Abstract
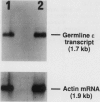
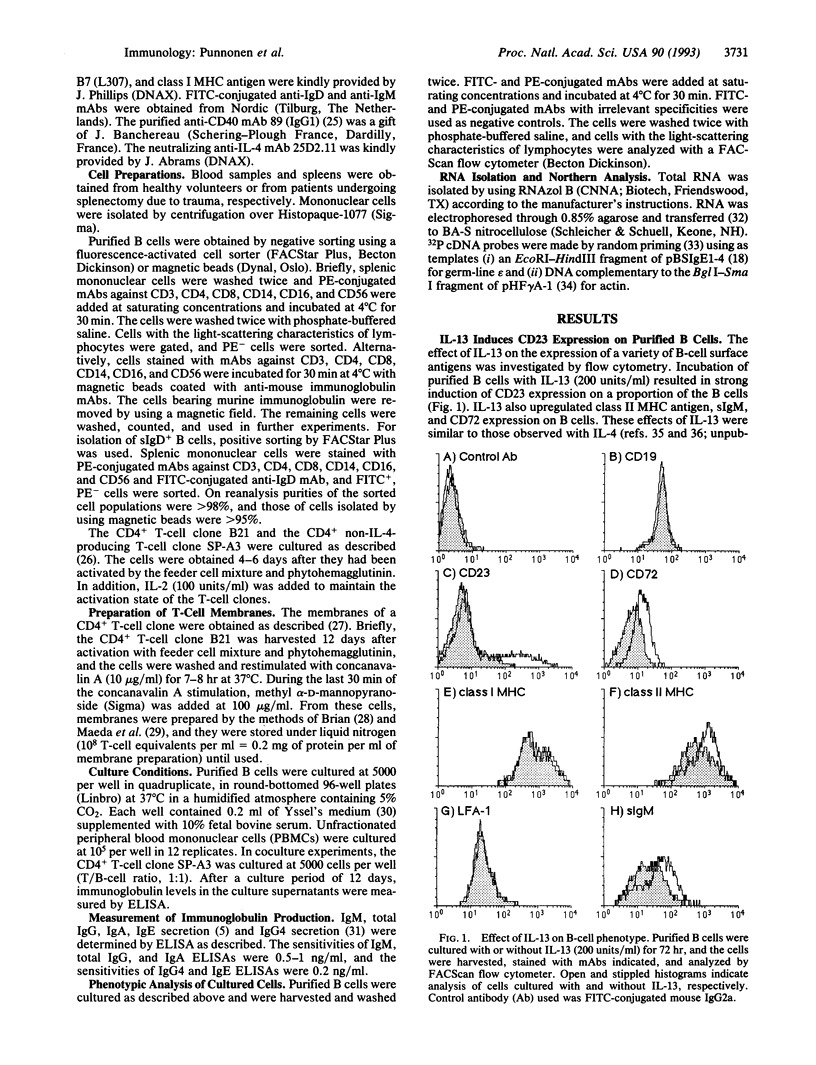
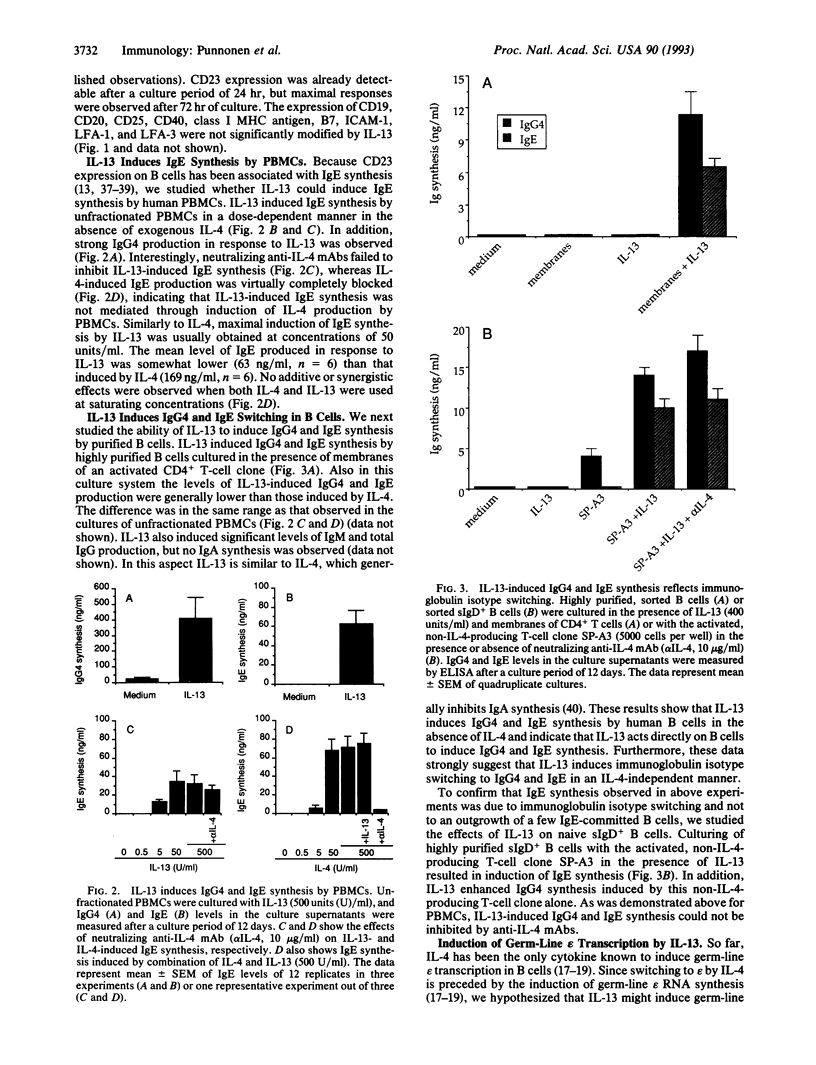
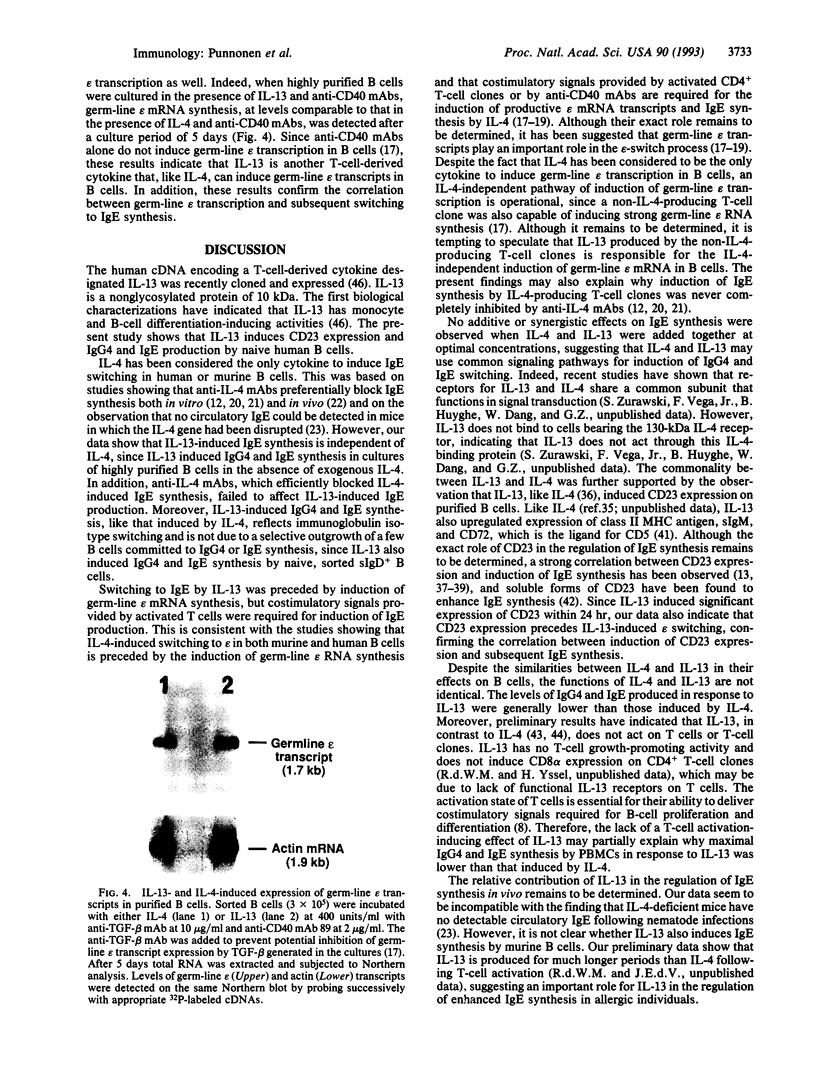
Recently the cDNA encoding interleukin 13 (IL-13), a T-cell-derived cytokine, was cloned and expressed. The present study demonstrates that IL-13 induces IgG4 and IgE synthesis by human B cells. IL-13-induced IgG4 and IgE synthesis by unfractionated peripheral blood mononuclear cells and highly purified B cells cultured in the presence of activated CD4+ T cells or their membranes. IL-13-induced IgG4 and IgE synthesis is IL-4-independent, since it was not affected by neutralizing anti-IL-4 monoclonal antibody. Highly purified, surface IgD+ B cells could also be induced to produce IgG4 and IgE by IL-13, indicating that the production of these isotypes reflected IgG4 and IgE switching and not a selective outgrowth of committed B cells. IL-4 and IL-13 added together at optimal concentrations had no additive or synergistic effect, suggesting that common signaling pathways may be involved. This notion is supported by the observation that IL-13, like IL-4, induced CD23 expression on B cells and enhanced CD72, surface IgM, and class II major histocompatibility complex antigen expression. In addition, like IL-4, IL-13 induced germ-line IgE heavy-chain gene transcription in highly purified B cells. Collectively, our data indicate that IL-13 is another T-cell-derived cytokine that, in addition to IL-4, efficiently directs naive human B cells to switch to IgG4 and IgE production.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armitage R. J., Fanslow W. C., Strockbine L., Sato T. A., Clifford K. N., Macduff B. M., Anderson D. M., Gimpel S. D., Davis-Smith T., Maliszewski C. R. Molecular and biological characterization of a murine ligand for CD40. Nature. 1992 May 7;357(6373):80–82. doi: 10.1038/357080a0. [DOI] [PubMed] [Google Scholar]
- Aubry J. P., Pochon S., Graber P., Jansen K. U., Bonnefoy J. Y. CD21 is a ligand for CD23 and regulates IgE production. Nature. 1992 Aug 6;358(6386):505–507. doi: 10.1038/358505a0. [DOI] [PubMed] [Google Scholar]
