Abstract
Background
The contribution of individual subsets of dendritic cells (DC) to the generation of adaptive immunity is central to understanding immune homeostasis and protective immune responses.
Objective
We sought to define functions for steady-state skin DC.
Methods
Herein we present an approach in which we restrict antigen presentation to individual DC subsets in the skin and monitor the effects on endogenous antigen-specific CD4+ T and B cell responses.
Results
Presentation of foreign antigen by Langerhans cells (LC) in the absence of exogenous adjuvant led to a large expansion of T follicular helper cells (Tfh). This was accompanied by B cell activation, germinal center formation and protective antibody responses against influenza. The expansion of Tfh and antibody responses could be elicited by both systemic and topical skin immunization. Tfh induction was not restricted to LC and occurred in response to antigen presentation by CD103+ dermal DC. CD103+ DC despite inducing similar Tfh responses as LC, were less efficient in induction of GC B cells and humoral immune responses. We also found that skin DC are sufficient to expand CXCR5+ Tfh through an IL-6 and IFNAR independent mechanism, but B cells were required for sustained Bcl6+ expression.
Conclusions
These data demonstrate that a major unappreciated function of skin DC is their promotion of Tfh and humoral immune responses that potentially represent an efficient approach for vaccination.
Clinical Implications
Our findings suggest that targeting antigen without adjuvants to a specific skin DC subset either by systemic or topical application will be an efficient approach to generate protective, antibody-based vaccines.
Keywords: steady-state conditions, skin dendritic cells, T follicular helper cells, protective humoral immune responses
Introduction
The dendritic cell (DC) paradigm states that after a pathogen encounter DC become activated and migrate to the secondary lymphoid organs where they initiate expansion and differentiation of pathogen-specific T cell responses1. In the steady-state, DC at barrier sites acquire self and benign environmental antigens. The presentation of these antigen by immature DC results in T cell tolerance either by activation-induced cell death or by induction of anergic/regulatory T cells2. This is believed to be the mechanism for the maintenance of peripheral tolerance and been hypothesized to be a targetable pathway to induce immune tolerance for the treatment of autoimmune disease.
In mouse skin there are two distinct populations of DC that express C-type lectin Langerin, Langerhans cells (LC) that reside in the epidermis and CD103+ DC in the dermis1. We have previously demonstrated that LC and CD103+ DC induced opposing Th responses against C. albicans. As such LC were necessary and sufficient for in vivo induction of Th17 responses, while CD103+ DC were required for cross presentation to CD8 T cells and Th1 responses3. The role of CD103+ DC in cross-presentation has been supported by other studies using different models and also antigen targeting3–6. In the setting of contact hypersensitivity the function of LC and CD103+ remains controversial7. 2,4-dinitrocholrobenzene (DNCB)-induced tolerance was dependent on LC-induced Treg expansion8. In addition, LC have been reported to promote deletion of antigen-specific CD4+ T cells after CFA-peptide immunization 9 and expansion of Treg during Leishmania infection 10. LC are also required for the induction of protective antibody responses after epicutaneous patch immunization11.
The function of Langerin-expressing cells in the steady-state can be examined by using i.p. injection of low amounts of anti-Langerin mAb/antigen conjugates. Since ligation of Langerin does not activate LC and CD103+ DC, this technique assays the effect of antigen presentation of Langerin+ DC in the absence of exogenous adjuvants. Anti-mouse Langerin/MOG conjugates induced expansion of antigen-specific transgenic Tregs and provided subsequent protection from EAE12. This finding suggests that Langerin-expressing DC (LC and CD103+ DC) promote tolerance through Treg expansion and is consistent with earlier studies using DEC-205 mAb to target antigen to other DC subsets under homeostatic conditions13.
The contribution of individual subsets of dendritic cells to the generation of adaptive immunity is central to understanding immune homeostasis and protective immune responses. To date, DC function has been studied either in vitro or using adoptive transfer of TCR transgenic T cells. To determine the functional consequence of foreign antigen presentation without adjuvants exclusively by LC or CD103+ DC we developed an approach in which we restrict antigen presentation to these individual DC subsets and monitor the effects on endogenous antigen-specific CD4+ T cells responses using MHC-II tetramers 14. We also developed a novel system for concomitant analysis of endogenous B cell responses. Using these techniques we defined new functions for LC and CD103+ DC, in Tfh induction and humoral immune responses.
Materials and Methods
Mice
HuLangerin15, huLangerin-Cre-I-Aβfl16, Batf3−/−17 mice have been previously described. CD90.1 congenic TEa Rag1−/− Cd4 TCR-transgenic to I-Eα52–68 on the C57BL/6 background18 were obtained from M. Jenkins (University of Minnesota), μMT and CD11c-Cre-MHCII from K. Hogquist (University of Minnesota), and IFNAR−/− from M. Mescher (University of Minnesota). IL-6−/− mice on C57BL/6 background were purchased from The Jackson Laboratory. All experiments were performed with 6- to 12-week-old female mice. Mice were housed in microisolator cages and fed irradiated food and acidified water. The University of Minnesota institutional care and use committee approved all mouse protocols.
Antibodies and Reagents
Fluorochrome-conjugated antibodies to CD4, CD11b, CD11c, CD40, B220, CD44, CD86, CD90.1, CD90.2, CD103, Gr-1, F4/80 and I-A/I-E were purchased from BioLegend (San Diego, CA). Antibodies to Foxp3, T-bet, PD-1, CXCR5, Bcl-6, Gata-3, RORγt and Live/Dead dye were acquired from eBioscience (San Diego, CA). Anti-Langerin (929F3) was from Dendritics (Lyon, France). Anti-human/anti-mouse Langerin mAb and conjugates (2G3-Eα, 2G3-2W1S, 4C7-2W1S and 2G3-FluHA1) were generated in house as previously described3,19. Eα: I-Eα52–68 well-characterized immune-dominant T cell epitope recognized by transgenic TEα cells in the context of I-Ab18. The 2W1S is a variant of peptide 52–68 from the I-E alpha chain14. 2W1S binds to the I-Ab MHCII molecule expressed in C57BL/6 (B6) mice and is immunogenic in this strain. FluHA-1: hemagglutinin HA [Influenza A virus (A/Puerto Rico/8/34(H1N1))] residues 18–33119.
Adoptive T Cell Transfer
T cells were adoptively transferred as previously described3. Briefly, skin-draining lymph nodes, spleen and mesenteric lymph node of TEα TCR transgenic mice were disrupted through a cell strainer and washed with sterile HBSS and labeled with CFSE (Invitrogen, Carlsbad, CA) in accordance with the manufacturer’s instructions. The cells were re-suspended in sterile PBS at a concentration of 1×106 cell/ml and 300 μl (3×105 cells) injected i.v. into different mouse strains.
LC and CD103+ DC Targeting with Anti-Langerin Antibody by Intraperitoneal Injection
Cohorts of mice were injected with either 10 μg of 2G3-AF647 or 10 μg of 4C7-AF647. Mice were sacrificed 16 hours later and the presence of the AF647 signal was analyzed by flow cytometry in cells isolated from the epidermis, lymph node and spleen as described3. In separate experiments, cohorts of mice were adoptively transferred with 3×105 TEα cells. Twenty-four hours later mice were immunized by intraperitoneal injection of 1 μg of 2G3-Eα and skin draining lymph nodes harvested at day 4. To define endogenous T cell responses, cohort of mice were injected with either 1 μg of 2G3-2W1S or 1 μg of 4C7-2W1S. Six skin-draining LNs and spleen were harvested at the indicated time points and analyzed by flow cytometry.
