Abstract
Background
The chemokine Stromal cell-derived factor 1α (SDF1α, CXCL12) is currently under investigation as a biomarker for various cardiac diseases. The correct interpretation of SDF1α levels is complicated by the occurrence of truncated forms that possess an altered biological activity.
Methodology
We studied the immunoreactivities of SDF1α forms and evaluated the effect of adding a DPP4 inhibitor in sampling tubes on measured SDF1α levels. Using optimized sampling, we measured DPP4 activity and SDF1α levels in patients with varying degrees of heart failure.
Results
The immunoreactivities of SDF1α and its degradation products were determined with three immunoassays. A one hour incubation of SDF1α with DPP4 at 37°C resulted in 2/3 loss of immunoreactivity in each of the assays. Incubation with serum gave a similar result. Using appropriate sampling, SDF1α levels were found to be significantly higher in those heart failure patients with a severe loss of left ventricular function. DPP4 activity in serum was not altered in the heart failure population. However, the DPP4 activity was found to be significantly decreased in patients with high SDF1α levels
Conclusions
We propose that all samples for SDF1α analysis should be collected in the presence of at least a DPP4 inhibitor. In doing so, we found higher SDF1α levels in subgroups of patients with heart failure. Our work supports the need for further research on the clinical relevance of SDF1α levels in cardiac disease.
Introduction
In recent years, the chemokine Stromal cell-Derived Factor 1α (SDF1α or CXCL12) has been shown to play a role in cardiovascular diseases [1] and to be a promising biomarker [2,3]. Together with its receptor, CXCR4, SDF1α is involved in the homing of progenitor/stem cells thereby favoring the repair of injured myocardium through angiogenesis [1,4–6]. In addition, there is a growing interest in SDF1α as a cardiovascular biomarker. Elevated levels are associated with a risk of heart failure [2], the extent of coronary artery disease [3], and right ventricular dysfunction in patients with idiopathic pulmonary hypertension [7].
Similar to other chemokines, an intact N-terminus is essential for its biological activity [8]. Work by Crump et al. showed that loss of the N-terminal lysine, generating SDF1α2–68, results in a complete loss of bioactivity [9]. In vivo, N-terminal trimming is often initiated by dipeptidyl peptidase 4 (DPP4). Trimming by DPP4 results in the formation of SDF1α3–68 which not only lacks chemotactic properties but is also a powerful antagonist of the CXCR4 [9,10]. In this regard, Broxmeyer et al. showed that DPP4 inhibition significantly increases homing and engraftment of hematopoietic stem cells [11]. Other enzymes that might play a role in N-terminal cleavage are leukocyte elastase, matrix metalloproteases 1, 2, 3, 9, 13 and 14, and cathepsin G generating SDF1α4–68, SDF1α5–68 and SDF1α6–68 respectively. As mentioned, these cleavage products lack biological activity [12–14].
All these findings clearly demonstrate a crucial role for DPP4 and other proteases in modulating the biological activity of SDF1α. Moreover, DPP4 inhibitors or protease-resistant SDF1α analogs might become novel therapies in pathologies such as ischemic heart disease and heart failure [1,15–18]. In this case, the distinction between intact and cleaved SDF1α will become increasingly important to assess the biologically active SDF1α levels. Unfortunately, at present no commercially available immunoassay claims to discriminate between the intact and cleaved, and thus inactive, forms of SDF1α.
In this study, we first report on the difference in immunoreactivity between intact SDF1α and its cleavage products in commercial immunoassays. The addition of a DPP4 inhibitor to plasma tubes, as a means to prevent ex vivo proteolysis, profoundly affected the measurements. Secondly, the use of SDF1α and DPP4 as biomarkers were analyzed in patients with varying degrees of heart failure [19,20].
Methods
Enzymes and Inhibitors
Soluble human DPP4 was purified from seminal fluid as described previously [21]. One unit (U) of activity is described as the amount of enzyme required to catalyze the conversion of 1 μmol of substrate per minute (0.5 mM Gly-Pro-p-nitroanilide in 50-mM Tris buffer; pH 8.3) at 37°C. Diisopropyl fluorophosphate (DFP), an irreversible serine protease inhibitor, was purchased from Acros. Sitagliptin (SG), a specific DPP4 inhibitor (DPP4-I), was extracted from Januvia tablets (Merck). Vildagliptin (VG) another DPP4-I was custom-synthesized by GLSynthesis, Inc. Complete protease inhibitor cocktail tablets were purchased from Roche and used according to manufacturer’s instructions. These tablets inhibit a broad range of proteases including serine, cysteine as well as metalloproteases.
DPP4 activities
DPP4 activities were measured using the fluorogenic substrate Gly-Pro-4-methyl-β-Naphtylamide as reported earlier [15]. In short, 10 μl sample was mixed with 100 μl of a 50-mM Tris buffer (pH 8.3) containing 0.5 mM Gly-Pro-4-methyl-β-Naphtylamide. The release of 4-methyl-β-Naphtylamide was measured for 10 min at 37°C (ʎex = 340 nm; ʎem = 430 nm).
ELISA and Antibodies
Two different lot numbers of CXCL12/SDF-1 DuoSet were purchased from RnDsystems (catalog N° DY460). The kit consists of a mouse anti-human/mouse SDF1α capture antibody, a biotinylated goat anti-human/mouse SDF1α detection antibody, and a Streptavidin-horseradish-peroxidase-(HRP) conjugate.
