Abstract
In liver cancer tumor-infiltrating regulatory T cells (Ti-Treg) are potent suppressors of tumor-specific T-cell responses and express high levels of the Treg-associated molecules cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and glucocorticoid-induced tumor necrosis factor receptor (GITR). In this study, we have evaluated the capacity of GITR-ligation, CTLA-4-blockade and a combination of both treatments to alleviate immunosuppression mediated by Ti-Treg. Using ex vivo isolated cells from individuals with hepatocellular carcinoma (HCC) or liver metastases from colorectal cancer (LM-CRC) we show that treatment with a soluble form of the natural ligand of GITR (GITRL), or with blocking antibodies to CTLA-4, reduces the suppression mediated by human liver tumor-infiltrating CD4+Foxp3+ Treg, thereby restoring proliferation and cytokine production by effector T cells. Importantly, combined treatment with low doses of both molecules exhibited stronger recovery of T cell function compared with either treatment alone. Our data suggest that in patients with primary and secondary liver cancer both GITR-ligation and anti-CTLA-4 mAb can improve the antitumor immunity by abrogating Ti-Treg mediated suppression.
Keywords: cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), glucocorticoid-induced tumor necrosis factor receptor (GITR), hepatocellular carcinoma (HCC), liver metastases from colorectal cancer (LM-CRC), regulatory T cells (Treg)
Introduction
HCC, the sixth most frequent tumor in the world 1 and the third most common cause of cancer-related death, 2 is an aggressive tumor with poor prognosis. HCC accounts for most of primary liver malignancies. However colorectal cancer (CRC), the third most common cancer worldwide, commonly metastasizes to the liver, which is the leading cause of CRC mortality. 3,4 The current therapeutic options for HCC and liver metastasis from CRC (LM-CRC) include surgery (resection and liver transplantation) and local (ablative) therapy. Unfortunately, less than 20% of HCC patients are eligible for curative procedures 5 because most of them have advanced disease at the time of diagnosis. Furthermore, over 50% of LM-CRC patients that undergo surgery develop recurrence within 2 y. 6,7 Chemotherapy has showed limited efficacy and provides a survival advantage of only 2.3–2.8 mo in advanced HCC. 8,9 An attractive alternative to these current therapeutic options is immunotherapy, which is based on the sensitivity, specificity and memory of the immune system. However, immune regulation in the liver and tumor environment may contribute to tumor outgrowth and limit the efficacy of immunotherapeutic strategies. 10,11 In support of this we and others have described the presence of regulatory CD4+Foxp3+ T cells in liver tumors, that suppress local antitumor immunity and that are associated with poor patient prognosis. 12-16 Liver Ti-Treg are characterized by high expression of glucocorticoid-induced tumor necrosis factor receptor related gene (GITR), CTLA-4 and inducible T cell co-stimulator (ICOS). 12 These molecules are important for the immunosuppressive function of Treg and can be targets for immunotherapeutic interventions. Abrogation of Ti-Treg function may allow the induction of antitumor immunity at the tumor site and improve the outcome of immunotherapy aimed to activate antitumor responses. In advanced melanoma, CTLA-4-blocking has demonstrated a survival advantage 17,18 and CTLA-4-blockade was shown to induce antiviral immunity in HCC-patients infected with hepatitis C virus (HCV). 19 However, only limited tumor-responses were reported, emphasizing the need to improve this strategy. Combining several candidate-immunomodulators may achieve synergistic immunoregulatory activity.
In this study, we have evaluated ex vivo the capacity of GITR-ligation, CTLA-4-blockade and a combination of both to alleviate immunosuppression mediated by Ti-Treg isolated from patients with primary and secondary liver cancer.
Results
GITR+CTLA-4+ Treg accumulate in liver tumors and have an increased suppressive capacity
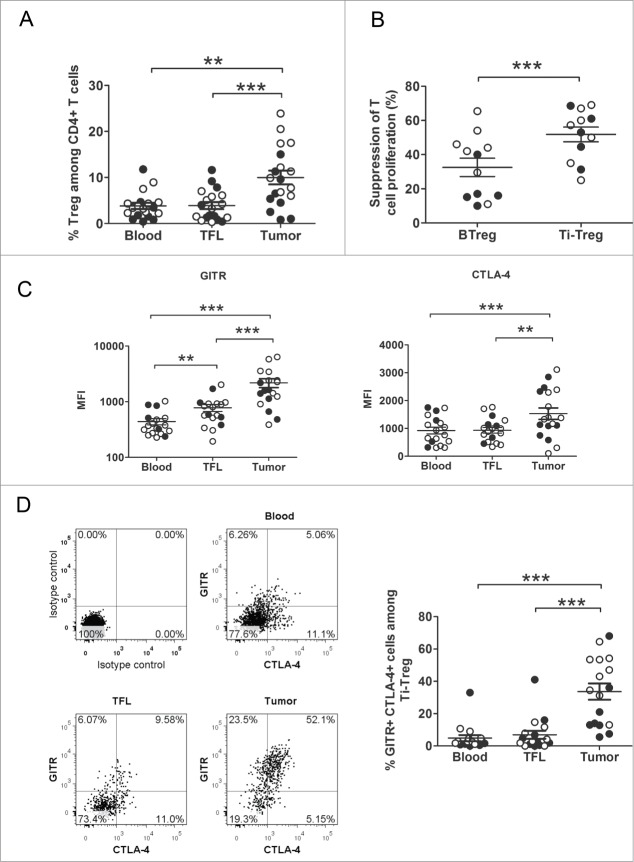
In order to confirm our previous finding showing that activated CD4+Foxp3+Treg are sequestered at the liver tumor site, we analyzed Treg in lymphocytes isolated from fresh liver tumors, tumor-free liver (TFL) tissues, and peripheral blood (PB) in a new cohort of HCC and LM-CRC patients by flow cytometry. Treg were present in all the three compartments analyzed, but were significantly more concentrated in the tumor areas compared with TFL (p = 0.0004) and blood (p < 0.0001) (Fig. 1A). We also corroborate in this new cohort that Ti-Treg are more suppressive than circulating Treg by analyzing their impact on T cell proliferation of autologous CD4+CD25− T cells stimulated with CMV-activated dendritic cells (DC). Ti-Treg showed a stronger suppression of T cell proliferation compared with blood Treg (p = 0.0005) (Fig. 1B). In addition, we analyzed the surface expression level of GITR and intracellular expression of CTLA-4 (Fig. 1C). GITR expression was significantly higher on tumor Treg than on Treg isolated from TFL (p = 0.0005) and blood (p = 0.0002). Tumor Treg were also distinguishable from blood and TFL Treg by their elevated intracellular expression of CTLA-4, which is a key negative regulator of T-cell activation (p = 0.0004 and 0.0018 respectively). Moreover, we found that a big proportion of Ti-Treg expressed both molecules, in contrast with blood or TFL derived Tregs that have a very low proportion of double positive cells (Fig. 1D). Thus, Ti-Treg derived from liver tumors express high levels of GITR and CTLA-4 and have an enhanced suppressive capacity.
