Abstract
Objective
To utilize a nonhuman primate model to examine the impact of maternal high-fat diet (HFD) consumption and pre-pregnancy obesity on offspring intake of palatable food. We will also examine whether maternal HFD consumption impaired development of the dopamine system, critical for the regulation of hedonic feeding.
Methods
The impact of exposure to maternal HFD and obesity on offspring consumption of diets of varying composition was assessed after weaning. We also examined the influence of maternal HFD consumption on the development of the prefrontal cortex-dopamine system at 13 months of age.
Results
During a preference test, offspring exposed to maternal obesity and HFD consumption displayed increased intake of food high in fat and sugar content relative to offspring from lean control mothers. Maternal HFD consumption suppressed offspring dopamine signaling (as assessed by immunohistochemistry) relative to control offspring. Specifically, there was decreased abundance of dopamine fibers and of dopamine receptor 1 and 2 protein.
Conclusion
Our findings reveal that offspring exposed to both maternal HFD consumption and maternal obesity during early development are at increased risk for obesity due to overconsumption of palatable energy-dense food, a behavior that may be related to reduced central dopamine signaling.
Keywords: macaque, childhood obesity, maternal obesity, high-fat, prefrontal cortex, dopamine
Introduction
Childhood obesity is a serious public health concern in developed countries (1, 2). Obesity is detrimental to a child's health and places a financial burden on the healthcare system (3, 4, 5). Thus, interest has arisen concerning its etiology. Maternal pre-pregnancy obesity, pregnancy obesity and excessive weight gain are major risk factors in the development of childhood obesity (6, 7, 8, 9). Developmental exposure to these factors may program behavioral changes that impact feeding behavior, facilitating development of offspring obesity. We developed a nonhuman primate (NHP) model of diet-induced obesity to examine the impact of maternal high-fat diet (HFD) consumption and obesity on offspring feeding behavior and brain development. NHPs develop the full spectrum of metabolic disease and have similar brain ontogeny to humans, thus, providing clinical insight.
Feeding behavior is regulated by two systems (10). First, homeostatic feeding, is mediated by a need to replenish energy deficiency, whereas the second, hedonic feeding, is regulated by a craving for highly, palatable energy-dense food. Hedonic feeding is one of the contributors to the development of childhood obesity by overriding homeostatic feeding (10). In our NHP model, exposure to maternal HFD consumption and maternal obesity during pregnancy impair homeostatic feeding in offspring (11, 12). Offspring exposed to maternal HFD display abnormalities in the development of melanocortinergic and serotoninergic neurotransmitter systems (13, 14). These juvenile offspring were also heavier (11) and had increased adiposity (12).
Rodent studies provide evidence that maternal HFD consumption and maternal obesity enhance hedonic feeding in offspring (15, 16), a finding presumably mediated by abnormalities in the development of the dopamine (DA) system (16, 17). Presently, we examine the impact of maternal HFD consumption and maternal obesity on palatable food consumption in NHP offspring. Maternal obesity was determined by measuring pre-pregnancy adiposity and weight and pregnancy weight. Furthermore, we determined the impact of maternal HFD consumption on the development of the DA system in the prefrontal cortex (PFC), implicated in higher-level executive functions. The use of this model is critical as NHPs, like humans, have a greater abundance of DA fiber projections (18) and complex DA fiber organization (19, 20). We examined the quantity of DA fibers (with the rate-limiting enzyme tyrosine hydroxylase) and of two dopamine receptor proteins (D1 and D2) across three layers of the PFC using immunohistochemistry.
Methods
Animals, Maternal Diet, and Maternal Obesity
Animal procedures were approved by the Oregon National Primate Research Center (ONPRC) Institutional Animal Care and Use Committee and conformed to NIH guidelines. Adult dams (Macaca fuscata), matched by weight (6-12.2 kg) and age (3.5-9.5 years), were fed either a control (CTR, 15% calories from fat) or HFD (37% calories from fat) ad libitum for up to 2-7 years; during gestation and lactation. Detailed dietary information has been described (11, 21). Pre-pregnancy dams were classified as lean or obese based on percent body fat obtained by dual energy X-ray absorptiometry. The baseline population used to define maternal obesity consisted of 48 adult females consuming a CTR diet. Percent body fat was 12.07 ± 1.87 (mean ± SD). Maternal obesity was defined as >15.8% body fat (two standard deviations above baseline mean). Maternal body weight was assessed before pregnancy and during the third trimester of pregnancy. Weight gain during pregnancy was calculated by subtracting pre-pregnancy body weight from pregnancy body weight.
Female and male offspring remained with mothers until weaning (7.5 months of age) when all offspring were placed on a CTR diet. The intake of palatable food and body weight of offspring from both maternal diet groups (CTR or HFD) and both maternal obesity groups (lean or obese) were examined yielding four offspring groups: (CTR-lean dam n = 5, CTR-obese dam n = 9, HFD-lean dam n = 5, HFD-obese dam n = 13). Allowing us to distinguish between the impact of maternal HFD consumption and obesity. Offspring body weight was assessed at weaning and 13 months.
Food Preference Test
Consumption of diets of varying fat and sugar content were examined the week after weaning and measured using a pelleted dispenser (Med Associates, Inc., St. Albans, VT). Offspring were offered four novel diets (BioServ Flemington, NJ). Two diets were low in fat with high carbohydrate content (13% fat, 66% carbohydrate, and 21% protein), one with sucrose (low-fat/sucrose) and the other with cornstarch (low-fat/cornstarch). The other two diets were high in fat (51% fat, 33% carbohydrate, and 16% protein), one with sucrose (high-fat/sucrose) and the other with cornstarch (high-fat/cornstarch). The two carbohydrate diets allowed us to examine whether offspring consume fat and sucrose individually or combined. During testing, monkeys were not fed morning meals. Thirty minutes prior to test, animals were acclimated to cage and fed 1/3 banana to ensure equal satiation across animals. A peer was housed adjacent to each animal to reduce isolation stress. The number of pellets consumed during 30-minute trials was recorded for five consecutive days of testing between 0900 and 1100 hours. During the first four days, animals were being habituated to test conditions. Our analysis focused on diet consumption on Day 5 when NHP were fully acclimated to testing conditions.
