Abstract
Introduction
Over recent decades, the burden of breast cancer has been increasing at an alarming rate in Asia. Prognostic research findings from Western countries may not readily be adapted to Asia, as the outcome of breast cancer depends on a multitude of factors ranging from genetic, clinical and histological predictors, to lifestyle and social predictors. The primary aim of this study is to determine the impact of lifestyle (eg, nutrition, physical activity), mental and sociocultural condition, on the overall survival and quality of life (QoL) among multiethnic Malaysian women following diagnosis of breast cancer. This study aims to advance the evidence on prognostic factors of breast cancer within the Asian setting. The findings may guide management of patients with breast cancer not only during active treatment but also during the survivorship period.
Methods
This hospital-based prospective cohort study will comprise patients with breast cancer (18 years and above), managed in the University Malaya Medical Centre (UMMC). We aim to recruit 1000 cancer survivors over a 6-year period. Data collection will occur at baseline (within 3 months of diagnosis), 6 months, and 1, 3 and 5 years following diagnosis. The primary outcomes are disease-free survival and overall survival, and secondary outcome is QoL. Factors measured are demographic and socioeconomic factors, lifestyle factors (eg, dietary intake, physical activity), anthropometry measurements (eg, height, weight, waist, hip circumference, body fat analysis), psychosocial aspects, and complementary and alternative medicine (CAM) usage.
Ethics and dissemination
This protocol was approved by the UMMC Ethical Committee in January 2012. All participants are required to provide written informed consent. The findings from our cohort study will be disseminated via scientific publication as well as presentation to stakeholders including the patients, clinicians, the public and policymakers, via appropriate avenues.
Keywords: breast cancer, survivor, lifestyle, cohort, Malaysia
Background
The past two decades observed a steep increase in breast cancer incidence in most Asian countries.1–4 However, this finding is in contrast to Western countries, where breast cancer incidence has stabilised or even decreased.5–7 While early detection and new treatment regimes have both contributed to significant improvement in breast cancer survival rates in developed countries,8 mortality rates due to breast cancer have been escalating in most parts of Asia.9 10 Amidst economic improvement and westernisation of Asian countries, distinct changes have been noticed in the lifestyle of women, in their reproductive factors and in the amount of exposure to environmental toxins.11–13 Although it has been established that risk factors for breast cancer in Asian populations are similar to those in Western populations, the prognostic factors may be different from those in Western countries; these include ethnicity, physical activity, obesity, as well as nutritional factors/dietary intake.1 11 14 In addition, the diverse Asian culture, different religions, varied ethnicity and lifestyles may also influence patients with breast cancer and affect their survivorship and quality of life (QoL).15 16 Body fatness has been frequently associated with increased risk of breast cancer and lower subsequent survival in the West,17 but little is known on the possible impact of obesity on survival following breast cancer within Asian settings. Breast cancer survivors also tend to be younger in Asia than survivors in Western countries.18 Thus the factors associated with breast cancer and survival in Asian countries may differ from those in Western countries. It is therefore intriguing to understand the impact of lifestyle, health behaviour, genetics, tumour biology and psychosocial factors, on QoL and overall survival of women from different ethnic groups, diagnosed with breast cancer in Asian settings.
Breast cancer is the most common cancer among Malaysian women.19 Malaysia is a multiethnic country comprising of three major ethnic groups. The largest proportion of the population is represented by Malays, subsequently followed by Chinese and Indians.20 21 Age-standardised incidence rate of breast cancer is highest among the Chinese, followed by Indians and lastly by Malays. However, there is evidence that the Malay ethnic group had lowest 5-year overall survival compared to the Indian and Chinese groups. In multiethnic South East Asian settings, survival of women with breast cancer seems to be associated with ethnicity, independent of stage at diagnosis, tumour pathology and treatment.20–22 Since ethnicity and culture interact with lifestyle choices in Malaysia, breast cancer survivors can be a good source to identify associations among these factors, in order to study the impact of these factors on survival and other outcomes. Likewise, this study will also establish probable associations between lifestyle and psychosocial condition on disease-free survival, overall survival and QoL after diagnosis of breast cancer. As such, the Malaysian Breast Cancer Survivorship Cohort (MyBCC) study is expected to provide evidence to improve the understanding of factors influencing breast cancer survivorship in multiethnic settings, and hence guide the management of breast cancer survivors.