Topical Application of Antigen
The back skin of the mice was wet-shaved under anesthesia using razorblades. Hundred microgram of 2W1S or MOG35–55 (GenScript, Piscataway, NJ) diluted in 100 μl of endotoxin-free PBS was smeared on the top of the skin. Separate cohort of mice was painted with 1μg of 2G3-2W1S.
Flow Cytometry
Single-cell suspensions were obtained and stained as previously described3. All the flow cytometry plots presented in this manuscript were pre-gated on live (using live/dead stain) and singlet events. The intracellular transcription factor staining was performed with BD Bioscience Cytofix/Cytoperm kit (BD Biosciences, San Jose, CA) in accordance with the manufacturer’s instructions. Samples were analyzed on LSR-II flow cytometers (BD Biosciences). Data were analyzed with FlowJo software (TreeStar, Ashland, OR). Boolean gating was used to identify single transcription factor expressing cells, and the data is displayed as percentage of total in a pie graph.
2W1S Tetramer Enrichment
Single-cell suspensions of LNs and spleen were stained for 1 hour at room temperature with 2W1S:I-Ab-streptavidin-APC tetramers (generous gift of M. Jenkins). Samples were then enriched for bead-bound cells on magnetized columns as previously described14.
Endogenous B cell Responses
Single-cell suspension of LNs from MD4, naïve and 2G3-immunized WT and huLangerin+ mice were incubated ex vivo with AF647 conjugated 2G3 on ice for 30 minutes. After washing the cells were stained for live/dead, CD11c, CD90.2, CD45.2, Gr-1, MHC-II, B220, GL-7 and CD38 markers and analyzed on flow cytometer.
Assessment of Humoral Responses
WT and huLangerin mice were injected i.p. with 1 μg of 2G3 at day 0 and day 14. LC−/− mice were treated in similar way but using 4C7. Serum samples were obtained prior and 21 days post immunization using BD Mictrotainer SST tubes (BD, Franklin Lakes, NJ) and stored at −80°C. To de termine Ag-specific antibody titers, ELISA plates were coated with 0.25 μg hIgG4 protein. Serial dilutions of serum in blocking buffer (TBS; Pierce, Rockford, IL) were incubated in the wells overnight at 4°C. After washing, plates were incuba ted with HRP-conjugated goat anti-mouse IgG (Jackson ImmunoResearch, West Grove, PA) in blocking solution for 2 hours at 37°C, then washed and devel oped with HRP substrate and read at 405 nm. Ab titer data are plotted on log scales. Assays for isotyping Ag-specific Abs in the serum used biotinylated goat anti-mouse IgG1, IgG2b, IgG3 and IgA polyclonal reagents and biotinylated anti-mouse IgE monoclonal 23G3 (SouthernBiotech, Birmingham, AL) as detecting reagents, followed by development with NeutrAvidin HRP (Pierce, Rockford, IL).
Intranasal Influenza Infection
WT and huLangerin were injected i.p. with 1 μg of 2G3-FluHA1 at day 0 and day 14. On day 21 the mice were infected intranasally with 2×104 PFU PR8 influenza virus. Body weights and survival curves were obtained. Mice that lost 25% of their original weight were sacrificed.
Quantitative RT-PCR for Viral Load
Lung samples were collected 9 days post influenza infection, and the viral load was assessed using standard quantitative RT-PCR protocol. Briefly, total RNA from lung samples were extracted using RNeasy mini kit (Qiagen, Valencia, CA) according to manufacturer protocol. The reverse transcription was performed using Invitrogen Superscript III kit (Invitrogen, Carlsbad, CA), and the RT-PCR reaction with SYBR-green (Applied Biosystems, Grand Island, NY). For amplification PR8 NP-specific primers (ACGGCTGGTCTGACTCACAT and TCCATTCCGGTGCGAACAAG) were used. The data was normalized to GAPDH and expressed as fold change of the relative expression.
Statistical Analysis
Differences between two data sets were analyzed by two-tailed Student’s t test and ANOVA using Prism (Graphpad) software. *p<0.05, **p<0.005, ***p<0.0005, ****p<0.0001.
Results
Selective Antigen Targeting to Immature LC and CD103+ DC
Targeting antigen to LC using anti-mouse Langerin mAb delivers antigen to LC as well as to CD103+ DC12,20,21. To target antigen to LC only, we used recombinant fusion protein of a humanized mAb specific for the ectodomain of human Langerin (clone 2G3) and peptide epitopes derived from I-E52–68 and 2W1S (2G3-Ag)3,19. The humanized 2G3 mAb does not cross-react with mouse Langerin, and showed no unspecific binding to mouse Fc-receptors 3,19. HuLangerin-DTR BAC transgenic mice were previously generated to inducible ablate LC by administration of diphtheria toxin (DT)15. In these mice, transgenic human Langerin is efficiently expressed by LC but not by other DC expressing mouse Langerin15. Since we are using these mice as huLangerin transgenic mice and not ablating LC using DT, they will henceforth be referred to as huLangerin mice. To confirm the specificity of targeting LC, huLangerin and WT littermate control mice were injected with 10 μg i.p. of 2G3 that had been conjugated to AF647. LC isolated from the epidermis had efficiently acquired 2G3-AF647 (see Figure E1A in this article’s Online Repository at www.jacionline.org). Keratinocytes (KC), dendritic epidermal T cells (DETCs), and LC from littermate control mice did not acquire the antibody (see Figure E1A in this article’s Online Repository at www.jacionline.org). Similarly, 2G3-AF647 could only be detected in LC in lymph nodes harvested from huLangerin and not control mice (see Figure E1B in this article’s Online Repository at www.jacionline.org). No staining was detected in the spleen (see Figure E1C in this article’s Online Repository at www.jacionline.org). Importantly, targeting LC with 2G3 did not result in the activation of LC, as determined by measuring the surface expression of CD40, CD86 or MHC-II (see Figure E2A–B in this article’s Online Repository at www.jacionline.org). Expression of cytokines and chemokines indicative of DC activation were also unaltered by 2G3 targeting (see Figure E2C in this article’s Online Repository at www.jacionline.org). Thus, anti-huLangerin (2G3)-peptides fusion proteins efficiently and selectively target LC without activating LC, and therefore mimic Langerin-mediated acquisition of antigen during steady-state conditions.
To selectively target antigen to CD103+ DC we injected huLangerin-DTA mice (LC−/−) that lack LC22 with anti-muLangerin antibody conjugated to AF647 (4C7)19. HuLangerin-DTA mice, unlike muLangerin-DTR mice that deplete both LC and CD103+ DC, only have selective absence of epidermal LC7,22. As shown on Figure E1D (see Figure E1D in this article’s Online Repository at www.jacionline.org), in the absence of LC only CD103+ DC were targeted and no labeling was observed in the spleen (see Figure E1E in this article’s Online Repository at www.jacionline.org). Thus, 4C7 antibody can be used in LC−/− mice to deliver antigen to CD103+ DC.
