Abstract
Astroglia are a major cellular constituent of the central nervous system (CNS) and play crucial roles in brain development, function and integrity. Increasing evidence demonstrates that astroglia dysfunction occurs in a variety of neurological disorders ranging from CNS injuries to genetic diseases and chronic degenerative conditions. These new insights herald the concept that transplantation of astroglia could be of therapeutic value in treating the injured or diseased CNS. Recent technological advances in the generation of human astroglia from stem and progenitor cells have been prominent. We propose that a better understanding of the suitability of astroglial cells in transplantation, as well as of their therapeutic effects in animal models may lead to the establishment of astroglia-based therapies to treat neurological diseases.
1. Establishment of astroglia-based cell therapy
Cell therapies for injured or diseased CNS have promising clinical applications. To date, the types of cells used for transplantation have ranged from neural progenitor cells (NPCs) that still have a degree of developmental plasticity, to more restricted neural cells, including various populations of neurons and oligodendroglia [88]. Astrocytes, also called astroglia, are the most numerous cells in the mammalian CNS. More and more, astrocyte dysfunction has been known to play an important role in a wide range of neurological disorders, suggesting that astroglia transplantation could be of therapeutic value in treating the injured or diseased human CNS. Owing to the broad and diverse roles of astroglia in CNS function, astroglia-based therapy may possess many advantages. Transplanted astroglia may not only replace diseased astrocytes, but also regulate neuronal functions [2, 13, 42, 76, 99], oligodendroglia differentiation and myelination [4, 64], homeostasis of the CNS [21, 67, 71, 87, 91] and blood brain barrier maintenance [34, 41, 50]. However, compared to neuron and oligodendroglia-based cell therapies, developing astroglia-based therapy for neurological disorders is far less studied. This is likely because most of CNS injuries are associated with astrocyte reactivation [22, 75], raising concerns that astroglia transplanted into the injured niche might also become reactive, with astrocytes that contribute to glial scars exhibiting detrimental effects, such as inhibiting axonal regeneration. In addition, early transplantation studies using astroglia cultured from rodent fetal or neonatal tissue showed variable results, or only modest benefits [8, 33, 47, 62, 102].
Along with the recent progress in studying astroglial functions in development and disease, the successful derivation of subtype-specific astroglia from human stem and progenitor cells, astroglia-based cell therapy has emerged as a promising therapeutic strategy to treat CNS injury and disease. Recent studies have changed our view of the effect of reactive astrocytes in pathology, by demonstrating that in the acute phase of CNS injury, reactive astrocytes can recapitulate numerous processes involved in early development of immature astroglia, exhibiting neuroprotective and regenerative effects [35, 75, 95]. It appears that these reactivated processes often go awry later, turning endogenous astrocytes into reactive astrocytes. Cumulative studies [29, 39, 60, 68, 92, 94] have also demonstrated that transplanted immature astrocytes do not become reactive after CNS injury. Rather, transplanted immature – but not mature – astrocytes support neurite outgrowth and reduce glial scar formation in the injured CNS. Moreover, recent reports [15–17, 45, 68] suggest that not all subtypes of astroglia are equivalent in their ability to promote neural repair. Therefore, the potential of developing astroglia-based therapy is vast; if we better understand the generation of optimal types of astroglia for transplantation, as well as the mechanisms by which transplanted astroglia exert their therapeutic effects.
The purpose of this review is to summarize the generation of astroglia from embryonic glial progenitor cells and pluripotent stem cells and the possible mechanisms underlying the therapeutic benefit of transplanted stem/progenitor-derived astroglia in a variety of experimental animal models of neurological disorders. Lessons learned from the achievements of astroglial transplantation and constructive suggestions to improve astroglia-based therapy will be offered. With the ultimate goal of further propelling astroglia-based therapy into a translational path, this review will also discuss several crucial issues that remain to be addressed in the field.
2. Generation of astroglia from stem and progenitor cells
Over 30 years ago, the methods for isolating and culturing astrocytes from neonatal rodent CNS were established [63]. However, astrocytes maintained in vitro have limited proliferation capacity when cultured even for relatively short periods of time as they soon expressed inhibitory properties, such as inhibiting axon outgrowth, seen in glial scar tissue [93]. Various groups have focused on deriving astroglia from stem and progenitor cells to obtain astroglia that can be expanded in large quantities and that are suitable for cell transplantation. Table 1 summarizes the marker expression and functional analyses of astroglia differentiated from rodent and human stem and progenitor cells.
Table 1.
Generation of astroglia from rodent and human stem and progenitor cells.
| Reference | Cellular source |
Inducing factor(s) | Protein expression of astroglial markers and neurotrophic/neuroprot ective factors |
Functional Analyses |
|---|---|---|---|---|
|
Davie s et al., 2006; Davies et al 2008 [15, 16] |
Rat fetal GRPs |
BMP4 | GFAP | Promoted axon outgrowth and locomotor recovery in a rat model of spinal cord transection injury (See Table 2). |
| CNTF | GFAP, A2B5, NG2, phosphacan, Olig2 |
Unable to promote recovery in a rat model of spinal cord transection injury. |
||
|
Davie s et al., 2011; Proschel et al., 2014 [17, 79] |
Human fetal glial progenitor cells (hGPCs) |
BMP4 | GFAP, AQP4, S100β,CX43, EAAT-2, AKAP12, GDNF, BDNF, neurturin, IGF1, GCLC, GPx, Glutathione |
Promoted recovery in a rat model of spinal cord transection injury; provided multiple benefits in a rat model of Parkinson’s disease (See Table 2). |
| CNTF | GFAP, AQP4, S100β, OLIG2, CSPGs, CSPG4/NG2, ROS. |
Unable to promote recovery in a rat model of spinal cord transection injury. |
||
|
Kamn asaran et al., 2008; Kuegler et al., 2012[49] [56] |
mESCs | Fetal Bovine serum (FBS) |
GFAP, CD44, Vimentin, S100β, AQP4, GS, Nestin, Musashi-1 |
Reacted to inflammatory cytokines by producing inflammatory mediators such as, IL-6 and nitric oxide; supported neuronal growth. |
|
Krenc ik et al., 2011; Krencik and Zhang, 2011 [55] [54] |
hESCs hiPSCs |
CNTF LIF |
GFAP, S100β, CD44, ALDH1L1, EAAT-1, EAAT-2, NF1A,NF1X, CHL1, AQP4, Regional identity markers (HOXB4, NKX2.1, OTX2) |
Expressed functional voltage-gated potassium channels and glutamate receptors; presented glutamate uptake; propagated calcium wave; supported neuronal growth; promoted synaptogenesis; participated in blood-brain barrier structure formation after engrafted into normal mouse brain. |
|
Jiang et al., 2013 [45] |
Olig2-GFP hESCs |
BMP4 | GFAP, S1 00β, Vimentin, CD44, OTX2, EAAT-1 BDNF, GDNF, NT3, TSPs, GPCs, APOE, APP, GCLC, NFE2L2 |
Expressed functional voltage-gated potassium and sodium channels; presented glutamate uptake; attenuated oxidative neuronal injury; supported neuronal growth; promoted synaptogenesis; promoted recovery in a rat model of brain ischemic injury (See Table 2). |
|
Gupta et al., 2011[38] |
hESCs | BMP4 BMP2 LIF |
GFAP, S100β, Nestin, AQP4, EAAT-1 GCLC, NFE2L2, Glutathione |
Presented glutamate uptake; attenuated oxidative neuronal injury. |
|
Shalt ouki et al., 2013 [89] |
hESCs hiPSCs hGPCs |
CNTF BMP2 |
GFAP, S 100β, CD44, AQP4, ALDH1L1, NF1A, NF1X, TMOD, EAAT-1 |
Presented glutamate uptake; promoted synaptogenesis; expressed GFAP after engrafted into the striatium of normal adult mouse. |
|
Royb on et al., 2013[84] |
mESCs | FBS FGF1 or FGF2 |
Immature mESC-derived astroglia expressed: GFAP, S100β, AQP4, Vimentin, NF1A Mature mESC- derived astroglia further expressed: GLT1, GLAST, aldolase C, CX43, ALDH1L1. |
Presented glutamate uptake. |
| hESCs hiPSC |
CNTF FGF1 or FGF2 FBS |
Immature hPSC- derived astroglia expressed: GFAP, S100β, CD44, Vimentin, NF1A, CX43, aldolase C, EAAT2, BDNF, GDNF. Mature hPSC- derived astroglia showed decreased expression of GFAP, NF1A and Cx43 and increased expression of EAAT1. |
Presented glutamate uptake; supported neuronal growth; Responded to TNFα to become reactive astrocytes; Expressed GFAP and NF1A after engrafted into the striatium of normal adult rats. |
2.1 Generation of astroglia from fetal-derived glial progenitor cells
Astroglia suitable for transplantation were first differentiated from rat and human glial-restricted precursor/progenitor cells (GRPs) that were purified from the developing brain or spinal cord tissue by the cell-surface antigen A2B5 [19, 81, 86]. In an early study from Davies et al [15], rat GRPs were differentiated into astroglia by exposure to bone morphogenetic protein 4 (BMP4), a member of transforming growth factor-β (TGFβ) superfamily implicated in astroglia specification [36, 37]. GRP-derived astrocytes (GDAs) were characterized by expressing astroglial markers GFAP and FGF receptor 3, but not expressing A2B5 or oligodendroglial markers NG2 and proteolipid protein (plp)/DM20. Furthermore, transplantation of these purified GDAs into an adult rat model of spinal cord transection injury, significantly promoted axon regeneration and restored locomotor function, while transplantation of undifferentiated GRPs did not, indicating the potential of a novel astroglia-based cell therapy for neural repair after CNS injury.
