Abstract
Somatostatin receptor 2 (SSTR2) is a negative regulator of cell proliferation in human breast cancer. Since there is little information about SSTR2 in canine mammary gland tumor (MGT), we clarified its distribution and expression level in normal mammary gland, benign MGT and malignant MGT. SSTR2 expression determined by immunohistochemical staining was observed in the cytoplasm of luminal epithelial cells. The intensity was negatively correlated with malignancy: normal tissues and some of the benign tumors had the highest levels, while the malignant tumors had little or no SSTR2 expression. As for the Western blotting, SSTR2 protein level in benign tumors was significantly lower than the normal mammary gland. On the other hand, SSTR2 protein levels in two of three malignant tumors were higher than the other groups. These results suggest that SSTR2 expression alters according to the malignancy of canine MGT.
Keywords: canine, malignancy, mammary gland tumor, somatostatin receptor 2 (SSTR2)
Somatostatin (SST) is a peptide hormone produced in the hypothalamus, pancreas and intestine; it regulates both endocrine and exocrine gland function [17], and down-regulates cell proliferation in various tissues of many species including dogs [1, 3, 4, 10, 14]. Among the SST receptor (SSTR) superfamily, SSTR2 is the most dominant receptor, with the highest affinity to the ligand and the strongest anti-proliferative effect [3]. In human breast cancer, tumor tissues that express SSTRs are less aggressive and more differentiated than tumors with little SSTRs expression, which are massive and highly malignant [5]. These findings lead to the speculation that SSTR signal transduction is closely related to the malignancy of human breast cancer.
In dogs, all subtypes of SSTRs, except SSTR4, have been already identified, and their expression was confirmed in just several tissues, such as pituitary and pancreas [2, 16]. Although SSTRs are predicted to be a molecular target of breast cancer therapy, there is little information about SSTRs in dogs. Hence, in this study, we examined and compared the distribution and expression of SSTR2 in normal canine mammary tissues, benign mammary gland tumor (MGT) and malignant MGT. Immunohistochemistry and Western blotting methods were utilized.
All experiments were approved by the Committees on the Ethics of Animal Experiments of Kitasato University and the University of Tokyo. For immunohistochemistry, 48 samples of canine mammary gland tissue embedded in paraffin blocks were obtained from the histopathological files in the Veterinary Pathology Laboratory, Kitasato University. Although the classification method about simple carcinoma is under controversy [18], the diagnosis of all specimens was based on the WHO classification [11]. Immunohistochemical staining was performed using the rabbit polyclonal antibodies against human SSTR2 (1:300 dilution, bs-1138R, Bioss, Boston, MA, U.S.A.). Peroxidase-conjugated anti-rabbit immunoglobulin G (Histofine Simple Stain MAX-PO (R), Nichirei, Tokyo, Japan) was used as the secondary antibody. After immunoreaction, sections were colorized with diaminobenzidine and counterstained with hematoxylin. Canine pancreas tissues were used as positive controls. Negative controls were carried out by replacing the primary antibodies with antibody diluents.
For Western blotting, 12 canine mammary gland tissues (4 normal, 4 simple adenomas, one complex adenoma and 3 simple carcinomas), harvested and flash frozen in liquid nitrogen, were obtained either from experimental animals or from patients undergoing surgery for mammary gland tumors at the Kitasato University Veterinary Teaching Hospital. We performed Western blotting analysis according to a previously reported protocol [12]. Twelve micrograms of protein were loaded in each well. The human SSTR2 antibody used in immunohistochemistry analysis (1:300 dilution) was also used in Western blotting. Samples were visualized using horseradish peroxidase-conjugated secondary antibodies and the EZ-ECL system (Biological Industries, Kibbutz Beit Hesmek, Israel). The expression level of SSTR2 in each sample was normalized to total actin (1:500 dilution, MAB1501, Sigma-Aldrich, St. Louis, MO, U.S.A.) expression on the same membrane as an internal control.