Experiments were repeated with two other ELISA kits: the human SDF1α mini ELISA Development Kit (Peprotech; catalog N° 900-M92) which consists of an anti-human SDF1α capture antibody, a biotinylated anti-human SDF1α detection antibody and an avidin-HRP conjugate and the human SDF1α ELISA Kit (Raybiotech; catalog N° ELH-SDF1a) which includes a plate precoated with capture antibody, a biotinylated anti-human SDF1α detection antibody, and a streptavidin-HRP conjugate.
The analyses were performed in Nunc Maxisorp 96-well plates for the RnD and Peprotech kit and in the supplied plate for the Raybiotech kit, according to manufacturer’s instructions. The readout was performed in a Tecan Infinite M200 microtiter plate reader.
SDF1α Truncation
To study SDF1α truncation in vitro, the peptide provided by the CXCL12/SDF-1 RnD DuoSet was used for spiking (500 pg/ml). SDF1α was completely truncated through an incubation of one hour at 37°C in the presence of DPP4 [22]. As a control, DPP4 was first inhibited by a 10-min pre-incubation at 4°C with 1 mM DFP. To study ex vivo cleavage, serum (Bio-Rad; level 2 liquid assayed multiqual chemistry control serum) was spiked with SDF1α and incubated at room temperature (25°C) or 37°C for one hour. As a control, the serum was pre-incubated with protease inhibitors (100 μM SG and 1x complete protease inhibitor cocktail).
Study population
Consecutive patients (age 65 ± 11 years) with a diagnosis of HfpeF (heart failure with preserved ejection fraction, > 40% and evidence of a left-ventricular dysfunction; n = 28) or HfrEF (heart failure with reduced ejection fraction, ≤ 40%; n = 30) [23] and a recent episode of decompensated heart failure, necessitating IV diuretic therapy, referred for diagnostic left and right heart catheterization were included in the study. Patients with HfrEF were further divided in those with compensated (characterized by a normal preload reserve) or decompensated heart failure (characterized by an impaired preload reserve) [24]. In addition, patients were categorized according to the ejection fraction in those with ‘normal’ ≥ 60%; ‘slight loss’ 59–51%; ‘loss’ 50–35%; ‘severe loss’ <35%. Patients with renal insufficiency defined by an estimated GFR (according to the modification of diet in renal disease study equation) below 60 ml/min/1.73 m2 or patients that received DPP4 inhibitors at the time of the study were excluded. All patients gave oral informed consent, a procedure which, at 2006, was approved by the local medical ethical committee of the OLV hospital, Aalst, Belgium. The study complied with the declaration of Helsinki. Patients were informed that the blood could be stored for the subsequent analysis of biomarkers. The oral informed consent was documented in the electronic or paper patient file and the study was approved by the local ethical committee.
Before diagnostic catheterization when the patient was in a stable hemodynamic condition five milliliter of whole blood was drawn from the femoral vein for subsequent measurements. Blood was collected in 7.5-ml EDTA tubes (S-monovette; Sarstedt) with or without DPP4-I to prevent ex vivo cleavage (VG, 120 μM final concentration in the tube). The samples were centrifugated for 15 min (2000 x g) and were subsequently frozen at –80°C until further analysis without undergoing additional freeze-thaw cycles. The plasma platelet number was not determined. Blood collected from patients with heart failure symptoms and without HfpeF or HfreF collected were chosen as control samples.
Catheterization of the left and right sides of the heart was performed unblinded from the right femoral artery and vein. Pulmonary capillary wedge pressure was measured by use of a Swan-Ganz catheter whereas LV pressure was recorded with a catheter, positioned in the left ventricular cavity. LV angiograms were obtained in left and right anterior oblique position. Left ventricular volumes and EF were derived from the single plane angiogram using the area-length method. An impaired preload reserve was defined by the presence of LVEDP ≥ 16 mm Hg [24].
Statistics
Each measurement was performed 5 times and all measurements are reported as mean ± standard error of the mean (SEM). Specificity of the different ELISAs was compared using a Kruskall-Wallis analysis. When a significant difference was found, groups were compared with a Mann-Whitney-U test.
Cardiovascular parameters, SDF1α concentrations and DPP4 activities in the patient samples were analyzed using a one-way ANOVA followed by Bonferroni’s post-hoc tests if necessary.
All statistical analyses were performed by the Statistical Package for the Social Sciences (SPSS) version 20. Statistical differences were determined to be significant when the p-value was below 0.05.
