Abstract
Association between coffee consumption and gastric cancer risk remains controversial. Hence, we performed a meta-analysis to investigate and quantify the potential dose–response association between long-term coffee consumption and risk of gastric cancer.
Pertinent studies were identified by searching PubMed and Embase from January 1996 through February 10, 2015 and by reviewing the reference lists of retrieved publications. Prospective cohort studies in which authors reported effect sizes and corresponding 95% confidence intervals (CIs) of gastric cancer for 3 or more categories of coffee consumption were eligible. Results from eligible studies were aggregated using a random effect model. All analyses were carried out using the STATA 12.0 software.
Nine studies involving 15 independent prospective cohorts were finally included. A total of 2019 incident cases of gastric cancer were ascertained among 1,289,314 participants with mean follow-up periods ranging from 8 to 18 years. No nonlinear relationship of coffee consumption with gastric cancer risk was indentified (P for nonlinearity = 0.53; P for heterogeneity = 0.004). The linear regression model showed that the combined relative risk (RR) of every 3 cups/day increment of total coffee consumption was 1.07 (95% CI = 0.95–1.21). Compared with the lowest category of coffee consumption, the RR of gastric cancer was 1.18 (95% CI = 0.90–1.55) for the highest (median 6.5 cups/day) category, 1.06 (95% CI = 0.85–1.32) for the second highest category (median 3.5 cups/day), and 0.97 (95% CI = 0.79–1.20) for the third highest category (median 1.5 cups/day). Subgroup analysis showed an elevated risk in the US population (RR = 1.36, 95% CI = 1.06–1.75) and no adjustment for smoking (RR = 1.67, 95% CI = 1.08–2.59) for 6.5 cups/day.
Current evidence indicated there was no nonlinear association between coffee consumption and gastric cancer risk. However, high coffee consumption (more than 6.5 cups/day) might increase the risk of gastric cancer in the US population. More high quality studies were warranted to further investigate the association.
INTRODUCTION
Coffee is one of the most frequently consumed beverages around the world. Because of its popularity, to explore the association between long-term coffee consumption and chronic disease risk has important public health implications. Compounds in coffee, complex mixture of more than a thousand chemicals, may have either beneficial or unfavorable effects on human body,1 and animal studies suggest that coffee can both stimulate and restrain tumors depending on different animal species.2–4 These contrasting effects parallel the results of previous observational studies that revealed no definite effect of coffee ingestion on cancer. Several meta-analyses showed that coffee consumption may decrease risk of certain cancers such as prostate cancer, colorectal cancer, and liver cancer but maternal coffee consumption during pregnancy may increase the risk of childhood acute leukemia.5–8 However, the relationship between coffee consumption and risk of gastric cancer is different among individual studies and remains controversial.
In 2006, the result of a meta-analysis did not support an association between coffee consumption and cancer risk.9 Another drawback of the previous meta-analysis is the inclusion of case–control studies that are prone to recall and selection bias. In recent years, 2 meta-analyses were performed based on cohort studies with controversial results.10–11 The previous meta-analyses9–11 mainly focused on the relationship between the highest coffee consumption level and either the lowest coffee consumption level or nondrinkers. However, the range of coffee consumption and the cut-offs for the categories differed among studies. Therefore, the shape of the association remains uncertain. A population-based cohort study of Swedish women found a significantly elevated risk of 4 or more cups/day coffee consumption as compared with nondrinkers after multivariate adjustment.12 Another cohort study also found an elevated risk of 4 or more cups/day coffee consumption as compared with nondrinkers in United States (US) for gastric cardia cancer.13 A Japanese cohort study reported a 2.54 times elevated risk of coffee consumption among women compared to rare/never intake of caffeinated coffee.14 However, a Finland cohort study observed a reduced risk of 3 to 6 cups/day coffee consumption as compared with nondrinkers in men15 and The Singapore Chinese Health Study found a lower risk of 1 cup/day coffee consumption compared to nondrinkers in women.16
Therefore, considering the persisting controversy on this issue, it is necessary to summarize the results from individual prospective studies of the association between coffee consumption and gastric cancer risk. To examine the potential dose–response association of long-term coffee consumption with risk of gastric cancer, we conducted this dose–response meta-analysis of coffee consumption and incidence of gastric cancer.
METHODS
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement17 in reporting this meta-analysis. The meta-analysis was approved by the ethics committee of Taihe Hospital, Hubei University of Medicine.
Eligibility Criteria
Studies were eligible for inclusion in the present meta-analysis if they met the following criteria: the exposure of interest was coffee consumption, including total coffee, decaffeinated coffee, or caffeinated coffee; the study design was prospective cohort or nested case–control; the outcome was risk of gastric cancer; the researchers presented relative risks (RRs), hazard ratios (HRs), or odds risks (ORs) with 95% confidence intervals (CIs) for 3 or more quantitative categories of coffee ingestion. We excluded animal studies, commentaries, letters, reviews, case–control studies, cross-sectional studies, clinical studies, and studies that assessed other associations.
Search Strategy
We searched the PubMed and Embase databases for prospective studies that evaluated the association between coffee consumption and risk of gastric cancer between January 1966 and February 10, 2015. The computer-based searches included the key words “coffee,” “caffeine,” “stomach cancer,” “cardia cancer,” and “gastric cancer.” In addition, the “coffee” and “stomach neoplasms” of Medical Subject Headings terms were used. Reference lists of retrieved papers were manually scanned for further relevant additional studies. We limited the search to studies that were carried out on humans and were written in English.
Data Extraction
Two investigators (SBZ and HW) independently screened all papers by title or abstract and then by full content evaluation. Any discrepancy between the 2 researchers was solved by discussion. Then 1 author assessed study eligibility and extracted the data, and the other author independently double-checked the available data. The following data were extracted from each study: first author's surname, publication year, duration of follow-up period, geographical location, gender, age at baseline, number of participants/person-years of follow-up, number of gastric cancer events, coffee consumption categories, mean/median coffee consumption in each categories, covariates adjusted for in the multivariable analysis, the RRs and corresponding 95% CIs for all categories of coffee consumption, and any other information of study quality assessment. If researches based on the same cohort were published multiple times, only the most informative paper was included. Unadjusted results were extracted when no adjusted results were provided.18 For 4 studies that presented data separately for men and women15–16,19 or for gastric cardia cancer and gastric noncardia cancer13 we considered the analyses for each gender or cohort as independent reports and extracted data separately.
To carry out a dose–response meta-analysis, we assigned the median coffee ingestion in each category of consumption to the relevant RRs for each study. If medians were not presented, we used means instead. The midpoint of the upper and lower boundaries in each category was used to estimate the median of consumption category when medians and means were not presented. For highest consumption category, the assigned median was 25% higher than the lower boundary of that category if the upper boundary of highest consumption category was not reported. For lowest consumption category, the assigned median value was a half of the upper boundary of that category if the lower boundary of the lowest category was not supplied.
