Abstract
Background
The epithelial-mesenchymal transition (EMT) is critical in the development of invasive epithelial malignancies. EMT is accelerated by inflammation, and results in decreased E-cadherin expression. Diet-induced obesity is an inflammatory state that accelerates pancreatic carcinogenesis; its effect on EMT and E-cadherin expression in the development of pancreatic ductal adenocarcinoma is unclear.
Methods
Conditional KrasG12D mice were fed a control diet (CD) or a high fat, high calorie (HFCD) for 3 or 9 months (n = 10 each). Immunohistochemistry with anti-E-cadherin antibody was performed. E-cadherin expression was characterized by staining intensity, location, and proportion of positive cells. In vitro expression of E-cadherin and Slug in primary PanIN and cancer cells was determined by Western blot.
Results
The HFCD led to increased weight gain in both 3- (15.8 vs. 5.6g, p<.001) and 9-month (19.8 vs. 12.9g, p=.007) mice. No differences in E-cadherin expression among various stages of pre-invasive PanIN lesions were found—regardless of age or diet. In invasive cancer, E-cadherin expression was aberrant with loss of membranous staining and prominent cytoplasmic staining. This was associated with strong, cytoplasmic expression of β-catenin. In vitro expression of E-cadherin was highest in primary PanIN cells, accompanied by absent Slug expression. Cancer cell lines demonstrated significantly decreased E-cadherin expression in the presence of upregulated Slug.
Conclusion
Despite increased pancreatic inflammation and accelerated carcinogenesis, the HFCD did not induce changes in E-cadherin expression in PanIN lesions of all stages. Invasive lesions demonstrated aberrant cytoplasmic E-cadherin staining. Loss of normal membranous localization may reflect a functional loss of E-cadherin.
INTRODUCTION
The incidence of pancreatic cancer in the United States is rising, and despite advancement in diagnostic imaging, surgical management, and the rise of specialized centers in the treatment of pancreatic ductal adenocarcinoma (PDAC), the prognosis for the majority of these patients remains strikingly poor.1, 2 Complete surgical resection remains the only chance for cure, however 80–90% of patients present with evidence of metastatic or locally advanced disease precluding curative resection.3 Because early invasion and metastasis present such a barrier to treatment in PDAC, attention is increasingly geared towards understanding the mechanism and risk factors for the epithelial-mesenchymal transition (EMT), a critical process in the development of invasive disease. In the context of carcinoma progression, EMT is the process by which a transformed epithelial cell loses its normal attachments and inherent polarity—thereby exhibiting a ‘mesenchymal’ phenotype—and crosses the basement membrane. In addition to promoting invasiveness, EMT may play a role in resistance to cell death and senescence, resistance to chemo- and immunotherapy, and may impart cells with stem cell like properties.4, 5
The transmembrane glycoprotein E-cadherin is a prototypic type I cadherin that functions primarily to mediate cell-cell adhesion by way of adherens junctions. The loss of E-cadherin is highly indicative of a loss of an epithelial phenotype.6 EMT inducing transcription factors Snail and Slug actively repress E-cadherin expression.7 Loss of E-cadherin has been shown to be a key step in the progression from adenoma to carcinoma.8 In PDAC, reduced E-cadherin expression may be found in as many as 60% of resected tumors, and is correlated with tumor dedifferentiation, increasing stage, and lymph node involvement.9 Although tumors with total loss of E-cadherin are associated with the worst prognosis, partial E-cadherin loss has been shown an independent predictor of poor outcome, and the percentage of E-cadherin loss has been shown inversely related to overall survival.10, 11 Interestingly, there has been recent evidence in a mouse model of PDAC to suggest that EMT and dissemination may occur prior to bona fide tumor formation in the pancreas. Circulating pancreatic cells with a mesenchymal phenotype—demonstrating E-cadherin loss and/or upregulated upstream EMT transcription factors—were identified in the pancreatic stroma, blood, and liver in mice that did not have demonstrable carcinoma in the pancreas, only pre-invasive pancreatic intraepithelial neoplasia (PanIN). Furthermore, this early EMT was accelerated by inflammation.12
Obesity is now widely considered to promote a systemic, inflammatory state with elevations in reactive oxygen species, pro-inflammatory cytokines, eicosanoids, circulating growth factors (e.g. insulin IGF-1), and leptin.13 This pro-inflammatory state is a leading theory to explain the growing link between obesity and PDAC, as posited by epidemiological studies such as the National Cancer Institute Pancreatic Cancer Cohort Consortium.14, 15 We have previously shown in a mouse model of PDAC that a high fat, high calorie diet (HFCD) leads to obesity, pancreatic inflammation, and accelerated pancreatic carcinogenesis.16 Although data is limited, there is preliminary in vitro evidence that serum from obese mice and leptin can promote EMT, decreased E-cadherin expression, and invasiveness in prostate, breast, and lung cancer cell lines.17–19 The present study was undertaken to determine whether early loss of E-cadherin is part of the mechanism by which diet-induced obesity accelerates pancreatic carcinogenesis in the conditional KrasG12D mouse model.
