Abstract
The early transcriptional response and subsequent induction of anchorage-independent growth after exposure to particles of high Z and energy (HZE) as well as γ-rays were examined in human bronchial epithelial cells (HBEC3KT) immortalised without viral oncogenes and an isogenic variant cell line whose p53 expression was suppressed but that expressed an active mutant K-RASV12 (HBEC3KT-P53KRAS). Cell survival following irradiation showed that HBEC3KT-P53KRAS cells were more radioresistant than HBEC3KT cells irrespective of the radiation species. In addition, radiation enhanced the ability of the surviving HBEC3KT-P53RAS cells but not the surviving HBEC3KT cells to grow in anchorage-independent fashion (soft agar colony formation). HZE particle irradiation was far more efficient than γ-rays at rendering HBEC3KT-P53RAS cells permissive for soft agar growth. Gene expression profiles after radiation showed that the molecular response to radiation for HBEC3KT-P53RAS, similar to that for HBEC3KT cells, varies with radiation quality. Several pathways associated with anchorage independent growth, including the HIF-1α, mTOR, IGF-1, RhoA and ERK/MAPK pathways, were over-represented in the irradiated HBEC3KT-P53RAS cells compared to parental HBEC3KT cells. These results suggest that oncogenically progressed human lung epithelial cells are at greater risk for cellular transformation and carcinogenic risk after ionising radiation, but particularly so after HZE radiations. These results have implication for: (i) terrestrial radiation and suggests the possibility of enhanced carcinogenic risk from diagnostic CT screens used for early lung cancer detection; (ii) enhanced carcinogenic risk from heavy particles used in radiotherapy; and (iii) for space radiation, raising the possibility that astronauts harbouring epithelial regions of dysplasia or hyperplasia within the lung that contain oncogenic changes, may have a greater risk for lung cancers based upon their exposure to heavy particles present in the deep space environment.
Introduction
Lung carcinogenesis is a multi-step process through which normal lung epithelial cells undergo a series of genetic changes in specific proto-oncogenes and tumour suppressor genes which ultimately cause a cell to enter into a state of uncontrolled growth (1–5). This process has been described as consisting of different stages including initiation, promotion and progression to cancer. While exposure to the carcinogens in tobacco smoke is the leading cause of lung cancer, ionising radiation can act to initiate and/or promote the carcinogenic process.
Indeed, evidence from epidemiological studies support the notion that the lung is among the most susceptible tissues to radiation-induced carcinogenesis (6,7). Multiple factors, including radiation dose, dose rate, genetic background and number of cells at risk have been associated with this increased cancer risk. For radiation exposure a conventional linear-no-threshold model has been used to describe the dose-dependent lung cancer incidence amongst atomic bomb survivors and other cases of external radiation exposures (7–9). For internal radiation of inhaled radon and plutonium exposure, a threshold of 0.1 Gy was introduced using a two-step clonal expansion model for better interpretation of epidemiological data (9,10). Furthermore, early animal studies clearly showed the effectiveness of low and high linear energy transfer (LET) radiations at inducing lung cancers (11–14). Moreover, when neutron exposures were given at a low dose rate an inverse dose-rate effect was seen (15). Understanding the mechanisms of radiation-induced carcinogenesis, particularly from high LET radiations is also important for carcinogenic risk assessment when long-term space travel is considered due to the exposure to accelerated particles with high Z and energy (HZE). HZE particles produce dense ionisations along their trajectory and can cause complex and irreparable clustered DNA damage (16–18). As a result they are more effective than sparsely ionising radiation like γ-rays for cell killing as well as other end points, such as chromatid and chromosome aberrations, mutation and ultimately carcinogenesis (19–26). Because of the very low dose rates for HZE particles in free space there is some concern for the likelihood of an inverse dose rate effect for carcinogenesis as a result of space travel outside of the earth’s magnetic field including trips to Mars or other sub-planetary bodies. In addition, HZE particles including 12C are now used for radiotherapy in Europe and Asia. Hence, there is some concern for low dose exposures to normal tissues in a therapeutic treatment field, particularly where there is the potential for filed effects.
Conventional radiation exposure through diagnostic exposures should also be considered particularly with the advent of multiple CT screens for patients at risk for developing lung cancer. And while screening has been shown to bring forward early disease, there appeared to be no reduction in disease stage or outcome in a Dutch study (27) while results from the much larger National Lung Screen Trial suggested an increase in earlier stage cancers and a reduction in mortality from lung cancer when compared with standard radiography (28,29). Interestingly, the estimate of mortality reduction needed to outweigh the increased risk of lung cancer as a result of diagnostic CT exposures suggested that for screening before the age of 50, the mortality reduction from CT screens versus the risk for radiation-induced lung cancers may be unachievable (30).
The population base for all of these studies consists largely of current or former smokers and the KRAS gene is frequently found to be mutated in non-small cell lung cancers (NSCLC) of smokers and former smokers (31–33). Histopathologic examination of a variety of tissues has identified what are called field effects in that the number of independent tumours or regions of dysplasia and hyperplasia is much larger than what would be expected from random chance (34,35). This is certainly the case for lung cancer (36) where the carcinogens from smoking mutagenise the respiratory epithelium resulting in multiple clonally independent foci of premalignant cells as well as abnormalities in histologically normal appearing cells (37). Such carcinogenic exposure also leads to loss of p53 and the expression of mutant KRAS in lung epithelial cells. However, it does not appear that these changes are sufficient to develop a fully malignant phenotype even in non-oncogenically immortalised lung epithelial cells (1,38,39).
The question then becomes what is the risk for lung cancer in individuals who have developed regions of hyperplasia and perhaps dysplasia within the lung field from environmental carcinogenic exposures as described previously, including loss of p53 or gain of a KRAS mutation, and who are then exposed to radiation, particularly HZE radiation?
