Abstract
Objective
Vibration-controlled transient elastography (VCTE) is increasingly used to assess liver fibrosis in viral hepatitis and fatty liver disease populations. Because the accuracy of VCTE in HIV-monoinfected populations has not been established, we evaluated its performance in assessing liver fibrosis in a cohort of HIV-monoinfected adults undergoing liver biopsy as part of a recently published clinical trial.
Methods
HIV-infected adults with elevated aminotransferase levels for ≥6 months while receiving antiretroviral therapy, and without chronic viral hepatitis or other known causes of liver disease, were prospectively evaluated by VCTE, other non-invasive markers of fibrosis, and percutaneous liver biopsy as part of a cross-sectional study examining liver pathology.
Results
66 patients were evaluated by VCTE and liver biopsy. The cohort was majority male (92%) with median age 50 years (range 17–68). Biopsy identified bridging fibrosis in 14 (21%) and non-alcoholic steatohepatitis (NASH) in 38 (58%) participants. VCTE was unsuccessful or unreliable in 7 participants (11%). In the 59 participants with reliable results, median liver stiffness measurement (LSM) was 5.9kPa (range 3.3–29.2 kPa); 25 participants (42%) had a LSM >7.1kPa, a value consistent with increased liver stiffness in other populations. VCTE had good sensitivity and specificity with an area under the receiver operating characteristic curve (AUROC) of 93% for detection of moderate fibrosis (Ishak F≥2; 95% CI 86–99%).
Conclusions
In HIV-monoinfected adults with biopsy-proven liver disease, LSM by VCTE was the best non-invasive predictor of fibrosis. Our findings support the continued use of VCTE for fibrosis screening in HIV-monoinfected patients with elevated aminotransferases.
Keywords: hepatotoxicity, liver biopsy, liver stiffness measurements
Background
Aminotransferase elevations are common in HIV-infected adults receiving antiretroviral therapy and are associated with a high rate of liver disease, including non-alcoholic steatosis (NASH) and fibrosis, in those without hepatitis B or C co-infection [1]. Non-invasive markers that can reliably determine the severity of underlying pathology can play an important role in the management of such patients. However, there is considerable overlap in clinical markers between those with and without significant liver pathology and the performance of non-invasive markers has not been well-established in this population [1].
Liver biopsy, the gold standard for diagnosis and staging of NASH and fibrosis, has several limitations. Biopsy is invasive, has potentially serious complications [2], and sampling errors can result in inaccurate estimates of the degree of liver damage [3, 4]. A non-invasive test or combination of tests that could reliably identify patients with fibrosis would allow targeting of liver biopsy to those most likely to benefit. This may be even more critical in the management of HIV-infected patients who have a high rate of non-alcoholic fatty liver disease (NAFLD) and in whom co-existing liver disease cannot be excluded without biopsy.
Vibration-controlled transient elastography (VCTE) has been shown to have good sensitivity and specificity for detection of advanced fibrosis in HIV and viral hepatitis co-infected patients [5] as well as in HIV-negative NASH populations [6]. However, one limitation of VCTE is a high failure rate, usually in the setting of abdominal obesity and increased waist circumference [7]. Moreover, liver stiffness can vary with aminotransferase flares [8] and is unreliable in the setting of acute liver injury [9]. Despite these potential limitations and a lack of validation in HIV monoinfected patients, clinical trials are increasingly using VCTE to study liver stiffness in HIV-infected populations [10, 11].
To assess the accuracy of VCTE for the detection of fibrosis in HIV-moninfected adults, we performed VCTE in participants in a recently published study of HIV-infected adults with elevated aminotransferases and without chronic viral hepatitis who were undergoing liver biopsy.
Methods
Study Population
The methods of the observational cross-sectional study have been published [1]. HIV-infected adults with aspartate aminotransferase (AST) or alanine aminotransferase (ALT) elevations greater than the upper limit of normal (AST >34 U/L or ALT >41 U/L) on ≥ 3 occasions over at least six months while receiving combination ART for ≥1 year were eligible if they had no evidence of active viral hepatitis, hereditary or autoimmune liver disease, or hemochromatosis, no ongoing alcohol abuse, and no contraindication to liver biopsy.
