+ T lymphocytes of mice with allergic asthma. Indian J Exp Biol 2011; 49: 585–93. [PubMed] [Google Scholar]
- 81Liu Y, Wang GY, Liu SK, Yang MY, Ma LB, Li K, et al. beta-arrestin2 stimulates interleukin-17 production and expression of CD4+ T lymphocytes in a murine asthma model. Iran J Allergy Asthma Immunol 2011; 10: 171–82. [PubMed] [Google Scholar]
- 82Walker JK, Fong AM, Lawson BL, Savov JD, Patel DD, Schwartz DA, et al. Beta-arrestin-2 regulates the development of allergic asthma. J Clin Invest 2003; 112: 566–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83Manson ME, Corey DA, Bederman I, Burgess JD, Kelley TJ. Regulatory role of beta-arrestin-2 in cholesterol processing in cystic fibrosis epithelial cells. J Lipid Res 2012; 53: 1268–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84Manson ME, Corey DA, White NM, Kelley TJ. cAMP-mediated regulation of cholesterol accumulation in cystic fibrosis and Niemann-Pick type C cells. Am J Physiol Lung Cell Mol Physiol 2008; 295: L809–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85Wu Y, Dong J, Yuan L, Liang C, Ren K, Zhang W, et al. Nephrin and podocin loss is prevented by mycophenolate mofetil in early experimental diabetic nephropathy. Cytokine 2008; 44: 85–91. [DOI] [PubMed] [Google Scholar]
- 86Toyoda M, Suzuki D, Umezono T, Uehara G, Maruyama M, Honma M, et al. Expression of human nephrin mRNA in diabetic nephropathy. Nephrol Dial Transplant 2004; 19: 380–5. [DOI] [PubMed] [Google Scholar]
- 87Quack I, Rump LC, Gerke P, Walther I, Vinke T, Vonend O, et al. beta-Arrestin2 mediates nephrin endocytosis and impairs slit diaphragm integrity. Proc Natl Acad Sci U S A 2006; 103: 14110–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88Quack I, Woznowski M, Potthoff SA, Palmer R, Konigshausen E, Sivritas S, et al. PKC alpha mediates beta-arrestin2-dependent nephrin endocytosis in hyperglycemia. J Biol Chem 2011; 286: 12959–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89Ziogas DC, Gras-Miralles B, Mustafa S, Geiger BM, Najarian RM, Nagel JM, et al. Anti-melanin-concentrating hormone treatment attenuates chronic experimental colitis and fibrosis. Am J Physiol Gastrointest Liver Physiol 2013; 304: G876–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90Lee T, Lee E, Irwin R, Lucas PC, McCabe LR, Parameswaran N. beta-Arrestin-1 deficiency protects mice from experimental colitis. Am J Pathol 2013; 182: 1114–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91Fan H, Liao Y, Tang Q, Chen XY, Zhang LJ, Liu XX, et al. Role of beta2-adrenoceptor-beta-arrestin2-nuclear factor-kappaB signal transduction pathway and intervention effects of oxymatrine in ulcerative colitis. Chin J Integr Med 2012; 18: 514–21. [DOI] [PubMed] [Google Scholar]
- 92Luan B, Zhang Z, Wu Y, Kang J, Pei G. Beta-arrestin2 functions as a phosphorylation-regulated suppressor of UV-induced NF-kappaB activation. Embo J 2005; 24: 4237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93Archacki SR, Angheloiu G, Tian XL, Tan FL, DiPaola N, Shen GQ, et al. Identification of new genes differentially expressed in coronary artery disease by expression profiling. Physiol Genomics 2003; 15: 65–74. [DOI] [PubMed] [Google Scholar]
- 94Ishibashi S, Goldstein JL, Brown MS, Herz J, Burns DK. Massive xanthomatosis and atherosclerosis in cholesterol-fed low density lipoprotein receptor-negative mice. J Clin Invest 1994; 93: 1885–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95Tiu R, Kalaycio M. Targeted therapy for patients with chronic myeloid leukemia: clinical trial experience and challenges in inter-trial comparisons. Leuk Lymphoma 2012; 53: 1263–72. [DOI] [PubMed] [Google Scholar]
- 96Kotula JW, Sun J, Li M, Pratico ED, Fereshteh MP, Ahrens DP, et al. Targeted disruption of beta-arrestin 2-mediated signaling pathways by aptamer chimeras leads to inhibition of leukemic cell growth. PLoS One 2014; 9: e93441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97Bruck W. The pathology of multiple sclerosis is the result of focal inflammatory demyelination with axonal damage. J Neurol 2005; 252 Suppl 5: v3–9. [DOI] [PubMed] [Google Scholar]
- 98Moore CS, Abdullah SL, Brown A, Arulpragasam A, Crocker SJ. How factors secreted from astrocytes impact myelin repair. J Neurosci Res 2011; 89: 13–21. [DOI] [PubMed] [Google Scholar]
- 99Aldrich A, Kielian T. Central nervous system fibrosis is associated with fibrocyte-like infiltrates. Am J Pathol 2011; 179: 2952–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100Ohguro H, Chiba S, Igarashi Y, Matsumoto H, Akino T, Palczewski K. Beta-arrestin and arrestin are recognized by autoantibodies in sera from multiple sclerosis patients. Proc Natl Acad Sci U S A 1993; 90: 3241–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101Forooghian F, Cheung RK, Smith WC, O'Connor P, Dosch HM. Enolase and arrestin are novel nonmyelin autoantigens in multiple sclerosis. J Clin Immunol 2007; 27: 388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102Shi Y, Feng Y, Kang J, Liu C, Li Z, Li D, et al. Critical regulation of CD4+ T cell survival and autoimmunity by beta-arrestin 1. Nat Immunol 2007; 8: 817–24. [DOI] [PubMed] [Google Scholar]
- 103Tsutsui S, Vergote D, Shariat N, Warren K, Ferguson SS, Power C. Glucocorticoids regulate innate immunity in a model of multiple sclerosis: reciprocal interactions between the A1 adenosine receptor and beta-arrestin-1 in monocytoid cells. FASEB J 2008; 22: 786–96. [DOI] [PubMed] [Google Scholar]