Steady-State Langerhans Cells and CD103+ Dendritic Cells expand Naïve CD4+ T Cells
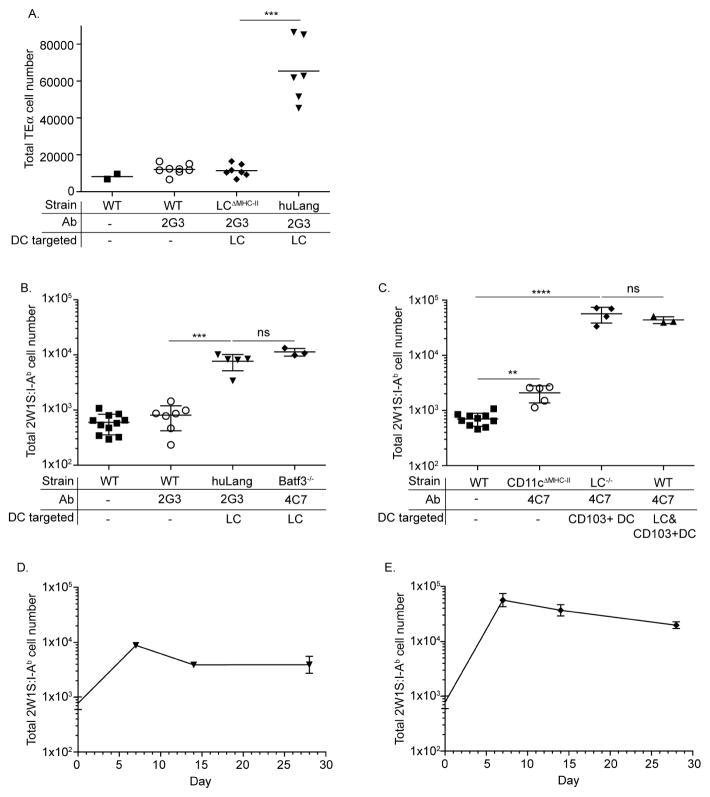
To determine the specificity of antigen targeting to LC, we adoptively transferred CD90.1 CFSE-labeled TEα cells into huLangerin and littermate control (WT). Mice were given 1 μg 2G3-Eα i.p. On day 4, TEα cells had expanded approximately 6-fold (Figure 1A) in huLangerin mice and had completely diluted their CFSE (see Figure E3A in this article’s Online Repository at www.jacionline.org). TEα cells failed to expand in control mice that lack huLangerin expression. HuLangerin-Cre I-Aβfl mice have a LC-specific ablation of MHC-II but do not express huLangerin since the insertion of Cre into the langerin gene in the human BAC disrupted the open reading frame16,23. To test whether LC directly present antigen to TEα cells, we generated huLangerin x huLangerin-Cre I-Aβfl (from now on LCΔMHC-II) mice. 2G3-Ag was efficiently targeted to LC in these mice (data not shown) but TEα failed to expand (Figure 1A) indicating that steady-state LC induce CD4+ T cell proliferation through direct, cognate interaction.
Figure 1. Steady-state LC and CD103+DC promote long lasting CD4+ T cells responses.
(A) TEα cell numbers after 2G3-Eα immunization is shown. (B–C) 2W1S-specific CD4+ T cells responses after 2G3-2W1S or 4C7-2W1S immunization is presented. (D–E) The kinetics of 2W1S-specific CD4+ T cell responses induced by LC (D) or CD103+ DC (E) is shown. Pooled data of two independent experiments is shown.
It has recently been demonstrated that TCR transgenic CD4+ T cells represent the responses of individual TCR clones and are not necessary indicative of the endogenous T cell response24. Thus, rather than using adoptive transfer of TCR transgenic T cells to analyze Th differentiation, we examined the response of endogenous CD4+ T cells using an I-Ab:2W1S tetramer/pull-down technique14. We immunized WT and huLangerin mice with 1 μg 2G3-2W1S i.p. and examined the expansion of 2W1S-specific CD4+ T cells using an I-Ab:2W1S tetramer. 2W1S-specific CD4+ T cells expanded approximately 10-fold in huLangerin mice, but failed to proliferate in WT mice (Figure 1B and see Figure E3B in this article’s Online Repository at www.jacionline.org).
HuLangerin is expressed as a transgene in huLangerin mice15. To confirm that targeting endogenous murine Langerin in LC would promote similar CD4+ T cell responses as huLangerin, we immunized Batf3−/− mice with 4C7-2W1S. Since these mice lack CD103+ DC17, only LC would be targeted by anti-muLangerin (4C7) antibody. We found no significant differences in T cell expansions by targeting endogenous murine Langerin or huLangerin (Figure 1B).
To define the T cell responses induced by steady-state CD103+ DC we injected WT, CD11c-Cre-MHC-IIfl/fl (CD11cΔMHC-II) and LC−/− mice with 1 μg 4C7-2W1S i.p. and examined the expansion of 2W1S-specific CD4+ T cells using an I-Ab:2W1S tetramer at day 7 post injection. We observed a robust expansion of 2W1S-specific CD4+ T cells by CD103+ DC (Figure 1C) that did not differ significantly of the responses induced by the joint effort of LC and CD103+ DC (Figure 1C). In CD11cΔMHC-II mice, where DC were not able to present antigen to CD4+ T cells we observed a minimal expansion of 2W1S-specific T cells (Figure 1C). Thus, direct presentation of targeted antigen by DC is required for optimal T cell responses, and CD103+ DC play a dominant role in induction of CD4 proliferation when both LC and CD103+ DC are targeted.
To determine the kinetics of CD4+ T cell responses initiated by LC and CD103+ DC we immunized huLangerin mice with 1 μg 2G3-2W1S i.p. and LC−/− mice with 1 μg 4C7-2W1S i.p. The mice were sacrificed at the indicated time points and the I-Ab:2W1S tetramer+ cells enumerated by flow cytometry. T cell responses induced by both LC and CD103+ DC peaked at day seven (Figure 1D–E). The initial peak was followed by a contraction with no signs of deletion. Thus, steady-state skin DC promote long-lasting CD4+ T cell responses.
Steady-state LC and CD103+ DC promote differentiation of T follicular helper cells
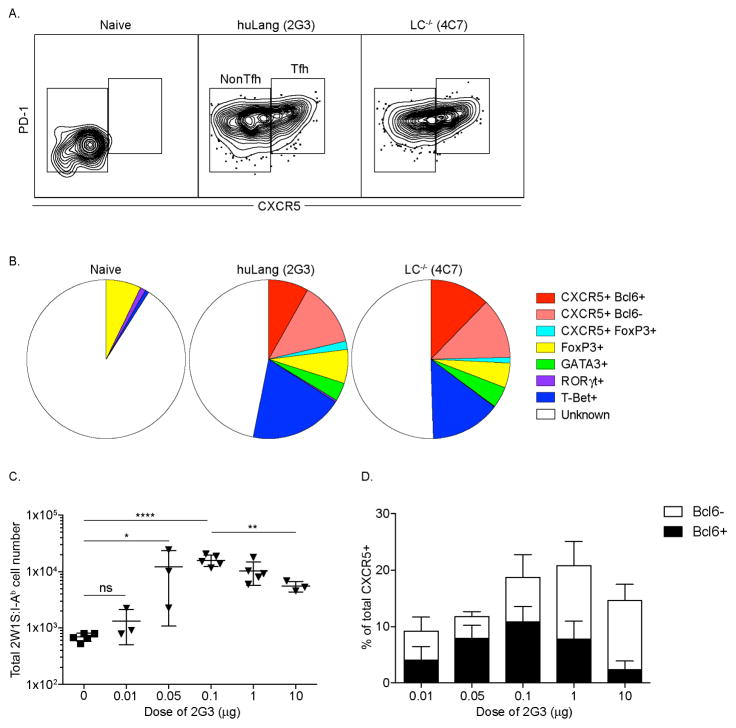
We next examined the phenotype of 2W1S cells expanded by LC or CD103+ DC, based on expression of PD-1 and CXCR5. We found that 15–30% of the responding CD4+ T cells expanded by both DC co-expressed PD-1 and CXCR5, identifying them as Tfh (Figure 2A). To further characterize the Th-phenotype, we performed intracellular stains for Bcl-6 (Tfh), T-bet (Th1), FoxP3 (Treg), Gata-3 (Th2) and RORγt (Th17) (see Figure E4 in this article’s Online Repository at www.jacionline.org). We found that around half of the CXCR5 positive cells also expressed Bcl-6 which is considered a specific marker of germinal center Tfh cells25,26. We noted no change in the percentage of Treg between naïve and immunized mice (p>0.05). Around 10–15 % of the cells expressed T-bet, and 3–5% were GATA-3 positive. No sizable population of RORγt expressing cells was detected (Figure 2B). No significant differences were detected between Th phenotypes induced by LC and CD103+ DC (p>0.05 for all subsets). Thus, antigen presentation by LC and CD103+ DC in the absence of inflammation leads to the generation of a mixed Th cell phenotype dominated by Tfh cells.
