Abstract
VRC-HIVMAB060-00-AB (VRC01) is a broadly neutralizing HIV-1 monoclonal antibody (mAb) isolated from the B cells of an HIV-infected patient. It is directed against the HIV-1 CD4 binding site and is capable of potently neutralizing the majority of diverse HIV-1 strains. This Phase I dose-escalation study in healthy adults was conducted at the National Institutes of Health (NIH) Clinical Center (Bethesda, MD, USA). Primary objectives were the safety, tolerability and pharmacokinetics (PK) of VRC01 intravenous (i.v.) infusion at 5, 20 or 40 mg/kg, given either once (20 mg/kg) or twice 28 days apart (all doses), and of subcutaneous (s.c.) delivery at 5 mg/kg compared to s.c. placebo given twice, 28 days apart. Cumulatively, 28 subjects received 43 VRC01 and nine received placebo administrations. There were no serious adverse events or dose-limiting toxicities. Mean 28-day serum trough concentrations after the first infusion were 35 and 57 μg/ml for groups infused with 20 mg/kg (n = 8) and 40 mg/kg (n = 5) doses, respectively. Mean 28-day trough concentrations after the second infusion were 56 and 89 μg/ml for the same two doses. Over the 5–40 mg/kg i.v. dose range (n = 18), the clearance was 0·016 l/h and terminal half-life was 15 days. After infusion VRC01 retained expected neutralizing activity in serum, and anti-VRC01 antibody responses were not detected. The human monoclonal antibody (mAb) VRC01 was well tolerated when delivered i.v. or s.c. The mAb demonstrated expected half-life and pharmacokinetics for a human immunoglobulin G. The safety and PK results support and inform VRC01 dosing schedules for planning HIV-1 prevention efficacy studies.
Keywords: HIV-1, monoclonal antibody, pharmacokinetics, Phase I clinical trial, passive immunization
Introduction
Many human viral infections and effective vaccines induce a humoral immune response that protects against future infection. This response is detected typically by in-vitro assays that demonstrate binding antibodies to viral surface proteins or by the prevention of viral infection at a cellular level mediated by neutralizing antibodies. Vaccine-induced virus-specific neutralizing antibodies are often considered a mechanistic correlate of protective immunity 1. To date, clinical trials of HIV-1 vaccine candidates have failed to show robust induction of neutralizing antibodies capable of recognizing the most commonly transmitted HIV-1 isolates 2–4. However, the sera from most HIV-1 infected individuals displays virus-neutralizing activity, and some sera are able to potently neutralize diverse viral strains 2,4,5.
In the early 1990s a few cross-reactive HIV-1 human neutralizing monoclonal antibodies (mAbs) were isolated. These mAbs targeted epitopes on the viral surface envelope glycoprotein (Env), a trimeric protein made up of three identical gp120 molecules associated non-covalently with three gp41 molecules. These first-generation human mAbs were limited in either breadth or potency of virus neutralization 6,7. Infusion of three mAbs (2G12, 2F5 and 4E10) into humans demonstrated, at best, a transient delay in rebounding virus in acutely infected individuals after anti-retroviral (ARV) treatment interruption, with rebounding virus often containing escape mutations 8–10. During the last 10 years, the development of panels of diverse HIV-1 isolates, along with reproducible Env-pseudovirus-based neutralization assays and testing of large clinical cohorts, has led to the identification of HIV-1 patients whose sera contain broadly reactive antibodies 11–16. Using new techniques for antigen-specific B cell sorting and recovery of immunoglobulin genes by polymerase chain reaction (PCR) 17,18, many new broadly reactive antibodies (bNAbs) have been isolated during the last 5–6 years 5,19,20. These antibodies target diverse epitopes on the HIV-1 Env 19,21, including the functionally conserved CD4 binding site (CD4bs) 22–25. Viral attachment to CD4 on a host target cell is an early requirement in the process of viral entry, thus antibody to this region can block HIV-1 entry. VRC-HIVMAB060-00-AB (VRC01) is representative of a class of bNAbs that interact with the CD4bs of HIV-1 Env and have been isolated from numerous donors 22–28. The ontogeny and structural mode of recognition of the VRC01 class of antibodies have been defined through genetic sequencing crystal structures. Members of this antibody class include VRC01, VRC07, 3BNC117, 12A12, VRC-PG04 and VRC-CH31 19,23. While the VRC01 class of antibodies are genetically diverse, with antibody sequence differences of more than 50%, their structural mode of recognition is similar, including reliance upon the antibody CDR H2 interaction with the CD4 binding site region of gp120. Thus, all VRC01 class antibodies contain heavy chain mimicry of the CD4 receptor, and have a heavy chain-derived from the IGHV1-2 germline gene and a light chain with a relatively short 5 amino acid CDR L3 23,26,29. Because they can neutralize more than 80% of diverse HIV-1 strains and target a conserved region of the virus necessary for function, candidates from the VRC01 class have been manufactured and advanced into clinical development for the prevention and treatment of HIV-1 infection 30,31.
VRC01 was isolated originally from an HIV-1-infected individual with controlled viral infection for more than 15 years in the absence of anti-retroviral therapy, using protein probes that select B cells with the appropriate binding specificity 25. VRC01 is highly somatically mutated from the germline precursor, with a nucleotide VH mutation frequency of 32% and VK mutation frequency of 17% 22,24. VRC01 is not self-reactive and lacks anti-phospholipid antibody activity, further supporting its clinical use 27. The B cell lineage of VRC01, as well as autologous virus, has been interrogated by evaluating longitudinal samples from the original donor 29,32. It is now understood that germline VRC01 can bind original Env sequence from the donor and that subsequent virus escape produced a fitness cost for virus replication 33. Subsequent somatic hypermutation (SHM) that occurred in B cells for more than 15+ years led to the expansion of a large VRC01 lineage.
Using in-vitro testing, the VRC01 bNAb has a half-maximal inhibitory concentration (IC50) of < 50 μg/ml against 91% and an IC50 of < 1 μg/ml against 72% of HIV-1 primary isolates in a panel of 190 Env-pseudotyped viral strains, representing all major circulating HIV-1 genetic subtypes including clades A, B, C, D, G and AG, AE and BC recombinants 25. Based on preclinical and in-vitro data, VRC01 may have the potential to prevent infection in those at risk of HIV-1, including in the setting of mother-to-child HIV-1 transmission during the intrapartum period and breastfeeding 34. VRC01 neutralized 78% of virus isolates obtained from infected infants in Zambian mother–infant pairs with HIV-1 transmission 35 and neutralized five of six clade C founder viruses cloned from HIV-1-infected infants in Malawi 36. Additionally, challenge studies have demonstrated the ability of VRC01 to protect non-human primates (NHP) from virulent chimeric simian–human immunodeficiency virus (SHIV) 37.
Based on preclinical protection and therapeutic data, the VRC01 drug product (VRC-HIVMAB060-00-AB) was developed by the Vaccine Research Center (VRC), National Institute of Allergy and Infectious Diseases (NIAID) at the National Institutes of Health (NIH) for evaluation in a series of clinical trials. The VRC 602 Phase I clinical trial represents the first evaluation of the safety, tolerability, pharmacokinetics (PK) and neutralizing potential of VRC01 administration in healthy uninfected adults.
Methods
Study design and procedures
VRC 602 was a single-site, Phase I dose-escalation study examining the safety and pharmacokinetics of the human monoclonal antibody VRC-HIVMAB060-00-AB (VRC01) in healthy, HIV-uninfected adults, aged 18–50 years, with a weight of 53–115 kg. The study was conducted at the NIH Clinical Center (CC) by the Vaccine Research Center (VRC) Clinical Trials Program (CTP), National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), Bethesda, MD (Clinicaltrials.gov NCT01993706). The Investigational New Drug (IND) application was sponsored by the NIAID Division of AIDS. The protocol was reviewed and approved by the NIAID Institutional Review Board. US Department of Health and Human Services guidelines for conducting clinical research were followed. All subjects gave written informed consent prior to participation.