METHODS
Mouse Model and Experimental Diet
To study the effects of diet-induced obesity on E-cadherin expression during pancreatic carcinogenesis, the conditional KrasG12D (LSL-KrasG12D; P48-Cre) mouse model described by Hingorani was used.20 Prior to implementation of the diet, presence of both Kras and Cre alleles were confirmed by PCR in all experimental mice, as previously described.21 Animals were fed either a control diet (CD) or the HFCD. Compared to the CD the HFCD contains increased calories (4,536 kcal/kg vs. 3,726 kcal/kg), a higher percentage of which come from corn oil based fat (40% vs 12%). The corn oil contains approximately 60% omega-6 polyunsaturated fatty acids, 27% monounsaturated fatty acids, 13% saturated fatty acids, and small amounts of omega-3 polyunsaturated fatty acids. Further details regarding the composition of the diet are described elsewhere.16 Mice were weighed weekly. Cohorts were sacrificed at 3 months and 9 months of age.
Histology and Immunohistochemistry
Formalin-fixed, paraffin-embedded pancreata were sectioned (4μm) and stained with hematoxylin and eosin (H&E). Murine PanIN lesions were classified according to current histopathologic criteria.22 Immunohistochemistry was performed on 4μm sections of paraffin-embedded pancreata. Paraffin was removed with xylene and graded alcohol. Heat-induced antigen retrieval was performed with citrate buffer (pH 6.0), and endogenous peroxidase activity was blocked with 3% hydrogen peroxide. Slides were then incubated overnight with primary antibody against E-cadherin (Cell Signaling, cat# 3195, 1:200 dilution) and β-catenin (Cell Signaling, cat# 8480, 1:100 dilution). Control images were prepared using isotype matched rabbit IgG (Cell Signaling, cat #3900, 1:200 dilution). Anti-rabbit secondary antibody conjugated with HRP was used (EnVision + R, Dako, cat# K-4003). Betazoid DAB Chromogen kit was used to visualize protein-antibody complexes (Biocare Medical, cat#BDB2004).
Immunoreactivity was semi-quantitatively analyzed in the following manner. The intensity of E-cadherin expression was graded as absent (0), weak (+), moderate (++), and strong (+++). The proportion of cells staining positive for E-cadherin were described as less than 25% (0), 25 to 50% (+), 50 to 75% (++), and greater than 75% (+++). The pattern of E-cadherin expression was described as membranous, cytoplasmic, or nuclear according to the predominant location of staining in each lesion. Final semi-quantitative description of immunoreactivity represents the consensus of two authors (GE; AS).
PanIN isolation and cell culture
Primary PanIN cells were harvested and isolated from 6-month old LSL-KrasG12D;P48-Cre mice using techniques scribed previously.23 At this age, the majority of ductal epithelial cells are of pathological grade PanIN-1a or higher, but no invasive cancer is present. We confirmed the presence of epithelial and absence of acinar and endocrine markers via immunohistochemistry and immunofluorescence. The KrasG12D mutation was confirmed in all PanIN lines via sequencing. These primary PanIN cells were plated on type I collagen coated plates, and cultured in 5% CO2 at 37°C using a DMEM/F12 based medium with serum and specific growth factors, also as previously described.23 Similarly, KC and KPC (immortalized murine PDAC lines derived from LSL-KrasG12D;Pdx-1-Cre and LSL-KrasG12D;Trp53;Pdx-1-Cre mice, respectively) cells were plated on type I collagen coated plates, and cultured in 5% CO2 at 37°C using an RPMI based medium with 10% fetal bovine serum.
Western Blotting
Cells were grown to 70% confluence, then lysed following extraction from the collagen matrix using collagenase. Samples were then centrifuged at 13,000 RPM for 10 minutes. Prior to Western blotting, the protein concentrations of cell lysate supernatants were measured using bicinchoninic acid protein assay (Pierce BCA Protein Assay Kit, Thermo Scientific, cat#23227). Whole cellular protein extracts were separated by electrophoresis on 4–15% polyacrylamide gets (Mini-PROTEAN TGX, Bio-Rad, cat#456-1084). Proteins were then transferred to a transfer membrane, and blocked at room temperature for 30 minutes with 5% milk diluted in tris-buffered saline and 0.01% Tween 20 (TBST). The membranes were then incubated overnight at 4°C with primary antibody to E-cadherin (Cell Signaling, cat#3195, 1:4000 dilution), Slug (Cell Signaling, cat# 9585, 1:1000 dilution), and glyceraldehyde-3 phosphate dehydrogenase (GAPDH, Cell Signaling, cat#5174, 1:4000 dilution) diluted in TBST with 5% bovine serum albumin as per manufacturer instruction. After washing, the membranes were incubated with HRP-linked anti-rabbit secondary antibody (Pierce, cat#31460). Signal intensities were analyzed and normalized relative to GAPDH. Densitometry analysis was performed using publically available ImageJ software, version 1.48.