Later studies from the same research groups identified that both rat [16] and human GRPs[17] could be differentiated into astroglia by exposure to either BMP4 or ciliary neurotrophic factor (CNTF), a member of the Interleukin-6 family that has been reported to induce astrogliogenesis both in vitro and in vivo [6, 44, 80]. As a result, two types of astroglia - GDAsBMP and GDAsCNTF- were generated. Both of them expressed astroglial markers including GFAP, AQP4, and S100β. However, these two types of astrocytes differed in expression of multiple genes thought to either promote or inhibit CNS homeostasis and regeneration, such as genes encoding connexin 43 (Cx43), glutamate transporter 1 (GLT-1), A-kinase anchoring protein-12 (AKAP12), and glia derived neurotrophic factor (GDNF).
2.2 Generation of astroglia from pluripotent stem cells
2.2.1 Generation of astroglia from murine embryonic stem cells
Pluripotent stem cells (PSCs), including embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), are capable of self-renewal and differentiation to all cell types in the body, including neural cells. Thus, PSCs provide an alternative and possibly better source of unlimited transplantable astroglia for developing cell therapies. In early studies [9, 30, 65, 108], astroglia were often generated as a byproduct, yielding a low percentage during directed neuronal or oligodendroglial differentiation of ESCs. Another study [49] reported the direct differentiation of astroglia from murine ESCs (mESCs) with high efficiency (>90%). The differentiation process comprised three sequential stages: an embryoid body (EB) stage, a glial progenitor cell stage, and an astroglia differentiation stage. A different group [56] developed a two-stage protocol to differentiate mESCs into astroglia. The first neural induction stage originated from a suspension of single cells in culture that developed cell aggregates. In the second astroglial differentiation stage, cell aggregates were plated to develop highly enriched astrocyte cultures. In both differentiation protocols, fetal bovine serum (FBS) was used to induce astroglial differentiation from mESC-derived neural or glial progenitor cells. These astrocytes were identified using astroglia markers, including GFAP, Vimentin, S100β, gluatamine synthetase (GS), and AQP4, and expressed astrocyte-associated receptors such as glutamate, serotonin, opiate, GABA, and adrenocortical receptors. Very few of the astrocytes expressed glial progenitor marker A2B5 and none of them expressed neuronal markers MAP2 and βIII-tubulin, or oligodendroglial markers CNPase and O4. Of note, comparative transcriptome profile analysis [49] demonstrated that these mESC-derived astroglia were markedly similar to those isolated from the cortex of embryonic and neonatal mice, which are enriched with astroglial progenitors or immature astroglia. In addition, the mESC-derived astroglia were able to self-renew (> 100 passages), expressed several stem cell markers, and exhibited differentiation plasticity. These are features of progenitor cells rather than fully differentiated astrocytes. Therefore, these results suggest that ESC-derived astroglia possess astroglia progenitor or immature astroglia cell-like characteristics.
2.2.2 Generation of astroglia from human pluripotent stem cells
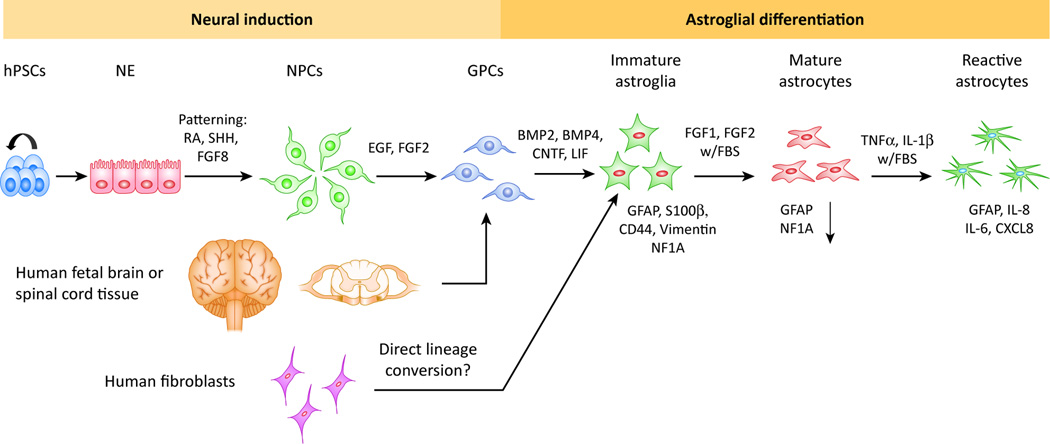
Human astrocytes possess unique complex hominid features that differentiate human brains from rodent brains [40, 69]. In general, longer and more complex procedures are required to derive astroglia from human pluripotent stem cells (hPSCs)[53]. As shown in Fig. 1, a schematic diagram summarizes the generation of human astroglia. The first study that differentiated hESCs into GFAP+ astroglia used a protocol involving the inhibition of sonic hedgehog (SHH) signaling at the neural induction stage [57]. Subsequently, the cells were induced to become astroglia lineage cells by culturing them in commercial human astrocyte medium. However, the components of this medium were not clearly defined and the function of the hESC-derived astroglia was not well characterized [57]. The first comprehensive chemically-defined protocol for differentiating hPSCs into astroglia was reported by Dr. Su-Chun Zhang’s laboratory [54, 55]. Their protocol contained three major stages: induction of neuroepithelial cells, generation of astroglial progenitors and immature astroglia, and maturation into astrocytes by maintaining astrospheres, floating cell aggregates composed of astroglial cells. Astroglia generated with this protocol were characterized by expressing astroglial markers GFAP, S100β, CD44, NF1A, ALDH1L1, and GLT-1. These astroglia exhibited functional properties such as glutamate uptake and promotion of neuronal synaptogenesis. One caveat of this method is that it necessitates 6 months of culture to generate a sufficiently pure population of astroglia. In order to circumvent this problem, subsequent studies have developed protocols yielding high purity astroglial cultures from hPSCs within 35 to 80 days by adding differentiation inducers, BMPs or CNTF, to NPCs cultured as dissociated cells at very early stages [24, 38, 45, 84, 89].
Figure 1. Generation of human astroglia.
Schematic diagram summarizing the generation of human astroglia with immature, mature and reactive phenotypes from hPSCs, human fetal tissue and potentially human fibroblasts via direct lineage conversion.
Subtypes of astroglia have been recognized based their marked phenotypic heterogeneity [109]. Different subtypes of astroglia have also been generated from hPSCs. Krencik et al. reported that in the first stage of induction of neuroepithelial cells, the addition of morphogens used for neuronal subtype specification such as retinoic acid (RA), SHH, and fibroblast growth factor 8 (FGF8) could pattern the neuroepithelial cells and subsequently generated subtypes of astroglia with different regional identities [54]. Astroglia derived from neuroepithelial cells treated with RA, SHH, or FGF8 expressed the hindbrain/spinal cord marker HOXB4, the mid-forebrain marker OTX2, and the ventral identity marker NKX2.1, respectively. In addition, different subtypes of astroglia can be derived from two subtypes of hESC-derived NPCs [45]. Human ESCs could be differentiated into OLIG2 positive and OLIG2 negative NPCs, which further gave rise to astroglia [45, 61, 103]. Despite similar marker expression, astroglia differentiated from OLIG2 positive and OLIG2 negative NPCs displayed distinct characteristics, including morphology, growth rate, gene expression profile, and electrophysiological properties[45, 61, 103].
