Abstract
Background
The preBötzinger Complex (preBC) plays an important role in respiratory rhythm generation. This study was designed to determine whether the preBC mediated opioid-induced respiratory rate depression at clinically relevant opioid concentrations in vivo and whether this role was age-dependent.
Methods
Studies were performed in 22 young and 32 adult New Zealand White rabbits. Animals were anesthetized, mechanically ventilated and decerebrated. The preBC was identified by the tachypneic response to injection of D,L-homoysteic acid. (1) The mu-opioid receptor agonist [D-Ala2,N-Me-Phe4,Gly-ol]-enkephalin (DAMGO, 100μM) was microinjected into the bilateral preBC and reversed with naloxone (1mM) injection into the preBC. (2) Respiratory depression was achieved with intravenous remifentanil (0.08–0.5 mcg/kg/min). Naloxone (1mM) was microinjected into the preBC in an attempt to reverse the respiratory depression.
Results
(1) DAMGO injection depressed respiratory rate by 6 ± 8 breaths/min in young and adult rabbits (mean ± SD, p <0.001). DAMGO shortened the inspiratory and lengthened the expiratory fraction of the respiratory cycle by 0.24 ± 0.2 in adult and young animals (p <0.001). (2) During intravenous remifentanil infusion, local injection of naloxone into the preBC partially reversed the decrease in inspiratory fraction/increase in expiratory fraction in young and adult animals (0.14 ± 0.14, p <0.001), but not the depression of respiratory rate (p = 0.19). PreBC injections did not affect respiratory drive. In adult rabbits, the contribution of non-preBC inputs to expiratory phase duration was larger than preBC inputs (3.5(−5.2–1.1), median (25%–75%), p = 0.04).
Conclusions
Systemic opioid effects on respiratory phase timing can be partially reversed in the preBC without reversing the depression of respiratory rate.
Introduction
Opioid-induced respiratory depression poses an important limitation for the use of opioids in perioperative pain control.1,2 This side effect is particularly pronounced in premature and young infants, which has been attributed to reduced opioid metabolism and a less effective blood-brain barrier.3 However, divergent results from adult canine 4,5 and neonatal rat preparations 6,7 point to important functional developmental differences in respiratory effects of opioids.
Studies investigating opioid effects on respiration have focused on the preBötzinger Complex (preBC) as the main region of respiratory rhythm/pattern generation 8 and by default as the main site of opioid-induced respiratory depression. 6,7 Neonatal in vitro studies support a role of the preBC in opioid-induced respiratory depression 7 but results from adult in vivo preparations are contradictory.
While microdialysis of the mu-opioid receptor agonist [D-Ala2,N-Me-Phe4,Gly-ol]-enkephalin (DAMGO, 5μM) into the brainstem of adult rats led to bradypnea 9, localized microinjection of DAMGO (100μM) into the preBC of adult dogs in vivo led to tachypnea. 5 In both studies local agonist concentrations were far higher (μM-mM) than the low nanomolar tissue concentrations generally achieved during systemic, intravenous opioid use (~20nM 10). When clinically relevant opioid doses were given, respiratory slowing from intravenous fentanyl (5 mcg/kg) was prevented in adult rats by microdialysis of naloxone into the brainstem administered for 45 min prior to fentanyl 9 but in adult dogs microinjection of naloxone directly into the functionally identified preBC region did not reverse respiratory depression from intravenous remifentanil. 5 We contend that these discrepant findings were due to the fact that localized microinjection in the dog solely affected the preBC while microdialysis of DAMGO and naloxone in rats was more diffuse and affected additional brainstem areas involved in respiratory rate control. 4 However, even if the preBC was not the site of opioid-induced respiratory depression in adult animals in vivo,5 respiratory depression from opioids was clearly observed in neonatal in vitro preparations that included only the preBC and used “near-clinical” agonist concentrations. 6,7 Thus developmental differences in clinical opioid effect on respiratory control sites seem to exist. A recently published point-counterpoint debate on this topic highlighted the ongoing controversy and emphasized the clinical relevance of identifying the site of opioid effect.11–14
The present study was designed to gain a comprehensive understanding of opioid effects on the preBC and respiratory pattern generation. We have developed a decerebrate rabbit model that allows studying developmental differences in respiratory control at the neuronal level in functionally defined areas of the brainstem in young and adult animals in vivo. Protocol 1 tested whether injection of pharmacological concentrations of the μ-opioid receptor agonist DAMGO into the preBC affected respiratory rate and pattern. Protocol 2 tested whether the depression of respiratory rate and the change in respiratory pattern that resulted from systemic opioid infusion at clinically relevant concentrations could be antagonized by localized injection of the opioid antagonist naloxone into the preBC. We hypothesized that there were developmental differences in the role of the preBC in clinical, opioid-induced respiratory depression between young and adult rabbits.
Materials and Methods
Surgical Procedures
This research was approved by the subcommittee on animal studies of the Zablocki Veterans Affairs Medical Center, Milwaukee, Wisconsin, in accordance with provisions of the Animal Welfare Act, the Public Health Service Guide for the Care and Use of Laboratory Animals, and Veterans Affairs policy. Adult (3–4 kg) and young (2–3 weeks, 110–550g) New Zealand White rabbits of either sex were induced with 5 vol% sevoflurane via facemask. Animals were ventilated via tracheotomy with an anesthesia machine (Ohmeda CD, GE, Datex Ohmeda, Madison, WI) or if weight was below 400g, with a small animal ventilator (SAR-830 ventilator, CWE, Colorado Springs, CO). Anesthesia was maintained with 1–2% (young) or 1.5–3% (adult) isoflurane. Inspiratory oxygen fraction, expiratory carbon dioxide concentration and expiratory isoflurane concentration were continuously recorded with an infrared analyzer (POET II, Criticare Systems, Waukesha, WI). Skin was infiltrated with lidocaine 1% before each skin incision. Femoral arterial and venous lines were used for blood pressure monitoring and infusion of solutions, respectively. Care was taken to increase anesthetic depth for any signs of “light anesthesia”, e.g., increase in blood pressure or lacrimation. Lactated Ringer’s solution with 2 mcg/ml epinephrine was continuously infused at 1 ml/h. At this rate, the infusion did not result in appreciable changes in heart rate and blood pressure. Infusion rate was increased as needed to counteract or prevent hypotension in response to drug injections or from blood loss. The animal was maintained at 37.0 +/− 0.5 °C with a warming blanket. The animal was placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA), and blunt precollicular decerebration with complete removal of the forebrain was performed through a parietal craniotomy. After decerebration isoflurane was discontinued or continued at subanesthetic levels (0.3–0.4 Vol%) for blood pressure control. Isoflurane concentration was not changed throughout the experimental protocol. The brainstem was exposed via occipital craniotomy and partial removal of the cerebellum and the volatile anesthetic was discontinued. Animals were paralyzed with vecuronium (initially 1 mg/kg and redosed as needed) to avoid motion artifacts during neural/neuronal recording. The vagal nerves were left intact. The phrenic nerve was recorded with fine bipolar electrodes through a posterior neck incision. Throughout the experiment animals were ventilated with a hyperoxic gas mixture (FiO2 0.6) to achieve functional denervation of the peripheral chemoreceptors and at mild hypercapnia (expiratory carbon dioxide: 45–55 mmHg) to ensure sufficient respiratory drive (i.e., above the apneic threshold) even during systemic opioid infusion. Blood pressure was maintained stable throughout the protocols by adjusting the intravenous infusion rate. At the end of the experiment the animals were euthanized with intravenous potassium chloride. In a subgroup of animals the microinjection site was marked with pontamine blue dye and the brainstem was removed after euthanasia and fixed for histological analysis.