Figure 1 (See previous page).
Tumor-infiltrating Treg are potent suppressors of T cell responses, and they are characterized by the expression of higher levels of CTLA-4 and GITR. (A) The proportions of Treg (CD3+CD4+CD25+FoxP3+) among CD4+ T cells were analyzed by flow cytometry in tumor, tumor-free liver tissue (TFL) and peripheral blood from patients with HCC or LM-CRC. Differences were analyzed by Wilcoxon matched pairs test. (B) CFSE-labeled CD4+CD25− T cells from PB were stimulated with autologous CMV-activated mDC (CMV-DC) for 5 d Autologous blood or tumor derived Treg from HCC-patients or LM-CRC patients were added in a ratio 1:5. Inhibition of T cell proliferation by Treg was determined by flow cytometry and reported as percentage of suppression of T cell proliferation. Data analyzed by paired t-test. (C) Differential expression of surface GITR and intracellular CTLA-4 was measured on Treg present in blood, TFL and tumor tissue. Differences were analyzed by Wilcoxon matched pairs test for GITR and by paired t-test for CTLA-4. (D) FACS analysis of the co-expression of CTLA-4 and GITR by Treg from blood, TFL and tumor tissue. One representative patient and the collective data analyzed by Wilcoxon matched pairs test. HCC (closed symbols) or LM-CRC (open symbols). Values are also depicted as means ±SEM. *p < 0.05, ** p < 0.01, *** p < 0.001.
GITR engagement reduces suppressive capacity of Ti-Treg
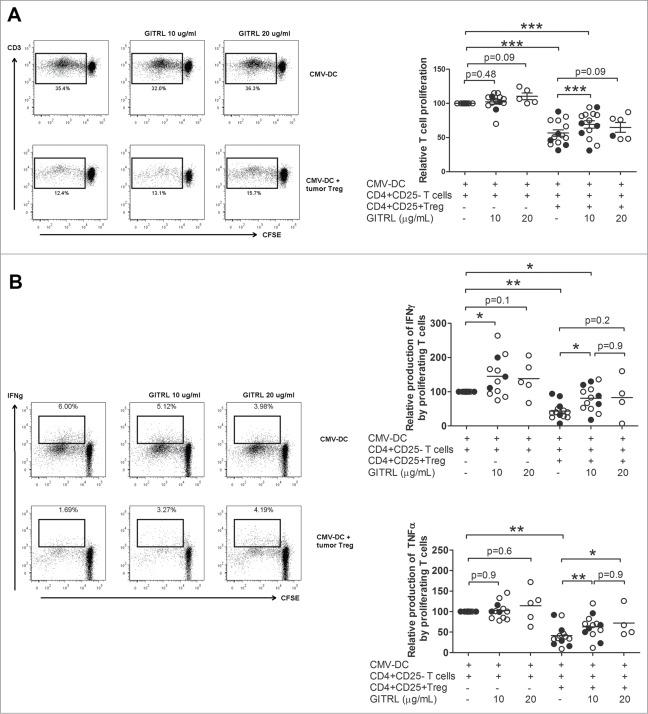
Soluble GITRL (sGITRL) was able to lower T cell suppression by Ti-Treg derived from liver tumors of patients with HCC or LM-CRC (Fig. 2). CD4+CD25− effector T cells proliferated robustly in response to autologous DC activated with CMV. This proliferative response, as well as the production of TNFα was not affected by the addition of 10 or 20 µg/mL of sGITRL. However, GITRL induced an increased amount of IFNγ suggesting a limited direct effect on CD4+CD25− T cells. CMV-specific T cell proliferation and cytokine production were inhibited by Ti-Treg derived from both groups of patients (Fig. 2A). Importantly, the addition of 10 µg/mL of sGITRL significantly reduced the suppression mediated by Ti-Treg on CMV-specific T cells, recovering both T-cell proliferation (57 ± 17 % vs. 69 ± 20 % proliferating CD4+ T cells, p = 0.0005) and cytokine production by proliferating cells (TNFα: 42 ± 25 % vs. 63 ± 28 %, p < 0.0001; IFNγ: 44 ± 24 % vs. 80 ± 37 %, p = 0.0001) (Fig. 2A, B). However, 10 µg/mL of sGITRL did not abrogate suppression of T cell proliferation completely, and a higher dose of sGITRL (20 µg/mL) could not further restore antigen-specific T cell proliferation nor cytokine production (Fig. 2A, B), and induced T cell death instead in most patients investigated (data not shown). Thus, sGITRL is able to partially decrease T cell suppression mediated by Ti-Treg isolated from liver cancer patients.
Figure 2.
GITR engagement partially abrogates suppression mediated by tumor-infiltrating Treg. CD4+CD25− effector T cells were isolated from peripheral blood and labeled with CFSE, and co-cultured during 5 d with autologous mDC activated with CMV antigens. Autologous tumor Treg from HCC-patients (closed symbols) or LM-CRC patients (open symbols) were added in the absence or presence of different concentrations of soluble GITRL (sGITRL). T cell proliferation (A) and cytokine (IFNγ and TNFα) production by proliferating cells (B) were measured by flow cytometry after re-stimulation with CMV-activated Mo-DC. Values are also depicted as means ±SEM, *p < 0.05, ** p < 0.01, *** p < 0.001. Differences were analyzed by paired t-test.