Methodological Quality Assessment
Newcastle–Ottawa scale (NOS) for cohort studies20 was used to assess the methodological quality of the included studies. The scale ranges from 0 to 9 points: 4 for selection of participants, 2 for comparability between groups, and 3 for assessment of outcome; with higher scores indicating higher study quality. We assigned points of 0 to 3, 4 to 6, and 7 to 9 for low, moderate, and high quality of studies, respectively.
Statistical Analysis
HRs were used as the common measure of association in all studies, which were equivalent to RRs in cohort studies. To analyze the trend of coffee ingestion and risk of gastric cancer, we performed both semiparametric and parametric methods as described in the previous meta-analysis.21 For the semiparametric method, 4 coffee ingestion groups were yielded – mean lowest, third highest, second highest, and highest. The lowest and highest coffee consumption categories corresponded to the lowest and highest group for each included reports.22,23 For studies with 3 coffee consumption categories, the middle category corresponded to either the third or the second highest group. For studies with 4 exposure categories, the third and second highest categories, respectively, corresponded to the third and second highest groups. If the report had more than 4 coffee consumption categories, third and second highest groups were chosen based on their similarity of the quantity of coffee consumption in that category to the 2 groups of the meta-analysis. A random-effects model was used to pool the RRs with corresponding 95% CIs of gastric cancer.24 Study heterogeneity in each groups was tested by Cochrane Q metric and I2 test, with P < 0.1 or I2 > 50% indicating statistical significant18,21,22,24,25
For the parametric method, a fixed effect dose–response meta-analysis was conducted, using the method recommended by Greenland and Longnecker.26 We used generalized least squares models with the maximum likelihood method to evaluate the coefficient for each study. If the P value for the goodness of fit/heterogeneity was less than 0.05, a random-effects generalized linear model was applied. Additionally, we investigated potential nonlinearity or curve linearity in the association between coffee consumption and risk of gastric cancer by a random-effects restrict cubic spline model with 3 knots at percentiles 1%, 25%, 50%, 75%, and 99% of the distribution.27 A P for nonlinearity or curve linearity was calculated by testing the null hypothesis that the coefficient of the second spline is equal to zero.
We did stratified analyses and meta-regression according to study location (US, Europe, and Asia), sex (men and women), study quality, and covariate adjustment (smoking and alcohol). We did not perform stratified analysis for type of coffee (caffeinated and decaffeinated) and type of gastric cancer due to insufficient data. We used Begg funnel plots and Egger tests to detect potential publication bias.28 All analyses were conducted with Stata 12.0 (StataCorp, College Station, TX) statistical software.
RESULTS
Study Search and Characteristics
The initial search identified 113 potentially relevant papers from the PubMed and 130 articles from the Embase. After screening titles and abstracts, we identified 45 studies for further evaluation (Figure 1). Finally, 9 studies12–13,15–16,19,29–32 were included in the meta-analysis. A manual search of reference cited by these articles did not identify new eligible reports. Among these 9 studies, 5 studies provided data separately for men and women,15,16,19,29,31 and 1 study for gastric cardia cancer and gastric noncardia cancer,13 and we treated them as independent studies. Therefore, we included 15 comparisons in the meta-analysis.
FIGURE 1.
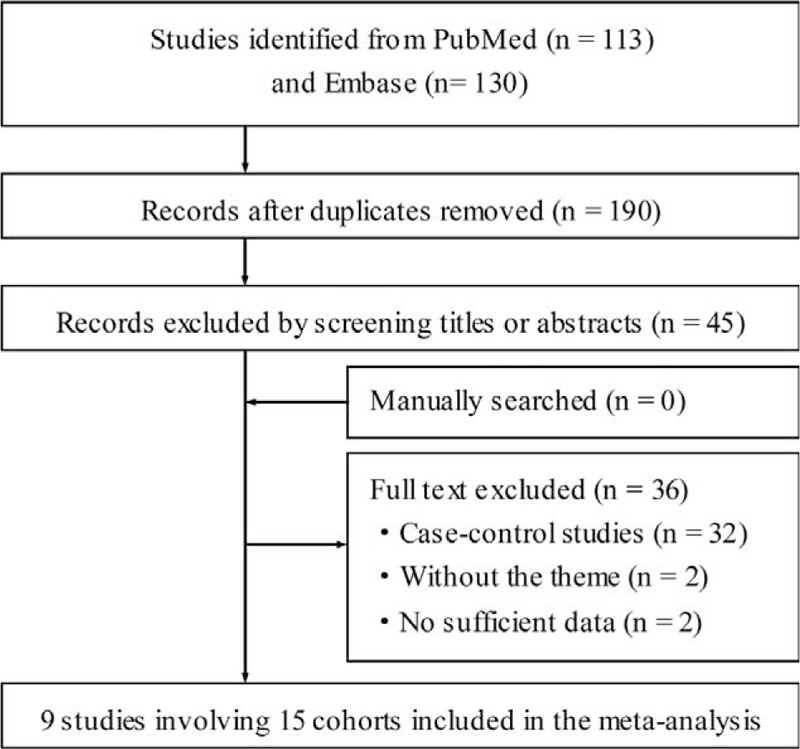
Flow diagram of literature search and study selection.
