Abstract
The potential for exposures to ionizing radiation has increased in recent years. Although advances have been made, understanding the global metabolic response as a function of both dose and exposure time is challenging considering the complexity of the responses. Herein we report our findings on the dose- and time-dependency of the urinary response to ionizing radiation in the male rat using radiation metabolomics. Urine samples were collected from adult male rats, exposed to 0.5 to 10 Gy γ–radiation, both before from 6 to 72 h following exposures. Samples were analyzed by liquid chromatography coupled with time-of-flight mass spectrometry, and deconvoluted mass chromatographic data were initially analyzed by principal component analysis. However, the breadth and complexity of the data necessitated the development of a novel approach to summarizing biofluid constituents after exposure, called Visual Analysis of Metabolomics Package (VAMP). VAMP revealed clear urine metabolite profile differences to as little as 0.5 Gy after 6 h exposure. Via VAMP, it was discovered that the response to radiation exposure found in rat urine is characterized by an overall net down-regulation of ion excretion with only a modest number of ions excreted in excess over pre-exposure levels. Our results show both similarities and differences with the published mouse urine response and a dose- and time-dependent net decrease in urine ion excretion associated with radiation exposure. These findings mark an important step in the development of minimally invasive radiation biodosimetry. VAMP should have general applicability in metabolomics to visualize overall differences and trends in many sample sets.
Keywords: Radiation, biodosimetry, bioinformatics
Introduction
Nuclear reactor complications in Fukushima resulting from the massive earthquake that occurred off the Pacific Coast of Japan in March of 2011 have served to remind us of how crucial it is that we expand and improve our ability to deal with large numbers of humans exposed to ionizing radiation (IR) (Christodouleas et al. 2011). The explosions that occurred at the Chernobyl reactor in Ukraine in 1986 released radioactive materials into the environment, resulted in 28 radiation-related deaths, and will likely be associated with long-term health effects that will only be known in time with diligent follow-up(Christodouleas et al. 2011). Further, our concern over the potential for hostile interests to deploy radiological weapons or improvised nuclear devices targeting civilian populations has increased dramatically in the past decade and has prompted the U.S. government to increase support for countermeasures research(Hafer et al. 2010). Among the most important need is rapid, high-throughput biodosimetry for accurately assessing radiation doses of large numbers of potentially exposed individuals to inform medical care (Grace et al. 2010; Hafer et al. 2010). The results presented here are from a study designed to extend the findings of earlier reports on radiation metabolomics using animal models (Tyburski et al. 2008; Lanz et al. 2009; Tyburski et al. 2009; Johnson et al. 2011; Khan et al. 2011; Tang et al. 2013; Zhang et al. 2014) and to further address the shortcomings of current radiation biodosimetry capabilities. We also provide a useful approach to visualize overall responses or changes in complex metabolomics datasets.
Through the development of a novel visualization procedure, called Visual Analysis of Metabolomics Package (VAMP), we attempted to characterize the global urine metabolome response to radiation. Rather than analyzing specific biomarkers, VAMP allows for a holistic qualitative evaluation of the entire metabolome detected in a biofluid, which may potentially reveal global trends that traditional approaches may overlook. This novel methodology has revealed an overall increasing down-excretion of urine metabolites with increasing radiation dose.
A variety of reports indicate that radiation metabolomics cam serve as a viable biomarker discovery platform for developing field-deployable biodosimetry through reports on (a) minimally invasive biomarkers, including several purine and pyrimidine metabolites, in the urine of mice and (b) the power of differential mobility spectrometry coupled with mass spectrometry (DMS-MS) for detecting specific target molecules in urine without the encumbrance of chromatographic separation as reviewed previously(Coy et al. 2010; Reisz et al. 2014). These accomplishments serve as proof of principle for measuring and defining urinary responses to radiation exposure in particular and exposure assessment in general (Roux et al. 2011). With a panel of five or more urine ions that are known to comprise a radiation exposure response in both a dose- and time-depend fashion, emergency response agencies may one day use portable DMS-MS or other deployable technology in the field for rapid radiation exposure assessment that informs medical triage decisions much sooner than current technology allows. A proposed strategy based on these experimental results for using urine biomarkers of radiation exposure with DMS-MS in field biodosimetry is reviewed by Coy and colleagues (Coy et al. 2011).
In this study, we expand upon earlier published observations of that have reported on the urinary and serum response of the mouse (Amendola et al. 2006; Lee et al. 2007; Tyburski et al. 2008; 2009; Ossetrova et al. 2010; Partridge et al. 2010; Chen et al. 2011) and rat(Randic & Supek 1961; Porciani et al. 2001; Lanz et al. 2009; Johnson et al. 2011; Tang et al. 2013; Zhang et al. 2014) to γ radiation exposures. Using Ultra-Performance® liquid chromatography (UPLC) in conjunction with time-of-flight mass spectrometry (TOFMS), we analyzed urine collected from rats prior to and at four time points after exposure to six different doses of gamma radiation ranging from 0.5 to 10 Gy at high dose rate. These represent a spectrum of biologic effects from minimal impact on the organism to doses producing major morbidity and some lethality, e.g. the LD50 for Sprague Dawley rats is approximately 8.75 to 9 Gy. The goals of this study were to explore and define the time- and dose-dependency of both specific urinary radiation biomarkers and the global urine metabolome response to IR exposure, as well as to demonstrate an approach to visualize overall changes in the urinary metabolome.
Materials & Methods
Compounds & Materials
All compounds used in this study for mass spectrometry were obtained from Sigma-Aldrich Co., St. Louis, Missouri. Reagents for liquid chromatography were of the highest grade available from Thermo Fisher Scientific, Pittsburgh, Pennsylvania. Plastic ware and other disposables were also obtained from Thermo Fisher Scientific.
Animals
Experiments in this study were conducted at the. Male Sprague Dawley (Hsd:SD) rats approximately 30 days old were purchased from Harlan Laboratories (Indianapolis, Indiana). Rats were allowed to acclimate in the vivarium for a minimum of two weeks prior to the start of experiments and were screened for common rodent pathogens during this period. Housing conditions were alternating 12-hour light and dark cycles with access to Teklad Global Rodent Diet 8604 (Harlan) and acidified water ad libitum. The rats were pair-housed in plastic microisolator cages on Teklad Sani-Chips bedding (Harlan) and individually in Nalgene metabolic cages (Thermo Fisher) during urine collection. The rats were randomly assigned to experimental groups and monitored for body weight and outward signs of distress. Any rats that appeared to develop adverse health effects were removed from the study. All procedures involving animals were (a) conducted with maximum possible well-being of the rats, (b) approved by the Institutional Animal Care and Use Committee prior to the start of the study, and (c) performed in compliance with the guidelines set forth in the Guide for the Care and Use of Laboratory Animals in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC).
Radiation Exposure
This study used a pre-post experimental design. After pre-exposure urine samples were collected, body weights were recorded and groups of rats were bilaterally exposed to 0.5, 1.0, 2.5, 5.0, 7.5, or 10 Gy gamma radiation (n = 20 per dose) at a rate of 0.6 Gy/min with a 60Co gamma source. For exposures, rats were placed into ventilated Plexiglass exposure boxes with total time in the boxes less than 30 min. Dosimetry rates were ascertained using an alanine/electron paramagnetic resonance system and acrylic rat phantoms with calibration factors traceable to the National Institute of Standards and Technology (NIST, Gaithersburg, Maryland). Each experimental group of 20 rats received a single, whole-body dose; dose-experiments were conducted in different calendar months. At the conclusion of exposure, rats were then immediately housed individually in metabolic cages for 6-h and 24-h urine collections.
Urine Collection
One pre-exposure 24-h urine sample was collected from each rat using individual housing in metabolic cages. Then groups of up to 20 rats were exposed to the one of the six doses listed above within one to two weeks later. After exposure, 10 of the 20 rats were housed individually for a 6-h post exposure urine, and the other 10 rats were housed for the first 24-h post exposure urine. The first 10 were then placed at 24 h post exposure for the 48 h post urine sample. Then the second set of 10 rats for the final urine collection, the 72 h post exposure sample, were placed in metabolic cages at 48 h post. The experimental design and urine sample collection schedule is illustrated in Figure 1. The volume of each urine sample was determined and the sample centrifuged (250 × g, 10 min, 4°C) to remove any debris before being aliquotted and stored at -80°C until use.
Figure 1.