Three open-label groups received intravenous VRC01 (group 1: 5 mg/kg, group 2: 20 mg/kg and group 3: 40 mg/kg) under a dose escalation plan (Fig. 1). The dosages in the trial were determined based on preclinical studies performed with VRC01, as well as results from human clinical trials with other mAbs developed for prevention or treatment of viral pathogens. In particular, data from the licensed product Synagis® (palivizumab) directed against the viral pathogen respiratory syncytial virus (RSV) helped to inform VRC01 dosing 38,39. Subjects were first randomized to the 5 mg/kg dose level with open-label intravenous (i.v.) administration, or blinded subcutaneous (s.c.) administration of VRC01 5 mg/kg or placebo. Safety reviews were conducted by a Data and Safety Monitoring Board at protocol-specified intervals for the two dose escalations. After five subjects were enrolled into each of the groups that specified days 0 and 28 product administration, an additional three subjects were enrolled into group 2 (20 mg/kg i.v.) to assess PK following a single infusion.
Figure 1.
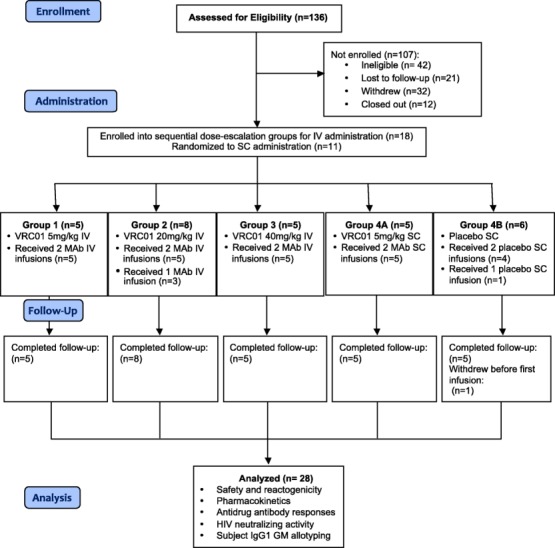
VRC602 Consolidated Standards of Reporting Trials (CONSORT) diagram with study enrolment, VRC-HIVMAB060-00-AB (VRC01) administration, subject follow-up and data analysis for the four study groups.
All product administrations were monitored by a study clinician. Safety laboratory tests were obtained prior to product administration and 2, 7, 14 and 28 days after each administration. Subjects kept a diary card of solicited systemic symptoms for 3 days after each dose and clinicians assessed the local site on the day of administration and on days 1, 2 and 7. All adverse events (AEs) were reported for 56 days after the second infusion, while serious adverse events and new chronic medical conditions were recorded for the duration of the study. AEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA), and severity was graded using the DAIDS table for Grading the Severity of Adult and Pediatric Adverse Events, version 1·0, December 2004 (with clarification in August 2009). Subjects were followed for safety for 12 weeks following their final administration of study product.
PK blood samples were collected pre-dose and timed from the end of product administration at 0, 1, 2, 4, 8, 12 and 24 h, as well as days 2, 7, 14, 21 and 28 after each administration and 56 days after the second administration. An additional PK blood draw was performed at 72 h post-administration in s.c. subjects. Blood samples for anti-VRC01 antibody evaluation were collected at study day 0 (baseline), day 28 (pre-second infusion), day 56 (28 days post-second infusion) and day 112 (12 weeks post-second infusion) and samples for VRC01 virus neutralization were collected at study days 0 (baseline), 2 (48 h post-first infusion), 28 (pre-second infusion), day (48 h post-second infusion) and 56 (28 days post-second infusion). Subject HIV enzyme immunoassay (EIA) response was monitored by standard diagnostic test (Ortho VITROS anti-HIV 1 + 2 assay; Ortho Clinical Diagnostics, Rochester, NY, USA) at screening and on study days 7 and 84.
Subject IgG1 GM (gamma marker) allotyping
In the present study subjects were evaluated for the GM3/17 IgG1 allotypes to determine allotype-specific effects on VRC01 (GM3) pharmacokinetics. In the human population there are four GM allotypes in the constant region of IgG1: GM1, 2, 3, and 17. Allelic GM3 and GM17 determinants are expressed in the Fd region (portion of the heavy chain included in the Fab fragment), and GM1 and GM2 are expressed in the Fc region 40. The presence of CH1 arginine at position 214 correlates with GM3, while CH1 lysine at 214 correlates with GM17 40. This panel was chosen based on the availability of quality GM molecular markers as well the likelihood of immunological impact. IgG1 markers GM3 and 17 (arginine to lysine) were determined by a predesigned TaqMan® genotyping assay from Applied Biosystems Inc. (Carlsbad, CA, USA) employing the following primers and probes: forward: 5′-CCCAGACCTACATCTGCAACGTGA-3′, reverse: 5′-CTGCCCTGGACTGGGACTGCAT-3′; reporter 1 (GM 17-specific): VIC-CTCTCACCAACTTTCTTGT-NFQ and reporter 2 (GM 3-specific): FAM-CTCTCACCAACTCTCTTGT-NFQ, as described previously 41.
Study product
To produce VRC-HIVMAB060-00-AB (VRC01), the heavy and light chains encoding VRC01 were cloned and sequenced allowing for the synthetic production of a codon-optimized variable region that was inserted into a proprietary immunoglobulin (Ig)G1 background sequence 25. The mammalian Glutamine Synthetase Gene Expression System developed by Lonza Biologics (Slough, UK) was used to produce VRC01 under cGMP using a stably transfected Chinese hamster ovary (CHO) cell line. The formulation buffer contains 25 mM sodium citrate, 50 mM sodium chloride and 150 mM L-arginine hydrochloride at pH 5·8. The placebo for s.c. administration was VRC-PLAMAB068-00-AB, a sterile, buffered aqueous solution of 25 mM sodium citrate, 50 mM sodium chloride, 150 mM L-arginine hydrochloride, 10% dextran 40 (w/w) and 0·005% polysorbate 80 (w/w) at pH 5·8. The purified product vials at 100 ± 10 mg/ml and the placebo vials were filled and labelled at the VRC Vaccine Pilot Plant operated by Leidos Biomedical Research, Inc. (Frederick, MD, USA).
A pharmacist prepared individual i.v. doses for subjects by adding the calculated volume of VRC01 needed to achieve the assigned mg/kg dose to a 100-ml bag of 0·9% sodium chloride injection USP; i.v. infusions were administered over at least 60 min; s.c. doses were administered using a s.c. infusion pump into one site in the abdomen or by direct needle and syringe injection with up to 2·5 ml per injection site.
Pharmacokinetic analysis
Quantification of VRC01 concentrations in subject serum was performed in 96-well plates on a Beckman Biomek-based automation platform (Beckman Coulter, Brea, CA, USA) utilizing the monoclonal antibody 5C9 for VRC01 detection. 5C9 is a mouse monoclonal antibody developed from mice immunized with VRC01. The 5C9 antibody was coated onto Immulon-4HXB microtitre plates overnight at 4°C. Plates were then washed and blocked [10% fetal bovine serum (FBS) in phosphate-buffered saline (PBS)] for 2 h at room temperature. Duplicate serial threefold dilutions covering the range of 100–24300 of the test sample were incubated for 2 h at 37°C followed by horseradish peroxidase-labelled goat anti-human antibody (1 h, 37°C) and 3,3′,5,5′-tetramethylbenzidine (TMB substrate) (15 min, room temperature). Colour development was stopped by the addition of sulphuric acid and plates were read within 30 min at 450 nm via the Molecular Devices Paradigm plate reader (Molecular Devices, Sunnyvale, CA, USA). Four-parameter logistic curve regression of a standard curve of VRC01 covering the range from 0·98 to 1000 ng/ml was utilized to quantitate sample concentrations based upon the average of sample dilutions within the range of the assay.
Individual-subject non-compartmental pharmacokinetic analysis was performed using Phoenix (version 6·3, Pharsight-Centara, Princeton, NJ, USA) with VRC01 concentration data from each subject. Calculated parameters included area under the curve (AUC), maximum concentration (Cmax), time to Cmax (Tmax), clearance (CL), terminal elimination rate constant (λz) and the terminal half-life (t1/2). Cmax and Tmax were taken directly from the observed concentration–time data. The terminal slope, λz, was determined from the log-linear portion of the curve and the t1/2 was calculated as 0·693/λz utilizing the following terminal phase equation to determine half-life: C(t2) = C(t1) × e[–λz×(t2–t1)], solved for C2; C2 = 0·5 × C1 and t1/2 = ln(2)/λz, where ln(2) = 0·693. The linear trapezoidal method was used to determine AUC following the first dose to day 28 (AUC0-D28) and AUC following the second dose to the final concentration (AUC0-Clast). The AUC after the final measured concentration (Clast) was estimated as Clast/λz. CL was calculated over both doses administered as (dose1 + dose2)/(AUCdose 1(0-D28)+ AUCdose 2(0-inf)). For subjects receiving only a single dose, CL was estimated as dose1/AUCdose1(0-inf).
