Summary
Hypercortisolism is associated with insulin resistance (IR) and diabetes mellitus (DM); however, to our knowledge prior studies have not examined the association of diurnal cortisol curve features with measures of glycemia or IR in a population-based setting. Using log-transformed salivary cortisol data on 850 ethnically diverse men and women from the Multi-Ethnic Study of Atherosclerosis, we investigated the cross-sectional association of cortisol curve features with (1) glycemia in those with and without DM and (2) IR, in non-diabetic subjects. The log-transformed salivary cortisol curve features included wake-up cortisol, cortisol awakening response (CAR), early decline slope (30 minutes to 2 hours post-awakening), late decline slope (2 hours post-awakening to bedtime), overall decline slope (0 minutes to bedtime, excluding 30 minute cortisol), bedtime cortisol and total area under the curve (AUC). Overall, following multivariable adjustment, among those with diabetes mellitus (DM), early decline slope, overall decline slope, bedtime cortisol, and AUC were significantly and positively associated with a 5.4% (95% CI: 1.3, 9.7), 54.7% (95% CI: 12.4, 112.9), 4.0% (95% CI: 1.6, 6.4), and 6.8% (95% CI: 3.3, 10.4) higher HbA1c per 1 unit increase in log cortisol feature, respectively. Cortisol curve features were not associated with HbA1c among non-diabetic participants; however, wake-up cortisol and AUC were associated with a 8.2% lower (95% CI: −13.3, −2.7) and 7.9% lower (95% CI: −14.6, −0.6) log HOMA-IR, respectively. This was attenuated by adjustment for waist circumference. Among participants with DM, cortisol curve parameters suggestive of higher hypothalamic-pituitary-adrenal (HPA) axis activity and dysfunction were associated with higher HbA1c. In non-diabetic participants, greater HPA activity was paradoxically associated with lower insulin resistance.
Keywords: Cortisol, Glycemia, Insulin Resistance, Type 2 Diabetes Mellitus, hypothalamic-pituitary-adrenal axis
Introduction
The hypothalamic-pituitary-adrenal (HPA) axis is altered by various psychological (e.g. stress, post-traumatic stress disorder, depression) and physiological (e.g. sleep insufficiency/disturbance) stressors, leading to HPA axis dysfunction. The normal HPA axis diurnal rhythm consists of high morning and low afternoon-evening cortisol levels with normal feedback control to lower elevated cortisol levels following acute stress. Repeated acute stresses lead to hippocampal and pituitary glucocorticoid receptor downregulation, poor feedback inhibitory control with increased cortisol secretion and loss of diurnal cortisol rhythm (Rosmond, 2003). Prior studies have shown that depressive disorders activate and alter the function of the HPA axis and increase the risk for type 2 diabetes (DM) (Stetler and Miller, 2011). Hypercortisolism in the setting of repeated psychological and physiological stresses leads to increased visceral adiposity, further promoting insulin resistance and hyperglycemia (Kahn et al., 2006).
The impact of cortisol on glycemia and insulin resistance can be assessed using glycated hemoglobin (HbA1c) and the homeostasis model assessment of insulin resistance (HOMA-IR), respectively. HbA1c is a measure of the average blood glucose levels over the prior 90 days with higher levels indicating worse glucose control (normal <5.7%, prediabetes 5.7–6.4%, DM ≥6.5%)(American Diabetes Association, 2010). The HOMA-IR is a model using steady-state (fasting) insulin and glucose to estimate insulin resistance, with higher levels indicating greater insulin resistance (Matthews et al., 1985). In previous analysis, higher fasting and mean overnight serum cortisol levels have been associated with higher HOMA-IR and fasting glucose (Anagnostis et al., 2009). Individuals with the metabolic syndrome have also been shown to have higher circulating cortisol concentrations in both the basal setting and in response to dynamic hypothalamic-pituitary-adrenal (HPA) axis testing (Duclos et al., 2005; Ward et al., 2003). Subclinical hypercortisolism has been documented in individuals with DM, with higher 24-hour urine free cortisol (Chiodini, 2005), higher dexamethasone suppressed cortisol (Chiodini, 2005; Godoy-Matos et al., 2006), higher basal plasma cortisol, (Chiodini, 2005) and larger adrenal gland volume (Godoy-Matos et al., 2006) than individuals without diabetes. One study showed a positive association between dexamethasone-suppressed cortisol and HbA1c, suggesting that hypercortisolism may also be related to glycemic control (Bruehl et al., 2007). We previously showed in the Multi-Ethnic Study of Atherosclerosis (MESA) Stress I Ancillary study that women with diabetes had higher total cortisol area under the curve (AUC) compared to women without diabetes whereas men with diabetes had a lower total cortisol AUC compared to men without diabetes (Champaneri et al., 2012). An important limitation in our prior cross-sectional analysis is that we lacked data on glycemic control at the time hormonal measures were assessed and were unable to assess its contribution to our observed associations. The only glycemic marker was a HbA1c value from 2–4 years prior to collection of salivary cortisol data. In this study, the objective was to use data from the MESA Stress II Ancillary Study, a diverse population-based study of adults, to assess whether glycemia, assessed via HbA1c, was cross-sectionally associated with diurnal cortisol curve features (wake-up cortisol, cortisol awakening response (CAR), early decline slope, late decline slope, overall decline slope, bedtime and total cortisol AUC). In our second analysis, we examined the association of cortisol curve features with insulin resistance, assessed via HOMA-IR, in non-diabetic individuals. To our knowledge, there are no prior studies examining the association of glycemia and insulin resistance with the full diurnal cortisol curve profile in diabetic and non-diabetic participants.
1. Methods
1.1 Study Population
We used data from MESA, a multi-center, longitudinal cohort study of the prevalence and correlates of subclinical cardiovascular disease and the factors that influence its progression (Bild, 2002). Between July 2000 and August 2002, 6,814 men and women without clinical cardiovascular disease who identified themselves as White, Black, Hispanic or Chinese, and were 45–84 years of age were recruited from six U.S. communities: Baltimore City and Baltimore County, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County; and St. Paul, Minnesota. Details on the sampling frames and the cohort examination procedures have been published previously (Bild, 2002). The MESA Stress II Study collected detailed measures of stress hormones, including salivary cortisol measures, on a subsample of 1,082 participants at the New York, Los Angeles and Baltimore MESA study sites between 2010–2012 during MESA Exam 5. These populations represent an ethnically and socioeconomically diverse group of participants. Written informed consent was obtained from each participant, and the study was approved by the Institutional Review Boards of each MESA institution.
1.2 Hormonal Measures
Salivary cortisol measures were collected over 2 days with 8 time points measured per day. The first sample was taken immediately after awakening (and before getting out of bed), the second sample 30 minutes later, and 6 additional timed samples throughout the day including a sample right before bedtime. Participants were instructed not to eat or drink or brush their teeth 15 minutes before collecting the salivary samples. They were also instructed to leave the cotton swab in their mouths for less than 2 minutes until soaked, moving it around inside their mouth. Participants were instructed to record the exact time of sample collection on a special card, which was facilitated by a provided alarm clock. Saliva samples were stored at −20° C until analysis. Before biochemical analysis, samples were thawed and centrifuged at 3000 rpm for 3 minutes to obtain clear saliva with low viscosity. Cortisol levels were determined using a commercially available chemiluminescence assay with a high sensitivity of 0.16 ng/mL (IBL, Hamburg, Germany). Intra- and inter-assay coefficients of variation were less than 8%.