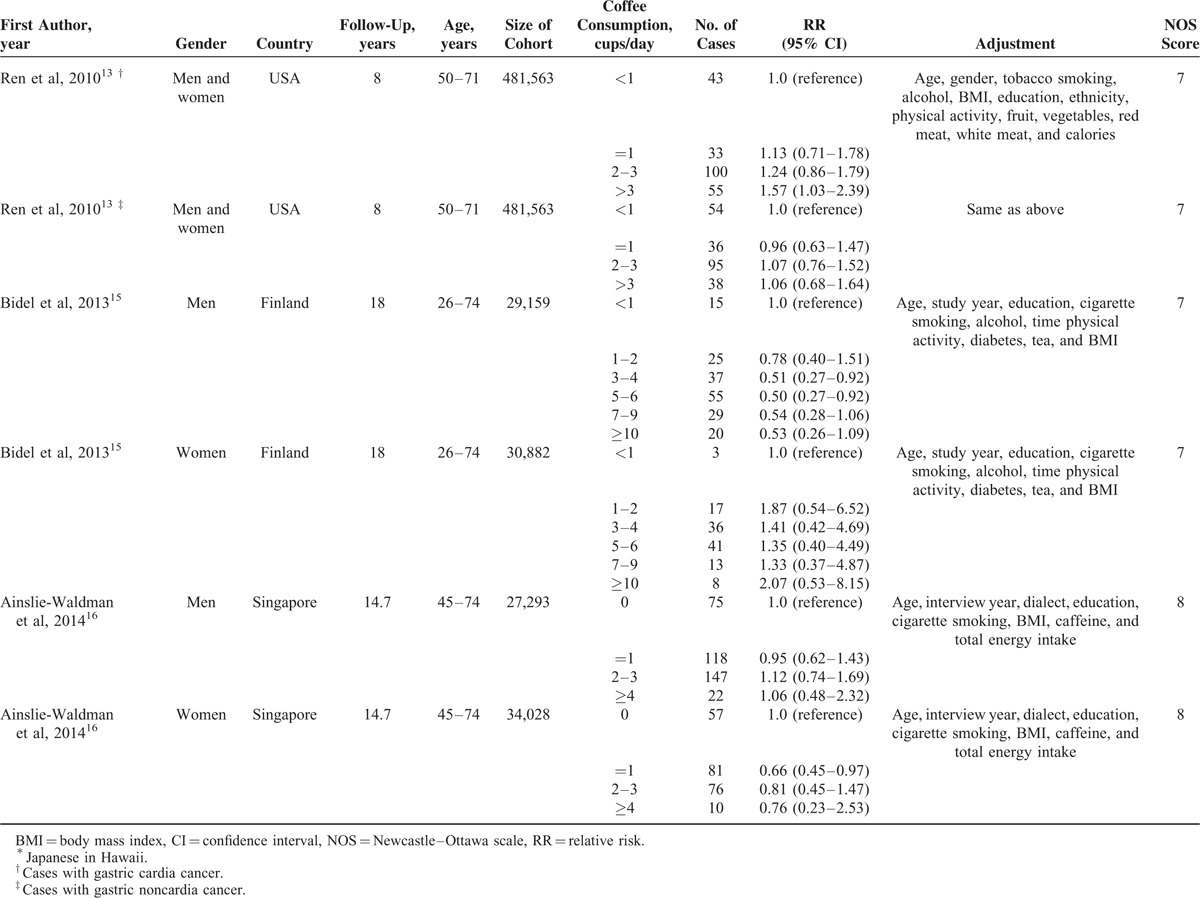
Characteristics of the included studies were shown in Table 1 . All 9 prospective cohorts with 15 studies in participants who were free of self-reported gastric cancer at baseline (total n = 1,289,314). Among the participants, 2019 incident cases of gastric cancer occurred during follow-up periods ranging from 8 to 18 years, with a median follow-up of 13.3 years. The 9 prospective cohort studies were published between 1986 and 2014. Of them, 3 studies were conducted in the US (1 for Japanese in Hawaii), 5 in Europe (Norway, Finnish, and Swedish), and 1 in Asia (Singapore Chinese). Both men and women were included in 7 studies, 1 study consisted men only, and 1 study consisted women only. In all studies, dietary intake was assessed by food frequency questionnaires (FFQs), but only 2 studies were validated against multiple day diet records or 24 hour recalls.12,16 No study distinguished caffeinated coffee and decaffeinated coffee. The quality assessment of all included studies was shown in Table 2. The mean NOS score was 6.7 (of a possible 9 points), suggesting a moderate quality of the reports included in the present meta-analysis. The points mainly lost in ascertainment of exposure and the percentage of loss to follow-up that 3 studies only used FFQs without further validation and all the studies did not address the loss to follow-up rate. The major adjusted confounders included age, smoking, education, body mass index (BMI), alcohol consumption, and physical activity. However, infection of the helicobacter pylori was unadjusted in all the included studies. The RRs and corresponding 95% CIs of 3 studies with 5 cohorts were estimated using data available in reports.
TABLE 1.
Characteristics of Prospective Cohort Studies of Coffee Consumption and Gastric Cancer Risk Included in a Meta-Analysis
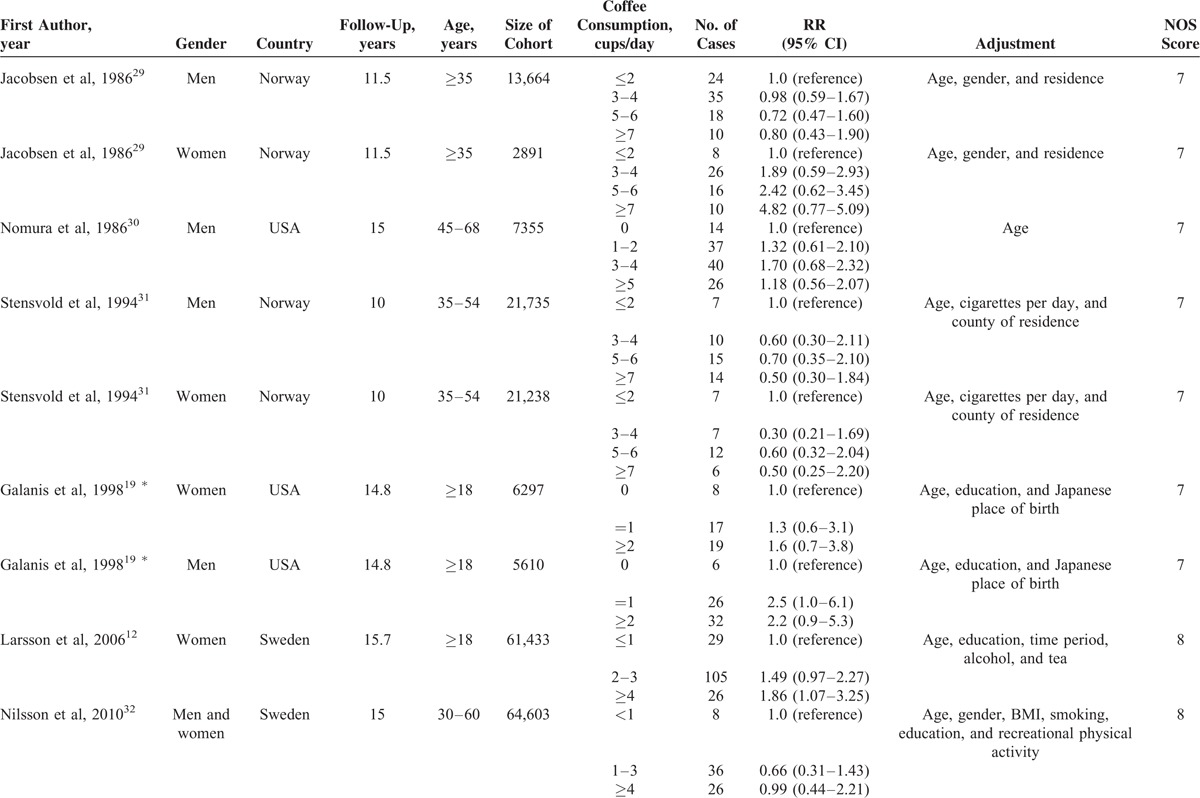
TABLE 1 (Continued).