Figure 2. Steady-state LC and CD103+ DC induce formation of Tfh cells in the endogenous antigen-specific CD4+ T cells.
(A) 2W1S-specific cells stained for PD-1 and CXCR5. (B) Th phenotype of 2W1S-specific cells. (C) Dose response in huLangerin mice. (D) Cells from (C) were stained for the indicated markers. Results are pooled data from three independent experiments.
Recently it has been shown that antigen dose can influence Tfh cell differentiation24,27,28. To determine whether induction of cells with Tfh-phenotype by LC depends on Ag dose, we immunized huLangerin mice with increasing amount of 2G3-2W1S ranging from 10 ng up to 10 μg. The cell expansion (Figure 2C) was determined as described above. At the lowest dose (10 ng) we observed a minimal expansion (≤2 fold) of the 2W1S-specific CD4+ T cells. The optimal dose was between 100ng and 1 μg with 12–15 fold expansion. At the highest dose measured (10 μg) the expansion started to drop significantly. To test whether the antigen dose affected the Tfh differentiation we stained the cells for CXCR5/PD-1 and Bcl-6. We observed Tfh cell induction by LC with different efficiencies at all the doses tested (Figure 2D). These data support the idea that LC-induced Tfh is not due to high antigen dose.
LC also promote Tfh cell generation in inflammatory conditions
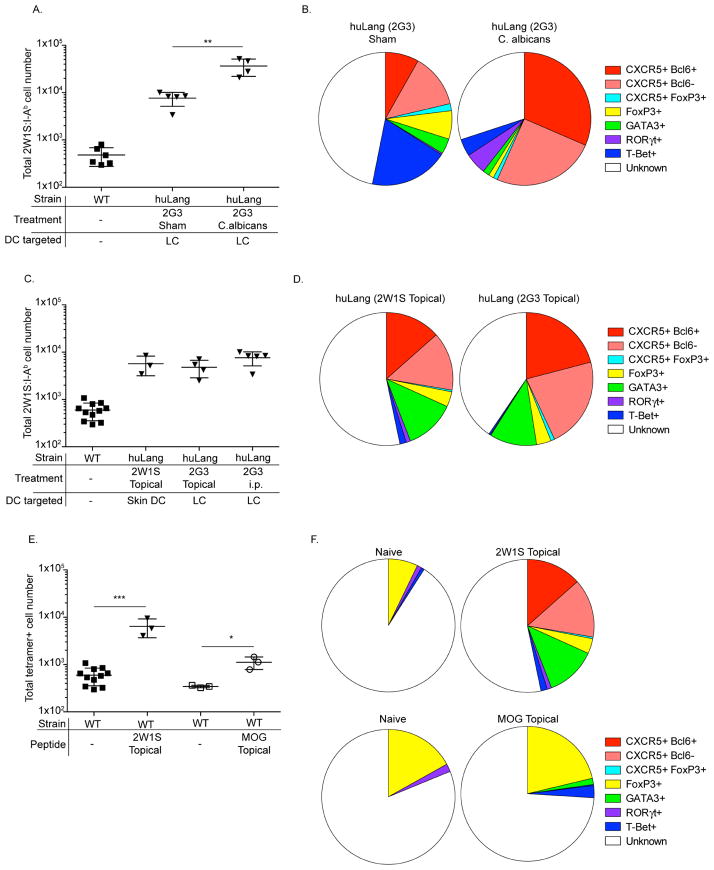
Previously we reported that targeting 2G3-Eα to LC during C. albicans infection resulted in expansion of Th173. To test whether LC also induce Tfh beside Th17, we immunized huLangerin mice with 1 μg 2G3-2W1S i.p. and infected the skin with WT C. albicans. Seven days later the CD4+ T cell responses were assessed as described above. C. albicans infection combined with targeting induced a more robust T cell response than the targeting alone (Figure 3A). C. albicans infection also had a significant effect on the Th phenotype. Comparing to targeting alone, C. albicans induced RORγt expression in naïve CD4+ T cells (p<0.05) and a more robust Tfh response (>50%, p<0.005) (Figure 3B). CD103+ DC also induced Tfh during C. albicans infection (see Figure E5 in this article’s Online Repository at www.jacionline.org). Thus, LC and CD103+ DC can promote Tfh cell responses during inflammatory conditions, and the Th phenotype is dependent on adjuvant.
Figure 3. Tfh induction by skin DC is not unique to steady-state targeting, but is limited to foreign antigen.
(A–D) The total number of 2W1S-specific T cells or their Th phenotype is shown in different conditions. (E) Total 2W1S and MOG-specific T cell numbers and their Th-phenotype (F) is shown. Results are pooled data from three independent experiments.
Tfh cell induction by LC is not unique to targeting
To determine whether Tfh induction by LC occurs only with Langerin-targeted antigen, we applied 2W1S peptide or 2G3-2W1S topically on the skin of wet-shaved WT and huLangerin mice, respectively. Topical application of 2G3-2W1S specifically targets epidermal LC and not LN LC (see Figure E6 in this article’s Online Repository at www.jacionline.org). We found that topical application of 2W1S and 2G3-2W1S promoted comparable T cell responses as i.p. targeting (Figure 3C). We also observed robust Tfh induction with topical application of the antigen, and unlike with i.p. targeting minimal T-bet expression and a sizable Gata-3 population (p<0.05; Figure 3D). Thus, these data suggest that Tfh induction by skin DC is not unique to targeting, and the T-bet expressing cells seen with i.p. injection might have been induced by LN LC.
Skin DC do not promote Tfh responses against MOG
Targeting self, MOG peptide to Langerin+ DC resulted in induction of FoxP3 in the adoptively transferred MOG-specific transgenic CD4+ T cells and provided resistance to development of EAE12. It was thus unexpected that targeting foreign antigen to skin DC using a similar technique yielded Tfh cells and no significant expansion of Treg in our system. To reconcile this obvious discrepancy, we topically applied on wet-shaved WT mice either 2W1S (foreign) or MOG (self) peptide. Seven days later we assessed the endogenous T cell responses using 2W1S and MOG-specific tetramers. We found that 2W1S-specific CD4+ T cells expanded approximately 10 fold over naïve numbers, while the MOG-specific T cells 2–3 fold (Figure 3E). Painting of 2W1S showed the already documented phenotype, with robust Tfh induction. However, presentation of MOG by skin DC did not induce Tfh, rather selectively expanded the FoxP3+ Treg population (Figure 3F). Thus, this likely reflects differences in the endogenous T cell repertoire to a foreign (2W1S) vs. self (MOG) antigen.
