Abstract
Background and Aims Recent global changes, particularly warming and drought, have had worldwide repercussions on the timing of flowering events for many plant species. Phenological shifts have also been reported in alpine environments, where short growing seasons and low temperatures make reproduction particularly challenging, requiring fine-tuning to environmental cues. However, it remains unclear if species from such habitats, with their specific adaptations, harbour the same potential for phenological plasticity as species from less demanding habitats.
Methods Fourteen congeneric species pairs originating from mid and high elevation were reciprocally transplanted to common gardens at 1050 and 2000 m a.s.l. that mimic prospective climates and natural field conditions. A drought treatment was implemented to assess the combined effects of temperature and precipitation changes on the onset and duration of reproductive phenophases. A phenotypic plasticity index was calculated to evaluate if mid- and high-elevation species harbour the same potential for plasticity in reproductive phenology.
Key Results Transplantations resulted in considerable shifts in reproductive phenology, with highly advanced initiation and shortened phenophases at the lower (and warmer) site for both mid- and high-elevation species. Drought stress amplified these responses and induced even further advances and shortening of phenophases, a response consistent with an ‘escape strategy’. The observed phenological shifts were generally smaller in number of days for high-elevation species and resulted in a smaller phenotypic plasticity index, relative to their mid-elevation congeners.
Conclusions While mid- and high-elevation species seem to adequately shift their reproductive phenology to track ongoing climate changes, high-elevation species were less capable of doing so and appeared more genetically constrained to their specific adaptations to an extreme environment (i.e. a short, cold growing season).
Keywords: Climate change, flowering phenology, phenotypic plasticity, global warming, drought, common garden, mid-elevation and high-elevation species, Swiss Alps
INTRODUCTION
In seasonal climates, the timing of flowering is crucial for plant reproductive success. Premature or late flowering can expose plants to adverse environmental conditions such as frost events (Inouye, 2008), can disrupt plant–pollinator interactions (Memmott et al., 2007) and can lead to failures in seed set or maturation. The timing of seasonal activities in plants has thus evolved to be triggered by reliable environmental cues such as date of snowmelt, photoperiod, temperature or soil moisture to guarantee reproductive success (Rathcke and Lacey, 1985). Recent global change has led to increased temperatures and to more frequent and more extreme floods and droughts in some areas (Hartmann et al., 2013) with repercussions on these environmental cues. Shifts in phenological events have been used as ‘fingerprints’ of ongoing climate change (Walther et al., 2002; Jentsch et al., 2009) and are well documented in numerous global-scale studies (Parmesan and Yohe, 2003; Peñuelas et al., 2004; Menzel et al., 2006; Cleland et al., 2007).
Phenotypic plasticity may play a crucial role in the short-term adjustment to novel conditions and can promote long-term adaptive evolution by buffering against rapid change (Price et al., 2003; Nicotra et al., 2010; Richter et al., 2012). Although a potential for rapid adaptive evolution in flowering phenology has been found (Franks et al., 2007; Haggerty and Galloway, 2011; Anderson et al., 2012) it remains unclear if natural selection can keep pace with the speed of ongoing changes (Visser, 2008; Shaw and Etterson, 2012). Alternatively, numerous plastic adjustments to current climate change such as advanced and accelerated phenophases in response to earlier snowmelt and spring warming have been documented worldwide (Abu-Asab et al., 2001; Fitter and Fitter, 2002; Cleland et al., 2007; Vitasse et al., 2013).
In Europe, springtime has advanced by 2·5 d per decade since the 1970s and delayed autumn events have led to an extension of the annual growing season (Menzel et al., 2006). Longer and warmer growing seasons could be associated with enhanced plant growth (Hudson et al., 2011), although limiting factors such as reduced water availability in summer could have negative effects. Indeed, summers in Switzerland have become drier over the past 30 years (Beniston et al., 1994; Kovats et al., 2014), and drought stress is known to influence plant growth, performance and reproductive success (Levitt, 1980) and is likely to also affect plant phenology (Peñuelas et al., 2004). While some studies report on advanced flowering dates in response to drought (Jentsch et al., 2009; Bernal et al., 2011; Franks, 2011) others found delayed flowering (Llorens and Peñuelas, 2005). Phenological responses to drought appear to be highly species specific (Bernal et al., 2011) as well as dependent upon the specific ecosystem (Peñuelas et al., 2004), and to follow complex spatiotemporal patterns (Peñuelas et al., 2004). Furthermore, little is known about the combined effect of warming and drought on flowering phenology (Dunne et al., 2003; Bloor et al., 2010).
In the Swiss Alps, the increase in temperature has been shown to be twice as high as that reported globally (Beniston et al., 1994), and summer droughts are predicted to become more frequent (Beniston et al., 1997; Kovats et al., 2014) making mountain biota in this region particularly exposed to climate change (Theurillat and Guisan, 2001; Körner, 2003). For alpine plants, reproduction is especially challenging and the timing of flowering even more central to reproductive success as the timeframe for growth and reproduction becomes progressively shorter with increasing elevation (Billings and Mooney, 1968; Körner, 2003). Few studies have examined the effect of drought on the phenology of alpine vegetation and generally found no shifts (Bloor et al., 2010; Cornelius et al., 2013). However, advanced flowering was found when plants were grown in warmer conditions (Scheepens and Stöcklin, 2013; Frei et al., 2014a), and other studies with similar findings debated whether phenological shifts were triggered by higher air temperatures or advanced snowmelt (Price and Waser, 1998; Dunne et al., 2003; Cornelius et al., 2013).
Furthermore, photoperiod plays a key role in protecting plants from hazardous sprouting before the typical last date of severe spring frosts. Keller and Körner (2003) found that half of 23 study species were highly sensitive to photoperiod, and a later publication from Basler and Koerner (2012) specified that particularly late-successional species are photoperiod sensitive, and may not react to periods of earlier snowmelt or higher temperatures. This high level of adaptation to the particular alpine conditions raises the question of whether high-elevation species harbour the same potential for phenological plasticity as mid-elevation species. As high-elevation species are adapted to short growing seasons and have evolved to avoid frost damage, the onset of flowering phenology is likely to be genetically fixed (Keller and Körner, 2003), constraining their capacity to respond plastically to changes in external conditions. While Vitasse et al. (2013) found lower phenological plasticity in high-elevation deciduous tree species, a reciprocal transplant experiment with three grassland species revealed no difference in plasticity between low- and high-elevation populations (Frei et al., 2014a). However, to our knowledge no study has examined if mid- and high-elevation herbaceous species harbour the same potential for phenotypic plasticity in flowering phenology on a larger scale.
