Abstract
Fructose consumption, which promotes insulin resistance, hypertension, and dyslipidemia, has increased by over 25% since the 1970s. In addition to metabolic dysregulation, fructose ingestion stimulates the hypothalamic-pituitary-adrenal (HPA) axis leading to elevations in glucocorticoids. Adolescents are the greatest consumers of fructose, and adolescence is a critical period for maturation of the HPA axis. Repeated consumption of high levels of fructose during adolescence has the potential to promote long-term dysregulation of the stress response. Therefore, we determined the extent to which consumption of a diet high in fructose affected behavior, serum corticosterone, and hypothalamic gene expression using a whole-transcriptomics approach. In addition, we examined the potential of a high-fructose diet to interact with exposure to chronic adolescent stress. Male Wistar rats fed the periadolescent high-fructose diet showed increased anxiety-like behavior in the elevated plus maze and depressive-like behavior in the forced swim test in adulthood, irrespective of stress history. Periadolescent fructose-fed rats also exhibited elevated basal corticosterone concentrations relative to their chow-fed peers. These behavioral and hormonal responses to the high-fructose diet did not occur in rats fed fructose during adulthood only. Finally, rats fed the high-fructose diet throughout development underwent marked hypothalamic transcript expression remodeling, with 966 genes (5.6%) significantly altered and a pronounced enrichment of significantly altered transcripts in several pathways relating to regulation of the HPA axis. Collectively, the data presented herein indicate that diet, specifically one high in fructose, has the potential to alter behavior, HPA axis function, and the hypothalamic transcriptome in male rats.
Keywords: High-fructose, diet, stress, adolescence, HPA axis, depressive-like behavior
1. Introduction
Fructose consumption has increased by at least 25% in the past 30 years (Havel, 2005) due to increases in added sweeteners such as sucrose and high-fructose corn syrup. Adolescents are the highest consumers of fructose at 72.8 g/day, with a quarter of adolescents consuming at least 15% of their daily caloric intake from fructose alone (Vos et al., 2008). This is part of a global energy imbalance, resulting in a growing epidemic of metabolic syndrome (Rutledge and Adeli, 2007). The epidemic is not restricted to adults, as today over 20% of American adolescents are obese (Elliott et al., 2002) and Type II diabetes’ rates are increasing among youth (Nadeau and Dabelea, 2008).
Diets high in fructose have implications beyond an excess caloric consumption. Such dietsalter insulin, blood pressure, and lipid profiles in animal models (Hwang et al., 1987, Catena et al., 2003) and humans (Stanhope et al., 2009, Teff et al., 2009). Fructose consumption also raises corticosterone levels in rats (Brindley et al., 1981, Brindley et al., 1985) and elevations in corticosterone may be responsible for fructose-induced hepatic gluconeogenesis (Kinote et al., 2012). The role of glucocorticoids in fructose metabolism is particularly relevant given the clinical data indicating an increased prevalence of depression among diabetic patients (Anderson et al., 2001). Altered hypothalamic-pituitary-adrenal (HPA) axis signaling is a classic feature of, and risk factor for, depression (Heim et al., 2008b).
Chronic stress, which disrupts HPA axis signaling (Bourke et al., 2013) and is associated with increased incidence of depression (Neigh et al., 2009) or depressive-like behavior (Bourke and Neigh, 2011), can exacerbate the effects of diet by promoting palatable food consumption (Pecoraro et al., 2004) and by inducing insulin resistance (Kaufman et al., 2007). A history of early life stress not only increases the risk of depression in adulthood (Neigh et al., 2009, Bale et al., 2010) but also increases the risk of metabolic dysfunction (Williamson et al., 2002). Adolescence is a “critical period” of development that shapes both stress responses (Romeo, 2010) and adult metabolism (Dietz, 1994). For these reasons, we designed our study to examine the interaction of adolescent stress and high-fructose diet on behavior, the HPA axis, and the hypothalamic transcriptome.
We hypothesized that fructose consumption beginning at weaning would induce metabolic disruption paralleling increases in anxiety-like and depressive-like behavior, and that fructose consumption would create a susceptibility to behavioral alterations in response to a subthreshold chronic adolescent stress. Further, we hypothesized that these behavioral and metabolic changes would correspond to alterations in HPA axis output both at baseline and in response to an acute stressor. Finally, we used whole-transcriptome RNA sequencing of the hypothalamus to determine the scope of changes in gene expression induced by a high-fructose diet during periadolescent development.
2. Materials and Methods
2.1 Animal Husbandry
Timed pregnant Wistar rats (n=22) were obtained on gestational day 12 to produce the periadolescent cohort from Charles River (Wilmington, MA), while male Wistar rats (n=16, PND 56) were obtained from Charles River (Wilmington, MA) to produce the adult cohort. Shipping stress during puberty can alter behavioral outcomes (Laroche et al., 2009) but shipping of pregnant dams has not been shown to alter developmental outcomes without a pharmacologic challenge (Ogawa et al., 2007); thus, shipping was conducted during in utero development to produce the periadolescent cohort. Animals for the adult-only diet exposure were obtained from Charles River as adults and acclimated to colony conditions for seven days prior to introduction of the high fructose diet (Capdevila et al., 2007). Rats were housed on a 14:10 reverse light:dark cycle in a facility controlled for humidity (60%) and temperature (20 °C–23 °C). For the periadolescent cohort, litters were culled on postnatal day (PND) 3 to eight pups per litter and weaned on PND 23 (n=134). Culled litters contained both male and female pups, but only male offspring were used in the current study. All experiments were performed in accordance with the Institutional Animal Care and Use Committee (IACUC) of Emory University and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2 Diet and Metabolic Measurement
Either two days post-weaning (Periadolescent Cohort; PND 25; Chow-Non-Stress, n=44; Chow-Stress, n=23; Fructose-Non-Stress, n=43; Fructose-Stress, n=24) or at PND 64 (Adult Cohort, Chow, n=8; Fructose, n=8, all Non-Stress), male rats were pair-housed and assigned to either the Lab Rodent Diet 5001 or a high-fructose diet. The numbers and endpoints for each cohort are further clarified in Supplemental Table 1. In addition, the experimental timelines for each cohort are visualized in Supplemental Figure 1.
The primary goal of using the high-fructose diet was to elicit physiologic changes typically associated with fructose consumption in humans, including altered lipid storage and hyperglycemia (Havel, 2005, Tappy and Le, 2010) and to examine concomitant effects on the brain and behavior but not to mimic common human consumption. This 55% high-fructose diet has previously been used to elicit such physiological changes in rodents, most notably increased adiposity, hyperglycemia, and hypertension. It is estimated that about 10% of total caloric intake for the United States population is from fructose with higher consumption among adolescents. One fourth of adolescents have been reported to consume at least 15% of daily calories from fructose (Vos et al., 2008). However, these percentages do not reflect the increase in mass of fructose consumed, as increased fructose consumption in humans has coincided with substantial increases in caloric consumption, primarily due to increases in carbohydrate consumption (Marriott et al., 2009). In addition, these estimates in humans are based on self-reported dietary recall, which typically underestimates consumption, particularly in obese subjects and adolescents. Obese adults underreport energy intake by an average of 47% and adolescents underreport by an average of 20% (Schoeller, 1995). Nonetheless, the diet’s effects should be understood in the context of an animal model useful for exploring potential effects of a given macronutrient (fructose) on energy homeostasis and stress response and not as a replica of the human condition.
The “periadolescent” cohorts were so named as their diet intake spanned the beginning of adolescence through adulthood, while the “adult” cohort consumed the high-fructose diet during adulthood only. All major outcomes were tested in adulthood for all cohorts. While adolescence is difficult to define precisely in rats as in humans, it is accepted that infancy and “childhood” end at weaning (PND21-23) and that adulthood begins at PND60 (Spear, 2000, McCormick and Mathews, 2007). The diet timelines were thus selected based on the aim to fully cover the adolescent period in the periadolescent cohorts and not in the adult cohorts; and additionally based on evidence from the literature that 8–10 weeks on a similar high-fructose diet will induce metabolic changes (Huang et al., 2004, Nakagawa et al., 2006).
Non-stressed rats were pair-housed throughout the study, while stressed rats remained pair-housed until the initiation of stress, and single-housed thereafter. The fructose diet used (Research diets D05111802) is 55% fructose while the standard chow (Lab Diet 5001) normally used is 0.30% fructose. Both diets were supplemented with comparable levels of vitamins and minerals deemed necessary for rodent health, and were reviewed by veterinary staff and approved by IACUC. The details of the macronutrients of each diet have been listed in Supplemental Table 2.
Metabolic measures were taken from a subset of the periadolescent cohort and the adult cohort. Blood glucose was tested near weekly after an overnight fast by tail prick using a Freestyle glucometer. Animal weights were also taken concurrently with glucose readings. Research assistants, carefully accounting for any spilled food, measured food consumption daily and caloric consumption was determined thereof. To determine caloric efficiency, the body mass gained per week per animal was divided by the mean weekly caloric consumption calculated per cage (of pair-housed animals) divided by two. While imprecise, this type of approximation should only serve to increase variability in caloric efficiency and thus increase probability of returning a false negative result as opposed to producing a false positive.
Fat pads were collected from only a subset of periadolescent animals after weeks on the diet (described below). Weight and fasting blood glucose were assessed in the stress cohorts prior to and after the mixed modality stress, and a subset of the stress & non-stressed animals in each diet cohort were submitted to a glucose tolerance test after eight weeks on the diet. These animals were not used for further behavioral testing.
2.3 Fat Pad Collection
After nine weeks on either the fructose or chow diets at PND89–90, fructose-fed (n=20) or chow-fed (n=16) animals were either rapidly decapitated (fructose: n=10; chow: n=8) or euthanized and perfused with saline for two minutes (fructose: n=10; chow). Epididymal fat pads were collected from both groups, and peri-renal fat pads were collected from the rapidly decapitated group as described by Casteilla et al (Casteilla et al., 2008). Both epididymal and peri-renal fat pads were weighed and normalized to total body weight. In addition, brains were collected and flash frozen on dry ice for later dissection. Trunk blood was collected in EDTA coated tubes and spun at 5400 rcf (3000 rpm in a Srovall SM-24 rotor) for 20 minutes for plasma collection.
