Abstract
There are quite a few controversies on surgical management of single-segment thoracic spinal tuberculosis (STB) with neurological deficits. The present study was to compare single-stage posterior-only transpedicular debridement, interbody fusion and posterior instrumentation (posterior-only surgery) with a combined posterior-anterior surgical approach for treatment of single-segment thoracic STB with neurological deficits and to determinethe clinical feasibility and effectiveness of posterior-only surgical treatment. Sixty patients with single-segment thoracic STB with neurological deficits were treated with one of two surgical procedures in our center from January 2003 to January 2013. Thirty patients were treated with posterior-only surgery (Group A) andthirty were treated with combined posterior-anterior surgery (Group B). The American Spinal Injury Association (ASIA) score system to evaluate the neurological deficits, thevisual analogue scale (VAS) to assess the degree of pain, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) to judge the activity of tuberculosis (TB), surgery duration, intraoperative blood loss, length of hospitalization, bonyfusion rates, and kyphosis correction of the two groups were compared. The average follow-up period was 36.5 ± 9.2 months for Group A and 34.6 ± 10.2 months for Group B. Under the ASIA score system, all patients improved with treatment. STB was completely cured and grafted bones were fused within 5-11 months in allpatients. There were no persistent or recurrent infections orobvious differences in radiological results between thegroups. The kyphosis deformity was significantly corrected after surgical management. The average operative duration, blood loss, length of hospital stay, and postoperative complication rateof Group A were lower than those of Group B. In conclusions, posterior-only surgery is feasible and effective, resulting in better clinical outcomes than combined posterior-anterior surgeries, especially in surgical time, blood loss, hospital stay, and complications.
Keywords: Tubercular spondylitis, single-segment, posterior-only approach, combined anterior and posterior surgery, comparision
Introduction
The past decades have witnessed the resurgence andeven increasing of tubercular disease attributed to theacquired immunodeficiency syndrome pandemic, surgeof immigration, and impoverished living and sanitaryconditions. Spinal tuberculosis (STB), which is a commonextra-pulmonary, is the most frequent and seriousform of skeletal tuberculosis. However, single-segment thoracic STB with neurological deficitshas rarely been reported in the literature in case of some cases reports in the mainstream academic journals. Although the ant-TB chemotherapy and external immobilization still play an irreplaceable role in treatment of STB in most cases, single-segment thoracic STB with neurological deficits is characterizedby kyphosis deformity, abscess formationand spinal cord compression, which usually beyond the chemotherapy function. Therefore, surgical invention will be necessary in these cases. To our knowledge, surgical treatment of single-segment thoracic STB with neurological deficits has rarely been reported.
It becomes obvious that single anterior debridement and bone grafting was often unsatisfactory in correction or prevention of progression of kyphosis deformity [1-3]. High incidence of progression of kyphosis has been observed following non-instrumented anterior fusion compared to non-operative treatment [4]. Some researchers demonstrated that posterior bone graft alone had either no evident benefit and was even harmful in the presence of active tuberculosis [2,5]. However, there has been a significant evolution in the treatment of spinal tuberculosis during the past several decades. Posterior pedicle screw system has become popular as a revolutionary technique for correction of angular deformity and stabilization of the unstable spine. It has been proven effective for the treatment of many thoracic spinal disorders that resulting in segmental instability and neurologic impairment [2]. Therefore, the treatment strategy for this disease entity has been revised and become more conservative and less invasive in recent years.
Posterior instrumentation and fusion, anterior debride mentand fusion, and anterior and posterior fusion have been described as effective in treatment of STB, but there is a lack of consensus as to the most effective means of managing single-segment thoracic STB with neurological deficits. Hence, the aimof our study was to compare the clinical outcomes of patients under going the posterior-only surgical procedure with those receiving combined posterior-anterior surgery for single-segment thoracic STB with neurological deficits.