In the present study, we focussed on the differences in cellular genetic background in radiation-induced carcinogenesis, with an emphasis on different radiation qualities, that is HZE radiation with different types of ion species and energies versus low LET radiation, in this case γ-rays from 137Cs exposure using a more oncogenically progressed cell line with specific genetic alterations associated with lung carcinogenesis (39). The parental cell line HBEC3KT has been non-oncogenically immortalised via overexpression of CDK4 and hTERT which in and of themselves are two of the earliest events in lung cancer pathogenesis. A previous study described the initial radioresponse of HBEC3KT cells and showed that the transcriptional responses of HBEC3KT were specific to the radiation quality (40). Here, in addition to CDK4 and hTERT manipulation, p53 expression and mutant KRAS expression were also altered via the integration of a short hairpin siRNA against p53 and the overexpression of KRASV12 by lenti-viral integration (38). This cell line is designated HBEC3KT-P53KRAS. Even though these two genetic alterations are seen in 50 and 30% of all NSCLC, respectively, these cell lines do not form tumours when injected into immune-suppressed mice. Others have used virally immortalised HBECs for studies of radiation-induced carcinogenesis. Such models are likely even more oncogenically progressed and the critical genetic alterations that lead to carcinogenesis are likely unknown (41–43). The radioresponse to radiations with different radiation qualities was determined, gene expression profiles of HBEC3KT-P53RAS were characterised in order determine whether signal transduction was altered, and the potential for promotion of anchorage-independent growth was determined for both HBEC3KT-p53RAS and the parental HBEC3KT after low- and high-LET radiation exposure to understand whether cells of a more progressed phenotype may harbour a greater risk for lung carcinogenesis.
Materials and methods
Cell lines and radiations
HBEC3KT is a normal bronchial epithelial cell line immortalised by over-expressing CDK4 and hTERT (39). HBEC3KT cells are non-tumourigenic, form soft agar colonies at a very low frequency (~10−6), and can differentiate into a ciliated epithelium (1,38,39). HBEC3KT-P53KRAS cells were created from HBEC3KT by suppressing p53 expression using an shRNA targeting p53, as well as the stable transfection of mutant K-RASV12. HBEC3KT-P53KRAS cells readily form colonies in soft agar but cannot form tumours in immune-compromised mice (38). The cells were grown in KSFM media and are mycoplasma free when tested using the e-Myco kit (Boca Scientific).
HZE particle radiations were carried out at the NASA Space Radiation Laboratory (NSRL) at Brookhaven National Laboratory. Irradiation with γ-rays was conducted using a 137Cs source within the Department of Biology at Brookhaven National Laboratory. Experiments were repeated at least twice for each radiation type over a 2-year period. The HZE particles used in this study were 56Fe (996.8 MeV/n, LET 151 keV/µ), and 28Si (990 MeV/n, 44keV/µ). Cells were irradiated by HZE particles at 0.5 and 1 Gy, and by γ-rays at 1 and 3 Gy. Cells were trypsinised, collected as a pellet of cells by centrifugation at 10000rpm and flash-frozen with dry ice at 1, 4, 12 and 24h after each radiation. At each time point, a mock cell sample without radiation was also collected as a cell pellet and flash-frozen. An extra mock sample before radiation as a common reference was also collected. Total RNA isolation was conducted at the time of sample processing for microarray analysis using the RNeasy Plus Mini Kit (Qiagen) according to manufacturer’s manual upon return to UT Southwestern.
Clonogenic assay
Relative cell survival was determined as follows. Four hours after irradiation, cells were trypsinised, counted and plated into 100mm dish. Both control and irradiated cells were cultured for 9–14 days, stained with 0.5% crystal violet in 1% formalin-PBS, and only the colonies that contained >50 cells were counted. Survival curves were plotted using a two-component survival fit. The relative biological effectiveness (RBE) was calculated using the ratio of doses between HZE and γ-ray at a survival level of 53%, which was the survival faction at the highest experimental dose (3 Gy) for γ-ray in HBEC3KT-P53RAS cell line. Clonogenic assays were performed over at least two different NSRL campaigns with each experiment performed in triplicate.
Soft agar colony formation assay
HBEC3KT cells (1.8×106 cells/100mm dish) or HBEC3KT-P53KRAS cells (2×105 cells/60mm dish) were plated in soft agar 6 days after radiation at a dose of 1 Gy for 56Fe, 28Si or γ-rays. The cells were suspended in a top layer of 0.3% agar and then were laid on a bottom layer of 0.5% agar. Cells were incubated in soft agar plates at 37°C for 3–4 weeks before stained with p-iodonitrotetrazolium violet (Sigma I8377). The soft agar assays were repeated three times for each radiation type in both cell lines.
RNA labelling and microarray hybridisation
Illumina HumanWG-6 V2 BeadChip (Illumina, Inc.) human whole-genome expression arrays were used in this study. Each RNA sample was amplified using the Ambion TotalPrep RNA amplification kit with biotin UTP (Enzo) labelling. The Ambion Illumina RNA amplification kit uses T7 oligo(dT) primer to generate single-stranded cDNA followed by a second strand synthesis to generate double stranded cDNA which is then column purified. In vitro transcription was used to synthesise a biotin-labelled cRNA using T7 RNA polymerase. The cRNA was then column purified, checked for size and yield using the Bio-Rad Experion system and then 1.5 µg of cRNA was hybridised for each array using standard Illumina protocols with streptavidin-Cy3 (Amersham). Slides were scanned on an Illumina Beadstation for Cy3 fluorescence.