Statistical analyses
Two-sided, Student’s t-test was performed to identify differences in the mean. Statistical significance was set at p < .05.
RESULTS
Effects of the HFCD on weight gain, inflammation, and PanIN progression
Implementation of the HFCD led to significant weight gain in both the 3-month (n =10) and 9-month (n = 10) cohorts (see Table 1). Consistent with previously published data,16 mice fed the HFCD for 3 months showed accelerated pancreatic neoplasia with more advanced PanIN lesions. This acceleration of PanIN progression was also evident in the 9-month cohort. Pancreatic inflammation as evident by acinar cell loss, fibrosis, and desmoplasia was increased in mice fed the HFCD (Figure 1). Two invasive tumors were found in the 9-month, HFCD-fed cohort. No cancers were found in the other cohorts.
Table 1.
HFCD Induced Weight Gain in 3- and 9-month Mice
| Age | Diet | Avg. weight gain (g) | p value |
|---|---|---|---|
| 3-month | CD (n = 5) | 5.6 | < .001 |
| HFCD (n = 5) | 15.8 | ||
| 9-month | CD (n = 5) | 12.9 | .007 |
| HFCD (n = 5) | 19.8 |
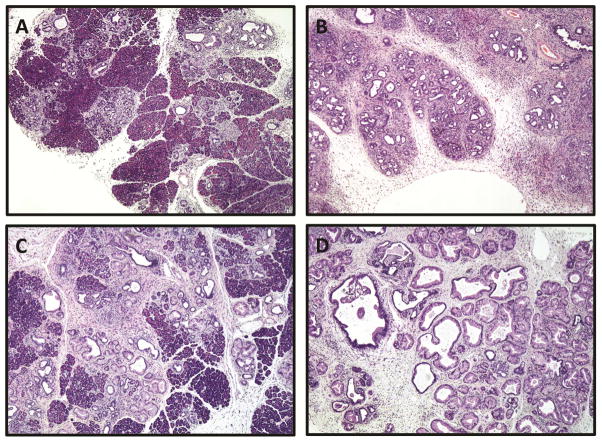
Figure 1. Age and HFCD increase the percentage of pancreatic acinar loss, desmoplasia, and extent of inflammation.
Representative H&E sections of mouse pancreata (age, diet): (A) 3-month, CD, (B) 3-month, HFCD, (C) 9-month, CD, and (D) 9-month, HFCD. For both 3-month and 9-month mice, those fed the HFCD demonstrate increased stigmata of pancreatic inflammation. All images 4× magnification.
E-cadherin expression in PanIN lesions and invasive cancer
E-cadherin was expressed in all PanIN lesions of all mice. Despite more severe pathology and a higher proportion of advanced PanIN lesions in the HFCD cohorts, there were no demonstrable differences in the E-cadherin staining pattern in PanIN lesions seen in all four cohorts. Regardless of diet, E-cadherin expression was of strong intensity, localized solely to the cell membrane, and ubiquitously present in PanIN lesions in 3-month and 9-month old mice (see Figure 2). In two 9-month old mice fed the HFCD, an invasive tumor was identified in the pancreas. In both cases, the E-cadherin expression in adjacent PanIN lesions remained strong, membranous, and ubiquitous. Overall, E-cadherin staining intensity in pancreatic cancers was diminished relative to PanIN lesions in the adjacent pancreas. In both cases, invasive lesions demonstrated aberrant E-cadherin expression with loss of membranous localization and prominent cytoplasmic staining (Figure 3). No invasive ducts demonstrated total E-cadherin loss. Table 2 summarizes the characteristics of E-cadherin expression in PanIN lesions and invasive cancer.