Neuronal Recording, Drug application, Measured Variables and Data Analysis
All neuronal recording and microinjection techniques have been well established by our research group and have been previously described in detail. 15,16 In short, extracellular neuronal recordings were obtained using multibarrel micropipettes (20–40 μm tip diameter) consisting of three drug barrels and a recording barrel containing a 7 μm thick carbon filament. Barrels were filled with the glutamate agonist D,L-homocysteic acid (DLH, 1 mM), the μ-opioid receptor agonist DAMGO (100 μM) and the opioid receptor antagonist naloxone (1 mM), which were dissolved in artificial cerebrospinal fluid. The microinjected volume was determined via height changes in the meniscus in the respective pipette barrel with a 100x monocular microscope and calibrated reticule (resolution ~3.5 nl). Respiratory neuron subtypes were classified according to their discharge patterns and their temporal relationships relative to the phrenic neurogram. The neuronal and phrenic neural activity, and pressure microejection marker signals were recorded using a digital acquisition system. These variables were also continuously displayed and recorded along with the phrenic neurogram, inspiratory time, expiratory time, discharge rate-meter, arterial blood and tracheal pressures, airway carbon dioxide and oxygen concentration on a computerized chart recorder (Powerlab/16SP; ADInstruments, Castle Hill, Australia). Post hoc analysis averaged respiratory cycles from the phrenic neurogram. Before and after drug injection, steady-state conditions were obtained for respiratory parameters. Between 10 and 50 consecutive respiratory cycles were averaged over 1–2 min with the number of cycles dependent on the respiratory rate. We determined peak phrenic activity (PPA), respiratory rate (RR) and inspiratory (Ti) and expiratory (Te) duration. Since changes in PPA closely reflect changes in respiratory tidal volume but the absolute value does not correspond with the absolute tidal volume,17 PPA was normalized to control values for all calculations. An estimate of respiratory drive was calculated as the rise in phrenic nerve amplitude during the inspiratory phase, i.e., (PPA/Ti). In order to discern the opioid effects on inspiratory and expiratory phase timing, we calculated the inspiratory fraction (Ti/Ttot) and expiratory fraction (Te/Ttot) of the total respiratory cycle (Ttot), i.e., Ttot=Ti+Te and Ti/Ttot + Te/Ttot = 1.
Sample Size and Statistical Analysis
Statistical procedures on the pooled data were performed using SigmaPlot 11 (Systat Software, Richmond, CA). Sample size was based on published in vivo studies using similar protocols. These studies had used 4 to 9 rats per protocol 9 and 10 to 21 dogs per protocol. 4,5 Initial data analysis was performed after 5–6 animals, and additional experiments were added for a protocol to either confirm or refute nonsignificant trends in the data. 18 Data sets were tested for normal distribution (Kolmogorov-Smirnov test). Statistical tests were performed on raw data except for respiratory drive, where peak phrenic activity is measured in arbitrary units and normalization to control is necessary to allow for comparison between animals. The effects of opioid agonists/antagonists on all respiratory parameters (RR, PPA, Ti, Te, PPA/Ti, Ti/Ttot) were determined separately for young and adult animals using one-way, repeated-measures analysis of variance (ANOVA) with Holm-Sidak correction for multiple comparisons for normally distributed data and Friedman repeated measures ANOVA on ranks with Tukey test for pair-wise multiple comparisons for not normally distributed data. Differences between opioid agonist/antagonist effects between young and adult animals were determined for RR, PPA/Ti and Ti/Ttot using two-way repeated measures ANOVA with treatment and developmental age as factors. To compare preBC and non-preBC inputs to Ti and Te, which were not normally distributed, Kruskal-Wallis ANOVA on ranks with Dunn’s method for multiple comparisons was used to test for differences between inputs and age groups. Two-tailed tests were performed to test for significant differences without regard to direction. P-values were not adjusted in response to the sequential testing that was conducted during the study. Differences were considered significant for P <0.05. Values are expressed as mean ± SD or median (25%–75% range) as appropriate.
Location of the preBötzinger Complex Region
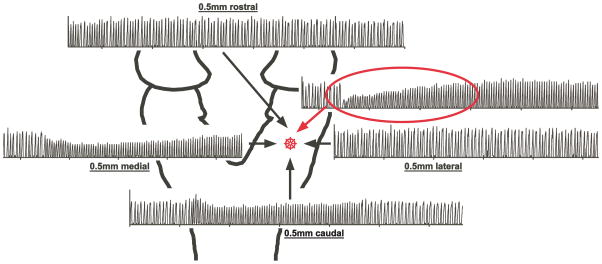
The preBC region was located according to three criteria: (1) Stereotaxic coordinates: Stereotaxic coordinates were available in the literature for adult rabbits.19,20 To establish the location in young rabbits we performed multiple preliminary experiments recording neuronal activity in a grid-wise fashion rostro-lateral of obex. The ventral respiratory column was identified by recording respiratory-related neuronal discharge activity. Within the ventral respiratory column the location of the preBC was determined using neuron types based on discharge patterns and DLH injection. We then injected pontamine blue dye into the preBC and verified the location histologically as immediately ventro-lateral to the nucleus ambiguous.7 On average, in young animals the preBC was located at 1.0 ± 0.5 mm rostral of obex, 2.2 ± 0.4 mm lateral of midline and 2.1 ± 0.2 mm ventral of the dorsal surface with part of the variance explained by differences in animal weight. (2) Mixture of inspiratory and expiratory neurons: The preBC is considered the part of the ventral respiratory column that contains a mixture of propriobulbar inspiratory and expiratory neuronal subtypes.21 This is in keeping with its presumptive role in respiratory rhythm generation.22 (3) Tachypneic response to injection of DLH: The tachypneic response to microinjection of DLH (fig. 1) has been accepted as a functional marker for the preBC by investigators using various animal models. 23,24 The tachypneic response to a glutamate agonist emphasizes the importance of this area in respiratory pattern generation. The preBC was defined as the area where DLH caused the largest increase in respiratory rate or, when the increase was identical in two neighboring areas, the area where the tachypneic effect lasted longest (fig. 1).
Figure 1.
Respiratory response to injection of D,L-homocysteic acid (DLH) into the brainstem. The preBötzinger Complex (star) was functionally identified as the area with recorded respiratory activity where DLH injection caused maximal tachypnea and had the fastest onset and longest effect as determined from the phrenic neurogram (red oval).
Protocol 1: Effects of pharmacological opioid concentrations on the preBC in young and adult rabbits
For all protocols, the experimenters were not blinded to the experimental conditions. Direct injection of high concentrations of the μ-opioid agonist DAMGO (100 μM) in small volumes into the preBC was performed to allow direct comparison with studies in other species (rodents, dogs) using this approach in in vitro and in vivo preparations. This protocol established the changes in respiratory pattern that could be achieved with maximal opioid receptor activation in the preBC. In addition, this protocol determined the drug volume required for maximal drug effect on the preBC. This was necessary to ensure that sufficient volumes of the local opioid antagonist naloxone were used in Protocol 2.
The preBC was identified on both sides of the brainstem as described in “Location of the preBC region”. To ensure that the entire preBC area was included and that a maximal effect of DAMGO on the respiratory pattern was achieved we microinjected DAMGO at the coordinate of maximal DLH effect as well as in the dorso-ventral center of respiratory activity 1mm rostral and 1mm caudal of this location on both sides of the brainstem. The injected volume was 70 nl per side in young animals. In adult animals, the initial injection was 140–210 nl per side and in those animals where no change in respiratory rate was observed an additional 140 nl was injected bilaterally. After the last injection the electrode was withdrawn and the tip cleaned. Then the opioid antagonist naloxone (140 nl in young, 350–700 nl in adults), dependent on the injected DAMGO volume) was injected at the same coordinates bilaterally. All injections were made in short sequence to avoid potential diffusion of the drugs into other areas. Ten minutes after localized naloxone injection, naloxone (30–80 mcg/kg) was given intravenously to completely reverse the DAMGO effect. To avoid confounding effects from previously injected drugs only one protocol was performed per animal.