Results
Specificity of SDF1α ELISAs
SDF1α and DPP4
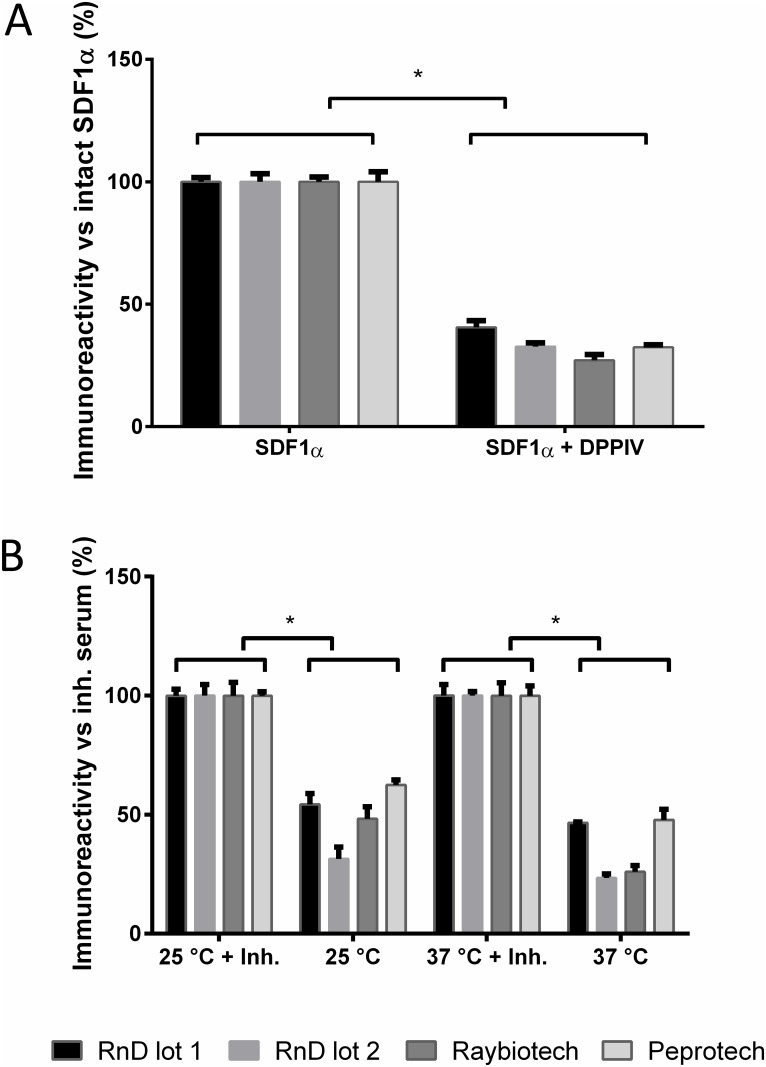
The immunoreactivity of intact vs cleaved SDF1α was tested by incubating SDF1α with DPP4. After one hour at 37°C, a significantly lower immunoreactivity was detected for the DPP4-generated SDF1α3–68. This effect was observed with different lot numbers (RnDsystems) and different commercial ELISA kits (Raybiotech, Peprotech) (Fig 1A). As expected, the incubation of SDF1α1–68 with inactivated DPP4 did not result in a difference in immunoreactivity (S1 Fig).
Fig 1. The SDF1α immunoreactivity measured by commercially available kits.
(A) SDF1α (500pg/ml) incubated in PBS for 1 h at 37°C was set at 100% immunoreactivity. Incubation in the presence of DPP4 (25 U/l) resulted in a significantly lower immunoreactivity compared to intact SDF1α (RnD lot 1: 40.5 ± 2.8%; RnD lot 2: 32.7 ± 1.5%; Raybiotech: 27.1 ± 2.4%; Peprotech 32.5 ± 1.2%; *p < 0.05; results ± SEM; n = 5). (B) The immunoreactivity of pure SDF1α (500pg/ml) spiked into serum and incubated for 1 h at 25°C or 37°C was measured with different commercial kits. As the 100% reference, SDF1α spiked into inhibited serum (DPP4-I and Roche protease inhibitor cocktail) was chosen. Ex vivo degradation in serum significantly decreased the immunoreactivity of SDF1α (25°C: RnD lot 1: 54.3 ± 4.1%; RnD lot 2: 31.4 ± 6.4%; Raybiotech: 48.3 ± 5.1%; Peprotech: 62.4 ± 2.3% and 37°C: RnD lot 1: 46.6 ± 0.4%; RnD lot 2: 23.5 ± 1.7%; Raybiotech: 25.9 ± 2.7%; Peprotech 47.8 ± 1.9%; *p < 0.05; results ± SEM; n = 5).
SDF1α in serum
To study the in vivo cleavage of SDF1α by serum proteases including DPP4, serum was spiked with SDF1α and incubated at 25°C and 37°C for one hour. As a control, serum was inhibited beforehand with a combination of DPP4-I and protease inhibitor cocktail. Compared to control a significantly lower SDF1α immunoreactivity was found at 25°C and 37°C for all kits (Fig 1B). No differences in SDF1α immunoreactivity could be detected between the different kits and 25°C or 37°C.