CTLA-4 blockade prevents T cell suppression mediated by Ti-Treg in a dose-dependent manner
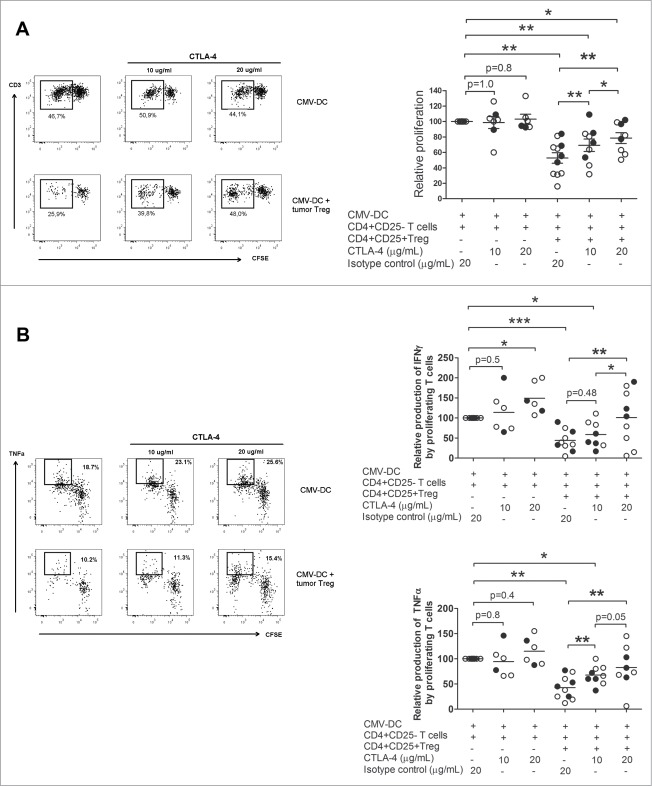
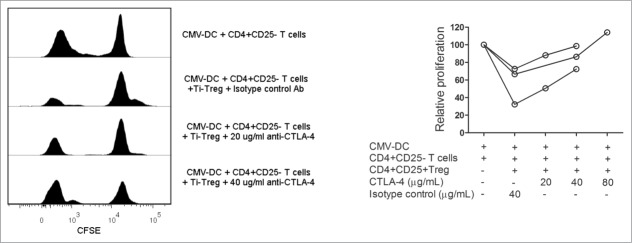
To address the impact of CTLA-4 in the suppression mediated by Ti-Treg we used a neutralizing antibody for CTLA-4 in our in vitro model of T cell proliferation. The anti-CTLA-4 antibody at a concentration of 10 µg/mL did not affect T cell proliferation or TNFα and IFNγ production in the absence of Treg, however treatment with 20 µg/mL of anti-CTLA-4 antibody induced an increase in the production of IFNγ (Fig. 3B), suggesting a direct effect of this antibody on CD4+CD25− effector T cells at this concentration. Anti-CTLA-4 treatment reduced the suppression mediated by Ti-Treg in a dose-dependent manner (Fig. 3A). Recovery of T cell function was observed in both T-cell proliferation (52.8 ± 22.5 % when Ti-Treg were present vs. 69.3 ± 23 % in the presence of Ti-Treg and 10 µg/mL of anti-CTLA-4, p = 0.008, vs. 78.6 ± 19.9 % with 20 µg/mL of anti-CTLA-4 antibody, p = 0.008) and cytokine production by proliferating cells (TNFα: 43 ± 23 % in the presence of Ti-Treg vs 67 ± 18 % with Ti-Treg and 10 µg/mL of anti-CTLA-4 antibody, p = 0.025, and 83 ± 42 % with Ti-Treg and 20 µg/mL of anti-CTLA-4 antibody, p = 0.008; IFNγ: 44 ± 26 % vs. 59 ± 33 %, p = 0.48, vs. 101 ± 69 %, p = 0.014, respectively) (Fig. 3A, B). Because T cell proliferation was not recovered completely with 20 µg/mL of anti-CTLA-4 antibody, as opposed to (near) full recovery of cytokine production, we were interested in investigating the effect of increasing the concentration of anti-CTLA-4 antibody further. Therefore, another set of antibody blocking experiments was performed with 40 and 80 µg/mL of anti-CTLA-4 antibody, which showed that higher concentrations of the neutralizing antibody for CTLA-4 resulted in complete recovery of T cell proliferation (Fig. 4).
Figure 3.
CTLA-4 blockage decreases (T)cell suppression mediated by tumor-infiltrating Treg in a dose dependent manner. CFSE-labeled CD4+CD25- T cells isolated from PB were stimulated with autologous CMV-DC and cultured with autologous tumor derived Tregs from HCC-patients (closed symbols) or LM-CRC (open symbols) in the absence or presence of 10 or 20 ug/mL of blocking anti-CTLA-4 mAb or an irrelevant isotype control antibody. Cell proliferation (A) and cytokine production by proliferating cells (B) were analyzed by flow cytometry after re-stimulation with CMV-activated Mo-DC. Values are also depicted as means ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001. All data were analyzed by paired t-test.
Figure 4.
High doses of anti-CTLA-4 mAb can completely abrogate (T)cell suppression mediated by tumor-infiltrating Treg. The effect of higher doses of anti-CTLA-4 mAb on Treg-mediated suppression was tested using lymphocytes isolated from three different LM-CRC patients. T cell proliferation analysis revealed that higher doses of the neutralizing anti-CTLA-4 antibody are able to completely abolish the suppression mediated by tumor-infiltrating Treg.