2.4 Mixed Modality Stress
As previously described, a mixed modality chronic stress paradigm that has been shown to elicit changes in adolescent rats (Bourke and Neigh, 2011, Bourke et al., 2013) was used in this study. Notably, this mixed modality chronic stress paradigm has previously elicited behavioral effects specifically in female rats and not in male rats (Bourke and Neigh, 2011). However, male rats subjected to this chronic stressor showed greater susceptibility to weight gain (Bourke and Neigh, 2011) and an increased neuroinflammatory response to lipopolysaccharide (Pyter et al., 2013) relative to female rats. Thus, this paradigm and the use of male animals were chosen to maximize the potential to observe an interactive effect of stress and diet without the potential for independent behavioral effects of the stress paradigm to obscure any potential behavioral effects of diet or the possibility of a synergistic effect of the two manipulations.
Animals receiving stress (n=47) were individually housed at PND 35 through the end of the study. These animals were also exposed randomly to either social defeat or restraint for 12 days (PND 37–49). Non-stressed rats remained pair-housed throughout the study (n=67). Social defeat stress was performed during the light phase in the home cage of a mature, territorial, Long-Evans rats for six of the 12 days of defeat. During the social defeat process, an intruder (experimental rat) was placed in the home cage of the resident. After the intruder was attacked by the resident five times on the first day, three times on the second day, and once each day thereafter, or after five minutes, a mesh barrier was placed in the cage, separating the intruder from the resident. This separation continued for 25 minutes. The intruder was then returned to its home cage. The pairings were randomly assigned to prevent stabilization of a dominance hierarchy.
For restraint stress during the remaining six days of stress, animals were placed in a clear acrylic rat restraint (BrainTree Scientific, Braintree, MA, USA) for 60 minutes during the light phase. These restraints prevented head-to-tail turns but did not compress the rat.
2.5 Glucose Tolerance Test
In order to evaluate the effects of a high-fructose diet initiated post-weaning in conjunction with adolescent stress on glucose metabolism, a subset of rats in each group from the adolescent study were submitted to a glucose challenge. In preparation for this challenge (PND79), a subset of periadolescent chow or fructose-fed stress and non-stress rats were fasted overnight. After weight and blood glucose readings were taken by tail prick using a Freestyle glucometer, rats were either given an intraperitoneal glucose bolus (2 g/kg dissolved in saline; n=31) or an equivalent volume of saline (n=29). After one hour, blood glucose was again assessed by tail prick using a Freestyle glucometer. Immediately thereafter, rats were rapidly decapitated, trunk blood was collected, and brains were removed and immediately frozen.
2.6 Insulin Analysis
Insulin was measured in plasma collected from saline- and glucose-treated stressed and non-stressed rats one hour after administration of the saline or glucose bolus, at the conclusion of the glucose tolerance test. Insulin was measured via ELISA (sensitivity 0.1 ng/ml, CrystalChem, Downers Grove, IL). All samples were run in duplicate and those with a CV of <15% were included for analysis.
2.7 Behavioral Testing
For the periadolescent cohort (n=8–14 per group), behavioral testing began in adulthood at PND 76, consisting sequentially of open field (PND 76), a 5-minute elevated plus maze test (PND 92), and a 10-minute forced swim test (PND 94). For the adult cohort, behavioral testing began at PND 116 with the same sequence (n=8 per group). The open field test and the swim test were conducted during the middle of the light cycle and the elevated plus maze was conducted two hours after the onset of the dark cycle. All behaviors were recorded by a video camera that was connected to an automated behavior analysis system (CleverSys, Inc, Reston, VA, USA). Open field: Rats from each group were placed in the center of an open field apparatus that consisted of a square field (75 cm × 75 cm) surrounded by approximately one-meter high plastic walls and allowed to explore for 10 minutes to assess baseline activity. Elevated Plus Maze: An elevated plus maze was used to model anxiety-like behavior in the rats by measuring the time spent in open arms vs the time spent in the closed arms (Pellow et al., 1985, Walf and Frye, 2007) (n=8–14 per group). The specifications for the San Diego Instruments elevated plus maze were as follows: 43 ½” long, 4” wide (arm width), 19 ½” high (open arms), and 31 ½” high (closed arms). During testing, animals were able to freely move from open to closed arms for 5 min. Forced Swim Test: The forced swim test has been utilized as a model for depressive-like behavior (Porsolt et al., 1978, Borsini and Meli, 1988). When used to determine whether chronic stress can elicit “behavioral despair” or depressive-like behavior, a single test session has been sufficiently sensitive (Lutter et al., 2008, Castro et al., 2010). In this test, floating was defined as the animal’s limbs remaining motionless for at least two seconds, and struggling was defined as the animal’s limbs in motion and its head above the surface. Rats were placed in a clear acrylic beaker (40 cm high X 18 cm diameter) filled with room temperature water. Immediately after the end of the single 10 min test, rats were removed from the beaker and rapidly decapitated.
2.8 Corticosterone Analyses
Corticosterone was measured in plasma from blood collected at baseline and immediately after the ten-minute forced swim test in both chow and fructose-fed periadolescent animals via ELISA (sensitivity 27 pg/mL, Enzo Life Sciences, Farmingdale, NY, USA). All samples were run in duplicate.
All blood samples were collected at least two hours before the onset of the animals’ dark cycle. For baseline samples, rats were transferred to a testing room two hours prior to the initiation of euthanasia for acclimation. After two hours, rats were rapidly decapitated within two minutes of handling in a different room separated by two doors from the testing room to prevent transfer of scent and noise and to ensure that decapitation occurred before the rise in plasma corticosterone. For forced swim samples, rats were also transferred to a testing room two hours prior to the initiation of the forced swim test. Subsequently, rats were subjected to the forced swim test for 10 minutes. Upon removal from the test, rats were briefly dried then rapidly decapitated within two minutes of removal from the test. Acclimation, swimming, and decapitation all occurred in separate rooms. Upon decapitation, trunk blood was collected in EDTA coated tubes, and brains were removed and immediately frozen. Blood was spun at 5400 rcf (3000 rpm in a Sorvall SM-24 rotor) for 20 minutes for plasma collection used in ELISAs.
2.9 Whole-Transcriptome RNA-Sequencing
Hypothalamic tissue at PND80 was collected from periadolescent fructose and chow-fed rats for evaluation of changes in gene expression. The brains used were from the saline-control-injected, non-stress rats in the glucose tolerance test as described in Supplemental Table 1. This cohort was used for RNA-seq analysis to ensure that all rats were in a fasting state, since none of the other cohorts were fasting at the time of euthanasia. This fasting state represents a challenge to energy homeostasis, which has been associated with enhancing neural differences in studies of sucrose and sugar consumption in rodents (Myers et al., 1988, Minano et al., 1989, Meguid et al., 2000, Avena et al., 2008). One brain from the fructose-fed cohort was not included in the RNA-seq for reasons of improper handling and storage between removal and RNA extraction. Thus the total number per group was chow, n=7; fructose, n=6.
Trizol RNA Extraction reagent (Life Technologies) and QiaShredder (Qiagen) were used to lyse and homogenize whole hypothalamus. An RNEasy kit from Qiagen was then used to extract RNA. An Agilent 2100 Bioanalyzer was used to assess RNA purity and quality. One microgram of total RNA was then used to build TruSeq (Illumina) mRNA sequencing libraries. A single end 100 base pair sequencing reaction was performed on an Illumina HiSeq 1000, generating ~25 million reads per sample. The raw sequence reads were mapped to the most recent RAT assembly (RGSC5.0) using the STAR aligner (Dobin et al., 2013). Data fragments per kilobase of exon per million fragments mapped (FPKM) were normalized and differential expression was examined using the Cufflinks software suite (Trapnell et al., 2010). All data discussed in this publication have been deposited in NCBI’s Gene Expression Omnibus (Edgar et al., 2002) and are accessible through GEO Series accession number GSE56238.
2.10 Pathway Analysis
Pathway analysis of differentially expressed genes established from RNA sequencing was performed using MetaCore (Thomson Reuters, New York City, NY), a web-based computational platform for analysis of high-throughput molecular data (Ekins 2007). The MetaCore database of pathways, networks, diseases, and individual protein-protein, protein-DNA, and protein-RNA interactions is one of the most comprehensive databases, containing over 4.5 million individual findings that are manually annotated based on literature reviews from over 2,700 peer-reviewed journals. Differentially expressed genes discovered through CuffDiff analysis were uploaded into MetaCore and mapped onto known pathways. Lists of pathways and networks that were significantly enriched with a greater than expected ratio of differentially expressed genes at the FDR < 0.05 were obtained, and specific genes in each pathway were probed for further analysis of function and expression.
2.11 Quantitative RT-PCR
To validate gene expression changes observed in RNA-sequencing and to determine whether these changes extended to either another brain region or the adult cohort, select targets were evaluated in the hypothalamus and the left hemisphere of the hippocampus for the periadolescent cohort used in RNA-sequencing as well as in these same regions of the adult cohort. For all samples, tissue was lysed and homogenized using Trizol RNA Extraction reagent (Life Technologies, Grand Island, NY) and QiaShredder (Qiagen, Valencia, MA). RNA was extracted with an RNEasy kit from Qiagen, then concentration and purity was assessed with a NanoDrop 2000 spectrophotometer (ThermoScientific, Wilmington, DE). RNA was standardized and then reverse-transcribed using the High Capacity RNA to cDNA kit (Life Technologies, Grand Island, NY). cDNA was quantified with the PicoGreen Assay (Invitrogen, Carlsbad, CA) and then standardized to 10 pg/µl. Rat TaqMan Gene Expression Assays were purchased from Life Technologies (Grand Island, NY) with probes labeled with 6-FAM and MGB (non-fluorescent quencher) at the 5’ and 3’ ends, respectively: Pomc (Rn00595020_m1), Crfr1 (Rn00578611_m1); Crfr2 (Rn00575617_m1) and the housekeeping genes Hprt1 (Rn01527840_m1), Ac tb (Rn00667869_m1), and Tfrc (Rn01474701_m1). After assessment of suitability, Tfrc was used as a housekeeping gene for all samples.
The following two-step RT-PCR cycling conditions were used on the 7900HT Sequence Detection System (Applied Biosystems): 50°C (2 min), 95°C (10 min), 40 cycles of 95°C (15 s) and 60°C (1 min). Relative gene expression of individual samples run in triplicate (with coefficient of variation cut-off set to 4%) was determined by the comparative ΔΔCT quantification method with fold change to standard chow of a given developmental cohort. All TaqMan gene expression assays are guaranteed to have 90 – 100% amplification efficiency as determined by the genome-aided probe and primer design pipeline and reported in the “Amplification Efficiency of TaqMan Gene Expression Assays” Application Note 127AP05-03 from Life Technologies.