In MESA Stress II, 98% of participants collected valid samples (i.e. valid cortisol sample and valid time of sample collection) on both days and 96% of participants collected at least 5 valid samples per day for both days. Collection rates were similar to MESA Stress I, where 97% of participants collected samples on all 3 days and 85% of participants collected at least 5 samples per day for all days on which they collected samples (Golden et al., 2014; Hajat et al., 2010). Participants recorded collection time on special cards. Based on prior work in our population, the median difference between the actual collection time and recorded times was between 2 and 4 min depending on the sample. The 25th and 75th percentiles were between 1 and 2 and 5 and 13 min, respectively, with the longest times corresponding to the last sample of the day. Overall, the first sample was taken within 5 min of wake-up for 78% of days across participants and the median difference between the first and second sample was 34 min. While lower compliance with the collection protocol was associated with a less pronounced CAR, compliance was not associated with any other cortisol features and adjustment for compliance did not affect the associations of cortisol features with sociodemographic characteristics (Golden et al., 2014).
1.3 Cortisol Features
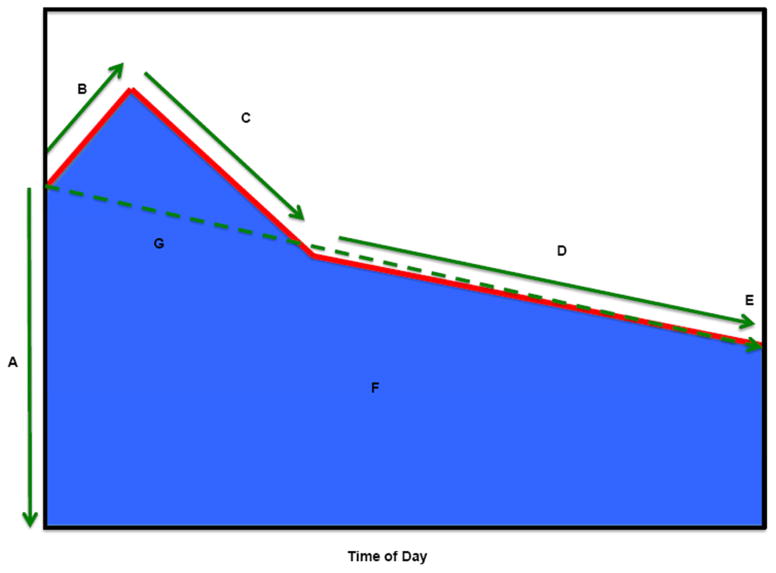
Normal diurnal cortisol regulation follows a circadian pattern, in which levels are typically high upon waking, increase by 50—75% during the 30—40 min post-awakening (CAR); and decline across the remainder of the day, reaching a nadir in the late evening some 18+ hours after awakening (Golden et al., 2014). We investigated seven features of the daily cortisol curve: Wake-up cortisol levels, CAR, standardized total cortisol AUC, early decline slope, late decline slope, overall decline slope and bedtime cortisol (Figure 1). Due to its positively skewed distribution, cortisol was log-transformed before the cortisol features were calculated (Adam et al., 2006; Champaneri et al., 2013, 2012; Hajat et al., 2010; Wang et al., 2014). Wake-up cortisol was defined as the salivary cortisol obtained at time 0. CAR was the cortisol rise from time 0 to 30 minutes post-awakening. Early decline in cortisol was defined as the decline in cortisol from 30 minutes post-awakening to 2 hours post-awakening. Late decline in cortisol was the decline in cortisol from 2 hours post-awakening to bedtime. The overall decline slope was calculated as the rate of decline using all samples except the 2nd sample (30 minutes post-awakening). To calculate the AUC, we used linear splines to connect the values from each of the sample times and then calculated the area under the linear spline based on the trapezoid rule (Yeh and Kwan, 1978), using all available data and restricting estimates to a 16-hour day duration for all participants (AUC was missing if less than 3 samples were collected for the exam day or the last 2 samples were missing). The AUC was then standardized by the length of duration (which is 16-hours in our analysis).
Figure 1. Diurnal Cortisol Profile.
Summary of diurnal cortisol parameters.
Key:
A. Wake-up cortisol (Time 0)
B. Cortisol awakening response (0 minutes to 30 minutes)
C. Early decline slope cortisol (30 minutes to 2 hours)
D. Late decline slope cortisol (2 hours to bedtime)
E. Bedtime cortisol
F. Total AUC (0 minutes to bedtime) cortisol
G. Overall decline slope cortisol (0 minutes to bedtime, excluding 30 minute cortisol)
1.4 Diabetes Status
Participants fasted for 12 hours and avoided smoking and heavy physical activity for 2 hours before each examination. Fasting blood samples were drawn between 7:30 and 10:30 AM. Serum was frozen and stored at −70°C as previously described (Bild, 2002). Diabetes status at Exam 5 was determined by 2003 ADA fasting criteria: diabetes (fasting glucose ≥ 126 mg/dL or takes insulin or oral diabetes medication); impaired fasting glucose if fasting glucose=100–125 mg/dL; and normal if fasting glucose is < 100 mg/dL according to the 2003 American Diabetes Association Criteria (American Diabetes Association, 2010).
1.5 HOMA-IR and HbA1c
HOMA-IR was calculated using fasting glucose and insulin variables collected at MESA Exam 5. HOMA-IR was calculated using the formula: HOMA-IR = (Glucose x Insulin)/405) (Matthews et al., 1985). HOMA-IR was only conducted in non-diabetic participants at Exam 5, as anti-diabetic medication use will alter insulin and glucose levels. HbA1c was collected at MESA Exam 5 in diabetic and non-DM participants.
1.6 Covariates
Demographic and socioeconomic factors previously shown to be associated with features of the cortisol curve were analyzed (Clow et al., 2004; Cohen et al., 2006; Hajat et al., 2010; Hansen et al., 2008; Ranjit et al., 2005). Data on age, race/ethnicity, sex, and cigarette smoking were self-reported using standard protocols previously described (Bild, 2002). Analysis of socioeconomic status was assessed by total gross family income in the past 12 months at MESA Exam 5. Prescription and over-the-counter medications were determined by transcription of medications brought into the clinic during each examination with particular attention to beta-blocker use, steroid use, hormone replacement therapy use, and aspirin use as covariates (Bild, 2002). Badrick et al, showed higher salivary cortisol levels in current smokers only and no differences among ex-smokers and never-smokers (Badrick et al., 2007), thus we categorized smoking as current smoking or non–current smoking status at MESA Exam 5. Adiposity measures at MESA Exam 5 included body mass index (BMI) and waist circumference. Depressive symptoms were assessed by the CES-D Scale at MESA visit 5 (Radloff, 1977).
