Abstract
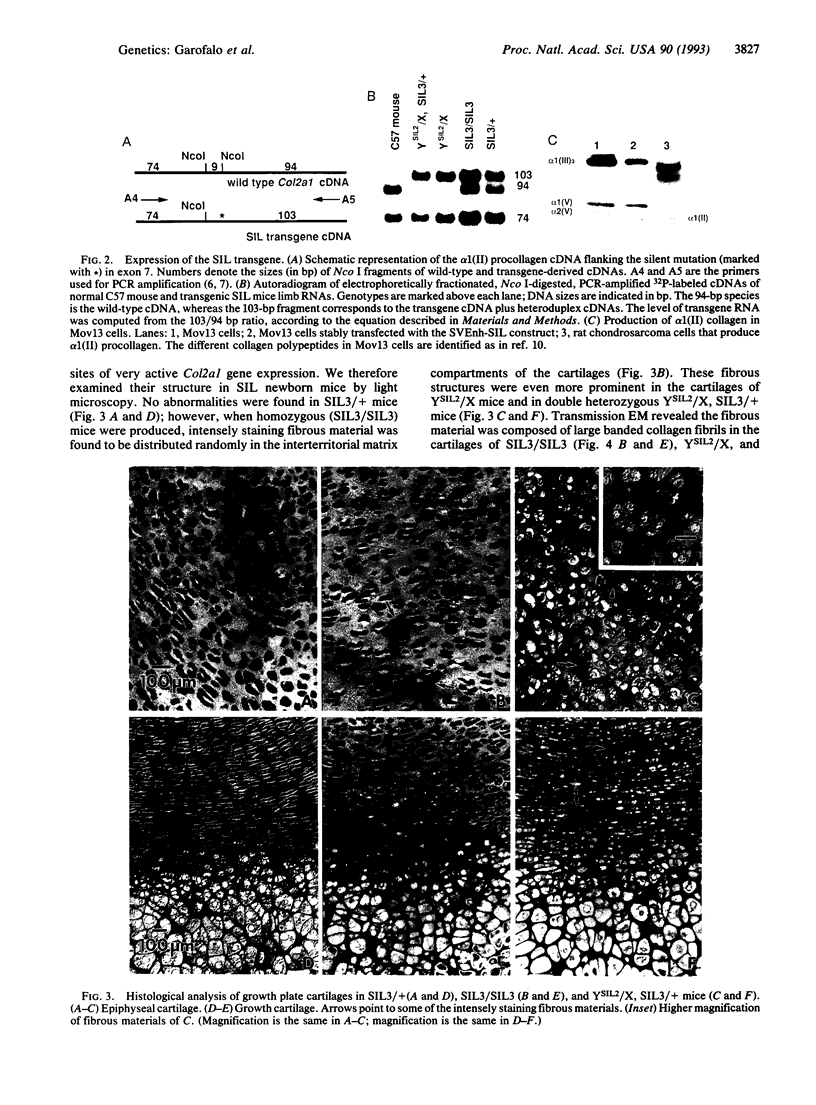
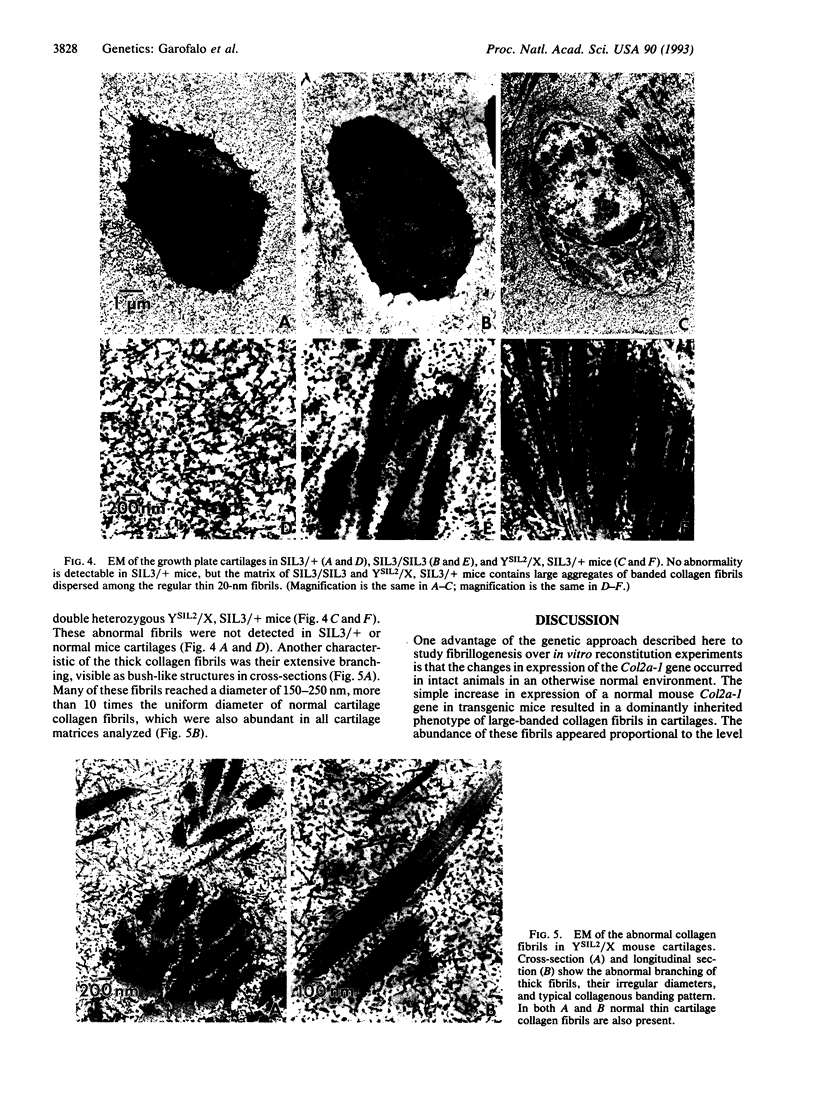
Cartilage collagen fibrils, which are characterized by their thin, uniform diameters, are formed of a multicomponent system of three collagen types (II, IX, and XI) and interacting proteoglycans. We have used a genetic approach to test whether the proper assembly of this multiprotein structure was altered by overexpression of one of its normal components. Here we show that in transgenic mice in which the normal mouse alpha 1(II) collagen is overexpressed, thick abnormal collagen fibrils are generated. Mice that showed the highest expression of the transgene also displayed a larger proportion of abnormal fibrils and died at birth. We propose that an imbalance among the constituents of the cartilage collagen fibrils disrupts the mechanism that controls their assembly. The results show the applicability of the transgenic mice system to studies of complex multicomponent protein assemblies in intact animals.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fjolstad M., Helle O. A hereditary dysplasia of collagen tissues in sheep. J Pathol. 1974 Mar;112(3):183–188. doi: 10.1002/path.1711120309. [DOI] [PubMed] [Google Scholar]
- Garofalo S., Vuorio E., Metsaranta M., Rosati R., Toman D., Vaughan J., Lozano G., Mayne R., Ellard J., Horton W. Reduced amounts of cartilage collagen fibrils and growth plate anomalies in transgenic mice harboring a glycine-to-cysteine mutation in the mouse type II procollagen alpha 1-chain gene. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9648–9652. doi: 10.1073/pnas.88.21.9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulmes D. J., Kadler K. E., Mould A. P., Hojima Y., Holmes D. F., Cummings C., Chapman J. A., Prockop D. J. Pleomorphism in type I collagen fibrils produced by persistence of the procollagen N-propeptide. J Mol Biol. 1989 Nov 20;210(2):337–345. doi: 10.1016/0022-2836(89)90335-5. [DOI] [PubMed] [Google Scholar]