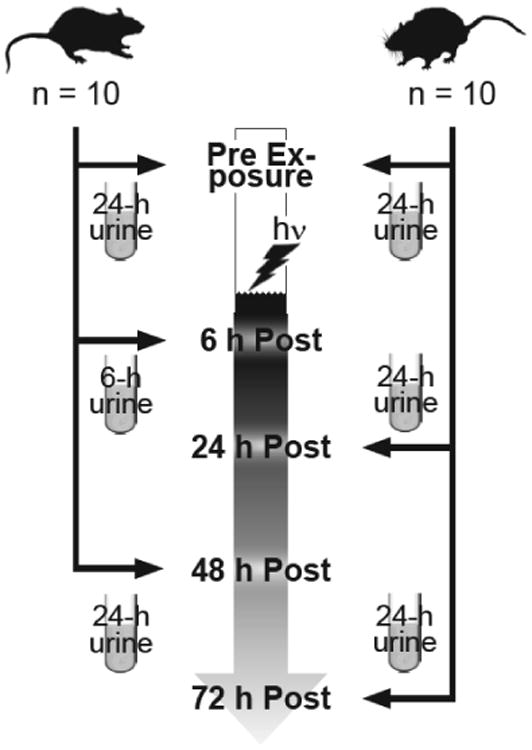
Experimental protocol for assessing the rat urine response to IR up to 72h post-exposure. Six experiments were conducted with a total of 120 rats; each experiment was conducted independently in separate calendar months to test each dose, one dose and 20 rats per experiment. Doses tested were 0.5, 1.0, 2.5, 5.0, 7.5, and 10 Gy with n = 20 age-matched male rats per dose. Twenty-four hour urine samples were collected in metabolic cages from each group of 20 rats one or more days preceding exposure and then from up to 10 rats per time point post-exposure. The 6h post-exposure time point consisted of urine collection over 6 h in metabolic cages beginning immediately post-exposure. All subsequent collections at 24 h, 48 h, and 72 h post-exposure were full 24-h collections with rats housed individually in metabolic cages.
UPLC-TOFMS Analyses
Urine samples were analyzed by UPLC-TOFMS with an injection each in ESI+ and ESI-modes according to previously described methods (Plumb et al. 2004; Tyburski et al. 2008; 2009). Briefly, urine aliquots were diluted 1:5 in 50% acetonitrile containing 4-nitrobenzoic acid at a final concentration of 40 μM and debrisoquine hemisulfate at a final concentration of 1 μM, vortex-mixed briefly at top speed, and centrifuged for 20 min at 13,000 × g at 4 °C. Five microliter-injections of the supernatants in auto-sampler vials were resolved for 10 min on a reversed-phase 2.1 × 50 mm ACQUITY UPLC® BEH C18 1.7-μm column (Waters Corp., Milford, Massachusetts) using an ACQUITY UPLC® system (Waters) operating with the parameters described elsewhere(Tyburski et al. 2009), interfaced using MassLynx® MS software (Waters) (Plumb et al. 2004).
The UPLC column eluent was introduced directly to TOFMS using a Q-TOF Premier® (Waters) operating in either positive or negative ESI mode with acquisition made in V mode under extended dynamic range. Details of the TOFMS operating parameters used in these analyses were previously reported by Tyburski and colleagues(Tyburski et al. 2009). Accurate mass was maintained via LockSpray® interface (Waters) using sulfadimethoxine (310.0736 Da) in 50% aqueous acetonitrile. Data were acquired in MS scanning centroid mode from 50 to 850 Da.
Data Processing and Multivariate Data Analyses
Chromatogram deconvolution, post-processing, and multivariate data analyses utilized common and standardized approaches. Briefly, MarkerLynx® software (Waters) was used to generate a multivariate data matrix from centroided and integrated mass chromatograms (Plumb et al. 2004; Castro-Perez et al. 2005; Tyburski et al. 2008; 2009). Relative abundance data for both ESI+ and ESI- modes were normalized by urine creatinine relative abundances acquired in ESI+ mode as described previously to compensate for potential confounding by variability in glomerular filtration (Dunn et al. 2004; Takahashi et al. 2007; Tyburski et al. 2009). These creatinine-normalized data were then Pareto-scaled and analyzed by principal component (PC) analysis (PCA) using SIMCA-P+ software (Umetrics, Inc., San Jose, California) (Pearson 1901; Noda 2008).
Visual Analysis of Metabolomics Package
The complexity and breadth of this data set necessitated the development of novel tools that permitted more effective and thorough analysis, leading to the development of the Visual Analysis of Metabolomics Package (VAMP). VAMP is a visualization tool that allows for metabolomics data to be qualitatively evaluated in a more holistic manner, and focuses on the entirety of the metabolome rather than specific metabolites. It consists of core components for making the basic three dimensional visualization graphs, as well as a series of simple statistically derived methods used for reducing spurious information from showing up on the plots. The 3D graphs produced represent global metabolome response in the form of ions detected by ESI-UPLC-TOFMS for a given post-exposure time point when compared to the pre-exposure time point for a given comparison. Each graph plots ions by their m/z (X-axis), retention time (Y-axis), and abundance percentage change (Z-axis) with respect to abundance levels measured during the pre-exposure time point urine samples. In addition, each point on the plot representing an ion is also colorized according to the magnitude of its abundance percentage change, with greater up-regulation represented by the red end of the spectrum, and greater down regulation by the blue end. The result is a holistic graphical representation of metabolome response that can be very easily interpreted by visual inspection. All programs, calculations, and graphs were produced using the MathWorks MATLAB numerical computing environment (MATLAB and Statistics Toolbox Release 2011a, The MathWorks, Inc., Natick, Massachusetts, United States). The code is available at https://sites.google.com/a/georgetown.edu/fornace-lab-informatics/home/vamp.
For a given ion (ionX), its change in concentration between pre- and post-exposure (referred to herein as Control and Experimental, respectively) metabolomic datasets is quantified by subtracting the mean Control abundance (μctrl, ionX) calculated by averaging across all samples within a time point, from the mean Experimental abundance (μexp, ionX). This value is then normalized by the mean Control abundance, yielding the value SionX:
This new value SionX quantifies any change in ion abundance in terms of a percentage change with respect to the mean Control. If the mean Experimental value exceeds the mean Control, the range of SionX lacks an upper bound. However, if the mean Control exceeds the mean Experimental, then the SionX value will be negative, with a range from 0 (non-inclusive) to -1 (i.e. the mean Experimental value is 0). Thus, the SionX value will not scale in the same manner as in the former case, making any meaningful qualitative and quantitative comparison between the two cases difficult. A separate equation is utilized for ions in the latter situation:
This complementary equation instead utilizes the (smaller) mean Experimental value as the normalizing factor, and the subsequent values of S′ionX will be in terms of percentage change with respect to the mean Experimental abundance, allowing for equivalent scaling to SionX . Though logarithms can also be used to achieve a similar result, doing so will eliminate the intuitive nature of interpreting simple percentages represented by the SionX and S′ionX values.
Utilizing these normalization methods, two separate 3D plots are constructed for up and down regulated ions. Ions are plotted according to their m/z (X axis), retention time (Y axis), and SionX or S′ionX values (Z axis). These two plots are combined into a single composite percentage change analysis (CPCA) graph by artificially treating the S′ionX values as negative (via multiplication by a -1 factor), creating a holistic visual representation of all ion concentration changes for a particular post-exposure time point when compared to the pre-exposure data.
Simple quantitative techniques were utilized for removing spurious information such as confounding factors and noise in the dataset. These techniques were applied to both the Control as well as the Experimental data sets. The standard deviation (σ) of an ion's abundance values was utilized for filtering ion features from the graph. Any ion in which a predefined fraction of its constituent sample ion abundances within a time point deviated from the mean by a predetermined threshold (i.e. 2σ) was excluded. A threshold was calculated based on the standard deviation of the ion's abundance value for a given group (i.e. pre- or post-exposure). For instance, if 80% of the data for an ion exceeded the threshold, then the ion was excluded from analysis. Furthermore, constituent sample ion abundances that exceeded the aforementioned threshold in the remaining ions were excluded, and the mean abundance recalculated to achieve a more representative mean abundance value. Finally, ions were excluded when a predefined fraction of its constituent sample ion abundances was zero (i.e. missing), described at length by Mak et al.(Mak et al. 2014) For instance, if an ion in the pre-exposure dataset only contained 2 non-zero data points out of the total sample size of 20, then its fraction of missing data would be 80%. If the maximum missing data threshold was for instance, 60%, then this ion would be excluded.