Protocol 2: Effects of clinical opioid concentrations on the preBC in young and adult rabbits
Recognizing that pharmacological (i.e., supraclinical) concentrations of μ-opioid receptor agonists may have different effects on the individual components of the respiratory network than tissue concentrations achieved with clinically relevant drug doses, we infused the μ-opioid agonist remifentanil intravenously at “clinical relevant” dose-rates for rabbits. 25 We then determined whether the resulting respiratory depression could be antagonized by localized injections of naloxone into the preBC, i.e., whether the preBC was the site of the opioid effect.
The preBC was identified on both sides of the brainstem as described in “Location of the preBC region”. Then remifentanil was infused intravenously at 0.08–0.5 mcg/kg/min until peak phrenic amplitude and respiratory rate were depressed by approximately 50%. Remifentanil was chosen since its rapid metabolism by plasma and tissue esterases ensures quick onset of the maximal dose effect and a context-sensitive half-life that is short (~4 min 26) and independent of the duration of the infusion. 25,27 After reaching steady-state respiratory depression for at least 5 min, naloxone (140 nl in young, 350 nl in adults) was injected bilaterally at the coordinates of maximal DLH effect as well as in the dorso-ventral center of respiratory activity 1 mm rostral and 1 mm caudal of this location. This volume of naloxone was based on results from Protocol 1 where it had reliably reversed the effects of DAMGO injection. Approximately 10 min after the last localized naloxone injection, naloxone (30–80 mcg/kg) was given intravenously to completely reverse the remifentanil effect. Only then was the remifentanil infusion terminated. To avoid confounding residual drug effects only one protocol was performed per animal.
Protocol 3: Potential endogenous opioid effects on the preBC in young and adult rabbits
To rule out that any naloxone effect observed in Protocol 2 was due to a reversal of endogenous opioid activity in the preBC rather than to a reversal of the intravenous remifentanil effect, we injected naloxone (140 nl in young, 350 nl in adults) into the preBC as well as in the dorso-ventral center of respiratory activity 1 mm rostral and 1 mm caudal of this location. This protocol was performed in a separate set of animals without remifentanil infusion. Ten minutes after the last injection, naloxone (30–80 mcg/kg) was given intravenously to completely reverse any potential endogenous opioid effect.
Model to assess Non-preBC and preBötzinger Complex contributions to phase timing
We developed a hypothetical model of preBC and non-preBC inputs to the respiratory pattern that can account for the opioid-induced pattern changes observed in Protocols 1 and 2. The model assumes that phase timing, i.e., the duration of inspiratory and expiratory phase within the respiratory cycle depends on three factors: (1) intrinsic timing properties of the respiratory neuronal network generating an inspiratory (Tiø) and expiratory phase (Teø); (2) non-preBC input affecting the inspiratory (Finon) and expiratory (Fenon) phase, and (3) preBC input affecting the inspiratory (FipreBC) and expiratory (FepreBC) phase. These inputs can either increase or decrease the respective phase duration. The model further assumes that both, preBC and non-preBC inputs, are reduced by systemic opioid agonists (e.g., intravenous remifentanil (r)), and that the opioid effect on the preBC inputs is fully reversed by local injection of the opioid antagonist naloxone into the preBC. For details on the computation, please see appendix 1. Mean values for Ti and Te obtained from Protocol 2 were used to develop the model including determination of the polarity of the preBC and non-preBC inputs. The model was then used to calculate the preBC and non-preBC inputs for each individual animal. Pooled values are presented in the Results section.
Results
Protocol 1: Effects of pharmacological opioid concentrations on the preBC in young and adult rabbits
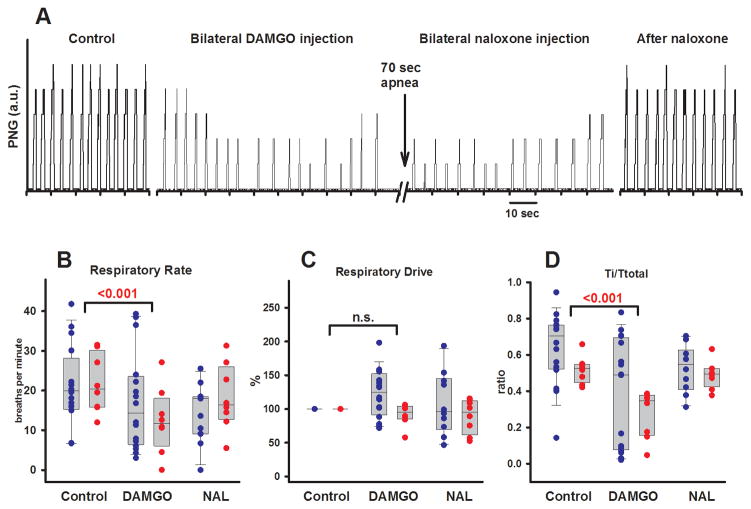
Eight protocols were completed in young rabbits (13–23 d, 200–500g). An example is presented in figure 2A. Univariate analysis of the effects of DAMGO injection into the preBC showed a significant depression of respiratory rate (−9.4 ± 5.7 breaths/min, p <0.001). This was due to an increase in Te (4.2 ± 4.9 s, p = 0.04) while Ti was unchanged (−0.1 (−0.4–0.15) s, p = 0.285). DAMGO injection increased the duration of the respiratory cycle (Ttot) by 3.6 ± 3.8 s (p = 0.03). The inspiratory fraction (Ti/Ttot) was reduced from 0.5 ± 0.1 to 0.3 ± 0.1 (p <0.001), and thus the expiratory fraction (Te/Ttot) was increased from 0.5 ± 1 to 0.7 ± 0.1 (p <0.001). PPA was not significantly changed (−2.0 ± 3.0 a.u., p = 0.074), and respiratory drive, calculated as PPA/Ti, was not affected (−8 ± 16%, p = 0.49). All DAMGO effects were reversed by local naloxone injection.
Figure 2.
Protocol 1. A: Phrenic neurogram of a young rabbit (20d, 325g). Bilateral [D-Ala2,N-Me-Phe4,Gly-ol]-enkephalin (DAMGO) microinjection (70 nl) into the preBötzinger Complex plus 1 mm rostral and 1 mm caudal to this area depressed peak phrenic amplitude and respiratory rate to the point of apnea. Local injection of naloxone (NAL) at the same coordinates reversed the DAMGO effect. B–D: Effects of localized microinjection of the μ-opioid agonist DAMGO in pharmacological concentrations (100 μM) into the bilateral preBötzinger Complex and reversal with localized injection of the opioid antagonist naloxone (1mM). Pooled data are shown separately for 16 adult (blue) and 8 young (red) rabbits. Box plots show median and range (10%, 25%, 75%, 90%); individual data points are superimposed. Levels of significance reflect the results of the 2-way analysis of variance. B: DAMGO injection depressed respiratory rate. C: Respiratory drive, calculated as PPA/Ti, was not affected. D: DAMGO injection depressed inspiratory fraction (Ti/Ttotal). The effects in young and adult animals were not significantly different. n.s. = not significant; PPA = peak phrenic activity; Ti = inspiratory duration; Ttotal = duration of the respiratory cycle.