As discussed earlier, previous studies have suggested that mESC-derived astroglia mimic astroglial progenitors or immature astroglia derived from the mouse brain [49, 56]. Generally, neural cells differentiated from hPSCs are also reflective of cells at very early human brain development [73, 74]. Particularly, hPSC-derived astroglia generated using chemically-defined serum-free protocols are immature astrocytes. Promoting maturation of hPSC-derived astroglia requires additional procedures. For instance, transplantation of hPSC-derived astroglia into the mouse brain could promote their maturation, as indicated by their participation in blood-brain barrier structure formation [54]. Moreover, a recent study [84] reported that treatment with FBS followed by FGF1 or FGF2 could induce hPSC-derived immature astroglia to exhibit a mature phenotype, characterized by increased glutamate transport activity and decreased expression of GFAP and NF1A. In contrast, treatment with tumor necrosis factor alpha or interleukin-1β (IL-1β) triggered the reactivity of hPSC-derived astroglia, as indicated by the expression of high levels of cytokines and chemokines such as IL-6, IL-8, and CXCL8 [84].
Human iPSCs (hiPSCs) are generated by reprogramming human somatic cells through forced expression of defined transcription factors [96, 106]. Human iPSCs are nearly indistinguishable from hESCs in their differentiation potential. Using identical protocols for hESCs, hiPSCs can also be differentiated into human astroglia. In the context of developing astroglial cell regenerative medicine, the use of hiPSCs circumvents the ethical and moral concerns of using hESCs because the generation of hiPSCs does not involve blastocyst destruction and oocyte donation. In addition, iPSC technology offers the possibility of generating patient-specific stem cell lines, thus providing a renewable source of autologous astroglia for transplantation [18]. Disease-specific hiPSCs can also be generated from patients with specific CNS diseases, providing new tools to study disease mechanisms and drug-screening with a therapeutic purpose. Astroglia differentiated from patients with amyotrophic lateral sclerosis (ALS) have been established as a new in vitro model to study the non-cell-autonomous effects of ALS astrocytes[12, 20, 23, 83]. Astroglia differentiated from Huntington’s disease-specific iPSCs has recapitulated features of Huntington’s disease patient cells, providing a novel in vitro disease model [48]. By differentiating Down’s syndrome hiPSCs into astroglia, a recent study revealed a novel pathogenic role of Down’s syndrome astrocytes and showed that abnormal phenotypes of the Down’s syndrome astrocytes can be corrected with a clinically available antibiotic drug, minocycline [11]. Another study [52] found that hiPSCs derived from patients with Costello syndrome, a developmental disorder with intellectual disability caused by mutant Harvey rat sarcoma viral oncogene homolog, showed accelerated development to astroglia compared to wild-type cell lines, as indicated by the higher percentage of cells labeled by astroglial markers S100β, CD44, and GFAP at different time points during differentiation. Moreover, the diseased astroglia dysregulated cortical maturation, by producing excessive extracellular matrix remodeling factors and proteoglycans. Thus, differentiation of astroglia from disease-specific hiPSCs provides new tools for modeling neurological diseases as well as providing new platforms for investigating therapeutics.
3. Application of stem and progenitor cell-derived astroglia in experimental animal models of neurological diseases
Astroglia respond to CNS injury by undergoing a process of reactive astrogliosis. However, there is growing appreciation that under appropriate conditions, astroglial cells, particularly those differentiated from PSCs and fetal tissue-derived progenitors, are also capable of promoting neuroprotection and repair. Table 2 summarizes the transplantation of astroglia using animal models of spinal cord injury and neurological disorders.
Table 2.
Transplantation of astroglia using animal models of spinal cord injury and neurological disorders.
| Disease | Animal model | Cell type(s) | Delivery method |
Key effects | Functional outcome | Reference |
|---|---|---|---|---|---|---|
| Spi nal cord injury |
Female athymic rats undergoing contusion lesion |
hGR Ps and hGDAs |
Intrath ecal injection |
Decreased glial scarring; decreased volume of fluid-filled cyst; No change in axon outgrowth. |
No significant improvements in motor function; Improved sensory function, and pain perception. |
Jin et al, 2011[46] |
| Spi nal cord injury |
Female rats undergoing unilateral spinal transection injury |
Froz en hGRPs, hGRPs, hGDABMP, and hGDACNTF |
Intrath ecal injection |
Continued expression of GFAP, nestin, and Ki67. Support of axonal growth into graft area. |
N/A | Haa s et al, 2013[39] |
| Spi nal cord injury |
Female rats undergoing unilateral spinal transection injury |
Hum an or rat GRPs, and human or rat hGDABMP and hGDACNTF |
Intrath ecal injection |
Increased neuronal survival; promoted axon outgrowth (GDAsBMP). |
Improved locomotion (GDAsBMP). |
Davi es et al, 2006; Davies et al, 2008; Davi es et al, 2011; [15– 17] |
| Spi nal cord injury |
Female rats undergoing cervical hemicontusion lesion |
GDA s derived from BAC- GLT1-eGFP mice infected with GLT1- expressing AAV8 vector |
Intrath ecal injection |
Decreased lesion size |
Improved diaphragm function |
Li et al, 2015[59] |
| Spi nal cord injury |
Adult rats undergoing contusion lesion |
Rat GDAs infected with D15A- expressing retroviral vector |
Intrath ecal injection |
Promoted axonal regeneration; Increased white matter sparing; decreased lesion size. |
Improved locomotion |
Fan et al, 2013[26] |
| Am yotrophic lateral sclerosis |
SOD1G9 3A rats |
Rat GRPs |
Intrath ecal injection |
Increased survival of cervical motor neurons |
Delayed decline in diaphragm function andmotor performance; increased survival of SOD1 transgenic rats |
Lep ore et al, 2008; [58] |
| Am yotrophic lateral sclerosis |
SOD1G9 3A mice |
hiPS C-derived glial-rich neural progenitor cells |
Intrath ecal injection |
Increased expression of neurotrophic factors |
Increased survival of SOD1 mutant mice |
Kon do et al, 2014[51] |
| Par kinson’s disease |
Male rats undergoing 6-OHDA injection into striatum |
Hum an or rat GDABMP |
Intrast riatal injection |
Increased neuronal cell survival in vitro (rat GDABMP); Rescued parvalbumin+ GABAergic neurons; increased synaptic density (hGDABMP). |
Reduced motor deficits |
Pros chel et al, 2014[79] |
| Par kinson’s disease |
Male rats undergoing 6-OHDA injection into striatum |
Rat astrocytes transfected with BDNF- expressing retrovirus |
Intrast riatal injection |
Increased survival of TH+ cells in the ventral tegmental area |
Prevented amphetamine-induced rotational behavior, a measure of neurobehavioral function due to striatal dopamine agonist depletion in Parkinson animal models. |
Yos himoto et al, 1995[105] |
| Par kinson’s disease |
SJL X C57 F1 mice undergoing 6- OHDA injection into striatum |
Mous e astrocytes transfected with GDNF- expressing retrovirus |
Intra- substantia nigral injection |
Protected nigral dopaminergic neurons; prevented dopamine depletion within the substantia nigra. |
Prevented amphetamine-induced rotational behavior |
Cun ningham et al, 2001[14] |
| Par kinson’s disease |
Female rats undergoing 6-OHDA injection into striatum |
Rat astrocytes transfected with GDNF- expressing lentivirus |
Intrast riatal or intra- substantia nigral injection |
Increased TH+ cell survival. |
Improved amphetamine-induced rotational behavior |
Eric son et al, 2005[25] |
| Isch emic stroke |
Male rats undergoing four-vessel occlusion |
Astro glia differentiated from hESC- derived Olig2+ and Olig2− NPCs |
Intrace rebro- ventricular injection |
Increased neuronal survival and reactive synaptogenesis in the hippocampus; decreased MBP disruption. |
Improved learning and memory (Olig2PC-derived astroglia). |
Jian g et al, 2013[45] |
| Alzh eimer’s disease |
Transge nic APdE9 mice |
Mous e astrocytes |
Intra- hippocampal injection |
Decreased Aβ burden; internalized deposited Aβ |
N/A | Pihl aja et al, 2008[77] |
3.1 Spinal cord injury
Traumatic spinal cord injury results in significant motor and sensory neurologic deficits. Astroglia transplantation has shown promise as a therapeutic modality for spinal cord injury. Previous studies showed that grafted hGDAs or hGRPs, which differentiated predominantly into astroglia after transplantation, suppressed glial scar formation in rat models of spinal cord injury [17, 39, 46]. Another sequelae from spinal cord injury in the animal model was the formation of fluid-filled cysts. Decreased cyst volume at the injury site was noted in both hGRP- and hGDA-treated animals [46]. In addition, injured rats treated with hGRPs and hGDAs showed improved neuronal survival and axon outgrowth at the site of injury when compared to untreated controls [17, 39, 46]. Despite these positive effects at a cellular level, discrepancies in behavioral recovery after cell transplantation have been reported. Studies from the Fisher group [39, 46] showed that transplantation of either hGRPs or hGDAs was not able to significantly promote motor function recovery. In contrast, the Davies group demonstrated that transplantation of a specific population of GDABMP supported locomotor recovery in a rat spinal cord transection injury model, when compared to transplantation of GDAsCNTF or undifferentiated hGRPs [17]. These findings indicate that the choice of appropriate astroglia subtypes for transplantation is crucial for achieving optimal therapeutic effects. A deeper understanding of the heterogeneity and maturation of progenitor cell-derived human astroglia may help resolve some of these discrepancies.