SDF1α in blood samples
Ex vivo cleavage of endogenous SDF1α
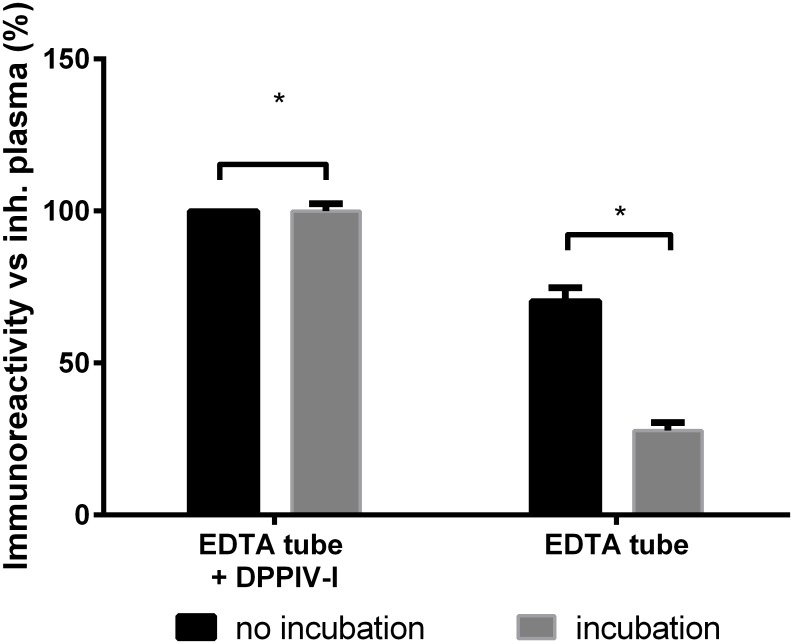
To examine the effect of ex vivo cleavage of endogenous SDF1α in plasma of healthy volunteers (n = 13). Blood was collected in EDTA tubes with or without DPP4-I, immediately processed and frozen at -80°C. The samples were thawed at 4°C and analyzed immediately. A significantly lower immunoreactivity was found in tubes that did not contain DPP4-I as compared to DPP4-inhibited samples (100%) (Fig 2).
Fig 2. The average SDF1α immunoreactivity of healthy plasma with the RnD SDF1α duoset when immediately analyzed (no incubation, n = 13, range [749–1776 pg/ml]) or after an incubation of 1 h at 37°C (n = 7, range [832–1776 pg/ml]).
Blood was collected in tubes with or without DPP4-I. A significantly lower immunoreactivity was found in regular tubes versus the DPP4-I containing tubes (no incubation: 67.6 ± 3.5%; incubation: 27.8 ± 2.8%; *p < 0.05; results ± SEM).
Since samples are often not immediately analyzed, we also determined the immunoreactivity of endogenous SDF1α after an incubation of one hour at 37°C (n = 7). As expected, the immunoreactivity of the samples without DPP4 inhibitor was significantly lower compared to the DPP4-inhibited samples (Fig 2).
Finally, the immunoreactivity observed in DPP4-I samples did not rise further after addition of Roche Protease Inhibitor cocktail on top of the DPP4-I. (S2 Fig).
SDF1α levels, DPP4 activity and hemodynamic parameters
Characteristics of the study population are summarized in Table 1. In the entire study population, SDF1α levels ranged from 491 to 2550 pg/ml, median 1033 [915–1143] pg/ml.
Table 1. Patients characteristics according to Tertiles of SDF1α levels.
| SDF1α Tertiles | |||||
|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | p-value | ||
| (n = 32) | (n = 32) | (n = 31) | |||
| Mean SDF1α | pg/ml | 836 | 1044 | 1369 | |
| range | 491–955 | 959–1119 | 1120–2550 | ||
| Age | years | 64 | 66 | 66 | 0.750 |
| SD | 8 | 12 | 10 | ||
| Men | n | 24 | 18 | 18 | 0.129 |
| % | 75 | 56 | 58 | ||
| Heart Rate | bpm | 73 | 67 | 72 | 0.386 |
| SD | 15 | 11 | 16 | ||
| Ejection Fraction | %max | 71 | 65 | 63 | 0.224 |
| SD | 18 | 17 | 20 | ||
| EDP | mmHg | 19 | 14 | 19 | 0.078 |
| SD | 8 | 4 | 16 | ||
| EDVI | ml/m2 | 71 | 76 | 72 | 0.709 |
| SD | 26 | 27 | 19 | ||
| ESVI | ml/m2 | 24 | 29 | 27 | 0.632 |
| SD | 21 | 21 | 21 | ||
| DPP4 activity | U/l | 24 | 23 | 20 | 0.042 |
| SD | 5 | 7 | 7 | ||
| 10-year survival | n | 22 | 24 | 20 | 0.399 |
| % | 69 | 75 | 65 |
DPP4: Dipeptidyl Peptidase 4
SDF1α: Stromal cell-Derived Factor 1 alpha
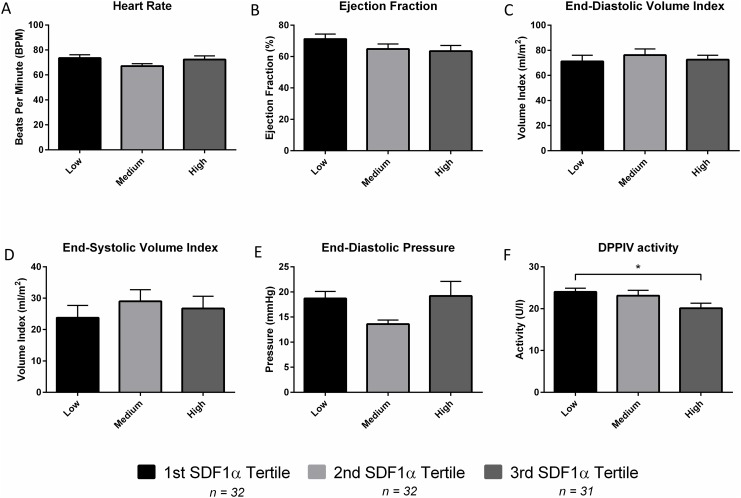
Patients were divided into tertiles of SDF1α. A significantly lower DPP4 activity was found in patients with low compared to those with high SDF1α levels. Other cardiovascular parameters did not differ between groups (Fig 3).
