Abstract
The onset of puberty is the result of the increased secretion of hypothalamic luteinizing hormone-releasing hormone (LHRH). The pubertal process can be altered by substances that can affect the prepubertal secretion of this peptide. Alcohol is one such substance known to diminish LHRH secretion and delay the initiation of puberty. The increased secretion of LHRH that normally occurs at the time of puberty is due to a decrease of inhibitory tone that prevails prior to the onset of puberty, as well as an enhanced development of excitatory inputs to the LHRH secretory system. Additionally, it has become increasingly clear that glial-neuronal communications are important for pubertal development because they play an integral role in facilitating the pubertal rise in LHRH secretion. Thus, in recent years attempts have been made to identify specific glial-derived components that contribute to the development of coordinated communication networks between glia and LHRH cell bodies, as well as their nerve terminals. Transforming growth factor-α and transforming growth factor-β1 are two such glial substances that have received attention in this regard. This review summarizes the use of multiple neuroendocrine research techniques employed to assess these glial-neuronal communication pathways involved in regulating prepubertal LHRH secretion and the effects that alcohol can have on their respective functions.
Keywords: alcohol, puberty, transforming growth factor-α, transforming growth factor-β1, glia
Introduction
Over the years it has been well documented that alcohol suppresses hypothalamic luteinizing hormone-releasing hormone (LHRH) secretion (Dees, Rettori, Kozlowski, & McCann, 1985; Dees, Srivastava, & Hiney, 2009; Dissen, Dearth, Scott, Ojeda, & Dees, 2004) and causes a delay in puberty-related events in rats (Dees & Skelley, 1990), rhesus monkeys (Dees, Dissen, Hiney, Lara, & Ojeda, 2000) and humans (Peck, Peck, Skaggs, Fukushima, & Kaplan, 2011; Richards & Oinonen, 2011). The hypothalamus plays the critical role in synchronizing events leading to the activation of mammalian puberty. This process requires the interaction of both glial and neuronal regulatory circuitries that serve to control the secretion of LHRH (Brann & Mahesh, 1994; Ojeda & Urbanski, 1994). Understanding mechanisms by which glial cells contribute to LHRH secretion and how alcohol can affect those actions is important for discerning the mechanism by which alcohol suppresses the pubertal process.
In mammals, the LHRH peptide is synthesized mainly in neurons within the preoptic area (POA), with the vast majority of the nerve processes coursing caudally into the medial basal hypothalamus (MBH) and ending near capillaries within the median eminence (ME). A difference between rats and primates is that the latter, including humans, also have LHRH cell bodies in the arcuate nucleus (ARC) of the MBH, whereas rats do not (Kozlowski & Dees, 1984). However, it is well accepted that the mechanisms governing the release of the peptide are very similar. The enhanced secretion of LHRH leads to increased pituitary gonadotropin secretion followed by an elevation in production of ovarian estradiol and subsequently, reproductive maturity. The secretory activity of LHRH neurons is triggered by several trans-synaptic inputs of both inhibitory and excitatory nature (Brann & Mahesh, 1994; Crowley, Parker, Sahu, & Kalra, 1995). Decreased secretion of inhibitory neurotransmitters (Terasawa, 1999; Terasawa & Fernandez, 2001), and the increased secretion of numerous excitatory neurotransmitters (Claypool, Kasuya, Saitoh, Marzban, & Terasawa, 2000; Hiney, Ojeda, & Dees, 1991; Hiney, Srivastava, Nyberg, Ojeda, & Dees, 1996; Hiney, Srivastava, Pine, & Dees, 2009; Lee, Hiney, Pine, Srivastava, & Dees, 2007; Navarro et al., 2004; Ojeda, Urbanski, Costa, Hill, & Moholt-Siebert, 1990) initiate the cascade of events that ultimately lead to the rise in pubertal LHRH release. Some of these transmitters are growth factors of glial origin and are important at the time of puberty because of their involvement in glial-neuronal signaling processes by which the glial cells, through their intimate association with the LHRH nerve terminals in the MBH, regulate LHRH secretion during mammalian puberty (Ma, Berg-von der Emde, Moholt-Siebert, Hill, & Ojeda, 1994; Ma, Costa, & Ojeda, 1994; Ojeda, Lomniczi, & Sandau, 2008). Two glial-derived members of the epidermal growth factor (EGF) family, transforming growth factor-α (TGFα) and transforming growth factor-β1 (TGFβ1), have been shown during the past decade to be significantly involved in the release of LHRH at puberty. Furthermore, their functions have been shown to be altered by alcohol. In this review, we will first describe the importance of their respective roles and physiological mechanisms of action pertaining to the control of LHRH secretion at puberty, and then detail the means by which alcohol alters their actions and disrupts glial-neuronal communications, hence, resulting in suppressed LHRH release. The material presented not only shows how TGFα and TGFβ1 contribute to normal puberty, but also provides insight as to how alcohol can detrimentally affect pubertal maturation.
ErbB receptor activation and prepubertal LHRH release
While EGF and TGFα can both stimulate LHRH release (Ojeda et al., 1990), TGFα plays the more pivotal role in regulation of LHRH neuronal function during puberty (Ma, Berg-von der Emde, et al., 1994; Ojeda et al., 2008). TGFα is highly expressed in both astrocytes and tanycytes of the MBH and increases markedly around the time of puberty (Ma, Junier, Costa, & Ojeda, 1992). TGFα binds to and activates the erbB1/erbB2 receptor complex on adjacent glial cells in MBH. Activation of these receptors results in the production of a glial substance, PGE2, which then induces the release of prepubertal LHRH secretion upon binding to specific receptors on nearby LHRH neuron terminals in the ME region of the MBH (Ma, Berg-von der Emde, Rage, Wetsel, & Ojeda, 1997). Studies have shown that TGFα stimulates LHRH release via an indirect mechanism that involves a paracrine effect of this growth factor on glial cells. In this regard, the receptors for TGFα have been shown only in glial cells (Ma, Berg-von der Emde, et al., 1994). In vitro studies have shown that exposure to TGFα stimulates secretion of PGE2 from hypothalamic glial cells into the medium, and that placing this conditioned medium on immortalized LHRH-secreting neurons referred to as GT1 cells causes LHRH release (Ma et al., 1997). Additionally, in hypothalamic glial cells, TGFα induced PGE2 formation, and the stimulatory effect of the TGFα-conditioned medium on LHRH release is prevented by erbB receptor inhibition or blockade of prostaglandin synthesis (Ma et al., 1997; Ojeda & Ma, 1999). Taken together, these studies demonstrate that TGFα acts by indirectly influencing hypothalamic glial-neuronal communication networks contributing to mammalian puberty.