2.12 Statistical Analysis
IBM SPSS Statistics (Version 20) and Graphpad Prism (Version 6.0) were used for statistical analysis and graphing of weight, blood glucose, and food consumption. Unpaired two-tailed Student’s t-tests or Analysis of Variance tests were performed with α=0.05. Holm-Sidak post-hoc testing was performed when appropriate. For analysis of RNA-seq data, differential expression was set as transcripts in the Fructose dataset that passed False Discovery Rate (FDR)<0.05 as determined using the Cufflinks software suite. This list of transcripts was then imported into the MetaCore™ Analysis Suite (Thomson Reuters, New York City, NY) for pathway analysis. Significant enrichment in pathway map folders and pathway maps was set at FDR<0.05.
3. Results
3.1 Periadolescent High-Fructose Diet Increases Caloric Efficiency, Fasting Glucose, and Visceral Fat Pad Mass
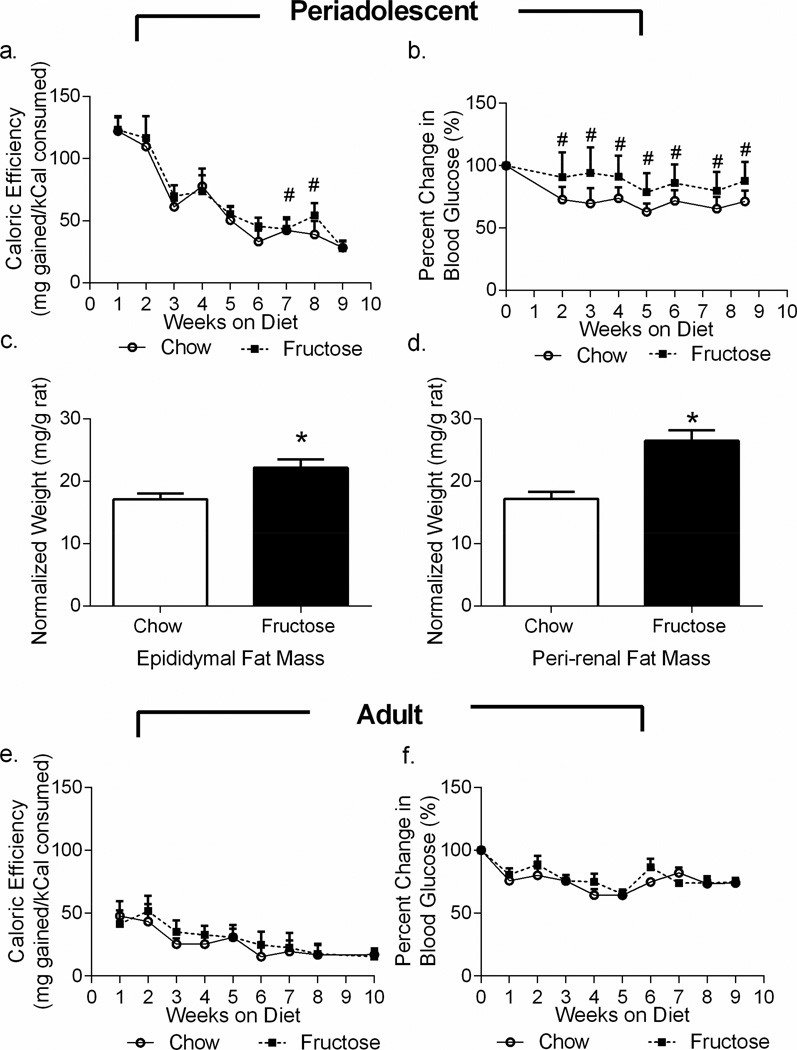
As determined by repeated-measures ANOVA, rats fed a high-fructose diet beginning at weaning had increased caloric efficiency relative to chow-fed rats, calculated as mg gained per kCal consumed (effect of diet; F1,34=6.855, p=0.0131; effect of time: F8,272=488.2, p<0.0001; Figure 1a), indicating that the fructose-fed rats gained more weight per kCal consumed. Neither the average weekly weight (effect of diet: F1,34=1.263, p=0.2690) nor the average kCal consumed per week (F1,16=0.2791, p=0.6045) differed significantly between groups when assessed by repeated measures ANOVA. For both weight and kCal, however, there were significant effects of time (time effect for weight: F9,306=2998, p<0.0001; time effect for kCal: F8,128=91.05, p<0.0001). Rats fed a high-fructose diet during periadolescence had elevated fasting glucose beginning only 20 days after initiating the diet (effect of diet: F1,34=17.02, p=0.0002; effect of time: F7,238=42.80, p<0.0001; Figure 1b). Raw values for periadolescent weight, kCal, and blood glucose are listed in Supplemental Tables 3, 4, and 5. In addition, both peri-renal fat mass (t16=4.270; p=0.0006, Figure 1c) and epididymal fat mass (t34=3.034; p=0.0046, Figure 1d) were increased in the periadolescent fructose-fed cohorts.
Figure 1. Periadolescent High-Fructose Diet Alters Metabolic Parameters.
a. Rats fed a high-fructose diet during periadolescence had increased caloric efficiency relative to chow-fed rats. b. Periadolescent fructose-fed rats had significantly higher fasting blood glucose relative to chow-fed rats beginning within 20 days on the diet. c, d. Both epididymal and retroperitoneal fat mass were increased in the periadolescent fructose-fed rats relative to their chow-fed controls. e, f. In rats fed the high-fructose diet during adulthood only, neither caloric efficiency nor blood glucose significantly differed from chow-fed rats. Data shown are mean ± SEM; asterisk indicates an effect of diet in the t-test and a hash tag indicates a significant post-hoc effect between diets at the same time point with p < 0.05.
We also evaluated changes in caloric efficiency and fasting glucose by repeated measures ANOVA in the cohort of rats fed either chow or the fructose diet in adulthood only (PND64-134). Diet had no effect on caloric efficiency (effect of diet: F1,12=1.475, p=0.2480, effect of time: F8,96=26.61, p<0.0001 Figure 1e). In addition, neither weight (weight effect: F1,14=2026, p=0.6596; time effect: F10,140=343.1, p<0.0001) nor kCal (kCal effect: F1,6=4.687, p=0.0736; time effect: F7,42=4.356, p=0.0010) significantly differed between diets when assessed by repeated measures ANOVA. Moreover, diet had no effect on fasting blood glucose in the adult cohorts over time (diet effect: F1,14=0.6751, p=0.4259; time effect: F9,126=12.25, p<0.0001, Figure 1f).Raw values for adult weight, kCal, and blood glucose are listed in Supplemental Tables 3, 4, and 5. Fat pads were not measured in the adult animals.
3.2 Periadolescent High-Fructose Diet and Stress Alter Weight Gain but Only Diet Increases Fasting Glucose
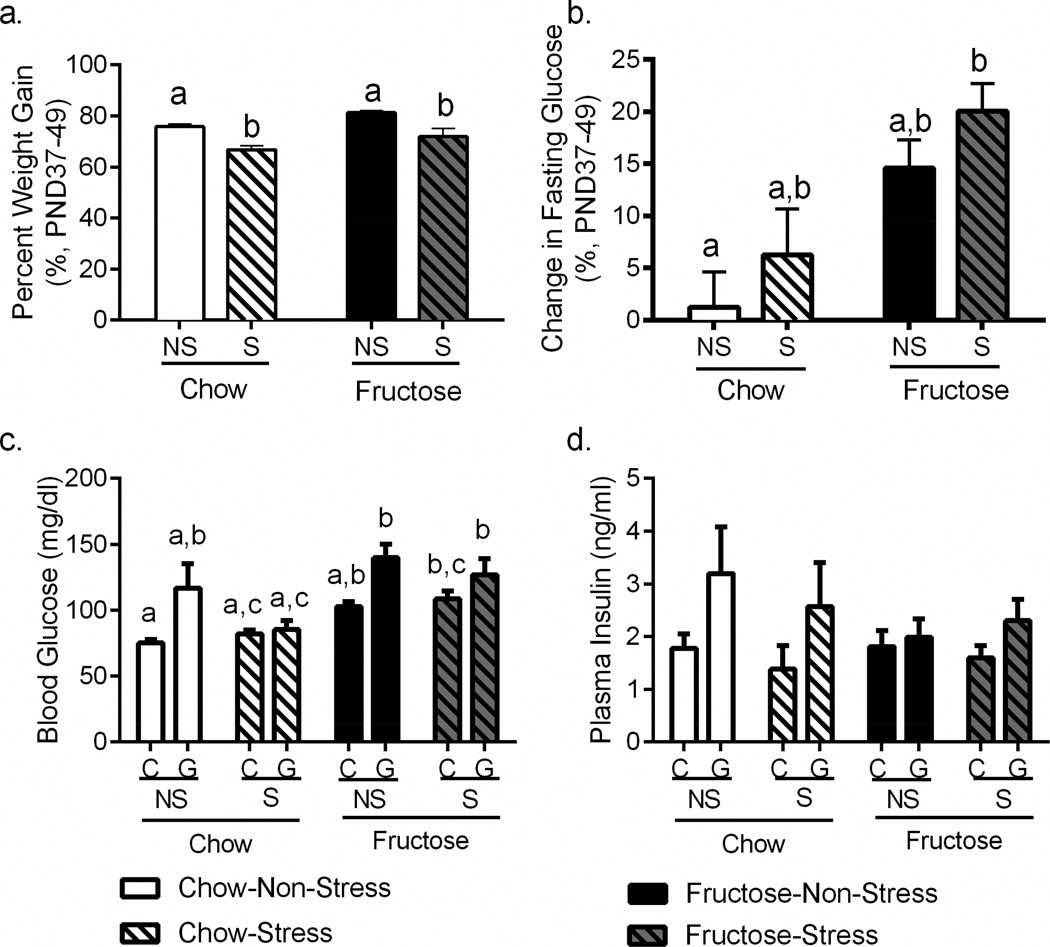
Though our primary metabolic endpoints were assessed in our non-stress cohorts, we analyzed weight gain in our stress cohorts to confirm the effects of stress and to evaluate potential interaction between stress and diet, as palatable food consumption has been shown to alter stress responses (Dallman et al., 2003). The percent of weight gained during the mid-adolescent stressor (PND37-49) was reduced in the stress cohorts (F1,135=28.63, p<0.0001; Figure 2a) and fructose-fed animals gained more weight during this period (F1,135=9.543, p=0.0024; Figure 2a), though post-hoc testing did not reveal individual significant differences between groups. The fructose diet also significantly increased fasting glucose during mid-adolescence (F1,131=11.37, p=0.0010; Figure 2b) irrespective of stress history, and post-hoc testing revealed a step-wise increase in change in fasting blood glucose across diet and stress groups. Glucose administration in the glucose tolerance test raised blood glucose in both chow and fructose stress & non-stress groups (main effect of glucose: F(1,24)=12.01, p=0.0020) as expected. Fructose diet exacerbated this effect and fructose-fed rats had significantly higher blood glucose in the glucose challenge than the standard chow fed rats (p<0.05; Figure 2c). No effect of stress was detected, nor an interaction with stress observed for either glucose administration or diet (p>0.40 for all effects). Only a main effect of glucose administration (F1,42=5.0697, p=0.0263, Figure 2d) was observed in analysis of plasma insulin; neither fructose nor stress, nor an interaction of either with the other or with glucose altered plasma insulin (all p>0.25).