Tissue Collection and Processing
At the time of this study, sufficient tissue was available in our brain bank to examine the impact of maternal HFD consumption on PFC-DA signaling. Animals were necropsied at 13 months of age and brain tissue was collected as described (14, 22). Offspring were deeply anesthetized with sodium pentobarbital (30 mg/kg i.v.); perfused with 0.9% saline followed by 4% paraformaldehyde. Coronal sections (35-μm-thick) were stored in cryoprotectant. Six equally spaced, anatomically-matched sections spanning entire PFC per animal were processed for immunohistochemistry. Six offspring were examined from each maternal diet group for TH, D1, and D2 immunohistochemistry.
TH Immunohistochemistry
TH in the PFC may arise from dopaminergic or noradrenergic neurons. A pilot study (CTR group, n = 3 animals) was conducted to examine the percent of TH co-localized with dopamine-β-hydroxylase (DBH). Less than 5% TH was co-localized with DBH, demonstrating PFC-TH fiber projections were dopaminergic. Thus, only TH was analyzed. Tissues were incubated in primary antibody mouse anti-TH (1:1,000, Millipore MAB318) for 48 hours at 4°C. Specificity of TH was validated by omission of primary antibody and by a previous preabsorption study (TH (23)). Negative control lacked immunostaining. After incubation, tissues were incubated for 1 hour in donkey anti-mouse/Alexa 568 secondary antibody (1:1,000 dilution, Life Technologies A10037, Carlsbad,CA).
D1 and D2 Immunohistochemistry
A second and third set of tissues was assayed for D1 and D2. For D1, tissues were incubated in primary antibody rat anti-D1 (1:1,000, Sigma Aldrich D2944) for 48 hours at 4°C. Specificity of D1 antibody was validated by omission of primary antibody and by a previous prebsorption and Western blot study (24). Tissues were incubated for 1 hour in donkey anti-rat/Alexa 488 (1:1,000, Life Technologies A21208) and counterstained with DAPI. For D2, tissues underwent antigen retrieval for 30 minutes in 10 mM sodium citrate buffer (pH 8.5) to unmask immunosignal and were incubated in primary antibody mouse anti-D2 (1:200, Santa Cruz sc-5303) for 48 hours at 4°C. Specificity of D2 antibody was validated by omission of primary antibody and by previous preabsorption and Western blot studies (25, 26). Tissues were incubated for 1 hour in donkey anti-mouse/Alexa 488 (1:1,000, Life Technologies A21202).
Confocal imaging of TH, D1, and D2
A Leica SP5 AOBS confocal microscope (Leica Microsystems, Buffalo Grove, IL) and a 20X glycerol-immersed objective (NA 0.7) were used to capture images, as described (14, 22). The previously observed bilaminar TH fiber innervation in PFC (19, 20) was confirmed. For TH, a single 3×7 montage containing 3 layers was captured at 224 × 224, 1-μm increments, zoom factor 1.7, and 700 Hz. These 3 layers were the superficial, medial, and deep layers. Two to three fields-of-view per layer were analyzed. Analysis was performed by individuals blind to treatment using Image J (NIH) software to determine density and intensity of TH-positive fibers. For D1 and D2, a montage was captured at a format of 224 × 224, 2-μm increments, zoom factor 1.7, and 400 Hz. D1 and D2 proteins were only expressed in medial and deep layers, therefore, only these layers were analyzed. Density and intensity of dopamine receptor-positive cells were measured. Percent of cells stained with dopamine receptor (total number of dopamine receptor-positive cells/total number of cells*100) and total number of cells (DAPI) were also assessed.
Statistical Analysis
Offspring body weight and caloric intake during preference testing was analyzed with 2-way ANOVAs (maternal diet × obesity). TH fiber innervation, D1, and D2 protein expression were analyzed with 2-way ANOVAs (maternal diet × PFC layer); group differences were examined by Fisher's LSD post-hoc tests. Correlations between maternal measures, offspring body weight at 13 months, and offspring body weight at weaning were examined with Pearson correlations. High-fat/sucrose intake was nonparametric. For high-fat/sucrose intake, maternal diet alone (CTR-lean mothers vs. HFD-lean mothers), maternal obesity alone (CTR-lean mothers vs. CTR-obese mothers), and a comparison between offspring from CTR-lean mothers and HFD-obese mothers were analyzed with Mann-Whitney tests. Associations between offspring intake of high-fat/sucrose diet and maternal obesity factors, and offspring body weight at weaning were determined with Spearman correlations. Statistical analyses were conducted using Prism v6.0 (Graph-Pad, San Diego, CA).
Results
Impact of Maternal HFD Consumption and Obesity on Body Weight and Intake of Palatable Food in Juvenile Offspring
At weaning, there was a main effect of maternal obesity (Figure 1A, F1,27 = 5.88, p<0.05) but not a main effect of maternal HFD (Figure 1A, F1,27 = 0.02, p=0.88) on offspring body weight. Posthoc tests demonstrated offspring from CTR-obese mothers weighed more than controls (offspring from CTR-lean mothers, p<0.05). Offspring from HFD-lean (p=0.69) and HFD-obese (p=0.10) mothers were not different than controls. Maternal pre-pregnancy body fat was positively correlated with offspring weight at weaning, such that offspring from mothers that were obese before pregnancy were heavier (Figure 1B, correlation, r = 0.39, p<0.05). Maternal pre-pregnancy body weight was positively correlated with maternal pre-pregnancy body fat (Supplementary Figure 1A, correlation, r = 0.86, p<0.0005) and offspring weight (Supplementary Figure 1B, correlation, r = 0.38, p<0.05). Maternal pregnancy weight was also positively correlated with maternal pre-pregnancy weight (Supplementary Figure 2A, correlation, r = 0.85, p<0.0005) and offspring weight (Supplementary Figure 2B, correlation, r = 0.45, p<0.05). Pregnancy weight gain was not associated with offspring weight (correlation, r = 0.09, p=0.62).