In vitro and in vivo effects of alcohol on erbB1 receptor activation and LHRH release
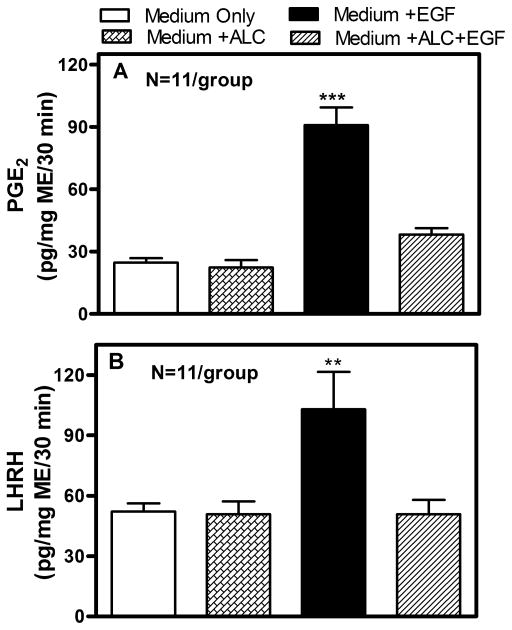
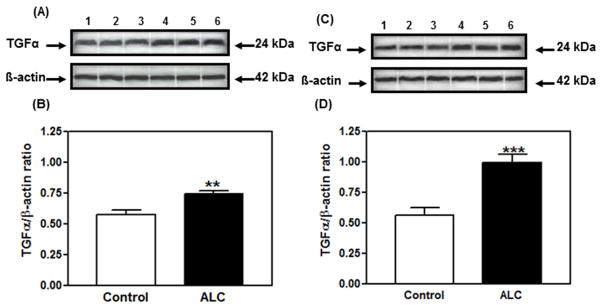
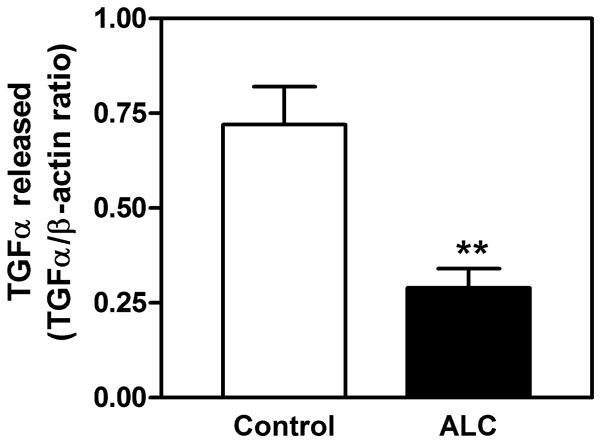
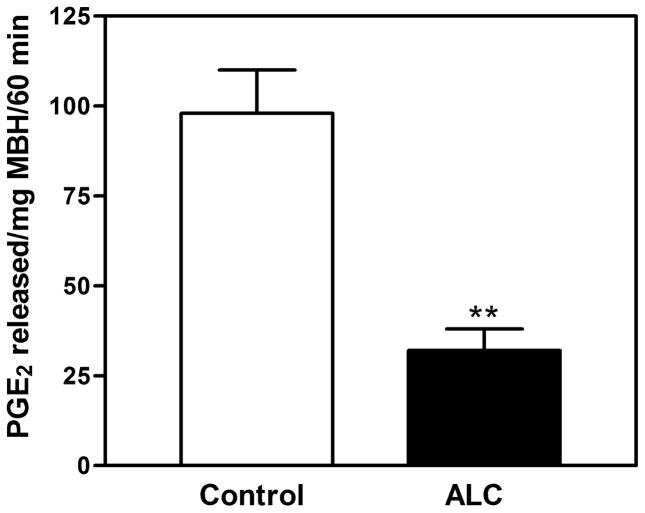
Understanding the mechanism of alcohol-induced suppression of LHRH release is important for determining how this drug disrupts pubertal development. Critical to this issue is the role of PGE2, which plays a major role in the LHRH secretory process in prepubertal animals (Ojeda, Urbanski, Katz, & Costa, 1988; Ojeda, Urbanski, Katz, Costa, & Conn, 1986). Furthermore, it is a critical component for the glial-dependent regulation of LHRH release (Ma et al., 1997; Prevot, Cornea, Mungenast, Smiley, & Ojeda, 2003). An earlier report (Hiney, Dearth, Srivastava, Rettori, & Dees, 2003) showed that acute in vitro exposure to alcohol blocks PGE2 and LHRH secretion from the same ME tissue fragments containing the LHRH nerve terminals (Fig. 1). Only recently, however, have the mechanisms of alcohol actions on the TGFα-PGE2 pathway been critically assessed with regard to prepubertal hypothalamic glial-neuronal communications (Srivastava, Hiney, & Dees, 2011). Specifically, it was shown in immature female rats that short-term alcohol exposure via a liquid diet feeding regimen for 4 and 6 days caused an increase in hypothalamic TGFα gene and protein expressions. TGFα gene expression was increased markedly at 4 days and was still elevated after 6 days (see Srivastava et al., 2011). This effect paralleled the increased TGFα protein expressions on both days (Fig. 2A–D). To determine whether the increased levels of TGFα protein were due to diminished release, basal TGFα secretion was assessed from MBHs incubated in vitro after 6 days of alcohol exposure in vivo. Results indicated that alcohol exposure suppressed TGFα release into the medium (Fig. 3). Taken together, these findings demonstrate that the alcohol suppressed the release of this glial peptide, resulting in an accumulation of hypothalamic TGFα mRNA and protein.
Fig. 1.
Effect of alcohol on EGF-induced PGE2 (Panel A) and LHRH (Panel B) release in vitro from the median eminence of prepubertal female rats. Open bars represent basal release of PGE2 and LHRH. Hatched bars represent basal release of PGE2 and LHRH in the presence of 50 mM alcohol, which would be approximately 230 mg/dL of serum in vivo. Solid bars represent EGF-induced PGE2 and LHRH release, and lined bars represent EGF-induced release of PGE2 and LHRH release in the presence of 50 mM alcohol. Note that EGF significantly stimulated both PGE2 and LHRH release, which was blocked by alcohol. Bars represent the mean ± SEM. **p < 0.01 vs. Medium only, Medium + alcohol, and Medium + alcohol + EGF; ***p < 0.001 vs. Medium only, Medium + alcohol, and Medium + alcohol + EGF. Modified from Hiney et al., 2003.
Fig. 2.
Effect of short-term alcohol exposure for 4 (panels A & B) and 6 days (panels C & D) on TGFα protein expression in the MBH of prepubertal female rats. (A & C) Representative Western immunoblot of TGFα and β-actin proteins in the MBH isolated from control (lanes 1–3) and alcohol-treated (lanes 4–6) animals. (B & D) Densitometric quantitation of all the bands from 2 blots assessing TGFα protein expression in the MBH. These data were normalized to the internal control β-actin protein, and the densitometric units represent the TGFα/β-actin ratio. Note that alcohol-treated animals showed increased TGFα protein expression on day 4 (panel B) and day 6 (panel D) compared with control animals. The respective bars illustrate the mean (± SEM) of an N of 7–8 per group. The mean blood alcohol levels after 4 and 6 days of treatment with the alcohol diet were 188 mg/dL and 210 mg/dL, respectively. **p < 0.01; ***p < 0.001 vs. control. Modified from Srivastava et al., 2011.