1.7 Statistical Analysis
We performed analyses of HbA1c samples from participants and estimated the effect separately for each diabetes group. 1,082 participants were enrolled in MESA Stress II. We restricted our analysis to participants with complete diurnal cortisol features. We excluded 13 participants with no valid daily cortisol samples (including samples with missing cortisol value or unreliable cortisol values (0 or >100 nmol/L) or missing time of sample collection) in any exam day. Participants with missing values in wakeup (N=10), CAR (N=24), early decline slope (N=17), late decline slope (N=9), AUC (N=29), bedtime cortisol (N=16) or the overall decline slope (N=1) were excluded. In addition, we further excluded participants who had missing values in diabetes (N=8), gross family annual income (N=21), waist circumference (N=2), CES-D (N=20), aspirin use (N=2), glucose (N=10), insulin (N=133) and HbA1c (N=14), which led to a further exclusion of 219 participants. We planned to use the income-wealth index as a socioeconomic status variable; however, the missing rate was high (136/1069=13%), so we used gross family annual income. The final analyses therefore included 850 participants.
In our first aim to assess the relation between glycemia and diurnal cortisol curve features in diabetic and non-diabetic participants, the exposure variable was the cortisol curve features (wakeup cortisol, CAR, early decline slope, late decline slope, overall decline slope, bedtime cortisol and total cortisol AUC) and the outcome variable was HbA1c. HbA1c was maintained as a continuous variable and was log-transformed to normalize the skewed distribution. A linear regression model was fitted with HbA1c as response variable and with cortisol features as exposure variable, adjusting for demographic information, adiposity measures, depressive symptoms, medications and behavioral variables. In addition, the model included an interaction term of diabetes and cortisol feature, which allows us to estimate the association of cortisol feature with HbA1c for each diabetes group as well as to test the difference in the association between diabetes groups. In Model 0, we examined HbA1c association with cortisol features with no additional adjustment. In Model 1, we examined HbA1c association with cortisol features adjusted for demographic variables. In Model 2, we examined the HbA1c association with cortisol features adjusted for demographic variables and waist circumference. In Model 3, we examined the HbA1c association with cortisol features adjusted for all variables. The regression coefficients derived from our linear regression models represent the change in HbA1c (%) per one unit increase in the value of the cortisol feature after adjusting for other factors (age, sex, race/ethnicity, gross family annual income, waist circumference, medications affecting hypothalamic-pituitary-adrenal axis function, current smoking status, and depressive symptoms score). To assess the association of insulin resistance, assessed via HOMA-IR, with diurnal cortisol curve features in non-diabetic participants, we performed similar analyses as our first aim with the exception that the outcome variable was log-transformed HOMA-IR, modeled continuously in non-diabetes participants only.
2. Results
The final analytic sample contained 850 participants with a mean age of 70 (SD: 9, range: 54—93). They were racially/ethnically diverse: Black (31.3%), Hispanic (43.4%), and White (25.3%) and were approximately evenly distributed across sexes (54.7% women and 45.3% men). The characteristics of the participants at MESA Exam 5 are displayed in Table 1. At the time of salivary cortisol collection 170 (20%) participants had prevalent diabetes of which 94% were on diabetic medications, 177 (20.8%) participants had impaired fasting glucose and 503 (59.2%) had normal glucose metabolism. As expected the DM group had a higher fasting blood glucose (135.6 mg/dL vs. 94.2 mg/dL), HbA1c (7.2% vs. 5.7%), waist circumference (107.5 cm vs. 99.5 cm) and BMI (31.7 kg/m2 vs. 29.0 kg/m2). While excluded participants were more likely to be White and take hormone replacement therapy, there were no other substantive differences between included and excluded individuals (Supplemental Table 1).
Table 1.
Characteristics of Participants at MESA Exam 5 (2010–2012) *
| Characteristic | Non-diabetes | Diabetes |
|---|---|---|
| Participants - n (%) | 680 (80) | 170 (20) |
| Demographic | ||
| Age - years | 69.6 ± 9.3 | 70.1 ± 8.1 |
| Female sex - n (%) | 370 (54) | 95 (56) |
| Male sex - n (%) | 310 (46) | 75 (44) |
| Race/Ethnicity - n (%) § | ||
| Non-Hispanic White | 200 (29) | 15 (9) |
| African-American | 193 (28) | 73 (43) |
| Hispanic | 287 (42) | 82 (48) |
| Gross family annual income (US Dollars) | 50085 ± 34113 | 40188 ± 31524 |
| Clinical | ||
| HbA1c – % | 5.7 ± 0.4 | 7.2 ± 1.7 |
| HbA1c (log-scale) – % | 1.7 ± 0.1 | 2 ± 0.2 |
| HOMA-IR€ | 246.3 ± 173.2 | N/A |
| HOMA-IR (log-scale) € | 5.3 ± 0.7 | N/A |
| Fasting glucose (mg/dL) | 94.2 ± 9.6 | 135.6 ± 54.6 |
| Fasting Insulin (pmol/L) | 57.5 ± 37.7 | 77.5 ± 45.3 |
| Body-mass index¶ | 29 ± 5.4 | 31.7 ± 5.5 |
| Waist circumference (cm) | 99.5 ± 13.8 | 107.5 ± 13.4 |
| Beta-blocker - n (%) | 105 (15) | 57 (34) |
| Steroid - n (%) | 31 (5) | 7 (4) |
| Hormone replace therapy - n (%) | 18 (3) | 0 (0) |
| Aspirin therapy - n (%) | 282 (41) | 103 (61) |
| Current Smoking - n (%) | 32 (5) | 14 (8) |
| CES-D score | 8.3 ± 7.9 | 9.3 ± 8 |
| Log Transformed Cortisol Features # | ||
| Wake-up Cortisol | 2.59 ± 0.85 | 2.54 ± 0.79 |
| Cortisol Awakening Response | 0.28 ± 0.72 | 0.24 ± 0.75 |
| Early Decline Slope | −0.45 ± 0.52 | −0.34 ± 0.49 |
| Late Decline Slope | −0.09 ± 0.07 | −0.09 ± 0.07 |
| Overall Decline Slope | −0.10 ± 0.06 | −0.09 ± 0.06 |
| Bedtime Cortisol | 1.10 ± 0.87 | 1.20 ± 0.85 |
| Total AUC (16-hours) Cortisol | 1.70 ± 0.65 | 1.78 ± 0.59 |
| Diabetes Status | ||
| Normal - n (%) | 503 (74) | |
| Impaired Fasting Glucose - n (%) | 177 (26) | |
| Untreated Diabetes - n (%) | 10 (6) | |
| Treated Diabetes - n (%) | 160 (94) |
Plus-minus values are means ± SD.
Race was self-reported.
HOMA-IR was not calculated for participants with diabetes.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Cortisol features were calculated using log-transformed cortisol values (unit: log(nmol/L)); for complete analytic data see Supplemental Table 2.