Sixteen protocols were completed in adult rabbits (3.8–4.8 kg). Univariate analysis showed that DAMGO injection into the preBC caused respiratory responses with large variability: Overall, DAMGO did not change respiratory rate (−3.8 ± 7.8 breaths/min, p = 0.11). There was a significant increase in Te (1.1 (0.2–5.6) s, p <0.05), and Ti was significantly reduced (−0.65 (−0.24– −1.3) s, p <0.05). Overall respiratory cycle duration was unchanged (0.1 (−0.4–4.8) s, p = 0.48), but inspiratory fraction was decreased from 0.6 ± 0.2 to 0.4 ± 0.3 (p <0.001) and expiratory fraction was thus increased from 0.4 ± 0.2 to 0.6 ± 0.3 (p <0.001). Since respiratory drive was not significantly changed (23 ± 36%, p = 0.09), the decrease in Ti resulted in a decrease in PPA (−4.1 ± 6.6 a.u., p = 0.03). Data for Ti, Te, Ttot, and PPA as well as the results of the univariate analysis are not shown in figures.
The interaction of the DAMGO effect on the preBC and animal age was analyzed for respiratory rate, respiratory drive and inspiratory fraction using 2-way, repeated measures ANOVA. DAMGO injection significantly depressed respiratory rate by 6 ± 8 breaths/min (p = 0.001, fig. 2B), did not affect respiratory drive (−12 ± 33%, p = 0.09, fig. 2C), but decreased inspiratory fraction (−0.24 ± 0.2, p <0.001, fig. 2D). There were no differences between the DAMGO effects in young and adult rabbits. All DAMGO effects were reversed by local naloxone injection (fig. 2B–D). Local naloxone reversal was obtained in all animals except for the first six adult rabbits where no change in amplitude or respiratory rate was observed with DAMGO injection during the experiments. However, off-line analysis revealed changes in inspiratory and expiratory fraction irrespective of changes in respiratory rate. Thus, subsequently local naloxone reversal was performed in all animals and completely reversed the effects of DAMGO injection. Since the change in Ti and Te during the initial experiments did not differ from the subsequent experiments where no change in respiratory rate was observed, we included these data in our analysis.
Protocol 2: Effects of clinical opioid concentrations on the preBC in young and adult rabbits
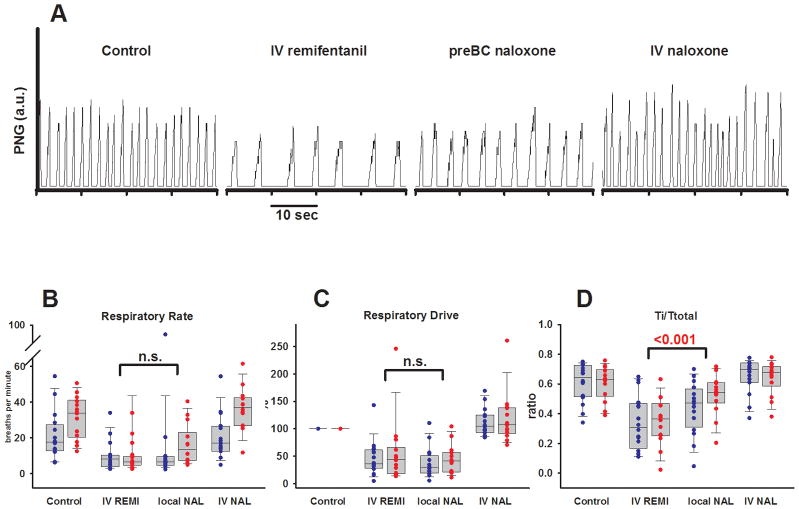
Fourteen protocols were completed in young rabbits (13–23 d, 110–550 g). An example is presented in figure 3A. Univariate analysis of the pooled data showed that intravenous remifentanil led to a significant decrease in respiratory rate (−18.3 ± 12.3 breaths/min, p <0.001), which was due to a large increase in Te (3.5 (1.1–5.7), p <0.05). Ti was not significantly changed (0.6 (−0.1–0.8) s, p = 0.06), resulting in a decrease in inspiratory fraction from 0.6 (0.5–0.7) to 0.4 (0.3–0.5) (p <0.05) and a corresponding increase in expiratory fraction from 0.4 (0.3–0.5) to 0.6 (0.5–0.7) (p <0.05). PPA was significantly decreased (−8 (−6 – −18) a.u., p <0.001) indicating a decrease in respiratory drive (−56 (−39– −81)%, p <0.001). Local injection of naloxone into the preBC led to a partial reversal of the Ti/Te ratio with an increase in Ti/Ttot and corresponding decrease in Te/Ttot by 0.18 ± 0.15 (p <0.05). This effect also resulted in a partial reversal (−300 ± 336%, p = 0.003) of the increase in normalized Te (+516 ± 434%, p <0.001). All remifentanil effects were completely reversed with intravenous naloxone, i.e., they were not different from control.
Figure 3.
Protocol 2. A: Phrenic neurogram (PNG, in arbitrary units) of a young rabbit (13d, 160g). Intravenous (IV) remifentanil depressed both peak phrenic amplitude and respiratory rate. Naloxone microinjection into the bilateral preBötzinger Complex (preBC) partially reversed the respiratory depression in this animal. IV naloxone achieved complete reversal of the remifentanil effect. B–D: Effects of intravenous remifentanil infusion (IV REMI), localized microinjection of the opioid antagonist naloxone (local NAL, 1mM) into the bilateral preBötzinger Complex and reversal of residual opioid effects with intravenous bolus of naloxone (IV NAL). Pooled data are shown separately for 16 adult (blue) and 14 young (red) rabbits. Box plots show median and range (10%, 25%, 75%, 90%); individual data points are superimposed. Levels of significance reflect the results of the 2-way analysis of variance. B: Local naloxone did not affect respiratory rate. C: Local naloxone did not affect respiratory drive, calculated as PPA/Ti. D: Local naloxone partially reversed the decrease in Ti/Ttotal. The effects in young and adult animals were not significantly different. n.s. = not significant; PPA = peak phrenic activity; Ti = inspiratory duration; Ttotal = duration of the respiratory cycle.
Sixteen protocols were completed in adult rabbits (3.6–5.6 kg). Univariate analysis of the pooled data showed that intravenous remifentanil significantly decreased respiratory rate (−10.3 (−7.2– −13.1) breaths/min, p <0.05) and PPA (−23 (−12– −36) a.u., p <0.05), and also led to a decrease in respiratory drive (−64 (−40– −71)%, p <0.05). These effects could not be antagonized by local naloxone injection but they were readily reversed by intravenous naloxone. Intravenous remifentanil did not significantly change Ti (0.5 (0–0.8) s, p = 0.36) but increased Te (3.8 (1.8–8.8) s, p <0.05). Remifentanil decreased the inspiratory fraction (−0.27 ± 0.19, p <0.001) and increased the expiratory fraction (0.27 ± 0.19, p <0.001), and this effect was partially reversed by local injection of naloxone into the preBC (0.1 ± 0.1, p = 0.01).
The interactions of drug effects and animal age were subsequently analyzed for respiratory rate, respiratory drive and inspiratory fraction using 2-way, repeated measures ANOVA (fig. 3B–D). Local naloxone injection did not change respiratory rate (−3 ± 16 breaths/min, p = 0.31) or respiratory drive (11 ± 44%, p = 0.22), but it significantly reversed the shortening of the inspiratory fraction (0.14 ± 0.14, p <0.001). There were no significant differences in drug effects between adult and young animals. These results suggest that in adult and young rabbits intravenous remifentanil causes changes in respiratory phase timing through direct action on the preBC. In addition, intravenous remifentanil has significant effects on areas outside the preBC, which ultimately mediate the depression of respiratory rate.