To examine how the combined effects of warming and drought affect the flowering phenology of mid- and high-elevation species as well as to examine whether phenotypic plasticity in flowering phenology differs between species origin, we reciprocally transplanted 14 congeneric pairs of herbaceous perennial mid- and high-elevation species between common gardens at 1050 and 2000 m a.s.l. Rain-shelters were used at each site to control the water input to our system to mimic severe drought events in summer. The study examined whether transplantation and drought events induced shifts in the flowering phenology of mid- and high-elevation species. Specifically, we tested the following expectations: (1) earlier onsets and expanded durations of phenophases at the lower (warmer) site taking advantage of a longer growing season, (2) delayed and shortened durations at the high-elevation site in accordance with later snowmelt and a shorter growing season, (3) earlier onsets and shortened durations of phenological stages in response to drought which acts to shorten the growing season, and (4) a lower phenological plasticity in high-elevation species, stemming from putative constrained adaptations to cold environments.
MATERIALS AND METHODS
Common gardens and study species
Two common gardens (Supplementary Data Fig. S1) were established in the Bernese Highlands in Switzerland, each accommodating four beddings delimited by a wooden frame (1 × 3 m). The high-elevation common garden is situated on the Schynige Platte (46°39′03·63″N, 7°54′32·76″E) at 2000 m a.s.l. on a southern slope. The snow-free period generally starts in June and lasts until October (approx. 150 d). The average annual temperature is 1 °C and the average annual amount of precipitation is approx. 1600–2000 mm, of which half falls as snow (MeteoSwiss, 2014). The lower elevation common garden is situated in Zweilütschinen (46°38′26·55″N, 7°54′15·20″E). This was at 1050 m a.s.l. with a south/south-western slope. The snow-free period usually lasts from mid-April to December (approx. 250 d). The average annual temperature is 7·2 °C and average annual precipitation is approx. 1100 mm, of which a quarter falls as snow (MeteoSwiss, 2014).
Twenty-eight perennial herbaceous species were included in this study, represented by 14 congeneric pairs of mid- and high-elevation species (Table 1). The species pairs were selected to cover a broad range of taxonomic groups and growth forms while avoiding an overlap in their altitudinal range of distribution. The ranges of mid-elevation species lie between approx. 300 and 1000 m.a.s.l, while the ranges of high-elevation species are mostly between approx. 1600 and 2400 m.a.s.l. (Table 1; Lauber and Wagner, 2001; Aeschimann et al., 2004). Seeds collected from flowers from wild populations were purchased from Swiss seed producers (Samen & Pflanzen AG Schutz, Filisur; UFA-Samen, fenaco Genossenschaft, Winterthur; Wildstaudengärtnerei, Eschenbach).
Table 1.
Overview of the congeneric pairs of mid- and high-elevation species included in our study with their main range limits in the literature having only been given in terms of altitudinal zonations as defined for the European Alps by Lauber and Wagner (2001) and Aeschiman et al. (2004): ‘colline’ = 300–900 m; ‘montane’ = 900–1500 m; ‘subalpine’ = 1600–2300 m; ‘alpine’ = 2300–3000 m; ‘mid-elevation’ species mainly ranged from the colline to the lower montane zones, while ‘high-elevation’ species mainly ranged from the subalpine to the alpine zones
| Family | Mid-elevation species | High-elevation species |
|---|---|---|
| Lamiaceae | Acinos arvensis (Lam.) Dandy | Acinos alpinus (L.) Moench |
| colline–montane | subalpine | |
| Poaceae | Anthoxanthum odoratum L. | Anthoxanthum alpinum Löve |
| colline–alpine | subalpine–alpine | |
| Fabaceae | Anthyllis vulneraria ssp. vulneraria L. s.l. | Anthyllis vulneraria ssp. alpéstris Schult |
| colline–montane | subalpine–alpine | |
| Brassicaceae | Arabis hirsuta L. | Arabis alpina L. s.l. |
| colline–montane | montane–alpine | |
| Campanulaceae | Campanula rotundifolia L. | Campanula scheuchzeri Vill. |
| Colline–subalpine | subalpine–alpine | |
| Asteraceae | Centaurea scabiosa L. s.l. | Centaurea montana L. |
| colline–montane | montane–subalpine | |
| Caryophyllaceae | Dianthus deltoides L. | Dianthus sylvestris Wulfen |
| colline–montane | colline–subalpine | |
| Rosaceae | Geum urbanum L. | Geum montanum L. |
| colline–montane | subalpine–alpine | |
| Fabaceae | Lotus corniculatus L. | Lotus alpinus Ramond |
| colline–subalpine | alpine | |
| Fabaceae | Onobrychis viccifolia Scop. | Onobrychis montana DC. |
| colline–montane | subalpine | |
| Poaceae | Phleum phleoides (L.) Karsten | Phleum alpinum L. |
| colline–montane | subalpine–alpine | |
| Plantaginaceae | Plantago lanceolata L. | Plantago alpina L. |
| colline–subalpine | subalpine–alpine | |
| Caryophyllaceae | Silene vulgaris ssp. vulgaris (Moench) Garcke s.l. | Silene vulgaris ssp. glareosa (Jord.) Marsd.-Jon & Turill |
| colline–subalpine | alpine | |
| Fabaceae | Trifolium pratense ssp. pratense L. | Trifolium pratense ssp. nivale (Koch) |
| colline–subalpine | alpine |
Experimental design
In spring 2012, seeds were germinated on moist blotting paper in the glasshouse of the Botanical Institute in Basel, Switzerland. Seedlings were individually transferred into multitrays (4 cm diameter, 6 × 9 = 54 pots) filled with low-nutrient soil (Anzuchterde Ökohum, Herrenhof, Switzerland). In mid June, plants were brought outside in the garden of the Botanical Institute to allow acclimation to outdoor conditions. At the beginning of July, plants were transported to the common gardens and transplanted into bigger pots (11·5 × 11·5 × 21·5 cm) filled with the same potting soil. At each site, 12 individuals of each species were randomized in the beddings previously enriched with potting soil and sunk to one-third depth into the soil. This design was systematically replicated in the beddings receiving rain-shelters, resulting in an experiment including a total of 1344 individuals across both sites and treatments (12 replicates × 2 sites × 2 treatments × 28 species = 1344 individuals; Fig. S1). The rain-shelters were installed after a week of acclimation and consisted of a triangular aluminium frame covered by an UV-B-transmissible greenhouse film (Luminance AF Window, Folitec, Germany) with a base area of 2·4 × 3·0 m and a height of 1·2 m. The tunnel shape with large openings allowed for constant wind flow preventing warming beneath the shelters. To minimize edge effects, the sheltered base was larger than the central 1 × 2·5 -m area occupied by plants. To avoid lethal consequences of the drought treatment, a minimal water input was provided. Twenty litres of rainwater was distributed per bedding every 2 weeks (approx. 0·12 L per individual). Accordingly, the difference in water availability between the beddings with and without rain-shelter equals the amount of precipitation. At the end of the first growing season, rain-shelters were removed and plants overwintered under snow.