2.1 Results for Hemoglobin A1C Analysis in Non-DM and DM Participants
In participants with DM, early decline slope, overall decline slope, bedtime cortisol, and total cortisol AUC were significantly and positively associated with a 5.4% (95% CI: 1.3, 9.7), 54.7% (95% CI: 12.4, 112.9), 4.0% (95% CI: 1.6, 6.4), and 6.8% (95% CI: 3.3, 10.4) higher HbA1c per 1 unit change in cortisol, respectively in multivariable adjusted analysis (Table 2). Wake-up cortisol, CAR, and late decline slope were not associated with HbA1c in DM participants. The results were not significantly changed by adjustment for sociodemographic variables, adiposity or full multivariable adjustment. Sensitivity analysis using raw unit HbA1c data showed a similar pattern of association as results using log-transformed HbA1c. In participants with DM compared with no DM, the difference in HbA1c % change per 1 unit change in early decline slope, overall decline slope, bedtime cortisol, and total cortisol AUC were significantly and positively associated with a 5.4% (95% CI: 0.8, 10.2), 53.9% (95% CI: 7.6, 120.2), 3.6% (95% CI: 1.0, 6.4), and 6.3% (95% CI: 2.5, 10.2) higher HbA1c per 1 unit change in cortisol in the DM group respectively in multivariable adjusted analysis. There was no association between any of the cortisol curve features and HbA1c in the non-DM participants.
Table 2.
Percent change in HbA1c per 1 unit increase in the selected diurnal cortisol features*
|
Model 0 % (95% CI) |
Model 1 % (95% CI) |
Model 2 % (95% CI) |
Model 3 % (95% CI) |
|
|---|---|---|---|---|
| Early Decline Slope | ||||
| Non-Diabetes | 0.7 (−1.3, 2.7) | 0.6 (−1.4, 2.6) | 0.1 (−1.8, 2.1) | 0.04 (−1.9, 2.0) |
| Diabetes | 7.5 (3.1, 12.1) | 7.0 (2.7, 11.4) | 6.3 (2.1, 10.6) | 5.4 (1.3, 9.7) |
| Overall Decline Slope | ||||
| Non-Diabetes | 10.1 (−7.4, 31.0) | 3.7 (−12.7, 23.2) | 0.8 (−14.8, 19.2) | 0.5 (−14.9, 18.7) |
| Diabetes | 78.1 (27.1, 149.6) | 65.0 (18.5, 129.7) | 57.1 (13.8, 116.9) | 54.7 (12.4, 112.9) |
| Bedtime Cortisol | ||||
| Non-Diabetes | 0.4 (−0.8, 1.7) | 0.5 (−0.7, 1.7) | 0.5 (−0.7, 1.7) | 0.3 (−0.8, 1.5) |
| Diabetes | 4.8 (2.2, 7.4) | 4.6 (2.1, 7.2) | 4.1 (1.7, 6.6) | 4.0 (1.6, 6.5) |
| Total AUC (16-Hours) Cortisol | ||||
| Non-Diabetes | −0.1 (−1.7, 1.53) | 0.4 (−1.2, 2.0) | 0.7 (−0.9, 2.2) | 0.5 (−1.0, 2.0) |
| Diabetes | 7.2 (3.5, 11.0) | 7.2 (3.5, 10.9) | 6.9 (3.4, 10.6) | 6.8 (3.3, 10.4) |
Model 0: HbA1c association with cortisol feature (no additional adjustment; include interaction term of diabetes and cortisol feature).
Model 1: HbA1c association with cortisol feature (adjustment only for demographic variable; include interaction term of diabetes and cortisol feature).
Model 2: HbA1c association with cortisol feature (adjustment for demographic + waist circumference; include interaction term of diabetes and cortisol feature).
Model 3: HbA1c association with cortisol feature (adjustment for all variables; include interaction term of diabetes and cortisol feature).
the analyses focused on both diabetes and non-diabetes samples was based on log-transformed cortisol and HbA1c. The result of percent change by 1 unit change in the cortisol feature was derived by the exponentiation of the model coefficient estimates.
Statistically significant findings are in bold. The difference between non-diabetes and diabetes was calculated and was significant throughout all analyses.
2.2 Results for HOMA-IR in Non-DM Participants
Among those without DM, wake-up cortisol was associated with a 9.4% lower (95% CI: −14.5, −4.2) HOMA-IR per 1 unit increase in cortisol feature, in the unadjusted model, 8.2% lower (95% CI: −13.3, −2.7) when adjusted for sociodemographic variables (Table 3). The association was attenuated and became non-significant when adjusted for sociodemographic variables and waist circumference (3.0% lower (95% CI: −7.7, 1.9)). Total cortisol AUC (16-hour) was significantly associated with HOMA-IR in the unadjusted and sociodemographic variables adjusted models with a 9.8% lower (95% CI: −16.4, −2.7) and 7.9% lower (95% CI −14.6, −0.6) HOMA-IR per 1 unit increase in cortisol feature. These results were attenuated and became non-significant when adjusted for waist circumference with a 4.2% lower (95% CI −10.1, 2.2) HOMA-IR per 1 unit increase in cortisol feature. To examine which feature of HOMA-IR, glucose or insulin, was associated with cortisol curve features, we created an XY scatterplot of glucose and insulin versus the individual cortisol curve features. Wake-up and total cortisol AUC were inversely associated with insulin with correlation coefficients of −0.148 (p-value < 0.001) and −0.128 (p–value < 0.001), whereas fasting glucose showed no statistically significant association with wake-up cortisol or total cortisol AUC with correlation coefficients of −0.009 (p-value = 0.824) and −0.074 (p-value = 0.053). Sensitivity analyses separating Non-DM into finer groups of impaired fasting glucose and normal fasting glucose, showed similar associations in HOMA-IR and cortisol features. Additionally, we found among normal fasting glucose participants the early decline slope was significantly associated with HOMA-IR in the unadjusted, sociodemographic variables adjusted model and sociodemographic and waist circumference adjusted models with a 14.9% higher (95% CI: 2.2, 29.1), 15.0% higher (95% CI: 2.3, 29.3), and 10.7% higher (95% CI: 0.31, 22.3) HOMA-IR per 1 unit increase in cortisol feature (data not shown).
Table 3.