Materials and methods
We analyzed the outcomes of sixty consecutive patients who underwent posterior-only surgery (Group A) or combined posterior-anterior surgery (Group B) performed by the same surgeons in the same institution from January 2003 to January 2013. Group A included 30 patients (18 male, 12 female), with a mean age on admission of 42.3 ± 10.1 years (range: 22-70 years). The mean follow-up period in Group A was 36.5 ± 9.2 months (range: 24-60 months). Group B included 30 patients (16 male, 14 female), with a mean age on admission of 38.5 ± 12.1 years (range: 20-68 months). The mean follow-up period in Group B was 34.6 ± 10.2 months (range: 24-68 months) (Table 1). The preoperative kyphosis anglewas 35.3 ± 11.3° and 36.5 ± 12.4°, respectively. The classification of the ASIA was used to assess the neurological deficit and 3 patients behaved with grade A, 4 with B, 13 with C, and 10 with D in group A; 4 patients behaved with grade A, 5 with B, 12 with C, and 9 with D in group B. The VAS, ESR and CRP of patients in group A upon admission was 7.5 ± 2.3, 42.1 ± 5.3 mm/h, 23.4 ± 4.6 mg/L, respectively; in group B, the VAS, ESR and CRP of patients upon admission was 7.8 ± 2.4, 43.3 ± 6.4 mm/h, 22.5 ± 5.4 mg/L, respectively (Table 2). Written informed consent was obtained from all patients and the study protocol was approved by our hospital ethics committee.
Table 1.
General data of study
| M/F | Age (years)# | Follow-up time (mon)# | Operation time (min)* | Amount of bleeding (ml)* | Hospitalization day (days)* | Bone fusion time (mon)# | |
|---|---|---|---|---|---|---|---|
| Group A | 18/12 | 42.3 ± 10.1 | 36.5 ± 9.2 | 140.2± 20.4 | 641.2 ± 148.2 | 11.8 ± 3.8 | 6.6 ± 2.9 |
| Group B | 16/14 | 38.5 ± 12.1 | 34.6 ± 10.2 | 248.4± 50.2 | 850.2 ± 200.5 | 17.4 ± 5.8 | 6.5 ± 3.0 |
M: male; F: female.
Independent-samples t test, compared age, follow-up time and bone fusion time between two groups, respectively, P>0.05.
Independent-samples t test, compared operation time, amount of bleeding and hospitalization day between two groups, respectively, P<0.05.
Table 2.
Clinical details of surgery groups (Group A/B)
| Schedule | Classification of neurological function by ASIA | VAS | Kyphosis angle (°) | ESR (mm/h) | CRP (mg/L) | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| A | B | C | D | E | |||||
| Pre-op | 3/4 | 4/5 | 13/12 | 10/9 | 7.5 ± 2.3/7.8 ± 2.4 | 35.3 ± 11.3/36.5 ± 12.4 | 42.1 ± 5.3/43.3 ± 6.4 | 23.4 ± 4.6/22.5 ± 5.4 | |
| Post-op | 2/3 | 3/3 | 12/13 | 13/11 | 3.9 ± 1.6/4.1 ± 1.7 | 8.6 ± 1.4/9.1 ± 1.6 | 20.3 ± 5.0/21.8 ± 2.4 | 10.4 ± 4.3/9.8 ± 4.9 | |
| TMP# | 1/0 | 2/3 | 4/5 | 23/22 | 2.2 ± 0.4/2.5 ± 0.5 | 9.1 ± 1.4/9.9 ± 1.4 | 11.5 ± 3.3/10.8 ± 1.3 | 4.8 ± 2.1/4.5 ± 1.2 | |
| FFU※ | 1/0 | 1/2 | 2/3 | 26/25 | 1.4 ± 0.5/1.5 ± 0.7 | 9.5 ± 1.8/10.1 ± 1.6 | 10.8 ± 2.6/6.8 ± 0.4 | 4.9 ± 1.1/5.8 ± 0.4 | |
ASIA, the American spinal injury association score system; VAS, visual analogue scale of pain; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; Pre-op, pre-operation; Post-op, post-operation; TMP, three months post-operation; FFU, final follow-up.
Wilcoxon signed rank test, compare classification of neurological function of pre-operation with final follow-up, P<0.05.
Independent-samples t test, compared kyphosis angle, VAS, ESR and CRP in 3 month post-operation with pre-operative groups, P<0.05.
There is no significant difference between group A and B in ASIA, VAS, correction of kyphosis angle, ESR and CRP, in parallel, P>0.05.
The diagnosis of thoracic TB was based on clinical symptoms such as weight loss, mild fever, night sweats, and fatigue; nonspecific laboratory data, including anemia, hypoproteinemia, and elevated ESR; and radiographic evidence, consisting of sequestered bone, subligamentous spread of infection, vertebral body destruction and collapse, and paraspinal abscess. Confirmation was based on the examination of surgical biopsy specimens, acid-fast staining, bacterial cultures, or polymerase chain reaction.