Objectives
The primary objective of our study is to determine the association between demographic, socioeconomic status, lifestyle factors (dietary intake, physical activity), body composition, psychosocial factors, return to work (RTW), as well as complementary and alternative medicine (CAM) use, and overall survival as well as quality of life (QoL) among multiethnic breast cancer survivors.
Specific objectives
To identify the ethnic differences in survival and QoL among Malaysian women after a diagnosis of breast cancer.
To assess the impact of socioeconomic inequality on survival and QoL among Malaysian breast cancer survivors.
To determine if (change of) body composition impacts survival and QoL of Malaysian women with breast cancer.
To study nutritional status and dietary intake among breast cancer survivors and their impact on survival and QoL.
To determine the level of physical activity and its effect on survival and QoL among breast cancer survivors.
To determine the prevalence and determinants of CAM use and its impact on survival and QoL based on breast cancer survivors.
To determine the level of distress, anxiety and depression, and its effect on survival and QoL among breast cancer survivors.
To assess financial difficulty and RTW rates and their effect on QoL among breast cancer survivors.
We hypothesise that demography, socioeconomic status, lifestyle (ie, nutrition, physical activity), body composition, work-related and psychosocial factors, and CAM use of the different ethnic groups, will impact the overall survival and QoL of Malaysian patients with breast cancer.
Methods
Study design
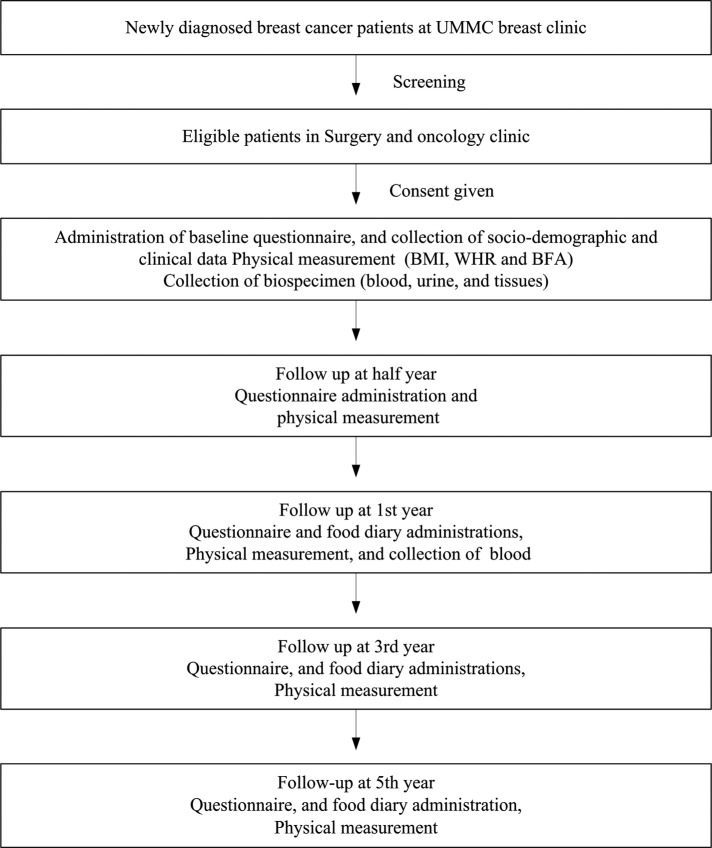
This hospital-based prospective cohort study will include those newly diagnosed patients with breast cancer (aged 18 years and above) managed in University Malaya Medical Centre (UMMC), Malaysia, who provide written-informed consent to participate. The UMMC is a tertiary academic hospital, situated in a relatively affluent part of Kuala Lumpur, which caters to a predominantly middle-class urban population. In 1993, this hospital established a prospective breast cancer registry. Based on a report of the National Cancer Registry in 2006, UMMC managed approximately 10% of newly diagnosed patients with breast cancer in Malaysia. For the current study, data on demography, clinical profile, pathological tumour characteristics and treatment will be obtained from the breast cancer registry. A baseline anthropometric measurement and body fat analysis (BFA) will be conducted by trained research assistants within 3 months of diagnosis. Figure 1 demonstrates the procedure of study recruitment and the overall flow of study. A survey in the form of a questionnaire will be conducted by trained interviewers at around the time of diagnosis (baseline) and at half year, first year, third year and fifth year follow-up, in order to collect data on QoL, socioeconomic status, physical activity, psychosocial support, RTW duration, and CAM usage among breast cancer survivors. A baseline assessment of dietary intake among survivors will be made using a 3-day food diary approximately 1 year after diagnosis. The rationale being to observe survivors’ habitual dietary intake after they are back to their normal life after completion of all conventional treatment. The survivors’ dietary intake assessment will be subsequently repeated at the third and fifth years after diagnosis (figure 1).