Data processing and visualisation
Expression values were extracted using BeadStudio v3.3. The data was background subtracted and quantile-normalised using the MBCB algorithm (44). Samples were then normalised to the references sample before radiation for each radiation quality at different NSRL runs. For sources of variation analysis and hierarchical clustering, the samples were further normalised to mock samples at each time point. The data then underwent a batch correction process using Partek Genomics Suite software to correct for the batch effect from sample collection during different NSRL campaigns as was done in prior experiments (40). Averaged log2 ratios of different runs were used for the analysis. The hierarchical clustering was done using Spearman absolute value dissimilarity metrics and Ward’s Method as the clustering algorithm.
Significance, gene function and pathway analysis
Significance analysis for differentially expressed genes between un-irradiated HBEC3KT and HBEC3KT-P53KRAS were performed using the SAM algorithm (45). The smallest median FDR used to determine significantly changed genes was 0.0025. Significance analysis for irradiated samples as a function of time was performed using the maSigPro package from Bioconductor (46). To increase statistical power, genes with Illumina Detection P-values >0.05 were excluded in all sample sets. These genes typically had low base-line expression and were considered as background signal. The significance cutoff used was a false discovery rate (FDR) <0.01 and an R-squared threshold of 0.33. For each radiation type, the common genes from the two radiation doses were subjected to a fold change cutoff (>1.3). Gene function, pathway and upstream analysis were performed using Ingenuity Pathway Analysis software. Activation z-score was calculated by Ingenuity Pathway Analysis based on gene expression data and the known up- or down-regulation of function for each gene. A z-score >2 was considered as significantly increased and a z-score <2 as significantly decreased for a particular gene function group.
Results
Radiation resistance in human bronchial epithelial cells induced by mutant KRAS and suppressed p53
We have reported previously that HZE particles resulted in significantly more cell killing than low-LET radiation in normal HBEC3KT cells (40). In the current study, we performed clonogenic assays and compared cell survival in HBEC3KT-P53KRAS and the parent HBEC3KT cell lines after HZE particles and γ-ray irradiation (Figure 1A). HBEC3KT-P53KRAS cells were more radioresistant than HBEC3KT cells irrespective of radiation type (Figure 1A). RBE in HBEC3KT cells was 4.29 for Fe versus γ-ray and 1.34 for Si versus γ-ray. The RBE for HBEC3KT-P53KRAS cells was 4.16 for Fe versus γ-ray and 1.75 for Si versus γ-ray.
Figure 1.
Clonogenic survival and anchorage-independent growth of HBEC3KT and HBEC3KT-P53KRAS cells that were irradiated by 56Fe, 28Si or γ-rays. (A) Survival curves were plotted using a two-component survival fit. Error bars: standard error. For all radiation types, HBEC3KT-P53KRAS cells were more radioresistant than parental HBEC3KT cells. (B) Plot of transformation frequencies that compares colonies grown in soft agar between irradiated and sham-treated HBEC3KT and HBEC3KT-P53KRAS cells, indicated enhanced anchorage independent growth of bronchial epithelial cells with p53 knock-down and mutant K-RAS after γ-ray and HZE radiations. (C) transformation frequencies corrected with survival fractions after radiation. Error bar: standard error. Lines indicate P < 0.05 between groups.
Ionising radiation increased anchorage independent growth in HBEC3KT-P53KRAS cells
There was no significant increase in the number of soft agar clones in HBEC3KT cells after any type of ionising radiation (P > 0.05) 6 days following exposure. The frequencies of transformation remained at background levels with all three radiation types. The frequency of soft agar growth for unirradiated HBEC3KT-P53KRAS cells was 2×10−5 while transformation in the parental HBEC3KT cell lines was 5×10−6. The frequency of soft agar growth for HBEC3KT-P53KRAS cells after irradiation was 5×10−5 after γ-ray, 1×10−4 after Si, 9.9×10−5 after Fe exposures which again was significantly higher than that seen for HBEC3KT (Figure 1B). If corrected for cell survival, the rate of cellular transformation for HBEC3KT becomes 9.5×10−6, 6.2×10−6 and 3.3×10−5 for γ-ray, Si and Fe, respectively. Only the corrected value for Fe irradiation is statistically significantly different from spontaneous (unirradiated) transformation or the other values for irradiated cells. Correcting for survival in HBEC3KT-P53RAS the transformation values become 5.5×10−5, 1.2×10−4 and 2.5×10−4 for γ-ray, Si and Fe, respectively. Transformation was significantly higher after Fe irradiation than Si irradiation after correcting for cell survival (Figure 1C).
Differential gene expression in oncogenically progressed cells
We compared gene expression profiles between HBEC3KT and the oncogenically progressed HBEC3KT-P53KRAS cells. HBEC3KT and HBEC3KT-P53KRAS transcriptome profiles for all probes (48 K) on the Illumina arrays did not significantly separate the two cell lines (Figure 2A). However, a subset of 382 genes selected from SAM analysis demonstrated significant expression changes as shown in principal component analysis (Figure 2B). Gene enrichment analysis identified several sub-functional groups under cell movement, including migration and invasion of cells, with increased functionality (z-score > 2) in HBEC3KT-P53KRAS, while signalling pathway analysis identified ATM and HIF-1α signalling as significantly different in HBEC3KT-P53KRAS cells (Figures 2C and D, Table 1).
Figure 2.
Comparison of gene expression profiles between HBEC3KT and HBEC-P53KRAS cells. (A) Principal component analysis of whole transcriptome profiles showed no clear segregation of the two cell lines. (B) PCA of 382 differentially expressed genes from SAM analysis separate two cell populations. (C) Gene functional categories that significantly changed in HBEC3KT-P53KRAS cells. (D) Signalling pathways that significantly changed in HBEC3KT-P53KRAS cells.
Table 1.