The indications for surgery in our study included; 1) persistent back pain unresponsive to chemotherapy for 2 months; 2) progressive neurological deficit and angular deformity or instability likely to appear; 3) radiologically, severe spinal cord decompression, significant vertebra destruction and/orparaspinal abscess with necrotic disc or inflammatory granulation tissue; 4) multilevel vertebras involved but only one center need to be debrided and performed short bone grafting fusion (less than two levels; 5) the elderly or the patients with poor health intolerant too much trauma; 6) patients who had undergone the anterior operation, in whom the anatomical structure was unclear. When the patients presented with following conditions were excluded; 1) presented with no neurological deficit; 2) multilevel lesions or several focus centers and required anterior long-segment bone fusion; 3) accompanied by paraspinal or deep multiple abscesses beyond its scope and severe kyphosis deformity (more than 50°). (In fact, it is difficult to randomly select a surgical treatment method clinically. Therefore, in our study, all cases in Group A (posterior-only) were collected in recent years, but the majority of cases in Group B (posterior-anterior) were collected earlier.)
Preoperative procedure
Chemotherapy was administrated soon after the clinical diagnosis was suspected. Anti-TB drugs with the HREZ chemotherapy regimen, consist of isoniazid (5-10 mg/kg/day with no more than 300 mg/day), rifampicin (5 to 10 mg/kg/day with no more than 300 mg/day), ethambutol (15 mg/kg/day with no more than 500 mg/day) and pyrazinamide (25 mg/kg/day with no more than 750 mg/day) 2-4 weeks before surgery. When progressive neurological deficits appear and severe back pain shows unresponsive to chemotherapy, we appropriately shorten the time of drug treatment. When the ESR, CRP and temperature returned to normal or had significantly decreased, and anemia and hypoproteinemia were rectified completely, we performed the surgical management.
Operative technique
Group A: posterior-only approach. The patients were in the prone position after administration of general endotracheal anesthesia. The patients involving T1-4 were placed in a halo with a weight of 3 kg during the operation. Through a midline incision, the posterior spinal elements including lamina, facet joints, and transverse processes were exposed (extraperiosteal dissection), extending one vertebrae above and below the involved segments. Transpedicular screws were allowed to use in the side of vertebral lamina based on preoperative symptoms and imaging. Generally, we preferred longer segmental fixation, at least two levels superior and inferior to the level of decompression. Transpedicular screws were also placed in the affected vertebrae if the upper part of the vertebrae was not destroyed by infection. Following transpedicular screws being implanted and C-arm X-ray confirming their accuracy, installed a temporary pre-bent rod on the mild side of the lesion to avoid spinal cord injury induced by instability of the spine during decompression and focal debridement and then selected the severe side of the lesion, which caused clinical symptoms or presented with paraspinal abscess as the decompression side. A unilateral facetectomy and a laminectomy up to the medial pedicle edge were performed. We cut off adjacent rib 1.0-1.5 cm beside the thoracic spine and sacrificed the thoracic nerve roots on the focal side for better exposure, if necessary. Generally, the decompression range was based on the extent of spinal canal stenosis and the scope of paraspinal abscess. And then, a suitable flush tube was plunged in paravertebral abscess to wash with appropriate pressure until no pus outflow following the removing the necrotic disc and the collapsed vertebrae by curettes through to healthy bleeding bone. When the abscess was beyond the flush tube extension, we controlled the patient’s position by changing operating table to facilitate the abscess into the focus (postural drainage). Sequentially, the rods were tightened and the kyphosis slowly and carefully rectified with the help of the compression and stretch of the internal fixation instrument. If the space created after focal debridement was too large, allograft bone would be selected for posterior fusion at the segment that underwent decompression and focal debridement. If necessary, the other sidewas treated in the same way. When the graft bone was loose, or the presence of bone defects, we embedded the relatively large bone particles or block into the gap by implementing impacted graft. Afterwards, administered locally with 1.0 g streptomycin and 0.2 g isoniazid and negative pressure drainage and incision sutures were performed postoperatively and resected specimens were collected for bacterial culture and pathological diagnosis (Figure 1).
Figure 1.