Selection of Candidate Markers
In addition to the mouse biomarkers that have previously been reported, we endeavored to identify novel, candidate biomarkers specific to the rat for urine radiation biodosimetry. The approach used in this study was very similar to previously published approaches(Tyburski et al. 2009). In short, in SIMCA-P+, correlation [p(corr), p scaled as correlation coefficient between ion abundances and component 1 scores] was calculated and plotted against importance (p, the loadings or coefficients with which the ion abundances are combined to form the component 1 scores) for the PC model built on the urine samples from rats prior to and after exposure to 2.5 Gy gamma radiation. Ions with p(corr) closest to 1.0 or -1.0 were individually inspected using traditional tests for significant differences relating to exposure status (described in more detail below).
Biomarker Identification and Quantification
In order to control for potential confounding by differential glomerular filtration, we normalized biomarker quantities by respective creatinine quantities as described (Dunn et al. 2004; Takahashi et al. 2007; Tyburski et al. 2009). To summarize, urine samples were diluted 1:1000 in 10 mM ammonium formate buffer (pH 3.5) and analyzed by high-performance LC (PerkinElmer Life and Analytical Sciences, Boston, Massachusetts) coupled to a API2000 SCIEX triple-quadrupole tandem mass spectrometer (Applied Biosystems/MDS Sciex, Foster City, California). Multiple reaction transitions were monitored in ESI+ mode for the target, creatinine (114.0→86.1 m/z), and the IS, debrisoquine hemisulfate (176.1 →134.2 m/z). Final creatinine concentrations (mM) were adjusted for the initial dilution made prior to LC-MS/MS analysis.
The identities and quantities of urinary radiation biomarkers were confirmed and measured, respectively, by UPLC-TOFMS/MS (Tyburski et al. 2008;2009). Briefly, exact masses of target biomarkers (creatine, thymidine, xanthine, xanthosine, 2′-deoxyuridine, 2′-deoxyxanthosine, taurine, and n-hexanoylglycine) were verified to be measured within ±10 parts per million (ppm) of calculated. A second criterion was a match within 0.1 min UPLC column retention time (Tret) for each biomarker. Exact masses and Tret were confirmed using pure commercial standards in solution when available (all but 2′deoxyxanthosine which was not commercially available at the time of this study), analyzed by UPLC-TOFMS. Tandem MS spectral matching was used as a final criterion for identifying targeted biomarkers. These confirmations were conducted by UPLC-TOFMSMS with collision energy ramped from 5 to 35V.
Statistical Analyses
Relative abundances of candidate radiation biomarkers, validated radiation biomarker quantities, urine volume information, and animal weight data were first tested for normal distribution using the Skewness and Kurtosis test. Candidate biomarkers whose relative abundances and validated biomarkers whose quantities were found to be normally distributed were then compared using calculated means according to exposure status with paired t tests where the comparisons were made pre-post in the same animals and unpaired t tests for different animals. Ions for which data were found to not follow a normal distribution were processed using nonparametric equivalents to paired and unpaired t-tests, namely the Mann-Whitney U test for comparing two groups and the Wilcoxon signed-rank test for more than two groups. Statistical analyses were performed with STATA software (Stata Corp LP, College Station, Texas). Graphical presentations were created in Prism software.
Results
To expand our understanding of the mammalian response to IR exposure and to further efforts toward minimally invasive radiation biodosimetry in humans, we analyzed urine samples collected from a total of 120 adult male rats before and at several time points after single exposures to doses of gamma radiation ranging from 0.5 to 10 Gy using LC-MS-based metabolomics. Relative ion abundances from deconvoluted mass chromatograms were normalized by corresponding urine creatinine concentrations to minimize potential confounding by individual variations in glomerular filtration. Principal component analyses were used to summarize the MS results and for candidate biomarker discovery. We also utilized VAMP for holistically exploring global perturbations in the urine metabolome. Our results expand current knowledge about the urinary response to IR by characterizing dose- and time-dependency of published and novel candidate radiation biomarkers.
The study population consisted of 120 adult male rats, split into groups of 20. Each group of 20 rats were exposed to a single dose of gamma radiation from a 60Co source at a rate of 0.6 Gy per minute, with total doses of 0.5, 1.0, 2.5, 5.0, 7.5, and 10 Gy. Each urine collection series and exposure occurred sequentially over four calendar months, and the rats always began at the same age for each exposure. During the course of each experiment, the rats exhibited increases in body weight commensurate with the feeding ad libitum of standard chow. Body weights, urine volumes, and urine creatinine concentrations for all dose-groups by time point are summarized in Supplemental Table S1. Most of the doses are sublethal except for 10 Gy where the expectation would be approximately 50-60% loss by 30 d. There were no deaths in the 10 Gy group over the 3 d post-irradiation time period; major morbidity and mortality typically occurs approximately 2 wk after irradiation at this dose
Principal Component Analysis
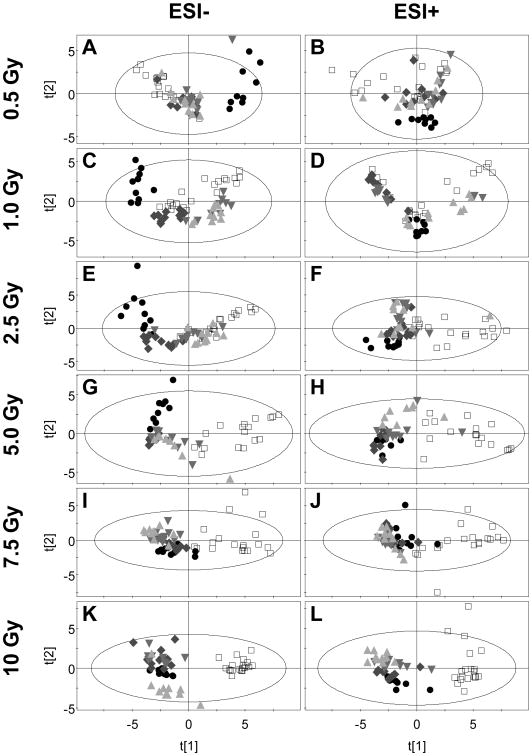
Creatinine-normalized relative abundances for urine ions measured in ESI- and ESI+ modes were pareto-scaled and modeled using PCA. Figure 2 shows the component 2 versus component 1 PCA scores for the two ionization modes by dose. The ellipse drawn in each panel indicates the boundary of Hotelling's T2 95% confidence interval. However, we chose to refrain from excluding outliers (scores that fell outside of the T2 ellipses). Open squares represent the pre-exposure urine samples, and shaded symbols represent the post-exposure samples at all time points.
Figure 2.
Principal components analysis shows dose- and time-dependent changes in urine metabolome after exposure to total body irradiation. Urine samples collected from rats before and after exposure to doses of total body gamma radiation ranging from 0.5 to 10 Gy were analyzed by UPLC-TOFMS. Shown are the principal component 2 (t[2]) versus principal component 1 (t[1]) scores for data acquired in both ESI- and ESI+ modes. Six-h (●) urine samples collected after exposure separated in the t[1] axis from pre exposure urine samples (□) at as little as 0.5 Gy (panels A and B). At dose of 1 Gy and above, the first 24-hour (◆) samples separate in the t[1] axis and at doses of 2.5 Gy and higher, the 48-h (▼) and 72-h (▲) urine samples separate in the t[1] axis from pre exposure as well. Ellipses are the boundaries of Hotelling's T2 95% confidence interval.
Spatial separation of scores in the component 1 plane associates with radiation exposure at all doses when considering the pre- versus 6h post-exposure samples. The 6h scores clearly and distinctly separate from pre-exposure scores at as low as a 0.5 Gy dose (Figure 2A, B). The 24h sample scores diverge from the pre-exposure sample scores at doses of 2.5 Gy and higher. Doses up to 2.5 Gy appear to affect PCA scores up to 24 h with a subsequent return to superimposition with pre-exposure scores. Figure 2D shows an exception to this general pattern for ESI+ data acquired from the 1.0 Gy dose group. At doses of 5 Gy and higher, the scores for all post-exposure time points diverge from pre-exposure scores, yielding stark spatial separation in the component 1 plane at 7.5 and 10 Gy doses. The results are similar when comparing data acquired in ESI- (Figure 2A, C, E, G, I, K) versus ESI+ (Figure 2B, D, F, H, J, L) TOFMS modes. Spatial separation in PCA scores is indicative of altered biofluid composition.