Increased cellular movement activity in HBEC3KT-P53KRAS cells
| Category | Diseases or functions annotation | Predicted activation state | Activation z-score | No. of molecules |
|---|---|---|---|---|
| Cellular movement | Migration of tumour cell lines | Increased | 3.201 | 30 |
| Cellular movement | Migration of cells | Increased | 3.101 | 83 |
| Cellular movement | Cell movement | Increased | 3.093 | 93 |
| Cellular movement | Invasion of cells | Increased | 2.897 | 40 |
| Cellular movement | Chemotaxis of cells | Increased | 2.828 | 22 |
| Cellular movement | Homing of cells | Increased | 2.628 | 25 |
| Cellular movement | Invasion of tumour cell lines | Increased | 2.505 | 34 |
| Cellular movement | Cell movement of tumour cell lines | Increased | 2.452 | 38 |
| Cellular movement | Invasion of tumour cells | Increased | 2.345 | 11 |
| Cellular movement | Leukocyte migration | Increased | 2.343 | 39 |
| Cellular movement | Chemotaxis of lymphocytes | Increased | 2.296 | 8 |
Altered p53, KRAS and HIF-1α signalling pathways in HBEC3KT-P53KRAS cell line
Upstream analysis using gene expression of p53 and KRAS downstream genes indicated activation of KRAS and inhibition of p53 functions. The analysis also predicted activation of several signalling networks that were regulated by p53 and KRAS, including AKT, NFκB, JUN, and MAP2K1 (Figure 3A and supplementary Table 1). Expression analysis suggested inhibition of HIF-1α ubiquitination due to down-regulation of PHD; and inhibition of HIF-1α degradation via p53 and MDM2, whereas HIF-1α activating molecules such as MAP kinases were over-expressed (Figure 3B and E). While there was no statistically significant increase of HIF-1α mRNA, western blotting confirmed higher protein levels of HIF-1α in HBEC3KT-P53KRAS cells (Figure 3C and D). Gene expression of HIF-1α targets which promoted cell migration and proliferation, such as MMP1 and PGF, were increased (Figure 3B and E). HIF-1α signalling has been shown to be involved in anchorage independent growth (47). This molecular evidence supports the increased anchorage independent growth seen in the HBEC3KT-P53KRAS cell line.
Figure 3.

Altered p53, KRAS and HIF-1α signalling pathways in HBEC-P53KRAS cells. (A) Upstream analysis based on gene expression profiles indicated activation of KRAS and suppression of TP53. The function of AKT, NFκB, JUN and MAP2K1 was also activated and was predicated based on the literature and consistent to the result of activated KRAS and suppressed p53. The predicted activation of p53 by KRAS was inconsistent to the finding that p53 was suppressed. (B) Scheme demonstrating the activation of HIF-1α pathway. Red: down-regulation. Green: up-regulation. (C) Western blot showed higher protein level of HIF-1α in HBEC3KT-P53KRAS. (D), Gene expression of HIF-1α showed no significant changes of transcripts between HBEC3KT and HBEC3KT-P53KRAS cells from microarray data. (E) Comparison of gene expression indicating up-regulation of HIF-1α-activating genes (top row) in HBEC3KT-P53KRAS and down-regulation of HIF-1α-degrading genes (bottom row). The boxplot shows median value, 75 percentile and the whisker represents 90 percentile. Dots indicate outliers.
Attenuated p53 pathway responses and distinct expression profiles associated with radiation types
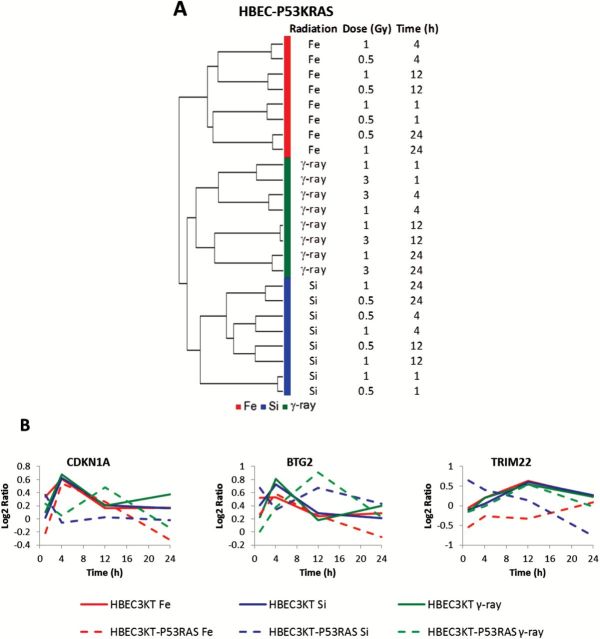
Transcriptome analysis of HBEC3KT-P53KRAS cells after radiation demonstrated distinct expression profiles that were specific to radiation quality (Figure 4A); the same phenomenon as we previously observed in parental HBEC3KT cells (40). As shown in our previous study, CDKN1A, BTG2 and TRIM22 were genes involved in p53 signalling and were consistently up-regulated in HBEC3KT regardless of the radiation types (40). By contrast, in HBEC3KT-P53KRAS cells, the responses of these genes were either weaker or delayed after radiation and showed radiation-type dependence, a condition expected because of the abrogation of p53 expression (Figure 4B).
Figure 4.
Gene expression profiling of HBEC3KT-P53KRAS cells that were irradiated by 56Fe, 28Si or γ-rays. (A) Unsupervised hierarchical clustering indicated distinct response of cells to different radiation qualities. (B) Temporal patterns of genes associated with p53-signalling were consistent and up-regulated in HBEC3KT cell lines after radiation by different radiation types. The up-regulations were weakened with smaller fold changes or delayed peaks and were radiation type dependent in HBEC3KT-P53KRAS cells.