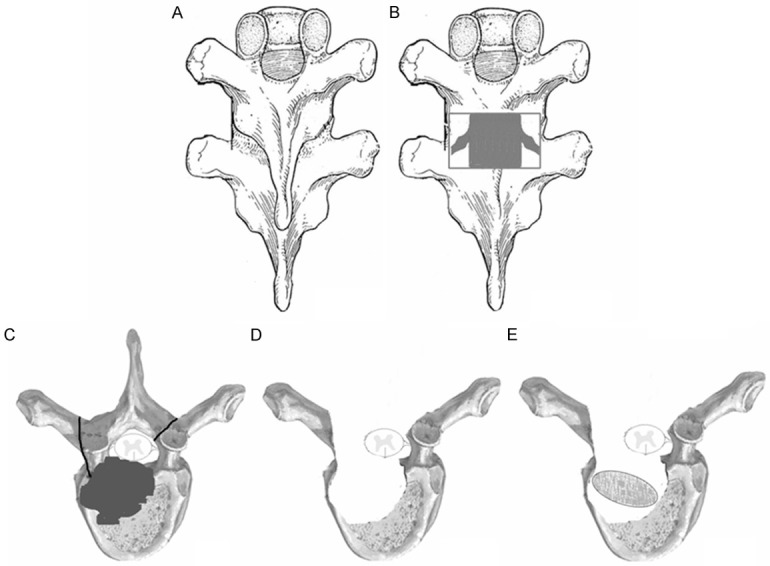
The schematic diagram of posterior-only surgery. A. Posterior exposure via extraperiosteal dissection; B, C. Facetectomy and laminectomy; D. Transpedicular thoracic debridement and decompression; E. Transpedicular posterior interbody bone grafting.
Group B: combined anterior and posterior approach. The detailed surgical process could refer to the reports by Zeng [6,7] and Wang [8].
Post-operative care
The drainage tube was pulled out when the volume of drainage was less 30 ml. Patients continued with the oral HREZ chemotherapy postoperatively. Six months later, pyrazinamide was discontinued. Patients then received nine- to twelve-month regimens of the HRE chemotherapy (6HREZ/9-12HRE). Ambulation was allowed one week after surgery with a brace. All of the patients were examined clinically and radiologically at one week, 3, 6 and 12 months after surgery and then once a year.
Follow-up index and statistical analysis
For Group A, the average follow-up period was 36.5 ± 9.2 months (range: 24-60 months). For Group B, the average follow-up period was 38.5 ± 12.1 years (range: 20-68 months). The following indices were recorded preoperatively, postoperatively, and during follow-up: (1) kyphosis angle, (2) neurological status, (3) VAS, and (4) ESR and CRP. The kyphosis angle, ESR and CRP were statistically analyzed by an independent-samples t test using SPSS 19.0 software. Surgery duration (min), blood loss (ml), and hospitalization (days) were also statistically analyzed between the two groups by an independent-samples t test. Discrepancy from the normal distribution was analyzed by a rank-sum test with a significance level of 0.05.
Results
The results of pathological examinations in all cases confirmed the spinal tuberculosis. The duration of follow-up was 36.5 ± 9.2 mon, 34.6 ± 10.2 mon; the operation time was 140.2 ± 20.4 min, 248.4 ± 50.2 min; the blood loss was 641.2 ± 148.2 ml, 850.2 ± 200.5 ml; the hospitalization time was 11.8 ± 3.8 days, 17.4 ± 5.8 days, and the fusion time was 6.6 ± 2.9 mon, 6.5 ± 3.0 mon, in two groups, respectively. Comparingthe age, follow-up time and bone fusion time between two groupsvia independent-samples t test, respectively, there was no significant difference (P>0.05). However, Comparing the operation time, amount of bleeding and hospitalization day between two groupsby dependent-samples t test, respectively, there was obvious difference (P<0.05). There were some complications, such as cerebrospinal fluid leakage (3 cases in group A and 6 cases in group B), water-electrolyte imbalance (5 cases in group A and 13 cases in group B), superficial infection (2 cases in group A and 5 cases in group B) and mild intestinal obstruction in (4 cases in group A and 7 cases in group B) after operation. No complication related to bone grafted and instrumentation was observed in both groups postoperatively; the symptoms disappeared after the patient was performed anti-inflammatory or symptomatic supportive treatment for 1-2 weeks.
Neurologic deficits in all patients were improved at final follow-up examination. The results were evaluated by ASIA classification and the detailed data could refer to ‘Table 2’. Statistical analysis demonstrated that there was significant difference between pre- and final follow-upin both groups (P<0.05). The VAS of pain were 7.5 ± 2.3 and 7.8 ± 2.4 preoperatively and drop to 3.9 ± 1.6 and 4.1 ± 1.7 postoperatively and 1.4 ± 0.5 and 1.5 ± 0.7 in the final follow-up, in both groups, respectively. All patients had no recurrence of tuberculosis and all had pain relief.