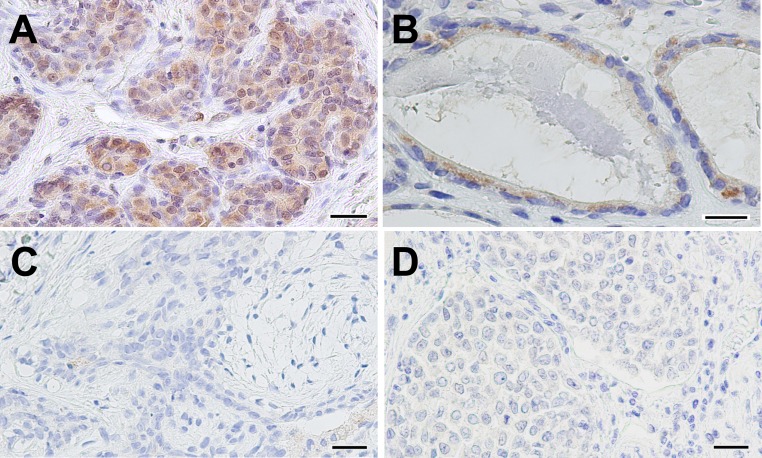
A summary of immunohistochemical data is shown in Fig. 1 and Table 1. In all samples examined, immunoreactivity was restricted to the luminal epithelial cells, although myoepithelial and stromal cells were present in the specimens. The samples are grouped into three groups in Table 1 based on their diagnoses. The first group contains tissues from normal or hyperplastic mammary glands. SSTR2 immunoreactivity in this group was readily observed in the cytoplasm of the luminal epithelial cells. The second group of tissues was from animals diagnosed with benign tumors. These were simple or complex mammary adenoma or mixed tumors. SSTR2 expression in approximately half of the tissues of this group was similar to the first group, with a similar immunoreactive pattern that was detected in the luminal epithelial cells; the other half of this group had little to no immunoreactivity. Samples from the third group were from animals diagnosed with malignant tumors: simple or complex mammary carcinoma or malignant mixed tumors. These tissues had negative or weakly positive immunoreactivity.
Fig. 1.
Representative images of immunoreactivity to human SSTR2 in canine mammary gland tissues. SSTR2 immunoreactivity in the cytoplasm of luminal epithelial cells in normal mammary gland tissue (A); Weakly positive signals for SSTR2 immunoreactivity in a simple adenoma (B); Negative immunoresponse in a complex adenoma (C) and a simple carcinoma (D). Scale bars indicate 25 µm in A, C and D; 20 µm in B.
Table 1. Expression levels of SSTR 2 in canine mammary glands, as determined by immunohistochemistry. Data represent the percentage of samples with that expression level.
| Diagnosis (total number of samples) |
Expression level | |||||
|---|---|---|---|---|---|---|
| + | ± | − | ||||
| Normal (5) | 3 | (60%) | 2 | (40%) | 0 | (0%) |
| Hyperplasia (2) | 1 | (50%) | 1 | (50%) | 0 | (0%) |
| Simple adenoma (12) | 2 | (16.7%) | 5 | (41.7%) | 5 | (41.7%) |
| Complex adenoma (7) | 1 | (14.3%) | 2 | (28.6%) | 4 | (57.1%) |
| Benign mixed tumor (5) | 1 | (20%) | 2 | (40%) | 2 | (40%) |
| Simple carcinoma (10) | 0 | (0%) | 5 | (50%) | 5 | (50%) |
| Complex carcinoma (5) | 0 | (0%) | 3 | (60%) | 2 | (40%) |
| Malignant mixed tumor (2) | 0 | (0%) | 1 | (50%) | 1 | (50%) |
+: Strongly positive; ±: Weakly positive; −: Negative.