Figure 2. Adolescent Stress and Periadolescent Fructose Affect Weight Gain and Blood Glucose.
a. The percent of weight gained during the mid-adolescent stressor (PND37-49) was reduced in the stress cohorts (p<0.05) and fructose-fed animals gained more weight during this period (p<0.05). b. The fructose diet also significantly increased fasting glucose during mid-adolescence (p<0.05) irrespective of stress history. c. Glucose administration in the glucose tolerance test raised blood glucose in both chow and fructose stress & non-stress groups (p<0.05) as expected. However, fructose diet exacerbated this effect and fructose-fed rats had significantly higher blood glucose in the glucose challenge than the standard chow fed rats (p<0.05). d. Only a main effect of glucose administration (p < 0.05) was observed in analysis of plasma insulin; neither fructose nor stress, nor an interaction of either with the other or with glucose altered plasma insulin. Data shown are mean ± SEM; different letters indicate significant differences (p < 0.05) as assessed by post-hoc testing. NS=Non-stressed; S=Stressed; C=Saline-injected control; G=Glucose-injected
3.3 Periadolescent High-Fructose Diet Increases Anxiety-like Behaviors
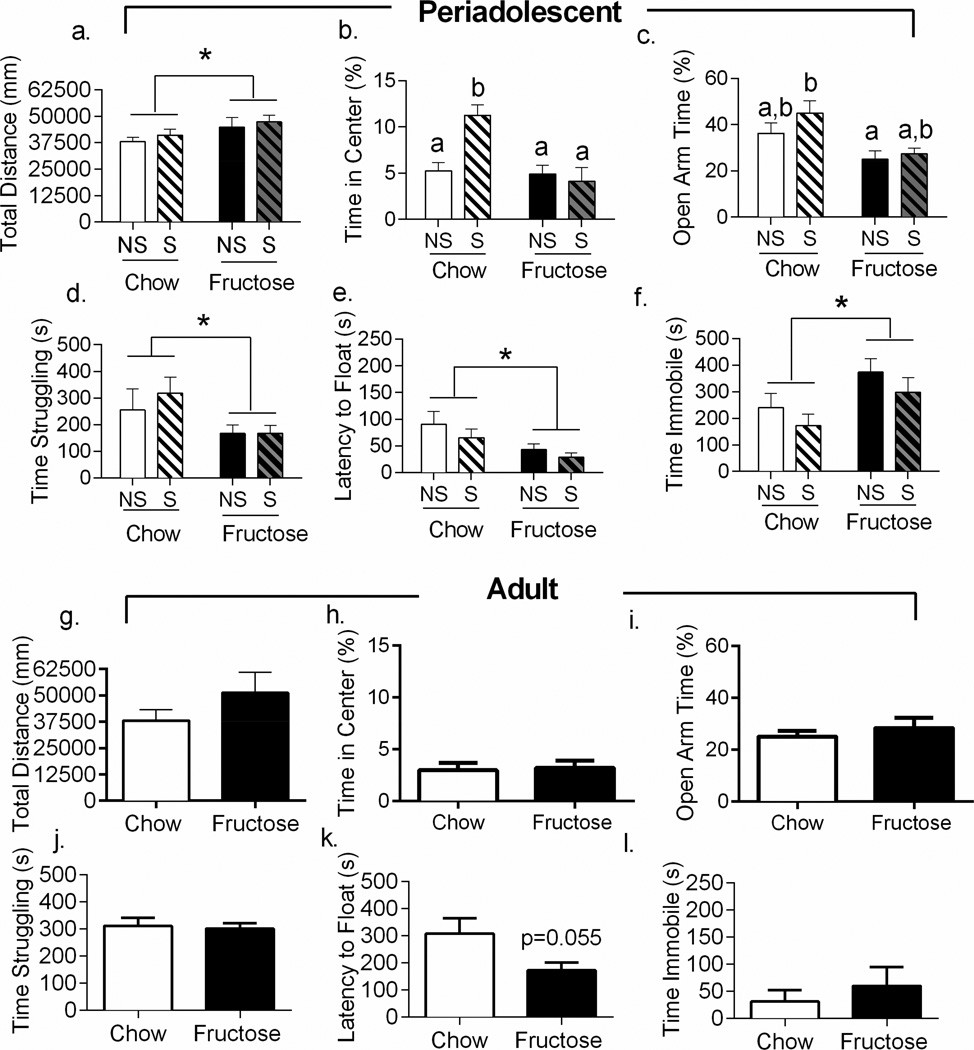
Rats that began consumption of the high-fructose diet at weaning showed elevated levels of anxiety-like behaviors in adulthood after 8–10 weeks on the diet (n=8–14 per diet & stress group). Anxiety-like behavior was assessed using the open field (Prut and Belzung, 2003) and elevated plus maze (Pellow et al., 1985). In the open field, periadolescent fructose-fed rats traveled farther (F1,34=4.814, p=0.0352, Figure 3a) and faster (F1,34=5.083, p=0.0307, data not shown) than the standard chow-fed rats in the open field test. Stress did not affect either of these measures (p>0.05 for all effects) nor was there an interaction between stress and diet (p>0.05). Diet (F1,34=10.86, p=0.0023; Figure 3b) and stress (F1,34=5.241, p=0.00284) both independently affected central tendency, and also interacted to affect central tendency (F1,34=8.974, p=0.0051). Post-hoc testing indicated that the stress effect was due to an increase in central tendency in the chow-fed stressed cohort (p=0.0017 versus non-stressed chow-fed cohort).
Figure 3. Periadolescent High-Fructose Diet Increases Anxiety-Like Behavior.
a. Periadolescent fructose-fed rats showed increased activity in the open field. b. Fructose-feeding during periadolescence reduced central tendency, while adolescent stress increased central tendency. c. Periadolescent fructose-fed rats reduced time spent in the open arms of the elevated plus maze. d, e, f. Periadolescent fructose-fed rats spent significantly less time struggling, had reduced latency to float, and spent more time immobile in the forced swim test than standard chow fed rats. g, h, i. Rats fed the high-fructose diet in adulthood only did not differ from chow controls in open field or elevated plus maze behavior. j, k, l. Rats that consumed the fructose diet in adulthood only did not change in either struggling (p>0.05) or immobility (p>0.05) in the forced swim. However, there was a numeric reduction in latency to float in the adult fructose-fed rats (t14=2.094 p=0.0549). Data shown are mean ± SEM; asterisk indicates a main effect of diet while letters indicate significant post-hoc effects with p < 0.05. NS=Non-stressed; S=Stressed
Consistent with an anxiogenic phenotype, rats fed a high-fructose diet throughout adolescence spent less percent time in the open arms of the elevated plus maze than standard chow-fed rats (F1,34=9.838, p=0.0035, Figure 3c). Chronic stress neither independently altered percent time in the open arms nor interacted with the high-fructose diet to modify behavior (p>0.05 for both). When assessing total arm entries, the high-fructose diet increased entries (F1,34=4.252, p=0.0469) and diet and stress interacted to affected number of arm entries (F1,34=8.543, p=0.0061, data not shown). Post-hoc testing revealed that this effect was due to a significantly greater number of total entries made by stressed high-fructose-fed rats than by stressed chow-fed rats (p=0.0125).
High-fructose diet consumption in adulthood only did not alter either locomotor activity (t14=1.183, p=0.2567, Figure 3g) or central tendency (t14=0.2562, p=0.8015, Figure 3h) in the open field. In the elevated plus maze, rats that started the fructose diet in adulthood also behaved similarly to chow-fed rats (open arm percent time: t14=0.7009, p=0.4948, total arm entries: t14=0.7658, p=0.4565; Figure 3i).
3.4 Periadolescent High-Fructose Diet Increases Depressive-like Behaviors
Rats that began consumption of the high-fructose diet at weaning showed elevated levels of depressive-like behaviors in adulthood after 8–10 weeks on the diet (n=8–14 per diet & stress group). In the forced swim test, fructose-fed rats spent less time struggling than standard chow fed rats (F1,22=4.501, p=0.0165; Figure 3d) and had reduced latency to float (F1,23=6.825, p=0.0156; see Figure 3e). Further, the fructose-fed rats spent more time immobile in the forced swim test (F1,22=6.745, p=0.0454, Figure 3f). Contrary to our original hypothesis, stress did not independently influence behavior nor did it interact with diet in the forced swim test in any measure regardless of diet (p>0.05 for all measures). The reduced activity in the forced swim test could not be attributed to reduced motor activity because, as previously described, fructose-fed rats traveled farther (F1,34=4.814, p=0.0352, Figure 3a) and faster (F1,34=5.083, p=0.0307, data not shown) than the standard chow-fed rats in the open field test.
Rats fed the high fructose diet during adulthood only did not change their behavior relative to their chow-fed controls in either struggling (t14=0.2769, p=0.7859, Figure 3j) or immobility (t14=0.7054, p=0.4921, Figure 3k) in the forced swim, and the reduction in latency to float in the adult fructose-fed rats did not reach significance (t14=2.094, p=0.0549, Figure 3l).