The National Institute of Allergy and Infectious Diseases Institutional Review Board approved the protocol and all participants provided written informed consent.
Laboratory Testing
Laboratory assessment prior to liver biopsy included complete blood counts, AST, ALT, alkaline phosphatase, gamma-glutamyl transpeptidase (GGT), bilirubin and albumin. HIV viral load was measured by PCR (RealTime HIV-1 Assay, Abbott Laboratories, Des Plaines, IL; lower limit of quantification 40 copies/mL) and CD4+ T-cell counts by flow cytometry.
The AST/platelet ratio index (APRI) [12], FIB-4 [13] and NAFLD fibrosis score [14] indices were calculated as non-invasive markers of fibrosis, using laboratory and clinical parameters at the time of study enrollment.
Transient Elastography
Liver stiffness was determined using VCTE (FibroScan®, Echosens, Paris, France) [15]. At least ten measurements were made using the M-probe according to the manufacturer’s recommendations and the median, expressed in kilopascal (kPa) units, determined [16]. Results were considered reliable if the success rate was ≥60% and the interquartile range/median ratio was ≤0.30.
Liver Histology
Percutaneous liver biopsy was performed under ultrasound guidance using an 18-gauge needle. One liver biopsy fragment was fixed in formalin. Paraffin-embedded sections were stained with hematoxylin and eosin, Masson’s trichrome, special stains for iron and copper, and for reticulin. Liver biopsies were read by a single liver pathologist (DEK). Liver biopsies with less than 10 portal tracts were excluded from analysis.
Fibrosis and inflammatory activity were scored according to the modified histology activity index (Ishak) scoring system [17]. Steatosis was graded on a scale of 0 to 4 based on the percentage of cells with fat. Histological features of NAFLD were also scored according to the NASH Clinical Research Network (CRN) scoring system [18].
Statistical Analyses
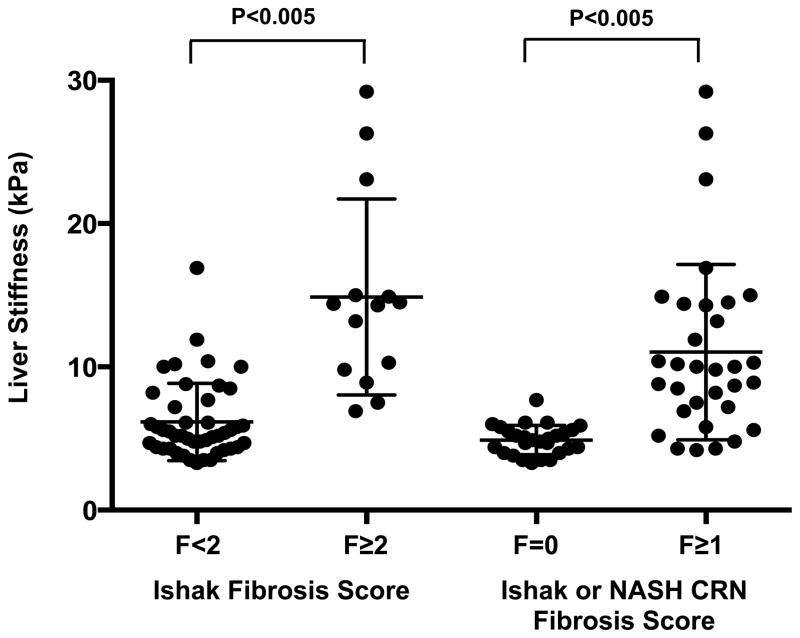
The diagnostic performance of VCTE, APRI, FIB-4 and NAFLD Fibrosis Score for the prediction of significant fibrosis (Ishak fibrosis score ≥2) was assessed by receiver operator characteristic (ROC) methodology. Sensitivity, specificity, positive predictive values (PPV), and negative predictive values (NPV) were calculated using cut-off values established in other liver disease populations [12, 14, 19]. Multivariate analyses were not undertaken because the relatively small number of cases (14) precluded stable estimates and valid inferences. Comparisons of those with and without fibrosis (Figure 1) were made using the Wilcoxon rank sum test with a Bonferroni adjustment for multiple comparisons.