Genetically engineered GDAs have also demonstrated an ability to mitigate injury. Rat GDAs overexpressing GLT-1, which acts to clear glutamate and attenuates excitotoxicity within the injured spinal cord, were shown to reduce lesion size and to improve diaphragm function after transplantation[59]. Injured animals transplanted with rat GDAs producing D15A, a neurotrophin with both neurotrophin-3 and Brain-derived neurotrophic factor (BDNF) activities, demonstrated improved locomotor function and an increased percentage of spared white matter, -- quantified by iron-eriochrome cyanine R staining at the injury site to identify myelinated white matter [26]. These results suggest a role for GDAs as vehicles, promoting transgenic-induced therapeutic changes in the potential treatment of spinal cord injury.
3.2 Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS) involves the degeneration of upper and lower motor neurons, resulting in progressive functional motor disability and death from respiratory failure. Exogenous astroglia transplantation has shown neuroprotective promise. In a transgenic human mutant SOD1 rat model of ALS, animals that received transplantation of GRPs, which efficiently differentiated into astrocytes in the animal model, displayed a delayed onset of disease symptoms, a diminished rate of motor performance decline, and increased survival time of the SOD1 transgenic rats [58]. A decreased rate of decline in peak response amplitudes of phrenic nerve compound muscle action potentials, (a measure of diaphragm function), was also observed in the GRP transplantation group. Additionally, an increased number of cervical motor neurons was observed after GRP transplantation, suggesting a neuroprotective effect [58]. Human iPSC-derived glial-rich neural progenitors may be another potential source for therapeutic astroglia transplantation. These cells have been shown to differentiate into astrocytes, upregulate expression of neurotrophic factors, and increase survival time in SOD1 mutant mice after transplantation [51]. Thus, astroglial transplantation may hold promise as a therapeutic option for ALS, a disease that currently has limited treatment options.
3.3 Parkinson’s disease
Parkinson’s disease (PD) is a disorder characterized by the degeneration of dopaminergic neurons in the substantia nigra and a deficiency in tyrosine hydroxylase (TH), a catalyst for the production of L-DOPA. Early studies demonstrated that transplantation of genetically engineered primary rodent astrocytes provided neuroprotection in animal models of PD [105]. After transplantation of virally transduced BDNF-producing rat astrocytes, 6-hydroxydopamine (6-OHDA)-lesioned rats showed improved survival of TH+ cells in the ventral tegmental area and improvement in amphetamine-induced rotational behavior, a measure of neurobehavioral function due to striatal dopamine agonist depletion in PD murine models[105]. Grafted primary astrocytes overexpressing GDNF into 6-OHDA-lesioned rodents also increased GDNF expression in the striatum and substantia nigra [14, 25]. The exogenous GDNF-expressing astrocytes offered neuroprotection by increasing the survival of TH+ cells in the substantia nigra [14, 25]. Moreover, the GDNF-producing astrocytes prevented the acquisition of amphetamine-induced rotational behavior in 6-OHDA-treated rodents and prevented dopamine depletion within the substantia nigra[14, 25]. The promise of transplanted hGDAs as a neuroprotective agent was reported in a study using 6-OHDA hemi-parkinsonian rat model, where hGDAsBMP transplantation into the striatum restored TH expression, rescued parvalbumin+ GABAergic neurons, increased synaptic density, and reduced functional motor deficits[79]. These findings highlight the potential for astroglial transplantation to slow or prevent neurodegeneration resulting from PD.
3.4 Brain ischemic stroke
Brain ischemic stroke is a common cause of morbidity and mortality resulting from the obstruction of an arterial supply to the brain. Only one study thus far has examined the therapeutic potential of transplanted astroglia in a rat model of global cerebral ischemia. Two different subtypes of human astroglia were differentiated from Olig2 negative and Olig2 positive hESC-derived NPCs. Both types of astroglia were observed to increase synapsin-1 expression, an indicator of reactive synaptogenesis, in the hippocampus of injured animals after transplantation [45]. Injured animals were also shown to have increase neuronal survival and decreased disruption of MBP staining after transplantation with either group, suggesting that astrocyte transplantation may be neuroprotective and may mitigate myelination injury in the animal stroke model [45]. Functionally, transplantation of astroglia differentiated from Olig2+ NPCs significantly improved learning and memory in injured animals, as demonstrated by improved performance in the Morris water maze neurobehavioral test in the astroglial transplantation group [45]. Functions of endogenous astrocytes change in response to stroke, both beneficially and detrimentally [32]. Thus, further understanding of astroglial function and influence on neuron survival during stroke is necessary to improve astroglial transplantation strategies for treating brain ischemic stroke injury.
3.5 Alzheimer’s disease
Alzheimer’s disease is the most common cause of dementia. Extracellular beta amyloid (Aβ) peptides are deposited, resulting in progressive neurodegeneration characterized by loss of memory and executive functions. So far, only one study has investigated astroglia transplantation as a therapy for Alzheimer’s disease. Ex vivo experiments have revealed that mouse astrocytes can reduce human Aβ burden when co-cultured with frontal cortical brain sections from a patient with Alzheimer’s disease [77]. Furthermore, transplantation of mouse astrocytes into a transgenic mouse model of Alzheimer’s disease demonstrated migration of the transplanted astrocytes to Aβ deposits and internalization of Aβ by the transplanted cells [77]. These results supported the role of astrocytes as active Aβ clearing cells in the CNS, suggesting that transplantation of astroglia may be a potential therapy for Alzheimer’s disease.
3.6 Clinical trials
A search of clinicaltrials.gov revealed only one clinical trial investigating the use of human astroglia-based transplantation therapy at the time of this writing. The proposed study is a phase 1/2 trial investigating the safety of hGRPs in patients with ALS (NCT02478450). Grants from California Institute for Regenerative Medicine (DR1-01471) and U.S. Army Medical Research and Materiel Command (W81XWH-10-1-0520) proposed to study the preclinical efficacy of transplantation of human astroglia differentiated from human fetal-derived or iPSC-derived GRPs in ALS. The therapeutic potential of astroglia transplantation for the treatment of neurodegenerative diseases in animal models is evident. The combined current lack of effective therapy and the relative paucity of translational studies and clinical trials at this time highlight the need for further experimental studies to validate the clinical use of human astroglia transplantation to mitigate the devastating morbidity and mortality resulting from neurological diseases and CNS injuries.
3.7 Mechanisms by which stem and progenitor cell-derived astroglia provide therapeutic benefits
Astrocytes play essential roles, both structurally and physiologically, in the normal CNS. Consistently, transplanted astroglia also exert therapeutic benefits through multifaceted mechanisms, as individually discussed below.