Fig. 3.
Effect of short-term alcohol exposure for 6 days on TGFα protein released in vitro from the MBH of prepubertal female rats. The content of TGFα released into the medium was determined by Western immunoblotting. Note that alcohol-treated animals showed a marked decrease in basal TGFα release compared with control animals. These data were normalized to the internal control β-actin protein, and the densitometric units represent the TGFα/β-actin ratio. The respective bars illustrate the mean (± SEM) of an N of 14 for control and an N of 9 for alcohol. **p < 0.01 vs. control. Modified from Srivastava et al., 2011.
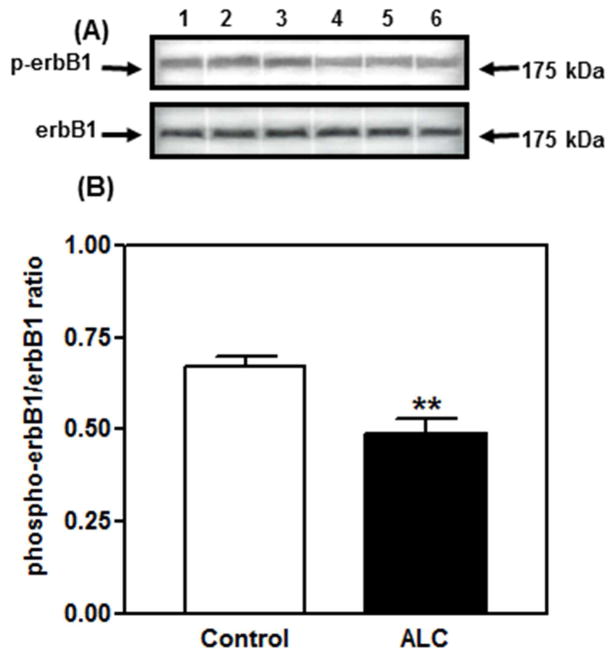
With regard to erbB1 receptors, alcohol exposure for 4 and 6 days did not elicit changes in erbB1 gene expression or the synthesis of total, non-phosphorylated erbB1 protein, but caused a marked decrease in the synthesis of the phosphorylated form of the receptor at 4 days (see Srivastava et. al., 2011), as well as at 6 days (Fig. 4). Apparently, the 4–6-day duration of alcohol exposure used was not long enough to down-regulate erbB1 gene expression; however, the translation of phosphorylated erbB1 protein was diminished. Interestingly, alcohol did not affect the synthesis of total and phosphorylated erbB2 (not shown), which further demonstrates a specific effect of alcohol on the erbB1 receptor. We suggest that because TGFα binding initiates autophosphorylation of the erbB1 receptor (Ebner & Derynck, 1991), it is plausible that the action of alcohol to suppress TGFα release (Fig. 3), at least in part, is a contributing factor to the alcohol-related decrease in erbB1 phosphorylation. However, we cannot rule out the possibility of a direct action of alcohol on the erbB1 autophosphorylation process that is independent of suppressed TGFα secretion. Several studies supporting this have demonstrated that chronic alcohol exposure disrupts phosphorylation of erbB1 by altering receptor affinity and/or tyrosine kinase activity (Tuma, Todero, Barak-Bernihagen, Casey, & Sorrell, 1998; Wang, Feng, & Wu-Wang, 1997).
Fig. 4.
Effect of short-term alcohol exposure for 6 days on phosphorylated erbB1 protein expressions in the MBH of prepubertal female rats. (A) Representative Western blot of phosphorylated erbB1 and total, nonphosphorylated erbB1 proteins in the MBH from controls (lanes 1–3) and animals exposed to alcohol for 6 days (lanes 4–6). (B) Densitometric quantitation of all of the bands from 2 blots assessing phosphorylated erbB1 protein in the MBH. Note that phosphorylated erbB1 protein expression decreased markedly in the animals treated 6 days with alcohol diet when compared to controls. These data were normalized to the total, nonphosphorylated erbB1 protein, and the densitometric units represent the phosphorylated erbB1/total, non-phosphorylated erbB1 ratio. The respective bars illustrate the mean (± SEM) of an N of 8 per group at 6 days. **p < 0.01 vs. control. Modified from Srivastava et al., 2011.
Because suppressed erbB1 phosphorylation was expected to result in decreased PGE2 release, rats were exposed to alcohol in vivo for 6 days. Their hypothalami were then removed and subsequently incubated in vitro for assessing the release of PGE2. In this regard, the in vivo alcohol exposure caused the suppression of PGE2 released in vitro (Fig. 5). This action was associated with the suppressed phosphorylation of the erbB1 receptor shown in Fig. 4.
Fig. 5.
Effect of short-term alcohol exposure for 6 days on prostaglandin-E2 (PGE2) release in vitro from the MBH of prepubertal rats. The content of PGE2 released into the medium was determined by ELISA. Note that alcohol-treated animals showed a marked decrease in the basal PGE2 release compared with control animals. The respective bars illustrate the mean (± SEM) of an N of 14 for control and an N of 9 for alcohol. **p < 0.01 vs. control. Modified from Srivastava et al., 2011.
Collectively, these results demonstrate the inhibitory effects of alcohol on the glial TGFα/erbB1 pathway contributing to the production and secretion of PGE2 within the MBH, hence, indicating that this is one of the mechanisms by which alcohol suppresses PGE2 and subsequently, LHRH secretion (Hiney & Dees, 1991; Lomniczi et al., 2000; Srivastava et al., 2011). The fact that alcohol can negatively affect the erbB1 receptor is important since it is known that an alteration in the function of this receptor is associated with delayed puberty (Apostolakis, Garai, Lohmann, Clark, & O’Malley, 2000).
TGFβ1 and prepubertal LHRH synthesis and release
In addition to TGFα regulation of LHRH secretion, another growth factor that is produced and secreted by hypothalamic astrocytes, TGFβ1, is now being shown to also influence LHRH synthesis and release by several methodologies, including astrocyte and neuronal cell line cultures, in vitro incubations of hypothalamic tissue, and in vivo studies.