Evaluating Global Urine Metabolome Trends
Via VAMP, we were able to discern a radiation response as early as 6 hours post-exposure at the lowest dose of 0.5 Gy, as well as observe both time- and dose-dependent global urine metabolome responses. For both the pre- and post-exposure groups, abundance values of ions from specific samples were excluded if they exceeded the standard deviation of the ion data by a factor of 2. Ion abundance averages were subsequently recalculated with this filtered subset. Furthermore, all data from an ion feature would be excluded entirely if 80% or more of the total data set for a given group exceeded the aforementioned threshold. Finally, ion features were excluded if 80% or more of the data had abundance values of zero. Though computationally simplistic, these basic filters were nonetheless sufficient for revealing clearly discernable trends in the data. All post-exposure ion abundance percentage changes were calculated with their respective pre-exposure data for each dose as the baseline. Furthermore, analysis via VAMP focused primarily on the ESI+ data set due to the intrinsic presence of creatinine in this ionization mode.
A transient response at the 6-hour post-exposure time point was apparent even at the lowest radiation dose of 0.5 Gy. This is clearly exhibited in Figure 3A, wherein a global down-regulation (blue-spectrum) of the metabolome is observed at 6h, but is far more subdued in the 24h-post exposure time-point. While the percentage of down-regulated ions has not significantly changed from the 6h (61.41% of the 1827 plotted ions) to the 24h (69.22% of the 1855 plotted ions) time points, the mean percentage of change of the 6h data is far higher in magnitude (-189.35%) than the 24h data (-69.22%). This transience disappears with an increase in radiation dose. In Figure 3B, the global metabolome response is represented for a 5 Gy dose at both 6h and 24h post-exposure, which bears far more similarity than the differential response seen at 0.5 Gy. Indeed, the number and magnitude of down-regulated ions at the 6h (76.90% of 1385 plotted ions, -422.19% down-regulation) and 24h (67.38% out of 1119 plotted ions, -392.89% down-regulation) is comparable, though there still is a small degree of attenuation in the latter time point. Notably, the overall magnitude of down-regulation is far greater in either time-point than the 0.5 Gy dose. At the highest radiation dose of 10 Gy (Figure 3C), down-regulation at both the 6h (76.96% of 1636 plotted ions, -330.67% down-regulation) and 24h (79.03% of 1798 plotted ions, -350.24%) time-points is not remarkably different than in the 5 Gy dose, though there is no attenuation at the 24h time point, and even an increase in the number of down-regulated ions.
Figure 3.
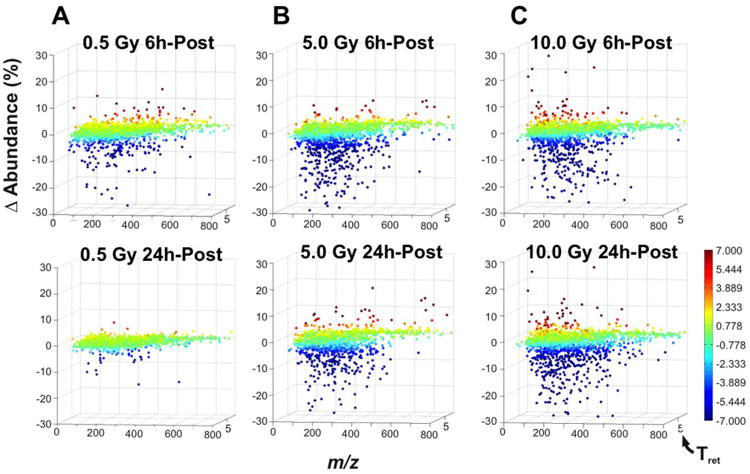
Comparing the 6 h and the 24 h post-exposure data for the (A) 0.5 Gy, (B) 5.0 Gy, and (C) 10 Gy doses via VAMP reveals a strong global down-regulation response at the 6 h time point that seems transient at 0.5 Gy, but generally maintained at the 5.0 and 10 Gy doses. Each plotted point in a graph represents an ion, which is defined by its m/z (X-axis), retention time (Y-axis), and the abundance percentage change after irradiation (Z-axis). Each ion is colorized according to the abundance percentage change, with up regulation represented by the red end of the spectrum, and the down regulation by the blue end. While this transience is clear at 0.5 Gy, even at the 5.0 Gy dose, the percentage (67.38% out of 1119 plotted ions) and magnitude (-392.89%) of down regulation at 24 h seems slightly attenuated when compared to the 6 h time point (76.90% of 1385 plotted ions, -422.19% down-regulation).
A clear time- and dose-dependent response can be observed when comparing global metabolome data from multiple time-points (24h, 48h, 72h) and doses (0.5, 2.5, 5.0 Gy) via VAMP (Figure 4). It can be broadly observed that there is an overall global down-regulation of the urine metabolome, irrespective of both dose and time-point. Both the fraction of the metabolome as well as the magnitude of this down-regulation increases with increasing dose as well as post-exposure time duration. Though there is indeed a minority of up-regulated ions as well, the time relationship is not immediately obvious from the graphs produced by VAMP, however it is clear from analyzing the highest two doses in Supplemental Figure 1 (7.5, 10.0 Gy) that there is a positive correlation with dose. Furthermore, while it seems that at the global metabolome perturbation peaks at the 48h time point for the 0.5 Gy dose before beginning to return to baseline, these effects do not exhibit signs of attenuation in the 2.5 Gy and 5 Gy doses (nor the 7.5 Gy and 10.0 Gy doses) at the measured time points. Finally, the visual similarity of all VAMP graphs for the highest two doses (Supplemental Figure 1) may indicate saturation in metabolome perturbation response.
Figure 4.
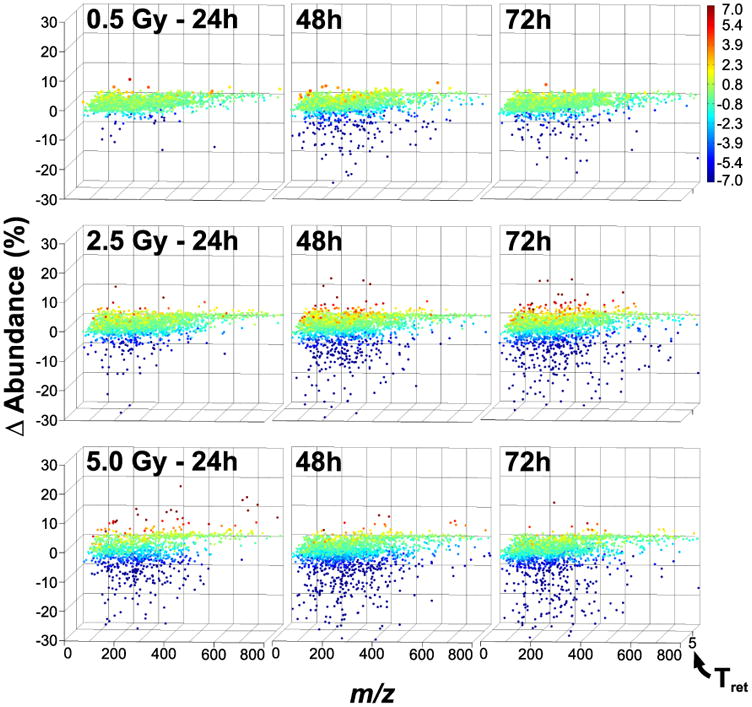
Analysis of the 24 h, 48 h, and 72 h post-exposure data for the 0.5 Gy, 2.5 Gy, and 5.0 Gy doses via VAMP reveals a clear time- and dose-dependent radiation response. As in Figure 3, there is a predominant down-regulation in the global metabolome response to radiation, regardless of time and dose. This response is exacerbated by both the dose and time postexposure, and it is not very apparent from the graphs that there is any clear attenuation of this response by the 72 h time point.