3.5 Periadolescent High-Fructose Diet Interacts with Acute Stress to Alter Plasma Corticosterone
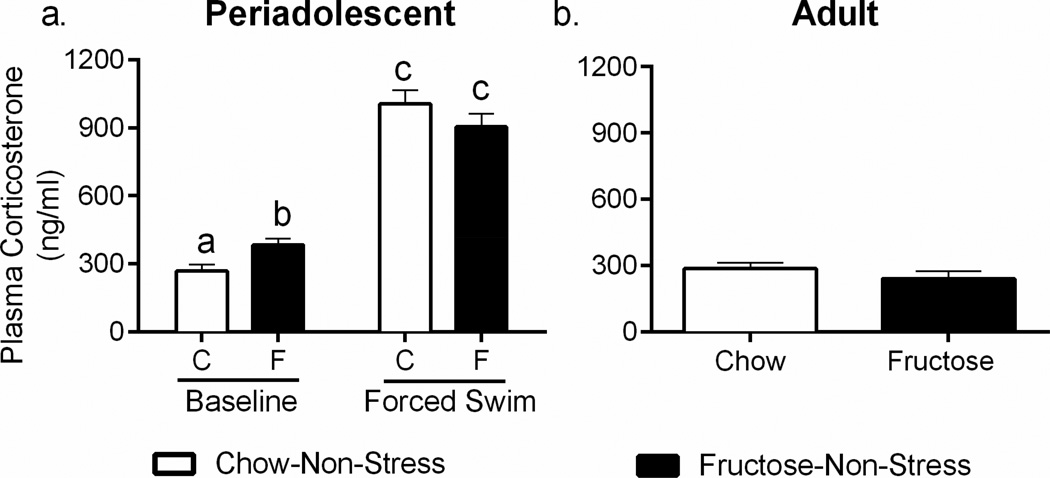
We evaluated basal and acute stress-induced HPA axis output through examination of terminal plasma corticosterone concentrations. Forced swim stress elevated plasma corticosterone as expected (F1,40-239.5; p<0.0001) and diet interacted with forced swim to affect plasma corticosterone (F1,40-7.042; p=0.01; Figure 4a). This interaction could be explained by a significant elevation of plasma corticosterone at baseline in the fructose-fed animals (mean difference 114.1 ng/ml, p<0.05), but no significant difference between chow-red and fructose-fed animals existed after the FST (mean difference −101.9 ng/ml, p=0.12).
Figure 4. Periadolescent High-Fructose Diet Elevates Baseline Corticosterone.
a. Plasma corticosterone was analyzed and demonstrated significant increases in basal corticosterone in the periadolescent fructose-fed cohort without differences in the forced swim test. b. Rats fed the high-fructose diet during adulthood only did not differ from chow-fed animals in plasma corticosterone. Data shown are mean ± SEM; different letters indicate significant effects in post-hoc testing with p < 0.05. C=Chow, F=Fructose
To determine whether the effects of fructose on plasma corticosterone were specific to developmental exposure, we examined the basal corticosterone concentrations in rats fed the high-fructose diet or chow in adulthood only. No effects of high-fructose diet consumption in the adult cohort were observed (t14=1.065, p=0.3048; Figure 4b).
3.6 Periadolescent High-Fructose Diet Remodels the Hypothalamic Transcriptome with Greatest Impact on POMC Processing
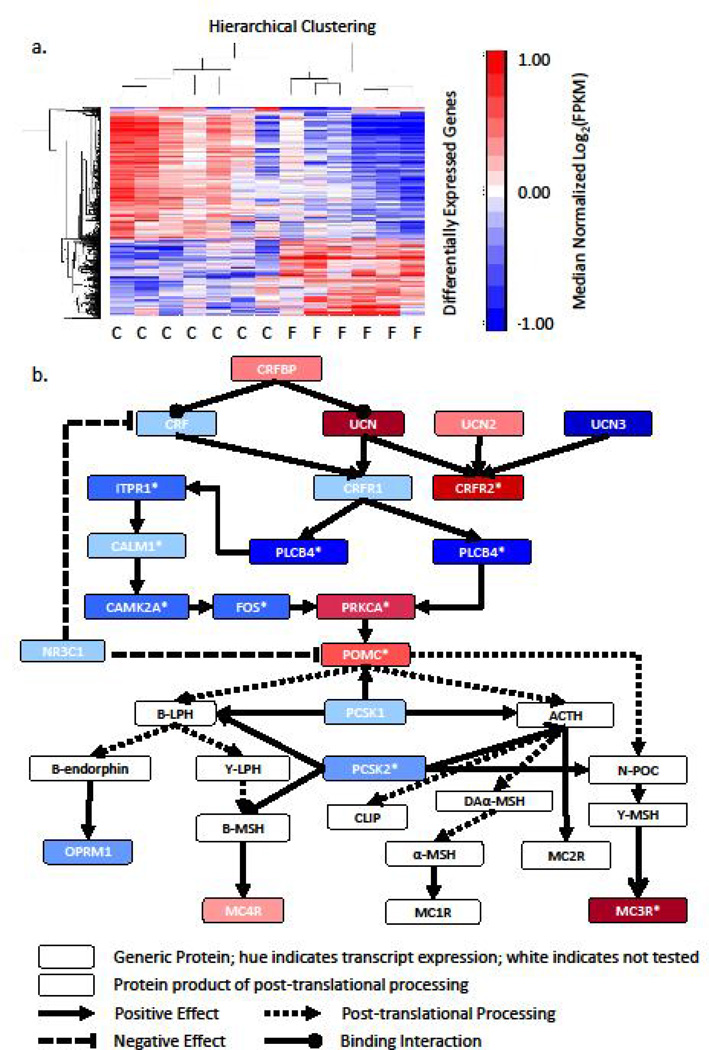
Given the robust effects of periadolescent fructose diet on behavior (Figure 3), the HPA axis (Figure 4), and metrics of metabolism (Figure 1 & Figure 2), we examined the consequences of a periadolescent high-fructose diet on the hypothalamic transcriptome. Hypothalamic tissue at PND80 was collected from periadolescent fructose and standard rats for evaluation of expression changes. RNA was extracted and used for whole-transcriptome RNA sequencing. Of the 17,366 transcripts assessed, 966 or 5.56% were significantly differently expressed between the standard chow cohort and the fructose chow cohort, after correction for the false discovery rate (Figure 5a). Analysis was performed using the MetaCore™ Analysis Suite (Thomson Reuters, New York City, NY) to determine pathways and networks that were significantly enriched with differentially expressed genes. Pathway analysis revealed significant enrichment of97 pathways with differentially expressed genes (all q<0.05; Supplementary Table 6, top 25 pathways). Notably, multiple pathways relating to HPA axis function, including “POMC Processing” (the pathway with the highest ratio of significantly altered transcripts), “Post-translational processing of neuroendocrine peptides,” and “Corticoliberin signaling via CRFR1” were significantly enriched with differentially expressed genes (all q<0.001). Network analysis, which can link multiple pathways through interacting genes, demonstrated significant enrichment of 25 networks with differentially expressed genes (all q<0.05, Supplementary Table 7), with the top ranking networks pertaining to synaptic contact and neuropeptide signaling. Significantly differentially expressed genes in the CRF receptor and POMC processing pathways are shown in Figure 5b by denotation with an asterisk.
Figure 5. Periadolescent High-Fructose Diet Remodels the Hypothalamic Transcriptome, Notably CRF Signaling and POMC Processing.
a. Heatmap of log2 transformed expression values (FPKM) of the 966 differently expressed transcripts between the two diet conditions (Chow: n=7; Fructose: n=6) in the periadolescent cohort. Zero roughly corresponds to mean expression level. b. Components of the POMC processing pathway and CRF signaling are combined to show altered genes in these pathways. Hues of the boxes indicate relative transcript expression (log2(FPKM)) in the fructose-fed animals. Differentially expressed genes that pass multiple testing corrections are denoted by an asterisk. C=Chow, F=Fructose
3.7 High-Fructose Diet Upregulates Hypothalamic Expression of Crfr2 when Consumed during Periadolescence
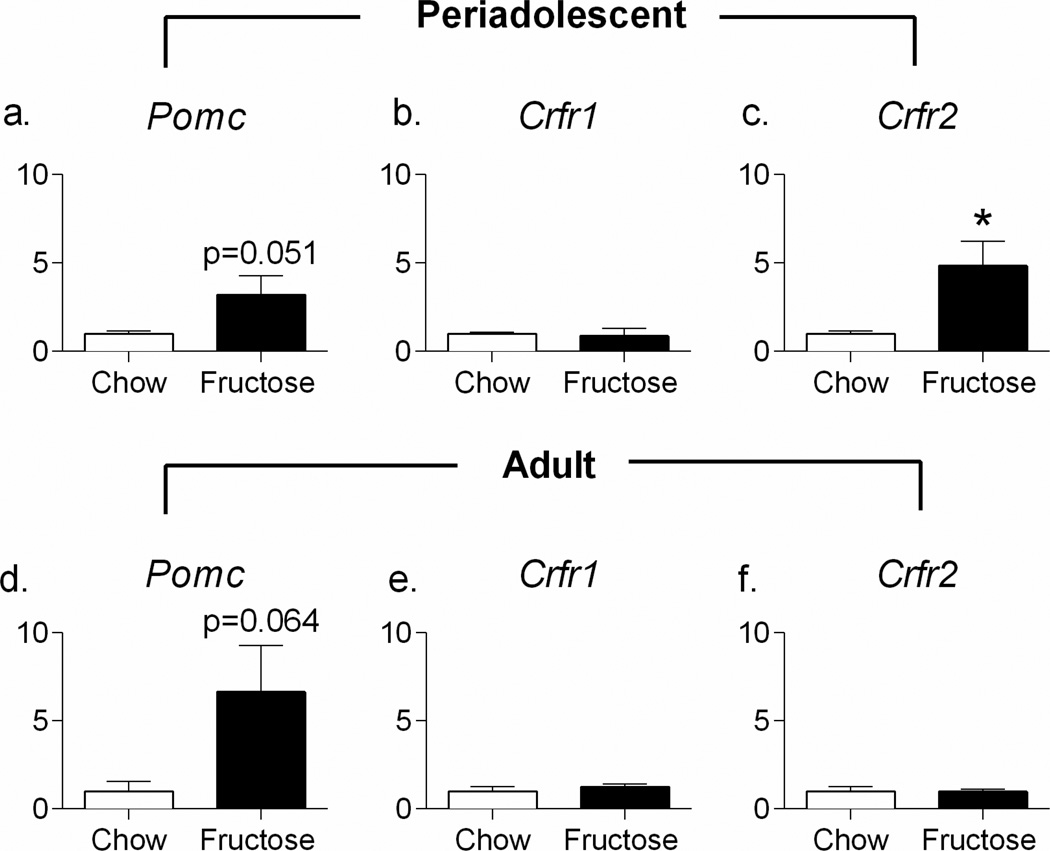
Quantitative RT-PCR was used to validate three genes, Pomc, Crfr1, and Crfr2, that were identified in RNA-sequencing as potentially altered due to periadolescent high-fructose diet. As shown in Figure 5b, hypothalamic Pomc and Crfr2 were significantly upregulated in the RNA-sequencing dataset, while Crfr1 was non-significantly downregulated. Consistent with these findings, RT-PCR indicated upregulation of hypothalamic Crfr2 (t11=2.978; p=0.0126, Figure 6c), while, despite greater than a 5-fold change in expression, Pomc did not reach significance (t11=2.192; p=0.0508, Figure 6a) in periadolescent fructose-fed animals, and there was no effect on Crfr1 (t11=0.3224; p=0.7532, Figure 6b). In animals fed fructose during adulthood only, the upregulation in Pomc was again greater than 5-fold, but did not reach significance (t10=2.087; p=0.0635, Figure 6d), and no effect was observed on either Crfr1 (t9=0.8502; p=0.4173, Figure 6e) or Crfr2 (t4=0.0732; p=0.9452, Figure 6f). In addition, fructose consumption had no effect on hippocampal Pomc, Crfr1, or Crfr2 in either the periadolescent or the adult cohort (all p>0.10, data not shown)
Figure 6. High-Fructose Diet Upregulates Hypothalamic Expression of Crfr1 when Consumed during Periadolescence.