Hypothalamic glia are not only associated with LHRH nerve terminals in the MBH/ME, but also have been demonstrated in the rat to have a connection with LHRH neuronal parakarya in the POA (Witkin, Ferin, Popilskis, & Silverman, 1991; Witkin & Silverman, 1985). TGFβ1 gene expression has been observed in hypothalamic astrocytes in culture (Buchanan, Mahesh, & Brann, 2000; Galbiati et al., 1996) and in both the POA and MBH in vivo (Bouret, De Seranno, Beauvillain, & Prevot, 2004). Furthermore, LHRH neurons in the POA express both TGFβ-receptor-1 (Prevot et al., 2000) and TGFβ-receptor-2 (Bouret et al., 2004). Taken together, these results suggest that this peptide may regulate LHRH synthesis and possibly release through direct activation of the LHRH neurons in the POA and may also contribute to release of the peptide, possibly through glial-nerve terminal interactions in the MBH/ME. Initial studies conducted in vitro demonstrated that TGFβ1 secreted from astrocytes grown in culture can act directly on GT1 neurons, a LHRH-secreting cell line, to stimulate LHRH gene expression and release of the peptide (Buchanan et al., 2000; Galbiati et al., 1996; Melcangi et al., 1995). GT1 cells and their processes/terminals are both present in the culture dish. Therefore, this does not take into account that, in the rat, for example, the LHRH is synthesized in neurons located in the POA, yet the area with the greatest concentration of its nerve terminals is located in the MBH. Thus, while the results using the GT1 cultures are interesting, they do not rule out the possibility that LHRH release in the live animal may occur not only following direct activation at the level of the nerve cell body, but also through actions, either direct or indirect, at the level of the nerve terminals. The nature of this question as to what are the sites and mechanisms of TGFβ1 actions on the control of LHRH has required the use of several experimental methods. The sections below will describe how the combination of cell culture, in vivo, and in vitro techniques, as well as assessing alcohol interactions, have contributed to our understanding of glial-neuronal communications regarding TGFβ1 control of LHRH synthesis and release.
Actions and interactions of TGFβ1 and alcohol on LHRH neurons
The TGFβ1 peptide has been shown (Srivastava, Hiney, & Dees, 2014) to induce LHRH gene expression in the POA within 6 h after a third ventricular injection, an action that was blocked by alcohol (Table 1). Hence, these data support the previous in vitro/cell-culture studies using GT1 cells, showing that LHRH gene expression is up-regulated by astrocyte-derived TGFβ1 (Galbiati et al., 1996). This information, along with results derived from rat POA tissues that show TGFβ1-expressing astrocytes are closely associated with LHRH neurons that are immunoreactive for both TGFβ receptors (Bouret et al., 2004; Prevot et al., 2000), provides in vivo evidence supporting the concept that TGFβ1 can up-regulate the LHRH gene.
Table 1.
The effects of transforming growth factor β1 (TGFβ1) and alcohol on LHRH gene expression in the preoptic area (POA) of prepubertal female rats. The central administration of TGFβ1 (100 ng) induced the expression of the LHRH gene at 6 h post-injection in animals that did not receive alcohol when compared to control animals. Note that this TGFβ1-induced LHRH gene expression was blocked in the alcohol-treated animals. N of 9 per group.
| Control | TGFβ1 | TGFβ1+ALC | |
|---|---|---|---|
| LHRH mRNA (relative expression) | 1.27±0.06 | 1.60±0.08* | 1.31±1.08 |
p < 0.05 vs. control and TGFβ1 + alcohol.
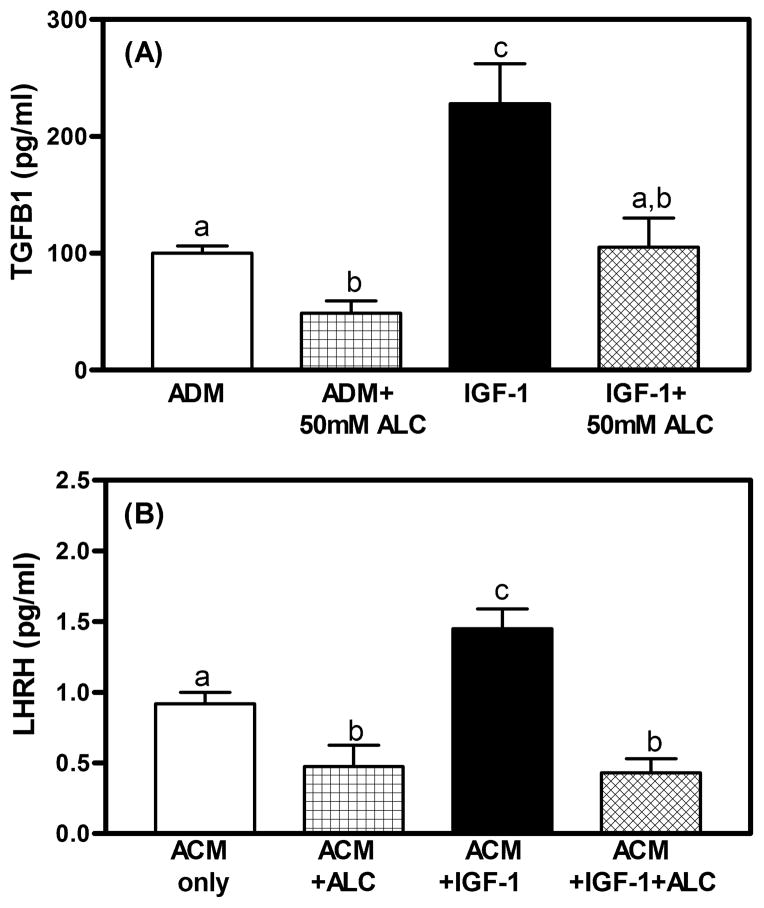
To further investigate glial-neuronal communications, we recently used a cell culture approach to assess the effects of alcohol and another glial-derived peptide, insulin-like growth factor-1 (IGF-1), on TGFβ1 and its potential action on LHRH neurons. In this regard, hypothalamic astrocytes were grown in culture and exposed to medium only, medium containing either alcohol or IGF-1, or medium with both alcohol and IGF-1. In Fig. 6A, astrocytes exposed to alcohol showed suppressed basal secretion of TGFβ1, while exposure to IGF-1 markedly stimulated the secretion of the peptide, and this action was blocked when alcohol was present in the medium. After this 18-h incubation, the medium from each of the four above groups was removed and used to replace the medium in which GN11 cells, another LHRH-secreting cell line, were growing in culture. In this regard, the medium that was originally exposed to the alcohol only, and then contained suppressed TGFβ1, caused a decrease in LHRH released from the GN11 cells (Fig. 6B). This figure also shows that the medium that was originally exposed to IGF-1 only, and then contained increased TGFβ1, caused a marked increase in LHRH released from the GN11 cells, whereas the medium that was originally exposed to both IGF-1 and alcohol and did not result in elevated TGFβ1, was unable to stimulate LHRH secretion from these cells. Collectively, the above studies using in vivo tissue assessments, as well as astrocyte cultures, along with two different LHRH cell lines, have provided convincing evidence for actions of glial-derived TGFβ1 on LHRH neurons.
Fig. 6.
A) TGFβ1 levels in conditioned medium of hypothalamic astrocytes following 18 h of exposure to astrocyte-defined medium (ADM), ADM + alcohol (50 mM), IGF-1 (100 ng/mL), and IGF-1 + alcohol. Alcohol inhibited TGFβ1 secretion from hypothalamic astrocytes over basal release (ADM). IGF-1 increased the amount of TGFβ1 released and alcohol blocked this increase. B) LHRH release from GN-11 neurons after 60-min incubation in astrocyte-conditioned media (ACM) from the groups in 6A. ACM was added to the wells but no additional alcohol was added to the wells. LHRH release was reduced when exposed to the ACM with alcohol as compared to ACM only. GN-11 cells exposed to ACM medium with IGF-1 have augmented LHRH release over basal release and this increase did not occur when neurons were exposed to ACM with IGF-1 + alcohol. N = 6 wells/group. a vs. c: p < 0.01; b vs. c: p < 0.01; a vs. b: p < 0.05.