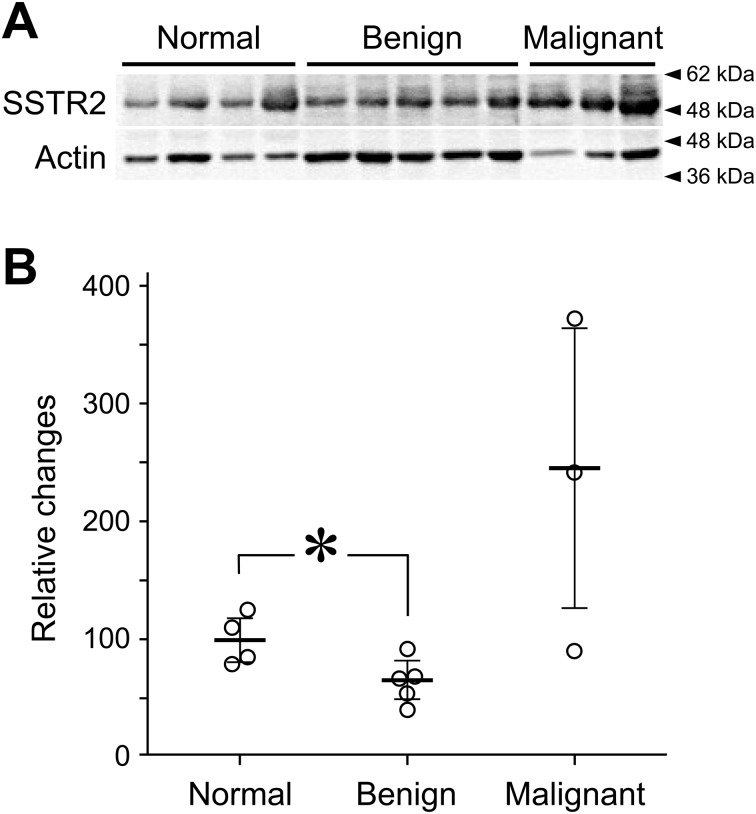
To evaluate the quantitative expression levels of SSTR2, we performed Western blotting (Fig. 2). In Western blotting, a specific band corresponding to approximately 50-kDa molecular weight was detected. In the benign MGT, the SSTR2 protein level was lower than in normal tissues. The levels of SSTR2 protein in two of three malignant MGTs were higher than in the other groups, while the level in one of three was around normal. In these malignant samples, there were also no clear signals of SSTR2 in immunohistochemistry (see the Supplementary Fig. 1), which is coincident with the immunohistochemical results in our other samples (Fig. 1). Further, there was no difference among tissue types: simple, complex and mixed tumors (data not shown).
Fig. 2.
Levels of SSTR2 proteins in canine mammary gland tissues as determined by Western blotting. (A) Images of Western blot analysis for SSTR2 and total actin. Samples from individuals with normal tissue, benign MGTs or malignant MGTs were loaded into each lane. (B) Levels of SSTR2 protein, normalized to actin. The mean SSRT2 protein level in normal tissue was set at 100. (Horizontal bars, mean ± SD; *P<0.05.)
SSTR2 is expressed in the luminal epithelial cells of the mammary tissues and breast cancers in humans [8]. In our study, SSTR2 expression in canine tumors was also limited to the luminal epithelial cells, specifically to the cytoplasm. SSTR expression in humans has also been reported to be down-regulated in more aggressive and less differentiated benign breast tumors [5]. The results of immunohistological staining agree with these human studies [5, 8]. Protein levels of the benign tumors were also less than the normal or hyperplastic tissue, as shown by Western blotting. These findings suggest that SSTR2 deficiency in canines may contribute to the development of benign tumors.
