Abstract
Immunoglubulin G (IgG) and its abnormal glycosylations are associated with carcinogenesis. The present study investigates the relationship between cancer-derived IgG and clinicopathological characteristics in hepatocellular carcinoma (HCC) and assesses the value of serum N-glycosylated IgG in diagnosing and monitoring hepatitis B virus (HBV)-related HCC. Tissue microarray analysis of 90 HCC tissues showed that HCC patients with IgG immunopositivity had higher levels of core-fucosylated α fetoprotein (AFP-L3), larger tumors, and a higher incidence of portal vein tumor thrombus. HCC-derived IgG stimulated the growth of liver cancer cells in vitro. HCC patients presented a significantly increased fraction of Lens culinaris agglutinin binding IgG (core-fucosylated IgG, IgG-L3) among total serum IgG. The clinical diagnostic performance of serum IgG-L3% was evaluated in 3 case-control studies (1 training set and 2 validation cohorts), including 293 patients with HCC, 131 with liver cirrhosis, 132 HBV carriers, and 151 healthy controls. IgG-L3% had better general diagnostic performance than AFP in the training set and validation cohort 1 (accuracy: 81.33–85.11% versus 63.33–78.61%). In validation cohort 2, where we aimed to assess the efficiency of IgG-L3% in patients with AFP-negative HCC, the diagnostic accuracy of IgG-L3% was 72.54–73.60%. Finally, a longitudinal evaluation based on 31 HCC patients demonstrated that IgG-L3% decreased in 24 patients after curative surgery. The remaining 7 patients showed elevated IgG-L3% and post-operative recurrence. HCC patients with higher IgG-L3% had poor survival during a 3-year follow up. We conclude that HCC-derived IgG is correlated with progressive behavior of HCC. Therefore, elevated core-fucosylated IgG is a new diagnostic and prognostic marker in HBV-related HCC.
Keywords: diagnosis, hepatocellular carcinoma (HCC), IgG, Lens culinaris agglutinin (LCA), non-invasive biomarker, tissue array
Abbreviations
- AFP
α-fetoprotein
- AFP-L3
core-α-1,6-fucosylated AFP
- DSA-FACE
DNA sequencer-assisted fluorophore-assisted carbohydrate electrophoresis
- ANTT
adjacent non-tumor tissue
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- IgG
immunoglobulin G
- IgG-L3
serum LCA-binding IgG
- LC
liver cirrhosis
- LCA
Lens culinaris agglutinin
- PVTT
portal vein tumor thrombus
- ROC
receiver operating characteristic
- TT
tumor tissue
- AUC
area under ROC curve.
Introduction
Glycans are an integral component of glycoproteins and significantly contribute to their structure and function.1 A recent comprehensive report endorsed by the US National Academies concluded that “glycans are directly involved in the pathophysiology of every major disease” and that “additional knowledge from glycoscience will be needed to realize the goals of personalized medicine.”2 Population-based studies have shown that the composition of the human plasma N-glycome varies significantly between individuals.3,4 Interestingly, the N-glycome from isolated immunoglubulin G (IgG) was more variable than the total plasma N-glycome,5 indicating that N-glycome analysis based on total plasma glycans, which are released from many different glycoproteins, might obscure signals from protein-specific regulation of glycosylation.
IgG is one of the best-understood glycoproteins. Each CH2 domain of the fragment crystallizable (Fc) region of IgG heavy chains carries a covalently attached biantennary N-glycan at the highly conserved asparagine 297 residue.6 Structural details of the attached glycans are of great physiological significance and are associated with many pathological conditions.6,7 For example, levels of galactose-deficient IgG are increased in liver fibrosis, cirrhosis, and ovarian and lung cancer8-10 and increased core fucosylated IgG was described as a diagnostic marker in ovarian cancer.11 Additionally, individual variation in IgG glycosylation changes during acute systemic inflammation was associated with increased mortality risk. These data suggest that N-glycans of IgG might be a new marker for disease diagnosis and therapeutic monitoring.
Although serum α-fetoprotein (AFP) has been widely accepted as a screening marker for hepatocellular carcinoma (HCC) in China, its diagnostic value is limited by rather poor sensitivity and specificity.12 Testing for a heterotype of AFP, Lens culinaris agglutinin (LCA)-reactive AFP (AFP-L3), can improve the specificity13 but obviously cannot improve the sensitivity of HCC diagnosis. Improved non-invasive biomarkers are still required for early diagnosis of HCC. Elevated levels of serum IgG, IgA, or IgM antibodies have been observed in patients with HCC.14,15 These tumor-reactive Igs were interpreted as humoral responses of the host to cancer growth.16 It has been confirmed that these tumor-reactive antibodies are capable of binding to normal and tumor associated antigens, including those of cell surface and intracellular proteins.16-18 IgGs with unidentified specificity might be directly secreted from cancer cells and are involved in cancer cell survival and growth.19 However, the potential association between IgG expression, its N-glycan changes, and HCC diagnosis in patients has not been investigated in greater depth.
In the present study, we investigated IgG expression in a tissue array from 90 HCC patients. To determine the function and N-glycan changes of circulating IgG in hepatitis B virus (HBV)-related HCC, we examined the effects of HCC-derived serum IgG on cell proliferation in vitro. We then analyzed serum IgG N-glycans using DNA sequencer-assisted fluorophore-assisted carbohydrate electrophoresis (DSA-FACE), LCA blotting, and immunoassay after LCA affinity chromatography. Finally, the diagnostic performance and prognostic value of N-glycosylated IgG were further assessed in cross-sectional and longitudinal studies.
Results
IgG expression in liver cancer tissues and cell lines
Immunohistochemical staining for IgG in a tissue array containing 90 HBV-related HCC paired tissues (tumor tissues [TT] versus paired adjacent non-tumor tissues [ANTT], Fig. 1A) revealed IgG immunopositivity in the cytoplasm in 42/90 cases (in either TT or ANTT).
Figure 1.