Percent change in HOMA-IR per 1 unit increase in the selected diurnal cortisol features*
| HOMA-IR (log scale) |
Model 0 % Change (95% CI) |
Model 1 % Change (95% CI) |
Model 2 % Change (95% CI) |
Model 3 % Change (95% CI) |
|---|---|---|---|---|
| Wake-up Cortisol | −9.4 (−14.5, −4.0) | −8.2 (−13.3, −2.7) | −3.0 (−7.68, 1.9) | −3.4 (−8.1, 1.6) |
| Cortisol Awakening Response | 0.0 (−6.7, 7.2) | 0.4 (−6.2, 7.5) | −0.8 (−6.4, 5.0) | −0.2 (−5.8, 5.8) |
| Early Decline Slope | 8.8 (−1.3, 19.8) | 9.1 (−0.8, 20.1) | 4.3 (−3.9, 13.1) | 4.0 (−4.2, 12.9) |
| Late Decline Slope | −7.1 (−53.9, 87.3) | −17.5 (−59.0, 66.0) | −20.4 (−55.9, 43.7) | −20.6 (−56.1, 43.6) |
| Overall Decline Slope | 39.7 (−39.0, 219.8) | 24.0 (−45.9, 184.4) | −8.9 (−54.9, 83.8) | −7.0 (−54.2, 88.8) |
| Bedtime Cortisol | −3.5 (−8.9, 2.2) | −2.6 (−8.1, 3.3) | −1.8 (−6.5, 3.2) | −1.9 (−6.7, 3.0) |
| Total AUC (16-hours) Cortisol | −9.8 (−16.4, −2.7) | −7.9 (−14.6, −0.6) | −4.2 (−10.2, 2.2) | −4.4 (−10.4, 2.0) |
Model 0: HbA1c association with cortisol feature (no additional adjustment; include interaction term of diabetes and cortisol feature).
Model 1: HbA1c association with cortisol feature (adjustment only for demographic variable; include interaction term of diabetes and cortisol feature).
Model 2: HbA1c association with cortisol feature (adjustment for demographic + waist circumference; include interaction term of diabetes and cortisol feature).
Model 3: HbA1c association with cortisol feature (adjustment for all variables; include interaction term of diabetes and cortisol feature).
the analysis only focused on non-diabetes participants and was based on log-transformed cortisol and HOMA-IR. The result of percent change by 1 unit change in the cortisol feature was derived by the exponentiation of the model coefficient estimates.
Statistically significant findings are in bold.
3. Discussion
The MESA Stress Study is one of only a few population-based cohort studies with well-characterized data on diurnal cortisol curve characteristics collected in a rigorous manner as well as measures of glycemia and a measure of insulin resistance. This enabled us to examine the association between subclinical hypercortisolism and hyperglycemia in all participants and insulin resistance, a precursor to DM, among non-diabetic individuals.
3.1 Association of HbA1c and Cortisol Curve Features
In this first report of the relation of the full diurnal cortisol curve and glycemia in DM, we found a positive association between glycemia and early decline slope, overall decline slope, bedtime cortisol, and total cortisol AUC in a multi-ethnic population. These findings indicate that an overall flattening of the cortisol curve and increased total daily cortisol exposure are associated with worsened glycemia in DM. This result corresponds with a prior cross-sectional analysis of glycemia in DM and cortisol in 190 participants, which showed a higher pre-lunch cortisol was associated with dose-dependent higher fasting blood glucose, urinary glucose, postprandial glucose and HbA1c (Oltmanns, 2006). Plausible explanations for our findings include, (1) the flattened cortisol profile with increased overall daily cortisol exposure leads to hyperglycemia in DM, (2) hyperglycemia causes HPA axis dysfunction and thus flattening of the cortisol profile or (3) a combined effect where hyperglycemia causes HPA axis dysfunction with flattening of the cortisol profile leading to further hyperglycemia in a cyclical pattern.
The first explanation is that elevated cortisol leads to hyperglycemia in DM. Clinical hypercortisolism can induce insulin resistance and lead to DM by promoting development of visceral adiposity, skeletal muscle insulin resistance, and activating lipolysis and free fatty acid release (Anagnostis et al., 2009). Previously, we analyzed the relationship between DM and diurnal cortisol curve features and found a blunted CAR which was statistically significant in men and slower early cortisol decline in men and women. Women had a higher total cortisol AUC with DM compared to women without DM and men with DM had a lower total cortisol AUC compared to men without DM(Champaneri et al., 2012). These results are consistent with two other studies showing a flattened diurnal cortisol rhythm in participants with DM (Hackett et al., 2014; Lederbogen et al., 2011), although neither study showed a blunted CAR, which was seen in our multi-ethnic analysis and in an analysis by Bruehl et al (Bruehl et al., 2009). However, the lack of association between the cortisol profile and HbA1c in non-diabetic and pre-diabetic individuals does not fully support this hypothesis and lends support to a second hypothesis that increased glycemia causes direct dysfunction of the HPA axis.
In a cross-sectional analysis of thirty DM participants and thirty matched controls, cortisol levels during dexamethasone suppression testing and corticotropin-releasing factor (CRF) stimulation testing were positively associated with HbA1c in DM participants (Bruehl et al., 2007). Bruehl et al, suggested that given that findings were independent of age, BMI, dyslipidemia and hypertension they were the result of a direct effect of hyperglycemia in DM on the HPA axis, possibly through damage to the hippocampus leading to alteration of the HPA axis (Bruehl et al., 2009). Supporting this finding is evidence from in-vitro analysis, revealing that hyperglycemia can activate the HPA axis through activation of the CRF promoter, activation of the CRF gene (Kageyama et al., 2012), and activation of pituitary proopiomelanocortin gene expression through free radical activation of transcription factors including NF-κB/AP1 (Asaba et al., 2007). There is also evidence that intracellular neuronal hyperglycemia leads to oxidative stress and advanced glycation end-products causing decreased plasticity and neuronal damage in a rodent model of depression and DM(Wang et al., 2009). These findings all support a role for the direct effect of hyperglycemia causing HPA axis dysfunction.
The third explanation is the possibility of a bi-directional effect of glycemia on the HPA axis and the HPA axis on glycemia. A rodent model of DM showed impaired hippocampus–dependent memory, synaptic plasticity and neurogenesis, which were reversed when cortisol levels were normalized with adrenalectomy and cortisol replacement (Stranahan et al., 2008), suggesting a dual role for hyperglycemia and cortisol in the pathogenesis of HPA axis dysfunction. (See Figure 2)
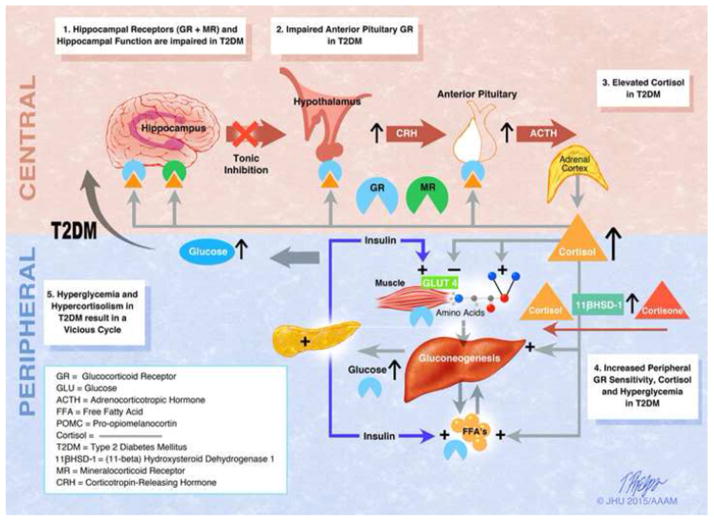
Figure 2. The Hypothetical Relationship Between Subclinical Hypercortisolism and Glycemia in T2DM.