Neutralizing antibody assay
Serum samples were evaluated to determine the relative concentration of HIV-1 neutralizing antibodies by evaluation of the capacity to prevent the infection of TZM-bl cells by single round infection pseudotyped virus. The pseudotyped virus expresses the envelope antigen and the luciferase reporter gene. Neutralization activity was quantitated by relative decrease in the luciferase activity compared to infection of TZM-Bl cells in the absence of samples. Pseudotyped viruses were generated by transfection of 293T/17 cells with optimized ratios of envelope-expressing plasmid and backbone vector (pSG3ΔEnv). A panel of six viruses was tested for neutralizing activity spanning a known range of VRC IC80s as well as negative controls. The tested viruses included Q23·17 subtype A, PVO·04 subtype B, MW965·26 subtype C, THRO·18 subtype B (poorly neutralized by VRC01), CAP210 subtype C (VRC01 resistant) and Moloney murine leukaemia virus (MuLV) (negative control). Neutralization assays were performed in 384-well plates using a Beckman Biomek liquid handling system 42. Additional assay details can be found in the supplemental methods in Supporting information.
Anti-drug antibody analysis (ADA)
To screen for the presence of VRC01 anti-idiotypic antibodies in the serum, a Meso Scale Discovery (MSD) electrochemiluminescence (ECL) bridging assay was developed. Detection of VRC01 anti-drug antibodies (ADA) was achieved by a homogeneous solution phase overnight incubation of diluted serum sample along with biotinylated and SULFO-TAG-labelled drug (VRC01). Any ADA present in the serum bound to biotinylated and SULFO-TAG-labelled drug and formed a complex. Biotin-labelled VRC01 served as a capture molecule on to a streptavidin precoated MSD plate and the SUFO-TAG-labelled VRC01 was the reporter used for detection. Additional assay details can be found in the supplemental methods in Supporting information.
Results
Study population
A total of 29 subjects were enrolled. Overall, the subject population was 76% male and 24% female, had a mean weight of 77 kg, and all subjects had an educational level of high school or higher, with 83% having a college or advanced degree (Table1). Twenty-seven subjects completed their scheduled infusions and 28 completed the protocol. One subject in group 4B (placebo) withdrew prior to the first infusion and one subject in group 4B (placebo) received only one infusion due to an intercurrent illness that did not resolve in time to receive the second infusion. Study participants were assessed for IgG GM allotype (Table2). No significant correlations between GM allotype and measured pharmacokinetic parameters were observed.
Table 1.
Demographic characteristics of study participants
| Category | Subcategory | Group 1 (n = 5) | Group 2 (n = 8) | Group 3 (n = 5) | Group 4A (n = 5) | Group 4B (n = 6) | Overall (n = 29) |
|---|---|---|---|---|---|---|---|
| n (%) | |||||||
| Gender | Male | 4 (80) | 7 (88) | 2 (40) | 5 (100) | 4 (67) | 22 (76) |
| Female | 1 (20) | 1 (13) | 3 (60) | 0 (0) | 2 (33) | 7 (24·1) | |
| Age (years)* | 21–30 | 1 (20) | 3 (38) | 4 (80) | 1 (20) | 5 (83) | 14 (48) |
| 31–40 | 3 (60) | 2 (25) | 0 (0) | 4 (80) | 1 (17) | 10 (35) | |
| 41–50 | 1 (20) | 3 (38) | 1 (20) | 0 (0) | 0 (0) | 5 (17) | |
| Race | Black/African | 1 (20) | 1 (13) | 0 (0) | 1 (20) | 1 (17) | 4 (14) |
| Ethnicity | Non-Hispanic/Latino | 5 (100) | 8 (100) | 5 (100) | 5 (100) | 5 (83) | 28 (97) |
| Mean Weight | kg (s.d.) | 68 (8·7) | 83 (12) | 72 (8·8) | 82 (9·2) | 77 (14) | 77 (12) |
| Education† | Secondary | 1 (20) | 1 (13) | 0 (0) | 2 (40) | 1 (17) | 5 (17) |
| College/university | 2 (40) | 2 (38) | 4 (80) | 2 (40) | 5 (83) | 16 (55) | |
| Advanced degree | 2 (40) | 4 (50) | 1 (20) | 1 (20) | 0 (0) | 8 (28) | |
There were no participants aged 18–20.
There were no participants with only a primary education level; s.d. = standard deviation.
Table 2.
Study participant immunoglobulin IgG gamma marker (GM) allotype
| GM3/3 | GM17/17 | GM3/17 | |
|---|---|---|---|
| 2 | 3 | 1 | |
| 5 | 10 | 4 | |
| 7 | 12 | 6 | |
| 11 | 27 | 8 | |
| 13 | 22 | 9 | |
| 16 | 14 | ||
| 17 | 15 | ||
| 19 | 18 | ||
| 20 | 23 | ||
| 21 | 26 | ||
| 25 | 28 | ||
| % of Study participants | 42·9 | 17·9 | 39·3 |
Product safety
There were 43 VRC01 and nine placebo administrations during the trial. VRC01 was safe and well tolerated and there were no serious adverse events. When present, local and systemic solicited reactogenicity (Tables3 and 4) was mild, with no moderate reactions in any group after either infusion.
Table 3.
Maximum local reactogenicity up to day 7 post-VRC-HIVMAB060-00-AB (VRC01) infusions*
| Symptoms intensity | All i.v. subjects groups 1, 2, 3 (n = 18) | s.c. VRC01 subjects group 4A (n = 5) | s.c. placebo subjects group 4B (n = 5) |
|---|---|---|---|
| n (%) | |||
| Pain/tenderness | |||
| None | 16 (89) | 3 (60) | 5 (100) |
| Mild | 2 (11) | 2 (40) | 0 (0) |
| Bruising | |||
| None | 18 (100) | 5 (100) | 5 (100) |
| Mild | 0 (0) | 0 (0) | 0 (0) |
| Swelling | |||
| None | 18 (100) | 5 (100) | 5 (100) |
| Mild | 0 (0) | 0 (0) | 0 (0) |
| Redness | |||
| None | 18 (100) | 4 (80) | 5 (100) |
| Mild | 0 (0) | 1 (20) | 0 (0) |
| Any local symptom | |||
| None | 16 (89) | 2 (40) | 5 (100) |
| Mild | 2 (11) | 3 (60) | 0 (0) |
There were no moderate or severe reactions throughout the trial; i.v. = intravenous; s.c. = subcutaneous.
Table 4.
Self-reported systemic reactogenicity day 3 post-VRC-HIVMAB060-00-AB (VRC01) infusions*
| Symptoms intensity | All i.v. subject groups 1, 2, 3 (n = 18) | s.c. VRC01 subjects group 4A (n = 5) | s.c. placebo subjects group 4B (n = 5) |
|---|---|---|---|
| n (%) | |||
| Malaise | |||
| None | 14 (78) | 4 (80) | 5 (100) |
| Mild | 2 (22) | 1 (20) | 0 (0) |
| Myalgia | |||
| None | 14 (78) | 4 (80) | 4 (80) |
| Mild | 4 (22) | 1 (20) | 1 (20) |
| Headache | |||
| None | 13 (72) | 4 (80) | 4 (80) |
| Mild | 5 (28) | 1 (20) | 1 (20) |
| Chills | |||
| None | 18 (100) | 5 (100) | 5 (100) |
| Mild | 0 (0) | 0 (0) | 0 (0) |
| Nausea | |||
| None | 17 (95) | 5 (100) | 5 (100) |
| Mild | 1 (5·6) | 0 (0) | 0 (0) |
| Temperature | |||
| None | 18 (100) | 5 (100) | 5 (100) |
| Mild | 0 (0) | 0 (0) | 0 (0) |
| Joint pain | |||
| None | 18 (100) | 5 (100) | 5 (100) |
| Mild | 0 (0) | 0 (0) | 0 (0) |
| Any systemic symptom | |||
| None | 10 (56) | 4 (80) | 3 (60) |
| Mild | 8 (44) | 1 (20) | 2 (40) |
There were no moderate or severe reactions throughout the trial; i.v. = intravenous; s.c. = subcutaneous.