The kyphosis angle were 35.3 ± 11.3° and 36.5 ± 12.4°, preoperatively; it significantly decreased to 8.6 ± 1.4° and 9.1 ± 1.6°, postoperatively, respectively (P<0.05). The kyphosis angle were 9.5 ± 1.8° and 10.1 ± 1.6° at final follow-up, whose loss of correction was only 0.9 ± 0.7° and 1.0 ± 0.5°, respectively. It still significantly improved in comparison to the preoperative measurements (P<0.05) (Figures 2, 3). Inter-vertebral bone graft and inter-transverses fusion were performed in all patients. Lateral X-ray or CT was used to assess the fusion and the formation of a bone bridge. All patients achieved bone fusion within 5-11 months after surgery, which were confirmed by two different surgeons based on the modified criteria of Lee et al [9] for radiological fusion. The average pretreatment ESR/CRP in both groups were 42.1 ± 5.3/43.3 ± 6.4 mm/h and 23.4 ± 4.6/22.5 ± 5.4 mg/L, respectively, which got normal during the final follow-up in all patients. There was a statistical difference between preoperative ESR and CRP and during the final follow-up ESR and CRP in both groups (P<0.05) (Tables 1, 2).
Figure 2.
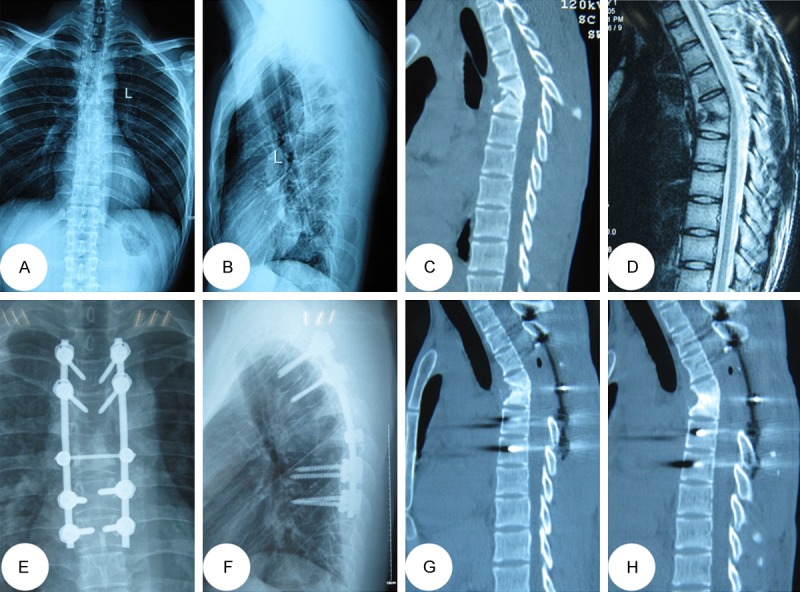
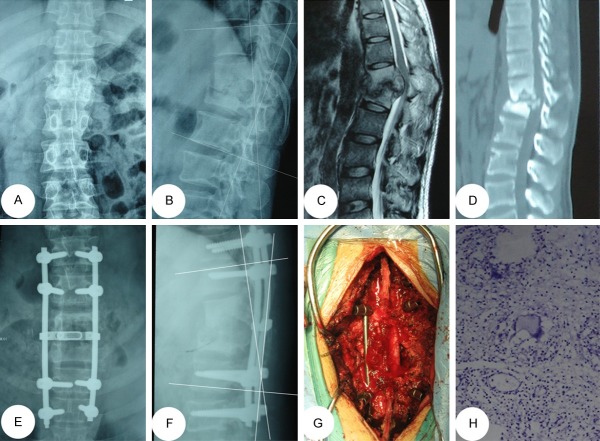
A 42-year-oldmale with T4/5 lesions was performed by posterior-only approach. (A-D) The pre-operative imaging data showed T4/5 vertebral bodies’ destructions with mild kyphosis deformity and spinal cord compressed. The postoperative anterior-posterior (E) and lateral X-ray (F) indicated that the kyphosis got obviously improved by posterior long-segment fixation. Sagittal and coronal CT-scan (G, H) showed satisfied bone fusion without relapse of Pott’s disease at the 12 months of post-operation.
Figure 3.
A 32-year-old male with T12/L1 lesions was performed by posterior-only approach. (A-D) The pre-operative imaging data showed T12/L1 presented with severe bone destructionand severe compression of spinal cord. The postoperative anterior-posterior (E) and lateral X-ray (F) indicated posterior long-segment fixation was in good position. (G) Intraoperative image indicates that debridement via posterior approach. (H) The postoperative histological result showing a mass of caseous necrosis.