(i) Neurotrophic and neuroprotective functions of astroglia
Astrocytes regulate the development and differentiation of neurons by secreting neurotrophic factors [7, 78], and facilitate the formation of functional synapses by producing modulators of synaptogenesis [1, 2, 76]. Stem and progenitor cell-derived astroglia cells abundantly express the neurotrophic factors GDNF, BDNF, and insulin-like growth factor (IGF), which contribute to the effects of transplanted astroglia on enhancing survival of neurons after CNS injury [15–17, 45, 51]. These astroglia also produce molecules that regulate synaptogenesis, such as thrombospondin and glypicans, which significantly promote the formation of new synapses in animal models of CNS injuries [39, 45, 46] and Parkinson’s disease [79]. Astrocytes exhibit neuroprotective effects by supporting neuronal antioxidant defenses and protecting neurons from oxidative stress in acute neuronal damage or chronic neurodegeneration [100]. A recent study [38] demonstrated that astroglia derived from hESCs promoted protective effects on hESC-derived neurons against oxidative damage through the activation of the transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) and release of glutathione. These antioxidant effects also contributed to the neuroprotection of transplanted astroglia in animal models of CNS injury [45] and neurodegenerative disease [79]. In addition, the expression of receptors, channels, and proton shuttling on the astroglial membranes could confer crucial roles in maintaining the pH, fluid and ion homeostasis of the synaptic interstitial fluid [28, 42, 71, 91, 99]. GDABMP expressed high levels of such astrocyte-related genes as AQP4, connexin 43, and AKAP12, which were relevant for tissue homeostasis maintenance after injury [15–17]. Moreover, one of the critical functions of astrocytes is to remove glutamate released from neurons during pathological conditions [71, 87]. Previous studies showed that the astrocytic transporter GLT-1 is down-regulated in response to stroke [82] and astrocytes in the hippocampal CA1 region lost glutamate transport activity within a few hours after forebrain ischemic injury, contributing to delayed neuronal damage [72]. Stem and progenitor cell-derived astroglia express glutamate transporters and are capable of glutamate uptake [45, 54, 58, 84, 89]. Transplanted astroglia also promoted neuronal survival via glutamate uptake thereby attenuating excitotoxicity after injury [17, 58].
(ii) Astroglial regulation of vascular remodeling
It is significant that pluripotent stem cell-derived astroglia highly express vascular endothelial growth factor (VEGF) [45, 51] because previous studies have shown that VEGF secreted by astroglia that differentiated from transplanted NPCs is important for vascular repair and neovascularization in the peri-infarct region in rodent models of brain stroke injury [43, 70]. Additionally, VEGF is also implicated as a mediator of human NPC-induced effects on dendritic sprouting, axonal plasticity, and axonal transport [3].
(iii) Immune regulatory function, anti-inflammatory role, and modulation of microglial function by astroglia
Astrocytes and their released mediators modulate both innate and adaptive immune reactions [21, 27]. Astrocytes are functionally involved in immune suppression. Astrocytes are the main cell type responsible for the production of immunosuppressive factors, such as TGFβ, in the CNS [27] and activation of astroglial dopamine D2 receptor suppresses neuroinflammation [90]. Recent studies also identified that the astroglia-secreted VEGF was important for the suppression of inflammation in animals that received transplantation of NPCs after brain stroke injury [43]. In addition, astroglia differentiated from transplanted GRPs in an animal model of ALS were also reported to reduce microgliosis [58].
(iv) Astroglial regulation of oligodendroglia differentiation and myelination
Astrocytes play important roles in supporting oligodendroglia development and maintaining CNS myelin [4, 64]. Previous studies have demonstrated that oligodendrocytes preferentially remyelinate axons in areas containing astrocytes in the demyelinating lesions in the rat spinal cord [5, 31, 97]. Transplanted hPSC-derived astroglia were also able to preserve myelin integrity in a rat model of global cerebral ischemic injury [45].
Of note, studies [15–17, 45, 92, 94] have reported that transplanted stem and progenitor cell-derived immature astroglia can suppress the activation of endogenous astrocytes and glial scar formation. However, the molecular mechanisms underlying these effects are largely unknown and remain to be investigated.
4. Concluding Remarks
Despite promising data indicating the clinical potential of stem/progenitor cell-derived astroglia for treatment of neurological diseases, several questions and challenges remain to be addressed, while this therapy proceeds along its translational path. To achieve an optimal outcome of astroglia-based therapy, it is imperative to derive a homogeneous population of astroglia at a defined immature stage from human stem and progenitor cells. Currently, there are no single markers available that reliably distinguish human immature and mature astroglia [84, 107]. Hence, there is a pressing need to identify markers, particularly cell surface markers that can be used to isolate developmentally synchronized human stem/progenitor cell-derived immature astroglia for cell transplantation. Moreover, it is crucial to better understand the heterogeneity of human astroglia and to determine whether specific subtypes of astroglia can be of better therapeutic use in different disease conditions. In combination with omics technologies such as transcriptomics, proteomics, and secretomics, hPSC-derived astroglia represent a solid opportunity to investigate the differential expression of biological markers and functional changes of human astroglia at specific immature and mature stages, the subtypes of human astroglia with different regional identities, and the subtypes of human astroglia derived from a different combination of inducing factors.
There is an emerging technique to directly convert somatic cells to other types of cells by ectopic expression of appropriate transcription factors[101]. Previous studies have reported direct lineage conversion of somatic cells, such as fibroblasts, to induce neural stem cells (iNSCs)[85], oligodendrocyte progenitor cells (iOPCs) [66, 104], or various neuron subtypes (iN)[98], by brief expression of specific transcription factors. This direct cell reprogramming technology, in which fibroblasts could also be potentially converted into astroglia, may provide a new alternative source of transplantable human astroglia. However, at this time, only one recent study [10] reported the success of generating induced astroglia, or iAstrocytes, from mouse fibroblasts. In the future, it will be interesting to examine how efficiently it is possible to generate functional human iAstrocytes (Fig. 1), and characterize their maturity and heterogeneity.
In acute CNS injuries, such as brain ischemic injury and spinal cord injury, intracranial or intrathecal cell transplantation is unlikely to be feasible within the therapeutic window of hours after insult, since patients may not arrive at a treatment facility within the therapeutic window, or may be too clinically unstable to tolerate such an invasive procedure. Astroglia-secreted soluble factors largely mediate the therapeutic benefits from transplanted astroglia in animal models. Thus, it would be interesting to examine whether the direct application of total concentrated factors collected from human immature astrocyte-conditioned medium via less invasive drug delivery methods, (e.g. intravenous or intranasal) could also have therapeutic effects. The translational potential of astroglia-based cell-free therapy could be promoted after a better understanding of the secretome of human progenitor and stem cell-derived astroglia. Moreover, CNS injuries and neurological diseases are often associated with damages to both neurons and glia. Delayed transplantation of neurons and oligodendroglia has been tested for decades. However, uncontrolled axon outgrowth from transplanted neurons and the lack of robustness of remyelination from transplanted oligodendroglia are still unresolved problems [88]. Therefore, transplantation of NPCs, neurons, or oligodendroglia, together with pre-differentiated immature astroglia might be worth exploring, as these could be capable of shaping axon outgrowth and accelerating remyelination.
OUTSTANDING QUESTIONS.
Why do immature - but not mature - astroglia not become reactive astrocytes after transplantation into diseased or injured CNS?
What are the molecular mechanisms by which transplanted immature astroglia reduce glial scar formation from endogenous astrocytes in diseased or injured CNS?
Are there single markers (particularly cell surface markers) that can discriminate human astroglia at immature and mature stages, subtypes of human astroglia with different regional identities, or subtypes of human astroglia derived from stem and progenitor cells?
How do human subtypes of astroglia, or astroglia at different developmental stages differ from each other in transcriptome, proteome and secretome characteristics? How are they functionally different?
In order to achieve optimal therapeutic effects, are specific subtypes of astroglia required for transplantation to treat different neurological disorders? For example, does treating brain and spinal cord injuries require the transplantation of astroglia with different spatial regional identities?
Can functional human iAstrocytes be generated from terminally differentiated somatic cells by direct reprogramming? How heterogeneous will the generated human iAstrocytes be? Will they be suitable for developing cell therapies?