Protocol 3: Potential endogenous opioid effects on the preBC in young and adult rabbits
In three young and three adult rabbits we injected naloxone into the preBC at volumes identical to Protocols 1 and 2. Localized bilateral injection of naloxone into the preBC did not affect PPA (p = 0.262), respiratory rate (p = 0.07), Ti (p = 0.18), Te (p = 0.68) or the Ti/Te ratio (p = 0.43), neither in the pooled data nor in separate data for young and adult rabbits. This suggests that in decerebrate rabbits there is no endogenous opioidergic tone in the preBC under the conditions of the experiment.
Model to assess Non-preBC and preBötzinger Complex contributions to phase timing
The model was developed using mean values for Ti and Te obtained from Protocol 2 and is presented in figure 4. In short, the model proposes that under control conditions input from the preBC prolongs and input from non-preBC sources shortens inspiratory duration while both, preBC and non-preBC inputs shorten expiratory duration. Systemic (IV) remifentanil reduces preBC and non-preBC inputs, which causes an increase in inspiratory and expiratory duration with a resulting decrease in respiratory rate. Selective local reversal of the remifentanil effect with naloxone in the preBC restores the preBC input to Ti (FipreBC) and to Te (FepreBC), which leads to an additional increase in inspiratory duration and a decrease in expiratory duration resulting in no change in respiratory rate. Additional reversal of the systemic opioid effects on the non-preBC sources with systemic (IV) naloxone reduces inspiratory duration and expiratory duration and restores respiratory rate to control values.
Figure 4.
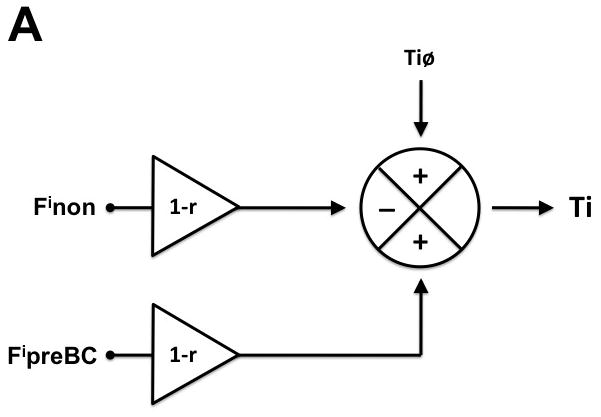
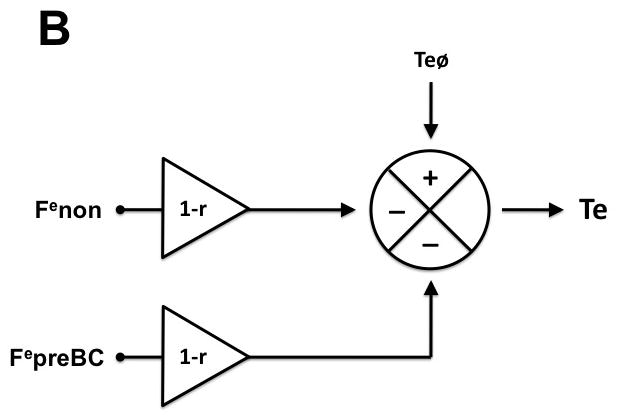
Hypothetical model of preBötzinger Complex (preBC) and non-preBC inputs to inspiratory and expiratory phase duration to explain the observed systemic opioid effects on respiratory phase duration. A: Intrinsic drive that maintains the inspiratory phase (Tiø) is modified by input from the preBC (FipreBC) as well as by input from outside the preBC (Finon) resulting in the observed inspiratory duration (Ti). Under control conditions, preBC input increases (+) and non-preBC input shortens (−) inspiratory duration. Intravenous (IV) remifentanil reduces both inputs by the factor (1-r) resulting in an increase in inspiratory duration. Local reversal of the remifentanil effect with naloxone in the preBC restores the preBC input to Ti (FipreBC), which leads to an additional increase in inspiratory duration. Additional reversal of the inhibition of non-preBC input (Finon) with IV naloxone reduces inspiratory duration to baseline. B: Intrinsic drive that maintains the expiratory phase (Teø) is modified by input from the preBC (FepreBC) as well as by input from outside the preBC (Fenon) resulting in the observed expiratory duration (Te). Under control conditions, both preBC input and non-preBC input shorten (−) expiratory duration. IV remifentanil reduces both inputs by the factor (1-r) resulting in an increase in expiratory duration. Local reversal of the remifentanil effect with naloxone in the preBC restores the preBC input to Te (FepreBC), which leads to a decrease in expiratory duration. Additional reversal of the inhibition of non-preBC input (Fenon) with IV naloxone reduces expiratory duration to baseline.
The pooled results for 16 adult and 14 young rabbits in Protocol 2 are presented in table 1. Statistical analysis showed no significant difference in the magnitude of non-preBC and preBC inputs to Ti and Te between adult and young rabbits. Of note, in adult animals the non-preBC input to Te was significantly larger than the preBC input (3.5 (−5.2 – 1.1), p = 0.04).
Table 1.
Pooled Data for preBC Complex and Non-preBC Inputs to the Inspiratory and Expiratory Phase in Young and Adult Rabbits
| Ti | p | Te | p | |||
|---|---|---|---|---|---|---|
| Fnon | FpreBC | Fnon | FpreBC | |||
| Adult | 2.9 (1.3–4.5) | 1.3 (0.2–4.1) | 0.57 | 7.0 (3.3–11.3) | 0.9 (−0.8–8.8) | 0.04 |
| Young | 1.3 (0.9–6.0) | 0.4 (−0.3–4.1) | 0.12 | 2.3 (1.3–5.2) | 3.2 (0.8–8.4) | 0.95 |
| p | 0.33 | 0.52 | 0.14 | 0.25 | ||
Computations used the equations outlined in appendix 1 and were based on the individual data for 16 adult and 14 young rabbits as obtained in Protocol 2; results are presented as median (25%–75%). Data for inspiratory (Ti) and expiratory duration (Te) were analyzed separately. Kruskal-Wallis analysis of variance on ranks with Dunn’s method for multiple comparisons was used to identify differences between inputs derived from outside the preBC (Fnon) and inputs derived from the preBC (FpreBC) in adult and young rabbits. In adult animals, inputs to Te derived from outside the preBC were significantly larger than inputs from the preBC.
preBC = preBötzinger.
Discussion
To our knowledge this is the first systematic attempt to compare the effects of systemic opioid administration resulting in clinically relevant effect site concentrations and the effects of local microinjection of pharmacological opioid doses on the preBC in young and adult animals of the same species in vivo. The results allow three important conclusions: (1) In young and adult rabbits, clinical opioid concentrations similarly affect inputs to respiratory phase timing that are mediated by the preBC. (2) In young and adult rabbits, systemic opioids also affect inputs to the respiratory rate and pattern generator that are derived from outside the preBC. (3) In adult rabbits, non-preBC input to expiratory phase duration is larger than the preBC input.