Targeted Candidate Biomarker Selection
Although a clear urinary response to radiation exposure is apparent at 6 h after 0.5 Gy exposure (Figures 2 and 3), we chose to further characterize the response to 2.5 Gy urine samples for candidate biomarker selection as we reasoned that the response is stronger and more long-lasting which relates positively to practical and functional biodosimetry requirements. In addition, distinguishing doses below or above 2 Gy is a priority for radiation dosimetry after a radiologic or nuclear event, so we chose a dose close to this. Using the PCA model constructed from the ESI- and ESI+ datasets acquired from all samples prior to (n = 20) and 6 (n = 9), 24 (n = 10), 48 (n = 10), and 72 (n = 10) h post-2.5 Gy exposure, we examined loadings S-plots of component 1 p(corr) (correlation, y-axis) values versus component 1 p (contribution, x-axis) values (Supplemental Figure 2). These are the plots we subsequently interrogated (detailed in Supplementary Information) for selecting candidate biomarkers, whose abundance across different doses when comparing pre- vs. 24 h post-exposure (Table 1 and Supplemental Table S2) as well as across different time points at 2.5 Gy exposure (Supplemental Tables S3 and S4) were explored. Statistical analysis was also conducted on the across-dose abundance data (Supplemental Tables S5-8). A subset of the most significant biomarkers was validated and includes carnitine, uric acid, cytosine, isocitrate, thymidine, and creatine. Thymidine and creatine levels were further examined across all dose- and time-points to provide a fuller picture of radiation response (Figure 5).
Table 1.
Up-regulated excretion of at urine metabolites after gamma radiation exposures ranging from 0.5 to 10 Gy.
| Pre-vs.24 h Post-2.5 Gy | Fold-Change (Pre-vs.24 Post-Exposure) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| m/z | Tret | Adduct | p(corr)[1] | Putative identity | 0.5 Gy | 1 Gy | 2.5 Gy | 5 Gy | 7.5 Gy | 10 Gy | |
| a | 135.0289 | 0.34 | [M-H]- | -0.776087 | Threonate | NS | NS | 17.3 (9.13, 32.1) | 19.9 (10.2, 95.8) | 2.25 (1.49, 3.87) | 3.49 (2.24, 6.57) |
| b | 165.0396 | 0.32 | [M-H]- | -0.731736 | Arabinonate | NS | -2.01 (-1.21, -3.59) | 4.55 (1.88, 10.3) | 11.6 (5.59, 96.0) | 3.86 (2.47, 7.55) | 5.36 (3.08, 11.4) |
| c | 128.9596 | 0.29 | [M-H]- | -0.723760 | ND | ND | > 10 | > 10 | > 10 | > 10 | |
| d | 355.0165 | 4.29 | [M-H]- | -0.712256 | NS | NS | > 10 | > 10 | 2.56 (1.82, 3.37) | 2.10 (1.38, 2.86) | |
| e | 241.0816 | 1.89 | [M-H]- | -0.690459 | Thymidine* | ND | ND | > 10 | > 10 | > 10 | > 10 |
| f | 112.9857 | 0.29 | [M-H]- | -0.680258 | Fragment of d | ND | ND | > 10 | > 10 | 2.43 (1.49, 3.79) | 2.38 (1.16, 4.08) |
| g | 209.0303 | 0.33 | [M-H]- | -0.663502 | Glucarate | ND | ND | > 10 | 12.5 (5.94, 32.6) | 24.4 (11.2, 398) | > 10 |
| h | 195.0490 | 0.32 | [M-H]- | -0.641262 | NS | NS | NS | 3.02 (2.07, 4.29) | 3.32 (2.43, 4.30) | 4.60 (2.81, 6.51) | |
| i | 251.0516 | 0.32 | [M-H]- | -0.629689 | ND | ND | > 10 | > 10 | > 10 | > 10 | |
| j | 176.9351 | 0.34 | [M-H]- | -0.533538 | ND | ND | > 10 | > 10 | 45.5 (17.3, 297) | > 10 | |
| k | 167.0195 | 0.36 | [M-H]- | 0.154537 | Uric Acid* | NS | NS | NS | 2.22 (1.18, 3.58) | > 10 | 15.4 (4.13, 49.9) |
| l | 201.9351 | 0.29 | [M+H]+ | -0.581992 | NS | NS | 11.6 (5.23, 113) | > 10 | > 10 | 6.60 (3.17, 415) | |
| m | 228.1009 | 0.36 | [M+H]+ | -0.527531 | NS | NS | > 10 | > 10 | 1.84 (1.42, 2.38) | 3.12 (1.87, 7.47) | |
| n | 207.1114 | 2.21 | [M+H]+ | -0.488026 | ND | NS | > 10 | > 10 | > 10 | > 10 | |
| o | 112.0502 | 0.35 | [M+H]+ | -0.48s7919 | Cytosine* | NS | NS | 5.49 (3.41, 12.2) | 5.18 (3.25, 10.8) | 2.56 (2.00, 3.29) | 3.43 (2.55, 4.76) |
| p | 455.1881 | 0.35 | [M+H]+ | -0.418781 | ND | ND | > 10 | > 10 | > 10 | > 10 | |
| q | 132.0769 | 0.35 | [M+H]+ | -0.389817 | Creatine* | > 10 | ND | > 10 | > 10 | > 10 | 3.43 (2.55, 4.76) |
| r | 162.1126 | 0.32 | [M+H]+ | -0.306065 | Carnitine* | NS | NS | 3.20 (1.41, 28.6) | 5.80 (3.01, 24.5) | 5.95 (2.72, 39.8) | 4.18 (1.78, 11.1) |
| s | 90.0543 | 0.34 | [M+H]+ | -0.292752 | Alanine or Sarcosine | NS | ND | > 10 | > 10 | > 10 | > 10 |
| t | 143.1176 | 0.36 | [M+H]+ | -0.199188 | NS | NS | 3.26 (1.40, 17.6) | 2.81 (1.35, 8.0) | 2.16 (1.33, 3.29) | 3.37 (2.31, 4.88) | |
| u | 141.0665 | 0.35 | [M+H]+ | -0.185771 | Methylimidazoleacetic acid | NS | NS | 1.67 (1.29, 2.16) | 1.52 (1.11, 2.21) | 1.55 (1.18, 2.02) | 1.70 (1.43, 2.00) |
Confirmed identity: CHEBI:17748 thymidine, CHEBI:27226 uric acid, CHEBI:16040 cytosine, CHEBI:16919 creatine, CHEBI:17126 carnitine
abbreviations: m/z, mass/charge; Tret, Retention Time; NS, Non-Significant; ND, Not Detected
Figure 5.
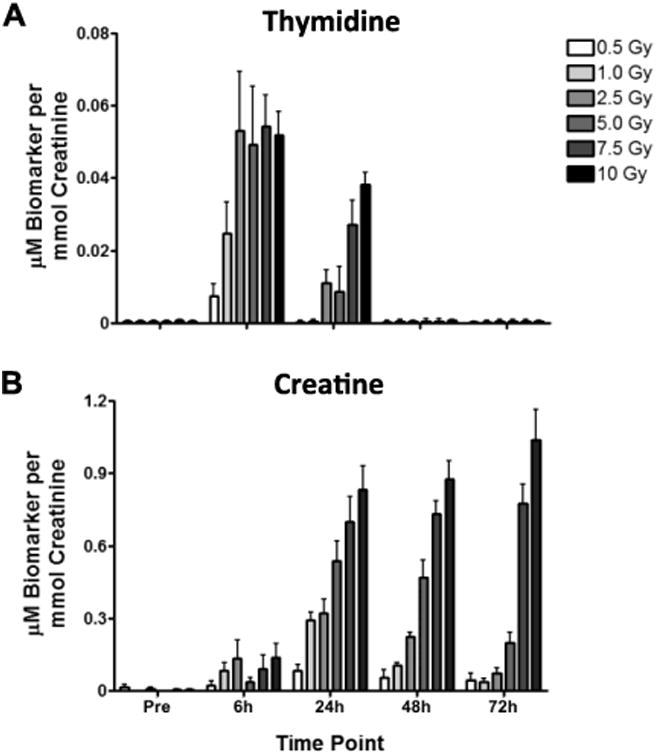
Exposure to total body gamma radiation results in dose- and time-dependent increases in urinary thymidine and creatine and decrease in urinary citrate. Urine was collected from male rats before (Pre) and at 6, 24, 48, and 72 h after exposure to 0.5, 1.0, 2.5, 5.0, 7.5, or 10 Gy. Samples were analyzed by UPLC-TOFMS in ESI- and ESI+ modes. Shown are means ± corresponding 95% confidence intervals (error bars; n = 20 per dose pre and 10 per dose-time post-exposure; alpha = 0.05). Panels A and B show mean (± 95% CIs) for thymidine and creatine using creatinine-normalized abundances. Asterisks indicate statistically significant difference in mean after exposure using paired t-tests assuming unequal variances.