Figure 1.
Liver stiffness measurements (n=59) as determined by vibration-controlled transient elastography, segregated by liver biopsy fibrosis score. Adjusted P-values remained highly significant.
Two sided P values <0.05 were considered significant. Analyses were performed using GraphPad Prism (version 6.0; GraphPad Software, Inc., La Jolla, CA).
Results
Participants
VCTE was performed on 58 of the previously reported 62 participants in the prospective biopsy cohort, as well as 8 additional patients with a history of non-alcoholic steatohepatitis on prior liver biopsy who enrolled in the natural history arm of the study for repeat liver biopsy and follow-up.
The characteristics of the 66 participants are shown in Table 1. The cohort was majority male (92%) and the median age of participants was 50 years (range 17–68 years). Median duration of HIV infection at biopsy was 17 years (range 1–28 years). Median ALT was 79 IU/mL (range 27–1244 IU/mL); 16 (24%) had ALT elevations greater than three times the upper limit of normal (>123 IU/mL).
Table 1.
Demographic and clinical parameters for the cohort (n=66)
| Characteristic | |
| Age, years | 50 (17–68) |
| Male sex, n (%) | 61 (92%) |
| Race (self-identified), n (%) | |
| White | 41 (62%) |
| Black | 4 (6%) |
| Asian | 2 (3%) |
| Not selected/mixed race | 19 (29%) |
| Ethnicity (self-identified), n (%) | |
| Hispanic/Latino | 22 (33%) |
| Not Hispanic/Latino | 44 (67%) |
| Time since HIV diagnosis, years | 17.3 (1.0–27.8) |
| CD4+ T-cell nadir, cells/mm3 | 180 (0–599) |
| CD4+ T-cell count at enrollment, cells/mm3 | 548 (105–1631) |
| HIV viral load, copies/ml | <40 (<40–726) |
| Total duration of antiretroviral therapy, years | 12.9 (1.7–27.0) |
| Lipodystrophy, clinical history, n (%) | 28 (42%) |
| Body mass index (BMI), kg/m2 | 27.6 (21.4–47.1) |
| Overweight (25 < BMI < 30), n (%) | 30 (45%) |
| Obese (BMI > 30), n (%) | 20 (30%) |
| Diabetes mellitus, n (%)a | 7 (11%) |
| AST, U/L (normal range 9–34) | 47 (19–411) |
| ALT, U/L (normal range 6–41) | 79 (27–1244) |
| Platelet count, x109 (normal range 150–400) | 208 (79–380) |
| Liver Biopsy Findings | |
| Fibrosis Score | |
| None (0) | 44 (67%) |
| Mild (1) | 8 (12%) |
| Moderate (2) | 1 (2%) |
| Bridging (3–4) | 13 (20%) |
| Cirrhosis (5–6) | 0 (0%) |
| Diagnosis | |
| Non-specific changes | 22 (33%) |
| Steatohepatitis | 38 (58%) |
| Steatohepatitis with any fibrosis, n (%) | 30 (45%) |
| Steatohepatitis with bridging fibrosis, n (%) | 12 (18%) |
| Fibrosis (F≥1) without steatohepatitis, n (%) | 3 (5%) |
| Bridging fibrosis without steatohepatitis, n (%) | 1 (2%) |
| Portal venopathy/nodular regenerative hyperplasia | 3 (5%) |
Median and range presented unless noted otherwise.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; HIV, human immunodeficiency virus
Three participants had a diagnosis of diabetes mellitus prior to study participation; 4 participants were diagnosed by oral glucose tolerance testing as part of the study.
Liver biopsy identified bridging fibrosis in 14 (21%) and NASH in 38 (58%). Portal venopathy was identified in 1 participant and nodular regenerative hyperplasia (NRH) in 2 participants. Non-specific changes, including mild-moderate steatosis and mild inflammation were seen in 22 (33%) patients.