Discussion
Tubercular spondylitis frequently happened, accounting for about 50% of the bone and joint tuberculosis [10]. While formal and sufficient anti-TB chemotherapy, strict bed rest and supportive therapy is the most basic methods of treatment of STB, when patients predisposed to bone destruction, sequestrum formation, paraspinal abscess and nerve compression, conservative regimes will yield to surgical approaches. Consequently, surgical indications of single-segment thoracic STB with neurological deficits should be lowered appropriately due to the neurol ogic impairment and late-onset paraplegia should be given priority. However, there are quite a few controversies on surgical management of single-segment thoracic STB with neurological deficits [11]. Some people recommend conservative treatment but others focused on anterior, posterior or combined surgeries. Some researchers insisted that the anterior approaches allows direct access to the focus, thorough debridement and convenient bone grafting [12], however, poor spinal stability, low fusion rate, high frequent pseudarthrosis, easy recurrence of TB, ineffective correction of kyphosis and maintenance of the correction and unsatisfactory neurological function after operation overwhelm its advantages. Moreover, anterior exposure of the upper thoracic spinal region which is blocked by thoracic bones, clavicle, costal bone and superior mediastinum organs presents a significant challenge to the spine surgeons, especially, when destruction by infection leads to kyphosis [2]. The combined anterior and posterior surgery becomes popular due to its satisfactory clinical outcomes [5,13], while the patients of ST usually with poor condition are difficult to tide over the terrible trauma, such as larger loss of blood, longer operation time and complications related to anterior approach [6,7,13]. However, there was quite few literature reported in surgical management of single-segment thoracic STB with neurological deficits via single-stage posterior transpedicular thoracic debridement, interbody bone grafting and posterior fixation.
The anterior column is prone to be affected by Mycobacterium tuberculosis and controversies about applying the posterior-only surgery in treatment of single-segment thoracic STB with neurological deficits focus in whether the surgeons could perform focal debridement and anterior decompression completely on the circumstance of limited visual field; whether it will affect the stability of spine and whether it will affect the anterior bony fuse and so on. The following characteristics of posterior approach can solve the above doubts: 1) This stand-alone posterior surgery creates enough operating space through resection of both side of the facet joint, diapophysis, laminectomy and nerve root allowing operation on the vertebral body at a 360-degree angle under direct visualization of the outside of the dura mater for thorough removal of the sequestrum, collapsed vertebras and intervertebral disk and complete spinal decompression without injuring the spinal cord, which helps to avoid the possible intra and post-operative complications that maybe associated with the anterior exposed and debrided. Paraspinal abscess, if there is, will be conquered by appropriate pressure washing and postural drainage. In our study, all patients had no recurrence of tuberculosis. It hasbeen queried that removing the TB focus via theposterior approach could cause spinal cord infection andcentral nervous system complications such as TB meningitis. However, none of the patients in our studywas complicated by TB meningitis, a finding consistent with otherreports [1,2]. 2) Weinstein [14] reported that vertebral pedicles provided at least 60% of pullout strength and 80% of axial pullout strength, while vertebral cancellous bone provided only 15-20% of pullout strength, indicating that the pullout strength of anterior instrumentation was less than posterior instrumentation. Moreover, based on the scope of vertebral destruction and the extent of vertebral osteoporosis, the surgeons were allowed to have multiple points of fixation along the spinal axis as opposed to anterior plating, which relies solely on endpoint fixation [3]. Furthermore, the thorax support is essential for effective load transferability. However, internal fixation is likely to cause fatigue damage and maybe lead to screws and rods loosening or fracture. So it is necessary to implanting autologous or allograft bone in the lateral facet joints of disease vertebrae and between the transverses, which provides bone support for the spine stability after inter-body fusion [1]. 3) STB is prone to involve the anterior column of a single motion segment i.e. two adjoining vertebral bodies and their intervening disc (peri-discal). Therefore, bone grafts into anterior and central column of the spine can play a major role in resistance to the vertical compressive stress, torsional force, shear force from the spine and sharing the part load of the internal fixation system, which avoid too stress to focus on the internal fixation screws and prevent the emergence of kyphosis recurrence and late-onset paraplegia. Interactionally, some pressure from internal fixation at graft-endplate interface will served as very relevant biomechanical indicators of biological phenomena, such as bone fusion [15], therefore, fusion combined with instrumentation was in full compliance with the requirements of the biomechanics of the spine. However, residual distance of interbody has direct impact on the survival rate of the bone graft after debridement [16]. The difficulty and key in the posterior-only surgical treatment of single-segment thoracic STB with neurological deficits lies in the relatively long inter-vertebral defects, which seriously affect the stability and support functions of the spine. Also, the long-segment bone graft is more prone to delayed stress fracture leading to severe loss of the correction of kyphosis, which makes the compressive stress form the upper and lower ends of vertebras concentrated too much on the screws, causing the screws loosening and breakage and resulting in further kyphosis and late-onset paraplegia [17]. Therefore, we reserved viable bone tissue as much as possible and trimmed appropriate graft being inserted in inter-vertebra. If the graft bone was loose, or the presence of bone defects, we embedded the relatively large bone particles or blockinto the gap by implementing impacted graft. In our study, all patients achieved bone fusion within 4.5 ± 3.2 months after surgery and there is no complication related to bone fusion. During operation, we scraped the surface of the sclerotic bone to the bleeding sub-healthy bone tissue without complete resection and performed all bone graft applying allogeneic bone rather than autologous iliac bone, which avoided the donor-site complications, such as pain, infection and wound unhealed.