- Banchereau J., de Paoli P., Vallé A., Garcia E., Rousset F. Long-term human B cell lines dependent on interleukin-4 and antibody to CD40. Science. 1991 Jan 4;251(4989):70–72. doi: 10.1126/science.1702555. [DOI] [PubMed] [Google Scholar]
- Brian A. A. Stimulation of B-cell proliferation by membrane-associated molecules from activated T cells. Proc Natl Acad Sci U S A. 1988 Jan;85(2):564–568. doi: 10.1073/pnas.85.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherwinski H. M., Schumacher J. H., Brown K. D., Mosmann T. R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987 Nov 1;166(5):1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark E. A., Lane P. J. Regulation of human B-cell activation and adhesion. Annu Rev Immunol. 1991;9:97–127. doi: 10.1146/annurev.iy.09.040191.000525. [DOI] [PubMed] [Google Scholar]
- DeKruyff R. H., Turner T., Abrams J. S., Palladino M. A., Jr, Umetsu D. T. Induction of human IgE synthesis by CD4+ T cell clones. Requirement for interleukin 4 and low molecular weight B cell growth factor. J Exp Med. 1989 Nov 1;170(5):1477–1493. doi: 10.1084/jem.170.5.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defrance T., Aubry J. P., Rousset F., Vanbervliet B., Bonnefoy J. Y., Arai N., Takebe Y., Yokota T., Lee F., Arai K. Human recombinant interleukin 4 induces Fc epsilon receptors (CD23) on normal human B lymphocytes. J Exp Med. 1987 Jun 1;165(6):1459–1467. doi: 10.1084/jem.165.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defrance T., Vanbervliet B., Brière F., Durand I., Rousset F., Banchereau J. Interleukin 10 and transforming growth factor beta cooperate to induce anti-CD40-activated naive human B cells to secrete immunoglobulin A. J Exp Med. 1992 Mar 1;175(3):671–682. doi: 10.1084/jem.175.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prete G., Maggi E., Parronchi P., Chrétien I., Tiri A., Macchia D., Ricci M., Banchereau J., De Vries J., Romagnani S. IL-4 is an essential factor for the IgE synthesis induced in vitro by human T cell clones and their supernatants. J Immunol. 1988 Jun 15;140(12):4193–4198. [PubMed] [Google Scholar]