Four adverse events assessed as possibly related to study product administration were mild in severity and resolved with no residual effects. Two of these events, one in group 4A (5 mg/kg s.c. VRC01) and one in 4B (placebo s.c.), were localized pruritus at the s.c. injection site on the day of infusion that resolved on the same day. One event was flushing (subject reported ‘warm sensation’ from abdomen to lower extremities) in the s.c. placebo group (4B) that resolved within an hour of the infusion, and one event was an elevated alanine amino transferase (ALT 54 IU/l) in the 5 mg/kg i.v. group (group 1) 28 days following infusion that also resolved with no residual effects within 7 days.
No subjects had a reactive HIV EIA response from the administered antibody throughout the study (tested at days 7 and 84).
Pharmacokinetics
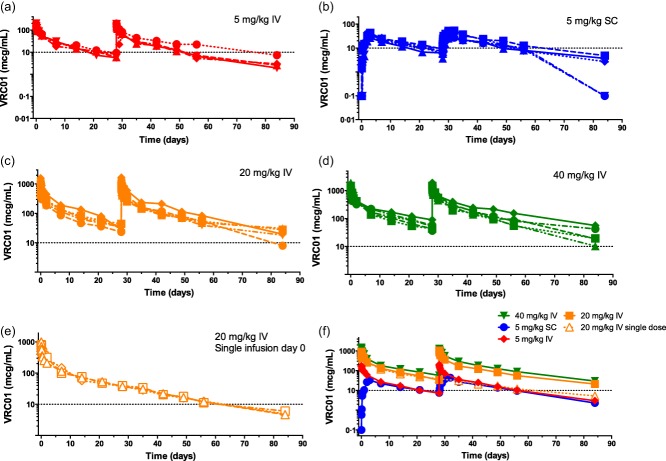
At 20 and 40 mg/kg, the mean [± standard deviation (s.d.)] maximum serum concentrations were 940 ± 320 (n = 8) and 1600 ± 230 (n = 5) μg/ml, respectively, after the first i.v. infusion and 1100 ± 360 (n = 5) and 1500 ± 400 (n = 5) μg/ml after the second i.v. infusion (Table5 and Fig. 2). At 20 and 40 mg/kg, mean 28-day trough serum concentrations were 35 ± 6·5 (n = 8) and 57 ± 19 μg/ml (n = 5) after the first i.v. dose and 56 ± 17 (n = 5) and 89 ± 40 (n = 5) μg/ml after the second, respectively, demonstrating a trend observed across dosing groups of higher trough values with repetitive doses (Fig. S1). VRC01 clearance for 20 mg/kg was 0·016 ± 0·0035 l/h, with a terminal half-life of 17 ± 4·0 days. VRC01 clearance for 40 mg/kg (n = 5) was 0·017 ± 0·0015 l/h, with a terminal half-life of 14 ± 2·9 days (Table5 and Fig. 2). For the i.v. groups overall (n = 18) the clearance was 0·016 ± 0·0033 l/h and terminal half-life was 15 ± 3·9 days.
Table 5.
VRC-HIVMAB060-00-AB (VRC01) mean pharmacokinetic parameter values
| Group and dose | Cmax | Tmax | CL | t1/2 | AUC | 28-day trough concentration |
|---|---|---|---|---|---|---|
| Mean (s.d.) | ||||||
| Group 1 (n = 5)i.v. 5 mg/kg | 190 (32) | 2·3 (0·7) | 0·016 | 14 | 45 000 (9700) | 7·2 (1·5) |
| Inf 1 | 210 (50) | 1·6 (0·9) | (0·0046) | (3·8) | 10 (6·9) | |
| Inf 2 | ||||||
| Group 2i.v. 20 mg/kg | 940 (320) | 2·1 (1·5) | 0·016* | 17* | 230 000 | 35 (6·5) |
| Inf 1 (n = 8) | 1100 (360) | 2·0 (5·9) | (0·0035) | (4·0) | (71 000) | 56 (17) |
| Inf 2 (n = 5) | ||||||
| Group 3 (n = 5)i.v. 40 mg/kg | 1600 (230) | 2·0 (0·6) | 0·0170 | 14 | 340 000 | 57 (19) |
| Inf 1 | 1500 (400) | 1·9 (0·6) | (0·0015) | (2·9) | 62 000 | 89 (40) |
| Inf 2 | ||||||
| Overalli.v. (n = 18) | 0·016 (0·0033) | 15 | ||||
| (3·9) | ||||||
| Group 4A (n = 5)s.c. 5 mg/kg | 34 (7·0) | 66 (9·4) | 0·029 | 17 | 30 000 | 7·54 (1·6) |
| Inf 1 | 38 (13) | 52 (9·7) | (0·0067) | (2·9) | (6400) | 9·4 (1·9) |
| Inf 2 | ||||||
Includes pharmacokinetic (PK) parameters from subjects who received one or two doses of VRC01. Cmax = maximum concentration (μg/ml); Tmax = time to Cmax (h); CL = clearance (l/h); t1/2 = terminal half-life (days); AUC = area under the curve, 0-inf (μg × h/ml); λz = terminal elimination rate constant (1/days), 28-day trough (μg/ml); i.v. = intravenous; s.c. = subcutaneous.
Figure 2.
VRC-HIVMAB060-00-AB (VRC01) concentration (μg/ml) shown per subject over time (days). (a) 5 mg/kg intravenous (i.v.), (b) 5 mg/kg subcutaneous (s.c.), (c) 20 mg/kg i.v., (d) 40 mg/kg i.v., (e) 20 mg/kg i.v. single dose and (f) subject means, all dose groups. A reference level of 10 μg/ml is indicated by the horizontal line on all plots. Doses were administered at days 0 and 28 (a–f) and at day 0 only (e).
Anti-VRC01 antibody responses were not detected in any subject at any time-point (Fig. 3).
Figure 3.
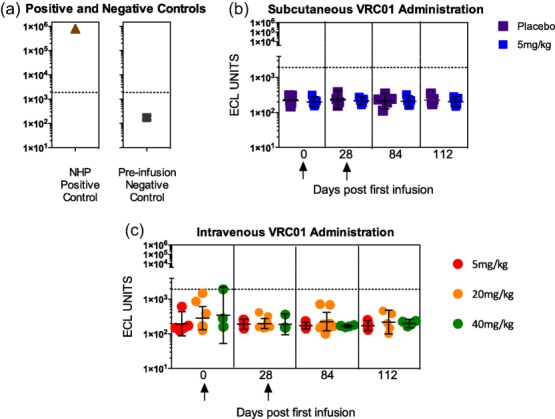
Evaluation of anti-VRC-HIVMAB060-00-AB (VRC01) antibodies following VRC01 infusion. The anti-drug antibody analysis (ADA) response is measured using a homogeneous bridging electrochemiluminescence (ECL) format. (a) Typical detection of ECL anti-VRC01 activity from a non-human primate 56 days following infusion with VRC01. The geometric mean values from 30 HIV-negative non-infused subjects demonstrate the negative control for the ECL bridging assay. (b) Longitudinal analyses following either subcutaneously delivered VRC01 at 5 mg/kg or placebo on days 0 and 28 (marked with arrows). (c) Longitudinal analyses following intravenous doses (5, 20 or 40 mg/kg) on days 0 and 28 (marked with arrows). All error bars indicate geometric mean with 95% confidence intervals. The horizontal line on panels a, b and c represents the upper bound of all known negative ADA responses from subjects never exposed to VRC01. No anti-VRC01 antibody was detected post-infusion on days 28, 56 or 112.