Figure 1.
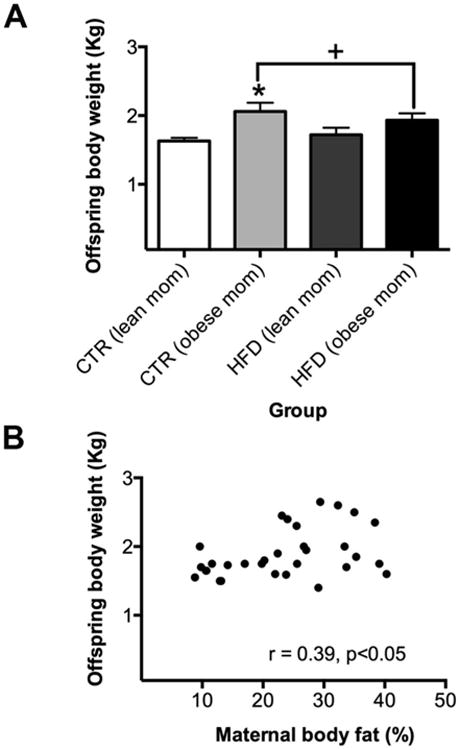
Maternal obesity, not maternal HFD consumption, altered offspring body weight at weaning. A) There was a main effect of maternal obesity (p<0.05) but not a main effect of maternal HFD consumption (p=0.88). Posthoc test results demonstrated that offspring from CTR-obese mothers weighed more than controls (offspring from CTR-lean mothers, p<0.05). Offspring from HFD-lean (p=0.69) and HFD-obese (p=0.10) mothers were not significantly different than controls. + Main effect of maternal obesity. * Greater than controls. B) Maternal pre-pregnancy body fat was positively correlated with offspring weight (p<0.05). Sample sizes for offspring from CTR-lean mothers n = 5 (n = 2 females; n = 3 males), offspring from CTR-obese mothers n = 9 (n = 5 females; n = 4 males), offspring from HFD-lean mothers n = 5 (n = 1 female; n = 4 males), and offspring from HFD-obese mothers n = 13 (n = 8 females; n = 5 males). Data shown as mean ± SEM.
The influence of maternal HFD consumption and maternal obesity on offspring intake of palatable food at weaning was examined. Offspring from HFD-obese mothers overconsumed high-fat/sucrose relative to control offspring (Figures 2A and 2B, U = 12.00, p<0.05). However, we did not detect a difference in high-fat/sucrose intake when either maternal diet (U = 10.00, p=0.66) or maternal obesity (U = 14.00, p=0.28) was examined alone (Figure 2A). This indicates the presence of an interaction between maternal diet and maternal obesity on offspring food preference. No difference in consumption was noted for the other three diets (Figure 2B). No significant effect of maternal diet (F1,28 = 2.11, p=0.16), obesity (F1,28 = 1.32, p=0.26), or interaction (F1,28 = 0.26, p=0.61) was observed on total caloric intake (Figure 2C). Additionally, maternal pre-pregnancy body fat was positively correlated with offspring intake of high-fat/sucrose, such that offspring from mothers with increased adiposity consumed more of high-fat/sucrose diet (Figure 2D, correlation, r = 0.36, p<0.05). Offspring weight was positively correlated with offspring intake of high-fat/sucrose, such that heavier offspring consumed more (Figure 2E, correlation, r = 0.48, p<0.01). In contrast, maternal pre-pregnancy weight (Supplementary Figure 1C, correlation, r = 0.25, p=0.17), pregnancy weight (Supplementary Figure 2C, correlation, r = 0.30, p=0.10), and pregnancy weight gain (correlation, r = 0.09, p=0.62) were not correlated with offspring intake of high-fat/sucrose. Offspring body weight at 13 months of age was positively correlated with offspring body weight at 6 months of age (Supplementary Figure 3, correlation, r = 0.54, p<0.005).
Figure 2.
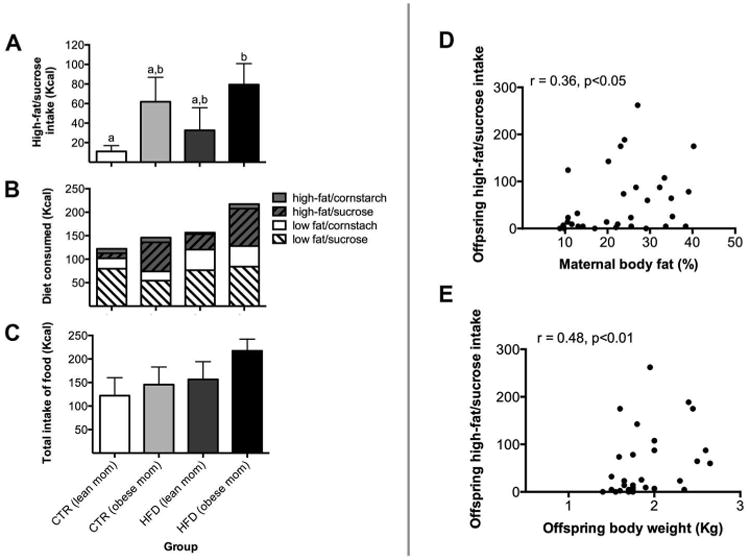
Both maternal HFD consumption and obesity impaired offspring absolute intake of palatable food at weaning. A) Offspring from HFD-obese mothers displayed increased consumption of palatable, energy-dense food (high-fat/sucrose) relative to offspring from CTR-lean mothers. Bars labeled with different letters indicate a significant difference (p<0.05), while bars with the same letter are not significantly different. B) Diet preference of each offspring group. C) No significant differences were apparent in total caloric intake between offspring groups. D) Maternal pre-pregnancy body fat was positively correlated with offspring intake of high-fat/sucrose (p<0.05). E) Offspring weight was positively correlated with offspring intake for high-fat/sucrose (p<0.01). Sample sizes for offspring from CTR-lean mothers n = 5, offspring from CTR-obese mothers n = 9, offspring from HFD-lean mothers n = 5, and offspring from HFD-obese mothers n = 13. Data shown as mean ± SEM.