- Erba H. P., Gunning P., Kedes L. Nucleotide sequence of the human gamma cytoskeletal actin mRNA: anomalous evolution of vertebrate non-muscle actin genes. Nucleic Acids Res. 1986 Jul 11;14(13):5275–5294. doi: 10.1093/nar/14.13.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Finkelman F. D., Katona I. M., Urban J. F., Jr, Snapper C. M., Ohara J., Paul W. E. Suppression of in vivo polyclonal IgE responses by monoclonal antibody to the lymphokine B-cell stimulatory factor 1. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9675–9678. doi: 10.1073/pnas.83.24.9675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascan H., Aversa G. G., Gauchat J. F., Van Vlasselaer P., Roncarolo M. G., Yssel H., Kehry M., Spits H., De Vries J. E. Membranes of activated CD4+ T cells expressing T cell receptor (TcR) alpha beta or TcR gamma delta induce IgE synthesis by human B cells in the presence of interleukin-4. Eur J Immunol. 1992 May;22(5):1133–1141. doi: 10.1002/eji.1830220505. [DOI] [PubMed] [Google Scholar]
- Gascan H., Gauchat J. F., Roncarolo M. G., Yssel H., Spits H., de Vries J. E. Human B cell clones can be induced to proliferate and to switch to IgE and IgG4 synthesis by interleukin 4 and a signal provided by activated CD4+ T cell clones. J Exp Med. 1991 Mar 1;173(3):747–750. doi: 10.1084/jem.173.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauchat J. F., Aversa G., Gascan H., de Vries J. E. Modulation of IL-4 induced germline epsilon RNA synthesis in human B cells by tumor necrosis factor-alpha, anti-CD40 monoclonal antibodies or transforming growth factor-beta correlates with levels of IgE production. Int Immunol. 1992 Mar;4(3):397–406. doi: 10.1093/intimm/4.3.397. [DOI] [PubMed] [Google Scholar]
- Gauchat J. F., Lebman D. A., Coffman R. L., Gascan H., de Vries J. E. Structure and expression of germline epsilon transcripts in human B cells induced by interleukin 4 to switch to IgE production. J Exp Med. 1990 Aug 1;172(2):463–473. doi: 10.1084/jem.172.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimata H., Yoshida A., Ishioka C., Lindley I., Mikawa H. Interleukin 8 (IL-8) selectively inhibits immunoglobulin E production induced by IL-4 in human B cells. J Exp Med. 1992 Oct 1;176(4):1227–1231. doi: 10.1084/jem.176.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiniwa M., Gately M., Gubler U., Chizzonite R., Fargeas C., Delespesse G. Recombinant interleukin-12 suppresses the synthesis of immunoglobulin E by interleukin-4 stimulated human lymphocytes. J Clin Invest. 