In Spring 2013, rain-shelters were reinstalled right after snowmelt (early May at the low common garden and mid-June at the high common garden) initiating the start of phenological recordings (plants did not reproduce in the first year). Air temperature was recorded hourly in each common garden and treatment at 0·5 m above the ground using sheltered data loggers (TidBit v.2 UTBI-001; Onset Computer Corp., Bourne, MA, USA). Similarly, light intensity loggers (Hobo pendant light data logger 64 K-UA-002-64; Onset Computer) were installed in each common garden at 1 m above the ground in both treatments. The drought treatment consisted of a minimal water input as in the previous year. Once a month, the volumetric soil moisture content (VSCM; m3 m–3) was measured randomly in 30 pots of each bedding with an HH2 Moisture Meter and a Theta Probe type ML2x (Delta-T Devices, Cambridge, UK).
Abiotic treatment effect
Averaged over the experimental period (May–October, Table 2), at the mid-elevation common garden, the daily temperature was 15·5 °C in control beddings and 15·9 °C in beddings topped by rain-shelters. In the high-elevation common garden, the average daily temperature was 11·2 °C in control beddings and 11·4 °C in beddings topped by rain-shelters. While there was a significant temperature difference between both common gardens, the rain-shelters increased the temperature at ground level only marginally by 0·25 °C.
Table 2.
Mean temperature, light intensity and volumetric soil moisture content (VSMC) for each treatment averaged over the experimental period (May–September)
| Temperature (°C) | Light intensity (lux) | VSMC (m3 m–3) | |
|---|---|---|---|
| Low site/Control | 15·5 | 115 323·5 | 0·4 |
| Low site/Dry | 15·9 | 84 554·8 | 0·06 |
| High site/Control | 11·2 | 139 846·9 | 0·48 |
| High site/Dry | 11·4 | 101 209·8 | 0·08 |
The recorded light intensity (measured in lux at 13:00 h) was higher at the high-elevation common garden and was significantly reduced by rain-shelters (Table 2). At both common gardens, the rain-shelters intercepted approx. 30 % of light but these values were not limiting for plant growth (see fig. 11·11 in Körner, 2003).
VSMC (Table 2) differed significantly between the control and the drought treatment in both the common gardens (W = 900, P = 10–4; W = 844·5, P = 10–4, respectively). At the mid-elevation site, the average VSMC of control pots equalled 0·40 ± 0·08 m3 m–3, while dry pots had a VSMC of 0·06 ± 0·02 m3 m–3. At the high-elevation site, control pots had an average VSMC of 0·48 ± 0·1 m3 m–3, while dry pots had an average VSMC of 0·08 ± 0·02 m3 m–3.
Phenology monitoring
Phenological stages were defined after Price and Waser (1998) and Dunne et al. (2003). Different stages were used for forbs and grasses to account for their morphological differences. Seven stages were defined for forbs: unopened buds, opened buds, opened flowers, old flowers, initiated fruits, enlarged fruits and dehisced fruit. For grasses, five stages were defined: beginning of heading, end of heading, exerted anthers or styles, dried and broken-off anthers/styles, and disarticulated seeds.
All observed stages were recorded weekly per individual and when 50 % or more of the flowers or inflorescences were in a particular stage it was identified as dominant. Once all plants had completed their reproductive cycle and the growing season came to an end, all plants were harvested. Above-ground biomass was cut at soil level and individuals were stored in parchment bags and transported to the laboratory within 24 h, dried for 72 h at 80 °C and weighed.
Phenological variables
Eight phenological variables were derived from the weekly recordings: onset of budding, onset of flowering, onset of fruiting, midpoint of flowering, duration of budding, duration of flowering, duration of fruiting and total duration of all three phenophases combined. Onset of budding, flowering and fruiting were defined as the date (day of the year) when the first bud, flower or fruit was observed. Midpoint of flowering was defined as the average date when opened flowers or exerted anthers/styles (for forbs and grasses, respectively) were dominant. The duration of a phenophase was defined as the number of days between the onset of said phenophase and the dominance of the following phenophase.
Phenotypic plasticity in flowering phenology
The degree of phenotypic plasticity in response to warming and drought was calculated as a phenotypic plasticity index (Piv) (Valladares et al., 2006). This index was calculated as the difference between the maximum and the minimum mean value of a given trait and species over all treatments divided by the maximum mean, which serves to standardize the index ranging from 0 (no plasticity) to 1 (maximum plasticity). Note that plasticity was considered at the species level rather than at the genotype level to compare the degree of plasticity between mid- and high-elevation species.
Statistical analysis
To test treatment effects, a linear mixed-effect model was used for all eight phenological variables. ‘Elevation’ (mid- or high-elevation site), ‘drought’ (control or drought treatment), ‘origin’ of species (mid-elevation or high-elevation) and their respective interactions were computed as fixed effects. To account for variances between species, they were nested in their respective genus and computed as random effects. The effects of ‘elevation’ and/or ‘drought’ indicate trait variation due to different environmental conditions (i.e. phenotypic plasticity), while the ‘origin’ of species effect indicates differences between mid- and high-elevation species. The interaction between ‘origin’ of species and ‘elevation’ and/or ‘drought’ indicates a difference in the responses to treatment conditions between mid- and high-elevation species. Above-ground dry mass was used as a covariate to correct for size effects on phenology, but was removed as it did not change the results or add value to the model. All linear mixed-effect models were implemented with the ‘lmerTest’ package for R software (Kuznetsova et al., 2013), based on type 3 errors and Satterthwaite approximation for denominator degrees of freedom. Post-hoc Tukey’s HSD tests for multiple comparisons were performed using the ‘multcomp’ package (Hothorn et al., 2014) for R software.
To test for differences in the degree of phenotypic plasticity of flowering phenology between mid- and high-elevation species, the Piv calculated for each species was analysed with a paired Wilcoxon signed rank test. All the analyses were performed in R version 3.0.2 software (R Development Core Team, 2013; https://www.r-project.org/).
RESULTS
Many individuals died over winter, were subjected to herbivory or were not reproductive, leading to the total exclusion of four genera (Centaurea, Geum, Onobrychis and Trifolium) from the analysis. For the remaining species, an average of 8·3 replicates per treatment combination were included in the final analysis with a total of 667 individuals (i.e. 20 out of 28 initial species and approx. 50 % of the initial sample size). Mortality, however, was independent of species’ origin and treatment combinations (Fisher’s exact test for count data: P = 0·85). In 2013, the average temperature during the growing season differed by 4·4 °C between common gardens and on average the drought treatment reduced VSMC by 0·37 m3 m–3. These changes in abiotic conditions induced highly species-specific shifts in the onsets and durations of phenophases but important patterns emerged when groups of mid- and high-elevation species were considered. To enhance clarity, we first report results from the control treatment, describing the shifts in reproductive phenology in response to temperature for mid- and high-elevation species and second drought effects.