Maternal HFD Consumption and Central DA Signaling in Juvenile Offspring
DA fiber innervation was examined in the PFC. As expected, bilaminar fiber innervation was observed (Figure 3A). Staining was concentrated in superficial and deep layers, but sparse in medial layer. Offspring from mothers consuming a HFD (p<0.001) exhibited a decrease in the density of TH-positive fibers relative to controls (Figures 3B and 3C, interaction, F2,20 = 9.19, p<0.005). No group difference was observed in intensity (interaction, F2,20 = 0.92, p=0.41). Density changes were apparent in the superficial layer (p<0.001), but not medial (p=0.29) or deep (p=0.13) layers.
Figure 3.
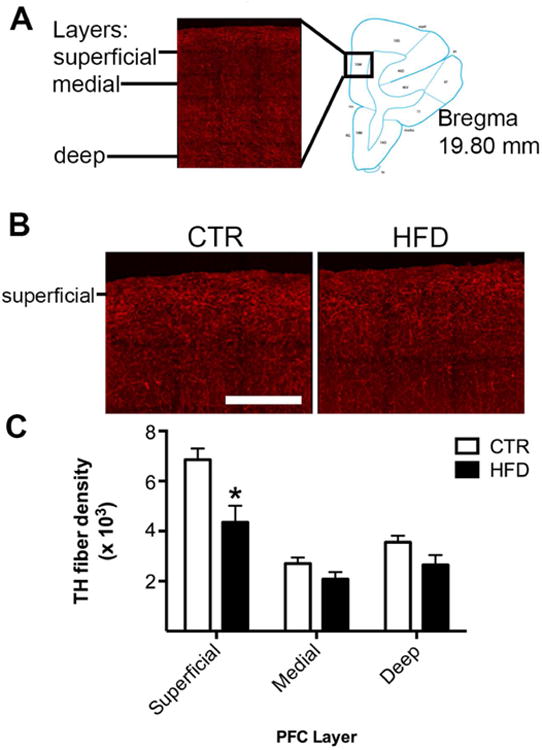
Maternal HFD consumption decreased DA fiber projections to PFC. A) DA fibers were concentrated in superficial and deep layers, but sparse in the medial layer. PFC diagram showing region analyzed (adapted from (40)). B) Representative images reveal a robust amount of TH-positive fibers in offspring from mothers consuming a HFD, but a comparative reduction in offspring from mothers consuming a CTR diet. Scale bar in image = 400 μm. C) Offspring from mothers consuming a HFD (p<0.001) exhibited a decrease in the abundance of TH-positive fibers (density) in the superficial layer, but not in the medial (p=0.29) or deep (p=0.13) layers. * Less than offspring from mothers consuming a CTR diet. Sample sizes for offspring from CTR mothers n = 6 (3 females; 3 males) and offspring from HFD mothers n = 6 (3 females; 3 males). Data shown as mean ± SEM.
The abundance of dopamine receptor proteins was also assessed. D1 and D2-positive cells were only evident in medial and deep layers (Figure 4A), therefore, these layers were analyzed. Offspring from mothers consuming a HFD displayed a decrease in the density of D1-positive cells relative to controls (Figures 4B and 4C, interaction, F1,10= 8.32, p<0.05). No group difference was observed in intensity (interaction, F1,10= 0.04, p=0.85). This change occurred in the deep layer (p<0.05), but not medial layer (p=0.20). Offspring from mothers consuming a HFD also displayed a decrease in the percent of cells stained with D1 relative to total number of cells (Figure 4B and 4D, main effect of maternal diet, F1,10= 5.47, p<0.05). The change in percent of cells stained with D1 was apparent in medial (p<0.05) and deep (p<0.05) layers. No group difference was observed in total number of cells in medial (p=0.14) or deep (p=0.24) layer (Figure 4E, interaction, F1,10= 0.82, p=0.39).
Figure 4.
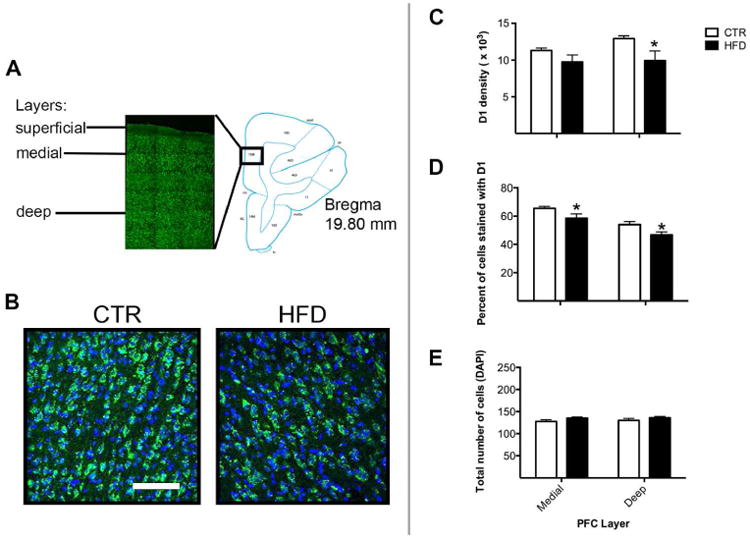
Maternal HFD consumption decreased dopamine receptor proteins (D1 and D2) in the PFC. A) D1- and D2-positive cells were evident in both medial and deep layers, but not in the superficial layer. PFC diagram showing region analyzed (adapted from (40)). B) Representative images reveal a robust amount of D1-positive cells in offspring from mothers consuming a CTR diet but a comparative reduction in offspring from mothers consuming a HFD. Scale bar in image = 100 μm. C) Offspring from mothers consuming a HFD (p<0.05) displayed a decrease in the abundance of D1-positive cells (density) in the deep layer, but not in the medial layer (p=0.20). * Less than offspring from mothers consuming a CTR diet. D) Offspring from mothers consuming a HFD displayed a decrease in the percent of cells stained with D1 in the medial (p<0.05) and deep (p<0.05) layers. * Less than offspring from mothers consuming a CTR diet. E) No group difference was observed in total number of cells in medial (p=0.14) or deep (p=0.24) layers. Sample sizes for offspring from CTR mothers n = 6 and offspring from HFD mothers n = 6. Data shown as mean ± SEM.