Vibration Controlled Transient Elastography (VCTE)
VCTE was unsuccessful in 3 participants with obesity (all with NASH) and unreliable in an additional 4 participants (2 with NASH, 2 with non-specific changes on biopsy), a failure rate of 11%. In the 59 participants with reliable results, median liver stiffness value was 5.9 kPa (range 3.3–29.2 kPa); 25 participants (42%) had score >7.1 kPa, a value consistent with increased liver stiffness in other liver disease populations [6, 20].
The sensitivity and specificity of VCTE for detection of moderate fibrosis was good, with an area under the receiver operating characteristic curve (AUROC) of 93% for F≥2 (95% CI 86–99%). A pre-specified cut-off for VCTE of 7.1 kPa, gave a sensitivity of 93% and specificity of 73% for F≥2 (Table 2). The negative predictive value (NPV) to exclude F≥2 was 97%. The liver stiffness cut-off that optimized performance in our cohort, 7.2 kPa, was similar to the pre-specified cut-off of 7.1 kPa, with a sensitivity of 93% and specificity 75% for detection of F≥2.
Table 2.
Performance of VCTE, APRI, FIB-4 and NAFLD FS for the detection of significant fibrosis in HIV-monoinfected adults with elevated aminotransferases and reliable liver stiffness measurements (n=59).
| VCTE | APRI [12] | FIB-4 [19] | NAFLD FS [14] | |
|---|---|---|---|---|
| AUROC (%, 95% CI) | 93 (86–99) | 61 (46–77) | 64 (49–79) | 70 (55–85) |
| Cut-offa | ≥7.1 kPa | >1.5 | >2.67 | >0.676 |
| Sensitivity (%) | 93 | 21 | 21 | 14 |
| Specificity (%) | 73 | 82 | 89 | 96 |
| Positive predictive value (%) | 52 | 27 | 38 | 50 |
| Negative predictive value (%) | 97 | 77 | 78 | 78 |
Values selected from previously published cut-offs that maximize sensitivity and specificity in other liver disease populations.
Abbreviations: APRI, AST-to-platelet ratio index; AUROC, area under the receiver-operating characteristic curve; CI, confidence interval; NAFLD FS, non-alcoholic fatty liver disease fibrosis score; VCTE, vibration-controlled transient elastography.
The sensitivity and specificity of VCTE for the detection of any fibrosis was also good with AUROC of 89% (95% CI 78–97%) for Ishak F>0 and/or NASH CRN F>0. A pre-specified cut-off for VCTE of 7.1 kPa gave a lower sensitivity of 75% and better specificity of 96%. The NPV to exclude any fibrosis was 76%; positive predictive value (PPV) to detect any fibrosis was 96%.
In an “intention to treat” analysis, all patients who underwent VCTE and liver biopsy were analyzed (n=66). Participants with unreliable results were categorized based on the reported liver stiffness value and those with unsuccessful VCTE were considered as not correctly classified. At the cut-off of 7.1 kPa, the NPV of VCTE in detecting F≥2 remained high at 97%. However, PPV declined slightly to 46%.
Liver stiffness was elevated (>7.1 kPa) in 21/33 (64%) participants with steatohepatitis, all with at least some fibrosis on biopsy (Ishak F>0 or NASH CRN F>0). One participant with advanced fibrosis (Ishak F4), not associated with NASH, had a VCTE of 6.9 kPa. VCTE was >7.1kPa in both participants with NRH.
Comparison with non-invasive laboratory markers of fibrosis
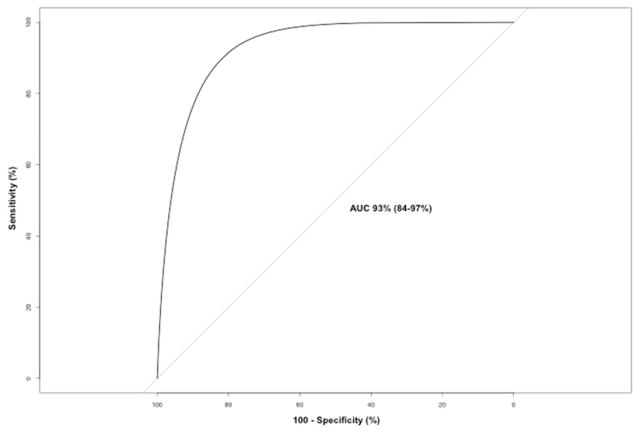
The performance of VCTE, APRI, FIB-4 and NAFLD Fibrosis Score for the detection of significant fibrosis are presented in Table 2. VCTE had the best diagnostic performance. Unlike the other predictors, VCTE had high values for both sensitivity and specificity, and had a very high AUROC (Figure 2).