(1)Hyperglycemia in diabetic mice leads to decreased hippocampal function due to astrogliosis and neuronal apoptosis (Stranahan et al., 2008). There is also evidence of decreased mineralocorticoid receptor stress responsivity in diabetic mice (Chan, 2005) and decreased glucocorticoid receptor content in sedentary mice (Campbell et al., 2010). These processes lead to hippocampal atrophy, which has been seen in humans (Bruehl et al., 2009) and a reduction in inhibition of the hypothalamus by the hippocampus.
(2)Human studies have shown decreased negative feedback via dexamethasone suppression testing in T2DM, suggesting abnormalities at the level of the anterior pituitary (Bruehl et al., 2009; Hudson et al., 1984). Glucocorticoid receptor dysfunction at the level of the anterior pituitary results in increased production of ACTH, which acts on the zona fasciculata of the adrenal gland.
(3)Human studies have shown elevated cortisol throughout the day in T2DM in women in a multi-ethnic study (Champaneri et al., 2012) and in both sexes in a study of Caucasians (Hackett et al., 2014).
(4)In the periphery, there is increased basal glucocorticoid receptor sensitivity in T2DM, which is associated with poorer glucose control (Carvalho et al., 2015) and a lack of stress induced modulation of glucocorticoid receptor sensitivity in diabetes mellitus (Rohleder et al., 2003). Peripheral cortisol synthesis from conversion of cortisone to cortisol is increased due to whole-body and liver cortisol regeneration by 11 beta-hydroxysteroid dehydrogenase type 1 in T2DM with obesity (Stimson et al., 2011). Blocking the glucocorticoid receptor with mifepristone or selective GR II antagonists specific to liver or adipose tissue, decrease glucose levels in T2DM (Beaudry et al., 2014; Watts et al., 2005). This data indicates that cortisol does have an effect of peripheral glycemia in T2DM.
(5)These processes individually and collectively may lead to worsening hyperglycemia and further blunting of the HPA axis, creating a vicious cycle.
3.2 Association of HOMA-IR and Cortisol Curve Features
A higher wake-up cortisol and total cortisol AUC were associated with lower insulin resistance as measured by the HOMA-IR in non-diabetic individuals. The lower HOMA-IR per 1 unit increase in cortisol was attenuated by waist circumference in the log-transformed analysis for both total cortisol AUC and wakeup cortisol. There was no association of the other diurnal cortisol curve features and HOMA-IR. We postulate that a higher wake-up cortisol contributing to a higher total cortisol AUC represents normal HPA axis plasticity in normoglycemic individuals without central adiposity. Previous studies have shown that a higher wake-up cortisol is indicative of improved HPA reactivity and lower odds of metabolic disturbances including central obesity (Duclos et al., 2005; Champaneri et al., 2013; Kumari et al., 2010), generalized obesity (Champaneri et al., 2013; Kumari et al., 2010), and metabolic syndrome (DeSantis et al., 2011). A higher wake-up cortisol is associated with higher educational level (Karlamangla et al., 2013) and White race (Cohen et al., 2006; Hajat et al., 2010; Karlamangla et al., 2013), whereas ethnic minority status is associated with a lower wake-up cortisol, possibly related to higher levels of discrimination (Zeiders et al., 2014). In regards to the elevated total cortisol AUC, a previous analysis in MESA found a similar association when examining metabolic syndrome and total cortisol AUC in non-DM participants, where a higher total cortisol AUC was associated with the lack of metabolic syndrome (DeSantis et al., 2011). Our data showed that the lower HOMA-IR was driven by the association of lower insulin levels with higher total cortisol AUC and wake-up cortisol, as there was no association of glucose levels with cortisol. In the normoglycemic state, cortisol inhibits insulin release (Delaunay et al., 1997; Ling et al., 1998), stimulates leptin (Newcomer et al., 1998; Wabitsch et al., 1996), and leptin also then further inhibits insulin release, while also blocking gluconeogenesis and glucagon and increasing peripheral insulin sensitivity (Park and Ahima, 2015). The association of lower HOMA-IR with higher wake-up cortisol and total cortisol AUC was fully blunted in a model including waist circumference, a correlate for visceral adiposity, a known mediator of insulin (Kahn et al., 2006) and leptin resistance (Sáinz et al., 2015). This suggests that as individuals gain weight/body fat through increased caloric intake and decreased physical expenditure, they may lose the beneficial effect of the interaction between cortisol and the adipoinsular axis and succumb to the detrimental effects of cortisol including insulin resistance, lipolysis and glycogenolysis leading to worsening hyperglycemia as seen in participants with DM in this study. This hypothesis is supported by a study of low dose hydrocortisone infusion for 3 hours to simulate a mild stress, in which abdominally obese participants developed markedly greater insulin resistance compared to normal BMI controls who did not develop insulin resistance (Darmon et al., 2006). In addition, there could also be an independent effect of elevated total cortisol AUC promoting abdominal adiposity and thus increasing insulin resistance and attenuating the association between higher wake-up/total cortisol AUC and improved insulin resistance as seen in clinical hypercortisolism. However, we feel this is unlikely in subclinical hypercortisolism given the prior cross-sectional analysis in MESA, which revealed an inverse association between wake-up and total cortisol AUC and BMI/waist circumference, especially in those with normal fasting glucose (Champaneri et al., 2013). This would suggest that higher wake-up and total cortisol AUC are associated with a lower BMI and waist circumference, which would not likely cause the attenuation in the association with wake-up and total cortisol AUC and HOMA-IR.
Our study has several strengths. First, MESA is a large population-based multi-ethnic cohort. Second, we used well-established criteria to determine DM status and did not rely on self-report. Third, salivary cortisol collection occurred over 2 days, allowing a more accurate determination of each participants diurnal cortisol pattern. Fourth, we were able to adjust for smoking, many medications, and other potential confounders using data collected in MESA. Finally, we had a large population of participants with normal glucose tolerance and impaired fasting glucose, allowing the assessment of glycemia in a non-diabetic population.
Our findings should be interpreted in light of some limitations. First, the cross sectional study design does not allow the assessment of the temporality of the association. Second, we were not able to assess DM duration, which may impact hormonal function in individuals with DM. Third, HbA1c assesses mean glycemia, but we were unable to assess post-prandial glycemia, which may also be associated with cortisol curve measures, given cortisol causes skeletal muscle insulin resistance, the major depot for post-prandial glucose. Finally, we were not able to assess leptin to test our hypothesis regarding insulin resistance in the non-diabetic participants.
In summary, this is the first study to examine the association of the full cortisol profile with glycemia in DM and insulin resistance in non-DM participants in a large multi-ethnic cohort. Glycemia is positively associated with the early decline slope, overall decline slope, bedtime cortisol, and higher total cortisol AUC. HOMA-IR is paradoxically inversely correlated with morning cortisol and total cortisol AUC and the association is attenuated following adjustment for waist circumference. We propose that in the normoglycemic and normal adiposity state, elevated wake-up and total cortisol AUC represent a healthier HPA axis with greater plasticity and that the cortisol-mediated effects leading to hyperglycemia vary by DM status and may be influenced by the adipoinsular axis through the actions of leptin and insulin. Recent studies have shown a direct link between adipokines and the HPA axis with leptin suppressing glucocorticoid secretion from the adrenal cortex by inhibiting CRF, pituitary ACTH release and direct inhibition at the adrenal gland (Roubos et al., 2012; Stieg et al., 2015). Adiponectin regulates glucocorticoids at the level of the hypothalamus and pituitary glands by increasing CRH and ACTH and decreasing cortisol secretion from the adrenal glands (Chen et al., 2014) Future studies should measure adipokines to allow further exploration of their role in the HPA axis, glucose metabolism and cardiometabolic disease.