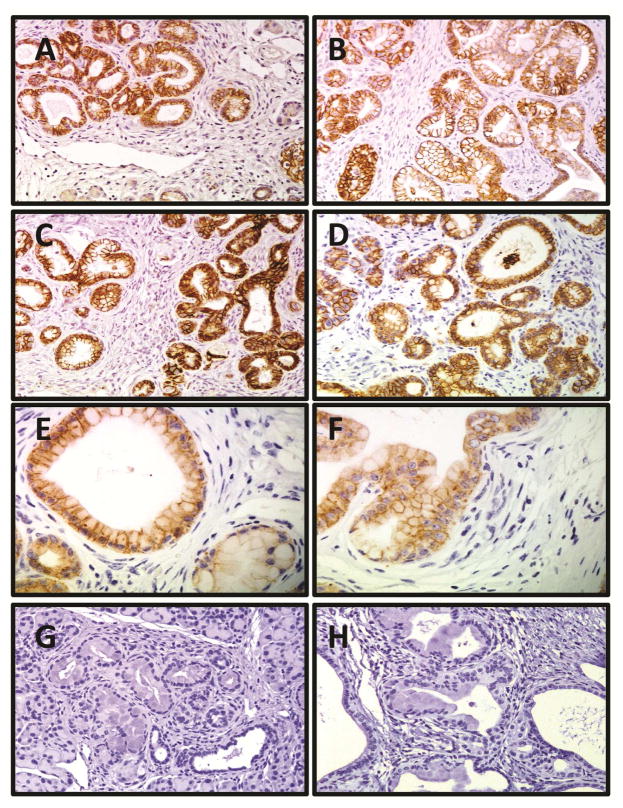
Figure 2. E-cadherin expression is strong, membranous, and ubiquitous in PanIN lesions regardless of age, diet, or PanIN stage.
Representative sections of IHC expression of E-Cadherin in mouse pancreata (age, diet): 20× magnification image of (A) 3-month, CD, (B) 3-month, HFCD, (C) 9-month, CD, and (D) 9-month, HFCD mice. (E) 40× magnification view of low-grade PanIN lesion in CD fed mouse, and (F) 40× magnification view of high-grade PanIN lesion in HFCD demonstrating similar E-cadherin staining pattern. 20× magnification images of (G) 3-month and (H) 9-month HFCD fed mice using isotype-matched control rabbit IgG demonstrating lack of nonspecific binding.
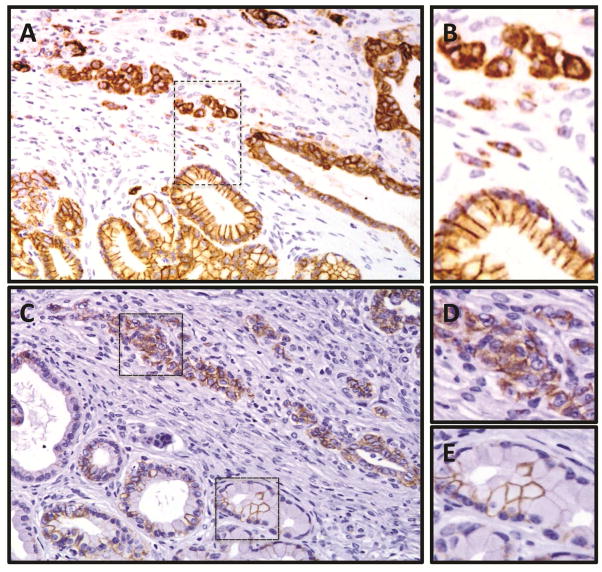
Figure 3. In invasive lesions, E-cadherin staining is cytoplasmic, with loss of membranous localization.
IHC expression of E-Cadherin in invasive pancreatic cancer found in a 9-month, HFCD fed mouse. (A) Invasive duct characterized by an aberrant and cytoplasmic E-Cadherin staining pattern, adjacent to PanIN lesion with preserved membranous expression. 20× magnification. (B) Magnified image of inset from (A). (C) IHC expression of β-catenin from the same specimen and location (deeper section) as shown in (A) and (B). In non-invasive PanIN lesions, expression of β-catenin is weak, absent or intermittent, and limited to the cell membrane when present. In invasive lesions corresponding to areas with cytoplasmic E-cadherin, β-catenin staining is moderate to strong, ubiquitous, and located in both the cytoplasm as well as on the cell membrane. (D) and (E); magnified images of insets from (C).
Table 2.
Semi-Quantitative Analysis of E-Cadherin Expression in Early PanIN lesions, Late PanIN lesions, and PDAC.
| PanIN1 | PanIN2, 3 | PDAC | |
|---|---|---|---|
| Staining Intensity | +++ | +++ | + to +++ |
| Proportion of Positive Cells | +++ | +++ | ++ to +++ |
| Staining Pattern | Membranous | Membranous | Cytoplasmic and membranous |
Immunohistochemistry with anti-β-catenin antibody was performed to investigate the expression of β-catenin in lesions with cytoplasmic E-cadherin (see Figure 3). In non-invasive PanIN lesions, β-catenin expression was found to be of weak intensity (+), absent to intermittent (0 – +), and limited to the cell membrane. In contrast, in invasive areas corresponding to cytoplasmic E-cadherin, β-catenin staining intensity was moderate to strong (++ – +++), ubiquitous (+++), and located in the cytoplasm in addition to the cell membrane. Isotype-matched control immunoglobulins were used for all IHC procedures (see Figure 2G, 2H) to confirm the absence of non-specific staining.