Effects of TGFβ1 and alcohol on LHRH release from nerve terminals within the MBH
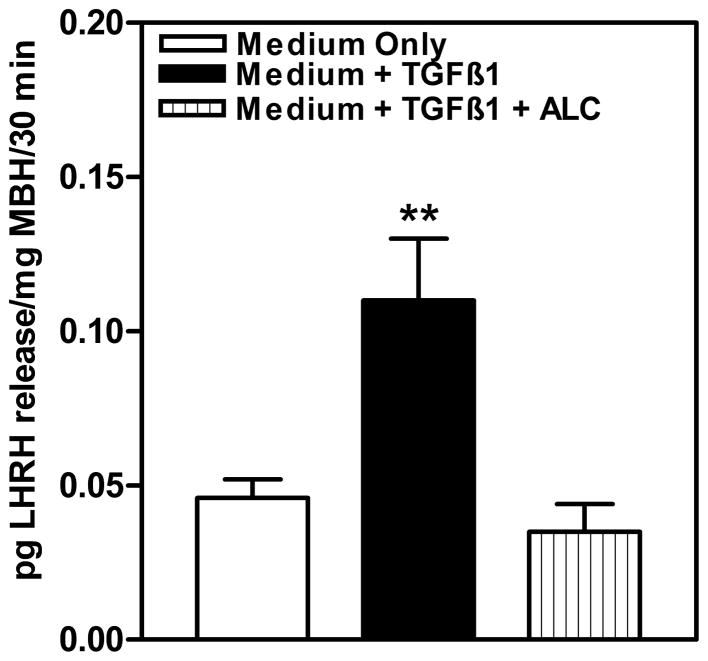
While the TGFβ1 action on the LHRH neuron is convincing, its ability to stimulate release of the peptide from nerve terminals when LHRH cell bodies are not present is less clear. An earlier report using the rat model indicated that TGFβ1 was ineffective in causing the release of the LHRH peptide from nerve terminals when only the ME was incubated in vitro (Ojeda et al., 1990). In a more recent study (Srivastava et al., 2014), we assessed the effect of TGFβ1 and alcohol on LHRH release in vitro from a tissue block that contained the entire MBH from prepubertal female rats, which includes both the ARC nucleus and the ME. In this regard, the TGFβ1 induced a marked increase in LHRH released from the MBH into the medium (Fig. 7). This figure also demonstrates that alcohol was capable of blocking the stimulatory effect of the TGFβ1 peptide on LHRH release. The difference between the results of this study compared to the previous one, indicating the lack of LHRH stimulation with the ME only, is the presence of the ARC nucleus in the tissue block along with the ME. Although more research is needed, it is likely that the TGFβ1 is acting indirectly by stimulating a neurotransmitter produced by neurons located in the ARC nucleus that, once secreted, is capable of inducing LHRH release from the nerve terminals in the ME. The potential for this action is supported by the fact that both TGFβ receptors are present in the ARC nucleus (Bouret et al., 2004; Prevot et al., 2000).
Fig. 7.
Effect of acute in vitro alcohol exposure on TGFβ1-induced LHRH release from the MBH of prepubertal female rats. Open bar indicates basal secretion of LHRH in medium only. Solid bar represents LHRH release in medium containing TGFβ1, and lined bar represents LHRH release in the presence of medium containing TGFβ1 plus 50 mM alcohol. Note that TGFβ1 induced the secretion of LHRH compared to the medium-only group and that the presence of alcohol in the medium blocked the TGFβ1-induced release of the peptide. The bars illustrate the mean (± SEM) of an N of 9 per group. **p < 0.01 vs. medium only and medium + TGFβ1 + alcohol. Modified from Srivastava et al., 2014.
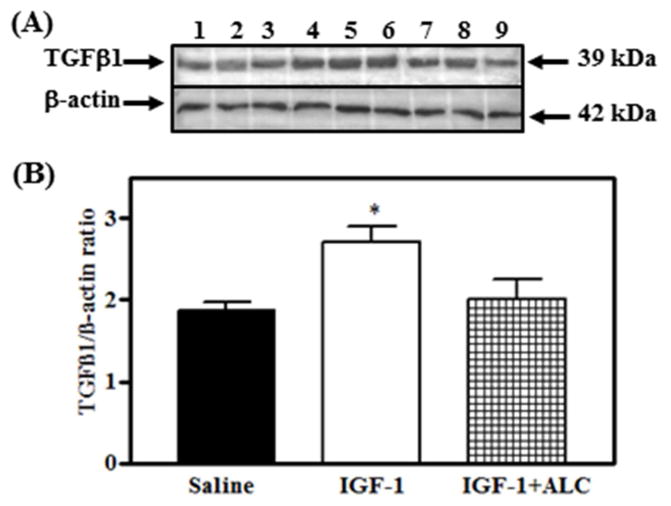
It is worth noting that TGFβ1 can facilitate LHRH release from the terminals within the MBH/ME via other mechanisms. For example, IGF-1 plays an important role at puberty (Hiney et al., 1996). Glial synthesis of this peptide increases in the hypothalamus as puberty approaches and furthermore, it is known that peripherally derived IGF-1 crosses the blood-brain barrier to enter this region, which contains the greatest number of IGF-1 receptors (Lesniak et al., 1988). This peptide can stimulate PGE2 release and subsequently, LHRH release in vitro (Hiney, Srivastava, Lara, & Dees, 1998) and LH in vivo (Hiney et al., 1996). Since alcohol inhibited IGF-induced TGFβ1 from cultured hypothalamic astrocytes, the question arose as to the ability of IGF-1 to stimulate TGFβ1 protein synthesis in MBH tissue from prepubertal female rats in vivo. Thus, we recently showed (Hiney, Srivastava, Volz, & Dees, 2014) that the central administration of IGF-1 stimulated an increase in TGFβ1 at 6 h post-injection, and that this action was blocked by alcohol (Fig. 8). This IGF-1 action was subsequently shown to be mediated by the transduction signal Akt, which was also suppressed by alcohol. In order to determine the mechanism of alcohol’s action on the IGF-1 signaling pathway in this brain region, we demonstrated that the IGF-1-stimulated phosphorylation of the IGF-1 receptor was inhibited by alcohol, thus resulting in the downstream alterations in Akt and TGFβ1 protein levels (Hiney et al., 2014).
Fig. 8.