Figure 1.
Flow chart of study recruitment process and overall study design (BFA, body fat analysis; BMI, body mass index; UMMC, University Malaya Medical Centre; WHR, waist-to-hip ratio).
Study participants
This study will recruit patients with breast cancer diagnosed and managed in the UMMC since February 2012 and will continue recruiting until February 2017. The participants will be followed up at 6 months, and 1, 3 and 5 years after the date of recruitment, and this will continue until 2022. All newly diagnosed patients with breast cancer in the UMMC breast clinic will be screened for eligibility. Only eligible patients who give consent will be recruited. Figure 1 shows detailed information on our recruitment strategy.
Eligibility criteria
Inclusion criteria
All women who are newly diagnosed with primary breast cancer in UMMC:
Aged 18 years and above
Diagnosed between February 2012 and December 2017
Within 3 months of diagnosis
TNM stage І–ІV breast cancer
Malaysian
Able to converse either in Malay, English, Mandarin or Tamil
Exclusion criteria
Patients with prior history of any other cancer
Patients whose attending physician certifies them as unfit due to other prevailing medical condition
Bedridden at time of recruitment
Ethics and dissemination
The study protocol and procedures were approved by the UMMC Ethical Committee (MEC number 896.150). We obtained full permission from the European Organization for Research and Treatment of Cancer (EORTC) to use the QoL questionnaires, EORTC QLQ-C30 and QLQ-BR23. Ethical approval was obtained from University Malaya Cancer Research Institute (MEC number 775.9) for collection of biospecimens. Eligible patients will be verbally informed, by trained research personnel, regarding the nature and purpose of the study, and given time to decide whether or not to participate. Written informed consent will be obtained from study participants prior to their enrolment and all collected information will be treated as confidential. We intend to present the results of our cohort study via scientific publication (peer-reviewed) as well as through presentation to stakeholders including the public, patients, clinicians and policymakers, via appropriate avenues.
Measures
Exposure measures
The exposures of interest are demographic variables (eg, age at diagnosis, ethnicity, age at marriage, age at first child birth, age at last child birth), socioeconomic variables (income, highest attained-education and occupation), anthropometric measurements (eg, height, weight, waist hip circumference, percentage of body fat), diet (habitual food intake), use of CAM (eg, used types of treatment other than hospital treatment), physical activity, work-related variables (type of work, working hours), health-related variables (eg, physical condition, appetite), psychosocial variables (eg, anxiety, depression) and biomarkers (eg, blood, tumour tissues).
Data on the exposure of interest will be obtained through personal interview (face-to-face), food diaries, and anthropometric and biological measurement, details of which are given in table 1.
Table 1.