Thymidine has been shown to be a sensitive urinary radiation biomarker in male mice and rats (Tyburski et al. 2008; Lanz et al. 2009; Tyburski et al. 2009; Johnson et al. 2011); and in case of the latter an increased urinary excretion of thymidine was observed in male rats sampled before and at one time point after a 3 Gy of x rays. Figure 5 A shows mean thymidine ± 95% confidence intervals, expressed as μM per mmol creatinine for all doses and time points. These estimates demonstrate that the male rat urine radiation response as a function of thymidine excretion is dose-dependent at doses ≤ 2.5 Gy up to 6 h post-exposure and ≤ 10 Gy at 24 h postexposure. Moreover, the dose dependency of the exposure-associated thymidine excretion is also time-dependent. The time-dependency is apparent in the comparison of mean normalized concentrations for all doses at 24 h versus 6 h post-exposure. Consistent with published reports that include time course measurements in the urine of mice (Tyburski et al. 2008; 2009), the increased excretion of thymidine in urine of irradiated rats is transient and undetectable by 48 h post-exposure.
We also observed among the ions detected in the ESI+ dataset that creatine excretion became detectable as early as 6 h post-exposure to as little as 0.5 Gy (Figure 5B). The excretion of creatine was also dose-dependent as illustrated by mean normalized concentrations at 24, 48, and 72 h post-exposure to all doses. Noteworthy is that even as late as 48 h, comparing the means across the doses reveals a significant trend, and the dose-dependency is near exponential. At doses below 7.5 Gy, there does seem to be a trend toward recovering to baseline excretion, whereas excretion post-7.5 Gy exposure is persistent. Creatine excretion after a 10-Gy exposure appears to still be increasing as late as 72 h.
Discussion
By utilizing both traditional modes of targeted analysis as well as novel methods that take a holistic approach to evaluating the metabolome, far more comprehensive conclusions can be drawn from the rich multi-dose and multi-time point radiation metabolomics data set generated in this study. As such, we have utilized tried and true techniques based on PCA as well as individually examining specific small molecules during targeted analysis. A more global approach to analyzing the data necessitated the development of VAMP, which allows for fluctuations in the entire metabolome to be visually evaluated in an easy to understand manner.
Despite the advent of technologies that allow for the comprehensive surveying of the small molecule constituents of metabolism via metabolomics, the focus in radiobiology thus far has been on specific mechanisms and known metabolites with well understood roles and functions. For the first time, we were able to characterize a global metabolome response via VAMP. This global metabolome down-regulation, which is positively correlated with both radiation dose and time post-exposure (at higher doses), would have otherwise been completely overlooked if only classical techniques such as PCA and targeted approaches were utilized. This global down-regulation may be indicative of a general trend of dysregulation in the metabolic networks, which is only exacerbated by increasing levels of IR (Figure 4). While this may be reversible in lower doses, as suggested by the early versus late post-exposure time point response at 0.5 Gy (Figure 3A), this becomes increasingly irrevocable with increasing dose (Figure 3B and 3C). Overall, these novel findings underscores the necessity of exploiting the strengths and advantages of newer technologies rather than restricting oneself to only tried and true techniques and methodologies.
Indeed, analysis of the data via VAMP revealed novel characteristics pertaining to the global metabolome response which would not have been revealed, and may have even been obfuscated with more established approaches. While PCA and similar multivariate approaches incorporate the entire data set during analysis, its approach to analysis treats metabolomics data like any other high-dimensionality data set, and as a result may obfuscate many of its defining qualities. In a sense, PCA potentially “over-processes” the data to the point where valuable information may be lost. At the opposite end of the spectrum is the traditional targeted approach to biomarker discovery, wherein ions are analyzed one at a time. In this scenario, the idiom of “missing the forest for the trees” is apt in that global trends in the metabolome can easily be missed when only focusing on individual metabolites. VAMP serves as a valuable methodology that offers a means of holistically visualizing the metabolome response for a given time point in its entirety, utilizing minimal and uncomplicated methods of data processing.
Utilizing VAMP during this study also revealed the general utility of our new approach for other metabolomics studies. VAMP utilizes a qualitative approach to analysis, eschewing classical statistical analysis techniques, in order to visualize metabolomics data in a manner that is easy to interpret, but at the same time is capable of illustrating the aggregate response of the entire metabolome. This simplistic approach to data processing allows the intrinsic features of metabolomics data (such as the m/z and retention time of ions) to play a role. This may be especially valuable in lipidomics, where the retention time plays a crucial role in initially identifying classes of lipids. While in no way is VAMP a comprehensive approach to conducting analysis for metabolomics data, it may nonetheless be a valuable tool that serves to complement more traditional methods of analysis in exposure biology as well as other applications of metabolomics profiling.
Targeted analysis yielded two especially strong candidates for biomarkers, thymidine and creatine. Thymidine has long been associated with IR exposure (Dewey & Humphrey 1965). More recently, thymidine uptake has been shown to be inhibited with increasing IR in in vitro studies with human endothelial cells (Kermani et al. 2001). Furthermore, thymidine's transient up-excretion within the first 24 h post-exposure has been described in male rats via GC-MS based metabolomics (Johnson et al. 2011). We expand on this finding by demonstrating dose dependence as well. As shown in Figure 5A, thymidine's transient, but strong response that is closely positively correlated to dose indicates that is may be an excellent biomarker for early detection of radiation exposure. While its levels return to baseline at the 48 and 72 h time points, the 6 and 24 h time point abundance levels are fairly robust. Creatine has also been similarly linked to radiation for many decades(Kurohara & Altman 1962). Recently, creatine up-excretion has been detected via metabolomics in non-human primate urine samples as a result of IR exposure, and is believed to be linked to the inability of muscle to utilize creatine, thus causing a buildup (Johnson et al. 2012). As with thymidine, creatine possesses a similar positive (and very consistent) correlation to radiation dose, but is more prevalent at the 24, 48, and 72 h time points When considered together, thymidine and creatine serve to complement one another, and are both worth further investigation as potential biomarkers for biodosimetry during potential radiological emergencies.
Targeted analysis also yielded isocitrate, uric acid, cytosine, and carnitine as potential biomarkers for radiation exposure. Isocitrate levels may offer clues to key events in radiation response, as mitochondrial NADP+ dependent isocitrate dehydrogenase (IDPm) has been found to play an important role in radiation induced apoptosis in an in vitro study (Lee et al. 2004;2007). IDPm, which catalyzes the oxidative decarboxylation of isocitrate to α-ketoglutarate, was found to be induced by γ-radiation exposure, which corroborates with the positively correlated dose-dependent magnitude of down-excretion found in our study. Uric acid was found to be up-excreted in our study as well as the aforementioned metabolomic study with non-human primate urine samples (Johnson et al. 2012). Along the lines of classical models for DNA damage via IR, cytosine crosslinking is a known to occur with γ radiation exposure (Dizdaroglu & Simic 1984) although the levels from direct damage by IR would be expected to be low at these doses. Finally, carnitine has been linked to radioprotective roles in the literature. In its acetylated form, acetyl-l-carnitine may protect against radiation induced oxidative stress (Mansour 2006), as well as radiation-induced damage in the brain and retina in rats(Sezen et al. 2008). Acetyl-l-carnitine is broken down in the blood by plasma esterases back into carnitine, which may explain its up-excretion in urine from our study (Visser et al. 2007).
Conclusions
Through the use of both novel and classical techniques, important aspects of radiation response were characterized in rats via analysis of the urine metabolome. The comprehensive nature of the study allowed the response to be characterized in both a time- and dose-dependent manner. While classical analysis approaches revealed several key biomarkers, most notably creatine and thymidine, the development of more novel methodologies, culminating in VAMP, allowed for the metabolome to be holistically evaluated. Through VAMP, an overall net down-regulation of the urine metabolome, whose magnitude is correlated with both dose and time post-exposure, was characterized. The increasing complexity of metabolomics studies emphasizes the need for specialized approaches such as VAMP, which may become a crucial factor in the advancement and evolution of metabolomics as a primary “-omics” platform.
Supplementary Material
Supplemental Figure 1. The visual similarity in the VAMP graphs produced from analysis of the 24 h, 48 h, and 72 h post-exposure data for the 7.5 Gy and 10.0 Gy doses may indicate a saturation in the metabolome perturbation response. However one clear trend in comparison to the lower doses (analyzed in Figure 4) is a more pronounced up-regulation of the metabolome, though indeed down-regulation still appears to be more dominant for all time-points and doses.