Are concentrated factors collected from human immature astrocyte-conditioned media effective in treating neurological disorders when delivered via a clinically relevant, non-invasive drug delivery method (e.g. intravenous or intranasal)? Can a more translational “astroglia-based cell-free therapy” be applied?
When transplanted together with NPCs, neurons or oligodendria, are astroglia capable of shaping axon outgrowth and accelerating/boosting remyelination? Could “combination cell-based therapy” be an advantageous treatment strategy?
TRENDS BOX.
Human astroglia are efficiently differentiated from human fetal-tissue derived progenitor cells and human pluripotent stem cells.
Transplanted stem and progenitor cell-derived astroglia exert therapeutic benefits in a variety of animal models of neurological disorders.
Transplanted astroglia promote neural repair and regeneration via multifaceted mechanisms including replacing diseased astrocytes, assisting oligodendria differentiation and myelination, regulating neuronal functions, and maintaining blood brain barrier/CNS homeostasis.
Astroglia-based cell therapy has emerged as a promising therapeutic strategy to treat CNS injury and neurological disease.
Glossary
- Alzheimer’s disease
A disorder involving the deposition of extracellular beta amyloid (Aβ) peptides, resulting in progressive loss of memory and executive functions
- Amyotrophic lateral sclerosis (ALS)
A disorder involving the degeneration of upper and lower motor neurons, resulting in progressive functional motor disability and eventual death
- Astrocytes or astroglia
Star-shaped CNS cells with functions including: synapse formation and removal, support of synaptic transmission, homeostatic regulation of the CNS, and blood brain barrier formation and maintenance
- Astrogliosis
An increase in the number of astrocytes resulting from CNS injury
- Brain ischemic stroke
The decrease of arterial blood supply to the brain leading to CNS cell death, resulting in neurologic deficits in the region of ischemia
- Central nervous system (CNS)
The division of the nervous system that includes the brain and spinal cord
- Embryonic stem cells (ESC)
Pluripotent cells derived from the inner cell mass of a blastocyst, able to self-renew and differentiate into multiple cell lineages
- Glial-derived astrocytes (GDA)
Astrocytes derived from glial-restricted progenitor cells (GRP)
- Glial-restricted precursor/progenitor cells (GRP)
Self-renewing cells that differentiate into cells of the glial lineage
- Glial scar
A cellular formation in response to CNS injury involving an increase in number and morphological alteration of glial cells, as the CNS attempts to protect and heal itself
- Induced pluripotent stem cells (iPSC)
Pluripotent cells generated from differentiated adult cells that are able to self-renew and differentiate into multiple cell lineages
- Microgliosis
An increase in number of microglia at the site of CNS injury
- Neural progenitor cells (NPCs)
Self-renewing cells that differentiate into cells that constitute the CNS
- Parkinson’s disease
A disorder involving the degeneration of dopaminergic neurons in the substantia nigra, resulting in motor dysfunction and dementia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allen NJ, Barres BA. Neuroscience: Glia - more than just brain glue. Nature. 2009;457:675–677. doi: 10.1038/457675a. [DOI] [PubMed] [Google Scholar]
- 2.Allen NJ, et al. Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature. 2012;486:410–414. doi: 10.1038/nature11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andres RH, et al. Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain. Brain : a journal of neurology. 2011;134:1777–1789. doi: 10.1093/brain/awr094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnett SC, Linington C. Myelination: do astrocytes play a role? The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2013;19:442–450. doi: 10.1177/1073858412465655. [DOI] [PubMed] [Google Scholar]
- 5.Blakemore WF, Crang AJ. The relationship between type-1 astrocytes, Schwann cells and oligodendrocytes following transplantation of glial cell cultures into demyelinating lesions in the adult rat spinal cord. Journal of neurocytology. 1989;18:519–528. doi: 10.1007/BF01474547. [DOI] [PubMed] [Google Scholar]
- 6.Bonni A, et al. Regulation of gliogenesis in the central nervous system by the JAK-STAT signaling pathway. Science. 1997;278:477–483. doi: 10.1126/science.278.5337.477. [DOI] [PubMed] [Google Scholar]
- 7.Bozoyan L, et al. Astrocytes control the development of the migration-promoting vasculature scaffold in the postnatal brain via VEGF signaling. J Neurosci. 2012;32:1687–1704. doi: 10.1523/JNEUROSCI.5531-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradbury EJ, et al. Astrocyte transplants alleviate lesion induced memory deficits independently of cholinergic recovery. Neuroscience. 1995;65:955–972. doi: 10.1016/0306-4522(94)00540-l. [DOI] [PubMed] [Google Scholar]
- 9.Brüstle O, et al. Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science. 1999;285:754–756. doi: 10.1126/science.285.5428.754. [DOI] [PubMed] [Google Scholar]
- 10.Caiazzo M, et al. Direct conversion of fibroblasts into functional astrocytes by defined transcription factors. Stem cell reports. 2015;4:25–36. doi: 10.1016/j.stemcr.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen C, et al. Role of astroglia in Down's syndrome revealed by patient-derived human-induced pluripotent stem cells. Nat Commun. 2014;5:4430. doi: 10.1038/ncomms5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen H, et al. Modeling ALS with iPSCs reveals that mutant SOD1 misregulates neurofilament balance in motor neurons. Cell stem cell. 2014;14:796–809. doi: 10.1016/j.stem.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung WS, et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 2013;504:394–400. doi: 10.1038/nature12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cunningham LA, Su C. Astrocyte delivery of glial cell line-derived neurotrophic factor in a mouse model of Parkinson's disease. Experimental neurology. 2002;174:230–242. doi: 10.1006/exnr.2002.7877. [DOI] [PubMed] [Google Scholar]
- 15.Davies JE, et al. Astrocytes derived from glial-restricted precursors promote spinal cord repair. J Biol. 2006;5:7. doi: 10.1186/jbiol35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies JE, et al. Transplanted astrocytes derived from BMP- or CNTF-treated glial-restricted precursors have opposite effects on recovery and allodynia after spinal cord injury. J Biol. 2008;7:24. doi: 10.1186/jbiol85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davies SJ, et al. Transplantation of specific human astrocytes promotes functional recovery after spinal cord injury. PLoS One. 2011;6:e17328. doi: 10.1371/journal.pone.0017328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng W. Induced pluripotent stem cells: paths to new medicines. A catalyst for disease modelling, drug discovery and regenerative therapy. EMBO Rep. 2010;11:161–165. doi: 10.1038/embor.2010.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dietrich J, et al. Characterization of A2B5+ glial precursor cells from cryopreserved human fetal brain progenitor cells. Glia. 2002;40:65–77. doi: 10.1002/glia.10116. [DOI] [PubMed] [Google Scholar]
- 20.Dimos JT, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 21.Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–190. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- 22.Eddleston M, Mucke L. Molecular profile of reactive astrocytes--implications for their role in neurologic disease. Neuroscience. 1993;54:15–36. doi: 10.1016/0306-4522(93)90380-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Egawa N, et al. Drug screening for ALS using patient-specific induced pluripotent stem cells. Sci Transl Med. 2012;4:145ra104. doi: 10.1126/scitranslmed.3004052. [DOI] [PubMed] [Google Scholar]
- 24.Emdad L, et al. Efficient differentiation of human embryonic and induced pluripotent stem cells into functional astrocytes. Stem Cells Dev. 2012;21:404–410. doi: 10.1089/scd.2010.0560. [DOI] [PubMed] [Google Scholar]
- 25.Ericson C, et al. Ex vivo gene delivery of GDNF using primary astrocytes transduced with a lentiviral vector provides neuroprotection in a rat model of Parkinson's disease. Eur J Neurosci. 2005;22:2755–2764. doi: 10.1111/j.1460-9568.2005.04503.x. [DOI] [PubMed] [Google Scholar]