Offspring from mothers consuming a HFD displayed decreased abundance of D2-positive cells (percent cells stained with D2 relative to total number of cells), compared to controls (Figures 5A and 5C, main effect of maternal diet, F1,10= 6.04, p<0.05). The change in percent of cells stained with D2 was apparent in the deep (p<0.05), but not medial (p=0.71) layer. Total number of cells was also increased (Figure 5D, main effect of maternal diet, F1,10= 11.68, p<0.01), a change evident in the deep (p<0.005), but not medial (p=0.10) layer. No group difference was observed in density (Figure 5B, interaction, F1,10= 0.03, p=0.87) in medial (p=0.63) or deep (p=0.56) layers. Lastly, no group difference was observed in intensity (F1,10= 0.05, p=0.83).
Figure 5.
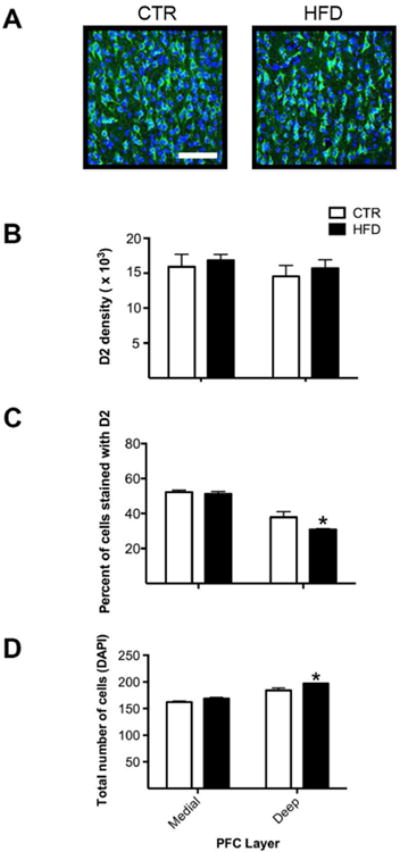
Maternal HFD consumption decreased D2 proteins in the PFC. A) Representative images reveal a robust amount of D2-positive cells in offspring from mothers consuming a CTR diet but a comparative reduction in offspring consuming a HFD. Scale bar in image = 100 μm. B) No group difference was observed in density in medial (p=0.63) or deep (p=0.56) layers. C) Offspring from mothers consuming a HFD displayed a decrease in the percent of cells stained with D2 in the deep (p<0.05), but not medial (p=0.71) layer. D) Offspring from mothers consuming a HFD displayed an increased total number of cells in the deep (p<0.005), but not medial (p=0.10) layer. * Greater than offspring from mothers consuming a CTR diet. Sample sizes for offspring from CTR mothers n = 6 and offspring from HFD mothers n = 6. Data shown as mean ± SEM.
Discussion
Our laboratory provides the first report that maternal HFD consumption and maternal obesity lead to increased intake of palatable, energy-dense food and reduced PFC-DA signaling in NHP offspring. First, maternal pre-pregnancy obesity and pregnancy weight increased offspring weight. Second, maternal HFD consumption combined with maternal pre-pregnancy obesity increased intake of palatable, energy-dense food. Third, maternal HFD consumption decreased the abundance of TH fiber projections and dopamine receptor (D1 and D2) protein. As NHPs possess similar brain ontogeny and capability to develop metabolic disease as humans, these findings may reflect human clinical populations.
Importantly, our NHP model differentiates between the influence of maternal HFD consumption and maternal obesity on offspring weight weaning, a distinction previously unstudied. Offspring exposed to maternal obesity displayed an increase in body weight. In addition, a positive correlation was observed between maternal obesity, pregnancy weight, and offspring body weight, indicating that offspring from obese and heavier mothers before pregnancy and during pregnancy were themselves heavier. These results align well with our previous study (11), where offspring exposed to maternal HFD and obesity were heavier than offspring from healthy mothers. Further, we expand our work by identifying maternal obesity as a risk factor for increased weight in NHP offspring and developmental time points implicated.
Maternal HFD consumption combined with maternal obesity increased offspring intake of highly palatable food. Offspring from HFD-obese mothers displayed an increased intake of the high-fat/sucrose diet relative to offspring from CTR-lean mothers, indicating that maternal diet and metabolic status acted synergistically to program intake of the high-fat/sucrose diet. Also, the positive correlation between maternal pre-pregnancy obesity and offspring high-fat/sucrose intake supports this assessment. We also observed a positive correlation between offspring weight and offspring intake of the diet high in fat and sugar content, where heavier animals consumed more palatable food. Similar evidence exists in rodent literature where maternal HFD consumption and obesity during gestation and lactation led offspring to overconsume palatable, energy-dense food during food preference tests (16). Our findings extend this previous report by distinguishing between maternal diet and maternal obesity. In contrast, another rodent study found maternal cafeteria-style diet consumption and obesity during gestation and lactation failed to increase intake of palatable food in offspring (15). Differences in the composition/duration of diets likely contribute to study differences.
Maternal HFD Consumption and Central DA Signaling
Abnormalities in the development of the DA system may contribute to this increased intake of palatable, energy-dense food. Offspring from mothers consuming a HFD displayed decreased amount of DA fiber projections to the PFC, which was primarily due to a decrease in density suggesting a reduced complexity of DA fiber projections; not a change in DA synthesis. Changes were apparent in the superficial layer indicating decreased cortico-cortical communication of DA fibers. Deterioration of cortico-cortical communication observed here may lead to problems with integration (27), such as sensory information from food, with motor onset to obtain more food. In contrast to our findings, a rodent study observed no impairments in the abundance of PFC-TH proteins in offspring from HFD-fed mothers (17). Differences between results could be attributed to the NHP PFC being more complex (18) or diet composition.