Activation of signalling pathways for cell proliferation and anchorage independent growth in response to radiation in HBEC3KT-P53KRAS cells
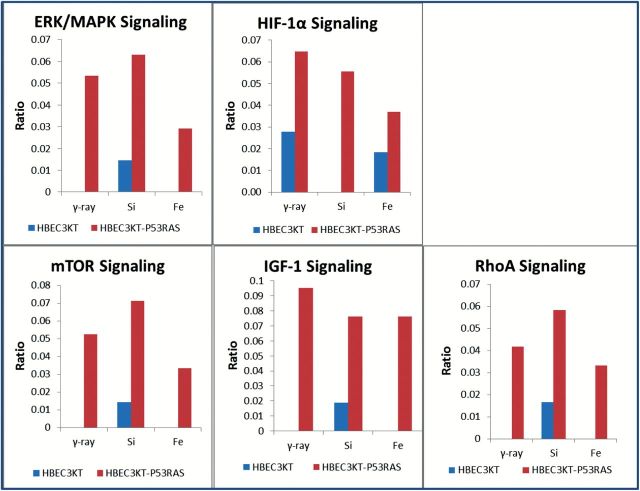
Molecular pathway analysis indicated strong pro-survival and pro-anchorage-independent growth signalling in HBEC3KT-P53KRAS cells in response to radiation. Comparisons of IPA scores suggested that the ERK/MAPK pathway activation is more significant in HBEC3KT-P53KRAS cells than in normal HBEC3KT cells for all three radiation types (Figure 5). The expression of the HIF-1α pathway, which was already aberrant in un-irradiated HBEC3KT-P53KRAS cells (Figure 5), was further augmented after radiation exposure. The mTOR, IGF-1 and RhoA pathways were also altered as a response to all three radiation qualities but were not observed as significantly changed in the parent HBEC3KT cell line (Figure 5) and all of these pathways are known to support anchorage independent growth and cellular transformation.
Fig. 5.
Signaling pathways associated with anchorage independent growth increased significantly in response to all radiation types in HBEC3KT-P53KRAS cells when compared with the syngeneic HBEC3KT cells. The ratio represents the significance of each pathways that changed in response to radiations.
Discussion
The incidence of radiation-induced cancer is affected by multiple factors including radiation dose, dose-rate and the genetic background of the target cells. The cellular transformation rate is also dependent on the length of time after irradiation over which oncogenically progressed cells may become enriched. Loss of p53 function and oncogenic RAS mutation are two genetic aberrations frequently observed in lung cancer. A previous study showed that the combination of p53 suppression and mutant K-RAS results in increased soft agar colony formation and partial loss of contact inhibition but does not result in oncogenesis when implanted into immune-compromised mice.(38) This particular K-RAS mutation, glycine to valine at codon 12, also specifically activates the PI3K and AKT survival pathways which are likely responsible to some degree to the radioresistance seen here. Interestingly, the radiation response as displayed by the transcriptome profiles in HBEC3KT-P53KRAS cell line showed a similar correlation of gene expression profiles and radiation types just as was seen in the normal HBEC3KT cells (40), in that the overall gene expression changes varied by radiation species used, each with very different energy deposition characteristics (radiation quality) and strongly correlated with post-radiation time and less affected by radiation dose. On the other hand, while genes associated with p53 signalling showed consistently up-regulated temporal patterns in HBEC3KT cells after all three radiation types these genes showed attenuated responses in HBEC3KT-P53KRAS cells with smaller fold differences, delayed maxima, and radiation type-dependent patterns, as would be expected because of suppression of p53 expression. Other pro-survival and proliferation pathways such as ERK/MAPK pathway were more significantly activated in HBEC3KT-P53KRAS cells than normal HBEC3KT cells, again consistent with the radioresistance of HBEC3KT-P53KRAS in response to both low- and high-LET radiations.
The spontaneous cellular transformation frequency in the HBEC3KTp53RAS cell line is 4-fold higher than that of the parental HBEC3KT cell line. The rate of cellular transformation in surviving cells is 10-fold higher after γ-ray exposure and 20-fold higher after HZE exposure. This is contrasted by the near complete lack of response seen in the surviving HBEC3KT cells.
Gene expression profiling supports the increased rate of cellular transformation in the HBEC3KTP53RAS cell line via the identification of cell movement as the most enriched molecular function followed by increased overrepresentation of cell migration and cell invasiveness. These findings supported the aggressive phenotype of the HBEC-P53KRAS cell line. Upstream analysis based gene expression data confirmed the activation of KRAS activity and suppression of p53. It also predicted activation of AKT and NFκB activities which indicated that HBEC3KT-P53KRAS cells were more proliferative than their parent cells. HIF-1α has been shown to play a central role in promoting anchorage-independent growth (47). Indeed, pathway analysis identified HIF-1α ubiquitination and degradation as suppressed and upon further examination, HIF-1α protein, which is usually undetectable in cells in normal oxygen environment because of fast ubiquitination and degradation, was found to be elevated in the HBEC3KT-P53RAS cells. This finding was bolstered by low levels of expression of the PHD, p53 (already knocked down) and MDM2 genes (Figure 3E). Expression analysis also revealed increased MAPK levels which likely phosphorylate the downstream HIF-1α protein and the HIF-1α transcriptional targets MMP and VEGF families, which in turn facilitate cell migration and proliferation, were up-regulated in surviving HBEC3KT-P53KRAS cells (Figure 3E). The overall results support an increased activity of HIF-1α due to phosphorylation of HIF-1α protein and inhibition of its degradation, but not by an apparent enhancement of transcription.