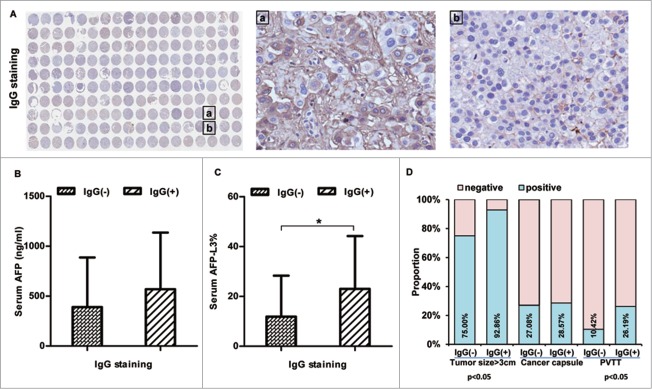
IgG expression is associated with serum AFP levels, tumor size, and incidence of portal vein tumor thrombus in patients with HCC. (A) Immunohistochemical staining for IgG was performed in tissue array of HBV-related HCC including 90 paired samples of HCC carcinoma and adjacent noncancerous liver tissue; the right panel shows representative tissues with positive IgG (a) and negative IgG (b) staining. (B, C) Serum AFP levels (B) and serum AFP-L3% (C) were measured in HCC patients included in the tissue array with different levels of IgG. (D) The association between IgG immunopositivity and tumor size/tumor capsule/PVTT was analyzed. *P < 0.05
Next, we measured serum AFP levels and the percentage of AFP-L3 compared to total AFP (AFP-L3%) in these HCC patients. Compared to patients with negative IgG immunostaining in liver tissue, patients with liver tissue positive for IgG (in either TT or ANTT) had higher levels of serum AFP (Fig. 1B) and AFP-L3% (P < 0.05, Fig. 1C).
In addition, we assessed the potential association between IgG immunopositivity in liver tissue and 3 tumor clinical parameters (tumor size, tumor capsule, and portal vein tumor thrombus [PVTT]) in these HCC patients. Patients with liver tissue positive for IgG in TT/ANTT had a larger tumor size and a higher incidence of PVTT than those negative for IgG in liver tissues (Fig. 1D, P < 0.05).
Furthermore, IgG was detected in supernatants of cultured SMMC-7721, MHCC97-H, and CSQT-2 cells, indicating that these hepatoma cell lines can secrete IgG (Fig. 2). MHCC97-H and CSQT-2, 2 hepatoma cell lines with higher metastatic activity, secreted 3 times more IgG than SMMC-7721 cells (Fig. 2).
Figure 2.
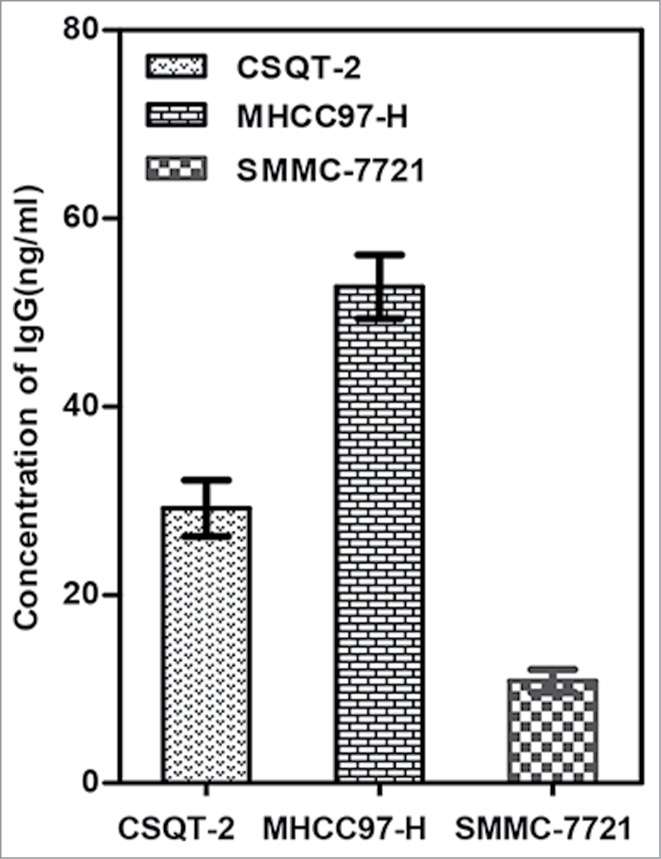
HCC cells secrete IgG. IgG in supernatants of 3 hepatoma cell lines, MHCC97-H, CSQT-2 and SMMC-7721, was measured as described in Patients and Methods.
IgG from sera of HCC patients promotes cancer cell proliferation in vitro
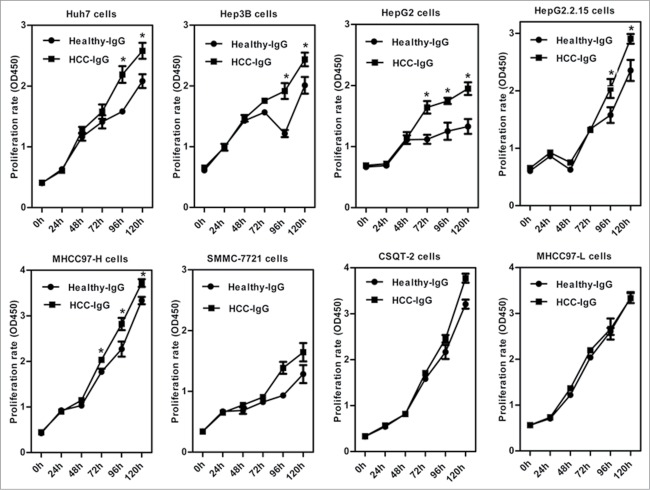
To investigate the effect of IgG on the proliferation of HCC cells, we treated 8 HCC cell lines (Huh7, Hep3B, HepG2, HepG2.2.15, MHCC97-H, SMMC-7721, CSQT-2, and MHCC97-L) with IgG isolated from sera of healthy volunteers and HCC patients. CCK8 assay analyses showed that, compared to treatment with IgG derived from sera of healthy individuals, incubation for 72–96 h with IgG derived from HCC patients significantly increased cell proliferation in Huh7, Hep3B, HepG2, HepG2.2.15, and MHCC97-H cells (P < 0.05, Fig. 3). Similar effects were observed in SMMC-7721 and CSQT-2 cells, but without statistical significance. There was no difference in proliferation of MHCC97-L cells after treatment with the different IgGs.
Figure 3.
IgG from HCC sera stimulates proliferation of HCC cells in vitro. Eight HCC cell lines were treated with IgG purified from sera of HCC patients (HCC-IgG) or healthy individuals (Healthy-IgG) by protein G/A spin columns. Cell proliferation was evaluated over 5 days by CCK8 assay. *P < 0.05
Expression of serum LCA-binding IgG (IgG-L3)is increased in patients with HCC
In this study, high-affinity LCA-binding IgG is called IgG-L3 to discriminate it from hetero-IgGs without LCA affinity (L1) and those with low affinity (L2). To validate the existence of a core-fucosylated structure in IgG-L3, N-glycan profiles of IgG, IgG-L3, and IgG-L3 lacking the core-fucose were analyzed by DSA-FACE as described previously.26,27 IgG showed 7 main biantennary glycans in the N-glycan profiling (Supplementary Fig. S1). After core fucosidase digestion, all of the peaks except for peak 5 (without core fucosylation) shifted one residue forward in the N-glycan profiling, confirming that IgG-L3 is a core-fucosylated IgG (Supplementary Fig. S1).