HIV-1 neutralizing activity
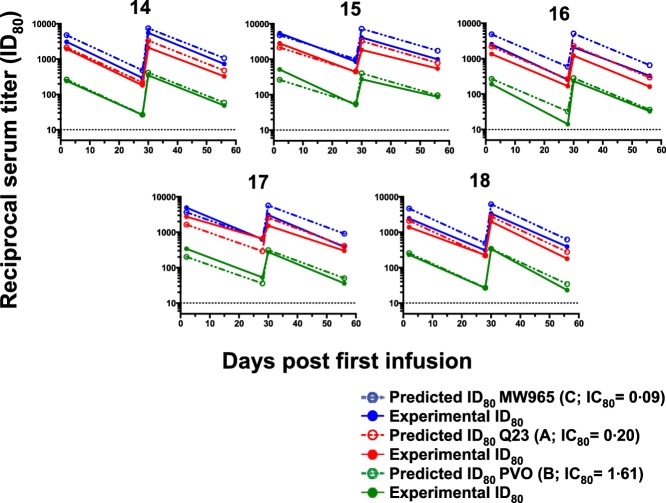
VRC01 sera concentrations and predicted and experimental reciprocal neutralization serum dilution (ID80) values were assessed for each VRC01 dose group during the course of the study (days 0, 2, 28, 30 and 56) (Table6). Predicted values across dose groups match the observed experimental values closely, and VRC01 retained broad neutralizing activity across HIV-1 subtypes A, B and C and a range of virus IC80 values (0·09–1·6 μg/ml) following i.v. and s.c. administration (Fig. 4). Across virus subtypes, predicted and experimental values show a trend of increasing reciprocal serum dilution values with increasing VRC01 doses and following the second infusion, indicating the increased availability of VRC01 for virus neutralization consistent with changes in VRC01 serum concentrations. As expected, there was no observed neutralizing activity in serum from any subject against the negative control MuLV or the VRC01 resistant virus CAP210, and poor neutralization for THRO·18 (data not shown).
Table 6.
Predicted and experimental reciprocal neutralization serum titres (ID80)
| Reciprocal neutralization serum titre (ID80) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Q23·17 (A)* | PVO·04 (B)* | MW965·26 (C)* | |||||||
| VRC01 Treatment | Subject | Day of study | VRC01 Sera Concentration (ug/ml) | Predicted | Experimental | Predicted | Experimental | Predicted | Experimental |
| 5 mg/kg i.v. | 1 | 0 | < 0·098 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 |
| 1 | 2 | 53·37 | 269·3 | 142·8 | 33·1 | 20·3 | 599·3 | 265·3 | |
| 1 | 28 | 8·01 | 40·4 | 24·1 | < 10·0 | < 10·0 | 89·9 | 32·5 | |
| 1 | 30 | 58·66 | 296·0 | 158·5 | 36·4 | 17·7 | 658·8 | 294·9 | |
| 1 | 56 | 5·31 | 26·8 | 24·3 | < 10·0 | < 10·0 | 59·6 | 36·1 | |
| 2 | 0 | < 0·098 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | |
| 2 | 2 | 53·25 | 268·7 | 237·8 | 33·1 | 21·5 | 598·1 | 297·6 | |
| 2 | 28 | 5·64 | 28·4 | 10·8 | < 10·0 | < 10·0 | 63·3 | 33·2 | |
| 2 | 30 | 58·40 | 294·7 | 274·6 | 36·3 | 22·2 | 655·9 | 634·1 | |
| 2 | 56 | 6·61 | 33·3 | 18·3 | < 10·0 | < 10·0 | 74·2 | 40·3 | |
| 3 | 0 | < 0·098 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | |
| 3 | 2 | 56·97 | 287·5 | 248·5 | 35·4 | 34·3 | 639·8 | 636·9 | |
| 3 | 28 | 5·93 | 29·9 | 21·5 | < 10·0 | < 10·0 | 66·6 | 42·7 | |
| 3 | 30 | 68·65 | 346·5 | 237·9 | 42·6 | 19·7 | 771·1 | 406·0 | |
| 3 | 56 | 7·29 | 36·8 | 27·3 | < 10·0 | < 10·0 | 81·9 | 66·5 | |
| 4 | 0 | < 0·098 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | |
| 4 | 2 | 66·32 | 334·7 | 339·6 | 41·2 | 26·1 | 744·8 | 553·2 | |
| 4 | 28 | 9·31 | 47·0 | 14·6 | < 10·0 | < 10·0 | 104·6 | 55·7 | |
| 4 | 30 | 83·07 | 419·2 | 223·3 | 51·6 | 26·8 | 933·0 | 461·3 | |
| 4 | 56 | 22·30 | 112·5 | 44·7 | 13·8 | < 10·0 | 250·5 | 86·2 | |
| 5 | 0 | < 0·098 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | |
| 5 | 2 | 29·44 | 148·6 | 146·9 | 18·3 | 26·9 | 330·6 | 247·1 | |
| 5 | 28 | 7·28 | 36·8 | 11·7 | < 10 | < 10·0 | 81·8 | 39·3 | |
| 5 | 30 | 35·22 | 177·7 | 215·7 | 21·9 | 25·5 | 395·6 | 407·9 | |
| 5 | 56 | 9·32 | 47·0 | 40·9 | < 10 | < 10·0 | 104·6 | 70·1 | |
| 20 mg/kg i.v. | 6 | 0 | < 0·098 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 |
| 6 | 2 | 203·03 | 1024·5 | 1151·9 | 126·1 | 115·3 | 2280·2 | 1686·6 | |
| 6 | 28 | 34·59 | 174·5 | 232·4 | 21·5 | 11·9 | 388·5 | 314·4 | |
| 6 | 30 | 253·90 | 1281·3 | 1382·6 | 157·7 | 233·2 | 2851·5 | 2209·7 | |
| 6 | 56 | 47·96 | 242·0 | 179·4 | 29·8 | 30·8 | 538·6 | 464·7 | |
| 7 | 0 | < 0·098 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | |
| 7 | 2 | 275·98 | 1392·7 | 1226·0 | 171·4 | 211·3 | 3099·5 | 1513·8 | |
| 7 | 28 | 36·17 | 182·5 | 150·1 | 22·5 | 22·8 | 406·2 | 225·8 | |
| 7 | 30 | 282·34 | 1424·8 | 1228·7 | 175·3 | 174·2 | 3170·9 | 2211·4 | |
| 7 | 56 | 39·71 | 200·4 | 220·8 | 24·7 | 31·2 | 446·0 | 367·2 | |
| 8 | 0 | < 0·098 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | |
| 8 | 2 | 248·80 | 1255·5 | 1307·9 | 154·5 | 131·2 | 2794·3 | 2226·3 | |
| 8 | 28 | 44·07 | 222·4 | 138·3 | 27·4 | 30·4 | 495·0 | 377·8 | |
| 8 | 30 | 271·20 | 1368·6 | 1308·3 | 168·4 | 195·0 | 3045·8 | 2994·7 | |
| 8 | 56 | 54·09 | 272·9 | 267·4 | 33·6 | 33·5 | 607·5 | 421·3 | |
| 9 | 0 | < 0·098 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | |
| 9 | 2 | 185·76 | 937·4 | 763·5 | 115·4 | 119·4 | 2086·3 | 1418·9 | |
| 9 | 28 | 23·44 | 118·3 | 52·5 | 14·6 | < 10·0 | 263·2 | 126·9 | |
| 9 | 30 | 172·63 | 871·1 | 1029·2 | 107·2 | 151·1 | 1938·7 | 1880·8 | |
| 9 | 56 | 18·90 | 95·4 | 77·8 | 11·7 | 11·4 | 212·2 | 244·6 | |
| 10 | 0 | < 0·098 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | |
| 10 | 2 | 220·30 | 1111·7 | 869·5 | 136·8 | 166·1 | 2474·2 | 1744·8 | |
| 10 | 28 | 26·08 | 131·6 | 165·8 | 16·2 | 29·1 | 292·9 | 233·8 | |
| 10 | 30 | 201·48 | 1016·7 | 1185·8 | 125·1 | 166·6 | 2262·8 | 2249·0 | |
| 10 | 56 | 47·33 | 238·8 | 87·2 | 29·4 | 17·3 | 531·5 | 217·3 | |
| 11 | 0 | < 0·098 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | |
| 11 | 2 | 291·975 | 1473·4 | 1437·7 | 181·3 | 201·5 | 3279·2 | 2456·0 | |