The average follow-upperiod was 36.5 ± 9.2 months (range: 24-60 months) in Group A and 38.5 ± 12.1 years (range: 20-68 months) in Group B. Patients had reached bone fusion by a mean of 6.6 ± 2.9 months in Group A and 6.5 ± 3.0 months in Group B, which is less time than others have reported [18,19]. The shorter fusion time in our study may be due tomost patients (50 cases) receiving autogenous iliac grafts.
The authors consider that the methods reported above are radical; therefore, the following points should be emphasized when adopting it: a) Effective chemotherapy is available at present to sterilize Mycobacterium tuberculosis without the need for aggressive anterior debridement; b) Complications related to anterior approach to thoracic spine can be avoided; c) The posterior instrumentation can fully serve to correct the angular deformity and minimize the loss of correction, combined with posterior interbody and posterolateral inter-transverse fusion; d) Multilevel vertebras involved but only one center need short bone grafting fusion (less than two levels) and unnecessary to get debridement in each lesion could got satisfied outcomes; e) Be carefully studied preoperative radiological imaging and CT scan to determine the feasibility of installing the screws because the pedicle structure and bone quality will also affect the placement of the screws; f) Application of the intraoperative electrophysiological monitoring, such as computer navigation monitoring system, so that some subjective factors can be ruled out; g) Make sure the security for spinal cord during debridement, decompression and interbody fusion; h) Master postural drainage technology during operation and place the drainage tube after operation in the treatment of abscess.
Conclusion
In our study, posterior-only surgery resulted in better clinical outcomes than combined posterior-anterior procedures and might be a better surgical therapy in managing single-segment thoracic STB with neurological deficits. The posterior-only approach had the advantages of less surgical invasion, less blood loss, shorteroperation time and hospital stay, and lower complication rate. Only when individual surgery is integrated with useful supportive care and standard chemotherapy can we obtain better outcomes in the treatment of TB. A large number of patients and longer follow-up will be required.
Acknowledgements
This publication was funded in part by the National Natural Science Foundation of China (81171736).
Disclosure of conflict of interest
None.
References
- 1.Pang X, Shen X, Wu P, Luo C, Xu Z, Wang X. Thoracolumbar spinal tuberculosis with psoas abscesses treated by one-stage posterior transforaminal lumbar debridement, interbody fusion, posterior instrumentation, and postural drainage. Arch Orthop Trauma Surg. 2013;133:765–72. doi: 10.1007/s00402-013-1722-9. [DOI] [PubMed] [Google Scholar]
- 2.Zhang HQ, Lin MZ, Shen KY, Ge L, Li JS, Tang MX, Wu JH, Liu JY. Surgical management for multilevel noncontiguous thoracic spinal tuberculosis by single-stage posterior transforaminal thoracic debridement, limited decompression, interbody fusion, and posterior instrumentation (modified TTIF) Arch Orthop Trauma Surg. 2012;132:751–757. doi: 10.1007/s00402-012-1473-z. [DOI] [PubMed] [Google Scholar]
- 3.Singh K, Vaccaro AR, Kim J, Lorenz EP, Lim TH, An HS. Biomechanical comparison of cervical spine reconstructive techniques after a multilevel corpectomy of the cervical spine. Spine (Phila Pa 1976) 2003;28:2352–2358. doi: 10.1097/01.BRS.0000085344.22471.23. discussion 2358. [DOI] [PubMed] [Google Scholar]
- 4.Rajasekaran S, Soundarapandian S. Progression of kyphosis in tuberculosis of the spine treated by anterior arthrodesis. J Bone Joint Surg Am. 1989;71:1314–1323. [PubMed] [Google Scholar]