The amount of D1 and D2, receptors involved in palatable food intake (28, 29, 30), were also examined. Offspring from mothers consuming a HFD displayed a decrease in the abundance of D1 protein. This change was mediated by a decrease in the area expressing D1-positive cells and in the percent of total cells stained with D1. Decreased abundance of D2 was attributed to a decrease in the percent of total cells stained with D2 combined with an increase in total number of cells. Proliferation of non D2-positive cells appears to have diluted the population of D2-positive cells. Decreased amount of D1 and D2 proteins in medial and deep layers may alter signaling to subcortical brain regions.
Impairments in DA neurocircuitry could be attributed to increased production of maternal obesity-associated inflammatory factors. Circulating levels of pro-inflammatory cytokines, such as interleukins and tumor necrosis factor α, are elevated in NHP HFD offspring (12) leading to an increase in interleukins in the brain (e.g. hypothalamus) (13). Furthermore, cytokine treatment has been shown to decrease survival of DA neurons (31). Therefore, laminar-specific impairments may be a result of a higher concentration of cytokine receptors exerting their influence on TH fibers and D1 and D2 in these layers.
Our present findings support the fetal origins of disease hypothesis (32). Our laboratory found that maternal HFD consumption programs the risk for food-related impulsivity in offspring. Impulsivity is a heightened drive for reward, associated with poor decision-making (33). Food-related impulsivity is a problem due to increased availability of palatable food. Previous research examined whether hedonic neural circuitry differs between obese and healthy individuals. Obese women (BMI > 30) were more anhedonic as compared to overweight women (BMI 25 - 30) (34). In a functional MRI study, obese adolescent girls exhibited increased neural activation of the insula in response to the anticipation of food, but weaker activation of the striatum during actual intake of palatable food (35). Lastly, Volkow et. al. reported an inverted-U relationship between D2 levels and hedonic responses to methylphenidate (36), where individuals with high D2 levels, perceived a small dose of the drug as pleasant, whereas individuals with low D2 levels required more drug to perceive a similar pleasant experience (36). This finding is in agreement with our present finding where low levels of D2 protein were observed in offspring from HFD-fed mothers suggesting they perceive this palatable food as less rewarding.
An important limitation is that the current study addresses intake of palatable food; not food addiction. Food addiction would be better targeted by examining operant-responses to palatable food. An additional limitation is that only the impact of maternal HFD was examined on PFC-DA signaling, whereas both maternal HFD and maternal obesity were assessed in preference test. Ongoing experiments with larger sample sizes will examine the impact of maternal obesity on PFC-DA signaling. Another limitation to our study is differences in developmental ages between the two studies may contribute to changes in DA immunosignal. However, striatal-TH immunosignal was similar at 6 and 12 months of age (37). Another caveat is intensity of DA immunosignal was measured. However, it provides an indirect measure of DA synthesis and would be better addressed with microdialysis. A final caveat is desensitization of dopamine receptors could contribute to overeating of palatable food in HFD offspring.
In summary, our research identifies maternal HFD consumption and maternal obesity as risk factors in programming increased weight and intake of palatable, energy-dense in offspring. Furthermore, these behavioral changes may be mediated by reduced PFC-DA signaling. Future work will determine whether CNS impairments extend to other neural components, such as the ventral tegmentum and nucleus accumbens. Our finding that maternal obesity drives offspring to overconsume palatable, energy-dense food agrees with evidence in humans that maternal obesity increases a child's intake of palatable food (38). Considering the persistent influence of exposure to a poor perinatal diet and maternal obesity on offspring behavior and physiology, public health policy should focus on improving maternal nutrition and metabolic health before and during pregnancy. Such interventions minimize the risk of childhood obesity and associated long-term health complications.
Supplementary Material
Supplementary Figure 1. Maternal weight before pregnancy and offspring weight after weaning. A) Maternal pre-pregnancy weight was positively correlated with maternal pre-pregnancy body fat (p<0.0005). B) Maternal pre-pregnancy weight was positively correlated with offspring weight (p<0.05). C) Maternal pre-pregnancy weight was not associated with offspring intake of high-fat/sucrose diet (p=0.17).
Supplementary Figure 2. Maternal weight during pregnancy and offspring intake of palatable food after weaning. A) Maternal pregnancy weight was positively correlated with maternal pre-pregnancy weight (p<0.0005). B) Maternal pregnancy body weight was positively correlated with offspring weight (p<0.05). C) Maternal pregnancy weight was not associated with offspring intake of high-fat/sucrose diet (p=0.10).
Supplementary Figure 3. Offspring weight at 6 and 13 months. A positive correlation was found between offspring weight at 13 months of age and offspring weights at 6 months of age (p<0.005).
What is already known about this subject?
Clinical research shows that maternal pre-pregnancy obesity, pregnancy obesity, and excessive pregnancy weight gain, are risk factors for the development of childhood obesity.
Maternal HFD consumption and obesity during pregnancy impair homeostatic feeding in nonhuman primate offspring.
Previous rodent studies demonstrate programming effects of maternal diet and obesity on hedonic feeding in offspring.
What does my study add?
Using a nonhuman primate model, which is highly translatable to humans, we demonstrate that both maternal high-fat diet consumption and maternal obesity increased offspring intake of palatable, energy-dense food.
Exposure to maternal high-fat diet consumption during early development programmed abnormalities in the development of the dopamine system in offspring.
Together, these findings show that both maternal high-fat diet consumption and maternal obesity impairs palatable, energy-dense food intake in NHP offspring, a finding never before observed.
Acknowledgments
Funding: The work in this article was supported by the Murdock Charitable Trust, Murdock College Research Program for Life Science, grant number 2011273:HVP (EL Sullivan) and by the following U.S. National Institutes of Health (NIH) Grants R01 MH107508R01 (EL Sullivan), R01 DK079194 (KL Grove), R24 DK090964 (KL Grove, JE Friedman, and KL Thornburg), Oregon Clinical and Translational Research Institute grant number UL1TR000128 (EL Sullivan) from the National Center for Advancing Translational Sciences, and P51 OD011092 for the operation of ONPRC and support of the Imaging and Morphology Core. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Trust or NIH. We appreciate the Director of the Imaging Core, Dr. Anda Cornea, for her assistance in confocal microscopy and analysis.