Measurement domains and data collection time points
| Theme | Instruments | Baseline | 6 Months | 1 Year | 3 Years | 5 Years |
|---|---|---|---|---|---|---|
| Demographic and socioeconomic characteristics | Sociodemographic | × | ||||
| Socioeconomy background (questionnaire) | × | × | ||||
| Quality of life | European Organization for Research and Treatment of Cancer Core Quality of Life Questionnaire (EORTC QLQ-C30) | × | × | × | × | × |
| EORTC of Cancer Breast Cancer Quality of Life Questionnaire (QLQ-BR23) | × | × | × | × | × | |
| Body weight and nutrition | Anthropometric measurement (body mass index, waist-to-hip ratio) | × | × | × | × | × |
| Food diary and mini-food album | × | × | × | |||
| Physical activity | Global Physical Activity Questionnaire (GPAQ) | × | × | × | × | × |
| Complementary and alternative medicine (CAM) | CAM questionnaire | × | × | × | × | × |
| Psychosocial factors | Hospital Anxiety and Depression Score (HADS) and | × | × | × | × | × |
| Distress Thermometer (questionnaire) | × | × | × | × | × | |
| Work-related factors | Return to work self-efficacy scale (RTW-SE) | × | × | × | × | × |
| Readiness for RTW scale | × | × | × | × | × | |
| Clinical, histological and biological factors | Clinical registry | × | × | × | × | × |
| Biobank (blood, urine and tissues) | × | × (only blood) |
Outcome measures
Patients will be monitored through follow-up in the breast cancer outpatient clinics in UMMC. Patients who are unable to attend clinics and those who are not scheduled for regular follow-up in UMMC will be contacted by phone.
The primary outcome is overall survival. Data on mortality will be obtained from the hospital’s medical records, as well as active follow-up through the next-of-kin of patients. In addition, we will regularly update the vital status of patients through direct linkage with the National Registration Department (NRD), Malaysia, which holds the death records in Malaysia.
The cohort will be followed up with scheduled phone calls and clinic visits in a systematic way to update patient's health status. Another outcome is QoL. QoL will be measured based on response to a QoL questionnaire obtained from the EORTC. The EORTC QLQ-C30 is comprised of 30 items including five functional scales (physical, role, emotional, cognitive and social) and 9 symptom scales (fatigue, nausea and vomiting, pain, dyspnoea, insomnia, appetite loss, constipation, diarrhoea, and financial difficulties) and Global health status scale. On the contrary, the QLQ-BR23 is a more detailed questionnaire, containing 23 questions designed to quantify the QoL of patients with breast cancer. It includes four functional scales (body image, sexual functioning, sexual enjoyment, future perspective) and four symptom scales (systemic therapy side effect, breast symptom, arm symptom, upset by hair loss). Information from the questionnaires will be scored accordingly. The raw score for each subscale will be calculated and subsequently linearly transformed to a level between 0 and 100 (standardised raw score) according to the guidelines of the EORTC scoring manual. A higher score for functional scale represents a high/healthy level of functioning. A high score for the global health status/QoL represents high QoL. However, a high score for a symptom scale represents a high level of symptom, indicating a worse QoL.
Tools used for measures
Anthropometry: Body mass index (BMI), body fat percentage and waist-to-hip ratio (WHR) will be used as indicators of body fatness. WHO definitions and classifications will be used. Patients’ nutritional status will be measured from BMI, BFA, WHR and food diaries. BMI and BFA will be measured at baseline, and during subsequent follow-up at 6 months, and 1, 3 and 5 years. WHR will be measured only at baseline. BMI and BFA, body fat percentages, will be measured using a portable body composition analyser (TANITA BC-418 Body Composition Analyser, Tokyo, Japan). This equipment will calculate body fat ratio, body fat mass, fat-free mass, estimated muscle mass and Basal Metabolic Rate, using data derived using Bioelectric impedance analysis (BIA). Hydration status, recent physical activity, consumption of food or beverages, temperature, menstrual status and body position are among the factors that can affect the validity and precision of the measurements. For example, participants should avoid alcohol and vigorous exercise for 12 h before testing, so that body fluids are not affected prior to the measurements. The measurements should be taken on participants approximately 3 h after eating, and within 30 min of voiding.
Food diary: Habitual food intake will be assessed using a self-administered 3-day food diary. A qualified trained dietician will provide information and give instructions on how to complete the 3-day dietary records. The food diary will be generalised to all ethnic groups (Malays, Chinese and Indians). A mini-food album detailing (multiethnic) Malaysian food items will be provided for guidance. Participants will be reassessed at 2 and 4 years after initial dietary assessment.