In-vitro E-cadherin and Slug expression in PanIN and invasive pancreatic cancer cell lines
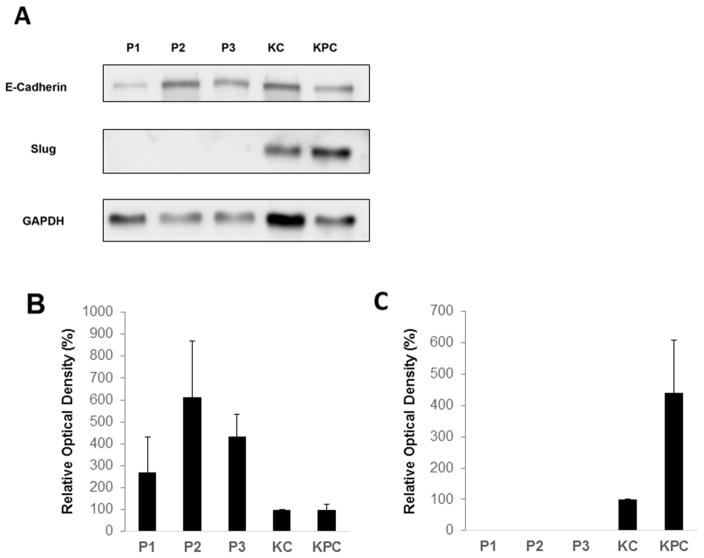
Having demonstrated that E-cadherin showed a different staining pattern in PanIN and invasive cancer lesions in vivo, we sought to characterize the in vitro expression of E-cadherin and EMT-inducing Slug in PanIN and invasive cell lines. Primary mouse PanIN cells, KC cells, and KPC cells expressed E-cadherin by Western blot analysis. PanIN lines (P1, P2, P3) derived from three different mice all expressed E-cadherin strongly, and all demonstrated higher E-cadherin expression than KC and KPC cells after normalization to GAPDH expression (Figure 4). By densitometry analysis, average E-cadherin expression in PanIN cells was about 2–6 fold higher than in KC and KPC cell lines (p<0.01). Slug expression is completely absent in all three PanIN lines, whereas it was present in both KC and KPC cell lines. The magnitude of Slug expression in KPC cells is more than 4-fold higher than in KC cells (p<0.01) (Figure 4).
Figure 4. In-vitro expression of E-cadherin and upstream EMT transcription factor Slug in primary mouse PanIN and pancreatic cancer cells.
(A) Representative Western blot demonstrating expression of E-cadherin, Slug, and GAPDH in PanIN cell lines P1, P2, and P3 relative to KC and KPC cancer cell lines. (B) Densitometric analysis of E-cadherin expression in PanIN, KC and KPC cells. (C) Densitometric analysis of Slug expression in PanIN, KC and KPC cells (no identifiable Slug expression present in PanIN cell lines). For (B) and (C), relative optical density is measured as an average of 4 separate western blots after normalization to GAPDH. Standard deviation bars are shown. Student’s t-test for the mean was performed to obtain the reported p-values.
DISCUSSION
Despite evidence that the HFCD leads to obesity, pancreatic inflammation, and accelerated PanIN progression,16 we did not identify a difference in E-cadherin expression in the PanIN lesions of mice fed the HFCD as compared to the CD. Regardless of age or diet, PanIN lesions strongly expressed E-cadherin in a membranous and ubiquitous fashion. This may indicate that early EMT is not part of the mechanism by which the HFCD accelerates pancreatic carcinogenesis. However, using IHC expression of E-cadherin as an indicator of EMT has one major limitation. IHC will necessarily fail to identify epithelial cells that have fully undergone EMT and migrated past the basement membrane, as these cells are located in the stroma and are likely to have total loss of E-cadherin. Indeed, Rhim et al demonstrated the presence of epithelial cells in the pancreatic stroma of PanIN mice by way of a novel, lineage labeled mouse model in which all pancreatic epithelial cells expressed yellow fluorescent protein (YFP) via Cre-Lox recombination (Pdx-Cre; LSL-KrasG12D; LSL-ROSAYfp). The authors defined a YFP expressing cell as having undergone EMT if it co-expressed Zeb1 and/or failed to express E-cadherin.12 Unfortunately, it appears the quantity of cells identifiable by this method is low. It appears that the HFCD does not directly accelerate E-cadherin loss in PanIN lesions, suggesting that changes in E-cadherin expression and/or EMT likely occurs at a given stage in pancreatic carcinogenesis that is independent from the disease promoting effects of diet-induced obesity.