Transplantation effect
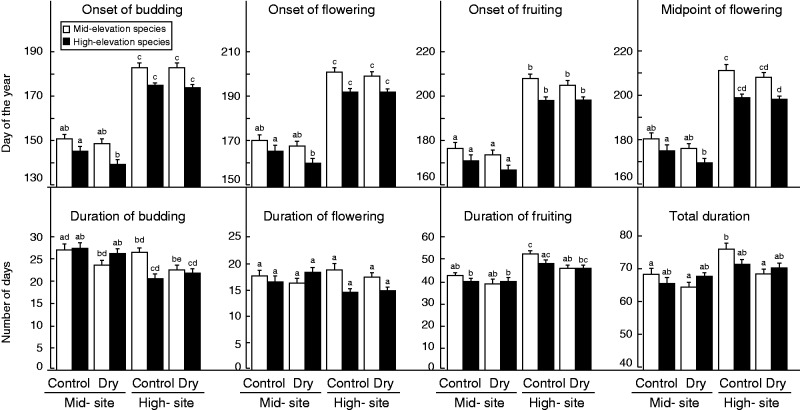
The reciprocal transplantation of species to a warmer or colder prospective climate induced major shifts in the time of initiation and the duration of reproductive phenology. The onsets of budding, flowering and fruiting were always initiated earlier at the low-elevation site by at least a month, but mid-and high-elevation species differed in their response to transplantations. While the differences between mid- and high-elevation species in phenological onsets were not always revealed by post-hoc multiple comparisons (Fig. 1), they were highly significant overall, as indicated by the significant interaction between elevation and origin treatments (Table 3; budding F = 7·64, P = 0·006; flowering F = 16·27, P < 10–4; fruiting F = 15·48, P < 10–4, respectively).
Fig. 1.
Responses of mid- and high-elevation species (as indicated in the key) to transplantational elevation and drought treatment (mean ± s.e.) in onset, midpoint and duration of phenophases. The average onsets of budding, fruiting and flowering and the midpoint of flowering are shown in absolute days of the year, while the average durations of phenophases are shown in number of days. The letters above each bar represent the results of post-hoc Tukey tests for multiple comparisons. While they often provide detailed information about the differences between treatment combinations, some interactions between main effects are not revealed by the analysis, although they are significant on average in the more powerful ANOVA.
Table 3.
Linear-mixed effect model for the responses of onsets and durations of phenological stages to the elevation (mid- vs. high-elevation site) and drought (control vs. dry) treatment, the origin of the species (mid- vs. high-elevation species) and their respective interactions; non-significant interactions were removed from the final model and significant P-values are shown in bold type*
| Onset of budding |
Onset of flowering |
Onset of fruiting |
Midpoint of flowering |
Duration of budding |
Duration of flowering |
Duration of fruiting |
Total duration |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| d.f. | F | P | d.f. | F | P | d.f. | F | P | d.f. | F | P | d.f. | F | P | d.f. | F | P | d.f. | F | P | d.f. | F | P | |
| Elevation | 1 | 1293·8 | <10–4 | 1 | 1206·9 | <10–4 | 1 | 1191·1 | <10–4 | 1 | 1099·4 | <10–4 | 1 | 37·63 | <10–4 | 1 | 2·29 | 0·13 | 1 | 44·87 | <10–4 | 1 | 11·53 | 0·0007 |
| Drought | 1 | 3·53 | 0·06 | 1 | 3·20 | 0·07 | 1 | 3·53 | 0·06 | 1 | 8·80 | 0·003 | 1 | 3·96 | 0·047 | 1 | 0·04 | 0·83 | 1 | 15·94 | <10–4 | 1 | 11·39 | 0·0008 |
| Origin | 1 | 2·62 | 0·14 | 1 | 2·97 | 0·12 | 1 | 3·57 | 0·10 | 1 | 4·30 | 0·07 | 1 | 0·06 | 0·81 | 1 | 1·80 | 0·22 | 1 | 0·22 | 0·65 | 1 | 0·00 | 0·95 |
| Elevation : drought | 1 | 7·55 | 0·006 | 1 | 7·15 | 0·008 | 1 | 3·92 | 0·048 | 1 | 6·51 | 0·01 | 1 | 0·06 | 0·81 | 1 | 0·15 | 0·70 | 1 | 3·45 | 0·06 | 1 | 4·80 | 0·03 |
| Elevation : origin | 1 | 7·64 | 0·006 | 1 | 16·27 | <10–4 | 1 | 15·48 | <10–4 | 1 | 29·47 | <10–4 | 1 | 6·03 | 0·01 | 1 | 3·73 | 0·54 | 1 | 0·00 | 1·00 | 1 | 0·48 | 0·49 |
| Drought : origin | 1 | 1·95 | 0·16 | 1 | 2·16 | 0·14 | 1 | 0·94 | 0·33 | 1 | 0·22 | 0·64 | 1 | 2·63 | 0·11 | 1 | 3·09 | 0·08 | 1 | 6·27 | 0·01 | 1 | 6·43 | 0·01 |
| Elevation : drought : origin | 1 | 0·15 | 0·70 | 1 | 0·83 | 0·36 | 1 | 0·49 | 0·49 | 1 | 0·22 | 0·64 | 1 | 1·61 | 0·21 | 1 | 0·75 | 0·39 | 1 | 0·03 | 0·85 | 1 | 0·25 | 0·62 |
*ANOVA was calculated on a total of 667 individuals including 20 species with an average number of 8·3 ± 2·85 replicates per treatment combination.
Indeed, high-elevation species consistently initiated the onset of budding, flowering and fruiting earlier than mid-elevation species, and these differences were particularly pronounced at the high-elevation site (Table 3; F = 7·64, P = 0·006; F = 16·27, P < 10–4; F = 15·48, P < 10–4, respectively). High-elevation species started budding 8·4 ± 2·2 d earlier than mid-elevation species when grown at the high-elevation site and 5·5 ± 2·2 d earlier when grown at the mid-elevation site (Fig. 1, Supplementary Data Table S1). High-elevation species also started flowering and fruiting earlier than mid-elevation species, with strongest responses at the high-elevation site (Fig. 1). The same was found for the midpoint of flowering, which was always reached earlier by high-elevation species relative to their lower elevation congeners, especially at the high-elevation site (Table 3; F = 29·47, P < 10–4). The midpoint of flowering was recorded 12·9 ± 2·3 d earlier for high-elevation species grown at the high-elevation site and 5·7 ± 2·6 d earlier when grown at the mid-elevation site (Fig. 1, Table S1).
Furthermore, differences in responses between mid- and high-elevation species were also revealed in the fact that advancement of the onset of phenophases in response to transplantation between sites was consistently greater for mid-elevation species relative to high-elevation species (again, not revealed by post-hoc tests). For mid-elevation species, the onset of budding at the mid- and the high-elevation sites differed by 32·5 ± 2·0 d, whereas for high-elevation species the difference was less (29·5 ± 2·1 d; Fig. 1). Similar trends were found for the onset of flowering and fruiting. The differences in responses between mid- and high-elevation species were particularly pronounced for advancement in midpoint of flowering. Going from the 2000 -m site down to the 1050 -m site, mid-elevation species advanced midpoint of flowering by 31·2 ± 2·4 d (Table 3; F = 29·47, P < 10–4), whereas high-elevation species advanced this stage by only 23·7 ± 2·2 d (Fig. 1, Supplementary Data Table S1).