- 26.Fan C, et al. Transplantation of D15A–expressing glial-restricted-precursor-derived astrocytes improves anatomical and locomotor recovery after spinal cord injury. Int J Biol Sci. 2013;9:78–93. doi: 10.7150/ijbs.5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farina C, et al. Astrocytes are active players in cerebral innate immunity. Trends in immunology. 2007;28:138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Fiacco TA, McCarthy KD. Astrocyte calcium elevations: properties, propagation, and effects on brain signaling. Glia. 2006;54:676–690. doi: 10.1002/glia.20396. [DOI] [PubMed] [Google Scholar]
- 29.Filous AR, et al. Immature astrocytes promote CNS axonal regeneration when combined with chondroitinase ABC. Dev Neurobiol. 2010;70:826–841. doi: 10.1002/dneu.20820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fraichard A, et al. In vitro differentiation of embryonic stem cells into glial cells and functional neurons. Journal of cell science. 1995;108:3181–3188. doi: 10.1242/jcs.108.10.3181. [DOI] [PubMed] [Google Scholar]
- 31.Franklin RJ, et al. Transplanted type-1 astrocytes facilitate repair of demyelinating lesions by host oligodendrocytes in adult rat spinal cord. Journal of neurocytology. 1991;20:420–430. doi: 10.1007/BF01355538. [DOI] [PubMed] [Google Scholar]
- 32.Gleichman AJ, Carmichael ST. Astrocytic therapies for neuronal repair in stroke. Neurosci Lett. 2014;565:47–52. doi: 10.1016/j.neulet.2013.10.055. [DOI] [PubMed] [Google Scholar]
- 33.Goldberg WJ, Bernstein JJ. Migration of cultured fetal spinal cord astrocytes into adult host cervical cord and medulla following transplantation into thoracic spinal cord. Journal of neuroscience research. 1988;19:34–42. doi: 10.1002/jnr.490190106. [DOI] [PubMed] [Google Scholar]
- 34.Gordon GR, et al. Astrocyte control of the cerebrovasculature. Glia. 2007;55:1214–1221. doi: 10.1002/glia.20543. [DOI] [PubMed] [Google Scholar]
- 35.Gotz M, et al. Reactive astrocytes as neural stem or progenitor cells: In vivo lineage, In vitro potential, and Genome-wide expression analysis. Glia. 2015;63:1452–1468. doi: 10.1002/glia.22850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gregori N, et al. The tripotential glial-restricted precursor (GRP) cell and glial development in the spinal cord: generation of bipotential oligodendrocyte-type-2 astrocyte progenitor cells and dorsal-ventral differences in GRP cell function. J Neurosci. 2002;22:248–256. doi: 10.1523/JNEUROSCI.22-01-00248.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gross RE, et al. Bone morphogenetic proteins promote astroglial lineage commitment by mammalian subventricular zone progenitor cells. Neuron. 1996;17:595–606. doi: 10.1016/s0896-6273(00)80193-2. [DOI] [PubMed] [Google Scholar]
- 38.Gupta K, et al. Human embryonic stem cell derived astrocytes mediate non-cell-autonomous neuroprotection through endogenous and drug-induced mechanisms. Cell Death Differ. 2011;19:779–787. doi: 10.1038/cdd.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haas C, Fischer I. Human astrocytes derived from glial restricted progenitors support regeneration of the injured spinal cord. Journal of neurotrauma. 2013;30:1035–1052. doi: 10.1089/neu.2013.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han X, et al. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell stem cell. 2013;12:342–353. doi: 10.1016/j.stem.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haseloff RF, et al. In search of the astrocytic factor(s) modulating blood-brain barrier functions in brain capillary endothelial cells in vitro. Cell Mol Neurobiol. 2005;25:25–39. doi: 10.1007/s10571-004-1375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiological reviews. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- 43.Horie N, et al. Transplanted stem cell-secreted vascular endothelial growth factor effects poststroke recovery, inflammation, and vascular repair. Stem cells. 2011;29:274–285. doi: 10.1002/stem.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hughes SM, et al. Ciliary neurotrophic factor induces type-2 astrocyte differentiation in culture. Nature. 1988;335:70–73. doi: 10.1038/335070a0. [DOI] [PubMed] [Google Scholar]
- 45.Jiang P, et al. hESC-derived Olig2+ progenitors generate a subtype of astroglia with protective effects against ischaemic brain injury. Nat Commun. 2013;4:2196. doi: 10.1038/ncomms3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jin Y, et al. Transplantation of human glial restricted progenitors and derived astrocytes into a contusion model of spinal cord injury. Journal of neurotrauma. 2011;28:579–594. doi: 10.1089/neu.2010.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joosten EA, et al. Collagen containing neonatal astrocytes stimulates regrowth of injured fibers and promotes modest locomotor recovery after spinal cord injury. Journal of neuroscience research. 2004;77:127–142. doi: 10.1002/jnr.20088. [DOI] [PubMed] [Google Scholar]
- 48.Juopperi TA, et al. Astrocytes generated from patient induced pluripotent stem cells recapitulate features of Huntington's disease patient cells. Molecular brain. 2012;5:17. doi: 10.1186/1756-6606-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamnasaran D, et al. Characterization and transformation potential of "Synthetic" astrocytes differentiated from murine embryonic stem cells. Glia. 2008;56:457–470. doi: 10.1002/glia.20631. [DOI] [PubMed] [Google Scholar]
- 50.Koehler RC, et al. Role of astrocytes in cerebrovascular regulation. J Appl Physiol (1985) 2006;100:307–317. doi: 10.1152/japplphysiol.00938.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kondo T, et al. Focal transplantation of human iPSC-derived glial-rich neural progenitors improves lifespan of ALS mice. Stem cell reports. 2014;3:242–249. doi: 10.1016/j.stemcr.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krencik R, et al. Dysregulation of astrocyte extracellular signaling in Costello syndrome. Sci Transl Med. 2015;7:286ra266. doi: 10.1126/scitranslmed.aaa5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krencik R, Ullian EM. A cellular star atlas: using astrocytes from human pluripotent stem cells for disease studies. Front Cell Neurosci. 2013;7:25. doi: 10.3389/fncel.2013.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krencik R, et al. Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nat Biotechnol. 2011;29:528–534. doi: 10.1038/nbt.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krencik R, Zhang SC. Directed differentiation of functional astroglial subtypes from human pluripotent stem cells. Nature protocols. 2011;6:1710–1717. doi: 10.1038/nprot.2011.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuegler PB, et al. GFAP-independent inflammatory competence and trophic functions of astrocytes generated from murine embryonic stem cells. Glia. 2012;60:218–228. doi: 10.1002/glia.21257. [DOI] [PubMed] [Google Scholar]
- 57.Lee DS, et al. Cyclopamine treatment of human embryonic stem cells followed by culture in human astrocyte medium promotes differentiation into nestin- and GFAP-expressing astrocytic lineage. Life sciences. 2006;80:154–159. doi: 10.1016/j.lfs.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 58.Lepore AC, et al. Focal transplantation-based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nature neuroscience. 2008;11:1294–1301. doi: 10.1038/nn.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li K, et al. Transplantation of glial progenitors that overexpress glutamate transporter GLT1 preserves diaphragm function following cervical SCI. Mol Ther. 2015;23:533–548. doi: 10.1038/mt.2014.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li K, et al. Human iPS cell-derived astrocyte transplants preserve respiratory function after spinal cord injury. Experimental neurology. 2015;271:479–492. doi: 10.1016/j.expneurol.2015.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Y, et al. OLIG gene targeting in human pluripotent stem cells for motor neuron and oligodendrocyte differentiation. Nature protocols. 2011;6:640–655. doi: 10.1038/nprot.2011.310. [DOI] [PubMed] [Google Scholar]
- 62.Lu SY, et al. Effect of fetal striatal and astrocyte transplants into unilateral excitotoxin-lesioned striatum. J Neural Transplant Plast. 1993;4:279–287. doi: 10.1155/NP.1993.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. The Journal of cell biology. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moore CS, et al. How factors secreted from astrocytes impact myelin repair. Journal of neuroscience research. 2011;89:13–21. doi: 10.1002/jnr.22482. [DOI] [PubMed] [Google Scholar]
- 65.Mujtaba T, Rao MS. Embryonic Stem Cells. Springer; 2002. Isolation of lineage-restricted neural precursors from cultured ES cells; pp. 189–204. [DOI] [PubMed] [Google Scholar]