Effects of IGF-1 and acute alcohol exposure on TGFβ1 protein in the MBH of prepubertal female rats. A) Representative Western blot of TGFβ1 and β-actin proteins from saline (lanes 1–3), IGF-1 (lanes 4–6), and IGF-1 + alcohol (lanes 7–9) -treated animals. B) Densitometric quantification of all bands assessing the TGFβ1 protein. These data were normalized to the internal control β-actin protein. IGF-1 (open bar) induced an increase in TGFβ1 over saline-treated (solid bar) animals. Animals were dosed by gastric gavage with a 3 g/kg injection of alcohol, which yielded a peak serum alcohol level of 150–180 mg/dL after 90 min. At this time, IGF-1 (200 ng/mL) was injected into a third ventricular cannula. An additional 2 g/kg dose of alcohol was given 4 h after the initial dose to maintain moderately elevated serum alcohol levels. The tissues were collected 6 h after the infusion of IGF-1 and the serum alcohol levels at that time were 154 mg/dL (Hiney et al. 2014). Note that exposure to alcohol blocked the IGF-1-induced expression of TGFβ1 protein (hatched bar). Each bar represents the mean ± SEM of the TGFβ1/β-actin ratio. The number of animals represented by each bar is 6. *p < 0.05 vs. saline-treated and IGF-1 + alcohol-treated animals. Modifed from Hiney et al., 2014.
The ability of IGF-1 to activate the glial production of prepubertal TGFβ1 is important with regard to the regulation of LHRH secretion at the time of puberty. Because IGF-1 and TGFβ1 are produced in glia, their glial to glial interrelationship is relevant. IGF-1 can affect TGFβ1 through activation of the above-mentioned TGFα-PGE2 pathway from adjacent glial cells. Oct 2 POU homeodomain genes are expressed in hypothalamic glia and increase during pubertal development (Ojeda et al., 1999). This gene is an upstream modulator of the TGFα, which, as stated earlier, is involved in LHRH release. We have shown that Oct 2 genes are a link between IGF-1 and TGFα (Dees, Srivastava, & Hiney, 2005). Specifically, centrally administered IGF-1 stimulated Oct 2c in the MBH and furthermore, this action was blocked by alcohol. Importantly, TGFα acts within the MBH through erbB1 receptors to stimulate glial-derived PGE2 release (Ma et al., 1997). Once released, PGE2 not only can induce LHRH release directly from its neuron terminals in the ME (Hiney & Dees, 1991; Ojeda, Negro-Vilar, & McCann, 1979), but it can also act on specialized glial cells called tanycytes that line the third ventricle of the hypothalamus (Prevot et al., 2003). With regard to the latter, PGE2 stimulates the release of TGFβ1, which then causes retraction of tanycyte processes within the ME to better allow for entry of LHRH into the hypophyseal portal blood (Prevot et al., 2003). Collectively, this information clearly depicts the importance of glial to glial communications in regulating prepubertal LHRH secretion. Also, an alcohol-induced suppression of TGFβ1 synthesis, either alone or coupled with the alcohol inhibition of IGF-1-induced PGE2 release (Hiney et al., 1998), would markedly affect tanycyte functions related to the LHRH secretory process.
Conclusions
A recent survey indicates that there has been a significant increase in alcohol use and abuse between adolescent and early teenage development (Miech, Johnston, O’Malley, Bachman, & Schulenberg, 2015). It is now well accepted that binge-type drinking is frequent and can easily produce BACs similar to those we have shown above. Thus, increased alcohol use poses a health concern for the young since they are vulnerable to its detrimental effects. As stated above, an indication of this is that girls who drink have four times the chance for showing delayed signs of pubertal maturation. Thus, identifying the underlying mechanisms by which alcohol can alter adolescent development is important and will help define treatment methods for aiding in recovery and lessening the impact of the adverse effects of alcohol during this critical stage of development.
In this review, we have focused on the contributions of two glial-neuronal communication pathways with regard to pubertal LHRH secretion and revealed how these interactions are influenced by alcohol. One of the pathways addressed is the TGFα-erbB receptor signaling system. Glial TGFα activates the erbB1/erbB2 receptor complex on adjacent glia in the MBH. This activation causes a cascade of events leading to the increased synthesis and release of PGE2, which in turn binds to its receptor on nearby LHRH nerve terminals, thus inducing release of the peptide. Additionally, evidence was presented using a combination of in vivo and in vitro methods showing that alcohol is capable of interfering with hypothalamic glial to glial signaling involved with prepubertal PGE2 synthesis/release, actions that ultimately contribute to the alcohol-induced decrease in LHRH secretion. The other communication network discussed is that of the influence of TGFβ1 on the LHRH system. With regard to the POA, we showed the potential for TGFβ1 to regulate LHRH synthesis and possibly release through a direct activation of LHRH neurons. We also discussed how TGFβ1 may contribute indirectly within the MBH to release LHRH peptide through potential glial-neuronal interactions in the ARC nucleus and furthermore, addressed the interactions between IGF-1, TGFα, PGE2, and TGFβ1 regarding their coordinated ability to facilitate LHRH release through their glial-glial and glial-nerve terminal interactions within the ME. Overall, this review has described the use of multiple research techniques to further our knowledge as to the importance of glial-LHRH neuronal communications at puberty, and how a toxic substance, such as alcohol, is capable of altering these important cell-cell interactions during an important time of development.
Highlights.
Increased hypothalamic TGFα following ALC exposure was due to decreased release of peptide.
ALC-induced decrease in TGFα secretion resulted in suppressed erbB1 activity, followed by suppression in prepubertal PGE2 release.
In POA, TGFβ1 stimulates LHRH synthesis and possibly release through a direct activation of LHRH neurons.
TGFβ1 may contribute indirectly within the MBH to release LHRH peptide through potential glial-neuronal interactions in the ARC nucleus.
IGF-1 induces TGFβ1 protein synthesis in the MBH area and this action is blocked by ALC.