1992 Jul;90(1):262–266. doi: 10.1172/JCI115846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn R., Rajewsky K., Müller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991 Nov 1;254(5032):707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- Lundgren M., Persson U., Larsson P., Magnusson C., Smith C. I., Hammarström L., Severinson E. Interleukin 4 induces synthesis of IgE and IgG4 in human B cells. Eur J Immunol. 1989 Jul;19(7):1311–1315. doi: 10.1002/eji.1830190724. [DOI] [PubMed] [Google Scholar]
- Lutzker S., Rothman P., Pollock R., Coffman R., Alt F. W. Mitogen- and IL-4-regulated expression of germ-line Ig gamma 2b transcripts: evidence for directed heavy chain class switching. Cell. 1988 Apr 22;53(2):177–184. doi: 10.1016/0092-8674(88)90379-0. [DOI] [PubMed] [Google Scholar]
- Maeda T., Balakrishnan K., Mehdi S. Q. A simple and rapid method for the preparation of plasma membranes. Biochim Biophys Acta. 1983 May 26;731(1):115–120. doi: 10.1016/0005-2736(83)90404-2. [DOI] [PubMed] [Google Scholar]
- McKenzie A. N., Culpepper J. A., de Waal Malefyt R., Brière F., Punnonen J., Aversa G., Sato A., Dang W., Cocks B. G., Menon S. Interleukin 13, a T-cell-derived cytokine that regulates human monocyte and B-cell function. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3735–3739. doi: 10.1073/pnas.90.8.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noelle R., Krammer P. H., Ohara J., Uhr J. W., Vitetta E. S. Increased expression of Ia antigens on resting B cells: an additional role for B-cell growth factor. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6149–6153. doi: 10.1073/pnas.81.19.6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliard X., Malefijt R. W., de Vries J. E., Spits H. Interleukin-4 mediates CD8 induction on human CD4+ T-cell clones. Nature. 1988 Oct 13;335(6191):642–644. doi: 10.1038/335642a0. [DOI] [PubMed] [Google Scholar]
- Punnonen J., Aversa G. G., Vandekerckhove B., Roncarolo M. G., de Vries J. E. Induction of isotype switching and Ig production by CD5+ and CD10+ human fetal B cells. J Immunol. 1992 Jun 1;148(11):3398–3404. [PubMed] [Google Scholar]