Supplemental Figure 2. Loadings S-plots of rat urine ions before and 6 h, 24 h, 48 h, and 72 h post exposure to 2.5 Gy gamma radiation. Urine ions measured by UPLC-TOFMS in ESI- or ESI+ modes are represented by PCA results for component 1 correlation (p(corr)[1]) versus component 1 weight (p[1]). These loadings, each represented by a circle (○), correspond to the ESI- (A) and ESI+ (B) PCA models for 2.5 Gy before and after exposure. The PCA model assembled in such a way that ions that were up regulated after exposure received negative correlation quantities (red arrow), whereas down regulated ions received positive quantities (black arrow). The red boxes in A and B indicate the zoomed views presented in panels C and D, respectively, of the urine ions positively correlating most with radiation exposure. The black boxes in A and B indicate the zoomed views presented in panels E and F, respectively, showing the ions negatively correlating with radiation exposure. Ions of confirmed identities are marked by red dots (
 ) and labeled: a, citrate; b, allantoin; c, thymidine; d, isocitrate; e, creatine; f, sodium-thymidine adduct. Marked by red dots only (panel A, no labels) are taurine, acetyl taurine, N-hexanoylglycine, xanthosine, and 2′-deoxyxanthosine. Indicated by dots (●) in panels C-F are the top 22-24 ions based on p(corr)[1] values. Details of these ions are given in Table 1. The variable p[1] is a calculated measure of relative abundance whereby ions of higher abundance in urine receive a higher p[1] quantity.
) and labeled: a, citrate; b, allantoin; c, thymidine; d, isocitrate; e, creatine; f, sodium-thymidine adduct. Marked by red dots only (panel A, no labels) are taurine, acetyl taurine, N-hexanoylglycine, xanthosine, and 2′-deoxyxanthosine. Indicated by dots (●) in panels C-F are the top 22-24 ions based on p(corr)[1] values. Details of these ions are given in Table 1. The variable p[1] is a calculated measure of relative abundance whereby ions of higher abundance in urine receive a higher p[1] quantity.
Acknowledgments
This study was supported by the National Institute of Health (National Institute of Allergy and Infectious Diseases) grant U19 A1067773. F.J.G. is supported by the National Cancer Instititue Intramural Research Program in the Center for Cancer Research. J.F.K. was supported in part by grant DARPA-FY08-0004 from the Defense Advanced Research Projects Agency. The views expressed are those of the authors and do not reflect the official policy or position of the Armed Forces Radiobiology Research Institute, the Uniformed Services University, the Department of Defense, or the United States Government. The authors would like to thank Drs. Andrew D. Patterson (Penn. State Univ.) and David J. Brenner for helpful discussions and their support.
Abbreviations
- DMS-MS
differential mobility spectrometry-mass spectrometry
- AFRRI
Armed Forces Radiobiology Research Institute
- MS
mass spectrometer
- UPLC-TOFMS
Ultra-Performance Liquid Chromatography-Time of Flight Mass Spectrometry
- ESI
Electrospray ionization
- ESI+
positive ESI
- ESI-
negative ESI
- PCA
principal component analysis
- PC
principal component
- IR
ionizing radiation
- IS
internal standard
- ppm
parts per million
- CI
confidence interval
- VAMP
Visual Analysis of Metabolomics Package
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to report.
Ethical Statement: All animal experiments were approved by the Armed Forces Radiobiology Research Institute's Animal Care and Use Committee prior to initiation. Animals were maintained in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International in accordance with the Guide for the Care and Use of Laboratory Animals.
References
- Amendola R, Basso E, Pacifici PG, Piras E, Giovanetti A, Volpato C, et al. Ret, Abl1 (cAbl) and Trp53 gene fragmentations in comet-FISH assay act as in vivo biomarkers of radiation exposure in C57BL/6 and CBA/J mice. Radiat Res. 2006;165:553–561. doi: 10.1667/RR3544.1. [DOI] [PubMed] [Google Scholar]
- Castro-Perez J, Plumb R, Granger JH, Beattie I, Joncour K, Wright A. Increasing throughput and information content for in vitro drug metabolism experiments using ultra-performance liquid chromatography coupled to a quadrupole time-of-flight mass spectrometer. Rapid Commun Mass Spectrom. 2005;19:843–848. doi: 10.1002/rcm.1859. [DOI] [PubMed] [Google Scholar]
- Chen C, Brenner DJ, Brown TR. Identification of urinary biomarkers from x-irradiated mice using NMR spectroscopy. Radiat Res. 2011;175:622–630. doi: 10.1667/RR2388.1. [DOI] [PubMed] [Google Scholar]
- Christodouleas JP, Forrest RD, Ainsley CG, Tochner Z, Hahn SM, Glatstein E. Short-term and long-term health risks of nuclear-power-plant accidents. N Engl J Med. 2011;364:2334–2341. doi: 10.1056/NEJMra1103676. [DOI] [PubMed] [Google Scholar]
- Coy SL, Cheema AK, Tyburski JB, Laiakis EC, Collins SP, Fornace AJ. Radiation metabolomics and its potential in biodosimetry. Int J Radiat Biol. 2011;87:802–823. doi: 10.3109/09553002.2011.556177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coy SL, Krylov EV, Schneider BB, Covey TR, Brenner DJ, Tyburski JB, et al. Detection of Radiation-Exposure Biomarkers by Differential Mobility Prefiltered Mass Spectrometry (DMS-MS) Int J Mass Spectrom. 2010;291:108–117. doi: 10.1016/j.ijms.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey WC, Humphrey RM. Increase in radiosensitivity to ionizing radiation related to replacement of thymidine in mammalian cells with 5-bromodeoxyuridine. Radiat Res. 1965;26:538–553. [PubMed] [Google Scholar]
- Dizdaroglu M, Simic MG. Radiation-induced crosslinking of cytosine. Radiat Res. 1984;100:41–46. [PubMed] [Google Scholar]
- Dunn SR, Qi Z, Bottinger EP, Breyer MD, Sharma K. Utility of endogenous creatinine clearance as a measure of renal function in mice. Kidney Int. 2004;65:1959–1967. doi: 10.1111/j.1523-1755.2004.00600.x. [DOI] [PubMed] [Google Scholar]
- Grace MB, Moyer BR, Prasher J, Cliffer KD, Ramakrishnan N, Kaminski J, et al. Rapid radiation dose assessment for radiological public health emergencies: roles of NIAID and BARDA. Health Phys. 2010;98:172–178. doi: 10.1097/01.HP.0000348001.60905.c0. [DOI] [PubMed] [Google Scholar]
- Hafer N, Cassatt D, Dicarlo A, Ramakrishnan N, Kaminski J, Norman MK, et al. NIAID/NIH radiation/nuclear medical countermeasures product research and development program. Health Phys. 2010;98:903–905. doi: 10.1097/HP.0b013e3181bbc4df. [DOI] [PubMed] [Google Scholar]
- Johnson CH, Patterson AD, Krausz KW, Kalinich JF, Tyburski JB, Kang DW, et al. Radiation Metabolomics. 5. Identification of Urinary Biomarkers of Ionizing Radiation Exposure in Nonhuman Primates by Mass Spectrometry-Based Metabolomics. Radiat Res. 2012;178:328–340. doi: 10.1667/rr2950.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CH, Patterson AD, Krausz KW, Lanz C, Kang DW, Luecke H, et al. Radiation metabolomics. 4. UPLC-ESI-QTOFMS-Based metabolomics for urinary biomarker discovery in gamma-irradiated rats. Radiat Res. 2011;175:473–484. doi: 10.1667/RR2437.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermani P, Leclerc G, Martel R, Fareh J. Effect of ionizing radiation on thymidine uptake, differentiation, and VEGFR2 receptor expression in endothelial cells: the role of VEGF(165) Int J Radiat Oncol Biol Phys. 2001;50:213–220. doi: 10.1016/s0360-3016(01)01445-6. [DOI] [PubMed] [Google Scholar]
- Khan AR, Rana P, Devi MM, Chaturvedi S, Javed S, Tripathi RP, et al. Nuclear magnetic resonance spectroscopy-based metabonomic investigation of biochemical effects in serum of gamma-irradiated mice. Int J Radiat Biol. 2011;87:91–97. doi: 10.3109/09553002.2010.518211. [DOI] [PubMed] [Google Scholar]
- Kurohara SS, Altman KI. Effect of exposure to ionizing radiation on creatine concentration in human and rat erythrocytes. Nature. 1962;196:151–153. doi: 10.1038/196285a0. [DOI] [PubMed] [Google Scholar]