- 66.Najm FJ, et al. Transcription factor-mediated reprogramming of fibroblasts to expandable, myelinogenic oligodendrocyte progenitor cells. Nat Biotechnol. 2013;31:426–433. doi: 10.1038/nbt.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nicoll JA, Weller RO. A new role for astrocytes: beta-amyloid homeostasis and degradation. Trends Mol Med. 2003;9:281–282. doi: 10.1016/s1471-4914(03)00109-6. [DOI] [PubMed] [Google Scholar]
- 68.Noble M, et al. Precursor cell biology and the development of astrocyte transplantation therapies: lessons from spinal cord injury. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2011;8:677–693. doi: 10.1007/s13311-011-0071-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oberheim NA, et al. Uniquely hominid features of adult human astrocytes. J Neurosci. 2009;29:3276–3287. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oki K, et al. Human-induced pluripotent stem cells form functional neurons and improve recovery after grafting in stroke-damaged brain. Stem cells. 2012;30:1120–1133. doi: 10.1002/stem.1104. [DOI] [PubMed] [Google Scholar]
- 71.Oliet SH, et al. Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science. 2001;292:923–926. doi: 10.1126/science.1059162. [DOI] [PubMed] [Google Scholar]
- 72.Ouyang YB, et al. Selective dysfunction of hippocampal CA1 astrocytes contributes to delayed neuronal damage after transient forebrain ischemia. J Neurosci. 2007;27:4253–4260. doi: 10.1523/JNEUROSCI.0211-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pasca AM, et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat Methods. 2015;12:671–678. doi: 10.1038/nmeth.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Patterson M, et al. Defining the nature of human pluripotent stem cell progeny. Cell research. 2012;22:178–193. doi: 10.1038/cr.2011.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pekny M, Pekna M. Astrocyte Reactivity and Reactive Astrogliosis: Costs and Benefits. Physiological reviews. 2014;94:1077–1098. doi: 10.1152/physrev.00041.2013. [DOI] [PubMed] [Google Scholar]
- 76.Pfrieger FW. Role of glial cells in the formation and maintenance of synapses. Brain research reviews. 2010;63:39–46. doi: 10.1016/j.brainresrev.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 77.Pihlaja R, et al. Transplanted astrocytes internalize deposited beta-amyloid peptides in a transgenic mouse model of Alzheimer's disease. Glia. 2008;56:154–163. doi: 10.1002/glia.20599. [DOI] [PubMed] [Google Scholar]
- 78.Powell EM, Geller HM. Dissection of astrocyte-mediated cues in neuronal guidance and process extension. Glia. 1999;26:73–83. doi: 10.1002/(sici)1098-1136(199903)26:1<73::aid-glia8>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 79.Proschel C, et al. Delayed transplantation of precursor cell-derived astrocytes provides multiple benefits in a rat model of Parkinsons. EMBO molecular medicine. 2014 doi: 10.1002/emmm.201302878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rajan P, McKay RD. Multiple routes to astrocytic differentiation in the CNS. J Neurosci. 1998;18:3620–3629. doi: 10.1523/JNEUROSCI.18-10-03620.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rao MS, et al. A tripotential glial precursor cell is present in the developing spinal cord. Proc Natl Acad Sci U S A. 1998;95:3996–4001. doi: 10.1073/pnas.95.7.3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rao VL, et al. Transient focal cerebral ischemia down-regulates glutamate transporters GLT-1 and EAAC1 expression in rat brain. Neurochemical research. 2001;26:497–502. doi: 10.1023/a:1010956711295. [DOI] [PubMed] [Google Scholar]
- 83.Richard JP, Maragakis NJ. Induced pluripotent stem cells from ALS patients for disease modeling. Brain Res. 2015;1607:15–25. doi: 10.1016/j.brainres.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roybon L, et al. Human stem cell-derived spinal cord astrocytes with defined mature or reactive phenotypes. Cell reports. 2013;4:1035–1048. doi: 10.1016/j.celrep.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ruggieri M, et al. Induced neural stem cells: methods of reprogramming and potential therapeutic applications. Progress in neurobiology. 2014;114:15–24. doi: 10.1016/j.pneurobio.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 86.Sandrock RW, et al. Isolation, characterization and preclinical development of human glial-restricted progenitor cells for treatment of neurological disorders. Regen Med. 2010;5:381–394. doi: 10.2217/rme.10.24. [DOI] [PubMed] [Google Scholar]
- 87.Sattler R, Rothstein JD. Regulation and dysregulation of glutamate transporters. Handb Exp Pharmacol. 2006:277–303. doi: 10.1007/3-540-29784-7_14. [DOI] [PubMed] [Google Scholar]
- 88.Selvaraj V, et al. Differentiating human stem cells into neurons and glial cells for neural repair. Front Biosci (Landmark Ed) 2012;17:65–89. doi: 10.2741/3916. [DOI] [PubMed] [Google Scholar]
- 89.Shaltouki A, et al. Efficient Generation of Astrocytes From Human Pluripotent Stem Cells in Defined Conditions. Stem cells. 2013 doi: 10.1002/stem.1334. [DOI] [PubMed] [Google Scholar]
- 90.Shao W, et al. Suppression of neuroinflammation by astrocytic dopamine D2 receptors via alphaB-crystallin. Nature. 2013;494:90–94. doi: 10.1038/nature11748. [DOI] [PubMed] [Google Scholar]
- 91.Simard M, Nedergaard M. The neurobiology of glia in the context of water and ion homeostasis. Neuroscience. 2004;129:877–896. doi: 10.1016/j.neuroscience.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 92.Smith GM, Miller RH. Immature type-1 astrocytes suppress glial scar formation, are motile and interact with blood vessels. Brain Res. 1991;543:111–122. doi: 10.1016/0006-8993(91)91054-5. [DOI] [PubMed] [Google Scholar]
- 93.Smith GM, et al. Maturation of astrocytes in vitro alters the extent and molecular basis of neurite outgrowth. Dev Biol. 1990;138:377–390. doi: 10.1016/0012-1606(90)90204-v. [DOI] [PubMed] [Google Scholar]
- 94.Smith GM, Silver J. Transplantation of immature and mature astrocytes and their effect on scar formation in the lesioned central nervous system. Prog Brain Res. 1988;78:353–361. doi: 10.1016/s0079-6123(08)60304-0. [DOI] [PubMed] [Google Scholar]
- 95.Sofroniew MV. Reactive astrocytes in neural repair and protection. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2005;11:400–407. doi: 10.1177/1073858405278321. [DOI] [PubMed] [Google Scholar]
- 96.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 97.Talbott JF, et al. Endogenous Nkx2.2+/Olig2+ oligodendrocyte precursor cells fail to remyelinate the demyelinated adult rat spinal cord in the absence of astrocytes. Experimental neurology. 2005;192:11–24. doi: 10.1016/j.expneurol.2004.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tsunemoto RK, et al. Forward engineering neuronal diversity using direct reprogramming. The EMBO journal. 2015;34:1445–1455. doi: 10.15252/embj.201591402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ullian EM, et al. Control of synapse number by glia. Science. 2001;291:657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- 100.Vargas MR, Johnson JA. The Nrf2-ARE cytoprotective pathway in astrocytes. Expert reviews in molecular medicine. 2009;11:e17. doi: 10.1017/S1462399409001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vierbuchen T, Wernig M. Direct lineage conversions: unnatural but useful? Nat Biotechnol. 2011;29:892–907. doi: 10.1038/nbt.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang JJ, et al. Effects of astrocyte implantation into the hemisected adult rat spinal cord. Neuroscience. 1995;65:973–981. doi: 10.1016/0306-4522(94)00519-b. [DOI] [PubMed] [Google Scholar]
- 103.Xue H, et al. A targeted neuroglial reporter line generated by homologous recombination in human embryonic stem cells. Stem cells. 2009;27:1836–1846. doi: 10.1002/stem.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang N, et al. Generation of oligodendroglial cells by direct lineage conversion. Nat Biotechnol. 2013;31:434–439. doi: 10.1038/nbt.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yoshimoto Y, et al. Astrocytes retrovirally transduced with BDNF elicit behavioral improvement in a rat model of Parkinson's disease. Brain Res. 1995;691:25–36. doi: 10.1016/0006-8993(95)00596-i. [DOI] [PubMed] [Google Scholar]
- 106.Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 107.Zhang SC. Defining glial cells during CNS development. Nature reviews. Neuroscience. 2001;2:840–843. doi: 10.1038/35097593. [DOI] [PubMed] [Google Scholar]
- 108.Zhang SC, et al. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19:1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- 109.Zhang Y, Barres BA. Astrocyte heterogeneity: an underappreciated topic in neurobiology. Curr Opin Neurobiol. 2010;20:588–594. doi: 10.1016/j.conb.2010.06.005. [DOI] [PubMed] [Google Scholar]