Abstract
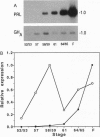
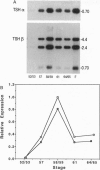
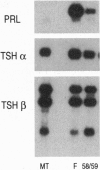
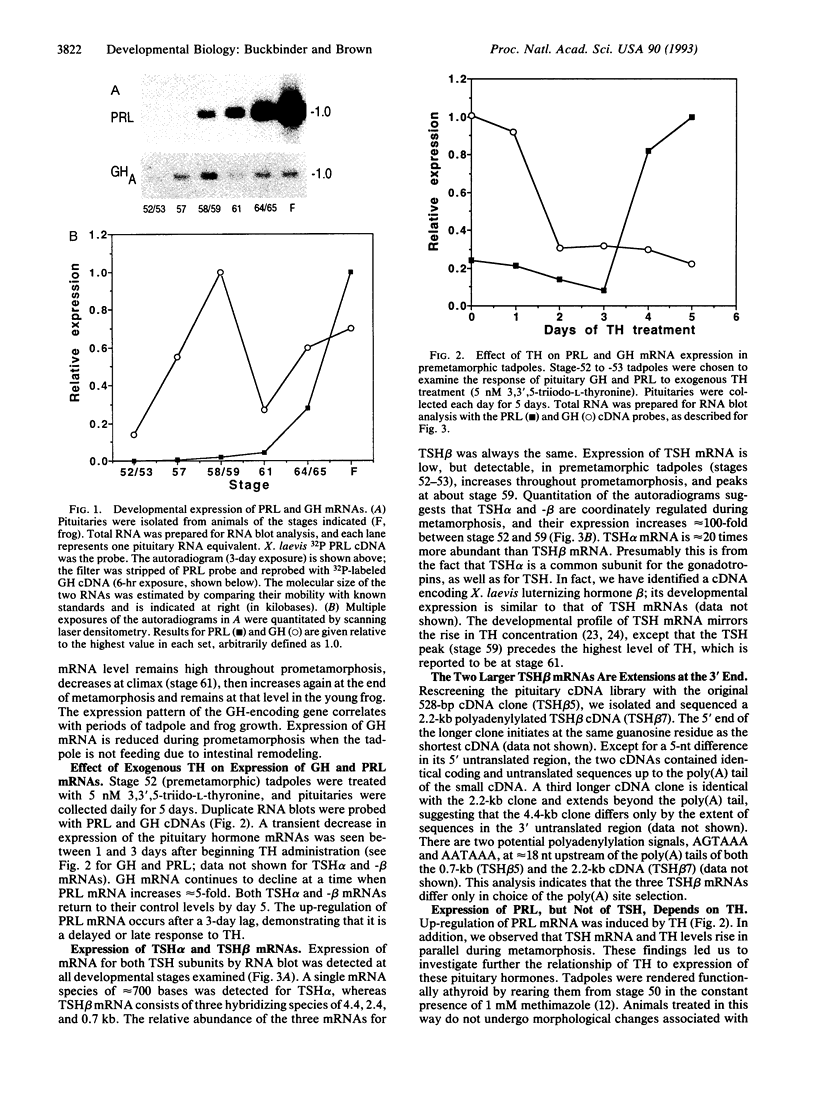
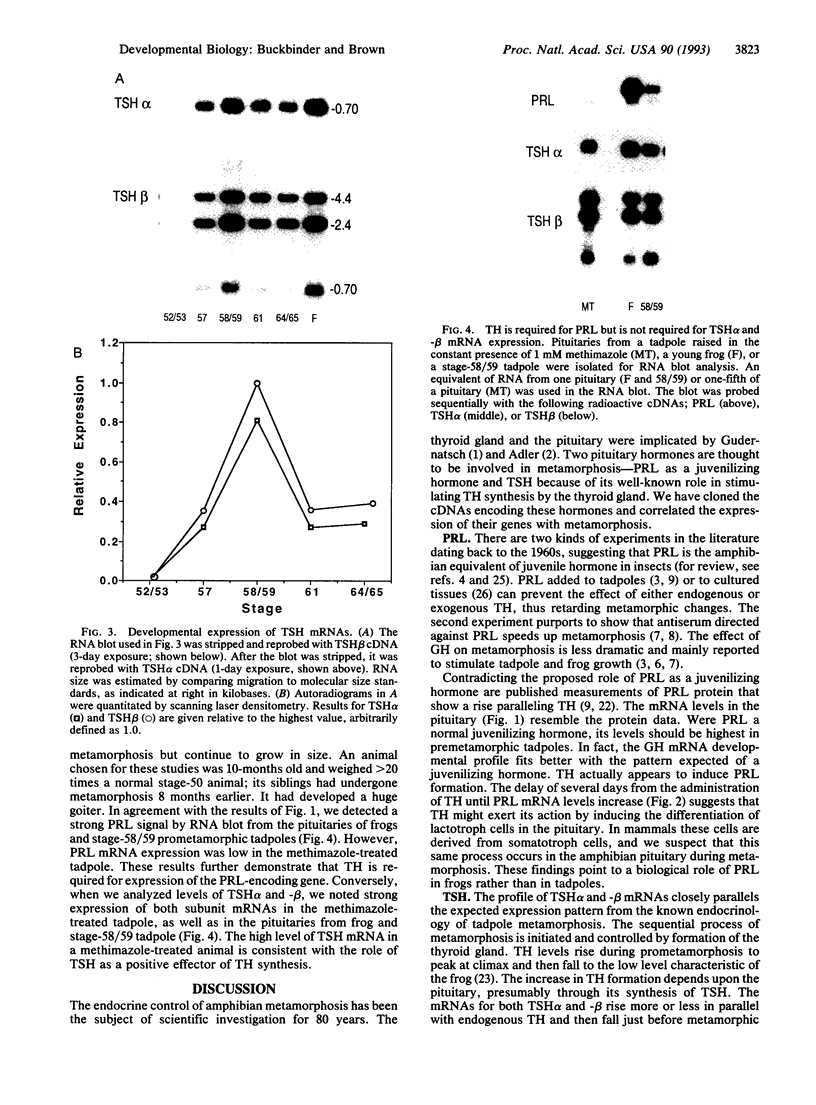
The cDNAs encoding Xenopus laevis prolactin (PRL) and the alpha and beta subunits of thyroid-stimulating hormone (TSH alpha and TSH beta, respectively) have been cloned from a pituitary library. Results of developmental RNA blot analysis contradict the long-held biological role for PRL as a juvenilizing hormone in amphibia. The pituitary gland of a premetamorphic tadpole expresses PRL mRNA at very low levels. The abundance of PRL mRNA increases late in metamorphosis as a response to thyroid hormone (TH), suggesting that PRL is more likely to have a function in the frog than in the tadpole. TSH alpha and -beta mRNA levels increase through prometamorphosis; this rise does not appear to be regulated directly by TH. At climax, both TH and TSH mRNA levels drop. The sequential morphological changes that characterize prometamorphosis depend upon the gradual increase of endogenous TH, which peaks at climax. This increase in TH in turn depends upon the lack of a traditional thyroid-pituitary negative-feedback loop throughout prometamorphosis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bern H. A., Nicoll C. S., Strohan R. C. Prolactin and tadpole growth. Proc Soc Exp Biol Med. 1967 Nov;126(2):518–520. doi: 10.3181/00379727-126-32493. [DOI] [PubMed] [Google Scholar]
- Bray T., Sicard R. E. Correlation among the changes in the levels of thyroid hormones, thyrotropin and thyrotropin-releasing hormone during the development of Xenopus laevis. Exp Cell Biol. 1982;50(2):101–107. doi: 10.1159/000163134. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clemons G. K., Nicoll C. S. Development and preliminary application of a homologous radioimmunoassay for bullfrog prolactin. Gen Comp Endocrinol. 1977 Aug;32(4):531–535. doi: 10.1016/0016-6480(77)90237-4. [DOI] [PubMed] [Google Scholar]
- Clemons G. K., Nicoll C. S. Effects of antisera to bullfrog prolactin and growth hormone on metamorphosis of Rana catesbeiana tadpoles. Gen Comp Endocrinol. 1977 Apr;31(4):495–497. doi: 10.1016/0016-6480(77)90042-9. [DOI] [PubMed] [Google Scholar]
- Cooper D. S., Kieffer J. D., Saxe V., Mover H., Maloof F., Ridgway E. C. Methimazole pharmacology in the rat: studies using a newly developed radioimmunoassay for methimazole. Endocrinology. 1984 Mar;114(3):786–793. doi: 10.1210/endo-114-3-786. [DOI] [PubMed] [Google Scholar]