Alteration of SSTR2 expression in the MGT is not fully understood. Some studies in human breast cancer report that the SSTR2 immunohistochemical reaction was less in malignant tumors than in normal tissue, but others report the opposite relationship for SSTR2 mRNA. For instance, Reubi et al. detected SSTR2-positive immunoreactivity in only 3/39 (8%) of human malignant breast tumors [15], while Orlando et al. performed q-PCR of 169 human breast cancers and concluded that the SSTR2 mRNA level was tremendously higher in human breast cancers than in unaffected tissues [13]. Evans et al. found SSTR2 mRNA expression in almost all breast carcinoma, but in only approximately half of benign tumors or normal breast tissues [7]. In the present study, all of SSTR2 immunohistochemical intensity in malignant tumors was lower or negative compared to normal tissues, whereas SSTR2 protein levels in two of three malignant tumors were higher than the other groups in the Western blotting analysis. Although mRNA levels were not determined in these specimens, our standings tended to be compatible with the previous results and this is the first report presenting the paradoxical results within one study.
We had conflicting results between immunohistochemical reactivity and protein level of SSTR2 in malignant tumors. In this study, the same primary antibody to SSTR2 was used for both immunohistochemistry and Western blotting. However, very little reactivity was observed in immunohistochemistry, while Western blotting detected a large amount of protein. Indeed, it is not so rare that there is some discrepancy between the results of immunohistochemistry and Western blotting [6, 9]. Although further studies are needed to understand the reasons for these results, there are several hypotheses that could account for them. 1) In immunohistochemistry, the tissue is fixed in 10% neutral buffered formalin, and the epitope may be obscured or cross-linked due to experimental conditions. 2) Because Western blotting was performed after the reduction treatment, a conformational change in the protein may alter the intensity of immunoreactivity.
In conclusion, we examined the distribution and expression of SSTR2 in canine mammary gland tissues. SSTR2 was mainly observed in the cytoplasm of luminal epithelial cells. There was less expression of SSTR2 in benign MGTs than in normal tissues. In malignant MGT, immunohistochemical intensity of SSTR2 was low, although SSTR2 protein levels were high. It is suggested that SSTR2 expression changes with malignancy of canine MGT.
Supplementary Material
REFERENCES
- 1.Ben-Shlomo A., Melmed S.2008. Somatostatin agonists for treatment of acromegaly. Mol. Cell. Endocrinol. 286: 192–198. doi: 10.1016/j.mce.2007.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Bruin C., Meij B. P., Kooistra H. S., Hanson J. M., Lamberts S. W., Hofland L. J.2009. Cushing’s disease in dogs and humans. Horm. Res. 71Suppl 1: 140–143. doi: 10.1159/000178058 [DOI] [PubMed] [Google Scholar]
- 3.Buscail L., Estève J. P., Saint-Laurent N., Bertrand V., Reisine T., O’Carroll A. M., Bell G. I., Schally A. V., Vaysse N., Susini C.1995. Inhibition of cell proliferation by the somatostatin analogue RC-160 is mediated by somatostatin receptor subtypes SSTR2 and SSTR5 through different mechanisms. Proc. Natl. Acad. Sci. U.S.A. 92: 1580–1584. doi: 10.1073/pnas.92.5.1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buscail L., Vernejoul F., Faure P., Torrisani J., Susini C.2002. Regulation of cell proliferation by somatostatin. Ann. Endocrinol. (Paris). 63: 2S13–2S18. [PubMed] [Google Scholar]
- 5.Cameron Smith M., Orlando C., Serio M., Maggi M.2003. Somatostatin receptors and breast cancer. J. Endocrinol. Invest. 26Suppl: 125–130. [PubMed] [Google Scholar]