Questionnaire: Questionnaires will be used to obtain detailed information on concurrent illness, work-related factors, CAM use, physical activity and QoL23–25 (described in detail in table 1). Questionnaires for RTW self-efficacy scale (RTW-SE), readiness for RTW scale and CAM use have undergone linguistic, content and face validation. Physical activity will be measured using the Global Physical Activity Questionnaire (GPAQ). Presence of anxiety and depression will be assessed using the Hospital Anxiety and Depression Score (HADS). To measure QoL, two modules will be used: EORTC QLQ-C30 and EORTC QLQ-BR23, which have been translated to the local languages and validated. The validity and reproducibility of EORTC QLQ-C30, GPAQ and HADS have been proven satisfactory.26–28
Clinical registry: There is already an established prospective breast cancer registry in UMMC,11 which includes: data on reproductive factors and family history; tumour characteristics, namely, size, grade, TNM stage, receptor status (oestrogen receptor, progesterone receptor, human epidermal growth factor receptor 2); and treatment details, those being treatment modalities (surgery, radiotherapy, chemotherapy and hormonal therapy). We will regularly link the current study variables to the registry.
Data collection point
Baseline
At the time of recruitment to the study, the following information will be recorded:
Demographic factors and clinical data
Information on other illnesses/comorbidities
- Details on
- QoL
- Psychosocial factors
- Work-related factors
- Physical activity
- CAM use
Biospecimens collected (blood, urine and tissues) will be preserved
BMI, WHR and BFA measurement
Follow-up
The following information will be collected at 6 months, and 1, 3 and 5 years after diagnosis:
Clinical data and particulars on concurrent illness
- Details on
- QoL
- Psychosocial factors
- Physical activity
- CAM use
- Work-related factors
- Food diaries (during 1st, 3rd and 5th year follow-up period)
BMI and BFA measurement
Figure 1 demonstrates the overall flow of the study.
Data analysis
Statistical analysis
The results of survivors’ demographics and baseline outcome variables will be summarised using descriptive summary measures, expressed as mean or median for continuous variables and proportions for categorical variables. We will report the model coefficient for continuous outcomes, OR for binary outcomes, or HR for time to event outcomes, with the corresponding 95% CIs, and associated p values. To determine the QoL among patients with breast cancer at different stages of the illness, we will use scores obtained from the EORTC QLQ-C30 and EORTC QLQ-BR23 instruments. A higher score represents a ‘better’ level of functioning and global health status, or a ‘worse’ level of symptoms. Median and other quartiles with 95% CI will be obtained for all the domains of QoL index according to the stage of breast cancer. The analysis of variance test will be conducted to determine the QoL score for each domain, according to respective cancer stages and ethnicity, p value <0.05 will be considered to be statistically significant.
Important prognostic factors will be identified by fitting the Cox’s proportional hazard model. Overall survival will be estimated using the Kaplan-Meier method and compared using the log-rank test. Multivariable Cox regression analysis will be used to estimate the relative risk of all-cause mortality (HR) associated with the various prognostic factors that we are studying. We will also assess whether ethnicity modifies the association between various prognostic factors and overall survival, as well as QoL.
Statistical power
We estimate that we will be able to recruit about 1000 women during the 6 years of enrolment, that is, 150–200 patients per year in UMMC. With reports showing Malaysia to have the highest prevalence of obesity in Asia,29 we hypothesise that change in body weight may explain most of the survival disparities associated with lifestyle. Previous studies in UMMC recorded a mean BMI of patients with breast cancer at diagnosis as 23.8 kg/m2 (SD=4.12) and overall 5-year survival at 75%.20 21 A Cox regression of the log HR on BMI of these women with an SD of 4 kg/m2 based on a sample of 1000 participating women achieves an output of 90% statistical power at a 5% significance level to detect a regression coefficient of BMI (kg/m2) equivalent to 0.0468. Recruitment of even 750 patients will be able to achieve 80% statistical power at 5% significance to detect a significant difference.