Characteristics of Prospective Cohort Studies of Coffee Consumption and Gastric Cancer Risk Included in a Meta-Analysis
Total Coffee Consumption and Gastric Cancer
Figure 2 summarizes the results of different levels of total coffee consumption compared with the lowest category. The pooled RR for incident gastric cancer for individuals in the highest category of coffee consumption (median consumption 6.5 cups/day) was 1.18 (95% CI = 0.90–1.55, I2 = 50.9%, P for heterogeneity = 0.01) compared with the lowest category. The summary RRs were 1.06 (95% CI = 0.85–1.32, I2 = 42.8%, P for heterogeneity = 0.06) for the second highest category (3.5 cups/day) and 0.97 (95% CI = 0.79–1.20, I2 = 37.6%, P for heterogeneity = 0.08) for the third highest category (1.5 cup/day) of coffee consumption. The between-study heterogeneity was moderate to high among the studies.
FIGURE 2.
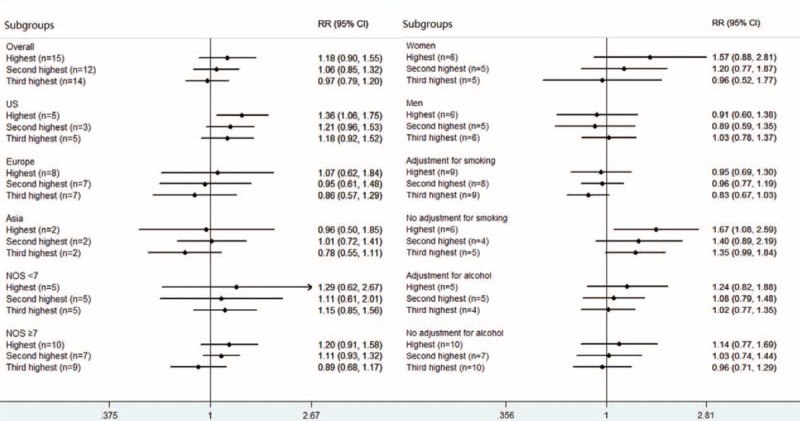
Forest plot of the association between highest (median 6.5 cups/day), second highest (3.5 cups/day), and third highest (1.5 cup/day) level of coffee consumption and risk of gastric cancer compared to the lowest level (0 cup/day).
Dose–Response Analyses of Total Coffee Consumption and Gastric Cancer
In a random-effects cubic spline model that included all reports, we did not observe evidence suggesting any curve or nonliner relation between coffee consumption and risk of gastric cancer (P for nonlinearity = 0.53; P for heterogeneity = 0.004; Figure 3). We then applied a linear regression model to fit the association. The combined RR of every 3 cups/day increment of total coffee consumption was 1.07 (95% CI = 0.95–1.21, P for heterogeneity = 0.004; Figure 4)
FIGURE 3.
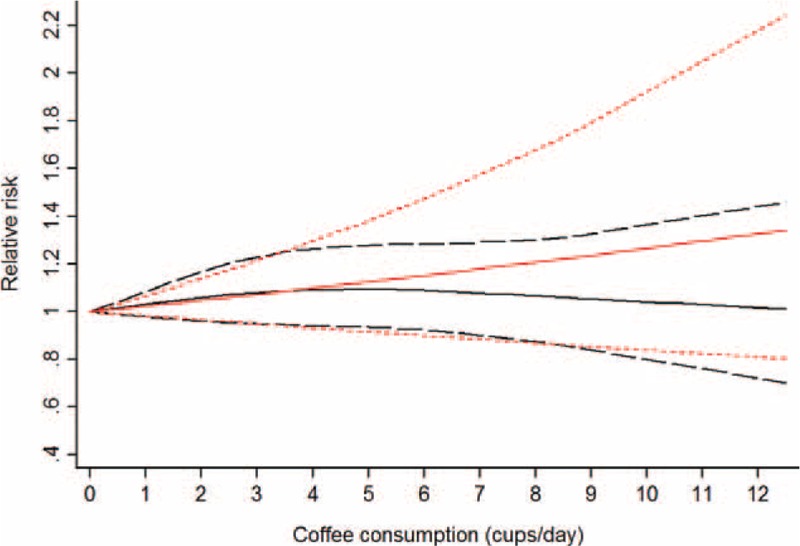
Dose–response analyses of 3 cups/day increment of total coffee consumption and risk of gastric cancer. The black solid line and the black long dashed line represent the estimated RRs and corresponding 95% CIs for the nonlinearity. The red solid line and the red short dashed line represent the estimated RRs and corresponding 95% CIs for the linearity. CI = confidence interval, RR = relative risk.
FIGURE 4.
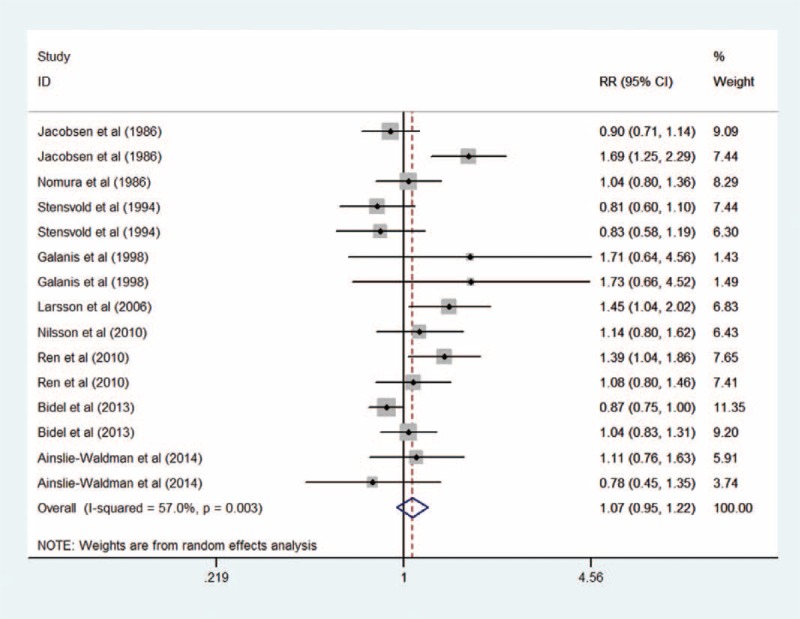
Forest plot of the association between 3 cups/day increment of total coffee consumption and risk of gastric cancer.