- Lapière C. M., Lenaers A., Kohn L. D. Procollagen peptidase: an enzyme excising the coordination peptides of procollagen. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3054–3058. doi: 10.1073/pnas.68.12.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. L., Piez K. A. Type II collagen from lathyritic rat chondrosarcoma: preparation and in vitro fibril formation. Coll Relat Res. 1983 Mar;3(2):89–103. doi: 10.1016/s0174-173x(83)80036-3. [DOI] [PubMed] [Google Scholar]
- Meeks-Wagner D., Hartwell L. H. Normal stoichiometry of histone dimer sets is necessary for high fidelity of mitotic chromosome transmission. Cell. 1986 Jan 17;44(1):43–52. doi: 10.1016/0092-8674(86)90483-6. [DOI] [PubMed] [Google Scholar]
- Mendler M., Eich-Bender S. G., Vaughan L., Winterhalter K. H., Bruckner P. Cartilage contains mixed fibrils of collagen types II, IX, and XI. J Cell Biol. 1989 Jan;108(1):191–197. doi: 10.1083/jcb.108.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsäranta M., Garofalo S., Decker G., Rintala M., de Crombrugghe B., Vuorio E. Chondrodysplasia in transgenic mice harboring a 15-amino acid deletion in the triple helical domain of pro alpha 1(II) collagen chain. J Cell Biol. 1992 Jul;118(1):203–212. doi: 10.1083/jcb.118.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsäranta M., Toman D., de Crombrugghe B., Vuorio E. Mouse type II collagen gene. Complete nucleotide sequence, exon structure, and alternative splicing. J Biol Chem. 1991 Sep 5;266(25):16862–16869. [PubMed] [Google Scholar]
- Novick P., Botstein D. Phenotypic analysis of temperature-sensitive yeast actin mutants. Cell. 1985 Feb;40(2):405–416. doi: 10.1016/0092-8674(85)90154-0. [DOI] [PubMed] [Google Scholar]
- Nusgens B. V., Verellen-Dumoulin C., Hermanns-Lê T., De Paepe A., Nuytinck L., Piérard G. E., Lapière C. M. Evidence for a relationship between Ehlers-Danlos type VII C in humans and bovine dermatosparaxis. Nat Genet. 1992 Jun;1(3):214–217. doi: 10.1038/ng0692-214. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Setoyama C., de Crombrugghe B. Regulation of a collagen gene promoter by the product of viral mos oncogene. Nature. 1985 Mar 21;314(6008):286–289. doi: 10.1038/314286a0. [DOI] [PubMed] [Google Scholar]