Extensive in vivo studies performing neuronal recordings with cross-correlation analysis22,28,29 and in situ studies performing repeat sectioning of the brainstem30 have delineated the Central Pattern Generator as a network of neurons that converts tonic excitatory chemodrive31,32 into a respiratory pattern with distinct inspiratory and expiratory phases. The Central Pattern Generator includes the parabrachial and Kölliker-Fuse nuclei in the pons and the preBC and Bötzinger Complex in the medulla oblongata. Prior studies in neonatal rodent models generally pointed to an important role of the preBC in opioid-induced respiratory depression,6,7 while adult in vivo studies found no reversal of the respiratory depression from clinical opioid concentrations in the preBC.5 However, the latter study did not evaluate for opioid-induced changes in relative phase duration, i.e., changes in the Ti/Te ratio. The present study provides evidence that both, preBC and non-preBC inputs to the Central Pattern Generator are depressed by systemic opioids at clinical concentrations. However, these inputs – and thus opioid-induced depression of these inputs - have separate and partly opposite effects on inspiratory and expiratory phase duration. Such differential effects were exposed when reversal of the opioid effect on preBC inputs changed the respiratory pattern but did not reverse the depression of respiratory rate (fig. 3). An area outside the preBC that mediates opioid effects on respiratory pattern may be located in the pons. In vivo studies in adult cats have described an opioid sensitive area in the pons33 where morphine injections decreased respiratory rate.34 Recently, an in vivo study in adult dogs demonstrated that injection of naloxone into the parabrachial nucleus reversed respiratory rate depression from intravenous remifentanil.4 Alternatively, Zhang et al. described in an adult in vivo rat preparation that injections of the opioid antagonist CTAP into the caudal medullary raphe partially reversed the decrease in respiratory rate.35 This suggests that respiratory chemoreceptor discharge may not only determine peak phrenic amplitude but also affect on respiratory rate. It will be important to establish whether these areas have similar relevance in the developmental rabbit model.
Our hypothetical model showed that non-preBC input to expiratory phase duration was larger than preBC input in adult but not in young rabbits, however, the age difference was not statistically significant (table 1). In Protocol 2, expiratory duration could be partially reversed with naloxone in the preBC in young rabbits (one-way ANOVA) but the difference between young and adult rabbits was not statistically significant (2-way repeated measure ANOVA). Greater importance of non-preBC inputs in adult animals has been postulated as the respiratory pattern changes from a neonatal, pacemaker driven pattern originating in the preBC to a more mature, network-generated pattern that depends on excitatory drive from the pons.36,37 This may also explain divergent results from neonatal rodent studies describing an important role of the preBC in opioid-induced respiratory depression6,7 and adult in vivo studies that located the effect in the pons.4 Our study was underpowered to detect subtle differences between the two age groups, however, it shows the importance to repeat these studies in other areas of the Central Pattern Generator.
Determining the dominant locations of opioid-induced respiratory depression may allow the development of drugs that specifically target the depressed areas and prevent this serious side effect. So far, studies have focused on substances that would stimulate the preBC, e.g., Ampakines,38 D1 agonists,39 5HT4A agonists40 and 5HT1A agonists41 though with limited success. Our data suggest that opioid-induced respiratory depression likely cannot be reversed by drug action targeted exclusively at a single area.
Methodological Considerations
Choice of species and age
The choice of rabbits allowed conducting identical protocols in young and adult animals of the same species with young animals large enough to allow for our complex instrumentation and differential drug injections. The technical difficulty limited us to using “young” rabbits between 2–3 weeks of age. This may explain the less pronounced respiratory depressant effect of opioids on the preBC we observed compared to neonatal rat preparations (≤7d)7 as well as the lack of difference between adult and young animals. However, rabbits are born at a rather premature stage. Immaturity of the ventilatory pattern, i.e., apnea in response to hypoxia, was observed in rabbits up to 22 days of age in our preparation (unpublished data describing the hypoxic ventilatory response, Astrid Stucke, M.D., Milwaukee, WI, 2008) and up to 6 weeks by Waites et al.42
Identification of the preBC
We used functional identification of the preBC and also injected DAMGO and naloxone at 1mm rostral and caudal of the presumed preBC location. Assuming spherical spread, a spread of ~ 450 μm in all directions would be expected for an injection volume of 70 nl, 540 μm for 140 nl and 740 μm for 350 nl.43 Injection beyond the borders of the preBC into closely adjacent areas of the ventral respiratory column should not have confounded the results, because the area caudal of the preBC does not mediate opioid-induced changes in respiratory pattern44 and injection of endomorphine-1 into the area rostral of the preBC, i.e., the Bötzinger Complex, affected the respiratory pattern similarly to injections into the preBC.45 DAMGO injection outside this area did not cause any changes in respiratory pattern (unpublished data describing DAMGO injection outside the “tachypneic area”, Astrid Stucke, M.D., Milwaukee, WI, 2011). Even if respiratory rate was unchanged, injections into the preBC changed the respiratory pattern in all animals suggesting that the injections were made in the correct area. Despite a major increase in body weight between 2 weeks and adulthood, brainstem dimensions increase by only ~60%, which made it reasonable to employ the same injection pattern in young and adult animals.
Sufficient injection volume
Volumes for DAMGO and naloxone injections were chosen to achieve maximal local effect without affecting chemoreception in adjacent areas.35 The effects of DAMGO injection and subsequent naloxone reversal were generally observed within a minute after each injection suggesting that the effects were truly localized. In those adult animals where DAMGO decreased respiratory rate the effect was achieved with the first injection (140–210 nl). In animals without effect on respiratory rate additional DAMGO injection (140 nl) did not change the result.
Statistical power to detect age differences
The large variability in the effects of DAMGO injection into the preBC in adult rabbits was not present in young rabbits. A power analysis for 2-way repeated measures ANOVA46 suggested that given the large variance approximately 40 young and 40 adult animals would be necessary to establish statistically significant differences, i.e., our study was likely underpowered to detect smaller age-dependent differences. The change in respiratory rate in adult rabbits ranged between −81% and +68%. Such variance has also been reported in urethane-anesthetized, spontaneously breathing adult rats where endomorphine-1 injection into the preBC caused changes in respiratory rate between −45% and +45%.45 The changes in adult animals were qualitatively different from the pattern observed in young animals and from the pattern observed with intravenous opioids in young and adult animals.5,9,39
Quantitative assessment of preBC and non-preBC inputs
Table 1 presents an estimate of the magnitude of preBC and non-preBC contributions to the duration of inspiratory and expiratory phase. Inputs were computed using values for the intrinsic timing properties Tiø and Teø derived for r = 0.5. However, the actual depression of respiratory rate in Protocol 2 varied between animals around the target of 50%. This would have resulted in a range of actual r-values and consequently in greater variability of the opioid effects on preBC and non-preBC inputs. The resulting variance in the pooled data likely reduced our ability to identify all but the largest differences. This does not diminish our finding that the non-preBC contribution to expiratory duration was larger than the preBC contribution and that this was specific for adult rabbits.
In conclusion, this is the first study to show that systemic opioids at clinically relevant concentrations affect respiratory phase timing and depress respiratory rate by actions on both, preBC and non-preBC inputs to the respiratory pattern generator. The magnitude of these effects was similar in young and adult rabbits. Non-preBC inputs to expiratory phase duration were larger than preBC inputs in adult rabbits.
Final Boxed Summary Statement.
What we already know about this topic
Opioid-induced respiratory depression is a critical but incompletely understood problem
The preBötzinger Complex (preBC) is an important regulator of respiratory rhythm
What this article tells us that is new
In an in vivo rabbit model the preBC partially mediates opioid effects on respiratory phase timing
The preBC does not mediate the opioid-induced depression of respiratory rate
Acknowledgments
Funding Sources: This work was supported by the Foundation for Anesthesia Education and Research, Schaumburg, Illinois (FAER-MRTG-BS-02-15-2010-Stucke); Dr. Zuperku is funded by the Department of Veterans Affairs, Washington, DC (VA merit grant 2 I01 BX000721-05).
The authors thank Dr. Aniko Szabo, Medical College of Wisconsin, for her help with the statistical analysis and Jack Tomlinson, Biological Laboratory Technician, for excellent technical assistance.