Stratified Analysis, Sensitivity Analysis, and Meta-Regression
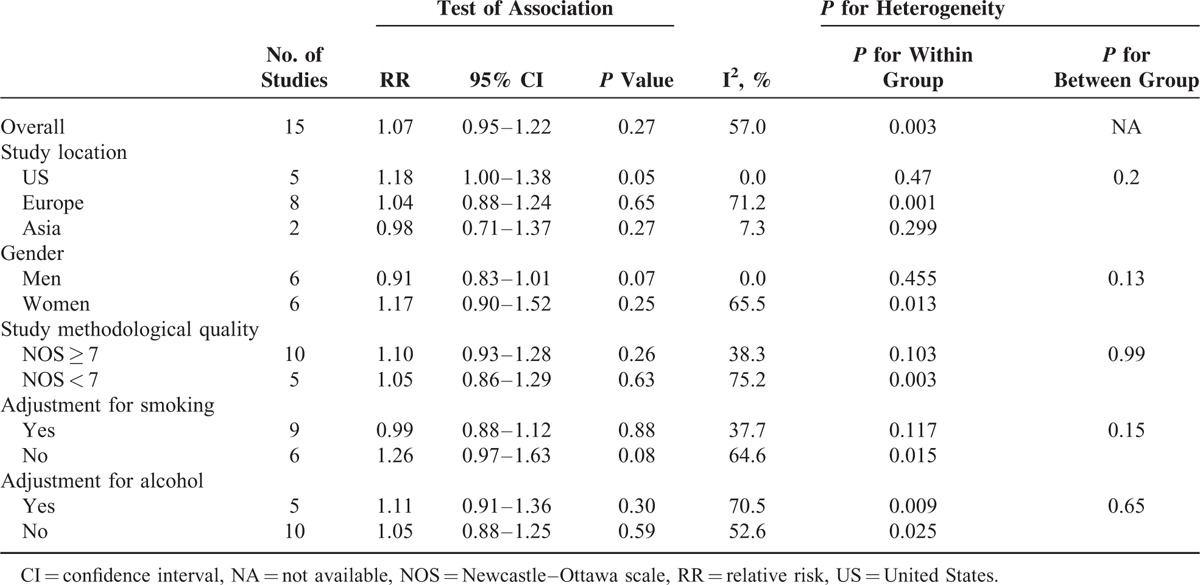
Figure 2 and Table 3 presented the results of subgroup analyses of semiparametric method and dose–response analysis, respectively. In stratified analyses, the null association between coffee consumption and risk of gastric cancer was similar for the second highest and third highest category by study location, sex, study quality, and covariate adjustment (smoking and alcohol) (Figure 2). For the highest category, an elevated risk was found among US population (RR = 1.36, 95% CI = 1.06–1.75; Figure 2) and no adjustment for smoking (RR = 1.67, 95% CI = 1.08–2.59; Figure 2). No significant association was observed in other subgroups. The results of stratified analyses for RRs estimated directly from the linear regression model were similar to the primary results except studies conducted in the US in which a marginal difference was observed (RR = 1.18, 95% CI = 1.00–1.38; Table 3). To test the robustness of our findings, we performed sensitivity analyses by excluding studies that did not provide multivariable-adjusted RRs of gastric cancer and the result did not change significantly. Further analysis detecting the influence of a single study on the summary RRs by omitting each 1 study yielded similar results. Meta-regression according to study location, sex, study quality, covariate adjustment (smoking and alcohol) did not reveal any significant association between groups.
TABLE 2.
The Quality Assessment of All Included Studies
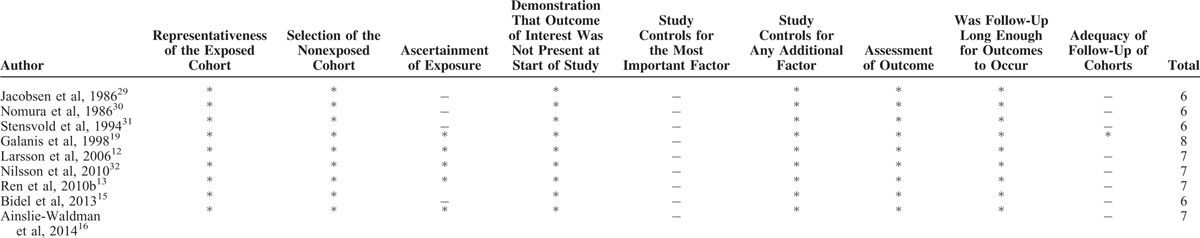
TABLE 3.
Overall Results and Subgroup Analyses of 3 cups/day Increment of Total Coffee Consumption
Publication Bias
Funnel plots suggest symmetry of all the results. The Egger regression test provided no evidence of publication bias for 3 cups/day increment (P = 0.10, Figure 5), highest (P = 0.65, Figure 6), second highest (P = 0.53, Figure 7), and third highest (P = 0.49, Figure 8) level of total coffee consumption.
FIGURE 5.
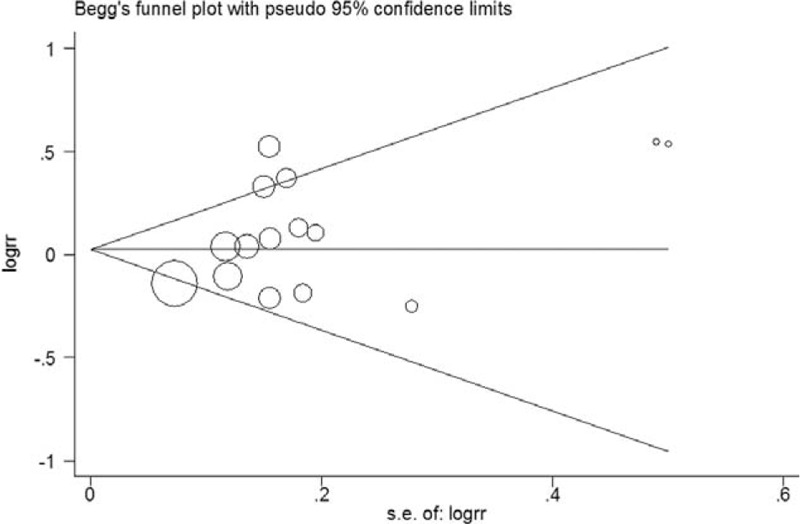
Funnel plot for the 3 cups/day increment of total coffee consumption.
FIGURE 6.