Discussion
Prognoses of breast cancer among Asian women vary considerably from those of Western women and may be attributable to difference in lifestyle, socioeconomic profile, health beliefs, culture and genetic backgrounds.10 Previous studies in selected populations (Malaysian) have suggested that ethnicity is an independent prognostic factor of breast cancer survival.21 Development of biomarkers for prognosis, an expanding biobank and, furthermore, outcome measures and QoL measures among cancer survivors, are relatively scarce in Asia.
To the best of our knowledge, this study is the first, in a multiethnic Asian setting, to determine the association between sociodemographic and lifestyle factors on overall survival and QoL in breast cancer survivors. Notably, previous cohort studies11 30 only conducted survival analysis utilising clinical data. Results from our study can provide insight into association between patients’ nutrition, BMI, body composition, physical, mental and socioeconomic status, QoL and overall survival. We will also be able to assess the influence of ethnicity on these associations. A major strength of this study is that we are using well-validated and reliable tools (ie, GPAQ and portable TANITA)31–33 to measure modifiable risk factors (ie, physical activity, body composition). This study will add evidence on the impact of body fatness and its changes on overall survival and QoL of Asian women following the diagnosis of breast cancer. In addition, there will be long-term storage of patients’ blood, serum, plasma, urine and tissues in the UMMC Biobank, for future research.
There are some limitations to the current study. It is expected that there will be some ambiguity in assessing the overall improvement or decrement in QoL of the survivors, as perception varies from one individual to another. Nevertheless, the validity and reproducibility of QoL has been satisfactory27 and was adopted from the EORTC. Given that we have chosen to address a cohort comprising mostly of urban residents attending a tertiary hospital, our findings may not necessarily reflect the overall situation of breast cancer survivors in Malaysia. Another limitation is that we will be using a 3-day food diary that depends on the respondent’s full co-operation. However, diet history will be taken from 2 weekdays and 1 weekend day and, as such, should reflect more valid estimates of patients’ habitual dietary intakes.34
On the basis of the cohort study proposed here, this knowledge will be important for developing an effective strategy for the improvement of overall survival and QoL of patients with breast cancer in middle-income countries such as Malaysia, where resources for healthcare are limited. This study will enable relevant parties to address prognostic factors that affect survival and enable a thorough focus to be placed on differences among ethnicity. Hence, we hope results from our study will contribute to the advancement in evaluation and appraisal methods for assessment of prognostic factors in breast cancer. In the long run, we aim to improve the survival and QoL following the diagnosis of breast cancer in Asian women.
Acknowledgments
The authors thank the doctors, nurses, technical staff and hospital administration staff at UMMC for the daily administration of the Malaysian Breast Cancer Survivorship Cohort Study.
Footnotes
Collaborators: Members of MyBCC working group: Taib NA, Bhoo-Pathy N, Su TT, Majid HA, Nahar AM, Ng CG, Dahlui M, and Hussain S, from University of Malaya, and Cantwell M, and Murray L from Queen's University Belfast.
Contributors: NAT, TI, NB-P and TTS designed the study and drafted the manuscript. MD, LM, HAM and AMN made substantial contributions to the conception and design of the project. CGN, MC and SH were involved in drafting the manuscript. TI and NB-P were in charge of the statistical analysis. All the authors critically revised the manuscript for important intellectual content and provided approval of the final version to be published.
Funding: The post-doctoral research fellow position for this project (UMQUB 3C-2011) was jointly funded by University of Malaya and Queen's University of Belfast. The study was supported by a High Impact Research Grant (UM.C/HIR/MOHE/06) from the Ministry of Higher Education, Malaysia.
Competing interests: None declared.
Ethics approval: The protocol was approved by the local ethics committee of UMMC, in January 2012.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Further information such as statistical examination can be obtained from the first author.