Appendix 1. Model to Assess preBC Complex and Non-preBC Contributions to Respiratory Phase Timing
Data from Protocol 2 provide values for inspiratory (Ti) and expiratory duration (Te), which can be used to assess the relative contributions of the preBC and areas outside the preBötzinger Complex (preBC) to phase duration as far as they are affected by opioids. We assume that during control conditions (Ticon, Tecon) the remifentanil effect is “0”, during systemic remifentanil infusion (Tiremi, Teremi) the remifentanil effect is “r”, and during local naloxone injection into the preBC during systemic remifentanil infusion (TiremiN, TeremiN) the remifentanil effect is “r” for non-preBC inputs and “0” for preBC inputs because of the local opioid antagonism.
Inspiratory phase duration (Ti) can be calculated according to
| E0 |
For the individual conditions, this yields the following relationships
| E1 |
| E2 |
| E3 |
Using the values for Ticon, Tiremi and TiremiN obtained in each individual animal we performed repeat computation with various assumed values for Tiø until r=0.5, i.e., a 50% reduction of all inputs by remifentanil. The r-value was chosen to match the ~50% decrease in respiratory rate that was targeted in Protocol 2. This yielded values for Finon, FipreBC and Tiø for each individual animal. Pooled data from all animals indicated a negative value for the factor Finon * (1-r), i.e., non-preBC input shortened inspiratory duration (fig. 4A).
Like computations were performed for expiratory phase duration (Te) using individual values for Te resulting from Protocol 2. Repeat computation showed that both, pontine and medullary inputs shortened Te (fig. 4B).
| E4 |
The resulting pooled values for Finon, FipreBC, Fenon, and FepreBC for young and adult animals are presented in table 1.
Finon: inputs to Ti from outside the preBC; FipreBC: inputs to Ti from the preBC; Fenon: inputs to Te from outside the preBC; FepreBC: inputs to Te from the preBC; Teø: intrinsic expiratory duration; Tiø: intrinsic inspiratory duration.
Footnotes
The work was presented in part at the American Society of Anesthesiologists Annual Meeting (October 15, 2012, Washington, DC) and the Annual Meeting of the Association of University Anesthesiologists (April 5, 2013, Miami, Florida)
The authors declare no competing interests.
References
- 1.Dahan A, Aarts L, Smith TW. Incidence, reversal, and prevention of opioid-induced respiratory depression. Anesthesiology. 2010;112:226–38. doi: 10.1097/ALN.0b013e3181c38c25. [DOI] [PubMed] [Google Scholar]
- 2.Hanna MH, Elliott KM, Fung M. Randomized, double-blind study of the analgesic efficacy of morphine-6-glucuronide versus morphine sulfate for postoperative pain in major surgery. Anesthesiology. 2005;102:815–21. doi: 10.1097/00000542-200504000-00018. [DOI] [PubMed] [Google Scholar]
- 3.McRorie TI, Lynn AM, Nespeca MK, Opheim KE, Slattery JT. The maturation of morphine clearance and metabolism. Am J Dis Child. 1992;146:972–6. doi: 10.1001/archpedi.1992.02160200094036. [DOI] [PubMed] [Google Scholar]
- 4.Prkic I, Mustapic S, Radocaj T, Stucke AG, Stuth EA, Hopp FA, Dean C, Zuperku EJ. Pontine mu-opioid receptors mediate bradypnea caused by intravenous remifentanil infusions at clinically relevant concentrations in dogs. J Neurophysiol. 2012;108:2430–41. doi: 10.1152/jn.00185.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mustapic S, Radocaj T, Sanchez A, Dogas Z, Stucke AG, Hopp FA, Stuth EA, Zuperku EJ. Clinically relevant infusion rates of mu-opioid agonist remifentanil cause bradypnea in decerebrate dogs but not via direct effects in the pre-Botzinger complex region. J Neurophysiol. 2010;103:409–18. doi: 10.1152/jn.00188.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mellen NM, Janczewski WA, Bocchiaro CM, Feldman JL. Opioid-induced quantal slowing reveals dual networks for respiratory rhythm generation. Neuron. 2003;37:821–6. doi: 10.1016/s0896-6273(03)00092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBötzinger complex. Science. 1999;286:1566–8. doi: 10.1126/science.286.5444.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires preBötzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci. 2001;4:1–4. doi: 10.1038/nn0901-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montandon G, Qin W, Liu H, Ren J, Greer JJ, Horner RL. PreBotzinger complex neurokinin-1 receptor-expressing neurons mediate opioid-induced respiratory depression. J Neurosci. 2011;31:1292–301. doi: 10.1523/JNEUROSCI.4611-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michelsen LG, Salmenpera M, Hug CC, Jr, Szlam F, VanderMeer D. Anesthetic potency of remifentanil in dogs. Anesthesiology. 1996;84:865–72. doi: 10.1097/00000542-199604000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Lalley PM, Pilowsky PM, Forster HV, Zuperku EJ. Rebuttal from Peter M. Lalley, Paul M. Pilowsky, Hubert V. Forster and Edward J. Zuperku. J Physiol. 2014;592:1169. doi: 10.1113/jphysiol.2013.268318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lalley PM, Pilowsky PM, Forster HV, Zuperku EJ. CrossTalk opposing view: The pre-Botzinger complex is not essential for respiratory depression following systemic administration of opioid analgesics. J Physiol. 2014;592:1163–6. doi: 10.1113/jphysiol.2013.258830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montandon G, Horner R. Rebuttal from Gaspard Montandon and Richard Horner. J Physiol. 2014;592:1167. doi: 10.1113/jphysiol.2013.268300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montandon G, Horner R. CrossTalk proposal: The preBotzinger complex is essential for the respiratory depression following systemic administration of opioid analgesics. J Physiol. 2014;592:1159–62. doi: 10.1113/jphysiol.2013.261974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dogas Z, Krolo M, Stuth EA, Tonkovic-Capin M, Hopp FA, McCrimmon DR, Zuperku EJ. Differential effects of GABAA receptor antagonists in the control of respiratory neuronal discharge patterns. J Neurophysiol. 1998;80:2368–77. doi: 10.1152/jn.1998.80.5.2368. [DOI] [PubMed] [Google Scholar]
- 16.Krolo M, Stuth EA, Tonkovic-Capin M, Dogas Z, Hopp FA, McCrimmon DR, Zuperku EJ. Differential roles of ionotropic glutamate receptors in canine medullary inspiratory neurons of the ventral respiratory group. J Neurophysiol. 1999;82:60–8. doi: 10.1152/jn.1999.82.1.60. [DOI] [PubMed] [Google Scholar]
- 17.Eldridge FL. Quantification of electrical activity in the phrenic nerve in the study of ventilatory control. Chest. 1976;70:154–7. doi: 10.1378/chest.70.1_supplement.154. [DOI] [PubMed] [Google Scholar]
- 18.Wood J, Freemantle N, King M, Nazareth I. Trap of trends to statistical significance: Likelihood of near significant P value becoming more significant with extra data. BMJ. 2014;348:g2215. doi: 10.1136/bmj.g2215. [DOI] [PubMed] [Google Scholar]
- 19.Mutolo D, Bongianni F, Carfi M, Pantaleo T. Respiratory responses to thyrotropin-releasing hormone microinjected into the rabbit medulla oblongata. Am J Physiol. 1999;277:R1331–8. doi: 10.1152/ajpregu.1999.277.5.R1331. [DOI] [PubMed] [Google Scholar]