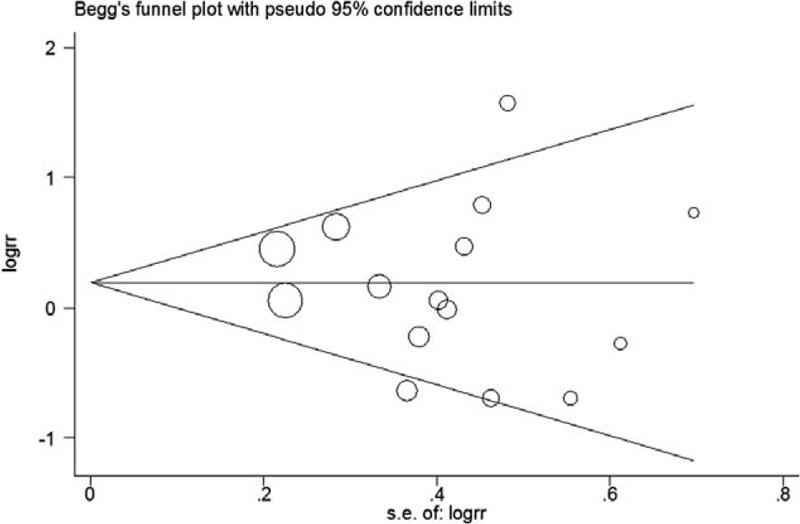
Funnel plot for the highest coffee consumption.
FIGURE 7.
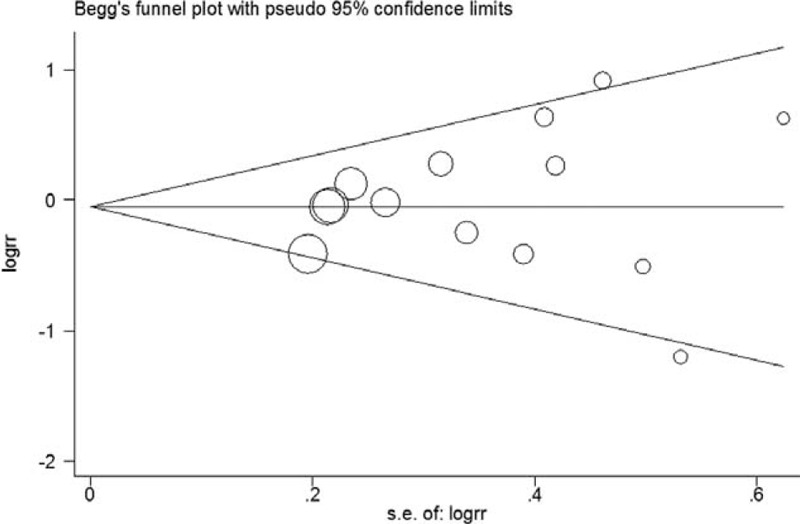
Funnel plot for the second highest coffee consumption.
FIGURE 8.
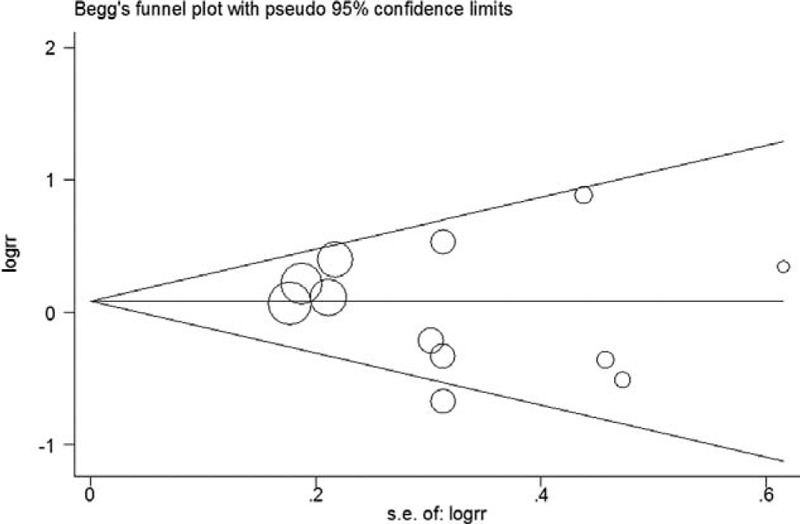
Funnel plot for the third highest coffee consumption.
DISCUSSION
The finding from this meta-analysis, based on 1,289,314 study participants and 2019 incident cases of gastric cancer, found no significant association between coffee consumption and risk of gastric cancer. For highest coffee consumption (median 6.5 cups/day), an elevated risk of gastric cancer was observed in the US population and no adjustment for smoking; and linear regression model showed a marginal significant increased risk in the US population. Therefore, high coffee consumption (more than 6 cups/day) may increase the risk of gastric cancer in Americans. This result should be treated with caution due to the limited number of included studies.
Our meta-analysis included studies conducted over different countries since 1986, but all the included studies were without stratification by coffee type (caffeinated and decaffeinated) and did not describe the characteristics of the coffee consumed. The type of coffee beans, roasting procedure, and specific method of coffee preparation can influence composition.33–36 These factors might contribute to the different risk in each population. It is known that gastric cancer is a complex and heterogeneous disease which is noted for marker global variations in etiology, natural course, incidence, and management.37,38 As a consequence, there might be certain amount of clinical heterogeneity even though we detected no statistical heterogeneity through our study. Additionally, meta-analysis is considered hypothesis-generating and is not conducted to test a hypothesis or establish a standard of care.39 Meta-analysis is secondary study that is based on primary studies and this defect is inevitable.20 The quality and applicability of any meta-analysis are dependent on the quality and comparability of information from the primary studies.39 If individual information were available, a more precise analysis such as individual patient data meta-analysis should be carried out rather than conventional meta-analysis. This is a big project and it needs authors of all published papers to share their data.40
The results from semiparametric method were basically consistent with parametric method among total population in the present meta-analysis. Semiparametric method suggested that for the highest group (median 6.5 cups/day) the risk of gastric cancer was not associated with coffee consumption; but in the stratum of US population and unadjusted for smoking, the elevated risks were observed (36% and 67%, respectively). As we all know, smoking has been identified as the most important behavioral risk factor for gastric cancer.41 Hence, the significant result of the group that did not adjust smoking might be confounded by this important risk factor. On the other hand, the dose–response results of parametric method indicated that coffee consumption had no adverse effect in overall population, but it had a marginal significant elevated risk of 18% (3 cups/day) for US population; for US population, therefore, 2 methods gave a similar elevated risk between coffee consumption and gastric cancer. However, the result should be considered with some caution because of potential confounding. This controversial result might indicate that ethnicity might play a critical role in the relationship between coffee consumption and risk of gastric cancer, which may be caused by gene effects, as shown in a genome-wide association study which observed a highly significant association between a mutation on CYP1A2 and coffee ingestion.42 Recently, a meta-analysis indicated that CYP1A2 gene polymorphisms were significant with gastric cancer risk.43 Therefore, the meta-analyses should be performed according to different genotypes of CYP1A2. But none of the included reports tested the genotypes and it was unlikely to conduct such a possibility of residual confounding cannot be ruled out. Lastly, possible language bias could occur because we excluded papers not in English. However, covered a wide range of non-English regions and the number of large cohort in other non-English countries is limited. Only 5 reports focused on US population, 8 on Europe population, and 2 on Asians. The different risk of coffee consumption and gastric cancer among diverse regions could due to the limited quantity of included studies.