RT-PCR indicated upregulation of hypothalamic Crfr2 (c) and a non-significant 5-fold upregulation of Pomc (a) in periadolescent fructose-fed animals without an effect on Crfr1 (b). In animals fed fructose during adulthood only, there was a non-significant 5-fold upregulation of Pomc (d) but no effect was observed on either Crfr1 (e) or Crfr2 (f).
4. Discussion
Consumption of a diet high in fructose altered behavior, physiology, and gene expression, irrespective of the rats’ stress histories, yet dependent on the developmental stage during which the diet was consumed. Only rats fed the high-fructose diet beginning at weaning showed increased anxiety-like and depressive-like behaviors in adulthood; rats that began the diet after reaching adulthood did not manifest the same behaviors. Moreover, rats fed fructose since weaning had elevated basal corticosterone relative to chow-fedrats. Surprisingly, the combination of the high-fructose diet with exposure to chronic adolescent stress did not produce any additional effects. Finally, the periadolescent high-fructose diet induced remodeling of the hypothalamic transcriptome, with 966 transcripts significantly altered and a dramatic enrichment of altered transcripts in the POMC pathway.
Chronic periadolescent fructose exposure induced both anxiety-like and depressive-like behaviors when assessed in adulthood. Periadolescent fructose-fed ratstraveled less in the open arms of the elevated plus maze (Figure 3c), a validated test of anxiety-like behavior in the rat (Pellow et al., 1985). Similarly, diet reduced central tendency (Figure 3b), another metric of anxiety (Prut and Belzung, 2003) in the animals exposed during periadolescence. While reductions in open field activity are sometimes associated with anxiety-like behavior, hyperactivity in the open field (Figure 3a) has also been linked to anxiety-like behavior (Murphy et al., 1996, Ito et al., 2010). In the forced swim test, a validated test of depressive-like behavior (Porsolt et al., 1977), periadolescent fructose-fed rats struggled less, exhibited a decreased latency to float, and increased floating time in the forced swim test, consistent with a depressive-like phenotype (Figure 3d–f). Adolescent stress had no effect on any of these metrics. In addition, the increased immobility was observed alongside increased activity in the open field, demonstrating that consumption of a high-fructose diet did not cause a global reduction in motor behavior (Figure 3a). Moreover, hyperactivity in the open field has been linked to anxiety-like behavior (Ito et al., 2010).
As the animals that consumed fructose during only adulthood had no difference in elevated plus maze behavior or immobility and struggle behavior in the forced swim test (Figure 3), the effects on anxiety- and depressive-like behavior induced by the high-fructose diet appeared to be developmentally specific. We did observe a non-significant decrease in the latency to float (p = 0.055) for the adult males fed a high fructose diet, but because this behavioral shift was not reflected in the other parameters of forced swim behavior this suggests that, if a behavioral effect is present after adult fructose, it is substantially attenuated compared to when the high fructose diet is initiated in periadolecence. In terms of metabolic abnormalities, rats fed the high-fructose diet during adolescent development also appeared uniquely susceptible relative to rats fed the diet during adulthood only. Periadolescent rats also had increased adiposity, though this effect was not tested in adult animals. Interestingly, though the effects of stress on behavior were not significant, both stress and diet affected energy homeostasis. During the stress period, stress reduced weight gain, while the fructose diet promoted increased weight gain (both main effects; Figure 2). Though only fructose had a significant effect on blood glucose both during the stress period (Figure 2b) and during the glucose tolerance test (Figure 2c), it is notable that the combined effects of stress and fructose induced a step-like pattern to increase blood glucose during the stress period. The single time point in the glucose tolerance test is a limitation of this study, as we cannot determine whether changes in blood glucose in the fructose-fed rats are due to a prolonged return to baseline or an elevated peak. Other studies have done such a time-course study using male rats fed a high-fructose diet (60–66% fructose) for two (Catena et al., 2003), eight (Huang et al., 2004), or ten weeks (Nakagawa et al., 2006) in adulthood only. Similar to the present study, none of these experiments observed baseline differences in fasting blood glucose in the animals fed in adulthood; however, after two weeks on the diet, differences in blood glucose were observed at 15 and 30 minutes after administration of a glucose bolus (Catena et al., 2003). After eight weeks on the diet, blood glucose differed between chow and fructose-fed rats at 120 minutes after glucose administration; but after ten weeks on the diet, blood glucose was equivalent at all time points between the two diet groups. All of these studies, however, observed significant differences in plasma insulin by the thirty-minute time point (Catena et al., 2003, Huang et al., 2004, Nakagawa et al., 2006). Conversely, no differences in plasma insulin were observed in the present study, indicating that insulin resistance had not developed at the time point examined. Future studies will be necessary to determine the dynamics of the response in periadolescent stressed and non-stressed animals and to further assess the dynamics in adult animals.
Unlike rats fed high fructose in adulthood only, periadolescent fructose-fed rats also had elevated baseline plasma corticosterone relative to the standard chow-fed rats, irrespective of stress history (Figure 4) but consistent with earlier studies showing that acute fructose consumption raises corticosterone levels in rats (Brindley et al., 1981, Brindley et al., 1985). Dietary macronutrients are also known to affect peripheral glucocorticoid metabolism, such that higher fat/lower carbohydrate diets can alter hepatic and adipose 11β hydroxysteroid dehydrogenase (11βHSD) as well as 5α- and 5β-reductase (Stimson et al., 2010). Similar changes in glucocorticoid metabolism have been observed in the context of obesity, with notably lower hepatic 11βHSD in obese patients resulting in impaired activation of cortisone to cortisol in the liver (Stewart et al., 1999) and potentially leading to an increased drive of the HPA axis (Rask et al., 2001). In addition, changes in corticosterone output have frequently been observed in chronic stress models (Plotsky and Meaney, 1993) and in adolescent stress models specifically (McCormick et al., 2011, Bourke et al., 2013). Such dysfunctional HPA axis activity has similarly been linked to clinical mood disorders (Heim et al., 2008a).
It is possible that the periadolescent animals exhibited greater effects from the high-fructose diet because it was administered across a period of vulnerability or a potential “critical period”. This concept of “critical periods” of development has been extensively examined across multiple developmental periods, including both prenatal (McCormick et al., 1995, Pankevich et al., 2009, Tamashiro et al., 2009) and adolescent (McCormick and Mathews, 2007, Bourke and Neigh, 2011) periods. Hormonal changes that occur during adolescence include adrenarche, or the increase in output of adrenal hormones, and gonadarche, the pubertal increase in output of gonadal hormones (Spear, 2000), which may be partially responsible for the vulnerability of this period. Puberty is also highly linked to metabolic changes because the onset of puberty is more highly linked to body weight and food intake than chronological age (Kennedy and Mitra, 1963), likely mediated by neuroendocrine factors such as leptin (Mantzoros et al., 1997). In the present study, the dietary manipulation extending throughout adolescence and into adulthood had greater effects on behavioral, hormonal, and genetic outcomes than when the diet was administered during adulthood only. While it is impossible to pinpoint the exact timing of such a critical period for the dietary administration from the given evidence, the data do indicate an age-related vulnerability in this younger cohort.
In the present study, periadolescent high-fructose diet, though damaging on metabolic and behavioral outcomes, did not create a susceptibility to behavioral effects of chronic adolescent stress exposure. Consistent with previous reports on the effects of chronic mixed modality stress in adolescent male rats (Bourke and Neigh, 2011), this chronic adolescent stress paradigm had only minor effects on behavior. This particular stressor was selected to facilitate observation of the additive effects of diet and stress, and to determine whether an additional “hit” in the form of diet could precipitate stress effects on behavior not previously observed in male rats on a standard diet. While no interaction was observed between diet and stress on depressive- and anxiety-like behavioral outcomes in this study, it is possible that a stress paradigm that has been established to impact male behavior may interact with diet (McCormick et al., 2012). Nonetheless, the data presented indicate that a periadolescent high-fructose diet has a greater impact on male rat behavior than chronic adolescent stress, and that the dietary impact on behavior may be specific to the developmental period of exposure.