Supplementary Material
Highlights.
We examined the association of the cortisol curve parameters with glycemia and insulin resistance.
In those with DM, cortisol curve parameters suggestive of higher HPA axis activity and dysfunction were associated with higher HbA1c.
In non-diabetic participants, greater HPA activity was associated with lower insulin resistance.
Acknowledgments
Grants: MESA was supported by contracts NO1-HC-95159 through NO1-HC-95165 and NO1- HC-95169 from the National Heart, Lung, and Blood Institute. MESA Stress Study was supported by RO1 HL10161-01A1 and R21 DA024273 (PI: Dr. Diez-Roux). Dr. Joshua J. Joseph MD was supported by an institutional training grant from the National Institute of Diabetes, Digestive, and Kidney Diseases (T32 DK062707). The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
Conflict of Interest
The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience–cortisol associations in a population-based sample of older adults. Proc Natl Acad Sci. 2006;103:17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2010;33:S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostis P, Athyros VG, Tziomalos K, Karagiannis A, Mikhailidis DP. The Pathogenetic Role of Cortisol in the Metabolic Syndrome: A Hypothesis. J Clin Endocrinol Metab. 2009;94:2692–2701. doi: 10.1210/jc.2009-0370. [DOI] [PubMed] [Google Scholar]
- Asaba K, Iwasaki Y, Asai M, Yoshida M, Nigawara T, Kambayashi M, Hashimoto K. High glucose activates pituitary proopiomelanocortin gene expression: possible role of free radical-sensitive transcription factors. Diabetes Metab Res Rev. 2007;23:317–323. doi: 10.1002/dmrr.677. [DOI] [PubMed] [Google Scholar]
- Badrick E, Kirschbaum C, Kumari M. The Relationship between Smoking Status and Cortisol Secretion. J Clin Endocrinol Metab. 2007;92:819–824. doi: 10.1210/jc.2006-2155. [DOI] [PubMed] [Google Scholar]
- Bild DE. Multi-Ethnic Study of Atherosclerosis: Objectives and Design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- Bruehl H, Rueger M, Dziobek I, Sweat V, Tirsi A, Javier E, Arentoft A, Wolf OT, Convit A. Hypothalamic-Pituitary-Adrenal Axis Dysregulation and Memory Impairments in Type 2 Diabetes. J Clin Endocrinol Metab. 2007;92:2439–2445. doi: 10.1210/jc.2006-2540. [DOI] [PubMed] [Google Scholar]
- Bruehl H, Wolf OT, Convit A. A blunted cortisol awakening response and hippocampal atrophy in type 2 diabetes mellitus. Psychoneuroendocrinology. 2009;34:815–821. doi: 10.1016/j.psyneuen.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champaneri S, Xu X, Carnethon MR, Bertoni AG, Seeman T, DeSantis AS, Roux AD, Shrager S, Golden SH. Diurnal salivary cortisol is associated with body mass index and waist circumference: The multiethnic study of atherosclerosis. Obesity. 2013;21:E56–E63. doi: 10.1002/oby.20047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champaneri S, Xu X, Carnethon MR, Bertoni AG, Seeman T, Roux AD, Golden SH. Diurnal salivary cortisol and urinary catecholamines are associated with diabetes mellitus: the Multi-Ethnic Study of Atherosclerosis. Metabolism. 2012;61:986–995. doi: 10.1016/j.metabol.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Wang Z, Zhan M, Liu R, Nie A, Wang J, Ning G, Ma Q. Adiponectin regulates ACTH secretion and the HPAA in an AMPK-dependent manner in pituitary corticotroph cells. Mol Cell Endocrinol. 2014;383:118–125. doi: 10.1016/j.mce.2013.12.007. [DOI] [PubMed] [Google Scholar]
- Chiodini I. Association of subclinical hypercortisolism with type 2 diabetes mellitus: a case-control study in hospitalized patients. Eur J Endocrinol. 2005;153:837–844. doi: 10.1530/eje.1.02045. [DOI] [PubMed] [Google Scholar]
- Clow A, Thorn L, Evans P, Hucklebridge F. The Awakening Cortisol Response: Methodological Issues and Significance. Stress Int J Biol Stress. 2004;7:29–37. doi: 10.1080/10253890410001667205. [DOI] [PubMed] [Google Scholar]
- Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T. Socioeconomic Status, Race, and Diurnal Cortisol Decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosom Med. 2006;68:41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- Darmon P, Dadoun F, Boullu-Ciocca S, Grino M, Alessi MC, Dutour A. Insulin resistance induced by hydrocortisone is increased in patients with abdominal obesity. AJP Endocrinol Metab. 2006;291:E995–E1002. doi: 10.1152/ajpendo.00654.2005. [DOI] [PubMed] [Google Scholar]
- Delaunay F, Khan A, Cintra A, Davani B, Ling ZC, Andersson A, Ostenson CG, Gustafsson J, Efendic S, Okret S. Pancreatic beta cells are important targets for the diabetogenic effects of glucocorticoids. J Clin Invest. 1997;100:2094–2098. doi: 10.1172/JCI119743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis AS, DiezRoux AV, Hajat A, Golden SH, Jenny NS, Sanchez BN, Shea S, Seeman TE. Associations of Salivary Cortisol Levels with Metabolic Syndrome and Its Components: The Multi-Ethnic Study of Atherosclerosis. J Clin Endocrinol Metab. 2011;96:3483–3492. doi: 10.1210/jc.2011-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclos M, Pereira PM, Barat P, Gatta B, Roger P. Increased cortisol bioavailability, abdominal obesity, and the metabolic syndrome in obese women. Obes Res. 2005;13:1157–1166. doi: 10.1038/oby.2005.137. [DOI] [PubMed] [Google Scholar]
- Godoy-Matos AF, Vieira AR, Moreira RO, Coutinho WF, Carraro LM, Moreira DM, Pasquali R, Meirelles RMR. The potential role of increased adrenal volume in the pathophysiology of obesity-related type 2 diabetes. J Endocrinol Invest. 2006;29:159–163. doi: 10.1007/BF03344090. [DOI] [PubMed] [Google Scholar]