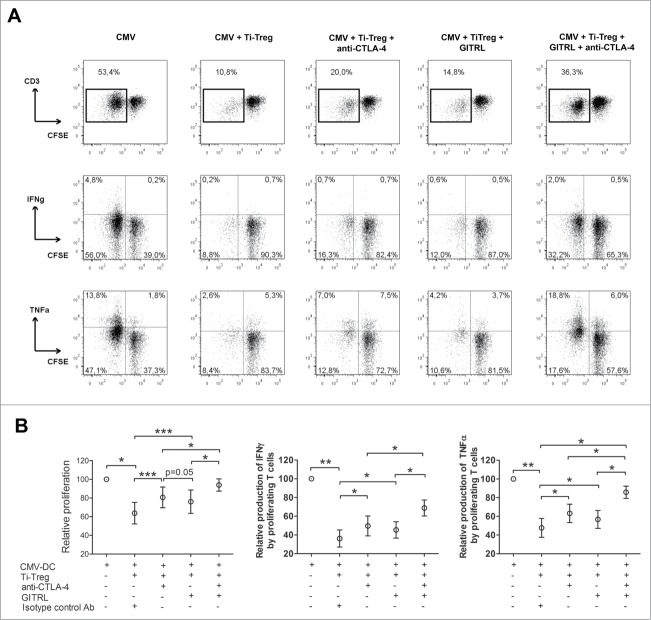
GITRL and anti-CTLA-4 antibody combination additively abolish T cell suppression mediated by Ti-Treg
We next asked whether a low dose of GITRL combined with a low dose of neutralizing anti-CTLA-4 antibody could fully recover T cell function in the presence of Ti-Treg. Blood derived CFSE-labeled CD4+CD25− stimulated with autologous DC activated with CMV were cultured in the presence of Ti-Treg and treated with 10 µg/mL of GITRL and anti-CTLA-4 antibody, either as monotherapy or in combination. Monotherapy with GITRL or anti CTLA-4 antibody induced partial recovery of T cell proliferation and cytokine production as observed in the experiments mentioned above (Figs. 2, 3 and 5). In comparison with the effect of each treatment alone, the combination of low doses of anti-CTLA-4 and GITRL resulted in marked enhancement of T cell proliferation and cytokine production (Fig. 5A, B). Importantly, the combination of low doses of both molecules provided similar effects as those observed with a high dose of anti-CTLA-4 antibody, restoring T cell proliferation and TNFα production completely.
Figure 5.
GITRL and CTLA-4 blockage additively abolish (T)cell suppressive capacity of tumor-infiltrating Treg. Blood-derived CFSE-labeled CD4+CD25− T cells from HCC-patients or LM-CRC patients were co-cultured with autologous CMV-DC for 5 d Autologous tumor derived Treg were added in a ratio 1:5. Cells were treated with 10 µg/mL of anti-CTLA-4 mAb or GITRL or a combination of both. Cell proliferation and cytokine production were analyzed by flow cytometry after re-stimulation with CMV-activated Mo-DC. (A) Depicts a representative experiment showing T cell proliferation, IFNγ and TNFα production after co-culture and re-stimulation. (B) Collective data of 5 patients tested (3 LM-CRC and 2 HCC) showing the relative T cell proliferation or cytokine production (IFNγ and TNFα) by proliferating cells. Values are means ± SEM, * p < 0.05, ** p < 0.01, *** p < 0.001. Comparison between groups was made by paired t-test.
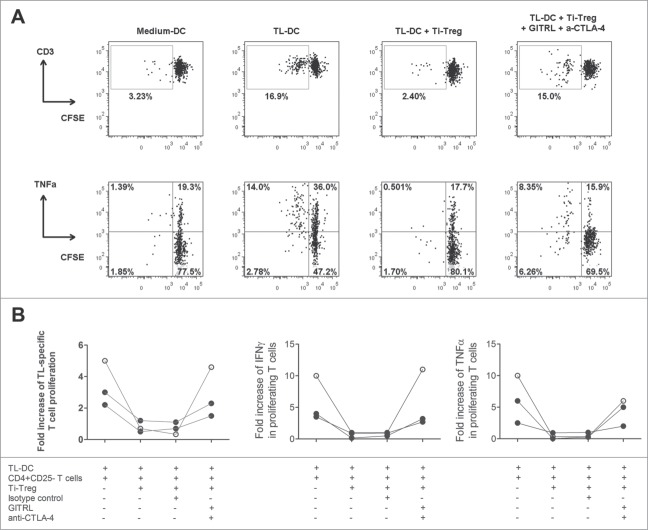
Combination of GITRL and anti-CTLA-4 antibody restored tumor-specific T cell response
To investigate whether the combination of GITRL and anti-CTLA-4 antibody is able to reestablish tumor-specific T cell responses, we used blood derived CFSE-labeled CD4+CD25− T cells stimulated with DC cultured with Tumor Lysates (TL-DC). Cells were co-cultured in the presence of Ti-Treg and treated with 10 µg/mL of GITRL and 10 µg/mL of anti-CTLA-4 antibody. All cell types and the TL were autologous. After one week of culture we observed that Ti-Treg suppressed efficiently cell proliferation and cytokine production of T cells against TL (Fig. 6). However, when the cells were treated with both GITRL and anti-CTLA-4 antibodies the tumor specific response can be completely restored.
Figure 6.
Treatment with GITRL and anti-CTLA-4 antibody can recover ex vivo antitumor T-cell immunity. Blood mDC activated with autologous TL were used to stimulate CFSE-labeled autologous peripheral CD4+CD25− T cells for one week. In some cultures autologous Ti-Treg were added and cells were treated with 10 µg/mL of an isotype control antibody or with a mixture of 10 µg/mL of GITRL and 10 µg/mL of anti-CTLA-4 antibody. T cell proliferation and cytokine production were analyzed by flow cytometry after re-stimulation with PMA and ionomycin. Proliferation and cytokine production are reported as fold increase of specific T cell proliferation or cytokine production, calculated by dividing the percentage of proliferation or cytokine production (TNFα or IFNγ) in the mDC + TL condition by that in the control condition without TL (medium DC). (A) A representative analysis from one patient and (B) Collective data from 3 different patients tested. HCC (closed symbols) or LM-CRC (open symbols).