Dr. Kievit reports a grant from National Institute of Health (NIH) during the conduct of the study; grants from Novo Nordisk, Janssen Research and Development, Rhythm Pharmaceuticals, ERX Pharma, Ember Therapeutics, Sanofi-Aventis Deutschland GmbH, and Leidos Biomedical Research Institute, outside the submitted work. Dr. Smith reports grants from NIH during the conduct of the study; grant from Biomeasure/Ipsen, outside the submitted work. Dr. Grove reports grants from NIH during the conduct of the study; grants from Novo Nordisk, Janssen, Merck, Acceleron Pharma, Biomeasure/Ipsen, Rhythm Metabolic, ERX Pharma, Ember Therapeutics, outside the submitted work. Dr. Sullivan reports grants from Murdock Charitable Trust, NIH, and Bill and Melinda Gates Foundation, during the conduct of the study.
Footnotes
Conflict of Interest: All other authors declare no conflict of interest.
References
- 1.Wang Y, Lobstein T. Worldwide trends in childhood overweight and obesity. International journal of pediatric obesity: IJPO: an official journal of the International Association for the Study of Obesity. 2006;1:11–25. doi: 10.1080/17477160600586747. [DOI] [PubMed] [Google Scholar]
- 2.Crombie IK, Irvine L, Elliott L, Wallace H. Targets to tackle the obesity epidemic: a review of twelve developed countries. Public health nutrition. 2009;12:406–413. doi: 10.1017/S1368980008002292. [DOI] [PubMed] [Google Scholar]
- 3.Dietz WH, Bellizzi MC. Introduction: the use of body mass index to assess obesity in children. The American journal of clinical nutrition. 1999;70:123S–125S. doi: 10.1093/ajcn/70.1.123s. [DOI] [PubMed] [Google Scholar]
- 4.Janssen I, Katzmarzyk PT, Srinivasan SR, Chen W, Malina RM, Bouchard C, et al. Combined influence of body mass index and waist circumference on coronary artery disease risk factors among children and adolescents. Pediatrics. 2005;115:1623–1630. doi: 10.1542/peds.2004-2588. [DOI] [PubMed] [Google Scholar]
- 5.Finkelstein EA, Graham WC, Malhotra R. Lifetime Direct Medical Costs of Childhood Obesity. Pediatrics. 2014 doi: 10.1542/peds.2014-0063. [DOI] [PubMed] [Google Scholar]
- 6.Leng J, Li W, Zhang S, Liu H, Wang L, Liu G, et al. GDM Women's Pre-Pregnancy Overweight/Obesity and Gestational Weight Gain on Offspring Overweight Status. PLoS One. 2015;10:e0129536. doi: 10.1371/journal.pone.0129536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitaker RC. Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics. 2004;114:e29–36. doi: 10.1542/peds.114.1.e29. [DOI] [PubMed] [Google Scholar]
- 8.Olson CM, Strawderman MS, Dennison BA. Maternal weight gain during pregnancy and child weight at age 3 years. Maternal and child health journal. 2009;13:839–846. doi: 10.1007/s10995-008-0413-6. [DOI] [PubMed] [Google Scholar]
- 9.Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Gillman MW. Gestational weight gain and child adiposity at age 3 years. American journal of obstetrics and gynecology. 2007;196:322 e321–328. doi: 10.1016/j.ajog.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lutter M, Nestler EJ. Homeostatic and hedonic signals interact in the regulation of food intake. The Journal of nutrition. 2009;139:629–632. doi: 10.3945/jn.108.097618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Comstock SM, Pound LD, Bishop JM, Takahashi DL, Kostrba AM, Smith MS, et al. High-fat diet consumption during pregnancy and the early post-natal period leads to decreased alpha cell plasticity in the nonhuman primate. Molecular metabolism. 2012;2:10–22. doi: 10.1016/j.molmet.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCurdy CE, Bishop JM, Williams SM, Grayson BE, Smith MS, Friedman JE, et al. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. The Journal of clinical investigation. 2009;119:323–335. doi: 10.1172/JCI32661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grayson BE, Levasseur PR, Williams SM, Smith MS, Marks DL, Grove KL. Changes in melanocortin expression and inflammatory pathways in fetal offspring of nonhuman primates fed a high-fat diet. Endocrinology. 2010;151:1622–1632. doi: 10.1210/en.2009-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sullivan EL, Grayson B, Takahashi D, Robertson N, Maier A, Bethea CL, et al. Chronic consumption of a high-fat diet during pregnancy causes perturbations in the serotonergic system and increased anxiety-like behavior in nonhuman primate offspring. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:3826–3830. doi: 10.1523/JNEUROSCI.5560-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bayol SA, Farrington SJ, Stickland NC. A maternal ‘junk food’ diet in pregnancy and lactation promotes an exacerbated taste for ‘junk food’ and a greater propensity for obesity in rat offspring. The British journal of nutrition. 2007;98:843–851. doi: 10.1017/S0007114507812037. [DOI] [PubMed] [Google Scholar]
- 16.Vucetic Z, Kimmel J, Totoki K, Hollenbeck E, Reyes TM. Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology. 2010;151:4756–4764. doi: 10.1210/en.2010-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naef L, Srivastava L, Gratton A, Hendrickson H, Owens SM, Walker CD. Maternal high fat diet during the perinatal period alters mesocorticolimbic dopamine in the adult rat offspring: reduction in the behavioral responses to repeated amphetamine administration. Psychopharmacology. 2008;197:83–94. doi: 10.1007/s00213-007-1008-4. [DOI] [PubMed] [Google Scholar]
- 18.Puig MV, Rose J, Schmidt R, Freund N. Dopamine modulation of learning and memory in the prefrontal cortex: insights from studies in primates, rodents, and birds. Frontiers in neural circuits. 2014;8:93. doi: 10.3389/fncir.2014.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldman-Rakic PS, Lidow MS, Smiley JF, Williams MS. The anatomy of dopamine in monkey and human prefrontal cortex. Journal of neural transmission Supplementum. 1992;36:163–177. doi: 10.1007/978-3-7091-9211-5_8. [DOI] [PubMed] [Google Scholar]