- Golden SH, Sánchez BN, DeSantis AS, Wu M, Castro C, Seeman TE, Tadros S, Shrager S, Diez Roux AV. Salivary cortisol protocol adherence and reliability by socio-demographic features: The Multi-Ethnic Study of Atherosclerosis. Psychoneuroendocrinology. 2014;43:30–40. doi: 10.1016/j.psyneuen.2014.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett RA, Steptoe A, Kumari M. Association of diurnal patterns in salivary cortisol with type 2 diabetes in the Whitehall II study. J Clin Endocrinol Metab. 2014;99:4625–4631. doi: 10.1210/jc.2014-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajat A, Diez-Roux A, Franklin TG, Seeman T, Shrager S, Ranjit N, Castro C, Watson K, Sanchez B, Kirschbaum C. Socioeconomic and race/ethnic differences in daily salivary cortisol profiles: The Multi-Ethnic Study of Atherosclerosis. Psychoneuroendocrinology. 2010;35:932–943. doi: 10.1016/j.psyneuen.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen ÅM, Garde AH, Persson R. Sources of biological and methodological variation in salivary cortisol and their impact on measurement among healthy adults: A review. Scand J Clin Lab Invest. 2008;68:448–458. doi: 10.1080/00365510701819127. [DOI] [PubMed] [Google Scholar]
- Kageyama K, Yamagata S, Akimoto K, Sugiyama A, Murasawa S, Suda T. Action of glucagon-like peptide 1 and glucose levels on corticotropin-releasing factor and vasopressin gene expression in rat hypothalamic 4B cells. Mol Cell Endocrinol. 2012;362:221–226. doi: 10.1016/j.mce.2012.06.023. [DOI] [PubMed] [Google Scholar]
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- Karlamangla AS, Friedman EM, Seeman TE, Stawksi RS, Almeida DM. Daytime trajectories of cortisol: Demographic and socioeconomic differences—Findings from the National Study of Daily Experiences. Psychoneuroendocrinology. 2013;38:2585–2597. doi: 10.1016/j.psyneuen.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari M, Chandola T, Brunner E, Kivimaki M. A Nonlinear Relationship of Generalized and Central Obesity with Diurnal Cortisol Secretion in the Whitehall II Study. J Clin Endocrinol Metab. 2010;95:4415–4423. doi: 10.1210/jc.2009-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederbogen F, Hummel J, Fademrecht C, Krumm B, Kühner C, Deuschle M, Ladwig KH, Meisinger C, Wichmann HE, Lutz H, Breivogel B. Flattened Circadian Cortisol Rhythm in Type 2 Diabetes. Exp Clin Endocrinol Amp Diabetes. 2011;119:573–575. doi: 10.1055/s-0031-1275288. [DOI] [PubMed] [Google Scholar]
- Ling ZC, Khan A, Delauny F, Davani B, Östenson CG, Gustafsson J-\AA, Okret S, Landau BR, Efendic S. Increased glucocorticoid sensitivity in islet beta-cells: effects on glucose 6-phosphatase, glucose cycling and insulin release. Diabetologia. 1998;41:634–639. doi: 10.1007/s001250050961. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Newcomer JW, Selke G, Melson AK, Gross J, Vogler GP, Dagogo-Jack S. Dose-Dependent Cortisol-Induced Increases in Plasma Leptin Concentration in Healthy Humans. Arch Gen Psychiatry. 1998;55:995. doi: 10.1001/archpsyc.55.11.995. [DOI] [PubMed] [Google Scholar]
- Oltmanns KM. Cortisol correlates with metabolic disturbances in a population study of type 2 diabetic patients. Eur J Endocrinol. 2006;154:325–331. doi: 10.1530/eje.1.02074. [DOI] [PubMed] [Google Scholar]
- Park HK, Ahima RS. Physiology of leptin: energy homeostasis, neuroendocrine function and metabolism. Metabolism. 2015;64:24–34. doi: 10.1016/j.metabol.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl Psychol Meas. 1977;1:385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Ranjit N, Young EA, Raghunathan TE, Kaplan GA. Modeling cortisol rhythms in a population-based study. Psychoneuroendocrinology. 2005;30:615–624. doi: 10.1016/j.psyneuen.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Rosmond R. Stress induced disturbances of the HPA axis: a pathway to Type 2 diabetes. Med Sci Monit. 2003;9:35–39. [PubMed] [Google Scholar]
- Roubos EW, Dahmen M, Kozicz T, Xu L. Leptin and the hypothalamo-pituitary–adrenal stress axis. Gen Comp Endocrinol. 2012;177:28–36. doi: 10.1016/j.ygcen.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Sáinz N, Barrenetxe J, Moreno-Aliaga MJ, Martínez JA. Leptin resistance and diet-induced obesity: central and peripheral actions of leptin. Metabolism. 2015;64:35–46. doi: 10.1016/j.metabol.2014.10.015. [DOI] [PubMed] [Google Scholar]
- Stetler C, Miller GE. Depression and Hypothalamic-Pituitary-Adrenal Activation: A Quantitative Summary of Four Decades of Research. Psychosom Med. 2011;73:114–126. doi: 10.1097/PSY.0b013e31820ad12b. [DOI] [PubMed] [Google Scholar]
- Stieg MR, Sievers C, Farr O, Stalla GK, Mantzoros CS. Leptin: A hormone linking activation of neuroendocrine axes with neuropathology. Psychoneuroendocrinology. 2015;51:47–57. doi: 10.1016/j.psyneuen.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat Neurosci. 2008;11:309–317. doi: 10.1038/nn2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wabitsch M, Jensen PB, Blum WF, Christoffersen CT, Englaro P, Heinze E, Rascher W, Teller W, Tornqvist H, Hauner H. Insulin and cortisol promote leptin production in cultured human fat cells. Diabetes. 1996;45:1435–1438. doi: 10.2337/diab.45.10.1435. [DOI] [PubMed] [Google Scholar]
- Wang S-h, Sun Z-l, Guo Y-j, Yuan Y, Yang B-q. Diabetes Impairs Hippocampal Function via Advanced Glycation End Product Mediated New Neuron Generation in Animals with Diabetes-Related Depression. Toxicol Sci. 2009;111:72–79. doi: 10.1093/toxsci/kfp126. [DOI] [PubMed] [Google Scholar]
- Wang X, Sánchez BN, Golden SH, Shrager S, Kirschbaum C, Karlamangla AS, Seeman TE, Diez Roux AV. Stability and predictors of change in salivary cortisol measures over six years: MESA. Psychoneuroendocrinology. 2014;49:310–320. doi: 10.1016/j.psyneuen.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward A, Fall CH, Stein CE, Kumaran K, Veena SR, Wood PJ, Syddall HE, Phillips DI. Cortisol and the metabolic syndrome in South Asians. Clin Endocrinol (Oxf) 2003;58:500–505. doi: 10.1046/j.1365-2265.2003.01750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh KC, Kwan KC. A comparison of numerical integrating algorithms by trapezoidal, Lagrange, and spline approximation. J Pharmacokinet Biopharm. 1978;6:79–98. doi: 10.1007/BF01066064. [DOI] [PubMed] [Google Scholar]
- Zeiders KH, Hoyt LT, Adam EK. Associations between self-reported discrimination and diurnal cortisol rhythms among young adults: The moderating role of racial–ethnic minority status. Psychoneuroendocrinology. 2014;50:280–288. doi: 10.1016/j.psyneuen.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.