Adding to these behavioral and physiological changes, the periadolescent high-fructose diet also reprogrammed hypothalamic transcript expression, affecting over 5% of all transcripts, with the POMC pathway and other pathways relating to processing of neuroendocrine peptides particularly affected (Figure 5; Supplementary Tables 6, 7). POMC is a precursor polypeptide primarily produced in the hypothalamic arcuate nucleus and anterior pituitary that is cleaved enzymatically into multiple downstream hormones, and adipose tissue-derived leptin acts on POMC neurons in the arcuate nucleus to promote satiety (Cowley et al., 2001). RNAseq analysis as well as qRT-PCR validation indicated that Pomc was upregulated 5-fold in the hypothalamus of both periadolescent and adult fructose-fed animals, although this change did not reach significance (Figures 5 & 6). This is directionally consistent with evidence from high-fat diet studies in mice indicating that exposure to a high-fat diet induces upregulation in Pomc as a potential defense against obesity (Ziotopoulou et al., 2000), and indicates potential susceptibility to hypothalamic remodeling in both the periadolescent and adult animals. However, remodeling appeared to be more extensive in the periadolescent animals, as RNAseq analysis and PCR validation revealed that Crfr2 was upregulated in the periadolescent high-fructose cohort but not the adult high-fructose cohort. In response to stress, secreted CRF stimulates CRFR1, which has a higher affinity for CRF than CRFR2 (Charmandari et al., 2005) and is traditionally associated with depressive-like and anxiety-like behavior (reviewed in (Bale and Vale, 2004)). Consistent with this finding that periadolescent fructose consumption alters CRFR2, the urocortins, which activate CRFR2 and may be involved in modulating the stress response (Bale and Vale, 2004, Charmandari et al., 2005), were also altered in RNAseq analysis (Figure 5b). While deficiency in CRFR2 is associated with increased stress sensitivity (Bale et al., 2000), upregulation in the periadolescent fructose-fed animals may be a compensatory mechanism to combat the increases in baseline corticosterone, depressive- and anxiety-like susceptibility, and divergence from the HPA axis’ homeostatic norm. It is possible that increases in Pomc expression in both periadolescent and adult animals indicate hypothalamic remodeling to counteract the metabolic effects of the diet, while only the periadolescent animals demonstrate remodeling to counteract the anxiogenic effects of the diet. However, both of these changes may underlie behavioral, metabolic, and hormonal outcomes seen in the fructose-fed rats.
Taken together, the results in this study indicate that consumption of a high-fructose diet from weaning throughout adolescent development and into adulthood is sufficient to alter metabolic indices, increase basal plasma corticosterone, induce anxiety- and depressive-like behaviors, and promote widespread changes in the hypothalamic transcriptome, particularly affecting genes and pathways related to stress and feeding. These results are consistent with the literature indicating that disruptions to energy homeostasis, such as in states like obesity and diabetes, are associated with depressive-like behavior and altered HPA axis function. Future research will be necessary to disentangle the effects of the metabolic effects of fructose from any potential direct effects of this monosaccharide on neural and hormonal function. Pair-feeding could be one way to address this issue that would shed light on the effect of food intake and shifts in basal metabolism on outcome. However, in light of the reduced consumption yet equivalent weight gain (consistent with the increased caloric efficiency) in the periadolescent animals, “pair-starving” the chow group, or force-feeding the fructose group, might only exacerbate the effects. Alternative methods to address this confound between the direct versus indirect effects of fructose could be to induce increased adiposity or hyperglycemia through other means, potentially by comparing diets (i.e., introduction of a high-fat diet); or by pharmacologically inducing hyperglycemia or genetically inducing obesity. While dietary reversal is outside the scope of the current study, it is a fascinating question for future research. The literature regarding reversibility of diet or metabolic dysfunction after consumption during a critical stage is mixed. For example, maternal high-fat diet can induce epigenetic changes associated with changes in body size and insulin sensitivity lasting up to two generations (Dunn and Bale, 2009). However, weaning onto a chow diet after exposure to a maternal high-fat diet reverses body mass differences in non-stressed rats (Tamashiro et al., 2009). The persistence of effects after consumption during adolescent development remains to be determined and is an important future direction of study.
In conclusion, we expand on prior studies that have examined stress responses in diet models to demonstrate effects of high-fructose diet and stress on behavior, physiology, and gene expression. This study indicates that a high-fructose diet promotes depressive- and anxiety-like behavior independent of chronic adolescent stress in male rats. The data corroborate the importance of the periadolescent period, as the effects of the high-fructose diet were evident only when consumed throughout adolescence. We also demonstrate a substantial shift (more than 5% of genes affected) in hypothalamic gene expression after consumption of a high-fructose diet during adolescence, most notably in genes and pathways regulating stress and feeding. Collectively, the data presented herein indicate a strong potential for diet, specifically high-fructose consumption, to alter behavior and reprogram the HPA axis both in terms of function and at the level of the transcriptome in the male rat.
Supplementary Material
Highlights.
Periadolescent high-fructose diet induces depressive- and anxiety-like behavior.
Periadolescent diet alters HPA axis output while promoting metabolic dysfunction.
Reduced adulthood susceptibility to the diet indicates developmental vulnerability.
Periadolescent high-fructose diet alters expression of 966 hypothalamic genes.
POMC processing and CRF signaling pathways are enriched with altered genes.
ACKNOWLEDGEMENTS
The authors wish to thank Emily Hardy, Jordan Kohn, Nirav Patel, Greg Tharp, and James Walton for their technical assistance. Funding for the studies in this manuscript was provided by R21MH091321-01 (GNN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors CSH, JB, SDK, ZPJ, and GNN declare that they have no conflicts of interest, financial or otherwise. The authors CSH, JB, SDK, ZJ, and GNN declare that they have no conflicts of interest, financial or otherwise.
REFERENCES
- Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes care. 2001;24:1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- Avena NM, Bocarsly ME, Rada P, Kim A, Hoebel BG. After daily bingeing on a sucrose solution, food deprivation induces anxiety and accumbens dopamine/acetylcholine imbalance. Physiol Behav. 2008;94:309–315. doi: 10.1016/j.physbeh.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, Nemeroff CB, Reyes TM, Simerly RB, Susser ES, Nestler EJ. Early life programming and neurodevelopmental disorders. Biol Psychiatry. 2010;68:314–319. doi: 10.1016/j.biopsych.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, Koob GF, Vale WW, Lee KF. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety like behaviour and are hypersensitive to stress. Nat Genet. 2000;24:410–414. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Borsini F, Meli A. Is the forced swimming test a suitable model for revealing antidepressant activity? Psychopharmacology (Berl) 1988;94:147–160. doi: 10.1007/BF00176837. [DOI] [PubMed] [Google Scholar]
- Bourke CH, Neigh GN. Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Hormones and behavior. 2011;60:112–120. doi: 10.1016/j.yhbeh.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke CH, Raees MQ, Malviya S, Bradburn CA, Binder EB, Neigh GN. Glucocorticoid sensitizers Bag1 and Ppid are regulated by adolescent stress in a sex-dependent manner. Psychoneuroendocrinology. 2013;38:84–93. doi: 10.1016/j.psyneuen.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindley DN, Cooling J, Glenny HP, Burditt SL, McKechnie IS. Effects of chronic modification of dietary fat and carbohydrate on the insulin, corticosterone and metabolic responses of rats fed acutely with glucose, fructose or ethanol. Biochem J. 1981;200:275–283. doi: 10.1042/bj2000275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindley DN, Saxton J, Shahidullah H, Armstrong M. Possible relationships between changes in body weight set-point and stress metabolism after treating rats chronically with D-fenfluramine. Effects of feeding rats acutely with fructose on the metabolism of corticosterone, glucose, fatty acids, glycerol and triacylglycerol. Biochem Pharmacol. 1985;34:1265–1271. doi: 10.1016/0006-2952(85)90504-0. [DOI] [PubMed] [Google Scholar]
- Capdevila S, Giral M, Ruiz de la Torre JL, Russell RJ, Kramer K. Acclimatization of rats after ground transportation to a new animal facility. Lab Anim. 2007;41:255–261. doi: 10.1258/002367707780378096. [DOI] [PubMed] [Google Scholar]
- Casteilla L, Penicaud L, Cousin B, Calise D. Choosing an Adipose Depot for Sampline. In: Yang K, editor. Adipose Tissue Protocols. Totowa, NJ: Humana Press; 2008. pp. 23–38. [Google Scholar]
- Castro JE, Varea E, Marquez C, Cordero MI, Poirier G, Sandi C. Role of the amygdala in antidepressant effects on hippocampal cell proliferation and survival and on depression like behavior in the rat. PloS one. 2010;5:e8618. doi: 10.1371/journal.pone.0008618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catena C, Giacchetti G, Novello M, Colussi G, Cavarape A, Sechi LA. Cellular mechanisms of insulin resistance in rats with fructose-induced hypertension. American journal of hypertension. 2003;16:973–978. doi: 10.1016/s0895-7061(03)01002-1. [DOI] [PubMed] [Google Scholar]
- Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol. 2005;67:259–284. doi: 10.1146/annurev.physiol.67.040403.120816. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, Bell ME, Bhatnagar S, Laugero KD, Manalo S. Chronic stress and obesity: a new view of “comfort food”. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz WH. Critical periods in childhood for the development of obesity. Am J Clin Nutr. 1994;59:955–959. doi: 10.1093/ajcn/59.5.955. [DOI] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn GA, Bale TL. Maternal high-fat diet promotes body length increases and insulin insensitivity in second-generation mice. Endocrinology. 2009;150:4999–5009. doi: 10.1210/en.2009-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic acids research. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott SS, Keim NL, Stern JS, Teff K, Havel PJ. Fructose, weight gain, and the insulin resistance syndrome. Am J Clin Nutr. 2002;76:911–922. doi: 10.1093/ajcn/76.5.911. [DOI] [PubMed] [Google Scholar]