Discussion
New treatment modalities are required to prolong survival in patients with liver cancer. Immunotherapy is an attractive option for HCC patients and patients with liver metastasis of CRC because increasing evidence suggests that immune responses play an important role in the control of these types of cancer. It has been reported that the composition of the tumor-infiltrating lymphocytes (TILs) is associated with the prognosis of both diseases, for example increased numbers of Treg are correlated with disease progression 20-24 and recurrence. 25 Furthermore, Treg frequencies have been associated with increased numbers of intra-tumoral macrophages, 13,26 circulating myeloid-derived suppressor cells (MDSC), 27 and with a compromised CD8+ T cell response. 14,15 Altogether these observations suggest that depletion or inhibition of Treg and concomitant stimulation of effector cells may be effective to reduce recurrence and prolong survival in patients with liver cancer. Indeed, depletion of CD25+ T cells in patients or animals models can result in induction of tumor-specific responses. 28,29 Moreover, in a small clinical trial in advanced HCC patients, treatment with low doses of cyclophosphamide resulted in reduced numbers of Treg and unmasking of circulating tumor-specific CD4+ T cell responses. 30
We previously reported the presence of highly activated CD4+Foxp3+ Treg in liver tumors of individuals with HCC or LM-CRC. 12 These Ti-Treg are characterized by the expression of high levels of GITR and CTLA-4, which are important molecules for their regulatory function and which can be targets for immunotherapy.12,16 GITR activation by its ligand acts as a co-stimulatory molecule for effector T cells that confers them less susceptible to Treg mediated suppression, whereas engagement of GITR expressed by Treg transiently inhibits their suppressive capacity (reviewed in 31). The GITR agonist antibody DTA-1 represents a very effective antitumor therapy in murine tumor models by increasing antitumor CD4+ and CD8+ T-cell effector functions as well as destabilizing and causing apoptosis of Tregs in the tumor microenvironment.32,33 Importantly, our findings support a role for GITR engagement as (part of) an immunotherapy for liver cancer patients, since treatment with sGITRL reduced Treg mediated inhibition of T cell functions. However, the observed effect was only partial and could not be enhanced by dose escalation. Indeed, a high dose of sGITRL had a cytotoxic effect on effector T cells in our in vitro system. In preclinical animal models, treatment with agonistic antibodies for GITR has been shown to cause anaphylaxis upon repeated doses.34 These observations warrant careful design and dosing strategies of drugs engaging GITR to prevent serious side effects during immunotherapy for patients with liver tumors.
On the other hand, CTLA-4 is constitutively expressed at high levels in CD4+Foxp3+ Treg and is a negative regulator of T-cell activation. By binding the B7 molecules CD80 and CD86, CLTA-4 reduces the T-cell stimulatory capacity of antigen-presenting cells such as DC. 35 Therefore, by using monoclonal antibodies (mAb) which block CTLA-4, it is possible to alleviate inhibition of DC function by Treg and thereby restore conventional T-cell proliferation after activation. 36 Recent studies have demonstrated that anti-CTLA-4 mAb administration augments clinical antitumor responses and improves survival in patients with metastatic melanoma 18,37 resulting in FDA approval of Ipilimumab, an anti-CTLA-4 antibody, for the treatment of unresectable or metastatic melanoma. The promising clinical activity of anti-CTLA-4 mAb in melanoma encourages to explore its therapeutic applicability in other malignancies, especially because it does not require specific targets expressed on tumor cells. However, there is scarce evidence for efficacy in liver cancer. Only one clinical trial using the anti-CTLA-4 mAb tremelimumab in HCC patients infected with HCV has been conducted, but it showed a partial tumor response in merely 17% of patients.19 Even though the clinical response was limited, the results point to a potential use for anti-CTLA-4 mAb in immunotherapeutic regimens for these patients. In our study, 20 µg/mL of anti-CTLA-4 antibody (BNI3) showed a 60% recovery of T cell proliferation, whereas a concentration of 10 µg/mL only provided a recovery of 35% of T cell proliferation. Higher doses were able to recover T cell functions completely. However, therapy with anti-CTLA-4 mAb ipilimumab has induced life-threatening adverse events, 17,38,39 which is a major limitation in exploring higher doses for the treatment. The current clinically approved dosing for anti-CTLA-4 antibody (Ipilimumab) is 3 mg/kg which results in a steady state serum concentration (Cmin) of about 10 µg/mL,18,40 and an overall response rate of 4% in patients with advanced melanoma with 3% of patients experiencing severe immune-related adverse events. A higher dose of Ipilimumab of 10 mg/kg (Cmin 32 µg/mL) showed a better response rate of 11% but also higher frequency (15%) of severe adverse events. 18
Based upon results from murine models, it has previously been suggested that GITR stimulation and CTLA-4 inhibition can be combined to enhance the induction of immune responses and might even work synergistically.41 Importantly, combination of low doses of anti-CTLA-4 antibody and GITRL resulted in a complete recovery of in vitro T cell proliferation and TNFα production. Even though the mechanisms by which these molecules exert their immunomodulatory effects is still elusive, it has been suggested that they can act either by abrogating the immunosuppressive function of Treg or by rendering effector T cells resistant to Treg mediated suppression. In our experiments we observed that both molecules had limited direct effects on effector T cells, only inducing an increase in the production of IFNγ, but this effect was not evident for TNFα and T cell proliferation, suggesting that both mechanisms can play a role in abrogation of the suppression mediated by Ti-Treg. It remains to be seen whether combination therapy of anti-CTLA-4 mAb and GITRL has a more beneficial safety profile compared to treatment with a high dose of either compound alone, but our data show that the combination of both treatments at low doses exhibited stronger inhibition of ex vivo Treg-mediated suppression compared with either treatment alone, suggesting that this combination may be more effective to induce antitumor immunity in patients with liver cancer.
In conclusion our data suggest that in patients with primary and secondary liver cancer both GITR-ligation and anti-CTLA-4 mAb can improve antigen-specific T cell responses in the tumors by protecting against Ti-Treg mediated suppression of their function. However, as part of an immunotherapeutic strategy, combining low doses of both drugs may be at least as effective as monotherapy with higher doses of either of the drugs.
Materials and Methods
Patients
Between June 2011 and November 2013 a total of 28 individuals who were eligible for surgical resection of HCC (n = 10 ) or LM-CRC (n = 18 ) were enrolled. Paired samples of fresh liver tumor tissue and TFL tissue obtained at the maximum distance from the tumor (>1 cm), were used for isolating TILs and intra-hepatic lymphocytes. In addition, PB was collected. None of the patients was treated with chemotherapy or radiation prior to resection. The clinical characteristics of the patients are summarized in Table 1. The study was approved by the local ethics committee and all patients in the study gave informed consent before tissue donation.
Table 1.
Patient characteristics
| HCC (n = 10 ) | LM-CRC (n = 18 ) | |
|---|---|---|
| Sex (male/female) | 8 /2 | 12/6 |
| Age (years) | 55.1 ± 6 .8 | 67.4 ± 1 .9 |
| Race (Caucasian/Asian/African) | 9/0/1 | 18/0/0 |
| ALT (units/L) | 48.2 ± 8.6 | 27.9 ± 3.6 |
| Bilirubin (µmol/L) | 14.0 ± 4.5 | 8.7 ± 3 .4 |
| Prothrombin time (INR) | 1.2 ± 0.03 | 1.0 ± 0 .01 |
| Liver fibrosis (metavir score) F0-F1 /F2 /F3-F4-cirrhosis | 9 /0 /1 | 18 /0 /0 |
| Stage of disease (TNM) | St I n = 7 | |
| St II n = 3 | St IVa n = 18 |
Etiology of liver disease in HCC patients: 6 no known liver disease, 1 hemochromatosis, 1 porphyria, 1 hepatitis B virus, 1 cirrhosis ECI.