Loss or decrease in E-cadherin expression is well described in the progression of epithelial malignancies, including PDAC. Decreased expression of E-cadherin has been reported previously in a mouse model of PDAC.24 In this study we report not a loss of E-cadherin, but rather a prominent alteration in the E-cadherin staining pattern seen in invasive disease. Alteration in the pattern of E-cadherin expression in PDAC as compared to PanINs has been described, but not clearly characterized.25 Rather than its location on the cell membrane, we found that E-cadherin was expressed prominently in the cytoplasm. Such aberrant E-cadherin expression has been previously described in the literature. In human tissue specimens, cytoplasmic E-cadherin has been found to be significantly more common in both PanIN lesions and PDAC in comparison to normal pancreatic ducts.26 Another study of human PDAC identified an association between aberrant, cytoplasmic localization of E-cadherin, loss of tumor differentiation, and increasing stage.27 Loss of membranous E-cadherin is also reported in other pancreatic malignancies, including solid pseudopapillary neoplasm, in which cytoplasmic E-cadherin is frequently observed.28 In pancreatic neuroendocrine tumors, aberrant nuclear E-cadherin expression has been described, and may correspond with higher rates of lymph node and liver metastasis.29
Because we were unable to identify early changes in E-cadherin expression regardless of age or diet, but noticed a different staining pattern in PanIN and invasive lesions, we sought to investigate the expression of E-cadherin and other EMT markers in vitro using PanIN and invasive ductal cells. Our in vitro data supports our in vivo observations. Not only is E-cadherin expression by Western blot analysis strongest in PanIN cells, but also the complete absence of Slug in these cells contrasts sharply with its upregulated presence in the invasive KC and KPC cell lines. Part of the EMT-inducing properties of Slug include transcriptional repression of E-cadherin; total absence of Slug in PanIN cells may in part explain why we found strong and ubiquitous expression of E-cadherin in all PanIN lesions regardless of age or diet. Furthermore, the relative decrease in E-cadherin expression in the presence of upregulated Slug strongly suggests the presence of an EMT program in the invasive cell lines. Unfortunately, we were unable to reliably detect and determine Slug expression in vivo. This may have been related to low levels of Slug expression in the tissue, but technical factors and poor quality of tested antibodies could not be ruled out.
Our results, along with the data described above, raise an important question regarding the interpretation of E-cadherin expression as a marker of EMT. The question is, namely, whether E-cadherin loss is the true phenotype of cells having undergone EMT, or whether it is more fundamentally the loss of membranous E-cadherin that signifies EMT has occurred. One study has specifically reported that loss of membranous E-cadherin is associated with lymph node metastasis, advanced stage, and high grade in human PDAC.30 As shown in Figure 3, we identified areas of clearly invasive disease where epithelial cells with cytoplasmic E-cadherin had invaded past the basement membrane into the stroma and no longer formed ducts, which indicate that these cells had undergone some degree of EMT.
The change in the localization of E-cadherin from the cell membrane to the cytoplasm may indicate a functional loss of E-cadherin. As the key component of adherens junction located at the cell membrane, E-cadherin maintains cellular polarity, cell-cell adhesion, and the epithelial phenotype. Disruption of the adherens junction and loss of the adhesive properties of E-cadherin have been shown to facilitate tumor progression.31, 32 Another important component of the adherens junction is β-catenin, which is associated with E-cadherin and the actin cytoskeleton near the cell membrane. β-catenin typically localizes to the plasma membrane in normal pancreatic ducts, but aberrant nuclear and cytoplasmic β-catenin has been identified in high-grade human PanIN lesions and in PDAC. More specifically, it has been reported that human PanIN lesions and PDAC are more likely to exhibit both aberrant E-cadherin and β-catenin staining relative to normal pancreatic ducts.26 Active Wnt/β-catenin signaling has been demonstrated in human PDAC specimens, may be involved in tumor initiation, and is likely to play a critical role in tumor progression.33, 34 E-cadherin has been posited a role in the sequestration of β-catenin at the plasma membrane, thus limiting to an extent the oncogenic activity of β-catenin.35, 36 Membranous E-cadherin may therefore act as a negative suppressor of canonical Wnt signaling.32 We identified stronger staining intensity and markedly increased cytoplasmic localization of β-catenin in areas of invasive disease known to have cytoplasmic E-cadherin. While this observation cannot be taken as proof that β-catenin activity is altered in areas with cytoplasmic E-cadherin, it provides a rationale for further investigation into the relationship between loss of membranous E-cadherin and WNT/β-catenin activity in PDAC models. The absence of E-cadherin on the cell membrane may therefore contribute to pancreatic carcinogenesis in two distinct ways, namely by loss of the epithelial phenotype and by the loss of a tumor suppressor effect on the canonical Wnt/β-catenin signaling pathway.