Fig 3. Comparison of cardiovascular parameters between SDF1α tertiles (Low = First Tertile, Medium = Second Tertile and High = Third Tertile).
No significant differences were observed in the Heart Rate (A), Ejection Fraction (B), End-Diastolic Volume Index (C), End-Systolic Volume Index (D) and End-Diastolic Pressure (E). The first and third tertile had significantly different DPP4 activities (F). *p < 0.05.
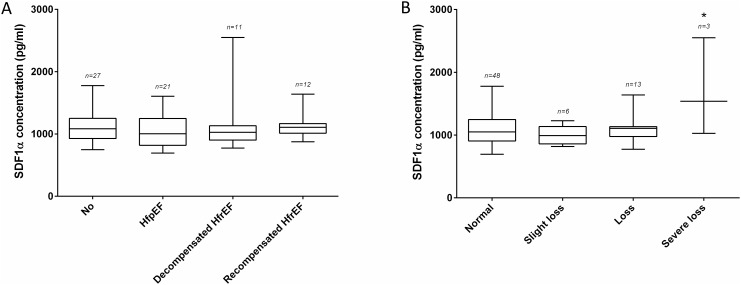
Although no difference in SDF1α levels was observed between controls, HfpEF and HfrEF patients (Fig 4A). Patients with severe LV dysfunction had significantly higher SDF1α concentrations (Fig 4B) DPP4 activity was similar in all of the investigated heart failure subgroups (S3 Fig).
Fig 4. SDF1α concentrations in patient samples collected in tubes with DPP4-I.
(A) No difference was found between patients with a different type of LV dysfunction (none 1096 ± 47 pg/ml; HfpEF 1043 ± 55 pg/ml; decompensated HfrEF 1201 ± 145 pg/ml; recompensated HfrEF 1109 ± 51 pg/ml). (B) For the different severities of LV dysfunction a significant difference was found in patients with a severe loss of LV function (normal 1076 ± 36 pg/ml; slight loss 1002 ± 62 pg/ml; loss 1090 ± 55 pg/ml; severe loss 1705 ± 447 pg/ml; *p < 0.05).
Discussion
Quantification of the in vivo circulating SDF1α
SDF1α currently receives a lot of interest within cardiovascular research [2,5,18]. Although current clinical immunoassays have a high sensitivity and reproducibility, it remains unknown which kind of fragments these antibodies exactly recognize. As this leads to misinterpretation, a method to quantitate the in vivo circulating intact SDF1α was developed using commercially available immunoassays.
Our study revealed that frequently used commercially available immunoassays react differently towards intact SDF1α as compared to DPP4-truncated SDF1α. This might have clinical consequences as DPP4 circulates freely in the blood and is bound to the endothelial cell membrane. Consequently, DPP4 is able to lower the immunoreactivity both in vivo as well as ex vivo [25], resulting in an underestimation of physiological concentrations. In addition, other proteases might also contribute to SDF1α degradation [12–14]. However, the remaining immunoreactivity after incubation with DPP4 and serum was similar, which suggests that the observed ex vivo loss in signal is due to N-terminal truncation. The relative contribution of DPP4 was confirmed ex vivo with healthy plasma samples and is in line with previous findings of Kanki et al. [1]. This loss in immunoreactivity most likely reflects the immunogenic properties of the highly basic N-terminus. Even though we found this to be true for all tested immunoassays, these characteristics are often poorly specified by the manufacturer.
Experimental and Clinical Implication
The ex vivo truncation, which occurs during sample handling, storage or even incubation of the ELISA plate, results in an underestimation of the physiological SDF1α concentrations. Of note, even under optimal pre-analytical conditions, there is a significant (more than 30%) loss of SDF1α- immunoreactivity in samples that did not contain a DPP4 inhibitor.
Our results show that ex vivo cleavage of SDF1α by DPP4 can lead to misinterpretations of experimental results. This is especially relevant when dealing with patients that are treated with DPP4 inhibitors or that have aberrant DPP4 activities, for example due to hyperglycemia or hypoxia [26, 27]. To overcome this problem, we highly recommend collecting all samples in tubes containing at least a DPP4 inhibitor to block any additional ex vivo splicing. The importance of this procedure is illustrated by the fact that an intact N-terminus is linked to SDF1α’s cardioprotective effects [1].
Our data is in line with other in vivo studies utilizing DPP4 inhibitors and measuring SDF1α levels. Most studies use the RnD immunoassay and all groups reported significantly higher SDF1α levels upon treatment with DPP4-I (S1 Table) [15,28,29]. Taking our results into account, these findings are suggestive of higher levels of intact and thus active circulating SDF1α.