INR= international normalized ratio.
Where applicable: mean ± SEM.
Cell preparation
Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll density gradient centrifugation. Single cell suspensions from TFL and tumor tissue were obtained by tissue digestion. Fresh tissue was cut into small pieces and digested with 0.5 mg/mL of collagenase (Cat. C5138, Sigma-Aldrich) and 0.2 mg/mL of DNase I (Cat. 10104159001, Roche), for 30 min at 37°C. Cell suspensions were filtered through 100 and 70 µm pore cell-strainers (BD, biosciences) and mononuclear cells (MNC) were obtained by Ficoll density gradient centrifugation. Viability was determined by trypan blue exclusion.
Flow cytometry analysis
PBMC and MNC isolated from TFL or tumor tissue were analyzed for expression of surface and intracellular markers using the following anti-human antibodies: PE-labeled anti-IFNγ (B27, BD Biosciences) and anti-CD25 (BC96) from ebiosciences; APC-labeled anti-FoxP3 (236A/E7) and anti-TNFa (Mab11) from e-biosciences; APC-H7-labeled anti-CD4+ (SK3, BD Biosciences); PeCy7-labeled anti-CD3 (UCTH1); eFluor®450-labeled anti-CD127 (eBioRDR5) from e-biosciences. Cells were incubated with the antibodies 10 min at room temperature in the dark, then washed and fixed with 1% paraformaldehyde. For intracellular cytokine staining, cells were permeabilized using the BD Cytofix/cytoperm fixation/permeabilization kit, and for FoxP3, cells were treated with the FoxP3 staining buffer set from e-biosciences. Dead cells were excluded by using the LIVE/DEAD fixable dead cell stain kit with aqua fluorescent reactive dye (Invitrogen). Cells were analyzed using a FACSCanto II system (BD Biosciences).
Suppression assay
Myeloid dendritic cells (mDC) were isolated from PBMC by positive selection (BDCA-1 DC isolation kit, Cat. 130–090–506, Miltenyi Biotec). mDC were cultured overnight with 10 µg/mL of Cytomegalovirus antigens (CMV, Cat. EL-01–02, Microbix biosystems, Canada). Autologous CD4+CD25− cells were isolated from PBMCs that were kept overnight at 4°C in medium supplemented with 10% of bovine fetal serum, by magnetic sorting (Cat. 130–091–301, Miltenyi Biotec). CD4+CD25− T cells were labeled with 0.1 µM of carboxyfluorescein diacetate succinimidyl ester (CFSE, C34554, Invitrogen) and co-cultured with the autologous mDC activated with CMV, at a ratio of 1 to 10 for 5 d in round-bottom 96-well plates with at least 5 × 104 CD4+CD25− T cells. Proliferation was measured by dilution of CFSE. CD4+CD25+ Treg were isolated by magnetic sorting as previously described. 12 Treg were added at 1:5 ratio versus CD4+CD25− T cells, and co-cultured for 5 d Treg were labeled with CellTrace Violet (Invitrogen) in order to be excluded from proliferating T cells.12 Furthermore, after co-culture T cells were re-stimulated overnight with autologous monocyte-derived DC activated with CMV, in the presence of brefeldin A and monensin (BD Biosciences). Proliferation and cytokine production were analyzed by flow cytometry and reported as relative proliferation or relative cytokine production where the numbers of proliferating and cytokine-producing cells induced by CMV-DCs were considered 100%. In some experiments azide-free and low endotoxin soluble GITRL (R and D systems, Cat. 6987-GL/CF), anti-CTLA-4 neutralizing (BNI3, Beckman Coulter, Cat. IM2070) or isotype control (IgG2a, Biolegend) antibodies were added to the co-cultures. Monocyte-derived DC were obtained by culturing monocytes with 10 ng/mL IL-4 and 50 ng/mL GM-CSF for 5 d, then immature DC were activated with CMV as described previously for mDC.
Tumor-specific T cell proliferation and suppression assay
Tumor lysates (TL) were generated from freshly dissected tumors by five cycles of freezing and thawing in phosphate buffered saline, followed by filtration (0.2 μm). mDC isolated from PBMC were cultured overnight with media or 20 μg/mL of autologous TL in the presence of 10 ng/mL of granulocyte-macrophage colony-stimulating factor (GM-CSF) (Miltenyi Biotec) and 0.5 μg/mL of polyinosinic:polycytidylic acid (InvivoGen, San Diego, CA). CD4+CD25− cells were isolated from PBMC that were kept overnight at 4°C in medium supplemented with 10% of bovine fetal serum, by magnetic sorting (Miltenyi Biotec). CD4+CD25− T cells were labeled with 0.1 μM of CFSE (Invitrogen) and co-cultured with autologous mDC cultured with media or TL, at a ratio of 1:10 for 5 d in round-bottom 96-well plates with at least 5 × 104 CD4+CD25− T cells. Proliferation was measured by dilution of CFSE. CD4+CD25+ Treg were isolated by magnetic sorting as described above. Treg were added at 1:5 ratio vs. CD4+CD25− T cells, and co-cultured for 5 d Treg were labeled with CellTrace Violet (Invitrogen) in order to be excluded from proliferating T cells. Furthermore, after co-culture T cells were re-stimulated by PMA (40 ng/mL) and ionomycin (1μg/mL) for 5 h in the presence of Brefeldin (Sigma, 5 μg/mL) during the last 4 h. Proliferation and cytokine production were analyzed by flow cytometry and reported as fold increase of specific T cell proliferation or cytokine production, calculated by dividing the percentage of proliferation or cytokine production (TNFα or IFNγ) in the TL condition by that in the control condition (media cultured DC). In all three experiments soluble GITRL (R and D systems, 10 μg/mL), neutralizing anti-CTLA-4 (BNI3, Beckman Coulter, 10 μg/mL) or isotype control (IgG2a, Biolegend, 10 μg/mL) antibodies were added to the co-cultures.