- Eddy L., Lipner H. Acceleration of thyroxine-induced metamorphosis by prolactin antiserum. Gen Comp Endocrinol. 1975 Apr;25(4):462–466. doi: 10.1016/0016-6480(75)90157-4. [DOI] [PubMed] [Google Scholar]
- Godine J. E., Chin W. W., Habener J. F. alpha Subunit of rat pituitary glycoprotein hormones. Primary structure of the precursor determined from the nucleotide sequence of cloned cDNAs. J Biol Chem. 1982 Jul 25;257(14):8368–8371. [PubMed] [Google Scholar]
- Kikuyama S., Yamamoto K., Mayumi M. Growth-promoting and antimetamorphic hormone in pituitary glands of bullfrogs. Gen Comp Endocrinol. 1980 Jun;41(2):212–216. doi: 10.1016/0016-6480(80)90145-8. [DOI] [PubMed] [Google Scholar]
- Krotoski D. M., Reinschmidt D. C., Tompkins R. Developmental mutants isolated from wild-caught Xenopus laevis by gynogenesis and inbreeding. J Exp Zool. 1985 Mar;233(3):443–449. doi: 10.1002/jez.1402330313. [DOI] [PubMed] [Google Scholar]
- Martens G. J., Groenen P. J., Braks A. A., Bussemakers M. J. Expression of two growth hormone genes in the Xenopus pituitary gland. Nucleic Acids Res. 1989 May 25;17(10):3974–3974. doi: 10.1093/nar/17.10.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer R. A., Croyle M. L., Donelson J. E. The sequence of a cloned cDNA for the beta subunit of bovine thyrotropin predicts a protein containing both NH2-and COOH-terminal extensions. J Biol Chem. 1984 Apr 25;259(8):5024–5027. [PubMed] [Google Scholar]
- Miller W. L., Eberhardt N. L. Structure and evolution of the growth hormone gene family. Endocr Rev. 1983 Spring;4(2):97–130. doi: 10.1210/edrv-4-2-97. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp Z. D., Cao Z. Regulation of cell-type-specific transcription and differentiation of the pituitary. Bioessays. 1990 Feb;12(2):80–85. doi: 10.1002/bies.950120206. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Yoshihama K., Kikuyama S., Yamamoto K., Wakabayashi K., Kato Y. Molecular cloning and nucleotide sequence analysis of complementary DNA for bullfrog prolactin. J Mol Endocrinol. 1990 Dec;5(3):281–287. doi: 10.1677/jme.0.0050281. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Niinuma K., Kikuyama S. Synthesis and storage of prolactin in the pituitary gland of bullfrog tadpoles during metamorphosis. Gen Comp Endocrinol. 1986 May;62(2):247–253. doi: 10.1016/0016-6480(86)90115-2. [DOI] [PubMed] [Google Scholar]