Bcl-6+ Tfh are B cell dependent but independent of IL-6 and type I interferon
The differentiation of Tfh cells is B cell dependent and relies on the presence of inflammatory cytokines such as IL-6 and type I interferon29. The requirements of Tfh-induction in the absence of adjuvants have never been addressed. To test the role of B cells, IL-6 and type I interferon in generation of Tfh cells in homeostatic conditions, we immunized μMT (lack mature B cells), IL-6−/− and IFNAR−/− mice with 1 μg 4C7-2W1S i.p. and analyzed the T cell responses as presented above. We found that the overall expansion of 2W1S-specific CD4+ T cell was not affected by the absence of IL-6 and IFNAR, but B cell deficiency led to an exaggerated T cell expansion (see Figure E7A in this article’s Online Repository at www.jacionline.org). The overall percentage of Tfh cells defined by PD-1 and CXCR5 expression was unchanged along the different mouse lines (see Figure E7B in this article’s Online Repository at www.jacionline.org). Interestingly, the percentage of Bcl-6+ CXCR5+ Tfh was significantly decreased in mice lacking B cells, but not in IL-6−/− and IFNAR−/− mice (see Figure E7C in this article’s Online Repository at www.jacionline.org). Thus, DC are sufficient to expand CXCR5+ Tfh through an IL-6 and IFNAR independent mechanism, but B cells are required for sustained Bcl6+ expression.
Antigen presentation by LC promote B cell responses
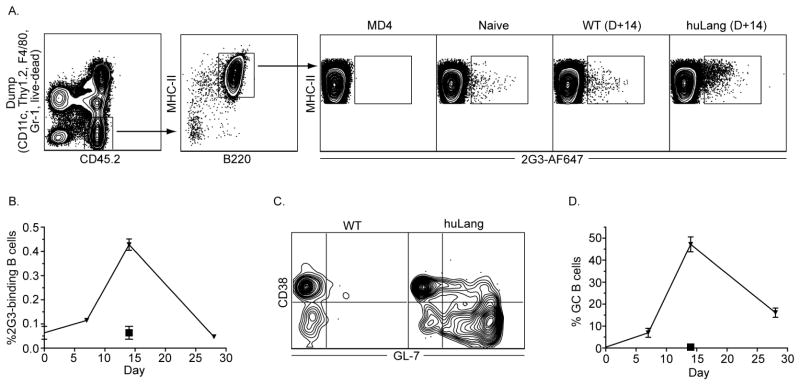
The generation of Tfh by skin DC prompted us to characterize the B cell responses. To define the endogenous B cell responses initiated by LC, we took advantage of the fact that our targeting antibodies are fully humanized. As such, in mice, B cells specific to human epitopes should be present. To test this, we incubated cell suspensions generated from LN of MD4 (with BCR specific to HEL), naïve, 2G3-immunized WT (D+14) and huLangerin (D+14) mice with 2G3-AF647 ex vivo. Antigen specific B cells were identified as dump negative (Thy1.2, Gr-1, CD11c, F4/80 and live/dead), CD45+, MHCII+, B220+ and AF647+ events (Figure 4A). No binding of 2G3-AF647 to MD4 cells was observed. We found that in a naïve mouse around 0.05% of total B cells bound specifically to 2G3-AF647, and that the 2G3-specific B cells failed to expand in littermate WT control mice (Figure 4A–B). However, as expected, in immunized huLangerin mice we observed a 10 fold expansion of the 2G3-specific B cells (Figure 4A–B). To determine the kinetics of the B cell responses, we immunized WT and huLangerin mice with 1 μg of 2G3 i.p. The mice were sacrificed at the indicated time points and the percentage of 2G3-specific B cells determined by flow cytometry. The B cell responses peaked around day 14 followed by a contraction (Figure 4B). To test whether antigen presentation by LC leads GC B cells formation, the 2G3-speciic B cells were stained for memory/naïve (CD38) and GC markers (GL-7). Antigen presentation by LC led to a robust GC B cell (CD38−, GL-7+) response by day 14 (Figure 4C), and that followed similar kinetics as the total B cell response (Figure 4D). Thus, these data suggest that antigen presentation by LC is necessary for initiation of B cell responses.
Figure 4. LC promote B cell responses.
(A) Gating strategy to identify 2G3-bindig B cells. (B) The kinetics of the 2G3-specific B cell responses in WT (square) and huLangerin (triangle) mice is shown. (C) The phenotype of 2G3-specific B cells 14 days post immunization. (D) as in (B), except that the kinetics of GC B cells (CD38−, GL-7+) is shown. Pooled data from three experiments.
Langerhans Cells and CD103+ DC Promote Humoral Immune Responses
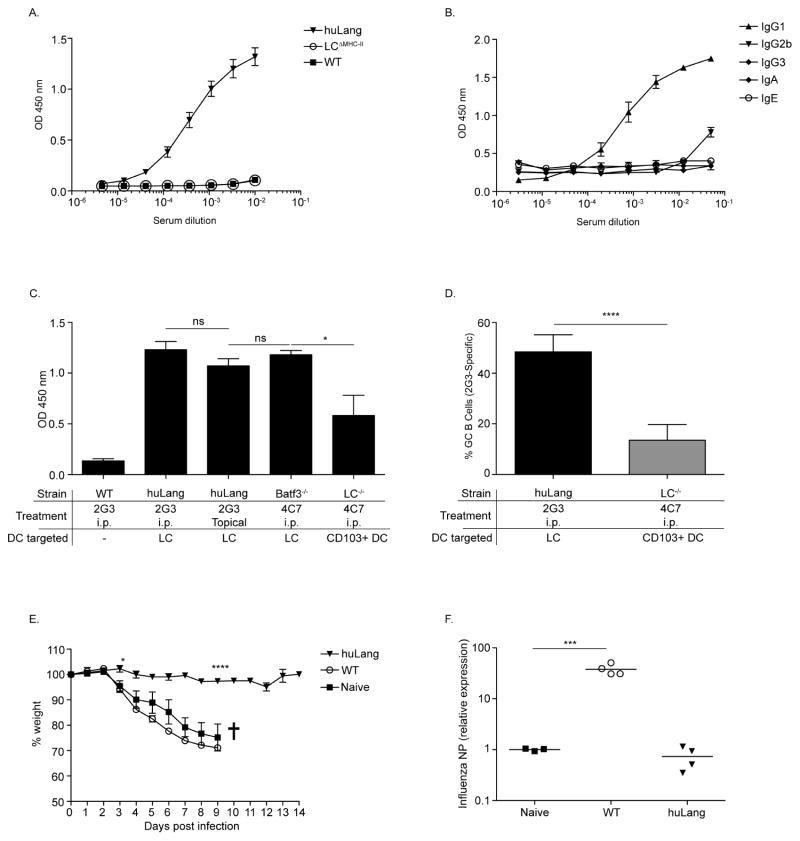
Since Tfh are critical for humoral immunity, including the generation of long-lived, high affinity plasma cells and memory cells25 we next examined the humoral immune responses induced by steady-state LC. To confirm the development of a humoral immune response upon antigen targeting to LC, huLangerin, LCΔMHC-II and WT mice were immunized with 1 μg of 2G3 i.p. and boosted with the same dose 14 days later. A strong anti-2G3 titer was observed in the serum of huLangerin mice, but not in LCΔMHC-II and WT, 21 days post immunization (Figure 5A). LC promoted an IgG response that was dominated by IgG1. Other isotypes such as IgG3, IgE and IgA, except IgG2b, were near the level of detection (Figure 5B). Thus steady-state LC promote IgG1 humoral immune responses through cognate interaction with CD4+ T cells
Figure 5. Skin DC induce humoral immune responses.