Several signalling pathways related to anchorage-independent growth were also activated only in HBEC3KT-P53KRAS cells after radiation, while not activated in the normal HBEC3KT cells. These pathways included the mTOR, IGF-1 and RhoA signalling pathways. A previous study showed that mTOR is essential for phosphorylation of eukaryotic translation initiation factor subunit I (eIF3i), which induces anchorage independent growth when over-expressed (48). IGF-1 has been shown, together with mutant KRAS, to induce anchorage independent growth through PI3K and NF-ΚB signalling (49), while inhibition of RhoA activity was shown to reduce the efficiency of Ras-induced anchorage independent growth (50). Lastly, the ERK/MAPK pathway is critical for extracellular signalling and adhesive-dependent growth (51). Most of these pathways were not significantly different when comparing un-irradiated HBEC3KT and HBEC3KT-P53KRAS cells (data not shown), indicating that radiation per se promoted anchorage independent growth. This is confirmed with soft agar assays in surviving cells where there was a significant increase in soft agar colonies in HBEC3KT-P53KRAS cells after radiation but particularly so after HZE exposures. Not seen was a radiation quality-dependent quantitative change in pathway representation. Pathway analysis identified these anchorage-independent growth pathways irrespective of radiation type. However, the extent to which pathway over-representation led to soft-agar clone formation was radiation quality dependent (Figure 1). The lack of a radiation quality-dependent quantitative change in pathway representation was not unexpected given the methodology underlying enrichment analysis as well as the complex nature of pathway regulation which involves post-translational modifications, which are not measured by transcriptome profiling, and gene enrichment analysis as performed in this study.
There are a number of questions yet to be resolved. For instance, is the level of KRAS mutant expression a factor? Sato et al. (1) suggests that this is the case. Is radiation sufficient to process HBEC3KT-P53RAS cells to oncogenesis? For Sato et al. (1) who did not examine radiation as an agent of oncogenic progression, overexpression of MYC was required as was the need for transformed (anchorage independence) HBEC3KT-P53RAS cells to undergo epithelial to mesenchymal transition. We are addressing these questions, as well as others, for both the HBEC3KT as well as HBEC3KT-P53RAS cell lines in ongoing studies of low and high LET radiation-induced cellular transformation and oncogenesis. Ultimately, we expect to understand the alterations necessary as well as the time scale over which these changes occur in normal and oncogenically progressed human bronchial epithelial cells.
In summary, our data clearly show that in oncogenically progressed human bronchial epithelial cells that have been altered to mimic and perhaps phenocopy hyperplastic/dysplastic epithelial cells in vivo, that is, cells where p53 expression has been suppressed and where a mutant K-RAS has been over-expressed, are more radioresistant. Furthermore, these cells are more susceptible to further oncogenic progression as expressed by anchorage independent growth, particularly after exposure to high LET radiations like those found in the space environment and indeed, for 28Si exposures at an LET seen in the clinical use of 12C particles for radiotherapy when a spread out Bragg Peak is utilised. (Virtually all 12C radiotherapy patients have been treated in this manner.) And it is noted that even though the LET for 28Si at 990 MeV/n is equivalent to that of 400 MeV/n 12C, their biological effectiveness may not be. Our results also showed that p53 suppression and mutant K-RAS activation results in radioresistance and that the gene expression profiles of HBEC3KT-p53RAS cells are specific to the quality of the radiation used, but are unique from that seen in the parental cell line. Furthermore, ionising radiation and especially HZE particle radiation exposure increased anchorage-independent growth in HBEC3KT-P53KRAS, whereas little to no increase was seen in the parental cells. Signal transduction analysis in HBEC3KT-p53RAS cells identified the activation of molecular signalling pathways that are associated with anchorage-independent cell growth, cell proliferation and cancer. These data suggest an increased risk for lung cancer for individuals who may have such genetic alterations within the epithelial cells of the lung and who receive low LET radiations. The risk for lung cancer if exposed to HZE particles may be higher still. These data support the notion that the screening of smokers in large numbers should be wary of the risk for inducing lung cancers (30), and that there is the possibility of increased risk for lung cancers after HZE particle radiotherapy. One could also support the concept of astronaut screening for markers of hyperplasia or dysplasia in order to estimate potential increased risk for long-term space missions outside the protection of the earth’s magnetosphere.
Supplementary data
Supplementary Table 1 is available at Mutagenesis Online.
Acknowledgements
We appreciate the efforts of the Brookhaven National Laboratory Medical Branch liaison team, led by Peter Guida, PhD, and the NSRL Physics team led by Adam Rusek, PhD. We also thank Richard Sautkulis and Paul Wilson in the BNL Biology Department for γ-ray irradiations. We would like to acknowledge the assistance of the Genomics Shared Resource at the Harold C. Simmons Cancer Center. This work was supported by NASA NSCOR (NNJ05HD36G, NNX11AC54G); and the NCI Cancer Center Support Grant (1P30 CA142543).