- Pène J., Rousset F., Brière F., Chrétien I., Bonnefoy J. Y., Spits H., Yokota T., Arai N., Arai K., Banchereau J. IgE production by normal human lymphocytes is induced by interleukin 4 and suppressed by interferons gamma and alpha and prostaglandin E2. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6880–6884. doi: 10.1073/pnas.85.18.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pène J., Rousset F., Brière F., Chrétien I., Paliard X., Banchereau J., Spits H., De Vries J. E. IgE production by normal human B cells induced by alloreactive T cell clones is mediated by IL-4 and suppressed by IFN-gamma. J Immunol. 1988 Aug 15;141(4):1218–1224. [PubMed] [Google Scholar]
- Pène J., Rousset F., Brière F., Chrétien I., Wideman J., Bonnefoy J. Y., De Vries J. E. Interleukin 5 enhances interleukin 4-induced IgE production by normal human B cells. The role of soluble CD23 antigen. Eur J Immunol. 1988 Jun;18(6):929–935. doi: 10.1002/eji.1830180615. [DOI] [PubMed] [Google Scholar]
- Roncarolo M. G., Yssel H., Touraine J. L., Betuel H., De Vries J. E., Spits H. Autoreactive T cell clones specific for class I and class II HLA antigens isolated from a human chimera. J Exp Med. 1988 May 1;167(5):1523–1534. doi: 10.1084/jem.167.5.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman P., Lutzker S., Cook W., Coffman R., Alt F. W. Mitogen plus interleukin 4 induction of C epsilon transcripts in B lymphoid cells. J Exp Med. 1988 Dec 1;168(6):2385–2389. doi: 10.1084/jem.168.6.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarfati M., Delespesse G. Possible role of human lymphocyte receptor for IgE (CD23) or its soluble fragments in the in vitro synthesis of human IgE. J Immunol. 1988 Oct 1;141(7):2195–2199. [PubMed] [Google Scholar]
- Sarfati M., Rector E., Wong K., Rubio-Trujillo M., Sehon A. H., Delespesse G. In vitro synthesis of IgE by human lymphocytes. II. Enhancement of the spontaneous IgE synthesis by IgE-binding factors secreted by RPMI 8866 lymphoblastoid B cells. Immunology. 1984 Oct;53(2):197–205. [PMC free article] [PubMed] [Google Scholar]