(A) Total 2G3-specific IgG in serum samples from WT, LCΔMHC-II and huLangerin mice were determined by ELISA. (B) Serum samples from (A) were analyzed for isotypes. (C) Total IgG at 1:100 dilution is depicted. (D) Percent of 2G3-specific GC B cells. (E) The weight loss and viral load (F) is shown. Pooled data from two independent experiments.
To compare humoral immune responses induced by epidermal LC vs. LN LC, we immunized huLangerin mice by either topical application of 2G3 or i.p. injection. To assess the humoral immune responses triggered by targeting hu vs. muLangerin, we injected Batf3−/− mice with 4C7. We found similar antibody responses in all three cases (Figure 5C). Thus, epidermal and LN LC promote comparable humoral immune responses independent of the nature of Langerin targeted.
To define the antibody responses initiated by CD103+ DC we immunized LC−/− with 4C7. We found that CD103+ DC also promoted humoral immune responses, but were less efficient than LC (Figure 5C). Consistent with the lower antibody levels, CD103+ DC induced significantly less GC B cells (Figure 5D). Antibody isotypes induced by CD103+ DC did not differ significantly from ones induced by LC (data not shown). Thus steady-state LC and CD103+ DC promote similar humoral immune responses, but LC are more efficient than CD103+ DC.
Langerhans Cells Promote Protective Humoral Immune Responses
The hallmark of humoral immune responses that involve Tfh cells is the presence of long-lived protective antibodies25. Therefore, we sought to determine whether steady-state LC-promoted humoral immune responses could confer systemic protection against a viral infection. LC, unlike CD103+ DC do not cross-present the targeting construct to CD8 T cells3,5, and such the role of antibodies in protection can be directly assessed. Since LC also promote a dominant IgG1 response to 2G3-FluHA conjugate19 (and data not shown), we decided to assess the efficiency of humoral immune response induced by LC in an influenza PR8 (H1N1) infection model. For this purpose we immunized huLangerin and WT mice with 1 μg of 2G3-FluHA1 i.p. Fourteen days later the mice were boosted and seven days later the mice were challenged intranasally with 2×104 live, PR8 influenza virus. Naïve and WT mice immunized with 2G3-FluHA1 lost ~25% of their weight during the course of the first week post infection (Figure 5E). In contrast, the immunized huLangerin mice did not lose weight (Figure 5E). Consistent with the failure to lose weight, immunized huLangerin mice had an undetectable viral load (Figure 5F). Thus, antigen presentation by LC induces the production of an effective humoral immune response that can confer systemic protection.
Discussion
Herein we have demonstrated that antigen presentation by LC and CD103+ DC under steady-state conditions promoted a robust expansion of endogenous antigen-specific CD4+ T cells. Antigen targeted using anti-Langerin antibody-antigen conjugates and topical application of peptide or protein efficiently induced Tfh differentiation. Tfh expansion was further increased by a concomitant C. albicans skin infection. Contrary to our expectation, significant FoxP3+ Treg expansion was not detected to a foreign peptide (2W1S) but the self-peptide (MOG35–55) did expand a population of Treg. We also found that skin DC were sufficient to expand CXCR5+ Tfh through an IL-6 and IFNAR independent mechanism, but B cells were required for sustained Bcl6+ expression. The expansion of Tfh cells by LC was accompanied by activation and expansion of antigen-specific B cells and the development of a robust antibody response that provided systemic protection against influenza infection. Despite inducing more robust CD4+ T cell responses than LC, the CD103+ DC were less effective in induction of GC B cells and humoral immune responses.
Antigen presentation by LC has been suggested to result in suppressive T cells responses and to promote peripheral tolerance8–10. Targeting MOG antigen to Langerin expressing cells resulted in a large expansion of MOG-specific FoxP3+ Treg cells and provided resistance to development of EAE12. It was thus unexpected that targeting antigen selectively to either LC or CD103+ DC using a similar technique failed to yield increased Treg in our system. In a side-by-side comparison we found that skin DC promoted Tfh responses against 2W1S but not MOG. This likely reflects differences in the endogenous T cell repertoire to a foreign (2W1S) vs. self (MOG) antigen.
Langerin is an antigen uptake receptor that binds a wide variety of ligands and can mediate uptake of numerous pathogens including HIV30. We found that targeting Langerin with antibody did not induce activation of Langerin-expressing cells. We also showed that targeting antigen through transgenically expressed huLangerin and endogenous murine Langerin promoted similar Th-responses in LC. Notably, the topical application of foreign antigen without targeting led to Tfh induction by skin DC, arguing that Tfh induction to skin antigens appears independent of Langerin targeting.
The differentiation of Tfh cells in infectious models depends on availability of inflammatory cytokines such as IL-6 and type I interferons25,29,31. Little is known about the requirements of Tfh differentiation in the absence of inflammatory signals. How Langerin-expressing skin DC and splenic DC32 promote Tfh remains to be determined. We did not observe maturation of skin DC upon targeting, measured by co-stimulatory markers and cytokine expression, suggesting that the upregulation of these factors might not be required for Tfh induction in homeostatic conditions. We also noted that the absence of IL-6, IFNAR or co-housing with conventional mice (data not shown) does not affect induction of Tfh cells in our system. Thus, it is tempting to hypothesize that Tfh induction by steady-state DC reflects a default pathway that is initiated against foreign antigens, which can be further augmented by the presence of inflammatory cytokines. The maintenance of Bcl-6 expression in Tfh was dependent on the presence of B cells. However, the mechanism of how B cells regulate Bcl6 expression in Tfh is not completely understood. A role for ICOS-L on germinal center B cells to maintain Bcl6 expression in Tfh has been shown33,34. Another interesting candidate to regulate Bcl6 expression in Tfh could be B cell derived IL-10. This cytokine has been shown as critical to the Tfh-cell help of B cells35; it will be interesting to address whether the reverse could also be true and whether IL-10 is a key tolerogenic cytokine supporting Tfh cell development under steady-state conditions.
Presentation of antigen acquired by B cells was initially thought to be the key event driving differentiation of naïve CD4+ T cells towards the Tfh lineage25. More recently, however, Tfh induction has also been shown to depend on antigen presentation by DC as well34,36,37. In our model, Tfh expansion and humoral immune responses were absent in control mice lacking expression of huLangerin and in huLangerin+ mice in which LC lacked expression of MHC-II. In these mice antigen-specific B cells had access to the antigen through direct binding to BCR, but they were not sufficient to initiate Tfh-differentiation, which is in accordance with a recently published data38. However, targeting antigen to skin DC in μMt mice resulted in significant decrease of Bcl6+ Tfh, supporting the role of B cells in terminal differentiation of Tfh cells. Thus, while B cells may sustain Bcl6+ expression, antigen presentation by skin DC to naïve T cells is sufficient for initiation of Tfh differentiation. Whether direct antigen acquisition through BCR and/or antigen transport/presentation by DC contribute to B cell responses remains to be determined. Topical application of antigen triggered comparable humoral responses as i.p. targeting. With topical application the antigen availability is limited to DC in the skin. As such, active antigen transport, presentation and possible activation of B cells by DC should take place. These data therefore support a model where DC beside inducing Tfh cells, they also regulate humoral immune responses by transporting and presenting antigen to B cells.