References
- 1. Sato M., Larsen J. E., Lee W., et al. (2013) Human lung epithelial cells progressed to malignancy through specific oncogenic manipulations. Mol. Cancer Res., 11, 638–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ding L., Getz G., Wheeler D. A., et al. (2008) Somatic mutations affect key pathways in lung adenocarcinoma. Nature, 455, 1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Imielinski M., Berger A. H., Hammerman P. S., et al. (2012) Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell, 150, 1107–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rudin C. M., Durinck S., Stawiski E. W., et al. (2012) Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat. Genet., 44, 1111–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Govindan R., Ding L., Griffith M., et al. (2012) Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell, 150, 1121–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cardis E., Vrijheid M., Blettner M., et al. (2007) The 15-Country Collaborative Study of Cancer Risk among Radiation Workers in the Nuclear Industry: estimates of radiation-related cancer risks. Radiat. Res., 167, 396–416. [DOI] [PubMed] [Google Scholar]
- 7. Preston D. L., Ron E., Tokuoka S., Funamoto S., Nishi N., Soda M., Mabuchi K., Kodama K. (2007) Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat. Res., 168, 1–64. [DOI] [PubMed] [Google Scholar]
- 8. Preston D. L., Shimizu Y., Pierce D. A., Suyama A., Mabuchi K. (2003) Studies of mortality of atomic bomb survivors. Report 13: Solid cancer and noncancer disease mortality: 1950–1997. Radiat. Res., 160, 381–407. [DOI] [PubMed] [Google Scholar]
- 9. Sokolnikov M. E., Gilbert E. S., Preston D. L., Ron E., Shilnikova N. S., Khokhryakov V. V., Vasilenko E. K., Koshurnikova N. A. (2008) Lung, liver and bone cancer mortality in Mayak workers. Int. J. Cancer, 123, 905–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jacob P., Meckbach R., Kaiser J. C., Sokolnikov M. (2010) Possible expressions of radiation-induced genomic instability, bystander effects or low-dose hypersensitivity in cancer epidemiology. Mutat. Res., 687, 34–39. [DOI] [PubMed] [Google Scholar]
- 11. Heidenreich W. F., Jacob P., Paretzke H. G., Cross F. T., Dagle G. E. (1999) Two-step model for the risk of fatal and incidental lung tumors in rats exposed to radon. Radiat. Res., 151, 209–217. [PubMed] [Google Scholar]
- 12. Ullrich R. L. (1983) Tumor induction in BALB/c female mice after fission neutron or gamma irradiation. Radiat. Res., 93, 506–515. [PubMed] [Google Scholar]
- 13. Ullrich R. L., Jernigan M. C., Adams L. M. (1979) Induction of lung tumors in RFM mice after localized exposures to X rays or neutrons. Radiat. Res., 80, 464–473. [PubMed] [Google Scholar]
- 14. Ullrich R. L., Storer J. B. (1979) Influence of gamma irradiation on the development of neoplastic disease in mice. III. Dose-rate effects. Radiat. Res., 80, 325–342. [PubMed] [Google Scholar]
- 15. Ullrich R. L., Jernigan M. C., Storer J. B. (1977) Neutron carcinogenesis. Dose and dose-rate effects in BALB/c mice. Radiat. Res., 72, 487–498. [PubMed] [Google Scholar]
- 16. Belli M., Campa A., Dini V., Esposito G., Furusawa Y., Simone G., Sorrentino E., Tabocchini M. A. (2006) DNA fragmentation induced in human fibroblasts by accelerated (56)fe ions of differing energies. Radiat. Res., 165, 713–720. [DOI] [PubMed] [Google Scholar]
- 17. Prise K. M., Ahnström G., Belli M., et al. (1998) A review of dsb induction data for varying quality radiations. Int. J. Radiat. Biol., 74, 173–184. [DOI] [PubMed] [Google Scholar]
- 18. Taucher-Scholz G., Kraft G. (1999) Influence of radiation quality on the yield of DNA strand breaks in SV40 DNA irradiated in solution. Radiat. Res., 151, 595–604. [PubMed] [Google Scholar]
- 19. Blakely E. A., Tobias C. A., Yang T. C., Smith K. C., Lyman J. T. (1979) Inactivation of human kidney cells by high-energy monoenergetic heavy-ion beams. Radiat. Res., 80, 122–160. [PubMed] [Google Scholar]
- 20. Borek C., Hall E. J., Rossi H. H. (1978) Malignant transformation in cultured hamster embryo cells produced by X-rays, 460-keV monoenergetic neutrons, and heavy ions. Cancer Res., 38, 2997–3005. [PubMed] [Google Scholar]
- 21. Cox R., Thacker J., Goodhead D. T., Munson R. J. (1977) Mutation and inactivation of mammalian cells by various ionising radiations. Nature, 267, 425–427. [DOI] [PubMed] [Google Scholar]
- 22. Eguchi K., Inada T., Yaguchi M., Satoh S., Kaneko I. (1987) Induction and repair of DNA lesions in cultured human melanoma cells exposed to a nitrogen-ion beam. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med., 52, 115–123. [DOI] [PubMed] [Google Scholar]
- 23. Raju M. R., Blakely E., Howard J., Lyman J. T., Kalofonos D. P., Martins B., Yang C. H. (1976) Letter: Human cell survival as a function of depth for a high-energy neon ion beam. Radiat. Res., 65, 191–194. [PubMed] [Google Scholar]
- 24. Skarsgard L. D., Kihlman B. A., Parker L., Pujara C. M., Richardson S. (1967) Survival, chromosome abnormalities, and recovery in heavy-ion and x-irradiated mammalian cells. Radiat. Res. Suppl., 7, 208–221. [PubMed] [Google Scholar]
- 25. Suzuki M., Watanabe M., Suzuki K., Nakano K., Kaneko I. (1989) Neoplastic cell transformation by heavy ions. Radiat. Res., 120, 468–476. [PubMed] [Google Scholar]
- 26. Tobias C. A., Blakely E. A., Chang P. Y., Lommel L., Roots R. (1984) Response of sensitive human ataxia and resistant T-1 cell lines to accelerated heavy ions. Br. J. Cancer. Suppl., 6, 175–185. [PMC free article] [PubMed] [Google Scholar]