- Snapper C. M., Paul W. E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987 May 22;236(4804):944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- Spiegelberg H. L., O'Connor R. D., Simon R. A., Mathison D. A. Lymphocytes with immunoglobulin E Fc receptors in patients with atopic disorders. J Clin Invest. 1979 Sep;64(3):714–720. doi: 10.1172/JCI109514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spits H., Yssel H., Takebe Y., Arai N., Yokota T., Lee F., Arai K., Banchereau J., de Vries J. E. Recombinant interleukin 4 promotes the growth of human T cells. J Immunol. 1987 Aug 15;139(4):1142–1147. [PubMed] [Google Scholar]
- Van de Velde H., von Hoegen I., Luo W., Parnes J. R., Thielemans K. The B-cell surface protein CD72/Lyb-2 is the ligand for CD5. Nature. 1991 Jun 20;351(6328):662–665. doi: 10.1038/351662a0. [DOI] [PubMed] [Google Scholar]
- Vercelli D., Jabara H. H., Arai K., Yokota T., Geha R. S. Endogenous interleukin 6 plays an obligatory role in interleukin 4-dependent human IgE synthesis. Eur J Immunol. 1989 Aug;19(8):1419–1424. doi: 10.1002/eji.1830190811. [DOI] [PubMed] [Google Scholar]
- Yssel H., De Vries J. E., Koken M., Van Blitterswijk W., Spits H. Serum-free medium for generation and propagation of functional human cytotoxic and helper T cell clones. J Immunol Methods. 1984 Aug 3;72(1):219–227. doi: 10.1016/0022-1759(84)90450-2. [DOI] [PubMed] [Google Scholar]
- Zhang K., Clark E. A., Saxon A. CD40 stimulation provides an IFN-gamma-independent and IL-4-dependent differentiation signal directly to human B cells for IgE production. J Immunol. 1991 Mar 15;146(6):1836–1842. [PubMed] [Google Scholar]
- de Vries J. E., Gauchat J. F., Aversa G. G., Punnonen J., Gascan H., Yssel H. Regulation of IgE synthesis by cytokines. Curr Opin Immunol. 1991 Dec;3(6):851–858. doi: 10.1016/s0952-7915(05)80003-2. [DOI] [PubMed] [Google Scholar]
- van Vlasselaer P., Gascan H., de Waal Malefyt R., de Vries J. E. IL-2 and a contact-mediated signal provided by TCR alpha beta + or TCR gamma delta + CD4+ T cells induce polyclonal Ig production by committed human B cells. Enhancement by IL-5, specific inhibition of IgA synthesis by IL-4. J Immunol. 1992 Mar 15;148(6):1674–1684. [PubMed] [Google Scholar]
- van Vlasselaer P., Punnonen J., de Vries J. E. Transforming growth factor-beta directs IgA switching in human B cells. J Immunol. 1992 Apr 1;148(7):2062–2067. [PubMed] [Google Scholar]