- 20.Bongianni F, Mutolo D, Cinelli E, Pantaleo T. Neurokinin receptor modulation of respiratory activity in the rabbit. Eur J Neurosci. 2008;27:3233–43. doi: 10.1111/j.1460-9568.2008.06295.x. [DOI] [PubMed] [Google Scholar]
- 21.Chitravanshi VC, Sapru HN. Phrenic nerve responses to chemical stimulation of the subregions of ventral medullary respiratory neuronal group in the rat. Brain Res. 1999;821:443–60. doi: 10.1016/s0006-8993(99)01139-7. [DOI] [PubMed] [Google Scholar]
- 22.Rybak IA, O’Connor R, Ross A, Shevtsova NA, Nuding SC, Segers LS, Shannon R, Dick TE, Dunin-Barkowski WL, Orem JM, Solomon IC, Morris KF, Lindsey BG. Reconfiguration of the pontomedullary respiratory network: A computational modeling study with coordinated in vivo experiments. J Neurophysiol. 2008;100:1770–99. doi: 10.1152/jn.90416.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krolo M, Tonkovic-Capin V, Stucke A, Stuth E, Hopp F, Dean C, Zuperku E. Subtype composition and responses of respiratory neurons in the pre-Botzinger region to pulmonary afferent inputs in dogs. J Neurophysiol. 2005;93:2674–87. doi: 10.1152/jn.01206.2003. [DOI] [PubMed] [Google Scholar]
- 24.Solomon IC, Edelman NH, Neubauer JA. Patterns of phrenic motor output evoked by chemical stimulation of neurons located in the pre-Bötzinger complex in vivo. J Neurophysiol. 1999;81:1150–61. doi: 10.1152/jn.1999.81.3.1150. [DOI] [PubMed] [Google Scholar]
- 25.Ma D, Chakrabarti MK, Whitwam JG. The combined effects of sevoflurane and remifentanil on central respiratory activity and nociceptive cardiovascular responses in anesthetized rabbits. Anesth Analg. 1999;89:453–61. [PubMed] [Google Scholar]
- 26.Burkle H, Dunbar S, Van Aken H. Remifentanil: A novel, short-acting, mu-opioid. Anesth Analg. 1996;83:646–51. doi: 10.1097/00000539-199609000-00038. [DOI] [PubMed] [Google Scholar]
- 27.Michelsen LG, Hug CC., Jr The pharmacokinetics of remifentanil. J Clin Anesth. 1996;8:679–82. doi: 10.1016/s0952-8180(96)00179-1. [DOI] [PubMed] [Google Scholar]
- 28.Segers LS, Nuding SC, Dick TE, Shannon R, Baekey DM, Solomon IC, Morris KF, Lindsey BG. Functional connectivity in the pontomedullary respiratory network. J Neurophysiol. 2008;100:1749–69. doi: 10.1152/jn.90414.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ezure K, Tanaka I. Distribution and medullary projection of respiratory neurons in the dorsolateral pons of the rat. Neuroscience. 2006;141:1011–23. doi: 10.1016/j.neuroscience.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 30.Smith JC, Abdala AP, Koizumi H, Rybak IA, Paton JF. Spatial and functional architecture of the mammalian brain stem respiratory network: A hierarchy of three oscillatory mechanisms. J Neurophysiol. 2007;98:3370–87. doi: 10.1152/jn.00985.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nattie E, Julius H. Comroe, Jr. distinguished lecture: Central chemoreception: Then … and now. J Appl Physiol (1985) 2011;110:1–8. doi: 10.1152/japplphysiol.01061.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guyenet PG. The 2008 Carl Ludwig Lecture: Retrotrapezoid nucleus, CO2 homeostasis, and breathing automaticity. J Appl Physiol. 2008;105:404–16. doi: 10.1152/japplphysiol.90452.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hurle MA, Mediavilla A, Florez J. Differential respiratory patterns induced by opioids applied to the ventral medullary and dorsal pontine surfaces of cats. Neuropharmacology. 1985;24:597–606. doi: 10.1016/0028-3908(85)90100-5. [DOI] [PubMed] [Google Scholar]
- 34.Eguchi K, Tadaki E, Simbulan D, Jr, Kumazawa T. Respiratory depression caused by either morphine microinjection or repetitive electrical stimulation in the region of the nucleus parabrachialis of cats. Pflugers Arch. 1987;409:367–73. doi: 10.1007/BF00583790. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Z, Xu F, Zhang C, Liang X. Activation of opioid μ receptors in caudal medullary raphe region inhibits the ventilatory response to hypercapnia in anesthetized rats. Anesthesiology. 2007;107:288–97. doi: 10.1097/01.anes.0000270760.46821.67. [DOI] [PubMed] [Google Scholar]
- 36.Funk GD, Feldman JL. Generation of respiratory rhythm and pattern in mammals: Insights from developmental studies. Curr Opin Neurobiol. 1995;5:778–85. doi: 10.1016/0959-4388(95)80106-5. [DOI] [PubMed] [Google Scholar]
- 37.Richter DW, Ballanyi K, Schwarzacher S. Mechanisms of respiratory rhythm generation. Curr Opin Neurobiol. 1992;2:788–93. doi: 10.1016/0959-4388(92)90135-8. [DOI] [PubMed] [Google Scholar]
- 38.Ren J, Poon BY, Tang Y, Funk GD, Greer JJ. Ampakines alleviate respiratory depression in rats. Am J Respir Crit Care Med. 2006;174:1384–91. doi: 10.1164/rccm.200606-778OC. [DOI] [PubMed] [Google Scholar]
- 39.Lalley PM. Opiate slowing of feline respiratory rhythm and effects on putative medullary phase-regulating neurons. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1387–96. doi: 10.1152/ajpregu.00530.2005. [DOI] [PubMed] [Google Scholar]
- 40.Manzke T, Guenther U, Ponimaskin EG, Haller M, Dutschmann M, Schwarzacher S, Richter DW. 5-HT4(a) receptors avert opioid-induced breathing depression without loss of analgesia. Science. 2003;301:226–9. doi: 10.1126/science.1084674. [DOI] [PubMed] [Google Scholar]
- 41.Manzke T, Dutschmann M, Schlaf G, Morschel M, Koch UR, Ponimaskin E, Bidon O, Lalley PM, Richter DW. Serotonin targets inhibitory synapses to induce modulation of network functions. Philos Trans R Soc Lond B Biol Sci. 2009;364:2589–602. doi: 10.1098/rstb.2009.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waites BA, Ackland GL, Noble R, Hanson MA. Red nucleus lesions abolish the biphasic respiratory response to isocapnic hypoxia in decerebrate young rabbits. J Physiol. 1996;495(Pt 1):217–25. doi: 10.1113/jphysiol.1996.sp021586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nicholson C. Diffusion from an injected volume of a substance in the brain tissue with arbitrary volume fraction and tortuosity. Brain Res. 1985;333:325–9. doi: 10.1016/0006-8993(85)91586-0. [DOI] [PubMed] [Google Scholar]
- 44.Stucke AG, Zuperku EJ, Sanchez A, Tonkovic-Capin M, Tonkovic-Capin V, Mustapic S, Stuth EA. Opioid receptors on bulbospinal respiratory neurons are not activated during neuronal depression by clinically relevant opioid concentrations. J Neurophysiol. 2008;100:2878–88. doi: 10.1152/jn.90620.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lonergan T, Goodchild AK, Christie MJ, Pilowsky PM. Mu opioid receptors in rat ventral medulla: Effects of endomorphin-1 on phrenic nerve activity. Respir Physiol Neurobiol. 2003;138:165–78. doi: 10.1016/s1569-9048(03)00173-3. [DOI] [PubMed] [Google Scholar]
- 46.Day SJ, Graham DF. Sample size and power for comparing two or more treatment groups in clinical trials. BMJ. 1989;299:663–5. doi: 10.1136/bmj.299.6700.663. [DOI] [PMC free article] [PubMed] [Google Scholar]