- 27. Saghir Z., Dirksen A., Ashraf H., et al. (2012) CT screening for lung cancer brings forward early disease. The randomised Danish Lung Cancer Screening Trial: status after five annual screening rounds with low-dose CT. Thorax, 67, 296–301. [DOI] [PubMed] [Google Scholar]
- 28. Aberle D. R., DeMello S., Berg C. D., et al. ; National Lung Screening Trial Research Team. (2013) Results of the two incidence screenings in the National Lung Screening Trial. N. Engl. J. Med., 369, 920–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. National Lung Screening Trial Research Team. Aberle D. R., Adams A. M., et al. (2011) Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med., 365, 395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Berrington de Gonzalez A., Kim K. P., Berg C. D. (2008) Low-dose lung computed tomography screening before age 55: estimates of the mortality reduction required to outweigh the radiation-induced cancer risk. J. Med. Screen., 15, 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barbacid M. (1987) ras genes. Annu. Rev. Biochem., 56, 779–827. [DOI] [PubMed] [Google Scholar]
- 32. Bos J. L. (1989) ras oncogenes in human cancer: a review. Cancer Res., 49, 4682–4689. [PubMed] [Google Scholar]
- 33. Sekido Y., Fong K. M., Minna J. D. (2003) Molecular genetics of lung cancer. Annu. Rev. Med., 54, 73–87. [DOI] [PubMed] [Google Scholar]
- 34. Slaughter D. P., Southwick H. W., Smejkal W. (1953) Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer, 6, 963–968. [DOI] [PubMed] [Google Scholar]
- 35. Chai H., Brown R. E. (2009) Field effect in cancer-an update. Ann. Clin. Lab. Sci., 39, 331–337. [PubMed] [Google Scholar]
- 36. Franklin W. A., Gazdar A. F., Haney J., Wistuba I. I., La Rosa F. G., Kennedy T., Ritchey D. M., Miller Y. E. (1997) Widely dispersed p53 mutation in respiratory epithelium. A novel mechanism for field carcinogenesis. J. Clin. Invest., 100, 2133–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Walser T., Cui X., Yanagawa J., Lee J. M., Heinrich E., Lee G., Sharma S., Dubinett S. M. (2008) Smoking and lung cancer: the role of inflammation. Proc. Am. Thorac. Soc., 5, 811–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sato M., Vaughan M. B., Girard L., et al. (2006) Multiple oncogenic changes (K-RAS(V12), p53 knockdown, mutant EGFRs, p16 bypass, telomerase) are not sufficient to confer a full malignant phenotype on human bronchial epithelial cells. Cancer Res., 66, 2116–2128. [DOI] [PubMed] [Google Scholar]
- 39. Ramirez R. D., Sheridan S., Girard L., et al. (2004) Immortalization of human bronchial epithelial cells in the absence of viral oncoproteins. Cancer Res., 64, 9027–9034. [DOI] [PubMed] [Google Scholar]
- 40. Ding L. H., Park S., Peyton M., Girard L., Xie Y., Minna J. D., Story M. D. (2013) Distinct transcriptome profiles identified in normal human bronchial epithelial cells after exposure to γ-rays and different elemental particles of high Z and energy. BMC Genomics, 14, 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hei T. K., Piao C. Q., Sutter T., Willey J. C., Suzuki K. (1996) Cellular and molecular alterations in human epithelial cells transformed by high LET radiation. Adv. Space Res., 18, 137–148. [DOI] [PubMed] [Google Scholar]
- 42. Hei T. K., Zhao Y. L., Roy D., Piao C. Q., Calaf G., Hall E. J. (2001) Molecular alterations in tumorigenic human bronchial and breast epithelial cells induced by high LET radiation. Adv. Space Res., 27, 411–419. [DOI] [PubMed] [Google Scholar]
- 43. Suzuki M., Kase Y., Yamaguchi H., Kanai T., Ando K. (2000) Relative biological effectiveness for cell-killing effect on various human cell lines irradiated with heavy-ion medical accelerator in Chiba (HIMAC) carbon-ion beams. Int. J. Radiat. Oncol. Biol. Phys., 48, 241–250. [DOI] [PubMed] [Google Scholar]
- 44. Ding L. H., Xie Y., Park S., Xiao G., Story M. D. (2008) Enhanced identification and biological validation of differential gene expression via Illumina whole-genome expression arrays through the use of the model-based background correction methodology. Nucleic Acids Res., 36, e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tusher V. G., Tibshirani R., Chu G. (2001) Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. U. S. A., 98, 5116–5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Conesa A., Nueda M. J., Ferrer A., Talón M. (2006) maSigPro: a method to identify significantly differential expression profiles in time-course microarray experiments. Bioinformatics, 22, 1096–1102. [DOI] [PubMed] [Google Scholar]
- 47. Jinka R., Kapoor R., Pavuluri S., Raj A. T., Kumar M. J., Rao L., Pande G. (2010) Differential gene expression and clonal selection during cellular transformation induced by adhesion deprivation. BMC Cell Biol., 11, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ahlemann M., Zeidler R., Lang S., Mack B., Münz M., Gires O. (2006) Carcinoma-associated eIF3i overexpression facilitates mTOR-dependent growth transformation. Mol. Carcinog., 45, 957–967. [DOI] [PubMed] [Google Scholar]
- 49. Yin E., Nelson D. O., Coleman M. A., Peterson L. E., Wyrobek A. J. (2003) Gene expression changes in mouse brain after exposure to low-dose ionizing radiation. Int. J. Radiat. Biol., 79, 759–775. [DOI] [PubMed] [Google Scholar]
- 50. Fleming Y. M., Ferguson G. J., Spender L. C., Larsson J., Karlsson S., Ozanne B. W., Grosse R., Inman G. J. (2009) TGF-beta-mediated activation of RhoA signalling is required for efficient (V12)HaRas and (V600E)BRAF transformation. Oncogene, 28, 983–993. [DOI] [PubMed] [Google Scholar]
- 51. Howe A. K., Aplin A. E., Juliano R. L. (2002) Anchorage-dependent ERK signaling—mechanisms and consequences. Curr. Opin. Genet. Dev., 12, 30–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.