- Havel PJ. Dietary fructose: implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr Rev. 2005;63:133–157. doi: 10.1301/nr.2005.may.133-157. [DOI] [PubMed] [Google Scholar]
- Heim C, Mletzko T, Purselle D, Musselman DL, Nemeroff CB. The dexamethasone/corticotropin-releasing factor test in men with major depression: role of childhood trauma. Biol Psychiatry. 2008a;63:398–405. doi: 10.1016/j.biopsych.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008b;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Huang BW, Chiang MT, Yao HT, Chiang W. The effect of high-fat and high-fructose diets on glucose tolerance and plasma lipid and leptin levels in rats. Diabetes, obesity & metabolism. 2004;6:120–126. doi: 10.1111/j.1462-8902.2004.00323.x. [DOI] [PubMed] [Google Scholar]
- Hwang IS, Ho H, Hoffman BB, Reaven GM. Fructose-induced insulin resistance and hypertension in rats. Hypertension. 1987;10:512–516. doi: 10.1161/01.hyp.10.5.512. [DOI] [PubMed] [Google Scholar]
- Ito H, Nagano M, Suzuki H, Murakoshi T. Chronic stress enhances synaptic plasticity due to disinhibition in the anterior cingulate cortex and induces hyper-locomotion in mice. Neuropharmacology. 2010;58:746–757. doi: 10.1016/j.neuropharm.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Kaufman D, Banerji MA, Shorman I, Smith EL, Coplan JD, Rosenblum LA, Kral JG. Early-life stress and the development of obesity and insulin resistance in juvenile bonnet macaques. Diabetes. 2007;56:1382–1386. doi: 10.2337/db06-1409. [DOI] [PubMed] [Google Scholar]
- Kennedy GC, Mitra J. Body weight and food intake as initiating factors for puberty in the rat. The Journal of physiology. 1963;166:408–418. doi: 10.1113/jphysiol.1963.sp007112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinote A, Faria JA, Roman EA, Solon C, Razolli DS, Ignacio-Souza LM, Sollon CS, Nascimento LF, de Araujo TM, Barbosa AP, Lellis-Santos C, Velloso LA, Bordin S, Anhe GF. Fructose-induced hypothalamic AMPK activation stimulates hepatic PEPCK and gluconeogenesis due to increased corticosterone levels. Endocrinology. 2012;153:3633–3645. doi: 10.1210/en.2012-1341. [DOI] [PubMed] [Google Scholar]
- Laroche J, Gasbarro L, Herman JP, Blaustein JD. Reduced behavioral response to gonadal hormones in mice shipped during the peripubertal/adolescent period. Endocrinology. 2009;150:2351–2358. doi: 10.1210/en.2008-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, Birnbaum S, Yanagisawa M, Elmquist JK, Nestler EJ, Zigman JM. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nature neuroscience. 2008;11:752–753. doi: 10.1038/nn.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantzoros CS, Flier JS, Rogol AD. A longitudinal assessment of hormonal and physical alterations during normal puberty in boys. V. Rising leptin levels may signal the onset of puberty. J Clin Endocrinol Metab. 1997;82:1066–1070. doi: 10.1210/jcem.82.4.3878. [DOI] [PubMed] [Google Scholar]
- Marriott BP, Cole N, Lee E. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. The Journal of nutrition. 2009;139:1228S–1235S. doi: 10.3945/jn.108.098277. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ. HPA function in adolescence: role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacology, biochemistry, and behavior. 2007;86:220–233. doi: 10.1016/j.pbb.2006.07.012. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Smythe JW, Sharma S, Meaney MJ. Sex-specific effects of prenatal stress on hypothalamic-pituitary-adrenal responses to stress and brain glucocorticoid receptor density in adult rats. Brain research Developmental brain research. 1995;84:55–61. doi: 10.1016/0165-3806(94)00153-q. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Thomas CM, Sheridan CS, Nixon F, Flynn JA, Mathews IZ. Social instability stress in adolescent male rats alters hippocampal neurogenesis and produces deficits in spatial location memory in adulthood. Hippocampus. 2011 doi: 10.1002/hipo.20966. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Thomas CM, Sheridan CS, Nixon F, Flynn JA, Mathews IZ. Social instability stress in adolescent male rats alters hippocampal neurogenesis and produces deficits in spatial location memory in adulthood. Hippocampus. 2012;22:1300–1312. doi: 10.1002/hipo.20966. [DOI] [PubMed] [Google Scholar]
- Meguid MM, Fetissov SO, Varma M, Sato T, Zhang L, Laviano A, Rossi-Fanelli F. Hypothalamic dopamine and serotonin in the regulation of food intake. Nutrition. 2000;16:843–857. doi: 10.1016/s0899-9007(00)00449-4. [DOI] [PubMed] [Google Scholar]
- Minano FJ, Peinado JM, Myers RD. Profile of NE, DA and 5-HT activity shifts in medial hypothalamus perfused by 2-DG and insulin in the sated or fasted rat. Brain Res Bull. 1989;22:695–704. doi: 10.1016/0361-9230(89)90089-0. [DOI] [PubMed] [Google Scholar]
- Murphy CA, DiCamillo AM, Haun F, Murray M. Lesion of the habenular efferent pathway produces anxiety and locomotor hyperactivity in rats: a comparison of the effects of neonatal and adult lesions. Behavioural brain research. 1996;81:43–52. doi: 10.1016/s0166-4328(96)00041-1. [DOI] [PubMed] [Google Scholar]
- Myers RD, Peinado JM, Minano FJ. Monoamine transmitter activity in lateral hypothalamus during its perfusion with insulin or 2-DG in sated and fasted rat. Physiol Behav. 1988;44:633–643. doi: 10.1016/0031-9384(88)90329-0. [DOI] [PubMed] [Google Scholar]
- Nadeau K, Dabelea D. Epidemiology of type 2 diabetes in children and adolescents. Endocr Res. 2008;33:35–58. doi: 10.1080/07435800802080138. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Hu H, Zharikov S, Tuttle KR, Short RA, Glushakova O, Ouyang X, Feig DI, Block ER, Herrera-Acosta J, Patel JM, Johnson RJ. A causal role for uric acid in fructose-induced metabolic syndrome. American journal of physiology Renal physiology. 2006;290:F625–F631. doi: 10.1152/ajprenal.00140.2005. [DOI] [PubMed] [Google Scholar]
- Neigh GN, Gillespie CF, Nemeroff CB. The neurobiological toll of child abuse and neglect. Trauma Violence Abuse. 2009;10:389–410. doi: 10.1177/1524838009339758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Kuwagata M, Hori Y, Shioda S. Valproate-induced developmental neurotoxicity is affected by maternal conditions including shipping stress and environmental change during early pregnancy. Toxicology letters. 2007;174:18–24. doi: 10.1016/j.toxlet.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Pankevich DE, Mueller BR, Brockel B, Bale TL. Prenatal stress programming of offspring feeding behavior and energy balance begins early in pregnancy. Physiol Behav. 2009;98:94–102. doi: 10.1016/j.physbeh.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress. Endocrinology. 2004;145:3754–3762. doi: 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain research Molecular brain research. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. European journal of pharmacology. 1978;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. European journal of pharmacology. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Pyter LM, Kelly SD, Harrell CS, Neigh GN. Sex differences in the effects of adolescent stress on adult brain inflammatory markers in rats. Brain, behavior, and immunity. 2013;30:88–94. doi: 10.1016/j.bbi.2013.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rask E, Olsson T, Soderberg S, Andrew R, Livingstone DE, Johnson O, Walker BR. Tissue-specific dysregulation of cortisol metabolism in human obesity. J Clin Endocrinol Metab. 2001;86:1418–1421. doi: 10.1210/jcem.86.3.7453. [DOI] [PubMed] [Google Scholar]
- Romeo RD. Adolescence: a central event in shaping stress reactivity. Dev Psychobiol. 2010;52:244–253. doi: 10.1002/dev.20437. [DOI] [PubMed] [Google Scholar]
- Rutledge AC, Adeli K. Fructose and the metabolic syndrome: pathophysiology and molecular mechanisms. Nutr Rev. 2007;65:S13–S23. doi: 10.1111/j.1753-4887.2007.tb00322.x. [DOI] [PubMed] [Google Scholar]
- Schoeller DA. Limitations in the Assessment of Dietary Energy-Intake by Self-Report. Metabolism-Clinical and Experimental. 1995;44:18–22. doi: 10.1016/0026-0495(95)90204-x. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and biobehavioral reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, McGahan JP, Seibert A, Krauss RM, Chiu S, Schaefer EJ, Ai M, Otokozawa S, Nakajima K, Nakano T, Beysen C, Hellerstein MK, Berglund L, Havel PJ. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. The Journal of clinical investigation. 2009;119:1322–1334. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PM, Boulton A, Kumar S, Clark PM, Shackleton CH. Cortisol metabolism in human obesity: impaired cortisone-->cortisol conversion in subjects with central adiposity. J Clin Endocrinol Metab. 1999;84:1022–1027. doi: 10.1210/jcem.84.3.5538. [DOI] [PubMed] [Google Scholar]
- Stimson RH, Lobley GE, Maraki I, Morton NM, Andrew R, Walker BR. Effects of proportions of dietary macronutrients on glucocorticoid metabolism in diet-induced obesity in rats. PloS one. 2010;5:e8779. doi: 10.1371/journal.pone.0008779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamashiro KL, Terrillion CE, Hyun J, Koenig JI, Moran TH. Prenatal stress or high-fat diet increases susceptibility to diet-induced obesity in rat offspring. Diabetes. 2009;58:1116–1125. doi: 10.2337/db08-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappy L, Le KA. Metabolic effects of fructose and the worldwide increase in obesity. Physiological reviews. 2010;90:23–46. doi: 10.1152/physrev.00019.2009. [DOI] [PubMed] [Google Scholar]
- Teff KL, Grudziak J, Townsend RR, Dunn TN, Grant RW, Adams SH, Keim NL, Cummings BP, Stanhope KL, Havel PJ. Endocrine and metabolic effects of consuming fructose-and glucose-sweetened beverages with meals in obese men and women: influence of insulin resistance on plasma triglyceride responses. J Clin Endocrinol Metab. 2009;94:1562–1569. doi: 10.1210/jc.2008-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nature biotechnology. 2010;28:511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos MB, Kimmons JE, Gillespie C, Welsh J, Blanck HM. Dietary fructose consumption among US children and adults: the Third National Health and Nutrition Examination Survey. Medscape journal of medicine. 2008;10:160. [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2:322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson DF, Thompson TJ, Anda RF, Dietz WH, Felitti V. Body weight and obesity in adults and self-reported abuse in childhood. Int J Obes Relat Metab Disord. 2002;26:1075–1082. doi: 10.1038/sj.ijo.0802038. [DOI] [PubMed] [Google Scholar]
- Ziotopoulou M, Mantzoros CS, Hileman SM, Flier JS. Differential expression of hypothalamic neuropeptides in the early phase of diet-induced obesity in mice. Am J Physiol Endocrinol Metab. 2000;279:E838–E845. doi: 10.1152/ajpendo.2000.279.4.E838. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.