- Lanz C, Patterson AD, Slavik J, Krausz KW, Ledermann M, Gonzalez FJ, et al. Radiation metabolomics. 3. Biomarker discovery in the urine of gamma-irradiated rats using a simplified metabolomics protocol of gas chromatography-mass spectrometry combined with random forests machine learning algorithm. Radiat Res. 2009;172:198–212. doi: 10.1667/RR1796.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Lee M, Kang CM, Jeoung D, Bae S, Cho CK, et al. Identification of possible candidate biomarkers for local or whole body radiation exposure in C57BL/6 mice. Int J Radiat Oncol Biol Phys. 2007;69:1272–1281. doi: 10.1016/j.ijrobp.2007.07.2336. [DOI] [PubMed] [Google Scholar]
- Lee SH, Jo SH, Lee SM, Koh HJ, Song H, Park JW, et al. Role of NADP+-dependent isocitrate dehydrogenase (NADP+-ICDH) on cellular defence against oxidative injury by gamma-rays.aaaa. Int J Radiat Biol. 2004;80:635–642. doi: 10.1080/09553000400007680. [DOI] [PubMed] [Google Scholar]
- Mak TD, Laiakis EC, Goudarzi M, Fornace AJ., Jr MetaboLyzer: A Novel Statistical Workflow for Analyzing Postprocessed LC-MS Metabolomics Data. Anal Chem. 2014;86:506–513. doi: 10.1021/ac402477z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour HH. Protective role of carnitine ester against radiation-induced oxidative stress in rats. Pharmacol Res. 2006;54:165–171. doi: 10.1016/j.phrs.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Noda I. Scaling techniques to enhance two-dimensional correlation spectra. J Molecular Structure. 2008;883:216–227. [Google Scholar]
- Ossetrova NI, Sandgren DJ, Gallego S, Blakely WF. Combined approach of hematological biomarkers and plasma protein SAA for improvement of radiation dose assessment triage in biodosimetry applications. Health Phys. 2010;98:204–208. doi: 10.1097/HP.0b013e3181abaabf. [DOI] [PubMed] [Google Scholar]
- Partridge MA, Chai Y, Zhou H, Hei TK. High-throughput antibody-based assays to identify and quantify radiation-responsive protein biomarkers. Int J Radiat Biol. 2010;86:321–328. doi: 10.3109/09553000903564034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson K. LIII. On lines and planes of closest fit to systems of points in space. The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science. 1901;2:559–572. [Google Scholar]
- Plumb R, Castro-Perez J, Granger J, Beattie I, Joncour K, Wright A. Ultra-performance liquid chromatography coupled to quadrupole-orthogonal time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 2004;18:2331–2337. doi: 10.1002/rcm.1627. [DOI] [PubMed] [Google Scholar]
- Porciani S, Lanini A, Balzi M, Faraoni P, Becciolini A. Polyamines as biochemical indicators of radiation injury. Phys Med. 2001;17(Suppl 1):187–188. [PubMed] [Google Scholar]
- Randic M, Supek Z. Urinary excretion of 5-hydroxyindolacetic acid after a single whole-body x-irradiation in normal and adrenalectomized rats. Int J Radiat Biol. 1961;4:151–153. doi: 10.1080/09553006114551071. [DOI] [PubMed] [Google Scholar]
- Reisz JA, Bansal N, Qian J, Zhao W, Furdui CM. Effects of ionizing radiation on biological molecules-mechanisms of damage and emerging methods of detection. Antioxid Redox Signal. 2014;21:260–292. doi: 10.1089/ars.2013.5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux A, Lison D, Junot C, Heilier JF. Applications of liquid chromatography coupled to mass spectrometry-based metabolomics in clinical chemistry and toxicology: A review. Clin Biochem. 2011;44:119–135. doi: 10.1016/j.clinbiochem.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Sezen O, Ertekin MV, Demircan B, Karslioglu I, Erdogan F, Kocer I, et al. Vitamin E and L-carnitine, separately or in combination, in the prevention of radiation-induced brain and retinal damages. Neurosurg Rev. 2008;31:205–13. doi: 10.1007/s10143-007-0118-0. discussion 213. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Boysen G, Li F, Li Y, Swenberg JA. Tandem mass spectrometry measurements of creatinine in mouse plasma and urine for determining glomerular filtration rate. Kidney Int. 2007;71:266–271. doi: 10.1038/sj.ki.5002033. [DOI] [PubMed] [Google Scholar]
- Tang X, Zheng M, Zhang Y, Fan S, Wang C. Estimation value of plasma amino acid target analysis to the acute radiation injury early triage in the rat model. Metabolomics. 2013;9:853–863. [Google Scholar]
- Tyburski JB, Patterson AD, Krausz KW, Slavik J, Fornace AJJ, Gonzalez FJ, et al. Radiation metabolomics. 1. Identification of minimally invasive urine biomarkers for gamma-radiation exposure in mice. Radiat Res. 2008;170:1–14. doi: 10.1667/RR1265.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyburski JB, Patterson AD, Krausz KW, Slavik J, Fornace AJ, Jr, Gonzalez FJ, et al. Radiation metabolomics. 2. Dose- and time-dependent urinary excretion of deaminated purines and pyrimidines after sublethal gamma-radiation exposure in mice. Radiat Res. 2009;172:42–57. doi: 10.1667/RR1703.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser W, Van Roermund C, Ijlst L, Waterham H, Wanders R. Metabolite transport across the peroxisomal membrane. Biochem J. 2007;401:365–375. doi: 10.1042/BJ20061352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhou X, Li C, Wu J, Kuo JE, Wang C. Assessment of early triage for acute radiation injury in rat model based on urinary amino acid target analysis. Mol Biosyst. 2014;10:1441–1449. doi: 10.1039/c3mb70526a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. The visual similarity in the VAMP graphs produced from analysis of the 24 h, 48 h, and 72 h post-exposure data for the 7.5 Gy and 10.0 Gy doses may indicate a saturation in the metabolome perturbation response. However one clear trend in comparison to the lower doses (analyzed in Figure 4) is a more pronounced up-regulation of the metabolome, though indeed down-regulation still appears to be more dominant for all time-points and doses.
Supplemental Figure 2. Loadings S-plots of rat urine ions before and 6 h, 24 h, 48 h, and 72 h post exposure to 2.5 Gy gamma radiation. Urine ions measured by UPLC-TOFMS in ESI- or ESI+ modes are represented by PCA results for component 1 correlation (p(corr)[1]) versus component 1 weight (p[1]). These loadings, each represented by a circle (○), correspond to the ESI- (A) and ESI+ (B) PCA models for 2.5 Gy before and after exposure. The PCA model assembled in such a way that ions that were up regulated after exposure received negative correlation quantities (red arrow), whereas down regulated ions received positive quantities (black arrow). The red boxes in A and B indicate the zoomed views presented in panels C and D, respectively, of the urine ions positively correlating most with radiation exposure. The black boxes in A and B indicate the zoomed views presented in panels E and F, respectively, showing the ions negatively correlating with radiation exposure. Ions of confirmed identities are marked by red dots (
 ) and labeled: a, citrate; b, allantoin; c, thymidine; d, isocitrate; e, creatine; f, sodium-thymidine adduct. Marked by red dots only (panel A, no labels) are taurine, acetyl taurine, N-hexanoylglycine, xanthosine, and 2′-deoxyxanthosine. Indicated by dots (●) in panels C-F are the top 22-24 ions based on p(corr)[1] values. Details of these ions are given in Table 1. The variable p[1] is a calculated measure of relative abundance whereby ions of higher abundance in urine receive a higher p[1] quantity.
) and labeled: a, citrate; b, allantoin; c, thymidine; d, isocitrate; e, creatine; f, sodium-thymidine adduct. Marked by red dots only (panel A, no labels) are taurine, acetyl taurine, N-hexanoylglycine, xanthosine, and 2′-deoxyxanthosine. Indicated by dots (●) in panels C-F are the top 22-24 ions based on p(corr)[1] values. Details of these ions are given in Table 1. The variable p[1] is a calculated measure of relative abundance whereby ions of higher abundance in urine receive a higher p[1] quantity.