Recently, a similar meta-analysis by Liu et al44 has been published online (http://www.ncbi.nlm.nih.gov/pubmed/26023935) during our manuscript under review. We read it in depth and we found the methodology of their work was well-designed; however, there were also some advantages of our meta-analysis. In their article, they concluded that coffee consumption was not associated with overall cancer risk and may be a risk factor for gastric cardia cancer. However, our results showed that coffee consumption might increase the risk of gastric cancer for the US population. In addition, the methodology assessment of all included studies in the present article was more detailed than their study (Table 2).44 Liu et al44 only presented the total scores of NOS, while the use of NOS is controversial as the summary scores involve inherent weight of component items.45
The present meta-analysis has several strengths. First, the large sample size allowed us to quantitatively assess the association between coffee consumption and risk of gastric cancer, thus making it more powerful than any individual research. Second, the nature of prospective cohort of the included studies diluted the influence of recall and selection bias. Third, we performed sensitivity analysis and meta-regression to detect the heterogeneity between and within groups. Ultimately, we performed both nonlinear and linear regression model to fit the association between coffee consumption and gastric cancer.
Limitations of observational cohorts included the problem of residual confounding, which also extends to meta-analysis of observation studies, and a causal relationship cannot be established with these data alone. Information of main confounders from most studies were not acquired for instance the history of infection with Helicobacter pylori, which is a strong and risk factor for gastric cancer, was not acquired from all the studies. All the study adjusted for age, sex, education, 3 studies with 6 reports adjusted for smoking status,13,15,16 and 3 studies with 5 reports adjusted for alcohol intake.12,13,15 In consequence, results from the present study should be interpreted with some caution because of many potential confounders. Fourth, all studies used FFQs to assess levels of coffee consumption. Moreover, although validation studies showed reasonable reproducibility and validity of self-reported coffee consumption, measurement error is inevitable.46 In addition, where errors exist in the measurement of confounders, the bias would in action in the direction of confounding, which may either attenuate or exaggerate the estimated association.47,48 Exposure misclassification of coffee consumption is the potential limitation. The range between the lowest and highest categories is much different among studies, which may lead to attenuation of true relationship in a prospective study, especially when the exposure was assessed before disease ascertained.46 Fifth, although all studies ruled out cases of self-reported gastric cancer at baseline, several undiagnosed cases might still be included in their statistical analysis. However, the effect of such a bias is unlikely to be big.
A recent cohort study by Bidel et al15 found that 3 to 6 cups/day of coffee consumption was associated with reduced risk and the association was only significant for male. Another cohort study by Ainslie-Waldman et al16 found that 1 cup/day of coffee consumption associated with decreased risk, and the relation was only significant for female. The results from these studies contradict from the present meta-analysis and the majority of studies in the literature. These discrepancies might be caused by possible reasons including a relatively small size, lack of updated dietary assessment, and strata analysis.
In summary, this dose–response meta-analysis suggests that coffee consumption is not associated with a significantly elevated risk of gastric cancer. As the coffee is one of the most frequently consumed beverages around the world especially for Western countries, the US population should avoid high coffee consumption since they may be more susceptible to the adverse effects of high consumption of coffee more than 6.5 cups/day. However, because of potential confounding, exposure misclassification, and other limitations of this study, this result should be considered with some caution.
Footnotes
Abbreviations: CI = confidence interval, FFQs = food frequency questionnaires, HR = hazard ratio, NOS = Newcastle–Ottowa Scale, OR = odds ratio, RR = relevant risk, US = United States.
S-BZ, HW, and MZ are the co-first author.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Butt MS, Sultan MT. Coffee and its consumption: benefits and risks. Crit Rev Food Sci Nutr 2011; 51:363–373. [DOI] [PubMed] [Google Scholar]
- 2.Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc 2008; 67:253–256. [DOI] [PubMed] [Google Scholar]
- 3.Nehlig A, Debry G. Potential genotoxic, mutagenic and antimutagenic effects of coffee: a review. Mutat Res 1994; 317:145–162. [DOI] [PubMed] [Google Scholar]
- 4.Porta M, Vioque J, Ayude D, et al. Coffee drinking: the rationale for treating it as a potential effect modifier of carcinogenic exposures. Eur J Epidemiol 2003; 184:289–298. [DOI] [PubMed] [Google Scholar]
- 5.Cao S, Liu L, Yin X, et al. Coffee consumption and risk of prostate cancer: a meta-analysis of prospective cohort studies. Carcinogenesis 2014; 35:256–261. [DOI] [PubMed] [Google Scholar]
- 6.Tian C, Wang W, Hong Z, et al. Coffee consumption and risk of colorectal cancer: a dose-response analysis of observational studies. Cancer Causes Control 2013; 24:1265–1268. [DOI] [PubMed] [Google Scholar]
- 7.Sang LX, Chang B, Li XH, et al. Consumption of coffee associated with reduced risk of liver cancer: a meta-analysis. BMC Gastroenterol 2013; 13:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng J, Su H, Zhu R, et al. Maternal coffee consumption during pregnancy and risk of childhood acute leukemia: a metaanalysis. Am J Obstet Gynecol 2014; 210:151.e1–151.e10. [DOI] [PubMed] [Google Scholar]
- 9.Botelho F, Lunet N, Barros H. Coffee and gastric cancer: systematic review and meta-analysis. Cad Saude Publica 2006; 22:889–900. [DOI] [PubMed] [Google Scholar]
- 10.Xie F, Wang D, Huang Z, et al. Coffee consumption and risk of gastric cancer: a large updated meta-analysis of prospective studies. Nutrients 2014; 6:3734–3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen Z, Liu H, Cao H. Coffee consumption and risk of gastric cancer: An updated meta-analysis. Clin Res Hepatol Gastroenterol 2015; 39:245–253. [DOI] [PubMed] [Google Scholar]
- 12.Larsson SC, Giovannucci E, Wolk A. Coffee consumption and stomach cancer risk in a cohort of Swedish women. Int J Cancer 2006; 119:2186–2189. [DOI] [PubMed] [Google Scholar]