- 5.Qureshi MA, Khalique AB, Afzal W, Pasha IF, Aebi M. Surgical management of contiguous multilevel thoracolumbar tuberculous spondylitis. Eur Spine J. 2013;4:618–23. doi: 10.1007/s00586-012-2459-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeng H, Shen X, Luo C, Xu Z, Zhang Y, Liu Z, Wang X. Comparison of three surgical approaches for cervicothoracic spinal tuberculosis: a retrospective case-control study. J Orthop Surg Res. 2015;10:100. doi: 10.1186/s13018-015-0238-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeng H, Wang X, Pang X, Luo C, Zhang P, Peng W, Wu P, Xu Z. Posterior only versus combined posterior and anterior approaches in surgical management of lumbosacral tuberculosis with paraspinal abscess in adults. Eur J Trauma Emerg Surg. 2014;40:607–616. doi: 10.1007/s00068-013-0367-2. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Pang X, Wu P, Luo C, Shen X. One-stage anterior debridement, bone grafting and posterior instrumentation vs. single posterior debridement, bone grafting, and instrumentation for the treatment of thoracic and lumbar spinal tuberculosis. Eur Spine J. 2014;23:830–837. doi: 10.1007/s00586-013-3051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee CK, Vessa P, Lee JK. Chronic disabling low back pain syndrome caused by internal disc derangements. The results of disc excision and posterior lumbar interbody fusion. Spine (Phila Pa 1976) 1995;20:356–361. doi: 10.1097/00007632-199502000-00018. [DOI] [PubMed] [Google Scholar]
- 10.Polley P, Dunn R. Noncontiguous spinal tuberculosis: incidence and management. Eur Spine J. 2009;18:1096–1101. doi: 10.1007/s00586-009-0966-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen X, Liu H, Wang G, Pang X, Luo C, Zeng H, Xu Z. The role of single-stage posterior debridement, interbody fusion with titanium mesh cages and short-segment instrumentation in thoracic and lumbar spinal tuberculosis. J Neurosurg Sci. 2015 doi: 10.23736/S0390-5616.16.03333-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Lan X, Liu XM, Ge BF. Debridement and bone grafting with internal fixation via anterior approach for treatment of cervicothoracic tuberculosis. Int Surg. 2011;96:358–362. doi: 10.9738/cc62.1. [DOI] [PubMed] [Google Scholar]
- 13.Zhang HQ, Guo CF, Xiao XG, Long WR, Deng ZS, Chen J. One-stage surgical management for multilevel tuberculous spondylitis of the upper thoracic region by anterior decompression, strut autografting, posterior instrumentation, and fusion. J Spinal Disord Tech. 2007;20:263–267. doi: 10.1097/01.bsd.0000211281.68400.1b. [DOI] [PubMed] [Google Scholar]
- 14.Weinstein JN, Rydevik BL, Rauschning W. Anatomic and technical considerations of pedicle screw fixation. Clin Orthop Relat Res. 1992:34–46. [PubMed] [Google Scholar]
- 15.Ferrara LA, Gordon I, Coquillette M, Milks R, Fleischman AJ, Roy S, Goel VK, Benzel EC. A preliminary biomechanical evaluation in a simulated spinal fusion model. Laboratory investigation. J Neurosurg Spine. 2007;7:542–548. doi: 10.3171/SPI-07/11/542. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell RM. Anterior spinal fusion in spinal tuberculosis using an iliac bone block. East Afr Med J. 1966;43:119–122. [PubMed] [Google Scholar]
- 17.Dvorak MF, Kwon BK, Fisher CG, Eiserloh HL 3rd, Boyd M, Wing PC. Effectiveness of titanium mesh cylindrical cages in anterior column reconstruction after thoracic and lumbar vertebral body resection. Spine (Phila Pa 1976) 2003;28:902–908. doi: 10.1097/01.BRS.0000058712.88053.13. [DOI] [PubMed] [Google Scholar]
- 18.Lee KB, Johnson JS, Song KJ, Taghavi CE, Wang JC. Use of Autogenous Bone Graft Compared With RhBMP in High-risk Patients: A Comparison of Fusion Rates and Time to Fusion. J Spinal Disord Tech. 2013;26:233–238. doi: 10.1097/BSD.0b013e3182440162. [DOI] [PubMed] [Google Scholar]
- 19.Harjeet K, Synghal S, Kaur G, Aggarwal A, Wahee P. Time of fusion of greater cornu with body of hyoid bone in Northwest Indians. Legal Medicine. 2010;12:223–227. doi: 10.1016/j.legalmed.2010.05.001. [DOI] [PubMed] [Google Scholar]