The stabilization of in vivo intact SDF1α by DPP4 inhibition can be a valuable therapeutic strategy after myocardial infarction. Zaruba et al. showed that genetic deletion or pharmacological inhibition of DPP4 in combination with Granulocyte-Colony-Stimulating Factor led to improved heart function and survival [16]. Another strategy is to locally inject a protease-resistant SDF1α. This improves cardiac function after cardial ischemia and might provide an additional therapy for heart failure [1,30]. Clinical trials also indicate the potential use of SDF1α in cardiovascular pathologies. SDF1α gene therapy has been shown to be beneficial in a phase I study [18]. In its follow-up study (STOP-HF trial, NCT01643590), intra-myocardial delivery of SDF1α suggested a dose-dependent change in LVEF [31]. In the SITAGRAMI trial (NCT00650143), DPP4 inhibition in high doses was shown to increase the biological half-life of SDF1α and resulted in an improved cardial regeneration after myocardial infarction [32].
DPP4 activity and SDF1α as biomarkers
In recent years, DPP4 activity and SDF1α have been suggested as possible biomarkers for heart failure [2,19,20]. Therefore, we evaluated both in patients referred for elective diagnostic cardiac catheterization.
We found DPP4 activities to be lower in patients with high SDF1α levels. It is of interest to note that the low DPP4 activities and high SDF1α levels might be related and that the difference in activity could point to the in vivo post-translational regulation of SDF1α [33]. In addition, patients with a severe loss of LV function showed a marked increase in SDF1α. Several mechanisms might be responsible for this observation. First, the higher wall stress with concomitant subendocardial ischemia may induce SDF1α production thereby mobilizing stem cells to the injured myocardium. Secondly, apart from beneficial effects, SDF1α might also have detrimental effects and depress LV function. It was recently shown that SDF1α has a negative inotropic effect through binding with its receptor CXCR4 [34]. Our results, in combination with a recent finding [2], point to the possible role of SDF1α as a biomarker in heart failure and warrant further investigation.
Surprisingly, no difference in DPP4 activities could be found between any of the investigated groups. This is in contrast with recent data demonstrating increased DPP4 activity levels in patients with heart failure [19,20]. One study focused on diastolic heart failure and only found a weak correlation with DPP4 activity in peripheral venous plasma [19], while a second study only included patients with a Left Ventricular Ejection Fraction lower than 45% [20]. As these studies had more stringent inclusion criteria for their heart failure patients, this could partially account for the observed discrepancy.
Conclusion
We demonstrated that the N-terminal truncation of SDF1α profoundly affects the immunoreactivity measured by ELISA irrespective of the commercially available kit used. Additionally, we found that in the absence of protease inhibitors ex vivo cleavage cannot be prevented and that more than a third of the immunoreactivity is lost. We therefore recommend collecting all samples in tubes with protease inhibitors, at least including a DPP4 inhibitor. The possible value of DPP4 activities and SDF1α levels as biomarkers for heart failure was also evaluated. DPP4 activities were found to be lower in patients with high SDF1α levels. DPP4 activities were similar in the investigated heart failure subgroups. In contrast, SDF1α plasma levels were significantly elevated in patients with a severe loss of LV function. The observation of lower DPP4 activity in patients with high SDF1α as well as the presence of elevated SDF1α in patients with severe LV dysfunction warrants further investigation. Therefore, additional research is needed into the use of SDF1α as a biomarker and the role of DPPV as a target in patients with heart failure.
Limitations of Our Study
The main limitation is the small number of severe heart failure patients included in our study. Therefore, data concerning this population should be interpreted with caution and confirmed with a larger population. A second limitation is the missing information regarding the epitopes recognized by the antibodies. Unfortunately, these could not be obtained from the suppliers as they considered it to be proprietary information. For the RnD duoset, we speculate that the mouse anti-SDF1 capture antibody is a monoclonal antibody that binds to an internal sequence. The goat anti-SDF1 antibody most probably is a polyclonal antibody that predominantly interacts with the N-terminus. As the polyclonal detection antibody is still able to interact with other parts of SDF1, the ELISA signal is strongly reduced, but not completely lost. The last limitation is that our proposed formula is based on a single time point and only gives a rough estimation of the circulating intact SDF1α.
Supporting Information
SDF1α in buffer was selected as the 100% reference.
(PPTX)
(PPTX)
(A) No difference was found between patients with a different type of LV dysfunction (B) or for the different severities of LV dysfunction.
(PPTX)
(DOCX)
Acknowledgments
We would like to thank all the personnel of the OLV hospital in Aalst who participated in the collection of samples. In addition, we would like to express our gratitude towards Nicole Lamoen for technical assistance.
Abbreviations
- DPP4
Dipeptidyl Peptidase 4
- DPP4-I
Dipeptidyl Peptidase 4 Inhibitor
- ELISA
Enzyme-Linked ImmunoSorbent Assay
- IH
Ischemic Heart Disease
- LV function
Left Ventricular function
- SDF1α
Stromal cell-Derived Factor 1 α
- SG
Sitagliptin
- VG
Vildagliptin
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the University of Antwerp Special research Fund (BOF; Grant FFB3551, https://www.uantwerpen.be/nl/) and the Flanders Research Foundation (FWO; Grant G0141.12, http://www.fwo.be/en/).