LC and CD103+ DC promote opposing Th responses against C. albicans3, and different antibody isotypes in a gene gun immunization model39. Here we report that selective antigen targeting to LC or CD103+ DC in steady-state induced comparable Th phenotypes and antibody isotypes. However, it is also worth noting that CD103+ DC promoted a more robust CD4+ T cell response, than LC, but were less effective in inducing antibody production. Consistent with this CD103+ DC also promoted a very weak GC B cell response in comparison to LC. Why these two DC induce different B cell responses remains to be determined. Multiple things could contribute, such as differences in numbers, localization, antigen storage etc. These data therefore suggest that DC, beside promoting Th-responses play a direct and an important role in B cell responses, and that should be taken in consideration at vaccine design.
The ability of LC to promote both Tfh induction and humoral immune responses raises the possibility that these cells may be specialized to generate antibody responses specific for cutaneous antigens such as commensal microorganisms and/or skin pathogens and their products. We further speculate that LC may participate in the pathogenesis of antibody-mediated cutaneous diseases such as pemphigus and allergic dermatitis.
The skin is a common site for immunization and DC vaccination using anti-lectin/Ag conjugates is emerging as a powerful new vaccination strategy40. Importantly, our model employs targeting of antigen using anti-human Langerin antibody/antigen conjugates in a human Langerin transgenic mouse. This is a pre-clinical model for anti-human Langerin targeting that is currently undergoing testing in non-human primates with the same reagent in anticipation of upcoming clinical trials. Thus, in addition to revealing an unexpected biology of dendritic cells, our findings suggest that targeting antigen to skin DC either by systemic or topical immunization without adjuvants will be an efficient approach to generate protective antibody responses.
E Figure 1. Antigen targeting to LC and CD103+ DC is specific. The gating strategies are shown on the figures (upstream gate: live/singlets). Shaded represent WT and black line huLangerin mice treated with 2G3-AF647 (A–C). In D and E LC−/− mice left untreated (shade) or injected (black line) with 4C7-AF647. One representative experiment out of three is shown.
E Figure 2. Targeting LC through Langerin does not induce maturation or alter cytokine profile. (A–B) WT (black line) and huLangerin (dashed line) mice. Shaded lines indicate isotype controls. (C) As in (A), except that LC were sorted from LNs of WT (open bar) and huLangerin (black bar) mice and cytokine profile tested. (D) Cytokine profile of LC sorted from C. albicans infected mice.
E Figure 3. Antigen targeting to LC and CD103+ DC induce antigen-specific T cell proliferation. (A) TEα proliferation profile in WT and huLang mice immunized with 2G3-Eα. (B) Endogenous 2W1S-specific T cell expansion induced by LC or CD103+ DC.
E Figure 4. Transcription profile of LC-expanded 2W1S-specific cells.
E Figure 5. CD103+ DC induce Tfh in the context of C. albicans infection.
E Figure 6. Epidermal application of 2G3-AF647 targets LC in the epidermis, but not LN LC. (A) Mice were left untreated (grey shaded), painted with 2G3-AF647 (dashed line) or injected i.p. with 2G3-AF647 (black line). Skin and LN were harvested 18 hours after treatment and the AF647-signal assessed in LC.
E Figure 7. B cells are required for Bcl-6 expression by Tfh cells, but not IL-6 and type I interferon signaling. μMT, IL-6−/− and IFNAR−/− mice were treated with 4C7-2W1S. Seven days later the expansion of 2W1S-specific cells (A) and phenotype (B–C) were determined by flow cytometry. One representative experiment out of two is shown.
Supplementary Material
Acknowledgments
This work was supported by grants from the Dermatology Foundation (B.Z. Igyártó), American Skin Association (B.Z. Igyártó) and National Institutes of Health (AR056632; D.H. Kaplan).
We thank M. Jenkins for 2W1S tetramer. We also thank the University of Minnesota Research Animal Resources staff for expert animal care and P. Champoux, T. Martin and J. Motl of the Flow Cytometry Core Facility at the Center for Immunology for assistance with flow cytometry experiments.
Abbreviations used
- CFA
Complete Freund adjuvant
- CFSE
Carboxyfluorescein succimidyl ester
- DC
Dendritic cells
- DETC
Dendritic epidermal T cells
- DNCB
2,4-dinitrocholrobenzene
- DTA
Diphtheria toxin subunit A
- DTR
Diphtheria toxin receptor
- EAE
Experimental autoimmune encephalomyelitis
- FluHA1
Influenza hemagglutinin A1
- GC
Germinal center
- KC
Keratinocytes
- LC
Langerhans cells
- MOG
Myelin oligodendrocyte glycoprotein
- TCR
T cell receptor
- Tfh
T follicular helper cells
- PFU
Plaque-forming unit
- PR8
Puerto Rico 8
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31(1):563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21(1):685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 3.Igyártó BZ, Haley K, Ortner D, Bobr A, Gerami-Nejad M, Edelson BT, et al. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity. 2011 Aug 26;35(2):260–72. doi: 10.1016/j.immuni.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bedoui S, Whitney PG, Waithman J, Eidsmo L, Wakim L, Caminschi I, et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol. 2009 May;10(5):488–95. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- 5.Flacher V, Tripp CH, Mairhofer DG, Steinman RM, Stoitzner P, Idoyaga J, et al. Murine Langerin+ dermal dendritic cells prime CD8+ T cells while Langerhans cells induce cross-tolerance. EMBO Mol Med. 2014;6(9):1191–204. doi: 10.15252/emmm.201303283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henri S, Poulin LF, Tamoutounour S, Ardouin L, Guilliams M, de Bovis B, et al. J Exp Med. 1. Vol. 207. Rockefeller Univ Press; 2010. Jan 18, CD207+ CD103+ dermal dendritic cells cross-present keratinocyte-derived antigens irrespective of the presence of Langerhans cells; pp. 189–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaplan DH. In vivo function of Langerhans cells and dermal dendritic cells. Trends Immunol. 2010 Dec;31(12):446–51. doi: 10.1016/j.it.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomez de Agüero M, Vocanson M, Hacini-Rachinel F, Taillardet M, Sparwasser T, Kissenpfennig A, et al. Langerhans cells protect from allergic contact dermatitis in mice by tolerizing CD8(+) T cells and activating Foxp3(+) regulatory T cells. J Clin Invest American Society for Clinical Investigation. 2012 May 1;122(5):1700–11. doi: 10.1172/JCI59725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shklovskaya E, O’Sullivan BJ, Ng LG, Roediger B, Thomas R, Weninger W, et al. Langerhans cells are precommitted to immune tolerance induction. Proc Natl Acad Sci USA National Acad Sciences. 2011 Nov 1;108(44):18049–54. doi: 10.1073/pnas.1110076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kautz-Neu K, Noordegraaf M, Dinges S, Bennett CL, John D, Clausen BE, et al. J Exp Med. 5. Vol. 208. Rockefeller Univ Press; 2011. May 9, Langerhans cells are negative regulators of the anti-Leishmania response; pp. 885–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ouchi T, Kubo A, Yokouchi M, Adachi T, Kobayashi T, Kitashima DY, et al. J Exp Med. 13. Vol. 208. Rockefeller Univ Press; 2011. Dec 19, Langerhans cell antigen capture through tight junctions confers preemptive immunity in experimental staphylococcal scalded skin syndrome; pp. 2607–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Idoyaga J, Fiorese C, Zbytnuik L, Lubkin A, Miller J, Malissen B, et al. J Clin Invest. 