Statistical analysis
All data set distributions were analyzed for normality using the Shapiro–Wilk normality test. The differences between paired groups of data were analyzed according to their distribution by either paired t-test or Wilcoxon matched pairs test using GraphPad Prism Software (version 5.0). p-values less than 0.05 were considered statistically significant (*p < 0.05; ** p < 0.01; ***p < 0.001).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grants to DS (Erasmus MC Fellowship) and AP-G (Erasmus MC Grant 2011) from Erasmus MC.
References
- 1.Anthony P. Hepatocellular carcinoma: an overview. Histopathology 2001; 39:109-18; PMID:11493326; http://dx.doi.org/ 10.1046/j.1365-2559.2001.01188.x. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. International journal of cancer 2001; 94:153-6; PMID:11668491 [DOI] [PubMed] [Google Scholar]
- 3.de Jong MC, Mayo SC, Pulitano C, Lanella S, Ribero D, Strub J, Hubert C, Gigot JF, Schulick RD, Choti MA. Repeat curative intent liver surgery is safe and effective for recurrent colorectal liver metastasis: results from an international multi-institutional analysis. Journal of Gastrointestinal Surgery 2009; 13:2141-51; PMID:19795176; http://dx.doi.org/ 10.1007/s11605-009-1050-0 [DOI] [PubMed] [Google Scholar]
- 4.House MG, Ito H, Gönen M, Fong Y, Allen PJ, DeMatteo RP, Brennan MF, Blumgart LH, Jarnagin WR, D'Angelica MI. Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1,600 patients during two decades at a single institution. Journal of the American College of Surgeons 2010; 210:744-52; PMID:20421043; http://dx.doi.org/ 10.1016/j.jamcollsurg.2009.12.040 [DOI] [PubMed] [Google Scholar]
- 5.El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology 2008; 134:1752-63; PMID:18471552; http://dx.doi.org/ 10.1053/j.gastro.2008.02.090 [DOI] [PubMed] [Google Scholar]
- 6.Kemeny N. Presurgical chemotherapy in patients being considered for liver resection. The Oncologist 2007; 12:825-39; PMID:17673614 [DOI] [PubMed] [Google Scholar]
- 7.de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD, Choti MA, Aldrighetti L, Capussotti L, Pawlik TM. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Annals of surgery 2009; 250:440; PMID:19730175 [DOI] [PubMed] [Google Scholar]
- 8.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. New England Journal of Medicine 2008; 359:378-90; PMID:18650514; http://dx.doi.org/ 10.1056/NEJMoa0708857 [DOI] [PubMed] [Google Scholar]
- 9.Siegel AB, Olsen SK, Magun A, Brown RS. Sorafenib: where do we go from here? Hepatology 2010; 52:360-9; PMID:20578152; http://dx.doi.org/ 10.1002/hep.23633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pedroza-Gonzalez A, Xu K, Wu TC, Aspord C, Tindle S, Marches F, Gallegos M, Burton EC, Savino D, Hori T et al.. Thymic stromal lymphopoietin fosters human breast tumor growth by promoting type 2 inflammation. J Exp Med 2011; 208:479-90; PMID:21339324; http://dx.doi.org/ 10.1084/jem.20102131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pardee AD, Butterfield LH. Immunotherapy of hepatocellular carcinoma: Unique challenges and clinical opportunities. Oncoimmunology 2012; 1:48-55; PMID:22720211; http://dx.doi.org/ 10.4161/onci.1.1.18344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pedroza-Gonzalez A, Verhoef C, Ijzermans JN, Peppelenbosch MP, Kwekkeboom J, Verheij J, Janssen HL, Sprengers D. Activated tumor-infiltrating CD4+ regulatory T cells restrain antitumor immunity in patients with primary or metastatic liver cancer. Hepatology 2013; 57:183-94; PMID:22911397; http://dx.doi.org/ 10.1002/hep.26013 [DOI] [PubMed] [Google Scholar]
- 13.Zhou J, Ding T, Pan W, Zhu LY, Li L, Zheng L. Increased intratumoral regulatory T cells are related to intratumoral macrophages and poor prognosis in hepatocellular carcinoma patients. Int J Cancer 2009; 125:1640-8; PMID:19569243; http://dx.doi.org/ 10.1002/ijc.24556 [DOI] [PubMed] [Google Scholar]
- 14.Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, Zhang Z, Yang H, Zhang H, Zhou C et al.. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology 2007; 132:2328-39; PMID:17570208; http://dx.doi.org/ 10.1053/j.gastro.2007.03.102 [DOI] [PubMed] [Google Scholar]
- 15.Unitt E, Rushbrook SM, Marshall A, Davies S, Gibbs P, Morris LS, Coleman N, Alexander GJ. Compromised lymphocytes infiltrate hepatocellular carcinoma: the role of T-regulatory cells. Hepatology 2005; 41:722-30; PMID:15791620; http://dx.doi.org/ 10.1002/hep.20644 [DOI] [PubMed] [Google Scholar]
- 16.Pedroza-Gonzalez A, Kwekkeboom J, Sprengers D. T-cell suppression mediated by regulatory T cells infiltrating hepatic tumors can be overcome by GITRL treatment. Oncoimmunology 2013; 2:e22450; PMID:23483229; http://dx.doi.org/ 10.4161/onci.22450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC et al.. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711-23; PMID:20525992; http://dx.doi.org/ 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, Waterfield W, Schadendorf D, Smylie M, Guthrie T Jr. et al.. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol 2010; 11:155-64; PMID:20004617; http://dx.doi.org/ 10.1016/S1470-2045(09)70334-1 [DOI] [PubMed] [Google Scholar]
- 19.Sangro B, Gomez-Martin C, de la Mata M, Inarrairaegui M, Garralda E, Barrera P, Riezu-Boj JI, Larrea E, Alfaro C, Sarobe P et al.. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol 2013; 59:81-8; PMID:23466307; http://dx.doi.org/ 10.1016/j.jhep.2013.02.022 [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi N, Hiraoka N, Yamagami W, Ojima H, Kanai Y, Kosuge T, Nakajima A, Hirohashi S. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res 2007; 13:902-11; PMID:17289884; http://dx.doi.org/ 10.1158/1078-0432.CCR-06-2363 [DOI] [PubMed] [Google Scholar]