Figure 2.
Receiver operator curve of vibration-controlled transient elastography measurement of liver stiffness for the prediction of significant hepatic fibrosis in HIV monoinfected adults. Area under the curve 93%.
Discussion
VCTE was the best non-invasive predictor of fibrosis in this cohort of HIV-monoinfected adults, with a high sensitivity and good specificity for detecting moderate to severe fibrosis in a population in which the most prevalent liver disease was NASH.
Our results mirror those seen in HIV-negative NAFLD patients. In one study of 246 adults with NAFLD, the AUROC for significant fibrosis was 0.93 [6]. At a liver stiffness cut-off of 7.9 kPa, the sensitivity, specificity, and positive and negative predictive values for significant fibrosis were 91%, 75%, 52% and 97% respectively. A failure rate of 13% was described. Similar performance is seen in studies of VCTE in HCV monoinfected and HIV/HCV co-infected cohorts, with mean AUROC for the diagnosis of significant fibrosis of 0.84 [5, 20, 21].
The non-invasive biomarkers APRI, FIB4 and NAFLD-FS performed poorly, with sensitivities ranging from 14–21%. The performance of these biomarkers can vary with disease state [22]. APRI, for example, performs best in hepatitis C monoinfected cohorts and may be less useful in the setting of HIV-related thrombocytopenia. Further, APRI performs poorly in studies of NASH in HIV-negative populations [6]. While potentially useful for identify advanced liver disease in certain settings, these markers failed to identify significant fibrosis in the current study of HIV-monoinfection and elevated aminotransferases and should be used cautiously in screening studies of HIV-monoinfected patients.
Our study was conducted only in HIV-infected adults with suppressed HIV viremia on ART and these results should be extended cautiously to untreated HIV-infected populations where HIV may directly promote hepatic inflammation and damage. Other limitations of our study include the small size of the study population and conduct at a referral center where the population is likely enriched for liver disease and fibrosis. However, VCTE can exclude severe fibrosis, with a specificity >70% in these patients, even in the setting of an 11% failure rate. Using the same cutoff (7.1 kPa), the specificity for any fibrosis (Ishak F>0) is 97%, potentially ruling out a need for further evaluation in patients with results below this value. The failure rate, similar to that seen in other similar studies, may be reduced in the future given the availability of an XL probe for use with obese patients. This probe was not available at our institution at the time of this study.
In a cohort of HIV-infected adults with biopsy-proven liver disease, liver stiffness measured by VCTE was the best non-invasive predictor of fibrosis. With an excellent negative predictive value and good positive predictive value, VCTE is a useful screening test to exclude advanced fibrosis in HIV-monoinfected patients with elevated aminotransferases. Staging of liver fibrosis is critical to the prognosis and management of liver disease in HIV-infected patients and VCTE can help identify those most likely to require liver biopsy for definitive diagnosis and targeted management.
Acknowledgments
This work is supported by the NIH Intramural Research Program, NIH Clinical Center, NIAID, NIDDK, NCI, and, in part, by an NIH Bench to Bedside Award funded by the Office of AIDS Research.
The authors thank the study volunteers who participated in this research project and the dedicated staff of the NIAID/CCMD Intramural HIV/AIDS research clinic (OP-8).
Footnotes
Author Contributions
Study concept and design: C.G.M., T.H., J.A.K; acquisition of data: C.G.M., M.M., C.K., D.E.K., T.H.; analysis and interpretation of data: C.G.M., C.K., D.E.K., T.H., J.A.K; drafting of the manuscript: C.G.M.; critical revision of the manuscript for important intellectual content: all authors; statistical analysis: M.P.