2. Vol. 123. American Society for Clinical Investigation; 2013. Feb 1, Specialized role of migratory dendritic cells in peripheral tolerance induction; pp. 844–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. Journal of Experimental Medicine. 2002 Dec 16;196(12):1627–38. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, et al. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007 Aug;27(2):203–13. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bobr A, Olvera-Gomez I, Igyártó BZ, Haley KM, Hogquist KA, Kaplan DH. Acute ablation of Langerhans cells enhances skin immune responses. J Immunol American Association of Immunologists. 2010 Oct 15;185(8):4724–8. doi: 10.4049/jimmunol.1001802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Igyártó BZ, Jenison MC, Dudda JC, Roers A, Müller W, Koni PA, et al. J Immunol. 8. Vol. 183. American Association of Immunologists; 2009. Oct 15, Langerhans cells suppress contact hypersensitivity responses via cognate CD4 interaction and langerhans cell-derived IL-10; pp. 5085–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edelson BT, KCW, Juang R, Kohyama M, Benoit LA, Klekotka PA, et al. J Exp Med. 4. Vol. 207. Rockefeller Univ Press; 2010. Apr 12, Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells; pp. 823–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grubin CE, Kovats S, deRoos P, Rudensky AY. Deficient positive selection of CD4 T cells in mice displaying altered repertoires of MHC class II-bound self-peptides. Immunity. 1997 Aug;7(2):197–208. doi: 10.1016/s1074-7613(00)80523-3. [DOI] [PubMed] [Google Scholar]
- 19.Flamar A-L, Zurawski S, Scholz F, Gayet I, Ni L, Li X-H, et al. J Immunol. 5. Vol. 189. American Association of Immunologists; 2012. Sep 1, Noncovalent assembly of anti-dendritic cell antibodies and antigens for evoking immune responses in vitro and in vivo; pp. 2645–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Idoyaga J, Cheong C, Suda K, Suda N, Kim JY, Lee H, et al. Cutting edge: langerin/CD207 receptor on dendritic cells mediates efficient antigen presentation on MHC I and II products in vivo. J Immunol. 2008 Mar 15;180(6):3647–50. doi: 10.4049/jimmunol.180.6.3647. [DOI] [PubMed] [Google Scholar]
- 21.Flacher V, Tripp CH, Stoitzner P, Haid B, Ebner S, Del Frari B, et al. Epidermal Langerhans cells rapidly capture and present antigens from C-type lectin-targeting antibodies deposited in the dermis. J Invest Dermatol. 2010 Mar;130(3):755–62. doi: 10.1038/jid.2009.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Immunity. 6. Vol. 23. Elsevier; 2005. Dec, Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity; pp. 611–20. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan DH, Li MO, Jenison MC, Shlomchik WD, Flavell RA, Shlomchik MJ. J Exp Med. 11. Vol. 204. Rockefeller Univ Press; 2007. Oct 29, Autocrine/paracrine TGFbeta1 is required for the development of epidermal Langerhans cells; pp. 2545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tubo NJ, Pagán AJ, Taylor JJ, Nelson RW, Linehan JL, Ertelt JM, et al. Single naive CD4+ T cells from a diverse repertoire produce different effector cell types during infection. Cell. 2013 May 9;153(4):785–96. doi: 10.1016/j.cell.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29(1):621–63. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 26.Tangye SG, Ma CS, Brink R, Deenick EK. Nat Rev Immunol. 6. Vol. 13. Nature Publishing Group; 2013. May 17, The good, the bad and the ugly — T; pp. 412–26. [DOI] [PubMed] [Google Scholar]
- 27.Baumjohann D, Preite S, Reboldi A, Ronchi F, Ansel KM, Lanzavecchia A, et al. Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity. 2013 Mar 21;38(3):596–605. doi: 10.1016/j.immuni.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 28.Deenick EK, Chan A, Ma CS, Gatto D, Schwartzberg PL, Brink R, et al. Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity. 2010 Aug 27;33(2):241–53. doi: 10.1016/j.immuni.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ballesteros-Tato A, Randall TD. Priming of T follicular helper cells by dendritic cells. Immunol Cell Biol. 2014 Jan;92(1):22–7. doi: 10.1038/icb.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Witte L, Nabatov A, Pion M, Fluitsma D, de Jong MAWP, de Gruijl T, et al. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat Med. 2007 Mar;13(3):367–71. doi: 10.1038/nm1541. [DOI] [PubMed] [Google Scholar]
- 31.Tangye SG, Ma CS, Brink R, Deenick EK. The good, the bad and the ugly - TFH cells in human health and disease. Nat Rev Immunol. 2013 Jun;13(6):412–26. doi: 10.1038/nri3447. [DOI] [PubMed] [Google Scholar]
- 32.Lahoud MH, Ahmet F, Kitsoulis S, Wan SS, Vremec D, Lee C-N, et al. J Immunol. 2. Vol. 187. American Association of Immunologists; 2011. Jul 15, Targeting antigen to mouse dendritic cells via Clec9A induces potent CD4 T cell responses biased toward a follicular helper phenotype; pp. 842–50. [DOI] [PubMed] [Google Scholar]
- 33.Choi YS, Yang JA, Crotty S. Dynamic regulation of Bcl6 in follicular helper CD4 T (Tfh) cells. Curr Opin Immunol. 2013 Jun;25(3):366–72. doi: 10.1016/j.coi.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011 Jun 24;34(6):932–46. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cai G, Nie X, Zhang W, Wu B, Lin J, Wang H, et al. J Immunol. 3. Vol. 189. American Association of Immunologists; 2012. Aug 1, A regulatory role for IL-10 receptor signaling in development and B cell help of T follicular helper cells in mice; pp. 1294–302. [DOI] [PubMed] [Google Scholar]
- 36.Goenka R, Barnett LG, Silver JS, O’Neill PJ, Hunter CA, Cancro MP, et al. J Immunol. 3. Vol. 187. American Association of Immunologists; 2011. Aug 1, Cutting edge: dendritic cell-restricted antigen presentation initiates the follicular helper T cell program but cannot complete ultimate effector differentiation; pp. 1091–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma CS, Deenick EK, Batten M, Tangye SG. J Exp Med. 7. Vol. 209. Rockefeller Univ Press; 2012. Jul 2, The origins, function, and regulation of T follicular helper cells; pp. 1241–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barnett LG, Simkins HMA, Barnett BE, Korn LL, Johnson AL, Wherry EJ, et al. J Immunol. 8. Vol. 192. American Association of Immunologists; 2014. Apr 15, B cell antigen presentation in the initiation of follicular helper T cell and germinal center differentiation; pp. 3607–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagao K, Ginhoux F, Leitner WW, Motegi S-I, Bennett CL, Clausen BE, et al. Proc Natl Acad Sci USA. 9. Vol. 106. National Acad Sciences; 2009. Mar 3, Murine epidermal Langerhans cells and langerin-expressing dermal dendritic cells are unrelated and exhibit distinct functions; pp. 3312–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romani N, Flacher V, Tripp CH, Sparber F, Ebner S, Stoitzner P. Curr Top Microbiol Immunol. Chapter 118. Vol. 351. Berlin, Heidelberg: Springer Berlin Heidelberg; 2012. Targeting skin dendritic cells to improve intradermal vaccination; pp. 113–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.