The duration of phenophases responded to transplantations, with the exception of the duration of flowering (Table 3). A significant interaction between elevation and origin was found for the duration of budding (Table 3; F = 6·03, P = 0·01), indicating a difference in response between mid- and high-elevation species. The duration of budding was generally shortened at the high-elevation site compared with the mid-elevation site, but this was significant only for high-elevation species, for which a contraction of 6·8 ± 1·1 d was recorded (Fig. 1, Table S1). For mid-elevation species, by contrast, this contraction was only of 0·5 ± 1·4 d. The duration of fruiting was significantly shorter at the mid-elevation site for both mid- and high-elevation species (Table 3; F = 44·87, P < 10–4). The maturation of fruits took 9·8 ± 1·3 d less at the mid-elevation site compared with the high-elevation site for mid-elevation species, and 8·1 ± 1·3 d less for high-elevation species (Fig. 1, Table S1).
Note that mid-elevation species had a particularly long duration of fruiting when grown at high elevation (Fig. 1). The total duration of reproductive phenology was also shortened at the mid-elevation site. However, this effect was only significant for mid-elevation species, which had a 7·5 ± 1·6-d shorter duration of reproduction when grown at the lower site. The effects of transplantation on the total duration of reproductive phenology were similar to those on the duration of fruiting (Fig. 1), reflecting that this last stage was proportionally the longest.
Finally, it is important to note that at the mid-elevation common garden, all reproductive individuals from mid- and high-elevation species reached the final fruit maturation stage (defined as 50 % or more flowers of one individual having reached stage 7: dehisced fruits for forbs and stage 5: disarticulated seeds for grasses). At the high-elevation common garden, 99 % of high-elevation species finished fruit maturation but only 85 % of mid-elevation individuals reached the final fruit maturation stage before final harvest.
Drought effect
Drought had a tendency to advance phenophases, but had the greatest effect at the low-elevation site. Drought consistently led to smaller advancement of phenophases than did transplantation to the warmer site (Fig. 1). Effects of drought on onset of budding, flowering, fruiting and midpoint of flowering varied depending on whether plants were grown at the mid- or high-elevation sites, as indicated by a significant interaction between elevation and drought (Table 3; E × D for budding P = 0·006; flowering P = 0·008; fruiting P = 0·048; and flowering mid-point P = 0·01). Drought initiated earlier phenophases at both sites but this effect was significant only at the mid-elevation site for high-elevation species (Fig. 1). At the mid-elevation site, drought-stressed high-elevation species initiated budding 6·1 ± 2·1 d earlier than individuals under control conditions, while drought-stressed mid-elevation species started budding only 2·1 ± 2·3 d earlier. In contrast, at high elevation, the onset of budding was only marginally advanced in the drought treatment, namely by 0·2 ± 1·7 d for mid-elevation species and by 0·9 ± 1·1 d for high-elevation species (Fig. 1, Table S1). The same results were found for the onset of flowering and fruiting, and for the midpoint of flowering (Table 3), although the difference between mid- and high-elevation species was not revealed by post-hoc comparisons for the onset of flowering (Fig. 1).
The durations of phenophases were unequally affected by drought and only the duration of flowering did not change in response to drought (Table 3, Fig. 1). The duration of budding was significantly shorter on average under dry conditions (Table 3; F = 3·96, P = 0·047), but this was not revealed by the post-hoc multiple comparisons (Fig. 1). For mid-elevation species, drought reduced the duration of budding by 3·3 ± 1·3 d at the mid-elevation site, and by 3·8 ± 1·2 d at the high-elevation site. This effect was less pronounced and less consistent in high-elevation species (Fig. 1, Table S1).
While the duration of fruiting was also generally shortened by drought at the lower site, a significant interaction between drought and origin was found (Table 3; F = 6·27, P = 0·01) meaning that mid- and high-elevation species responded differently to the drought treatment. At the lower site, drought affected the duration of fruiting only marginally for mid- and high-elevation species (Fig. 1). By contrast, at the high-elevation site, the duration of fruiting was significantly shortened by 6·4 ± 1·4 d for mid-elevation species under drought stress but only marginally by 2·1 ± 1·3 d for high-elevation species (Fig. 1, Table S1).
For the total duration, a significant interaction was found between drought and elevation, as well as between drought and origin (Table 3, F = 4·8, P = 0·03; F = 6·3, P = 0·01, respectively). Drought-induced shifts in the total duration of reproductive phenology were more pronounced at the high-elevation site than at the mid-elevation site and in mid-elevation species compared with high-elevation species. Drought significantly shortened the total duration of reproduction for mid-elevation species when growing at the high-elevation site, namely by 7·5 ± 1·7 d, but this effect was only marginal for mid-elevation species when grown at the lower elevation site and for high-elevation species at both sites (Fig. 1).
Piv of mid- and high-elevation species
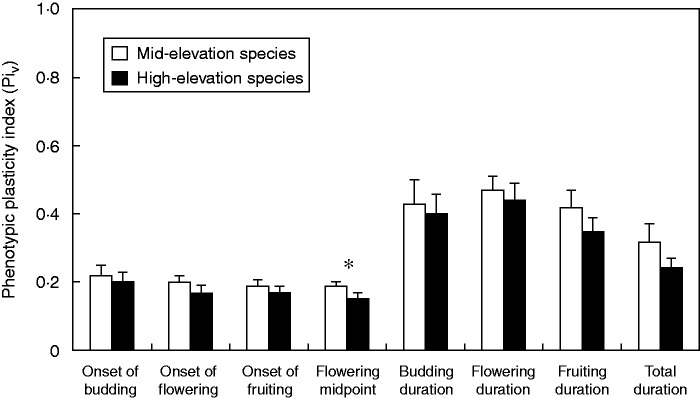
A significant difference in the Piv was found for the midpoint of flowering (Fig. 2; V = 40, P = 0·03). Mid-elevation species had a greater Piv than high-elevation species, indicating that the shift in midpoint of flowering in response to elevation and drought was greater for mid-elevation species than for high-elevation species (0·19 ± 0·04 and 0·15 ± 0·05, respectively). These results are consistent with the previously reported shifts in number of days. Furthermore, as the Piv of mid-elevation species was systematically greater (Fig. 2) we also compared the mean Piv across all traits between mid- and high-elevation species and found a significantly higher mean value for mid-elevation species (PivMid = 0·31 ± 0·1, PivHigh = 0·26 ± 0·1; V = 36, P = 0·01). This overall result indicates that mid-elevation species tended to have a greater degree of phenotypic plasticity in their reproductive phenology than high-elevation species and hence a greater capacity to adjust these traits to environmental changes in temperature and water availability.
Fig. 2.
Phenotypic plasticity index (Piv) of mid- and high-elevation species (as indicated in the key) calculated across all treatments for onsets and durations of phenophases. The error bars denote s.e. *P < 0·05.