The mechanisms by which the HFCD accelerate pancreatic carcinogenesis remain incompletely understood. Regardless of diet, E-cadherin expression in PanIN lesions is of strong intensity, localized to the cell membrane, and ubiquitously present. In our study PanIN lesions in mice fed the HFCD exhibit the same pattern of E-cadherin expression as PanIN lesions of similar grade in mice fed the CD. Diet-induced obesity may promote pancreatic carcinogenesis by a mechanism that is independent from the induction of EMT. Loss of E-cadherin is considered the hallmark of EMT, and is strongly associated with aggressive disease and poor prognosis. We report cytoplasmic localization of E-cadherin in invasive disease; whether such aberrant expression represents a tumor phenotype distinct from the “mesenchymal” phenotype of tumors with partial or complete E-cadherin loss remains unknown. However, our data and the published literature suggest that loss of membranous E-cadherin may be biologically equivalent to a total loss of E-cadherin. Further inquiry into the interpretation of E-cadherin expression as a marker of EMT is required, and may be beneficial in understanding the progression of PDAC.
Acknowledgments
Grant support: National Institutes of Health (P01CA163200), Hirshberg Foundation for Pancreatic Cancer Research
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Howlader NNA, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975–2011. National Cancer Institute; Bethesda, MD: Apr, 2014. based on November 2013 SEER data submission, posted to the SEER web site. [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: a cancer journal for clinicians. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Schneider G, Siveke JT, Eckel F, Schmid RM. Pancreatic cancer: basic and clinical aspects. Gastroenterology. 2005;128:1606–25. doi: 10.1053/j.gastro.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nature reviews Cancer. 2002;2:442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 7.Nieto MA. The snail superfamily of zinc-finger transcription factors. Nature reviews Molecular cell biology. 2002;3:155–66. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- 8.Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392:190–3. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- 9.Joo YE, Rew JS, Park CS, Kim SJ. Expression of E-cadherin, alpha- and beta-catenins in patients with pancreatic adenocarcinoma. Pancreatology : official journal of the International Association of Pancreatology. 2002;2:129–37. doi: 10.1159/000055903. [DOI] [PubMed] [Google Scholar]
- 10.Hong SM, Li A, Olino K, Wolfgang CL, Herman JM, Schulick RD, et al. Loss of E-cadherin expression and outcome among patients with resectable pancreatic adenocarcinomas. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2011;24:1237–47. doi: 10.1038/modpathol.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamada S, Fuchs BC, Fujii T, Shimoyama Y, Sugimoto H, Nomoto S, et al. Epithelial-to-mesenchymal transition predicts prognosis of pancreatic cancer. Surgery. 2013;154:946–54. doi: 10.1016/j.surg.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–61. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becker S, Dossus L, Kaaks R. Obesity related hyperinsulinaemia and hyperglycaemia and cancer development. Archives of physiology and biochemistry. 2009;115:86–96. doi: 10.1080/13813450902878054. [DOI] [PubMed] [Google Scholar]
- 14.Arslan AA, Helzlsouer KJ, Kooperberg C, Shu XO, Steplowski E, Bueno-de-Mesquita HB, et al. Anthropometric measures, body mass index, and pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan) Archives of internal medicine. 2010;170:791–802. doi: 10.1001/archinternmed.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krejs GJ. Pancreatic cancer: epidemiology and risk factors. Digestive diseases. 2010;28:355–8. doi: 10.1159/000319414. [DOI] [PubMed] [Google Scholar]
- 16.Dawson DW, Hertzer K, Moro A, Donald G, Chang HH, Go VL, et al. High-fat, high-calorie diet promotes early pancreatic neoplasia in the conditional KrasG12D mouse model. Cancer prevention research. 2013;6:1064–73. doi: 10.1158/1940-6207.CAPR-13-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan D, Avtanski D, Saxena NK, Sharma D. Leptin-induced epithelial-mesenchymal transition in breast cancer cells requires beta-catenin activation via Akt/GSK3- and MTA1/Wnt1 protein-dependent pathways. The Journal of biological chemistry. 2012;287:8598–612. doi: 10.1074/jbc.M111.322800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng H, Liu Q, Zhang N, Zheng L, Sang M, Feng J, et al. Leptin promotes metastasis by inducing an epithelial-mesenchymal transition in A549 lung cancer cells. Oncology research. 2013;21:165–71. doi: 10.3727/096504014X13887748696662. [DOI] [PubMed] [Google Scholar]