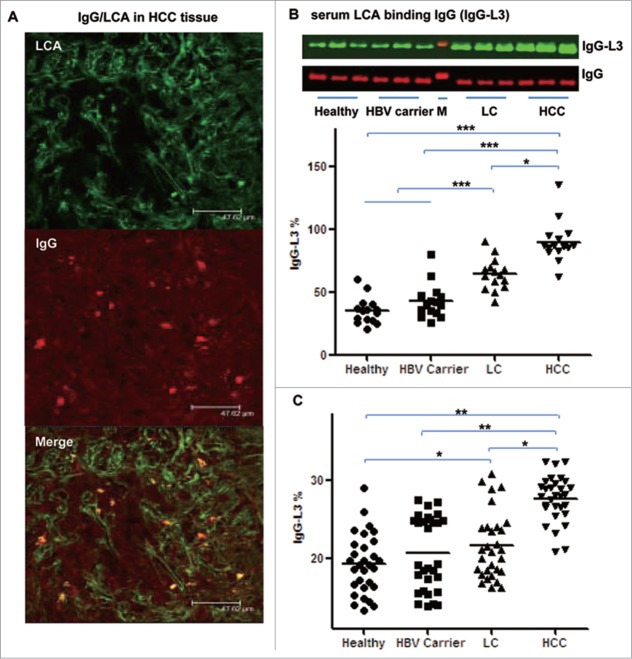
To assess the expression of IgG-L3 in patients with HCC, we performed co-staining for LCA and IgG in liver tissues from HCC patients. Significant co-staining of IgG and LCA was present in cancer cells (Fig. 4A). To determine the levels of core-fucosylated IgG (IgG-L3%, calculated as IgG-L3/total IgG × 100) in the circulation, we performed lectin (LCA) blotting for IgG purified from sera of healthy volunteers, HBV carriers, patients with liver cirrhosis (LC), and patients with HCC (Fig. 4B). LCA blotting analyses revealed that IgG-L3% in sera was significantly increased in patients with HCC compared to healthy volunteers, HBV carriers, and patients with LC (P < 0.05, Fig. 4B). Immunoassay performed after LCA affinity chromatography showed similar results to the LCA-blotting (Fig. 4C).
Figure 4.
IgG-L3% is increased in HCC. HCC tissues were co-stained for IgG and LCA. (A) A representative liver tissue sample. (B, C) Serum IgG-L3 was measured with IgG LCA-blotting (B) and scattering immunoassay after LCA chromatography (C) as described in Patients and Methods. *P < 0.05, **P < 0.01, ***P < 0.001.
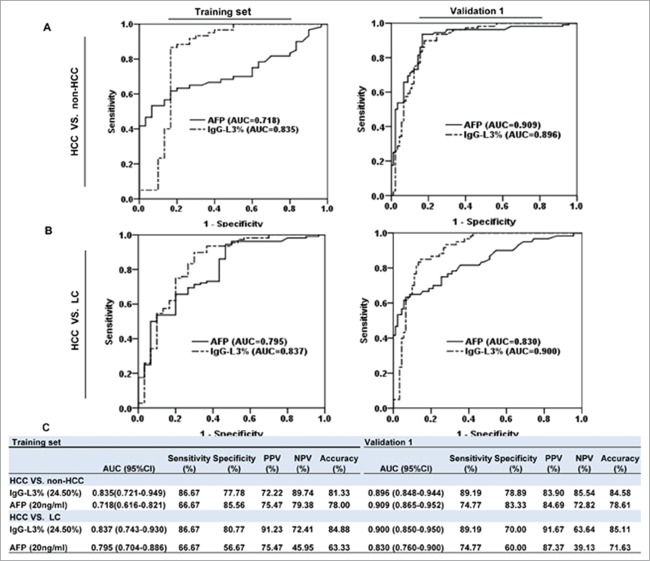
Serum IgG-L3% has better diagnostic performances than AFP
To evaluate whether IgG-L3% is a potential diagnostic marker for HCC, we measured serum IgG-L3% and AFP levels in a training cohort including 120 patients and 30 healthy volunteers (HCC versus non-HCC: 60 versus 90, Fig. 5). Receiver operating characteristic (ROC) curve analyses revealed that IgG-L3% demonstrated 81.3% diagnostic accuracy for segregating HCC from non-HCC, which was superior to AFP (78.0%). At the cutoff value of 24.5%, IgG-L3% had significantly higher diagnostic sensitivity compared to AFP (86.67 versus 66.67), but slightly lower diagnostic specificity (77.78 versus 85.56) (Fig. 6A left panel and Fig. 6C). Next, we compared the diagnostic efficiency of IgG-L3% and AFP to identify HCC from LC (60 versus 30) in this patient cohort. IgG-L3% displayed 86.67% sensitivity and 80.77% specificity compared with 66.67% and 56.67%, respectively, for AFP only (Fig. 6B left panel and Fig. 6C).
Figure 5.
Recruitment and exclusion process for the clinical study population.
Figure 6.
IgG-L3% has better HCC diagnostic efficacies than AFP. The area under the curve (AUC) shows diagnostic efficacies of IgG-L3% and AFP (A): HCC versus non-HCC; (B): HCC versus LC) in the training set (left panel) and validation 1 cohort (right panel). (C) Sensitivity, specificity, and accuracy of the 2 parameters. AFP, α-fetoprotein; HCC, hepatocellular carcinoma; LC, liver cirrhosis.
To confirm these primary findings from a training cohort, we generated a validation group including 170 patients and 30 healthy volunteers (validation set 1, Fig. 5). Consistent with the training cohort, IgG-L3% demonstrated significantly higher diagnostic accuracy than AFP in differentiating HBV-related HCC from non-HCC and LC (for HCC versus non-HCC: 84.58% for IgG-L3% compared with 78.61% for AFP, Fig. 6A right panel and Fig. 6C; for HCC versus LC: 85.11% for IgG-L3% compared with 71.63% for AFP, Fig. 6B right panel and Fig. 6C).