Acknowledgments
This work was supported by the NIH grant AA007216 (to WLD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apostolakis EM, Garai J, Lohmann JE, Clark JH, O’Malley BW. Epidermal growth factor activates reproductive behavior independent of ovarian steroids in female rodents. Molecular Endocrinology. 2000;14:1086–1098. doi: 10.1210/mend.14.7.0490. [DOI] [PubMed] [Google Scholar]
- Bouret S, De Seranno S, Beauvillain JC, Prevot V. Transforming growth factor beta1 may directly influence gonadotropin-releasing hormone gene expression in the rat hypothalamus. Endocrinology. 2004;145:1794–1801. doi: 10.1210/en.2003-1468. [DOI] [PubMed] [Google Scholar]
- Brann DW, Mahesh VB. Excitatory amino acids: function and significance in reproduction and neuroendocrine regulation. Frontiers in Neuroendocrinology. 1994;15:3–49. doi: 10.1006/frne.1994.1002. [DOI] [PubMed] [Google Scholar]
- Buchanan CD, Mahesh VB, Brann DW. Estrogen-astrocyte-luteinizing hormone-releasing hormone signaling: a role for transforming growth factor-beta(1) Biology of Reproduction. 2000;62:1710–1721. doi: 10.1095/biolreprod62.6.1710. [DOI] [PubMed] [Google Scholar]
- Claypool LE, Kasuya E, Saitoh Y, Marzban F, Terasawa E. N-methyl D,L-aspartate induces the release of luteinizing hormone-releasing hormone in the prepubertal and pubertal female rhesus monkey as measured by in vivo push-pull perfusion in the stalk-median eminence. Endocrinology. 2000;141:219–228. doi: 10.1210/endo.141.1.7231. [DOI] [PubMed] [Google Scholar]
- Crowley WR, Parker SL, Sahu A, Kalra SP. Interacting transmembrane signals regulating GnRH and LH secretion. In: Lee PA, Plant TM, editors. The Neurobiology of Puberty. Bristol, UK: John Wiley & Sons; 1995. pp. 41–54. [Google Scholar]
- Dees WL, Dissen GA, Hiney JK, Lara F, Ojeda SR. Alcohol ingestion inhibits the increased secretion of puberty-related hormones in the developing female rhesus monkey. Endocrinology. 2000;141:1325–1331. doi: 10.1210/endo.141.4.7413. [DOI] [PubMed] [Google Scholar]
- Dees WL, Rettori V, Kozlowski GP, McCann SM. Ethanol and the pulsatile release of luteinizing hormone, follicle stimulating hormone and prolactin in ovariectomized rats. Alcohol. 1985;2:641–646. doi: 10.1016/0741-8329(85)90139-9. [DOI] [PubMed] [Google Scholar]
- Dees WL, Skelley CW. Effects of ethanol during the onset of female puberty. Neuroendocrinology. 1990;51:64–69. doi: 10.1159/000125317. [DOI] [PubMed] [Google Scholar]
- Dees WL, Srivastava V, Hiney JK. Actions and interactions of alcohol and insulin-like growth factor-1 on female pubertal development. Alcoholism: Clinical and Experimental Research. 2009;33:1847–1856. doi: 10.1111/j.1530-0277.2009.01041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dees WL, Srivastava VK, Hiney JK. Alcohol alters insulin-like growth factor-1 activated oct 2 POU domain gene expression in the immature female hypothalamus. Journal of Studies on Alcohol. 2005;66:35–45. doi: 10.15288/jsa.2005.66.35. [DOI] [PubMed] [Google Scholar]
- Dissen GA, Dearth RK, Scott HM, Ojeda SR, Dees WL. Alcohol alters luteinizing hormone secretion in immature female rhesus monkeys by a hypothalamic action. Endocrinology. 2004;145:4558–4564. doi: 10.1210/en.2004-0517. [DOI] [PubMed] [Google Scholar]
- Ebner R, Derynck R. Epidermal growth factor and transforming growth factor-alpha: differential intracellular routing and processing of ligand-receptor complexes. Cell Regulation. 1991;2:599–612. doi: 10.1091/mbc.2.8.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbiati M, Zanisi M, Messi E, Cavarretta I, Martini L, Melcangi RC. Transforming growth factor-beta and astrocytic conditioned medium influence luteinizing hormone-releasing hormone gene expression in the hypothalamic cell line GT1. Endocrinology. 1996;137:5605–5609. doi: 10.1210/endo.137.12.8940390. [DOI] [PubMed] [Google Scholar]
- Hiney JK, Dearth RK, Srivastava V, Rettori V, Dees WL. Actions of ethanol on epidermal growth factor receptor activated luteinizing hormone secretion. Journal of Studies on Alcohol. 2003;64:809–816. doi: 10.15288/jsa.2003.64.809. [DOI] [PubMed] [Google Scholar]
- Hiney JK, Dees WL. Ethanol inhibits luteinizing hormone-releasing hormone release from the median eminence of prepubertal female rats in vitro: investigation of its action on norepinephrine and prostaglandin-E2. Endocrinology. 1991;128:1404–1408. doi: 10.1210/endo-128-3-1404. [DOI] [PubMed] [Google Scholar]
- Hiney JK, Ojeda SR, Dees WL. Insulin-like growth factor 1: a possible metabolic signal involved in the regulation of female puberty. Neuroendocrinology. 1991;54:420–423. doi: 10.1159/000125924. [DOI] [PubMed] [Google Scholar]
- Hiney JK, Srivastava V, Lara T, Dees WL. Ethanol blocks the central action of IGF-1 to induce luteinizing hormone secretion in the prepubertal female rat. Life Sciences. 1998;62:301–308. doi: 10.1016/s0024-3205(97)01111-9. [DOI] [PubMed] [Google Scholar]
- Hiney JK, Srivastava V, Nyberg CL, Ojeda SR, Dees WL. Insulin-like growth factor 1 of peripheral origin acts centrally to accelerate the initiation of female puberty. Endocrinology. 1996;137:3717–3728. doi: 10.1210/endo.137.9.8756538. [DOI] [PubMed] [Google Scholar]
- Hiney JK, Srivastava VK, Pine MD, Dees WL. Insulin-like growth factor-1 activates KiSS-1 gene expression in the brain of the prepubertal female rat. Endocrinology. 2009;150:376–384. doi: 10.1210/en.2008-0954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiney JK, Srivastava VK, Volz CE, Dees WL. Alcohol alters insulin-like growth factor-1-induced transforming growth factor β1 synthesis in the medial basal hypothalamus of the prepubertal female rat. Alcoholism: Clinical and Experimental Research. 2014;38:2572–2578. doi: 10.1111/acer.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski GP, Dees WL. Immunocytochemistry for LHRH neurons in the arcuate nucleus area of the rat: fact or artifact? The Journal of Histochemistry and Cytochemistry. 1984;32:83–91. doi: 10.1177/32.1.6690601. [DOI] [PubMed] [Google Scholar]
- Lee B, Hiney JK, Pine MD, Srivastava VK, Dees WL. Manganese stimulates luteinizing hormone releasing hormone secretion in prepubertal female rats: hypothalamic site and mechanism of action. The Journal of Physiology. 2007;578:765–772. doi: 10.1113/jphysiol.2006.123083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesniak MA, Hill JM, Kiess W, Rojeski M, Pert CB, Roth J. Receptors for insulin-like growth factors I and II: autoradiographic localization in rat brain and comparison to receptors for insulin. Endocrinology. 1988;123:2089–2099. doi: 10.1210/endo-123-4-2089. [DOI] [PubMed] [Google Scholar]
- Lomniczi A, Mastronardi CA, Faletti AG, Seilicovich A, De Laurentiis A, McCann SM, et al. Inhibitory pathways and the inhibition of luteinizing hormone-releasing hormone release by alcohol. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:2337–2342. doi: 10.1073/pnas.040569597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YJ, Berg-von der Emde K, Moholt-Siebert M, Hill DF, Ojeda SR. Region-specific regulation of transforming growth factor alpha (TGF alpha) gene expression in astrocytes of the neuroendocrine brain. The Journal of Neuroscience. 1994;14:5644–5651. doi: 10.1523/JNEUROSCI.14-09-05644.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YJ, Berg-von der Emde K, Rage F, Wetsel WC, Ojeda SR. Hypothalamic astrocytes respond to transforming growth factor-alpha with the secretion of neuroactive substances that stimulate the release of luteinizing hormone-releasing hormone. Endocrinology. 1997;138:19–25. doi: 10.1210/endo.138.1.4863. [DOI] [PubMed] [Google Scholar]