| 11 | 28 | 37·530 | 189·4 | 180·1 | 23·3 | 19·8 | 421·5 | 438·9 | |
| 11 | 56 | 12·592 | 63·5 | 53·1 | < 10·0 | < 10·0 | 141·4 | 180·8 | |
| 12 | 0 | < 0·098 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | |
| 12 | 2 | 317·500 | 1602·2 | 1290·6 | 197·2 | 193·7 | 3565·8 | 2854·6 | |
| 12 | 28 | 39·168 | 197·7 | 198·8 | 24·3 | 11·9 | 439·9 | 584·0 | |
| 12 | 56 | 11·178 | 56·4 | 45·9 | < 10·0 | < 10·0 | 125·5 | 182·6 | |
| 13 | 0 | < 0·098 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | |
| 13 | 2 | 207·325 | 1046·2 | 1522·6 | 128·7 | 245·1 | 2328·5 | 2502·4 | |
| 13 | 28 | 37·150 | 187·5 | 195·5 | 23·1 | 23·3 | 417·2 | 439·1 | |
| 13 | 56 | 12·091 | 61·0 | 55·2 | < 10·0 | < 10·0 | 135·8 | 148·0 | |
| 40 mg/kg i.v. | 14 | 0 | < 0·098 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 |
| 14 | 2 | 425·30 | 2146·2 | 1940·8 | 264·1 | 240·0 | 4776·5 | 3085·4 | |
| 14 | 28 | 42·97 | 216·8 | 181·0 | 26·7 | 26·4 | 482·5 | 293·5 | |
| 14 | 30 | 666·17 | 3361·7 | 2110·4 | 413·7 | 344·0 | 7481·7 | 5525·5 | |
| 14 | 56 | 94·75 | 478·1 | 325·0 | 58·8 | 48·6 | 1064·1 | 734·7 | |
| 15 | 0 | < 0·098 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | |
| 15 | 2 | 423·83 | 2138·8 | 2804·5 | 263·2 | 515·2 | 4760·0 | 5443·4 | |
| 15 | 28 | 89·39 | 451·1 | 436·5 | 55·5 | 51·0 | 1004·0 | 853·6 | |
| 15 | 30 | 648·20 | 3271·1 | 1839·0 | 402·5 | 272·9 | 7279·9 | 4086·6 | |
| 15 | 56 | 154·88 | 781·6 | 548·7 | 96·2 | 86·7 | 1739·5 | 991·0 | |
| 16 | 0 | < 0·098 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | |
| 16 | 2 | 434·48 | 2192·5 | 1348·4 | 269·8 | 190·1 | 4879·6 | 2555·7 | |
| 16 | 28 | 52·56 | 265·3 | 168·1 | 32·6 | 14·2 | 590·3 | 253·1 | |
| 16 | 30 | 455·65 | 2299·4 | 1206·5 | 283·0 | 231·4 | 5117·4 | 2080·3 | |
| 16 | 56 | 58·23 | 293·8 | 161·9 | 36·2 | 33·0 | 653·9 | 328·7 | |
| 17 | 0 | < 0·098 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | |
| 17 | 2 | 324·50 | 1637·5 | 2753·8 | 201·5 | 339·7 | 3644·4 | 4945·2 | |
| 17 | 28 | 57·53 | 290·3 | 660·8 | 35·7 | 52·8 | 646·2 | 632·0 | |
| 17 | 30 | 504·70 | 2546·9 | 1531·5 | 313·4 | 275·5 | 5668·3 | 3076·8 | |
| 17 | 56 | 81·13 | 409·4 | 300·2 | 50·4 | 35·9 | 911·1 | 384·1 | |
| 18 | 0 | < 0·098 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | |
| 18 | 2 | 415·33 | 2095·9 | 1385·6 | 257·9 | 230·9 | 4664·6 | 2436·7 | |
| 18 | 28 | 43·60 | 220·0 | 225·9 | 27·1 | 26·5 | 489·6 | 308·7 | |
| 18 | 30 | 549·23 | 2771·6 | 2000·9 | 341·1 | 337·5 | 6168·4 | 3373·6 | |
| 18 | 56 | 55·24 | 278·8 | 179·1 | 34·3 | 23·2 | 620·4 | 391·7 | |
| 5 mg/kg s.c. | 19 | 0 | < 0·098 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 |
| 19 | 2 | 22·780 | 115·0 | 88·8 | 14·1 | < 10·0 | 255·8 | 239·1 | |
| 19 | 28 | 5·911 | 29·8 | 12·1 | < 10·0 | < 10·0 | 66·4 | 41·3 | |
| 19 | 30 | 30·238 | 152·6 | 113·5 | 18·8 | 12·5 | 339·6 | 326·5 | |
| 19 | 56 | 7·733 | 39·0 | 36·2 | < 10·0 | < 10·0 | 86·8 | 55·3 | |
| 20 | 0 | < 0·098 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | |
| 20 | 2 | 31·330 | 158·1 | 299·2 | 19·5 | 25·6 | 351·9 | 358·7 | |
| 20 | 28 | 9·024 | 45·5 | 68·1 | < 10·0 | < 10·0 | 101·3 | 115·8 | |
| 20 | 30 | 47·112 | 237·7 | 519·0 | 29·3 | 58·0 | 529·1 | 1089·0 | |
| 20 | 56 | 12·410 | 62·6 | 104·5 | < 10·0 | < 10·0 | 139·4 | 224·7 | |
| 21 | 0 | < 0·098 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | |
| 21 | 2 | 19·305 | 97·4 | 96·2 | 12·0 | 13·1 | 216·8 | 165·1 | |
| 21 | 28 | 6·192 | 31·2 | 15·7 | < 10·0 | < 10·0 | 69·5 | 37·0 | |
| 21 | 30 | 25·190 | 127·1 | 120·1 | 15·6 | 11·5 | 282·9 | 260·5 | |
| 21 | 56 | 7·964 | 40·2 | 23·9 | < 10·0 | < 10·0 | 89·4 | 51·5 | |
| 22 | 0 | < 0·098 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | |
| 22 | 2 | 20·190 | 101·9 | 112·9 | 12·5 | 10·4 | 226·8 | 170·4 | |
| 22 | 28 | 7·188 | 36·3 | 20·3 | < 10·0 | < 10·0 | 80·7 | 72·0 | |
| 22 | 30 | 51·937 | 262·1 | 267·2 | 32·3 | 31·4 | 583·3 | 370·1 | |
| 22 | 56 | 8·780 | 44·3 | 38·4 | < 10·0 | < 10·0 | 98·6 | 62·5 | |
| 23 | 0 | < 0·098 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | < 10·0 | |
| 23 | 2 | 38·100 | 192·3 | 180·7 | 23·7 | 25·5 | 427·9 | 379·6 | |
| 23 | 28 | 9·383 | 47·3 | 25·3 | < 10·0 | < 10·0 | 105·4 | 59·5 | |
| 23 | 30 | 29·883 | 150·8 | 119·9 | 18·6 | 13·8 | 335·6 | 222·5 | |
| 23 | 56 | 10·253 | 51·7 | 40·2 | < 10·0 | < 10·0 | 115·2 | 79·9 | |
HIV subtype; i.v. = intravenous; s.c. = subcutaneous; VCR01 = VRC-HIVMAB060-00-AB.
Figure 4.
Serum neutralization titres after VRC-HIVMAB060-00-AB (VRC01) infusion of 40 mg/kg. Data for five subjects are shown. Solid lines and symbols show measured serum dilution that produced 80% neutralization (ID80) against the virus indicated in the legend. Predicted ID80 values were calculated based on the measured concentration of VRC01 in each sera and the established inhibitory concentration (IC80) of the monoclonal antibody against each virus. Predicted values are graphed as open circles with dotted lines. The viruses tested are Q23.17, clade A (red), PVO, clade B (green) and MW965, clade C (blue) The VRC01 IC80 values against each virus is shown in parenthesis in the legend.