DISCUSSION
Responses to transplantation and drought
Transplantation of high-elevation species to a site with earlier springtime resulted in advanced onset of reproductive phenology, an overall pattern in agreement with existing literature (Price and Waser, 1998; Dunne et al., 2003; Scheepens and Stöcklin, 2013). Mid- and high-elevation species initiated all reproductive phenophases approx. 1 month earlier at the lower elevation site, indicating the important role of temperature for phenophases. Interestingly, high-elevation species initiated budding prior to mid-elevation species at both sites, on average by 10 d at the high site and by 5 d at the low site. In contrast, other studies found that reproduction was always initiated first by low-elevation populations at the low-elevation sites (Haggerty and Galloway, 2011; Frei et al., 2014a, b). However, in those studies, experimental gardens were situated at lower elevations relative to our study sites (at 514 and 600 m compared with ours at 1050 m). This resulted in high-elevation populations in prior studies being exposed earlier in the year to days with higher temperatures, yet relatively shorter photoperiods than in our study, and may have driven the observed differences in results among our studies. As photoperiod is also a fundamental cue for a frost risk-free initiation of growth and reproduction for some alpine plants (Keller and Körner, 2003; Körner, 2003; Basler and Koerner, 2012), it is likely that high-elevation populations in the prior studies waited for days with a sufficiently long photoperiod and did not rely solely on temperature to initiate reproduction. However, in our study, photoperiod was similar between both common gardens at the time of reproductive onset. Hence, advanced initiation of reproductive phenology in high-elevation species at both of our study sites probably reflects other adaptations to cold climates and short growing seasons, for example low growing degree day requirements (Haggerty and Galloway, 2011) and preformation of buds (Sørensen, 1941; Billings and Mooney, 1968; Bliss, 1971).
At the mid-elevation site, most phenophases were shortened, which is in agreement with previous studies (Sherry et al., 2007; Post et al., 2008; Steltzer and Post, 2009). However, the duration of budding was longer at the lower, warmer site relative to the high-elevation site, which highlights the contrasting effects of warming on individual phenophases (Post et al., 2008; Haggerty and Galloway, 2011; Cornelius et al., 2013). Contracted phenophases have generally been explained as resulting from increased developmental rates in warm conditions (Sherry et al., 2007; Haggerty and Galloway, 2011). Alternatively, extended reproductive durations are often linked to an expanded growing season (Dunne et al., 2003). In our study, the average daily temperature during the budding phase was higher at the mid- than at the high-elevation site (14·1 and 12·3 °C, respectively). Thus, temperature alone cannot explain expanded budding duration, which is in contradiction with fast developmental rates expected under warm conditions. This result might be related mainly to the fact that high-elevation species significantly contracted this phenophase at high-elevation sites (Fig. 1) to guarantee sufficient time for flowering and fruit maturation, but it is also possible that plants tried to take advantage of a longer growing season at the lower site with advanced spring. The duration of fruiting was, however, highly accelerated for both groups of species by fast maturation rates under higher temperatures. As this last stage was proportionally the longest it resulted in a shorter total reproductive duration at the low-elevation site, which suggests that plants were not able to consistently prolong their reproductive cycle to take advantage of a longer growing season.
Limited water availability had considerable effects on plant reproductive phenology but drought-induced shifts were less extensive than those in response to temperature changes (shifts in the order of magnitude of a few days against a month, respectively). However, when drought stress was combined with higher temperatures, it generally emphasized the responses of species and consistently led to further advancements and shortenings of phenophases for mid-elevation species in responses to drought. This result is in line with a 4-d advancement in mid-flowering date recorded after a simulated drought in Central Europe (Jentsch et al., 2009) and with a study which revealed that an ‘escape strategy’ inducing earlier flowering was selected for in Brassica rapa following a natural drought (Franks et al., 2007; Franks, 2011). In our study, species responded to drought by plastic shifts congruent with such an ‘escape strategy’. When the growing season is shortened by drought, plants with late reproductive initiations might be unable to mature seeds before conditions become lethal. Hence, when water availability is limited, a shift towards rapid development and maturation of flowers is advantageous and allows the maintenance of reproductive success (Vasek and Sauer, 1971; Franks, 2011).
Mid- and high-elevation species generally advanced phenophases in response to drought but changes in the duration of phenophases were less pronounced in high-elevation species. While the total duration of reproductive phenology was mainly shortened for high-elevation species, a slight extension of budding and of fruiting was recorded at high- and mid-elevation sites, respectively. This result highlights the divergent effects of drought on certain phenophases (Peñuelas et al., 2004; Llorens and Peñuelas, 2005). In our case, high-elevation species are normally less exposed to drought periods than their congeners from lower elevations (Vasek and Sauer, 1971). Precipitation tends to increase with elevation and evapo-transpiration tends to decrease with elevation. Accordingly soil moisture availability generally increases with elevation (Körner, 2003). Consequently, the inconsistent responses of high-elevation species to drought at both sites suggest that although high-elevation species also tended towards an ‘escape strategy’ when facing drought, they might be less efficient in doing so then their mid-elevation congeners.
Constrained degree of phenotypic plasticity in high-elevation species
In line with our hypothesis, the differences between herbaceous mid- and high-elevation species affected their potential for phenological plasticity, as previously found for low- and high-elevation populations of deciduous tree species (Vitasse et al., 2013). Our results revealed that herbaceous high-elevation species tended to have a lower Piv than mid-elevation species for flowering phenology even though this difference was only significant for the midpoint of flowering and when averaged over all phenological variables. Nevertheless, both mid- and high-elevation species showed a notable capacity of tracking environmental changes through phenological shifts while maintaining a high performance. It is particularly interesting that high-elevation species were found to have a lower Piv specifically for the midpoint of flowering. The exact timing of flowering might be the most crucial phenophase for successful reproduction. The timing of flowering is even more crucial in cold environments, where short growing seasons (Billings and Mooney, 1968; Körner, 2003) and adverse conditions such as frost events (Inouye, 2008) pose additional challenges to reproductive success. Consequently, strong directional selection decreasing temperature sensitivity and increasing photoperiodic control (Basler and Koerner, 2012; Vitasse et al., 2013) may have shaped the evolution of reproductive phenology of high-elevation species to coincide with favourable environmental conditions, presumably contributing to local adaptation in heterogeneous landscapes (Hall and Willis, 2006; Verhoeven et al., 2008; Anderson et al., 2011).
The selective pressures controlling timing of reproduction become increasingly strong with elevation and thus we hypothesize that the difference in phenological plasticity would have been more pronounced if more strictly alpine species, from above treeline-elevation, had been chosen. This would have provided a more extreme contrast with congeneric mid-elevation species. Here, our results indicate that adaptation to short growing seasons in the alpine environment limits the potential for phenotypic plasticity in the reproductive phenology of high-elevation species in response to environmental changes, leading to a higher genetic canalization of the timing of peak flowering (Price et al., 2003; Pigliucci et al., 2006; Ghalambor et al., 2007).
Consequences of phenological shifts
For high-elevation species, transplantation to a lower elevation resulted in advanced phenophases, suggesting adaptive tracking of an advanced growing season (Cleland et al., 2012). However, higher temperatures also accelerated developmental rates and led to shortened phenophases, indicating that high-elevation plants were unable to take advantage of a longer growing season. Furthermore, in advanced growing seasons, the time frame for resource acquisition is abbreviated before environmental cues initiate reproduction. Consequently, advanced flowering could potentially lead to decreased fitness (Post et al., 2008; Scheepens and Stöcklin, 2013).