- Ma YJ, Costa ME, Ojeda SR. Developmental expression of the genes encoding transforming growth factor alpha and its receptor in the hypothalamus of female rhesus macaques. Neuroendocrinology. 1994;60:346–359. doi: 10.1159/000126769. [DOI] [PubMed] [Google Scholar]
- Ma YJ, Junier MP, Costa ME, Ojeda SR. Transforming growth factor-alpha gene expression in the hypothalamus is developmentally regulated and linked to sexual maturation. Neuron. 1992;9:657–670. doi: 10.1016/0896-6273(92)90029-d. [DOI] [PubMed] [Google Scholar]
- Miech RA, Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975–2014: Volume I, Secondary school students. Ann Arbor: Institute for Social Research, The University of Michigan; 2015. p. 599. Available at http://monitoringthefuture.org/pubs.html#monographs. [Google Scholar]
- Melcangi RC, Galbiati M, Messi E, Piva F, Martini L, Motta M. Type 1 astrocytes influence luteinizing hormone-releasing hormone release from the hypothalamic cell line GT1-1: is transforming growth factor-beta the principle involved? Endocrinology. 1995;136:679–686. doi: 10.1210/endo.136.2.7835301. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernández-Fernández R, Barreiro ML, Roa J, Sanchez-Criado JE, et al. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology. 2004;145:4565–4574. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Hill J, Hill DF, Costa ME, Tapia V, Cornea A, et al. The Oct-2 POU domain gene in the neuroendocrine brain: a transcriptional regulator of mammalian puberty. Endocrinology. 1999;140:3774–3789. doi: 10.1210/endo.140.8.6941. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Lomniczi A, Sandau US. Glial-gonadotrophin hormone (GnRH) neurone interactions in the median eminence and the control of GnRH secretion. Journal of Neuroendocrinology. 2008;20:732–742. doi: 10.1111/j.1365-2826.2008.01712.x. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Ma YJ. Glial-neuronal interactions in the neuroendocrine control of mammalian puberty: facilitatory effects of gonadal steroids. Journal of Neurobiology. 1999;40:528–540. doi: 10.1002/(sici)1097-4695(19990915)40:4<528::aid-neu9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Negro-Vilar A, McCann SM. Release of prostaglandin Es by hypothalamic tissue: evidence for their involvement in catecholamine-induced luteinizing hormone-releasing hormone release. Endocrinology. 1979;104:617–624. doi: 10.1210/endo-104-3-617. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Urbanski HF. Puberty in the rat. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. 2. New York: Raven Press; 1994. pp. 363–409. [Google Scholar]
- Ojeda SR, Urbanski HF, Costa ME, Hill DF, Moholt-Siebert M. Involvement of transforming growth factor alpha in the release of luteinizing hormone-releasing hormone from the developing female hypothalamus. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:9698–9702. doi: 10.1073/pnas.87.24.9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda SR, Urbanski HF, Katz KH, Costa ME. Prostaglandin E2 releases luteinizing hormone-releasing hormone from the female juvenile hypothalamus through a Ca2+-dependent, calmodulin-independent mechanism. Brain Research. 1988;441:339–351. doi: 10.1016/0006-8993(88)91412-6. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Urbanski HF, Katz KH, Costa ME, Conn PM. Activation of two different but complementary biochemical pathways stimulates release of hypothalamic luteinizing hormone-releasing hormone. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:4932–4936. doi: 10.1073/pnas.83.13.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck JD, Peck BM, Skaggs VJ, Fukushima M, Kaplan HB. Socio-environmental factors associated with pubertal development in female adolescents: the role of prepubertal tobacco and alcohol use. The Journal of Adolescent Health. 2011;48:241–246. doi: 10.1016/j.jadohealth.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevot V, Bouret S, Croix D, Takumi T, Jennes L, Mitchell V, et al. Evidence that members of the TGFbeta superfamily play a role in regulation of the GnRH neuroendocrine axis: expression of a type 1 serine-threonine kinase receptor for TGRbeta and activin in GnRH neurones and hypothalamic areas of the female rat. Journal of Neuroendocrinology. 2000;12:665–670. doi: 10.1046/j.1365-2826.2000.00508.x. [DOI] [PubMed] [Google Scholar]
- Prevot V, Cornea A, Mungenast A, Smiley G, Ojeda SR. Activation of erbB-1 signaling in tanycytes of the median eminence stimulates transforming growth factor beta1 release via prostaglandin E2 production and induces cell plasticity. The Journal of Neuroscience. 2003;23:10622–10632. doi: 10.1523/JNEUROSCI.23-33-10622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards MA, Oinonen KA. Age at menarche is associated with divergent alcohol use patterns in early adolescence and early adulthood. Journal of Adolescence. 2011;34:1065–1076. doi: 10.1016/j.adolescence.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Srivastava VK, Hiney JK, Dees WL. Prepubertal ethanol exposure alters hypothalamic transforming growth factor-α and erbB1 receptor signaling in the female rat. Alcohol. 2011;45:173–181. doi: 10.1016/j.alcohol.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava VK, Hiney JK, Dees WL. Actions and interactions of alcohol and transforming growth factor β1 on prepubertal hypothalamic gonadotropin-releasing hormone. Alcoholism: Clinical and Experimental Research. 2014;38:1321–1329. doi: 10.1111/acer.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa E. Hypothalamic control of the onset of puberty. Current Opinion in Endocrinology and Diabetes. 1999;6:44–49. [Google Scholar]
- Terasawa E, Fernandez DL. Neurobiological mechanisms of the onset of puberty in primates. Endocrine Reviews. 2001;22:111–151. doi: 10.1210/edrv.22.1.0418. [DOI] [PubMed] [Google Scholar]
- Tuma DJ, Todero SL, Barak-Bernihagen M, Casey CA, Sorrell MF. Chronic ethanol ingestion impairs TGF-alpha-stimulated receptor autophosphorylation. Alcohol. 1998;15:233–238. doi: 10.1016/s0741-8329(97)00127-4. [DOI] [PubMed] [Google Scholar]
- Wang SL, Feng J, Wu-Wang CY. Time-dependent alteration of epidermal growth factor receptor in rat stomach by ethanol feeding. Toxicology Letters. 1997;90:115–123. doi: 10.1016/s0378-4274(96)03836-2. [DOI] [PubMed] [Google Scholar]
- Witkin JW, Ferin M, Popilskis SJ, Silverman AJ. Effects of gonadal steroids on the ultrastructure of GnRH neurons in the rhesus monkey: synaptic input and glial apposition. Endocrinology. 1991;129:1083–1092. doi: 10.1210/endo-129-2-1083. [DOI] [PubMed] [Google Scholar]
- Witkin JW, Silverman AJ. Synaptology of luteinizing hormone-releasing hormone neurons in rat preoptic area. Peptides. 1985;6:263–271. doi: 10.1016/0196-9781(85)90050-6. [DOI] [PubMed] [Google Scholar]