- 13.Ren JS, Freedman ND, Kamangar F, et al. Tea, coffee, carbonated soft drinks and upper gastrointestinal tract cancer risk in a large United States prospective cohort study. Eur J Cancer 2010; 46:1873–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagata C, Takatsuka N, Kawakami N, et al. A prospective cohort study of soy product intake and stomach cancer death. Br J Cancer 2002; 87:31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bidel S, Hu G, Jousilahti P, et al. Coffee consumption and risk of gastric and pancreatic cancer – a prospective cohort study. Int J Cancer 2013; 132:1651–1659. [DOI] [PubMed] [Google Scholar]
- 16.Ainslie-Waldman CE, Koh WP, Jin A, et al. Coffee intake and gastric cancer risk: the Singapore Chinese health study. Cancer Epidemiol Biomarkers Prev 2014; 23:638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu C, Zeng XT, Liu TZ, et al. Fruits and vegetables intake and risk of bladder cancer: A PRISMA-Compliant Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. Medicine (Baltimore) 2015; 94:e759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galanis DJ, Kolonel LN, Lee J, et al. Intakes of selected foods and beverages and the incidence of gastric cancer among the Japanese residents of Hawaii: a prospective study. Int J Epidemiol 1998; 27:173–180. [DOI] [PubMed] [Google Scholar]
- 20.Zeng X, Zhang Y, Kwong JS, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med 2015; 8:2–10. [DOI] [PubMed] [Google Scholar]
- 21.Ding M, Bhupathiraju SN, Chen M, et al. Caffeinated and decaffeinated coffee consumption and risk of type 2 diabetes: a systematic review and a dose-response meta-analysis. Diabetes Care 2014; 37:569–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeng XT, Leng WD, Zhang C, et al. Meta-analysis on the association between toothbrushing and head and neck cancer. Oral Oncol 2015; 51:446–451. [DOI] [PubMed] [Google Scholar]
- 23.Leng WD, Zeng XT, Kwong JSW, et al. Periodontal disease and risk of coronary heart disease: an updated meta-analysis of prospective cohort studies. Int J Cardiol 2015; 201:469–472. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng XT, Liu DY, Kwong JS, et al. Meta-analysis of association between interleukin-1beta C-511T polymorphism and chronic periodontitis susceptibility. J Periodontol 2015; 86:812–819. [DOI] [PubMed] [Google Scholar]
- 26.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 1992; 135:1301–1309. [DOI] [PubMed] [Google Scholar]
- 27.Harrell FE, Jr, Lee KL, Pollock BG. Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst 1988; 80:1198–1202. [DOI] [PubMed] [Google Scholar]
- 28.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacobsen BK, Bjelke E, Kvale G, et al. Coffee drinking, mortality, and cancer incidence: results from a Norwegian prospective study. J Natl Cancer Inst 1986; 76:823–831. [PubMed] [Google Scholar]
- 30.Nomura A, Heilbrun LK, Stemmermann GN. Prospective study of coffee consumption and the risk of cancer. J Natl Cancer Inst 1986; 76:587–590. [DOI] [PubMed] [Google Scholar]
- 31.Stensvold I, Jacobsen BK. Coffee and cancer: a prospective study of 43,000 Norwegian men and women. Cancer Causes Control 1994; 5:401–408. [DOI] [PubMed] [Google Scholar]
- 32.Nilsson LM, Johansson I, Lenner P, et al. Consumption of filtered and boiled coffee and the risk of incident cancer: a prospective cohort study. Cancer Causes Control 2010; 21:1533–1544. [DOI] [PubMed] [Google Scholar]
- 33.Gross G, Jaccaud E, Huggett AC. Analysis of the content of the diterpenes cafestol and kahweol in coffee brews. Food Chem Toxicol 1997; 35:547–554. [DOI] [PubMed] [Google Scholar]
- 34.Lombaert GA, Pellaers P, Chettiar M, et al. Survey of Canadian retail coffees for ochratoxin A. Food Addit Contam 2002; 19:869–877. [DOI] [PubMed] [Google Scholar]
- 35.Romani S, Pinnavaia GG, Dalla Rosa M. Influence of roasting levels on ochratoxin a content in coffee. J Agric Food Chem 2003; 51:5168–5171. [DOI] [PubMed] [Google Scholar]
- 36.La Pera L, Avellone G, Lo Turco V, et al. Influence of roasting and different brewing processes on the ochratoxin A content in coffee determined by high-performance liquid chromatography-fluorescence detection (HPLC-FLD). Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2008; 25:1257–1263. [DOI] [PubMed] [Google Scholar]
- 37.Shah MA, Ajani JA. Gastric cancer – an enigmatic and heterogeneous disease. JAMA 2010; 303:1753–1754. [DOI] [PubMed] [Google Scholar]
- 38.Thrumurthy SG, Chaudry MA, Hochhauser D, et al. The diagnosis and management of gastric cancer. BMJ 2013; 347:f6367. [DOI] [PubMed] [Google Scholar]
- 39.Hennekens CH, Demets D. The need for large-scale randomized evidence without undue emphasis on small trials, meta-analyses, or subgroup analyses. JAMA 2009; 302:2361–2362. [DOI] [PubMed] [Google Scholar]
- 40.Zhou Y, Zhuang W, Hu W, et al. Consumption of large amounts of Allium vegetables reduces risk for gastric cancer in a meta-analysis. Gastroenterology 2011; 141:80–89. [DOI] [PubMed] [Google Scholar]
- 41.Ladeiras-Lopes R, Pereira AK, Nogueira A, et al. Smoking and gastric cancer: systematic review and meta-analysis of cohort studies. Cancer Causes Control 2008; 19:689–701. [DOI] [PubMed] [Google Scholar]
- 42.Cornelis MC, Monda KL, Yu K, et al. Genome-wide meta-analysis identifies regions on 7p21 (AHR) and 15q24 (CYP1A2) as determinants of habitual caffeine consumption. PLoS Genet 2011; 7:e1002033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xue H, Lu Y, Xue Z, et al. The effect of CYP1A1 and CYP1A2 polymorphisms on gastric cancer risk among different ethnicities: a systematic review and meta-analysis. Tumour Biol 2014; 35:4741–4756. [DOI] [PubMed] [Google Scholar]
- 44.Liu H, Hua Y, Zheng X, et al. Effect of coffee consumption on the risk of gastric cancer: a systematic review and meta-analysis of prospective cohort studies. PLoS One 2015; 10:e0128501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greenland S. Quality scores are useless and potentially misleading: reply to “Re: A critical look at some popular analytic methods”. Am J Epidemiol 1994; 140:300–301. [DOI] [PubMed] [Google Scholar]
- 46.Hu EA, Pan A, Malik V, et al. White rice consumption and risk of type 2 diabetes: meta-analysis and systematic review. BMJ 2012; 344:e1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Freedman LS, Schatzkin A, Midthune D, et al. Dealing with dietary measurement error in nutritional cohort studies. J Natl Cancer Inst 2011; 103:1086–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kipnis V, Freedman LS. Impact of exposure measurement error in nutritional epidemiology. J Natl Cancer Inst 2008; 100:1658–1659. [DOI] [PubMed] [Google Scholar]