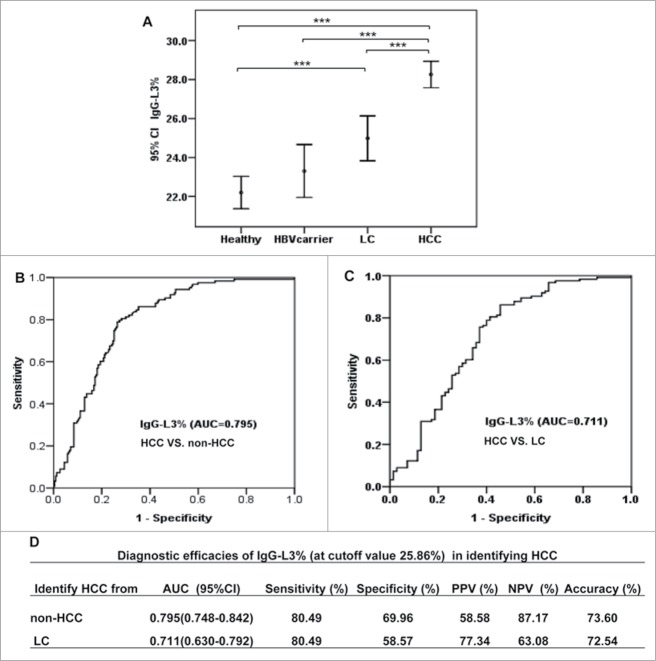
IgG-L3% has high diagnostic accuracy in HCC patients with low serum levels of AFP
To assess whether IgG-L3% has diagnostic potential in HCC patients with low serum levels of AFP, we enrolled validation study cohort 2 including 123 HCC patients with serum AFP levels ≤20 ng/mL, 71 patients with LC, 72 HBV carriers, and 91 healthy volunteers. In line with the training cohort and validation cohort 1, the serum IgG-L3% value gradually increased from lowest values in healthy volunteers and HBV carriers to patients with LC, and was significantly higher in HCC patients with serum AFP levels ≤20 ng/mL (Fig. 7A). ROC analyses revealed that the areas under the curves (AUCs) of IgG-L3% were 0.795 (95% confidence interval [CI]: 0.748–0.842, for HCC versus non-HCC Fig. 7B and 7D), and 0.711 (95% CI: 0.630–0.792, for HCC versus LC, Fig. 7C and 7D).
Figure 7.
IgG-L3% shows good diagnostic efficacy in identifying HCC with serum AFP ≤ 20 ng/ml. (A) IgG-L3% was determined in different groups of patients in validation 2 cohort: healthy volunteers, HBV carriers, LC, and HCC with AFP ≤ 20 ng/mL. (B-C) AUC shows the diagnostic efficacy of IgG-L3% in discriminating HCC from non-HCC (B) and LC (C). Detailed sensitivity, specificity and accuracy of the 2 parameters are presented in (D). ***P < 0.001. AFP, α-fetoprotein; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; LC, liver cirrhosis
HCC patients with a high value of serum IgG-L3% have poor prognosis
The final aim of this study was to assess whether IgG-L3% is a potential prognostic marker in a cohort of 31 HCC patients who had received surgery. Six months after radical operation, IgG-L3% values were reduced in 24 of 31 patients and increased in 7 patients (P = 0.003, Fig. 8A). All 7 patients with increased IgG-L3% value suffered postoperative recurrence.
Figure 8.
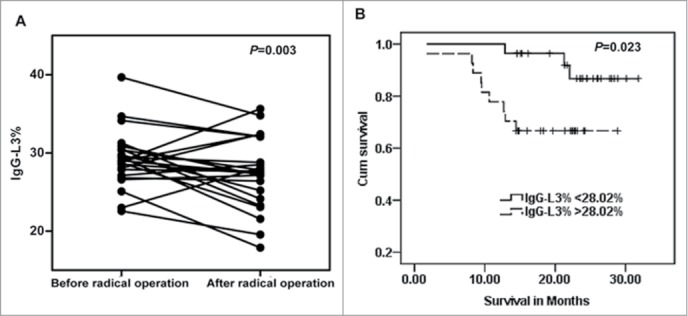
HCC patients with a high serum level of IgG-L3% show poor prognosis. (A) IgG-L3% was measured in 31 HCC patients before and 6 months after radical surgery. (B) Kaplan-Meier analysis of overall survival time in 55 HCC patients with 3-year follow-up.
We divided 55 HCC patients in the training set into 2 groups according to the median value of IgG-L3% (28.02%) before surgery. After 36-month follow up, Kaplan-Meier test showed that the overall survival rate in patients with high IgG-L3% value was significantly lower than that in patients with low IgG-L3% value (P = 0.023, Fig. 8B).
Discussion
Glycoproteins are predominantly produced by hepatocytes and B lymphocytes.20,21 Some hepatocyte-produced glycoproteins and their glycosylated forms have been intensively studied and used as disease markers.12,13,22 Among these proteins, a core-fucosylated heterogeneous form of AFP (AFP-L3) is used as a HCC surveillance marker.13 Although false positivity of serum AFP in HCC surveillance was partially improved by combined diagnosis of AFP and AFP-L3, there remains a 30–40% chance of false-negative findings using this combined marker in HCC identification.12,13 AFP is also unsatisfactory for monitoring HCC recurrence after curative surgery in AFP-negative cases. In this study, we focus on another major type of glycoprotein, IgG, which accounts for nearly half of the glycoproteins in plasma, and address the following questions: 1) Is IgG produced in HCC tissue? 2) What is the function of the HCC-derived IgG? 3) What are the structural features of IgG? 4) Is there an increase in core-fucosylated IgG (IgG-L3) HCC? 5) What is the clinical performance of IgG-L3 in HCC monitoring?
Using HCC tissue array and HCC cell lines, we confirmed that IgG was secreted from malignant hepatocytes. Functional analysis demonstrated that HCC-derived IgG promotes proliferation of several hepatoma cell lines. These findings are quite similar to a previous study showing that both autocrine and paracrine effects of IgG promote the growth of liver cancer cells.19 In addition, HCC patients with positive IgG staining had worse clinical pathological characteristics including a higher frequency of PVTT, greater tumor size, and higher levels of AFP-L3%. Consistent with these findings, HCC cells with high metastatic capacity, e.g., MCCH97-H, secreted more IgG. These results suggest that HCC-produced IgG is associated with the progression of HCC. It is therefore likely that measuring serum IgG levels in patients would be helpful in the diagnosis of HCC.
Our previous research confirmed a significant increase in core fucosylation catalyzed by α-1,6-fucosyltransferase (FUT8) in HCC tissues and portal vein tumor thrombus tissues.23 The expression level of Fut8 was highly correlated with tumor size and the presence of satellite nodules, whereas FUT8 knockdown suppressed the proliferation, migration, and invasion of MHCC97-H cells.23 This study indicated that structural changes mediate the procarcinogenic properties of glycoproteins. In line with this hypothesis, we found that the fraction of LCA-binding IgG (called IgG-L3 in this study) compared to total IgG was significantly elevated in sera of HCC patients compared to non-HCC controls (HBV carriers and patients with liver cirrhosis).