- 6.Daginakatte G. C., Chard-Bergstrom C., Andrews G. A., Kapil S.1999. Production, characterization, and uses of monoclonal antibodies against recombinant nucleoprotein of elk coronavirus. Clin. Diagn. Lab. Immunol. 6: 341–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans A. A., Crook T., Laws S. A., Gough A. C., Royle G. T., Primrose J. N.1997. Analysis of somatostatin receptor subtype mRNA expression in human breast cancer. Br. J. Cancer 75: 798–803. doi: 10.1038/bjc.1997.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frati A., Rouzier R., Lesieur B., Werkoff G., Antoine M., Rodenas A., Darai E., Chereau E.2014. Expression of somatostatin type-2 and -4 receptor and correlation with histological type in breast cancer. Anticancer Res. 34: 3997–4003. [PubMed] [Google Scholar]
- 9.Gomez-Sanchez C. E., de Rodriguez A. F., Romero D. G., Estess J., Warden M. P., Gomez-Sanchez M. T., Gomez-Sanchez E. P.2006. Development of a panel of monoclonal antibodies against the mineralocorticoid receptor. Endocrinology 147: 1343–1348. doi: 10.1210/en.2005-0860 [DOI] [PubMed] [Google Scholar]
- 10.Kumar U., Grigorakis S. I., Watt H. L., Sasi R., Snell L., Watson P., Chaudhari S.2005. Somatostatin receptors in primary human breast cancer: quantitative analysis of mRNA for subtypes 1–5 and correlation with receptor protein expression and tumor pathology. Breast Cancer Res. Treat. 92: 175–186. doi: 10.1007/s10549-005-2414-0 [DOI] [PubMed] [Google Scholar]
- 11.Misdorp W., Else R. W., Hellman E., Lipscomb T. P.1999. Histological Classification of Mammary Tumors of the Dog and the Cat, Armed Force Institute of Pathology and World Health Organization, Washington, D.C. [Google Scholar]
- 12.Morita T., Okada M., Yamawaki H.2014. Mechanisms underlying a decrease in KCl-induced contraction after long-term serum-free organ culture of rat isolated mesenteric artery. J. Vet. Med. Sci. 76: 963–969. doi: 10.1292/jvms.14-0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orlando C., Raggi C. C., Bianchi S., Distante V., Simi L., Vezzosi V., Gelmini S., Pinzani P., Smith M. C., Buonamano A., Lazzeri E., Pazzagli M., Cataliotti L., Maggi M., Serio M.2004. Measurement of somatostatin receptor subtype 2 mRNA in breast cancer and corresponding normal tissue. Endocr. Relat. Cancer 11: 323–332. doi: 10.1677/erc.0.0110323 [DOI] [PubMed] [Google Scholar]
- 14.Prévost G., Hosford D., Thomas F.1994. Receptors for somatostatin and somatostatin analogues in human breast tumors. Ann. N. Y. Acad. Sci. 733: 147–154. doi: 10.1111/j.1749-6632.1994.tb17264.x [DOI] [PubMed] [Google Scholar]
- 15.Reubi J. C., Maurer R., von Werder K., Torhorst J., Klijn J. G., Lamberts S. W.1987. Somatostatin receptors in human endocrine tumors. Cancer Res. 47: 551–558. [PubMed] [Google Scholar]
- 16.Robben J. H., Visser-Wisselaar H. A., Rutteman G. R., van Rijk P. P., van Dongen A. J., Voorhout G., van den Ingh T. S., Hofland L. J., Lamberts S. W.1997. In vitro and in vivo detection of functional somatostatin receptors in canine insulinomas. J. Nucl. Med. 38: 1036–1042. [PubMed] [Google Scholar]
- 17.Theodoropoulou M., Stalla G. K.2013. Somatostatin receptors: from signaling to clinical practice. Front. Neuroendocrinol. 34: 228–252. doi: 10.1016/j.yfrne.2013.07.005 [DOI] [PubMed] [Google Scholar]
- 18.Yoshimura H., Nakahira R., Kishimoto T. E., Michishita M., Ohkusu-Tsukada K., Takahashi K.2014. Differences in indicators of malignancy between luminal epithelial cell type and myoepithelial cell type of simple solid carcinoma in the canine mammary gland. Vet. Pathol. 51: 1090–1095. doi: 10.1177/0300985813516637 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.