- Seegmiller R., Fraser F. C., Sheldon H. A new chondrodystrophic mutant in mice. Electron microscopy of normal and abnormal chondrogenesis. J Cell Biol. 1971 Mar;48(3):580–593. doi: 10.1083/jcb.48.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillence D. O., Horton W. A., Rimoin D. L. Morphologic studies in the skeletal dysplasias. Am J Pathol. 1979 Sep;96(3):813–870. [PMC free article] [PubMed] [Google Scholar]
- Smith L. T., Wertelecki W., Milstone L. M., Petty E. M., Seashore M. R., Braverman I. M., Jenkins T. G., Byers P. H. Human dermatosparaxis: a form of Ehlers-Danlos syndrome that results from failure to remove the amino-terminal propeptide of type I procollagen. Am J Hum Genet. 1992 Aug;51(2):235–244. [PMC free article] [PubMed] [Google Scholar]
- Stacey A., Bateman J., Choi T., Mascara T., Cole W., Jaenisch R. Perinatal lethal osteogenesis imperfecta in transgenic mice bearing an engineered mutant pro-alpha 1(I) collagen gene. Nature. 1988 Mar 10;332(6160):131–136. doi: 10.1038/332131a0. [DOI] [PubMed] [Google Scholar]
- Stanescu V., Stanescu R., Maroteaux P. Pathogenic mechanisms in osteochondrodysplasias. J Bone Joint Surg Am. 1984 Jul;66(6):817–836. doi: 10.2106/00004623-198466060-00002. [DOI] [PubMed] [Google Scholar]
- Vaughan L., Mendler M., Huber S., Bruckner P., Winterhalter K. H., Irwin M. I., Mayne R. D-periodic distribution of collagen type IX along cartilage fibrils. J Cell Biol. 1988 Mar;106(3):991–997. doi: 10.1083/jcb.106.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuorio E., de Crombrugghe B. The family of collagen genes. Annu Rev Biochem. 1990;59:837–872. doi: 10.1146/annurev.bi.59.070190.004201. [DOI] [PubMed] [Google Scholar]
- van der Rest M., Garrone R. Collagen family of proteins. FASEB J. 1991 Oct;5(13):2814–2823. [PubMed] [Google Scholar]