Contributor Information
Collaborators: MyBCC study group, NA Taib, N Bhoo-Pathy, TT Su, HA Majid, AM Nahar, CG Ng, M Dahlui, S Hussain, M Cantwell, and L Murray
References
- 1.Hirabayashi Y, Zhang M. Comparison of time trends in breast cancer incidence (1973–2002) in Asia, from cancer incidence in five continents, vols IV-IX. Jpn J Clin Oncol 2009;39:411–12. 10.1093/jjco/hyp054 [DOI] [PubMed] [Google Scholar]
- 2.Medina VM, Laudico A, Mirasol-Lumague MR et al. Cumulative incidence trends of selected cancer sites in a Philippine population from 1983 to 2002: a joinpoint analysis. Br J Cancer 2010;102:1411–14. 10.1038/sj.bjc.6605640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sim X, Ali RA, Wedren S et al. Ethnic differences in the time trend of female breast cancer incidence: Singapore, 1968–2002. BMC Cancer 2006;6:261 10.1186/1471-2407-6-261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takiar R, Srivastav A. Time trend in breast and cervix cancer of women in India—(1990–2003). Asian Pac J Cancer Prev 2008;9:777–80. [PubMed] [Google Scholar]
- 5.Fontenoy AM, Leux C, Delacour-Billon S et al. Recent trends in breast cancer incidence rates in the Loire-Atlantique, France: a decline since 2003. Cancer Epidemiol 2010;34:238–43. 10.1016/j.canep.2010.03.007 [DOI] [PubMed] [Google Scholar]
- 6.Ravdin PM, Cronin KA, Howlader N et al. The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med 2007;356:1670–4. 10.1056/NEJMsr070105 [DOI] [PubMed] [Google Scholar]
- 7.Glass AG, Lacey JV Jr, Carreon JD et al. Breast cancer incidence, 1980–2006: combined roles of menopausal hormone therapy, screening mammography, and estrogen receptor status. J Natl Cancer Inst 2007;99:1152–61. 10.1093/jnci/djm059 [DOI] [PubMed] [Google Scholar]
- 8.Coleman MP, Quaresma M, Berrino F, et al. , CONCORD Working Group. Cancer survival in five continents: a worldwide population-based study (CONCORD). Lancet Oncol 2008;9:730–56. 10.1016/S1470-2045(08)70179-7 [DOI] [PubMed] [Google Scholar]
- 9.Leong SP, Shen ZZ, Liu TJ et al. Is breast cancer the same disease in Asian and Western countries? World J Surg 2010;34:2308–24. 10.1007/s00268-010-0683-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhoo-Pathy N, Yip CH, Hartman M et al. Breast cancer research in Asia: adopt or adapt Western knowledge? Eur J Cancer 2013;49:703–9. 10.1016/j.ejca.2012.09.014 [DOI] [PubMed] [Google Scholar]
- 11.Pathy NB, Yip CH, Taib NA et al. Breast cancer in a multi-ethnic Asian setting: results from the Singapore-Malaysia hospital-based breast cancer registry. Breast 2011;20(Suppl 2):S75–80. 10.1016/j.breast.2011.01.015 [DOI] [PubMed] [Google Scholar]
- 12.Coughlin SS, Ekwueme DU. Breast cancer as a global health concern. Cancer Epidemiol 2009;33:315–18. 10.1016/j.canep.2009.10.003 [DOI] [PubMed] [Google Scholar]
- 13.Saxena N, Hartman M, Bhoo-Pathy N et al. Breast cancer in South East Asia: comparison of presentation and outcome between a middle income and a high income country. World J Surg 2012;36:2838–46. 10.1007/s00268-012-1746-2 [DOI] [PubMed] [Google Scholar]
- 14.Islam T, Ito H, Sueta A et al. Alcohol and dietary folate intake and the risk of breast cancer: a case-control study in Japan. Eur J Cancer Prev 2013;22:358–66. 10.1097/CEJ.0b013e32835b6a60 [DOI] [PubMed] [Google Scholar]
- 15.Lin YH, Chiu JH. Use of Chinese medicine by women with breast cancer: a nationwide cross-sectional study in Taiwan. Complement Ther Med 2011;19:137–43. 10.1016/j.ctim.2011.04.001 [DOI] [PubMed] [Google Scholar]
- 16.Taib NA, Yip CH, Low WY. A grounded explanation of why women present with advanced breast cancer. World J Surg 2014;38:1676–84. 10.1007/s00268-013-2339-4 [DOI] [PubMed] [Google Scholar]