- 20.Martin KA, Spuhler IA. The fine structure of the dopaminergic innervation of area 10 of macaque prefrontal cortex. The European journal of neuroscience. 2013;37:1061–1071. doi: 10.1111/ejn.12124. [DOI] [PubMed] [Google Scholar]
- 21.Grant W, Gillingham MB, Batra AL, Fewkes NM, Comstock SM, Takahashi D, et al. Maternal high fat diet is associated with decreased plasma n-3 fatty acids and fetal hepatic apoptosis in nonhuman primates. PLoS One. 2011;6:e17261. doi: 10.1371/journal.pone.0017261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grayson BE, Allen SE, Billes SK, Williams SM, Smith MS, Grove KL. Prenatal development of hypothalamic neuropeptide systems in the nonhuman primate. Neuroscience. 2006;143:975–986. doi: 10.1016/j.neuroscience.2006.08.055. [DOI] [PubMed] [Google Scholar]
- 23.Dermitzaki E, Tsatsanis C, Minas V, Chatzaki E, Charalampopoulos I, Venihaki M, et al. Corticotropin-releasing factor (CRF) and the urocortins differentially regulate catecholamine secretion in human and rat adrenals, in a CRF receptor type-specific manner. Endocrinology. 2007;148:1524–1538. doi: 10.1210/en.2006-0967. [DOI] [PubMed] [Google Scholar]
- 24.Levey AI, Hersch SM, Rye DB, Sunahara RK, Niznik HB, Kitt CA, et al. Localization of D1 and D2 dopamine receptors in brain with subtype-specific antibodies. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:8861–8865. doi: 10.1073/pnas.90.19.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drescher MJ, Cho WJ, Folbe AJ, Selvakumar D, Kewson DT, Abu-Hamdan MD, et al. An adenylyl cyclase signaling pathway predicts direct dopaminergic input to vestibular hair cells. Neuroscience. 2010;171:1054–1074. doi: 10.1016/j.neuroscience.2010.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodman RL, Maltby MJ, Millar RP, Hileman SM, Nestor CC, Whited B, et al. Evidence that dopamine acts via kisspeptin to hold GnRH pulse frequency in check in anestrous ewes. Endocrinology. 2012;153:5918–5927. doi: 10.1210/en.2012-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conte A, Khan N, Defazio G, Rothwell JC, Berardelli A. Pathophysiology of somatosensory abnormalities in Parkinson disease. Nature reviews Neurology. 2013;9:687–697. doi: 10.1038/nrneurol.2013.224. [DOI] [PubMed] [Google Scholar]
- 28.Johnson PMK, J P. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nature neuroscience. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gore BB, Zweifel LS. Genetic reconstruction of dopamine D1 receptor signaling in the nucleus accumbens facilitates natural and drug reward responses. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:8640–8649. doi: 10.1523/JNEUROSCI.5532-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Land BB, Narayanan NS, Liu RJ, Gianessi CA, Brayton CE, Grimaldi DM, et al. Medial prefrontal D1 dopamine neurons control food intake. Nature neuroscience. 2014;17:248–253. doi: 10.1038/nn.3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jarskog LF, Xiao H, Wilkie MB, Lauder JM, Gilmore JH. Cytokine regulation of embryonic rat dopamine and serotonin neuronal survival in vitro. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 1997;15:711–716. doi: 10.1016/s0736-5748(97)00029-4. [DOI] [PubMed] [Google Scholar]
- 32.Barker DJ, Eriksson JG, Forsen T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. International journal of epidemiology. 2002;31:1235–1239. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- 33.Davis C. Psychobiological traits in the risk profile for overeating and weight gain. International journal of obesity. 2009;33 Suppl 2:S49–53. doi: 10.1038/ijo.2009.72. [DOI] [PubMed] [Google Scholar]
- 34.Davis C, Strachan S, Berkson M. Sensitivity to reward: implications for overeating and overweight. Appetite. 2004;42:131–138. doi: 10.1016/j.appet.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. Journal of abnormal psychology. 2008;117:924–935. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Gifford A, et al. Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. The American journal of psychiatry. 1999;156:1440–1443. doi: 10.1176/ajp.156.9.1440. [DOI] [PubMed] [Google Scholar]
- 37.Martin LJ, Cork LC. The non-human primate striatum undergoes marked prolonged remodeling during postnatal development. Frontiers in cellular neuroscience. 2014;8:294. doi: 10.3389/fncel.2014.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rising R, Lifshitz F. Relationship between maternal obesity and infant feeding-interactions. Nutrition journal. 2005;4:17. doi: 10.1186/1475-2891-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paxinos G, Huang XF, Petrides M, Toga A. The Rhesus Monkey Brain. In Stereotaxic Coordinates. 2008 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Maternal weight before pregnancy and offspring weight after weaning. A) Maternal pre-pregnancy weight was positively correlated with maternal pre-pregnancy body fat (p<0.0005). B) Maternal pre-pregnancy weight was positively correlated with offspring weight (p<0.05). C) Maternal pre-pregnancy weight was not associated with offspring intake of high-fat/sucrose diet (p=0.17).
Supplementary Figure 2. Maternal weight during pregnancy and offspring intake of palatable food after weaning. A) Maternal pregnancy weight was positively correlated with maternal pre-pregnancy weight (p<0.0005). B) Maternal pregnancy body weight was positively correlated with offspring weight (p<0.05). C) Maternal pregnancy weight was not associated with offspring intake of high-fat/sucrose diet (p=0.10).
Supplementary Figure 3. Offspring weight at 6 and 13 months. A positive correlation was found between offspring weight at 13 months of age and offspring weights at 6 months of age (p<0.005).