References
- 1. Kanki S, Segers VFM, Wu W, Kakkar R, Gannon J, Sys SU, et al. Stromal cell-derived factor-1 retention and cardioprotection for ischemic myocardium. Circ Heart Fail. 2011;4: 509–18. 10.1161/CIRCHEARTFAILURE.110.960302 [DOI] [PubMed] [Google Scholar]
- 2. Subramanian S, Liu C, Aviv A, Ho JE, Courchesne P, Muntendam P, et al. Stromal cell-derived factor 1 as a biomarker of heart failure and mortality risk. Arterioscler Thromb Vasc Biol. 2014;34: 2100–5. 10.1161/ATVBAHA.114.303579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ghasemzadeh N, Hritani AW, De Staercke C, Eapen DJ, Veledar E, Al Kassem H, et al. Plasma Stromal Cell-Derived Factor 1α/CXCL12 level predicts long-term adverse cardiovascular outcomes in patients with coronary artery disease. Atherosclerosis. Elsevier; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110: 3300–5. [DOI] [PubMed] [Google Scholar]
- 5. Lee B-C, Hsu H-C, Tseng W-YI, Chen C-Y, Lin H-J, Ho Y-L, et al. Cell therapy generates a favourable chemokine gradient for stem cell recruitment into the infarcted heart in rabbits. Eur J Heart Fail. 2009;11: 238–45. 10.1093/eurjhf/hfn035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aiuti BA, Webb IJ, Bleul C, Springer T. The Chemokine SDF-1 Is a Chemoattractant for Human CD34+ Hematopoietic Progenitor Cells and Provides a New Mechanism to Explain the Mobilization of CD34+ Progenitors to Peripheral Blood. J Exp Med. 1997;185: 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang T, Li Z-N, Chen G, Gu Q, Ni X-H, Zhao Z-H, et al. Increased levels of plasma CXC-Chemokine Ligand 10, 12 and 16 are associated with right ventricular function in patients with idiopathic pulmonary arterial hypertension. Heart Lung. 43: 322–7. 10.1016/j.hrtlng.2014.04.016 [DOI] [PubMed] [Google Scholar]
- 8. Baggiolini M. Chemokines in pathology and medicine. J Intern Med. 2001;250: 91–104. [DOI] [PubMed] [Google Scholar]
- 9. Crump MP, Gong JH, Loetscher P, Rajarathnam K, Amara A, Arenzana-Seisdedos F, et al. Solution structure and basis for functional activity of stromal cell-derived factor-1; dissociation of CXCR4 activation from binding and inhibition of HIV-1. EMBO J. 1997;16: 6996–7007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shioda T, Kato H. Anti-HIV-1 and chemotactic activities of human stromal cell-derived factor 1α (SDF-1α) and SDF-1β are abolished by CD26/dipeptidyl peptidase IV-mediated cleavage. PNAS. 1998;95: 6331–6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Christopherson KW, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305: 1000–3. [DOI] [PubMed] [Google Scholar]
- 12. McQuibban GA, Butler GS, Gong JH, Bendall L, Power C, Clark-Lewis I, et al. Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J Biol Chem. 2001;276: 43503–8. [DOI] [PubMed] [Google Scholar]
- 13. Delgado MB, Clark-Lewis I, Loetscher P, Langen H, Thelen M, Baggiolini M, et al. Rapid inactivation of stromal cell-derived factor-1 by cathepsin G associated with lymphocytes. Eur J Immunol. 2001;31: 699–707. [DOI] [PubMed] [Google Scholar]
- 14. Valenzuela-Fernández A, Planchenault T, Baleux F, Staropoli I, Le-Barillec K, Leduc D, et al. Leukocyte elastase negatively regulates Stromal cell-derived factor-1 (SDF-1)/CXCR4 binding and functions by amino-terminal processing of SDF-1 and CXCR4. J Biol Chem. 2002;277: 15677–89. [DOI] [PubMed] [Google Scholar]
- 15. Jungraithmayr W, De Meester I, Matheeussen V, Baerts L, Arni S, Weder W. CD26/DPP-4 inhibition recruits regenerative stem cells via stromal cell-derived factor-1 and beneficially influences ischaemia-reperfusion injury in mouse lung transplantation. Eur J Cardiothorac Surg. 2012;41: 1166–73. 10.1093/ejcts/ezr180 [DOI] [PubMed] [Google Scholar]
- 16. Zaruba M-M, Theiss HD, Vallaster M, Mehl U, Brunner S, David R, et al. Synergy between CD26/DPP-IV inhibition and G-CSF improves cardiac function after acute myocardial infarction. Cell Stem Cell. 2009;4: 313–23. 10.1016/j.stem.2009.02.013 [DOI] [PubMed] [Google Scholar]
- 17. Larocca TJ, Jeong D, Kohlbrenner E, Lee A, Chen J, Hajjar RJ, et al. CXCR4 gene transfer prevents pressure overload induced heart failure. J Mol Cell Cardiol. 2012;53: 223–32. 10.1016/j.yjmcc.2012.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Penn MS, Mendelsohn FO, Schaer GL, Sherman W, Farr M, Pastore J, et al. An open-label dose escalation study to evaluate the safety of administration of nonviral stromal cell-derived factor-1 plasmid to treat symptomatic ischemic heart failure. Circ Res. 2013;112: 816–25. 10.1161/CIRCRESAHA.111.300440 [DOI] [PubMed] [Google Scholar]