To evaluate the clinical diagnostic performance of IgG-L3%, 3 sets of case-control studies (training, validation 1, and validation 2) were designed. We focused on (1) the general diagnostic performance of IgG-L3% in HCC; (2) the capacity of IgG-L3% to discriminate HCC from LC, a disease in which AFP might be positive and nodular changes similar to HCC might be seen in imaging examination; (3) the efficacy of IgG-L3% in identifying AFP-negative HCC (validation 2). We demonstrated that IgG-L3% had better general diagnostic performance for HCC than AFP in both the training set and validation set 1. Furthermore, IgG-L3% was much better than AFP in discriminating HCC from LC. More interestingly, the diagnostic accuracy of IgG-L3% in detecting AFP-negative HCC was 70.9–72.5%. These results suggest that more than two-thirds of cases of AFP-negative HCC can be identified by determination of IgG-L3%. Therefore, IgG-L3% is an improved serum marker for HCC diagnosis in clinical practice.
In parallel to the above cross-sectional observations, we conducted a longitudinal evaluation. The decrease in IgG-L3% after curative surgery and elevation in IgG-L3% in cases of postoperative recurrence suggest that IgG-L3% is also a useful marker for monitoring the therapeutic response and recurrence of HCC. Furthermore, in a 36-month follow-up study, HCC patients with higher levels of IgG-L3% had a significantly worse overall survival rate. In 7 cases of HCC in which IgG-L3% was extraordinarily increased after curative surgery, a higher incidence of satellite nodules (3 of 7 cases) and adjacent tissue adhesion (3 of 7 cases) and a greater tumor size were detected. The worse clinicopathological characteristics of these 7 patients further support the prognostic value of IgG-L3% for HCC.
To examine clinical applicability, we performed a methodological evaluation of IgG-L3%. Both inter- and intra-coefficient of variation were less than 7%, indicating excellent stability and reproducibility (Supplementary Table 1).
This study has several interesting points: First, the autocrine effect of IgG in malignant hepatocytes correlated with clinical pathological behavior of HCC with the demonstration that IgG had a growth-factor like proliferative effect on liver cancer cells. Second, the method for detection of core-fucosylated IgG (IgG-L3) is more feasible than that for other circulating glycoproteins like fetuin A, haptoglobin, and α1-acid glycoprotein,24-26 because the amount of IgG-L3 is above the common detection limit after LCA affinity isolation. Third, the superiority of IgG-L3% in discriminating HCC from liver cirrhosis and in diagnosing AFP-negative HCC was clearly demonstrated. Furthermore, longitudinal monitoring of IgG-L3% after treatment and postoperative recurrence might help clinicians to assess the treatment response and establish a follow-up strategy.
There are some limitations of this study: 1) Isolation of total serum LCA-binding glycoproteins is required for IgG-L3% detection. This procedure is time consuming compared to highly automatized clinical detection; 2) Only 2 clinical medical centers were involved in this clinical evaluation. As a consequence, both the cohort size and the follow-up duration should be extended in future studies; 3) The regulatory mechanisms for IgG production by malignant hepatocytes and the signal transduction pathway of this HCC-derived IgG must be elucidated.
In summary, core-fucosylated IgG in HCC is correlated with worse clinical pathological features of HCC. HCC-derived IgG induces the proliferation of liver cancer cells. The elevation of core-fucosylated IgG (IgG-L3%) is a promising new alternative marker as a complement to AFP for HCC diagnosis and dynamic management. Expanded clinical validations and the mechanisms of production, function, and regulation of the altered N-glycosylated IgG require further investigation.
Patients and Methods
Patients
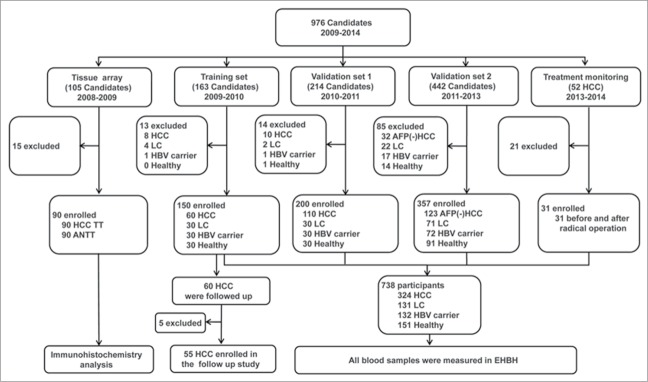
Patients and healthy volunteers were enrolled from Shanghai Eastern Hepatobiliary Surgery Hospital (EHBH) and Shanghai Changzheng (CZ) hospital. The protocol of this clinical study was approved by the Ethics Committees of EHBH and CZ hospital. Written informed consent was obtained from all participants. The clinical study population is shown in Figure 5. A total of 976 candidates participated in the present study.
Inclusion criteria were as follows: HCC was defined on the basis of abdominal ultrasonography, dynamic computed tomography (CT) scanning, or MRI characteristics combined with AFP, and was confirmed by histopathology.27 The diagnosis of LC was based on histopathology of liver biopsy and clinical, laboratory, or imaging evidences.28 Patients with cirrhosis who had increased serum AFP concentrations were required to have undergone imaging measurement by multiple methods (ultrasonography, CT, or MRI) to exclude hepatic mass for at least 3 months before enrollment. HBV carriers were defined by positive serum hepatitis B surface antigen (HBsAg) for at least 6 months with normal liver function before enrollment. The healthy controls were recruited from individuals undergoing a routine physical examination in the hospital who had normal liver biochemistry and no history of various liver diseases or malignant diseases.
A total of 148 patients were excluded according to the following exclusion criteria: patients infected with hepatitis A virus, hepatitis C virus, hepatitis D virus, hepatitis E virus, human immunodeficiency virus, Epstein-Barr virus, or cytomegalovirus; patients who had a history of other solid tumors, alcohol consumption >30 g/day, metastatic liver cancer, autoimmune liver disease, drug-related liver disease, alcoholic hepatitis, obstructive jaundice, other causes of chronic liver disease, renal inadequacy or blood diseases.