Discussion
Advances in HIV-1 virology and B cell technologies during the past decade have led to the isolation of many broadly neutralizing antibodies which target diverse epitopes on the HIV-1 Env. 5,19,20. VRC01 is a potent bNAb and is member of a class of HIV-1 mAbs that bind to the conserved CD4 binding site of gp120 by partially mimicking the structural interaction of the cellular CD4 receptor with gp120 23,26,29. VRC01 neutralizes up to 91% of HIV-1 primary isolates in a panel representing all major circulating HIV-1 genetic subtypes, and delivery has demonstrated complete protection in NHP SHIV challenge models 37.
In the clinical trial described here, intravenous and subcutaneous administration of VRC01 was safe and was well tolerated in healthy volunteers, without dose-limiting toxicity or serious adverse events. When present, local and systemic reactogenicity events associated with administration were mild and resolved with no residual effects. Subjects did not develop anti-VRC01 antibody responses and all subjects were negative for HIV-1 by EIA throughout the trial.
PK analysis from this clinical trial revealed a VRC01 terminal half-life of 15 days across all i.v.-infused dose groups, and 28-day trough levels after first infusion of 35 μg/ml and 57 μg/ml for 20 and 40 mg/kg dose groups, respectively. Following the second infusion, the 28-day trough values rose to 57 μg/ml and 89 μg/ml for 20 and 40 mg/kg dose, respectively. In non-human primate animal models, passive administration of VRC01 has provided complete protection against a high-dose mucosal challenge with two different SHIVs; SHIV-SF162P3, VRC01 IC50: 1·86 μg/ml and SHIV-BaLP4, VRC01 IC50: 0·02 μg/ml. For reference, in a 170 HIV panel the median VRC01 IC50 value is 0·3 μg/ml 25. In the SHIV infection model, viral challenge was performed 2 days after i.v. VRC01 infusion and plasma VRC01 levels were measured just prior to challenge 37. Using the more neutralization-sensitive SHIV BaLP4, complete protection against infection was observed after an infusion dose of 1·25 mg/kg and approximately 50% protection was observed after infusion of 0·3 mg/kg. The associated plasma VRC01 concentrations at the time of challenge were 4–5 μg/ml after the 1·25 mg/kg infusion (unpublished data) and 1–2 μg/ml after the 0·3 mg/kg infusion 37. Using the more neutralization-resistant SHIV SF162P3, complete protection was observed after infusion of 20 mg/kg and 50% protection was seen after 5 mg/kg infusion. The associated VRC01 plasma levels were 52–88 μg/ml on day 2 after 20 mg/kg infusion and were 18–28 μg/ml after the 5 mg/kg 37. In the trial reported here, following a single infusion of 20 mg/kg the mean VRC01 concentration remained above 20 μg/ml up to 6 weeks post-infusion. In the 20 mg/kg dose group which received two infusions, the mean VRC01 concentration remained above 20 μg/ml up to 8 weeks post-second infusion. Together, these data indicate that potentially protective VRC01 serum levels can be achieved for up to 8 weeks post-infusion. In addition, in-vitro virus neutralization assays demonstrated that passive infusion of VRC01 produced plasma viral neutralization across HIV-1 subtypes A, B and C, as expected.
As part of the overall PK assessment, subjects were genotyped for the most common IgG1 GM alleles (GM3 and GM17) to determine if subject allotype impacts upon the activity of the VRC01 drug product. VRC01 contains the GM3 allele in the constant region of the heavy chain (γ1), and GM determinants of IgG1 have the potential to be immunogenic and anti-allotype antibodies have been detected when individuals are exposed to an allotype they do not possess in their genome 43–45. No correlations to any of the PK or clinical parameters were seen based on subject GM allotyping; therefore, subject allotype, or the theoretical potential for an anti-GM3 response, had no influence on PK, virus neutralization or safety outcomes in this study.
Based on the initial clinical data reported here and available in-vitro and preclinical data, VRC01 has potential clinical use in three broad areas: (1) prevention of transmission from HIV-1-infected mothers to newborn and breastfeeding infants, (2) prevention of HIV infection by sexual transmission and (3) therapeutic applications in HIV-1-infected individuals. To further define the optimal regimen, an assessment of PK parameters including half-life and trough are being evaluated extensively in an ongoing Phase IB multi-site study in healthy adults using a range of doses, routes and administration intervals. VRC01 is also being evaluated for safety, pharmacokinetics and virological impact in infants at risk of infection and in a series of trials involving HIV-1-infected aviraemic and viraemic subjects. Given its PK profile, human safety data and ability to protect NHPs from infection, VRC01 will be assessed in passive immunization prevention studies in infants 34, adolescents and adults at high risk of infection. Ongoing trials will evaluate multiple dosing regimens, ARV treatment interruption following VRC01 administration and the potential impact of VRC01 on the viral reservoir. Both intravenous and subcutaneous administration routes will continue to be evaluated. Subcutaneous administration has practical advantages; however, for adults, weight-based subcutaneous dosing is limited by tolerability of the injected volume and may therefore be more appropriate for infant administration where the total volume of a weight-based dose would be lower. The data generated in expanded VRC01 trials will help to guide anti-CD4bs nNAb development and may provide a benchmark for the development of next generation HIV-1 vaccines designed to induce bNAbs.
Acknowledgments
The authors thank the VRC602 trial volunteers for their contribution and commitment to HIV research. We also acknowledge the contributions of our NIH Clinical Center, Mark Connors and other NIAID colleagues, the NIAID Institutional Review Board, the EMMES Corporation, Mary Marovich, Carl Dieffenbach and other colleagues in the NIAID Division of AIDS (DAIDS), the NIAID Intramural Data and Safety Monitoring Board, as well as Judy Stein, Abe Mittelman, Hillery Harvey, Hope Wilson, Jason Gall, Gary Nabel and our other current and prior colleagues at the NIAID Vaccine Research Center and the VRC Vaccine Pilot Plant, operated by the Vaccine Clinical Materials Program of Leidos Biomedical Research, Inc., Frederick, MD. The VRC 602 Study Team members not listed in the author line include Floreliz Mendoza, Laura Novik, Kathy Zephir, William Whalen, Brenda Larkin, Olga Vasilenko, Nina Berkowitz, Brandon Wilson, Iris Pittman, Gretchen Schieber, Hope Decederfelt, Judith Starling, John Gilly, Srinivas Rao, Florence Kaltovich, Phyllis Renehan, Meghan Kunchai, Sarah Romano, Katie Menard, Ly Diep, Chuka Anude and Mary Allen. This work was supported by the intramural research program of the Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health. The findings and conclusions in this report are those of the authors and do not necessarily reflect the views of the funding agency or collaborators.
Disclosure
Some authors are listed as inventors on pending patent applications for VRC01.
Supporting Information
Additional Supporting information may be found in the online version of this article at the publisher's web-site:
Fig. S1. Day 28 VRC-HIVMAB060-00-AB (VRC01) trough concentrations after first and second infusions by dose group. Across doses a trend of higher day 28 VRC01 trough concentrations is observed following the second VRC01 dose.