- 19.Price RS, Cavazos DA, De Angel RE, Hursting SD, deGraffenried LA. Obesity-related systemic factors promote an invasive phenotype in prostate cancer cells. Prostate cancer and prostatic diseases. 2012;15:135–43. doi: 10.1038/pcan.2011.54. [DOI] [PubMed] [Google Scholar]
- 20.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer cell. 2003;4:437–50. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 21.Funahashi H, Satake M, Dawson D, Huynh NA, Reber HA, Hines OJ, et al. Delayed progression of pancreatic intraepithelial neoplasia in a conditional Kras(G12D) mouse model by a selective cyclooxygenase-2 inhibitor. Cancer research. 2007;67:7068–71. doi: 10.1158/0008-5472.CAN-07-0970. [DOI] [PubMed] [Google Scholar]
- 22.Hruban RH, Adsay NV, Albores-Saavedra J, Compton C, Garrett ES, Goodman SN, et al. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. The American journal of surgical pathology. 2001;25:579–86. doi: 10.1097/00000478-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Reichert M, Takano S, Heeg S, Bakir B, Botta GP, Rustgi AK. Isolation, culture and genetic manipulation of mouse pancreatic ductal cells. Nature protocols. 2013;8:1354–65. doi: 10.1038/nprot.2013.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer cell. 2005;7:469–83. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 25.Ijichi H, Chytil A, Gorska AE, Aakre ME, Fujitani Y, Fujitani S, et al. Aggressive pancreatic ductal adenocarcinoma in mice caused by pancreas-specific blockade of transforming growth factor-beta signaling in cooperation with active Kras expression. Genes Dev. 2006;20:3147–60. doi: 10.1101/gad.1475506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Aynati MM, Radulovich N, Riddell RH, Tsao MS. Epithelial-cadherin and beta-catenin expression changes in pancreatic intraepithelial neoplasia. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10:1235–40. doi: 10.1158/1078-0432.ccr-03-0087. [DOI] [PubMed] [Google Scholar]
- 27.Karayiannakis AJ, Syrigos KN, Chatzigianni E, Papanikolaou S, Alexiou D, Kalahanis N, et al. Aberrant E-cadherin expression associated with loss of differentiation and advanced stage in human pancreatic cancer. Anticancer research. 1998;18:4177–80. [PubMed] [Google Scholar]
- 28.Audard V, Cavard C, Richa H, Infante M, Couvelard A, Sauvanet A, et al. Impaired E-cadherin expression and glutamine synthetase overexpression in solid pseudopapillary neoplasm of the pancreas. Pancreas. 2008;36:80–3. doi: 10.1097/mpa.0b013e318137a9da. [DOI] [PubMed] [Google Scholar]
- 29.Chetty R, Serra S, Asa SL. Loss of membrane localization and aberrant nuclear E-cadherin expression correlates with invasion in pancreatic endocrine tumors. The American journal of surgical pathology. 2008;32:413–9. doi: 10.1097/PAS.0b013e31813547f8. [DOI] [PubMed] [Google Scholar]
- 30.Pignatelli M, Ansari TW, Gunter P, Liu D, Hirano S, Takeichi M, et al. Loss of membranous E-cadherin expression in pancreatic cancer: correlation with lymph node metastasis, high grade, and advanced stage. The Journal of pathology. 1994;174:243–8. doi: 10.1002/path.1711740403. [DOI] [PubMed] [Google Scholar]
- 31.Behrens J, Mareel MM, Van Roy FM, Birchmeier W. Dissecting tumor cell invasion: epithelial cells acquire invasive properties after the loss of uvomorulin-mediated cell-cell adhesion. The Journal of cell biology. 1989;108:2435–47. doi: 10.1083/jcb.108.6.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmalhofer O, Brabletz S, Brabletz T. E-cadherin, beta-catenin, and ZEB1 in malignant progression of cancer. Cancer metastasis reviews. 2009;28:151–66. doi: 10.1007/s10555-008-9179-y. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Morris JPt, Yan W, Schofield HK, Gurney A, Simeone DM, et al. Canonical wnt signaling is required for pancreatic carcinogenesis. Cancer research. 2013;73:4909–22. doi: 10.1158/0008-5472.CAN-12-4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeng G, Germinaro M, Micsenyi A, Monga NK, Bell A, Sood A, et al. Aberrant Wnt/beta-catenin signaling in pancreatic adenocarcinoma. Neoplasia. 2006;8:279–89. doi: 10.1593/neo.05607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gottardi CJ, Wong E, Gumbiner BM. E-cadherin suppresses cellular transformation by inhibiting beta-catenin signaling in an adhesion-independent manner. The Journal of cell biology. 2001;153:1049–60. doi: 10.1083/jcb.153.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–7. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]