HCC tumor tissues (TT) and paired adjacent non-tumor tissues (ANTT) from 90 HBV-related HCC patients were used for tissue array studies. A total of 745 patients were enrolled in a training set and 2 validation cohorts. The training cohort of 150 patients (including 60 with HBV-related HCC, 30 with HBV-associated LC, 30 HBV carriers, and 30 healthy controls) was recruited in EHBH and CZ hospitals from 2009 to 2010. The 60 HCC patients were followed up for 36 months after radical surgery. Validation 1 cohort, including 110 patients with HBV-related HCC, 30 with LC, 30 HBV carriers, and 30 healthy controls, was recruited from 2010 to 2011. Validation cohort 2 enrolled 123 patients with HBV-related HCC with negative serum AFP (AFP ≤20 ng/mL), 71 with LC, 72 HBV carriers, and 91 healthy controls from 2011 to 2013. A further cohort of 31 HCC patients was recruited for monitoring of treatment by radical surgery from June 2013 to May 2014. Clinicopathological characteristics of these patients are summarized in Supplementary Tables 2–5.
Cells
Human liver cancer cell lines SMMC-7721, Huh7, Hep3B, HepG2, and HepG2.2.15 were obtained from the Shanghai Institute of Cell Biology, Chinese Academy of Sciences. MHCC97-H and MHCC97-L were purchased from the Liver Cancer Institute of Fudan University.29 CSQT-2 cell line derived from portal vein tumor thrombus of HCC was gifted from the cell bank of EHBH.30 All of the above cell lines were authenticated by detection of AFP in the supernatant of cell cultures. In addition, Hep3B and HepG2 were authenticated by short tandem repeat profiling by Beijing Microread Gene Technology Company. Cells were used within 6 months of receipt.
Tissue array construction, immunohistochemistry, and immunofluorescence analyses
Fresh HCC tumor tissue (TT) and paired adjacent non-tumor tissue (ANTT) were fixed immediately in 10% buffered formalin, dehydrated in increasing concentrations of alcohol, and embedded in paraffin. Tissue array chips were constructed containing 90 matched pairs of HCC TT (odd columns) and ANTT (even columns) with 1.5 mm/specimen.
Tissue array sections (4 μm) were deparaffinized in serial ethanol dilutions. After antigen retrieval, the slides were incubated in peroxidase blocking reagent (Dako, S2001) for 1 h and with rabbit anti-human IgG antibody (Abcam, ab6759) overnight at 4°C. For immunohistochemistry, slides were washed with PBS and EnVision peroxidase (Dako, K4001) was applied for 1 h at room temperature. Sections were developed with diaminobenzidine for 5 min. Hematoxylin was used for counterstaining. Two pathologists who were blinded to sample identities qualitatively and quantitatively assessed immunohistochemical staining. Immunohistochemical staining of IgG was further validated in routine HCC slides.
For co-immunofluorescence staining of IgG and LCA in HCC tissues, slides were incubated with FITC-labeled LCA (a specific core-α-1,6-fucosylated structure binding protein, Vector laboratories, FL-1041) and rabbit anti-human IgG (Abcam, ab6759) overnight at 4°C. Alexa633-donkey anti-rabbit IgG (Molecular Probes/Invitrogen, A-21082) was used as secondary antibody. Samples were mounted using Dako-Cytomation Fluorescent Mounting Medium (Dako, S302380–2). Confocal microscopy was performed as described previously.31
Enzyme-linked immunoabsorbent assay (ELISA)
IgG in supernatants of HCC cells was detected using a commercial ELISA kit (RayBiotech, Norcross, GA; ELH-IGG). Fresh culture medium was used as blank control. The experiments were performed in triplicate.
Sera IgG purification
Sera of HCC patients and healthy controls were collected from EHBH. Serum IgG was purified using protein G plus/protein A-agarose column suspension (Calbiochem-Novabiochem Corp., IP10) according to the manufacturer's instruction. The purity of the IgG was assessed by 10% SDS-PAGE.
Cell proliferation assay
Cancer cells were seeded in a 96-well plate at 1 × 103 cells/mL and incubated overnight at 37°C in Dulbecco's minimal essential medium (DMEM) with 2.5% FCS. The supernatant was discarded and IgG (1 g/mL) purified from HCC sera was added to the culture for a further 120 h. Cell proliferation was determined by CCK8 assay (Dojindo; CK04). IgG (1 g/mL) from healthy controls was used as control.
Quantitative analysis of serum IgG-L3 by lectin blotting
Serum total IgG was purified using protein G plus/protein A-agarose suspension (Calbiochem-Novabiochem Corp., IP10). Lectin blotting was performed as described previously.32 Briefly, 10 μg of IgG was subjected to 12% SDS-PAGE and then electrotransferred onto nitrocellulose membranes. The membranes were blocked overnight and incubated sequentially with 5 μg/mL biotinylated LCA and IRDye 800 cw conjugated streptavidin (LI-COR Biosciences, 926–32231) at room temperature. Fluorescent bands were detected and quantified using the Odyssey Infrared Imaging System (LI-COR Biosciences).
Quantitative analysis of serum IgG-L3 with immunoassay
LCA-binding glycoproteins were obtained using agarose-bound LCA affinity chromatography (Beijing Hotgen Biotech, China). Total IgG and IgG-L3 were quantified by nephelometric immunoassay (BNII, Siemens, Germany). The IgG-L3% was calculated as IgG-L3/total IgG × 100. In addition, the intra- and inter- assay variations were assessed at 2 levels (low, high) to confirm that this assay was reliable (Supplementary Table 1). All measurements were conducted in duplicate.
Validation of the core-fucosylated structure of IgG-L3 by DSA-FACE
The IgG-L3 fraction was obtained by LCA column affinity chromatography followed by protein G plus/protein A-agarose column chromatography. α-1, 6-Fucosidase (bovine kidney, GKX-5006; Glyko) was used to cleave the core-fucose of IgG-L3. Total serum glycoproteins, serum IgG, IgG-L3, and IgG-L3 lacking core-fucose were analyzed for N-glycan structure with DSA-FACE as described previously.33,34 The results were analyzed using the GeneMapper v3.7 software (Life Technologies Corporation).
Diagnostic performance evaluation of IgG-L3%
Diagnostic specificity, sensitivity, accuracy, and AUCs with 95% confidence interval were used to evaluate the diagnostic performance of IgG-L3% for HCC after ROC curve analyses. Longitudinal treatment monitoring and prognostic value of IgG-L3% for HCC patients were also evaluated.
Statistical analysis
Statistical analysis was performed with SPSS for Windows (version 16.0). Differences between 2 independent groups were tested with the Mann-Whitney U test (continuous variables and non-parametric analyses). ROC curves were constructed to assess sensitivity, specificity, and respective AUCs with 95% CI. We investigated the optimum cutoff value for diagnosis by maximizing the sum of sensitivity and specificity and minimizing the overall error (square root of the sum [1-sensitivity]2 + [1-specificity]2). We compared IgG-L3% before and after surgical resection in HCC using the independent samples t test and the paired t test. P values lower than 0.05 (2-sided) were considered to be significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors are grateful to Prof. Wei-Qiang Zheng from Changhai Hospital for technical support.