Conflicts of Interest: All authors: no conflicts declared.
Presented in part at the 15th Conference on Retroviruses and Opportunistic Infections (CROI) 2008, Boston, MA, and at the Liver Meeting (AASLD) 2010, Boston, MA.
References
- 1.Morse CG, McLaughlin M, Matthews L, Proschan M, Thomas F, Gharib AM, et al. Non-alcoholic Steatohepatitis and Hepatic Fibrosis in Human Immunodeficiency Virus-1 Mono-infected Adults with Elevated Transaminases on Antiretroviral Therapy. Clin Infect Dis. 2015 doi: 10.1093/cid/civ101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piccinino F, Sagnelli E, Pasquale G, Giusti G. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J Hepatol. 1986;2:165–173. doi: 10.1016/s0168-8278(86)80075-7. [DOI] [PubMed] [Google Scholar]
- 3.Maharaj B, Maharaj RJ, Leary WP, Cooppan RM, Naran AD, Pirie D, et al. Sampling variability and its influence on the diagnostic yield of percutaneous needle biopsy of the liver. Lancet. 1986;1:523–525. doi: 10.1016/s0140-6736(86)90883-4. [DOI] [PubMed] [Google Scholar]
- 4.Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449–1457. doi: 10.1016/j.hep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 5.Vergara S, Macias J, Rivero A, Gutierrez-Valencia A, Gonzalez-Serrano M, Merino D, et al. The use of transient elastometry for assessing liver fibrosis in patients with HIV and hepatitis C virus coinfection. Clin Infect Dis. 2007;45:969–974. doi: 10.1086/521857. [DOI] [PubMed] [Google Scholar]
- 6.Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le Bail B, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454–462. doi: 10.1002/hep.23312. [DOI] [PubMed] [Google Scholar]
- 7.Castera L, Foucher J, Bernard PH, Carvalho F, Allaix D, Merrouche W, et al. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology. 2010;51:828–835. doi: 10.1002/hep.23425. [DOI] [PubMed] [Google Scholar]
- 8.Coco B, Oliveri F, Maina AM, Ciccorossi P, Sacco R, Colombatto P, et al. Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases. J Viral Hepat. 2007;14:360–369. doi: 10.1111/j.1365-2893.2006.00811.x. [DOI] [PubMed] [Google Scholar]
- 9.Sagir A, Erhardt A, Schmitt M, Haussinger D. Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatology. 2008;47:592–595. doi: 10.1002/hep.22056. [DOI] [PubMed] [Google Scholar]
- 10.Bailony MR, Scherzer R, Huhn G, Plankey MW, Peters MG, Tien PC. Association of HIV infection, hepatitis C virus infection, and metabolic factors with liver stiffness measured by transient elastography. J Infect Dis. 2013;208:1776–1783. doi: 10.1093/infdis/jit357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vermehren J, Vermehren A, Mueller A, Carlebach A, Lutz T, Gute P, et al. Assessment of liver fibrosis and associated risk factors in HIV-infected individuals using transient elastography and serum biomarkers. BMC Gastroenterol. 2012;12:27. doi: 10.1186/1471-230X-12-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 13.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 14.Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 15.Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound in medicine & biology. 2003;29:1705–1713. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48:835–847. doi: 10.1016/j.jhep.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, et al. Histological grading and staging of chronic hepatitis. Journal of hepatology. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 18.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 19.Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104–1112. doi: 10.1016/j.cgh.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castera L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343–350. doi: 10.1053/j.gastro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 21.Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, et al. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134:960–974. doi: 10.1053/j.gastro.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 22.Sebastiani G, Castera L, Halfon P, Pol S, Mangia A, Di Marco V, et al. The impact of liver disease aetiology and the stages of hepatic fibrosis on the performance of non-invasive fibrosis biomarkers: an international study of 2411 cases. Aliment Pharmacol Ther. 2011;34:1202–1216. doi: 10.1111/j.1365-2036.2011.04861.x. [DOI] [PubMed] [Google Scholar]