References
- Plotkin SA, Gilbert PB. Nomenclature for immune correlates of protection after vaccination. Clin Infect Dis. 2012;54:1615–17. doi: 10.1093/cid/cis238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascola JR, Montefiori DC. The role of antibodies in HIV vaccines. Annu Rev Immunol. 2010;28:413–44. doi: 10.1146/annurev-immunol-030409-101256. [DOI] [PubMed] [Google Scholar]
- McElrath MJ, Haynes BF. Induction of immunity to human immunodeficiency virus type-1 by vaccination. Immunity. 2010;33:542–54. doi: 10.1016/j.immuni.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbaugh J, Morris L. The antibody response against HIV-1. Cold Spring Harb Perspect Med. 2012;2:a007039. doi: 10.1101/cshperspect.a007039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton DR, Poignard P, Stanfield RL, Wilson IA. Broadly neutralizing antibodies present new prospects to counter highly antigenically diverse viruses. Science. 2012;337:183–6. doi: 10.1126/science.1225416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton DR, Stanfield RL, Wilson IA. Antibody vs. HIV in a clash of evolutionary titans. Proc Natl Acad Sci USA. 2005;102:14943–8. doi: 10.1073/pnas.0505126102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantophlet R, Burton DR. GP120: target for neutralizing HIV-1 antibodies. Annu Rev Immunol. 2006;24:739–69. doi: 10.1146/annurev.immunol.24.021605.090557. [DOI] [PubMed] [Google Scholar]
- Stiegler G, Armbruster C, Vcelar B, et al. Antiviral activity of the neutralizing antibodies 2F5 and 2G12 in asymptomatic HIV-1-infected humans: a phase I evaluation. AIDS. 2002;16:2019–25. doi: 10.1097/00002030-200210180-00006. [DOI] [PubMed] [Google Scholar]
- Armbruster C, Stiegler GM, Vcelar BA, et al. Passive immunization with the anti-HIV-1 human monoclonal antibody (hMAb) 4E10 and the hMAb combination 4E10/2F5/2G12. J Antimicrob Chemother. 2004;54:915–20. doi: 10.1093/jac/dkh428. [DOI] [PubMed] [Google Scholar]
- Trkola A, Kuster H, Rusert P, et al. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat Med. 2005;11:615–22. doi: 10.1038/nm1244. [DOI] [PubMed] [Google Scholar]
- Binley JM, Wrin T, Korber B, et al. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J Virol. 2004;78:13232–52. doi: 10.1128/JVI.78.23.13232-13252.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binley JM, Lybarger EA, Crooks ET, et al. Profiling the specificity of neutralizing antibodies in a large panel of plasmas from patients chronically infected with human immunodeficiency virus type 1 subtypes B and C. J Virol. 2008;82:11651–68. doi: 10.1128/JVI.01762-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascola JR, D'Souza P, Gilbert P, et al. Recommendations for the design and use of standard virus panels to assess neutralizing antibody responses elicited by candidate human immunodeficiency virus type 1 vaccines. J Virol. 2005;79:10103–7. doi: 10.1128/JVI.79.16.10103-10107.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simek MD, Rida W, Priddy FH, et al. Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J Virol. 2009;83:7337–48. doi: 10.1128/JVI.00110-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Gao F, Mascola JR, et al. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol. 2005;79:10108–25. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Migueles SA, Welcher B, et al. Broad HIV-1 neutralization mediated by CD4-binding site antibodies. Nat Med. 2007;13:1032–4. doi: 10.1038/nm1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrammert J, Smith K, Miller J, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667–71. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiller T, Meffre E, Yurasov S, Tsuiji M, Nussenzweig MC, Wardemann H. Efficient generation of monoclonal antibodies from single human B cells by single cell RT–PCR and expression vector cloning. J Immunol Methods. 2008;329:112–24. doi: 10.1016/j.jim.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong PD, Mascola JR. Human antibodies that neutralize HIV-1: identification, structures, and B cell ontogenies. Immunity. 2012;37:412–25. doi: 10.1016/j.immuni.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong PD, Mascola JR, Nabel GJ. Broadly neutralizing antibodies and the search for an HIV-1 vaccine: the end of the beginning. Nat Rev Immunol. 2013;13:693–701. doi: 10.1038/nri3516. [DOI] [PubMed] [Google Scholar]
- Julien JP, Lee PS, Wilson IA. Structural insights into key sites of vulnerability on HIV-1 Env and influenza HA. Immunol Rev. 2012;250:180–98. doi: 10.1111/imr.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Georgiev I, Wu X, et al. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science. 2010;329:811–7. doi: 10.1126/science.1192819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid JF, Mouquet H, Ueberheide B, et al. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. 2011;333:1633–7. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Zhou T, Zhu J, et al. Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing. Science. 2011;333:1593–602. doi: 10.1126/science.1207532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Yang ZY, Li Y, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329:856–61. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Zhu J, Wu X, et al. Multidonor analysis reveals structural elements, genetic determinants, and maturation pathway for HIV-1 neutralization by VRC01-class antibodies. Immunity. 2013;39:245–58. doi: 10.1016/j.immuni.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, O'Dell S, Walker LM, et al. Mechanism of neutralization by the broadly neutralizing HIV-1 monoclonal antibody VRC01. J Virol. 2011;85:8954–67. doi: 10.1128/JVI.00754-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AP, Jr, Diskin R, Nussenzweig MC, Bjorkman PJ. Structural basis for germ-line gene usage of a potent class of antibodies targeting the CD4-binding site of HIV-1 gp120. Proc Natl Acad Sci USA. 2012;109:E2083–90. doi: 10.1073/pnas.1208984109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Wang C, O'Dell S, et al. Selection pressure on HIV-1 envelope by broadly neutralizing antibodies to the conserved CD4-binding site. J Virol. 2012;86:5844–56. doi: 10.1128/JVI.07139-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caskey M, Klein F, Lorenzi JC, et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature. 2015;522:487–91. doi: 10.1038/nature14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudicell RS, Kwon YD, Ko SY, et al. Enhanced potency of a broadly neutralizing HIV-1 antibody in vitro improves protection against lentiviral infection in vivo. J Virol. 2014;88:12669–82. doi: 10.1128/JVI.02213-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Zhang Z, Schramm CA, et al. Maturation and diversity of the VRC01-antibody lineage over 15 years of chronic HIV-1 infection. Cell. 2015;161:470–85. doi: 10.1016/j.cell.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch RM, Wong P, Tran L, et al. HIV-1 fitness cost associated with escape from the VRC01 class of CD4 binding site neutralizing antibodies. J Virol. 2015;89:4201–13. doi: 10.1128/JVI.03608-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronin Y, Mofenson LM, Cunningham CK, et al. HIV monoclonal antibodies: a new opportunity to further reduce mother-to-child HIV transmission. PLOS Med. 2014;11:e1001616. doi: 10.1371/journal.pmed.1001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura KJ, Cerini C, Sobrera ER, et al. Coverage of primary mother-to-child HIV transmission isolates by second-generation broadly neutralizing antibodies. AIDS. 2013;27:337–46. doi: 10.1097/QAD.0b013e32835cadd6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell ES, Ojeda S, Fouda GG, et al. Short communication: HIV type 1 subtype C variants transmitted through the bottleneck of breastfeeding are sensitive to new generation broadly neutralizing antibodies directed against quaternary and CD4-binding site epitopes. AIDS Res Hum Retroviruses. 2013;29:511–5. doi: 10.1089/aid.2012.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegu A, Yang ZY, Boyington JC, et al. Neutralizing antibodies to HIV-1 envelope protect more effectively in vivo than those to the CD4 receptor. Sci Transl Med. 2014;6:243ra88. doi: 10.1126/scitranslmed.3008992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groothuis JR. Safety and tolerance of palivizumab administration in a large Northern Hemisphere trial. Northern Hemisphere Expanded Access Study Group. Pediatr Infect Dis J. 2001;20:628–30. doi: 10.1097/00006454-200106000-00018. [DOI] [PubMed] [Google Scholar]
- Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study Group. Pediatrics. 1998;102:531–7. [PubMed] [Google Scholar]
- Jefferis R, Lefranc MP. Human immunoglobulin allotypes: possible implications for immunogenicity. MAbs. 2009;1:332–8. doi: 10.4161/mabs.1.4.9122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey JP, Kaur N, Costa S, et al. Immunoglobulin genes implicated in glioma risk. Oncoimmunology. 2014;3:e28609. doi: 10.4161/onci.28609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarzotti-Kelsoe M, Bailer RT, Turk E, et al. Optimization and validation of the TZM-bl assay for standardized assessments of neutralizing antibodies against HIV-1. J Immunol Methods. 2014;409:131–46. doi: 10.1016/j.jim.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudenberg HH, Fudenberg BR. Antibody to hereditary human gamma-globulin (Gm) factor resulting from maternal–fetal incompatibility. Science. 1964;145:170–1. doi: 10.1126/science.145.3628.170. [DOI] [PubMed] [Google Scholar]
- Kickler TS, Ness PM, Braine HG, Richardson L, Farkosh M. The expression of IgG allotypes on platelets and immunization to IgG allotypes in multitransfused thrombocytopenic patients. Blood. 1990;76:849–52. [PubMed] [Google Scholar]
- Allen JC, Kunkel HG. Antibodies to genetic types of gamma globulin after multiple transfusions. Science. 1963;139:418–9. doi: 10.1126/science.139.3553.418. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Day 28 VRC-HIVMAB060-00-AB (VRC01) trough concentrations after first and second infusions by dose group. Across doses a trend of higher day 28 VRC01 trough concentrations is observed following the second VRC01 dose.