Funding
This study was supported by National Natural Science Foundation of China No.81271925, 81301877, 81101639; State Key Projection Infectious Diseases of China No 2012ZX10002–016.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1.Gornik O, Pavic T, Lauc G. Alternative glycosylation modulates function of IgG and other proteins - Implications on evolution and disease. Biochim Biophys Acta 2012; 1820:1318-26; PMID:22183029; http://dx.doi.org/ 10.1016/j.bbagen.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Walt D, Aoki-Kinoshita KF, Bendiak B, Bertozzi CR, Boons GJ, Darvill A, Hart G, Kiessling LL, Lowe J, Moon R, et al.. Transforming glycoscience: A roadmap for the future. Nacional Academies Press, Washington: 2012. [PubMed] [Google Scholar]
- 3.Knezević A, Polasek O, Gornik O, Rudan I, Campbell H, Hayward C, Wright A, Kolcic I, O'Donoghue N, Bones J, et al.. Variability, heritability and environmental determinants of human plasma N-glycome. J Proteome Res 2009; 8:694-701; PMID:19035662; http://dx.doi.org/ 10.1021/pr800737u. [DOI] [PubMed] [Google Scholar]
- 4.Pucic M, Pinto S, Novokmet M, Knezevic A, Gornik O, Polasek O, Vlahovicek K, Wang W, Rudd PM, Wright AF, et al.. Common aberrations from normal human N-glycan plasma profile. Glycobiology 2010; 20:970-5; PMID:20378934; http://dx.doi.org/ 10.1093/glycob/cwq052. [DOI] [PubMed] [Google Scholar]
- 5.Pucic M, Knezevic A, Vidic J, Adamczyk B, Novokmet M, Polasek O, Gornik O, Supraha-Goreta S, Wormald MR, Redzic I, et al.. High throughput isolation and glycosylation analysis of IgG-variability and heritability of the IgG glycome in three isolated human populations. Mol Cell Proteomics 2011; 10:M111 010090; PMID:21653738; http://dx.doi.org/ 10.1074/mcp.M111.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huhn C, Selman MH, Ruhaak LR, Deelder AM, Wuhrer M. IgG glycosylation analysis. Proteomics 2009; 9:882-913; PMID:19212958; http://dx.doi.org/ 10.1002/pmic.200800715. [DOI] [PubMed] [Google Scholar]
- 7.Bakovic MP, Selman MH, Hoffmann M, Rudan I, Campbell H, Deelder AM, Lauc G, Wuhrer M. High-throughput IgG Fc N-glycosylation profiling by mass spectrometry of glycopeptides. J Proteome Res 2013; 12:821-31; PMID:23298168; http://dx.doi.org/ 10.1021/pr300887z. [DOI] [PubMed] [Google Scholar]
- 8.Mehta AS, Long RE, Comunale MA, Wang M, Rodemich L, Krakover J, Philip R, Marrero JA, Dwek RA, Block TM. Increased levels of galactose-deficient anti-Gal immunoglobulin G in the sera of hepatitis C virus-infected individuals with fibrosis and cirrhosis. J Virol 2008; 82:1259-70; PMID:18045939; http://dx.doi.org/ 10.1128/JVI.01600-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qian YF, Wang YS, Zhang XW, Zhou L, Zhang ZJ; Jie J. Quantitative analysis of serum IgG galactosylation assists differential diagnosis of ovarian cancer. J Proteome Res 2013;12:4046-55; PMID:23855414; http://dx.doi.org/ 10.1021/pr4003992. [DOI] [PubMed] [Google Scholar]
- 10.Holland M, Takada K, Okumoto T, Takahashi N, Kato K, Adu D, Ben-Smith A, Harper L, Savage CO, Jefferis R. Hypogalactosylation of serum IgG in patients with ANCA-associated systemic vasculitis. Clin Exp Immunol 2002; 129:183-90; PMID:12100039; http://dx.doi.org/ 10.1046/j.1365-2249.2002.01864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saldova R, Royle L, Radcliffe CM, Abd Hamid UM, Evans R, Arnold JN, Banks RE, Hutson R, Harvey DJ, Antrobus R, et al.. Ovarian cancer is associated with changes in glycosylation in both acute-phase proteins and IgG in the western world. Glycobiology 2007; 17:1344-56; PMID:17884841; http://dx.doi.org/ 10.1093/glycob/cwm100. [DOI] [PubMed] [Google Scholar]
- 12.Debruyne EN, Delanghe JR. Diagnosing and monitoring hepatocellular carcinoma with α-fetoprotein: new aspects and applications. Clin Chim Acta 2008; 395:19-26; PMID:18538135; http://dx.doi.org/ 10.1016/j.cca.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Zhang XF, Lai EC, Kang XY, Qian HH, Zhou YM, Shi LH, Shen F, Yang YF, Zhang Y, Lau WY, et al.. Lens culinaris agglutinin-reactive fraction of α-fetoprotein as a marker of prognosis and a monitor of recurrence of hepatocellular carcinoma after curative liver resection. Ann Surg Oncol 2011; 18:2218-23; PMID:21336512; http://dx.doi.org/ 10.1245/s10434-011-1613-7. [DOI] [PubMed] [Google Scholar]
- 14.Olubuyide IO, Salimonu LS, Adeniran SO. Soluble immune complexes and immunoglobulin (IgG, IgA and IgM) levels in Nigerians with primary liver cell carcinoma. Afr J Med Sci 1993; 22:57-62. [PubMed] [Google Scholar]
- 15.Manjula S, Aroor AR, Raja A, Rao SN, Rao A. Serum immunoglobulins in brain tumors. Acta Neurochir (Wien) 1992; 115:103-5; PMID:1605076; http://dx.doi.org/ 10.1007/BF01406366. [DOI] [PubMed] [Google Scholar]
- 16.Taylor DD, Gercel-Taylor C. Tumor-reactive immunoglobulins in ovarian cancer: diagnostic and therapeutic significance? (review) Oncol Rep 1998; 5:1519-24. [DOI] [PubMed] [Google Scholar]
- 17.Gure AO, Altorki NK, Stockert E, Scanlan MJ, Old LJ, Chen YT. Human lung cancer antigens recognized by autologous antibodies: definition of a novel cDNA derived from the tumor suppressor gene locus on chromosome 3p21.3. Cancer Res 1998;58:1034-41; PMID:9500467. [PubMed] [Google Scholar]