- 17.Kwan ML, John EM, Caan BJ et al. Obesity and mortality after breast cancer by race/ethnicity: the California Breast Cancer Survivorship Consortium. Am J Epidemiol 2013;179:95–111. 10.1093/aje/kwt233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahn E, Cho J, Shin DW et al. Impact of breast cancer diagnosis and treatment on work-related life and factors affecting them. Breast Cancer Res Treat 2009;116:609–16. 10.1007/s10549-008-0209-9 [DOI] [PubMed] [Google Scholar]
- 19.Lim G, Rampal S, Halimah Y, In cancer incidence in Penisular Malaysia, 2003–2005 National Cancer Registry. Kuala Lumpur: National Cancer Registry, 2008. [Google Scholar]
- 20.Taib NA, Akmal M, Mohamed I et al. Improvement in survival of breast cancer patients—trends in survival over two time periods in a single institution in an Asia Pacific Country, Malaysia. Asian Pacific J Cancer Prev 2011;12:345–9. [PubMed] [Google Scholar]
- 21.Bhoo-Pathy N, Hartman M, Yip CH et al. Ethnic differences in survival after breast cancer in South East Asia. PLoS ONE 2012;7:e30995 10.1371/journal.pone.0030995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohd Taib NA, Yip CH, Mohamed I. Survival analysis of Malaysian women with breast cancer: results from the University of Malaya Medical Centre. Asian Pac J Cancer Prev 2008;9:197–202. [PubMed] [Google Scholar]
- 23.Chen Z, Gu K, Zheng Y et al. The use of complementary and alternative medicine among Chinese women with breast cancer. J Altern Complement Med 2008;14:1049–55. 10.1089/acm.2008.0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 25.Lagerveld SE, Bültmann U, Franche RL et al. Factors associated with work participation and work functioning in depressed workers: a systematic review. J Occup Rehabil 2010;20:275–92. 10.1007/s10926-009-9224-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soo KL, Wan Abdul Manan WM, Wan Suriati WN et al. The Bahasa Melayu Version of the Global Physical Activity Questionnaire: Reliability and Validity Study in Malaysia. Asia Pac J Public Health 2015;27:NP184–93. [DOI] [PubMed] [Google Scholar]
- 27.Yusoff N, Low WY, Yip CH. The Malay Version of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC-QLQ C30): Reliability and Validity Study. Int Med J Malays 2010;9:45–50. [Google Scholar]
- 28.Yusoff N, Low WY, Yip CH. Psychometric properties of the Malay Version of the hospital anxiety and depression scale: a study of husbands of breast cancer patients in Kuala Lumpur, Malaysia. Asian Pac J Cancer Prev 2011;12:915–17. [PubMed] [Google Scholar]
- 29.Ng M, Fleming T, Robinson M et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384:766–81. 10.1016/S0140-6736(14)60460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdullah NA, Wan Mahiyuddin WR, Muhammad NA et al. Survival rate of breast cancer patients in Malaysia: a population-based study. Asian Pac J Cancer Prev 2013;14:4591–4. 10.7314/APJCP.2013.14.8.4591 [DOI] [PubMed] [Google Scholar]
- 31.Bull FC, Maslin TS, Armstrong T. Global physical activity questionnaire (GPAQ): nine country reliability and validity study. J Phys Act Health 2009;6:790–804. [DOI] [PubMed] [Google Scholar]
- 32.Barreira TV, Staiano AE, Katzmarzyk PT. Validity assessment of a portable bioimpedance scale to estimate body fat percentage in white and African-American children and adolescents. Pediatr Obes 2013;8:e29–32. 10.1111/j.2047-6310.2012.00122.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sim PY, Su TT, Abd Majid H et al. A comparison study of portable foot-to-foot bioelectrical impedance scale to measure body fat percentage in Asian adults and children. Biomed Res Int 2014;2014:475659 10.1155/2014/475659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cuenca-Garcia M, Artero EG, Sui X et al. Dietary indices, cardiovascular risk factors and mortality in middle-aged adults: findings from the Aerobics Center Longitudinal Study. Ann Epidemiol 2014;24:297–303.e2. 10.1016/j.annepidem.2014.01.007 [DOI] [PubMed] [Google Scholar]