Alternatively, for mid-elevation species, the upward transplantation resulted in delayed initiation and prolonged phenophases. While the later initiation of reproduction at the higher site might be adaptive, the prolonged phenophases suggest an entirely passive response to slower developmental rates in cold conditions (Sherry et al., 2007). At the final harvest in late autumn 15 % of mid-elevation plants had not yet started to disperse their seeds and we estimate that in total approx. 30 % of flowers from mid-elevation species would not have completed fruit maturation (E. Hamann, pers. obs.). A prolonged reproductive period of upward migrated mid-elevation species could thus have fitness costs if associated with uncompleted seed maturation before winter fall.
Limited water availability advanced and shortened phenophases, a result congruent with the aforementioned ‘escape strategy’ limiting the negative impact of drought stress on plant fitness (Franks, 2011). However, drought-induced phenological shifts were greater for mid-elevation species, suggesting that they were more capable of adopting an efficient ‘escape strategy’ than their high-elevation congeners. Phenotypic plasticity has been suggested to be adaptive only when the environmental fluctuations experienced by populations do not fall outside their native range (Ghalambor et al., 2007). While mid-elevation species are frequently exposed to dry summer periods, high-elevation species have rarely experienced such environmental conditions in the past (Körner, 2003), which could explain why they were unable to produce an ‘escape strategy’ as efficient as their mid-elevation congeners.
We conclude that while the direction of plastic responses in reproductive phenology tended to track environmental changes, adaptation of species to their native range seem to constrain adaptive plasticity in novel conditions and could potentially lead to maladaptive responses (Ghalambor et al., 2007).
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Figure S1: schematic overview of the experimental design. Table S1: average day of the year of initiation and midpoint of phenological stages, and durations of phenophases reported for mid- and high-elevation species and treatment combinations.
ACKNOWLEDGEMENTS
We thank Samuel Schmid and Michael Scherer-Lorenzen from the Agroscope, Switzerland, for the loan of the rain-shelters. We also thank the Schynige Platte Alpine Botanical Garden, Sophie Schmid, Georg Armbruster and Guy Villaume for technical support. We thank one anonymous reviewer and the handling editor for thorough revisions that considerably improved the manuscript. This work was supported by the Swiss National Science Foundation (project no. 3100A-135611).
LITERATURE CITED
- Abu-Asab MS, Peterson PM, Shetler SG, Orli SS. 2001. Earlier plant flowering in spring as a response to global warming in the Washington, DC, area. Biodiversity and Conservation 10: 597–612. [Google Scholar]
- Aeschimann L, Lauber K, Moser DM, Theurillat JP. 2004. Flora alpina. Bern: Haupt. [Google Scholar]
- Anderson JT, Willis JH, Mitchell-Olds T. 2011. Evolutionary genetics of plant adaptation. Trends in Genetics 27: 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JT, Inouye DW, McKinney AM, Colautti RI, Mitchell-Olds T. 2012. Phenotypic plasticity and adaptive evolution contribute to advancing flowering phenology in response to climate change. Proceedings of the Royal Society B-Biological Sciences 279: 3843–3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basler D, Koerner C. 2012. Photoperiod sensitivity of bud burst in 14 temperate forest tree species. Agricultural and Forest Meteorology 165: 73–81. [Google Scholar]
- Beniston M, Rebetez M, Giorgi F, Marinucci MR. 1994. An analysis of regional climate change in Switzerland. Theoretical and Applied Climatology 49: 135–159. [Google Scholar]
- Beniston M, Diaz HF, Bradley RS. 1997. Climatic change at high-elevation sites: an overview. Climatic Change 36: 233–251. [Google Scholar]
- Bernal M, Estiarte M, Penuelas J. 2011. Drought advances spring growth phenology of the Mediterranean shrub Erica multiflora. Plant Biology 13: 252–257. [DOI] [PubMed] [Google Scholar]
- Billings WD, Mooney HA. 1968. Ecology of arctic and alpine plants. Biological Reviews of the Cambridge Philosophical Society 43: 481–529. [Google Scholar]
- Bliss LC. 1971. Arctic and alpine plant life cycles. Annual Review of Ecology and Systematics 2: 405–438. [Google Scholar]
- Bloor JMG, Pichon P, Falcimagne R, Leadley P, Soussana J-F. 2010. Effects of warming, summer drought, and CO2 enrichment on aboveground biomass production, flowering phenology, and community structure in an upland grassland ecosystem. Ecosystems 13: 888–900. [Google Scholar]
- Cleland EE, Chuine I, Menzel A, Mooney HA, Schwartz MD. 2007. Shifting plant phenology in response to global change. Trends in Ecology & Evolution 22: 357–365. [DOI] [PubMed] [Google Scholar]
- Cleland EE, Allen JM, Crimmins TM, et al. 2012. Phenological tracking enables positive species responses to climate change. Ecology 93: 1765–1771. [DOI] [PubMed] [Google Scholar]
- Cornelius C, Leingartner A, Hoiss B, Krauss J, Steffan-Dewenter I, Menzel A. 2013. Phenological response of grassland species to manipulative snowmelt and drought along an altitudinal gradient. Journal of Experimental Botany 64: 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne JA, Harte J, Taylor KJ. 2003. Subalpine meadow flowering phenology responses to climate change: integrating experimental and gradient methods. Ecological Monographs 73: 69–86. [Google Scholar]
- Fitter AH, Fitter RSR. 2002. Rapid changes in flowering time in British plants. Science 296: 1689–1691. [DOI] [PubMed] [Google Scholar]
- Franks SJ. 2011. Plasticity and evolution in drought avoidance and escape in the annual plant Brassica rapa. New Phytologist 190: 249–257. [DOI] [PubMed] [Google Scholar]
- Franks SJ, Sim S, Weis AE. 2007. Rapid evolution of flowering time by an annual plant in response to a climate fluctuation. Proceedings of the National Academy of Sciences of the United States of America 104: 1278–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei ER, Ghazoul J, Matter P, Heggli M, Pluess AR. 2014a. Plant population differentiation and climate change: responses of grassland species along an elevational gradient. Global Change Biology 20: 441–455. [DOI] [PubMed] [Google Scholar]
- Frei ER, Ghazoul J, Pluess AR. 2014b. Plastic responses to elevated temperature in low and high elevation populations of three grassland species. PLoS ONE 9: e98677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghalambor CK, McKay JK, Carroll SP, Reznick DN. 2007. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Functional Ecology 21: 394–407. [Google Scholar]