- 18.Gure AO, Stockert E, Scanlan MJ, Keresztes RS, Jager D, Altorki NK, Oid LJ, Chen YT. Serological identification of embryonic neural proteins as highly immunogenic tumor antigens in small cell lung cancer. Proc Natl Acad Sci USA: 2000;97:4198-203; PMID:10760287; http://dx.doi.org/ 10.1073/pnas.97.8.4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qiu XY, Zhu XH, Zhang L, Mao YT, Zhang J, Hao P, Li GH, Lv P, Li ZX, Sun X, et al.. Human epithelial cancers secrete immunoglobulin G with unidentified specificity to promote growth and survival of tumor cells. Cancer Res 2003; 63:6488-95; PMID:14559841. [PubMed] [Google Scholar]
- 20.Arnold JN, Saldova R, Hamid UM, Rudd PM. Evaluation of the serum N-linked glycome for the diagnosis of cancer and chronic inflammation. Proteomics 2008; 8:3284-93; PMID:18646009; http://dx.doi.org/ 10.1002/pmic.200800163. [DOI] [PubMed] [Google Scholar]
- 21.Kam RK, Poon TC, Chan HL, Wong N, Hui AY, Sung JJ. High-throughput quantitative profiling of serum N-glycome by MALDI-TOF mass spectrometry and N-glycomic fingerprint of liver fibrosis. Clin Chem 2007; 53:1254-63; PMID:17510303; http://dx.doi.org/ 10.1373/clinchem.2007.085563. [DOI] [PubMed] [Google Scholar]
- 22.Lauc G, Huffman JE, Pučić M, Zgaga L, Adamczyk B, Mužinić A, Novokmet M, Polašek O, Gornik O, Krištić J, et al.. Loci associated with N-glycosylation of human immunoglobulin G show pleiotropy with autoimmune diseases and haematological cancers. PLOS Genet 2013; 9:e1003225; PMID:23382691; http://dx.doi.org/ 10.1371/journal.pgen.1003225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji J, Gu X, Fang M, Zhao Y, Yi C, Wang A, Gao C. Expression of α 1,6-fucosyltransferase 8 in hepatitis B virus-related hepatocellular carcinoma influences tumour progression. Dig Liver Dis 2013; 45:414-21; PMID:23352314; http://dx.doi.org/ 10.1016/j.dld.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Kalabay L, Jakab L, Prohászka Z, Füst G, Benkö Z, Telegdy L, Lörincz Z, Závodszky P, Arnaud P, Fekete B. Human fetuin/alpha2HS-glycoprotein level as a novel indicator of liver cell function and short-term mortality in patients with liver cirrhosis and liver cancer. Eur J Gastroenterol Hepatol 2002; 14:389-94; PMID:11943951; http://dx.doi.org/ 10.1097/00042737-200204000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Ang IL, Poon TC, Lai PB, Chan AT, Ngai SM, Hui AY, Johnson PJ, Sung JJ. Study of serum haptoglobin and its glycoforms in the diagnosis of hepatocellular carcinoma: a glycoproteomic approach. J Proteome Res 2006; 5:2691-700; PMID:17022640; http://dx.doi.org/ 10.1021/pr060109r. [DOI] [PubMed] [Google Scholar]
- 26.Hashimoto S1, Asao T, Takahashi J, Yagihashi Y, Nishimura T, Saniabadi AR, Poland DC, van Dijk W, Kuwano H, Kochibe N, et al.. Alpha l-acid glycoprotein fucosylation as a marker of carcinoma progression and prognosis. Cancer 2004;101:2825-36; PMID:15536618; http://dx.doi.org/ 10.1002/cncr.20713. [DOI] [PubMed] [Google Scholar]
- 27.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology 2005; 42:1208-36; PMID:16250051; http://dx.doi.org/ 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 28.Shen QJ, Fan J, Yang XR, Tan YX, Zhao WF, Xu Y, Wang N, Niu YD, Wu Z, Zhou J, et al.. Serum DKK1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: a large-scale, multicenter study. Lancet Oncol 2012; 13:817-26; PMID:22738799; http://dx.doi.org/ 10.1016/S1470-2045(12)70233-4. [DOI] [PubMed] [Google Scholar]
- 29.Tian J, Tang ZY, Ye SL, Liu YK, Lin ZY, Chen J, Xue Q. New human hepatocellular carcinoma (HCC) cell line with highly metastatic potential (MHCC97) and its expressions of the factors associated with metastasis. Br J Cancer 1999; 81:814-21; PMID:10555751; http://dx.doi.org/ 10.1038/sj.bjc.6690769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang T, Hu HS, Feng YX, Shi J, Li N, Guo WX, Xue J, Xie D, Liu SR, Wu MC, et al.. Characterisation of a novel cell line (CSQT-2) with high metastatic activity derived from portal vein tumour thrombus of hepatocellular carcinoma. Br J Cancer 2010; 102:1618-26; PMID:20461085; http://dx.doi.org/ 10.1038/sj.bjc.6605689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dooley S, Hamzavi J, Ciuclan L, Godoy P, Ilkavets I, Ehnert S, Ueberham E, Gebhardt R, Kanzler S, Geier A, et al.. Hepatocyte-specific Smad7 expression attenuates TGF-β-mediated fibrogenesis and protects against liver damage. Gastroenterology 2008; 135:642-59; PMID:18602923; http://dx.doi.org/ 10.1053/j.gastro.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 32.Miyoshi E, Ihara Y, Hayashi N, Fusamoto H, Kamada T, Taniguchi N. Transfection of N-acetylglucosaminyltransferase III gene suppresses expression of hepatitis B virus in a human hepatoma cell line, HB611. J Biol Chem 1995; 270:28311-5; PMID:7499330; http://dx.doi.org/ 10.1074/jbc.270.47.28311. [DOI] [PubMed] [Google Scholar]
- 33.Vanhooren V, Laroy W, Libert C, Chen C. N-glycan profiling in the study of human aging. Biogerontology 2008; 9:351-6; PMID:18431686; http://dx.doi.org/ 10.1007/s10522-008-9140-z. [DOI] [PubMed] [Google Scholar]
- 34.Fang M, Zhao YP, Zhou FG, Lu LG, Qi P, Wang H, Zhou K, Sun SH, Chen CY, Gao CF. N-glycan based models improve diagnostic efficacies in hepatitis B virus-related hepatocellular carcinoma. Int J Cancer 2010; 127:148-59; PMID:19904744; http://dx.doi.org/ 10.1002/ijc.25030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.