- 21.Chen KJ, Lin SZ, Zhou L, Xie HY, Zhou WH, Taki-Eldin A, Zheng SS. Selective recruitment of regulatory T cell through CCR6-CCL20 in hepatocellular carcinoma fosters tumor progression and predicts poor prognosis. PLoS One 2011; 6:e24671; PMID:21935436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen X, Li N, Li H, Zhang T, Wang F, Li Q. Increased prevalence of regulatory T cells in the tumor microenvironment and its correlation with TNM stage of hepatocellular carcinoma. J Cancer Res Clin Oncol 2010; 136:1745-54; PMID:20221638; http://dx.doi.org/ 10.1007/s00432-010-0833-8 [DOI] [PubMed] [Google Scholar]
- 23.Ling KL, Pratap SE, Bates GJ, Singh B, Mortensen NJ, George BD, Warren BF, Piris J, Roncador G, Fox SB et al.. Increased frequency of regulatory T cells in peripheral blood and tumour infiltrating lymphocytes in colorectal cancer patients. Cancer Immun 2007; 7:7; PMID:17388261 [PMC free article] [PubMed] [Google Scholar]
- 24.Brudvik KW, Henjum K, Aandahl EM, Bjornbeth BA, Tasken K. Regulatory T-cell-mediated inhibition of antitumor immune responses is associated with clinical outcome in patients with liver metastasis from colorectal cancer. Cancer Immunol Immunother 2012; 61:1045-53; PMID:22159472; http://dx.doi.org/ 10.1007/s00262-011-1174-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sasaki A, Tanaka F, Mimori K, Inoue H, Kai S, Shibata K, Ohta M, Kitano S, Mori M. Prognostic value of tumor-infiltrating FOXP3+ regulatory T cells in patients with hepatocellular carcinoma. Eur J Surg Oncol 2008; 34:173-9; PMID:17928188; http://dx.doi.org/ 10.1016/j.ejso.2007.08.008 [DOI] [PubMed] [Google Scholar]
- 26.Liu J, Zhang N, Li Q, Zhang W, Ke F, Leng Q, Wang H, Chen J, Wang H. Tumor-associated macrophages recruit CCR6+ regulatory T cells and promote the development of colorectal cancer via enhancing CCL20 production in mice. PLoS One 2011; 6:e19495; PMID:21559338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, Greten TF, Korangy F. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology 2008; 135:234-43; PMID:18485901; http://dx.doi.org/ 10.1053/j.gastro.2008.03.020 [DOI] [PubMed] [Google Scholar]
- 28.Jones E, Dahm-Vicker M, Simon AK, Green A, Powrie F, Cerundolo V, Gallimore A. Depletion of CD25+ regulatory cells results in suppression of melanoma growth and induction of autoreactivity in mice. Cancer Immun 2002; 2:1; PMID:12747746 [PubMed] [Google Scholar]
- 29.Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, Zhang A, Dahm P, Chao N, Gilboa E et al.. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest 2005; 115:3623-33; PMID:16308572; http://dx.doi.org/ 10.1172/JCI25947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greten TF, Ormandy LA, Fikuart A, Hochst B, Henschen S, Horning M, Manns MP, Korangy F. Low-dose cyclophosphamide treatment impairs regulatory T cells and unmasks AFP-specific CD4+ T-cell responses in patients with advanced HCC. J Immunother 2010; 33:211-8; PMID:20139774; http://dx.doi.org/ 10.1097/CJI.0b013e3181bb499f [DOI] [PubMed] [Google Scholar]
- 31.Nocentini G, Ronchetti S, Petrillo MG, Riccardi C. Pharmacological modulation of GITRL/GITR system: therapeutic perspectives. Br J Pharmacol 2012; 165:2089-99; PMID:22029729; http://dx.doi.org/ 10.1111/j.1476-5381.2011.01753.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen AD, Diab A, Perales MA, Wolchok JD, Rizzuto G, Merghoub T, Huggins D, Liu C, Turk MJ, Restifo NP et al.. Agonist anti-GITR antibody enhances vaccine-induced CD8(+) T-cell responses and tumor immunity. Cancer research 2006; 66:4904-12; PMID:16651447; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-2813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen AD, Schaer DA, Liu C, Li Y, Hirschhorn-Cymmerman D, Kim SC, Diab A, Rizzuto G, Duan F, Perales MA et al.. Agonist anti-GITR monoclonal antibody induces melanoma tumor immunity in mice by altering regulatory T cell stability and intra-tumor accumulation. PLoS One 2010; 5:e10436; PMID:20454651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy JT, Burey AP, Beebe AM, Gu D, Presta LG, Merghoub T, Wolchok JD. Anaphylaxis caused by repetitive doses of a GITR agonist monoclonal antibody in mice. Blood 2014; 123:2172-80; PMID:24558202; http://dx.doi.org/ 10.1182/blood-2013-12-544742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wing K, Suri-Payer E, Rudin A. CD4+ CD25+-Regulatory T Cells from Mouse to Man. Scandinavian journal of immunology 2005; 62:1-15; PMID:16091121; http://dx.doi.org/ 10.1111/j.1365-3083.2005.01634.x [DOI] [PubMed] [Google Scholar]
- 36.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science 2008; 322:271-5; PMID:18845758; http://dx.doi.org/ 10.1126/science.1160062 [DOI] [PubMed] [Google Scholar]
- 37.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC. Improved survival with ipilimumab in patients with metastatic melanoma. New England Journal of Medicine 2010; 363:711-23; PMID:20525992; http://dx.doi.org/ 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ et al.. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A 2003; 100:8372-7; PMID:12826605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beck KE, Blansfield JA, Tran KQ, Feldman AL, Hughes MS, Royal RE, Kammula US, Topalian SL, Sherry RM, Kleiner D et al.. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol 2006; 24:2283-9; PMID:16710025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trinh VA, Hagen B. Ipilimumab for advanced melanoma: a pharmacologic perspective. J Oncol Pharm Pract 2013; 19:195-201; PMID:23047236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pruitt SK, Boczkowski D, de Rosa N, Haley NR, Morse MA, Tyler DS, Dannull J, Nair S. Enhancement of anti-tumor immunity through local modulation of CTLA-4 and GITR by dendritic cells. Eur J Immunol 2011; 41:3553-63.; PMID:22028176 [DOI] [PMC free article] [PubMed] [Google Scholar]