- 19. Shigeta T, Aoyama M, Bando YK, Monji A, Mitsui T, Takatsu M, et al. Dipeptidyl peptidase-4 modulates left ventricular dysfunction in chronic heart failure via angiogenesis-dependent and -independent actions. Circulation. 2012;126: 1838–51. 10.1161/CIRCULATIONAHA.112.096479 [DOI] [PubMed] [Google Scholar]
- 20. Dos Santos L, Salles TA, Arruda-Junior DF, Campos LCG, Pereira AC, Barreto ALT, et al. Circulating dipeptidyl peptidase IV activity correlates with cardiac dysfunction in human and experimental heart failure. Circ Heart Fail. 2013;6: 1029–38. 10.1161/CIRCHEARTFAILURE.112.000057 [DOI] [PubMed] [Google Scholar]
- 21. De Meester I, Vanhoof G, Lambeir AM, Scharpé S. Use of immobilized adenosine deaminase (EC 3.5.4.4) for the rapid purification of native human CD26/dipeptidyl peptidase IV (EC 3.4.14.5). J Immunol Methods. 1996;189: 99–105. [DOI] [PubMed] [Google Scholar]
- 22. Lambeir a M, Proost P, Durinx C, Bal G, Senten K, Augustyns K, et al. Kinetic investigation of chemokine truncation by CD26/dipeptidyl peptidase IV reveals a striking selectivity within the chemokine family. J Biol Chem. 2001;276: 29839–45. [DOI] [PubMed] [Google Scholar]
- 23. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128: e240–327. 10.1161/CIR.0b013e31829e8776 [DOI] [PubMed] [Google Scholar]
- 24. Heymes C, Vanderheyden M, Bronzwaer JG, Shah AM, Paulus WJ. Endomyocardial nitric oxide synthase and left ventricular preload reserve in dilated cardiomyopathy. Circulation. 1999;99: 3009–16. [DOI] [PubMed] [Google Scholar]
- 25. Matheeussen V, Baerts L, De Meyer G, De Keulenaer G, Van der Veken P, Augustyns K, et al. Expression and spatial heterogeneity of dipeptidyl peptidases in endothelial cells of conduct vessels and capillaries. Biol Chem. 2011;392: 189–98. 10.1515/BC.2011.002 [DOI] [PubMed] [Google Scholar]
- 26. Pala L, Pezzatini A, Dicembrini I, Ciani S, Gelmini S, Vannelli BG, et al. Different modulation of dipeptidyl peptidase-4 activity between microvascular and macrovascular human endothelial cells. Acta Diabetol. 2012;49 Suppl 1: S59–63. 10.1007/s00592-010-0195-3 [DOI] [PubMed] [Google Scholar]
- 27. Eltzschig HK, Faigle M, Knapp S, Karhausen J, Ibla J, Rosenberger P, et al. Endothelial catabolism of extracellular adenosine during hypoxia: the role of surface adenosine deaminase and CD26. Blood. 2006;108: 1602–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fadini GP, Boscaro E, Albiero M, Menegazzo L, Frison V, de Kreutzenberg S, et al. The oral dipeptidyl peptidase-4 inhibitor sitagliptin increases circulating endothelial progenitor cells in patients with type 2 diabetes: possible role of stromal-derived factor-1alpha. Diabetes Care. 2010;33: 1607–9. 10.2337/dc10-0187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huang C-Y, Shih C-M, Tsao N-W, Lin Y-W, Huang P-H, Wu S-C, et al. Dipeptidyl peptidase-4 inhibitor improves neovascularization by increasing circulating endothelial progenitor cells. Br J Pharmacol. 2012;167: 1506–19. 10.1111/j.1476-5381.2012.02102.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Segers VFM, Lee RT. Protein therapeutics for cardiac regeneration after myocardial infarction. J Cardiovasc Transl Res. 2010;3: 469–77. 10.1007/s12265-010-9207-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chung ES, Miller L, Patel AN, Anderson RD, Mendelsohn FO, Traverse J, et al. Changes in ventricular remodelling and clinical status during the year following a single administration of stromal cell-derived factor-1 non-viral gene therapy in chronic ischaemic heart failure patients: the STOP-HF randomized Phase II trial. Eur Heart J. 2015;36: 2228–38. 10.1093/eurheartj/ehv254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Theiss HD, Gross L, Vallaster M, David R, Brunner S, Brenner C, et al. Antidiabetic gliptins in combination with G-CSF enhances myocardial function and survival after acute myocardial infarction. Int J Cardiol. 2013;168: 3359–69. 10.1016/j.ijcard.2013.04.121 [DOI] [PubMed] [Google Scholar]
- 33. Mortier A, Van Damme J, Proost P. Regulation of chemokine activity by posttranslational modification. Pharmacol Ther. 2008;120: 197–217. 10.1016/j.pharmthera.2008.08.006 [DOI] [PubMed] [Google Scholar]
- 34. Pyo RT, Sui J, Dhume A, Palomeque J, Blaxall BC, Diaz G, et al. CXCR4 modulates contractility in adult cardiac myocytes. J Mol Cell Cardiol. 2006;41: 834–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SDF1α in buffer was selected as the 100% reference.
(PPTX)
(PPTX)
(A) No difference was found between patients with a different type of LV dysfunction (B) or for the different severities of LV dysfunction.
(PPTX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.