- Haggerty BP, Galloway LF. 2011. Response of individual components of reproductive phenology to growing season length in a monocarpic herb. Journal of Ecology 99: 242–253. [Google Scholar]
- Hall MC, Willis JH. 2006. Divergent selection on flowering time contributes to local adaptation in Mimulus guttatus populations. Evolution 60: 2466–2477. [PubMed] [Google Scholar]
- Hartmann DL, Klein Tank AMG, Rusticucci M, et al. 2013. Observations: atmosphere and surface. In: Stocker TF, Qin D, Plattner GK, et al, eds. Climate Change 2013: The Physical Sciences Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press. [Google Scholar]
- Hothorn T, Bretz F, Westfall P, Heiberger RM, A. S. 2014. Simultaneous inference in general parametrics models. Biometrical Journal 50: 346–363. [DOI] [PubMed] [Google Scholar]
- Hudson JMG, Henry GHR, Cornwell WK. 2011. Taller and larger: shifts in Arctic tundra leaf traits after 16 years of experimental warming. Global Change Biology 17: 1013–1021. [Google Scholar]
- Inouye DW. 2008. Effects of climate change on phenology, frost damage, and floral abundance of montane wildflowers. Ecology 89: 353–362. [DOI] [PubMed] [Google Scholar]
- Jentsch A, Kreyling J, Boettcher-Treschkow J, Beierkuhnlein C. 2009. Beyond gradual warming: extreme weather events alter flower phenology of European grassland and heath species. Global Change Biology 15: 837–849. [Google Scholar]
- Keller F, Körner C. 2003. The role of photoperiodism in alpine plant development. Arctic Antarctic and Alpine Research 35: 361–368. [Google Scholar]
- Körner C. 2003. Alpine plant life: functional plant ecology of high mountain ecosystems. Berlin: Springer. [Google Scholar]
- Kovats RS, Valentini R, Bouwer LM, et al. 2014. Europe. In: Barros VR, Field CB, Dokken DJ, eds. Climate Change 2014: Impacts, Adaptation, and vulberability. Part B: Regional Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press. [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen RHB. 2013. lmerTest: Tests for random and fixed effects for linear mixed effect models (lmer objects of lme4 package). http://cran.r-project.org/package=lmerTest. [Google Scholar]
- Lauber K, Wagner G. 2001. Flora Helvetica. Bern: Haupt. [Google Scholar]
- Levitt J. 1980. Responses of plants to environmental stress. New York: Academic Press. [Google Scholar]
- Llorens L, Peñuelas J. 2005. Experimental evidence of future drier and warmer conditions affecting flowering of two co-occurring Mediterranean shrubs. International Journal of Plant Sciences 166: 235–245. [Google Scholar]
- Memmott J, Craze PG, Waser NM, Price MV. 2007. Global warming and the disruption of plant–pollinator interactions. Ecology Letters 10: 710–717. [DOI] [PubMed] [Google Scholar]
- Menzel A, Sparks TH, Estrella N, et al. 2006. European phenological response to climate change matches the warming pattern. Global Change Biology 12: 1969–1976. [Google Scholar]
- MeteoSwiss. 2014. Federal Office of Meteorology and Climatology. http://www.meteoswiss.admin.ch/. Last accessed 3 June 2015. [Google Scholar]
- Nicotra AB, Atkin OK, Bonser SP, et al. 2010. Plant phenotypic plasticity in a changing climate. Trends in Plant Science 15: 684–692. [DOI] [PubMed] [Google Scholar]
- Parmesan C, Yohe G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421: 37–42. [DOI] [PubMed] [Google Scholar]
- Peñuelas J, Filella I, Zhang XY, et al. 2004. Complex spatiotemporal phenological shifts as a response to rainfall changes. New Phytologist 161: 837–846. [DOI] [PubMed] [Google Scholar]
- Pigliucci M, Murren CJ, Schlichting CD. 2006. Phenotypic plasticity and evolution by genetic assimilation. Journal of Experimental Biology 209: 2362–2367. [DOI] [PubMed] [Google Scholar]
- Post ES, Pedersen C, Wilmers CC, Forchhammer MC. 2008. Phenological sequences reveal aggregate life history response to climatic warming. Ecology 89: 363–370. [DOI] [PubMed] [Google Scholar]
- Price MV, Waser NM. 1998. Effects of experimental warming on plant reproductive phenology in a subalpine meadow. Ecology 79: 1261–1271. [Google Scholar]
- Price TD, Qvarnstrom A, Irwin DE. 2003. The role of phenotypic plasticity in driving genetic evolution. Proceedings of the Royal Society of London Series B-Biological Sciences 270: 1433–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathcke B, Lacey EP. 1985. Phenological patterns of terrestrial plants. Annual Review of Ecology and Systematics 16: 179–214. [Google Scholar]
- Richter S, Kipfer T, Wohlgemuth T, Guerrero CC, Ghazoul J, Moser B. 2012. Phenotypic plasticity facilitates resistance to climate change in a highly variable environment. Oecologia 169: 269–279. [DOI] [PubMed] [Google Scholar]
- Scheepens JF, Stöcklin J. 2013. Flowering phenology and reproductive fitness along a mountain slope: maladaptive responses to transplantation to a warmer climate in Campanula thyrsoides. Oecologia 171: 679–691. [DOI] [PubMed] [Google Scholar]
- Shaw RG, Etterson JR. 2012. Rapid climate change and the rate of adaptation: insight from experimental quantitative genetics. New Phytologist 195: 752–765. [DOI] [PubMed] [Google Scholar]
- Sherry RA, Zhou XH, Gu SL, et al. 2007. Divergence of reproductive phenology under climate warming. Proceedings of the National Academy of Sciences of the United States of America 104: 198–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen TJ. 1941. Temperature relations and phenology of the northeast Greenland flowering plants. Meddelelser om Grønland 125: 1–305. [Google Scholar]
- Steltzer H, Post E. 2009. Seasons and life cycles. Science 324: 886–887. [DOI] [PubMed] [Google Scholar]
- Theurillat JP, Guisan A. 2001. Potential impact of climate change on vegetation in the European Alps: a review. Climatic Change 50: 77–109. [Google Scholar]
- Valladares F, Sanchez-Gomez D, Zavala MA. 2006. Quantitative estimation of phenotypic plasticity: bridging the gap between the evolutionary concept and its ecological applications. Journal of Ecology 94: 1103–1116. [Google Scholar]
- Vasek FC, Sauer RH. 1971. Seasonal progression of flowering in Clarkia. Ecology 52: 1038–1045. [Google Scholar]
- Verhoeven KJF, Poorter H, Nevo E, Biere A. 2008. Habitat-specific natural selection at a flowering-time QTL is a main driver of local adaptation in two wild barley populations. Molecular Ecology 17: 3416–3424. [DOI] [PubMed] [Google Scholar]
- Visser ME. 2008. Keeping up with a warming world; assessing the rate of adaptation to climate change. Proceedings of the Royal Society B-Biological Sciences 275: 649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitasse Y, Hoch G, Randin CF, et al. 2013. Elevational adaptation and plasticity in seedling phenology of temperate deciduous tree species. Oecologia 171: 663–678. [DOI] [PubMed] [Google Scholar]
- Walther GR, Post E, Convey P, et al. 2002. Ecological responses to recent climate change. Nature 416: 389–395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.